Introduction
Following initial tests in humans at the United Imaging Healthcare (UIH) factory in Shanghai and receipt of FDA 510(k) clearance, a UIH uEXPLORER Total-body (TB) PET/CT scanner was installed at the EXPLORER Molecular Imaging Center (EMIC), UC Davis in May 2019. EMIC is a satellite imaging and research clinic located approximately 1.5 miles from the UC Davis Medical Center (UCDMC). UCDMC contains a National Cancer Institute-designated Comprehensive Cancer Center, two medical cyclotrons and a commercial radiopharmaceutical production facility (PETNET), a specialized not-for-profit radiopharmaceutical production facility (Optimal Tracers) and a well-equipped radiochemistry research laboratory (the Sutcliffe lab). In the Spring of 2019, the existing clinical PET operation at UCDMC consisted of two standard PET/CT scanners providing oncologic, cardiac and neurological PET imaging services, with a volume of approximately 20 cases per day (of which approximately 8 were cardiac studies). The UCDMC patient enrollment area is large and extends from the Central Valley to the greater Northern California region outside the San Francisco Bay Area.
EMIC was created with the objective to provide both clinical and research imaging services to UC Davis and to the community, with the intention of allocating equal scanner time to the clinical and research missions. This paper will focus on the clinical implementation of TB PET/CT at EMIC (Fig.1)
Figure 1:
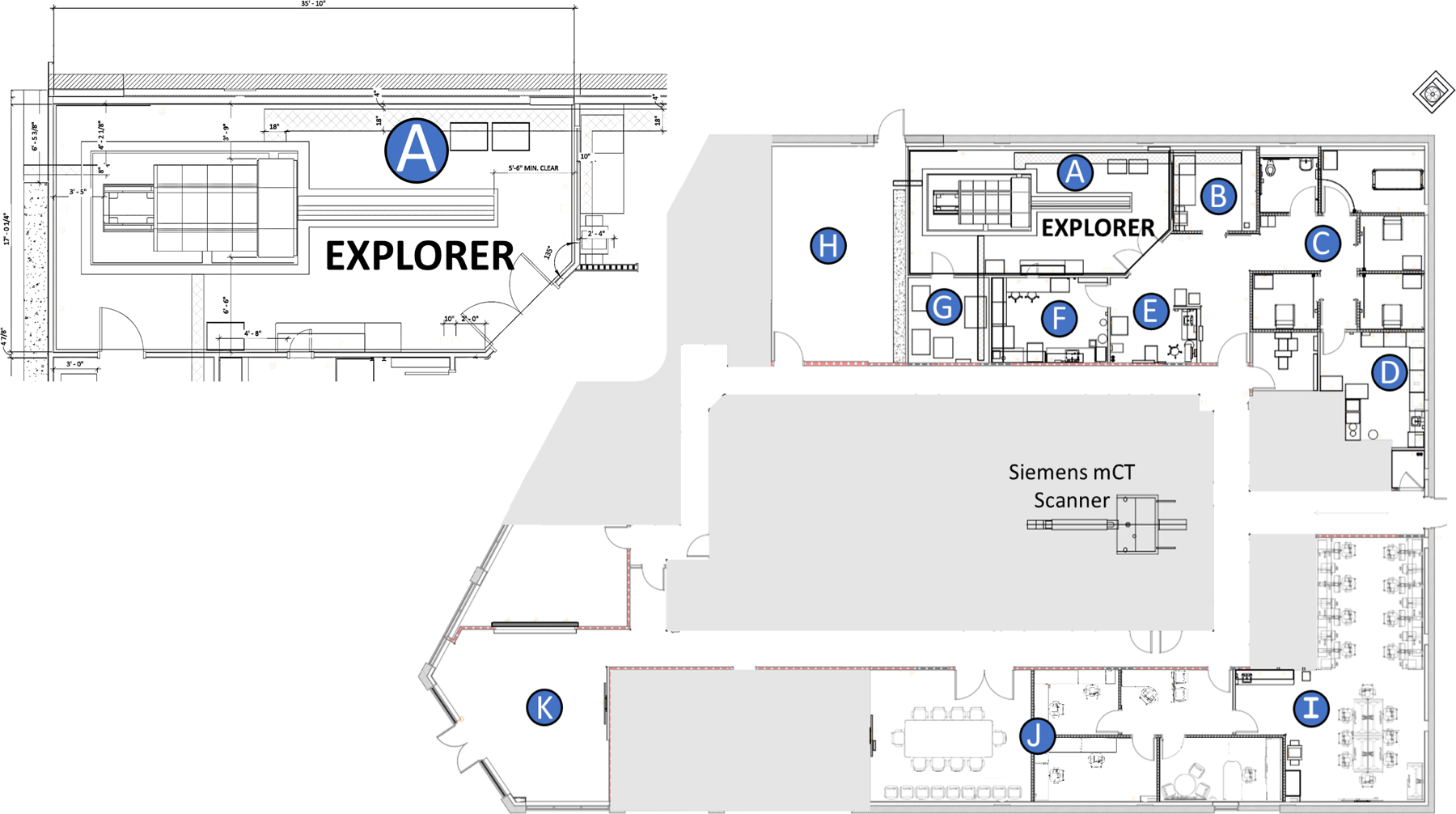
EXPLORER Molecular Imaging Center Site Layout. A) EXPLORER scanner room, B) Scanner control room, C) Uptake and Toilet, D) Radiopharmaceutical (Hot) lab, E) Nurse & Technologist Workstation, F) Metabolite Lab, G) Computing hardware room, H) Future Cyclotron Room, I) Research Area & Viewing Stations, J) Conference Room and Office Space, K) Patient Reception & Waiting Room. The gray area outlines the affiliated imaging center (Northern California PET Imaging Center) housing a Siemens mCT PET/CT scanner
Results from the initial tests in humans had demonstrated that this scanner was capable of delivering exceptional image quality with 18F-FDG, with high resolution and low noise1. Our clinical implementation philosophy evolved through extensive discussions, but we converged on a view that since EMIC had a rare opportunity to implement protocols that could deliver unprecedented image quality for clinical purposes, that we should do so. This presented both an opportunity and a risk for clinical implementation, since our radiologists could not have had prior experience in reading such high-fidelity PET images before. This is of course a persistent issue in the field of radiology due to the constant development of more sophisticated tools, e.g., the case of small pulmonary embolism detected with high resolution computed tomography2. In many ways, our challenge echoed that of the early pioneers of clinical PET in the late 1980’s and early 1990’s, although, of course, the basics of FDG-PET imaging were far better understood in 2019 than they were thirty years before that. Similar to what Maisey3 did when implementing clinical PET for the first time in the UK, we used a team approach, involving nuclear medicine physicians, radiologists, physicists, technologists and oncologists to develop the protocols and to understand the results 3.
18F-FDG: Studies in healthy subjects
Our first human imaging studies were performed on healthy subjects who had given informed consent under an IRB approved protocol. This protocol had multiple arms and was aimed at providing preliminary data to guide future research studies, but the arm of most interest to the development of our clinical protocols involved injecting 370 MBq (10 mCi) of 18F-FDG, performing an initial dynamic scan for 60 minutes, and following it with 20 minute scans at 1.5 h, 3 h, 6 h, 9 h and 12 h post-injection (Fig 2). The data acquired from the first subjects enrolled enabled us to develop our initial image reconstruction protocols, as well as allowing us to gain initial understanding of issues relating to minimization of patient motion 4 and how to perform bolus injections given the constraints of the long gantry 5. In particular, we learned that while the count density in the data supported image reconstructions with isotropic voxels of size of approximately 1 mm, handling these very large PET datasets, with their corresponding CT datasets, was a significant challenge to our data bandwidth capabilities and our PACS workstations. We also found that the expected significant reduction in blood pool activity at 3 h post-injection resulted in markedly clear images with high contrast, and without a major noise penalty. Correlation of results from human subjects data with additional experiments performed on large cylindrical phantoms 6 suggested that injecting a somewhat smaller amount of activity might achieve a better trade-off between effective dose and data quality at 1 h post-injection.
Figure 2:
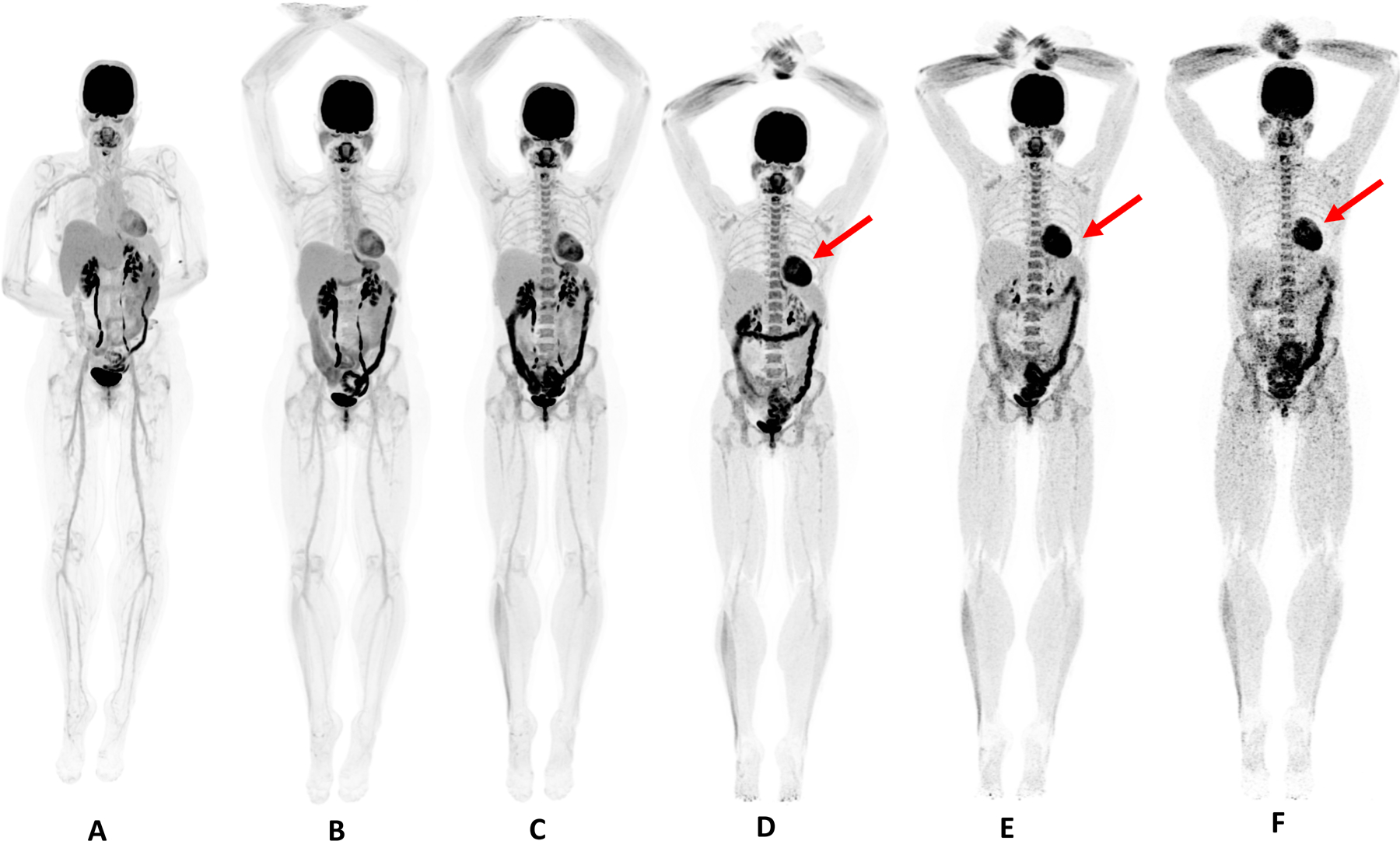
51 year-old female, healthy volunteer. Dynamic scan was acquired for 60 minutes after injection of 370 MBq of 18F-FDG. A) Reconstruction of the last 20 minutes of dynamic acquisition; B, C, D, E, F are 20 minute duration scans obtained at 90 min, 3, 6, 9 and 12 hours post-injection. Patient was fed with high-protein snack after the 3 hour scan; this explains the more intense myocardial uptake (red arrow) on the following scans.
Interactions with Oncologists
We used a gradual approach to the clinical implementation of TB PET/CT. The first step involved educating the clinical oncologists in the advantages and possible risks of performing TB PET/CT with our high-performance protocols. Our understanding of the risks primarily involved the possibility of false positive results due to the increased potential for detection of smaller or milder FDG uptake foci than might be seen on conventional PET/CT, the significance of which might be open to misinterpretation. We then worked with a select team of providers who were allowed to order TB PET/CT scans, but only after a discussion between the radiologist and patient. In addition, all the initial cases were presented and further discussed at interdisciplinary conferences where the images were closely scrutinized.
At this point, the referring physicians appeared to divide into two groups – those who were enthusiastic about the image quality, and those who were concerned about decreased confidence in interpretation due to the high level of detail that the images provided. Again, this is a common problem encountered as radiological instrumentation and methods improve, and we heard similar stories from colleagues who had been involved in the early adoption of the prior generation of high-performance PET scanners. Both positions have merit and we forged an approach that was a synthesis of optimism tempered by caution and humility. As a matter of caution, we stress the possibility of encountering the risk of false positives during discussions with the referring physicians, in particular when selecting patients to be scanned with TB PET/CT. The direct and continuous collaboration between the radiologists and the oncologists mitigates this risk to acceptable levels for both parties. On the positive side, we note that we are much less prone to error due to the significant reduction in image noise which improves confidence in regions such as the liver. Furthermore, we find ourselves able to more confidently characterize small lesions with CT correlates as benign or malignant.
After approximately 100 clinical cases, we felt comfortable enough to expand the pool of referring physicians, but we still insist on a direct conversation between the referring physician and a radiologist before accepting a new referral.
Moving forward, we have begun a National Cancer Institute-funded clinical study to directly compare TB PET/CT with conventional PET/CT in the initial staging of patients with a range of different cancers. In our early results, we find, as expected, that the difference in image quality is astonishing, but the aim of the study is to determine whether the improvement in image quality actually translates to improved patient management. We will learn more as clinical outcomes become apparent.
18F-FDG: Protocol Selection
On the strength of our analysis of the human images and of our phantom studies, we began by injecting 185 MBq (5 mCi) of 18F-FDG, and imaging for 20 minutes starting at 90 minutes post-injection (Fig. 3). However, while imaging at 90 minutes post-injection has plenty of precedent (e.g.,Ref 7,8 ), we found ourselves feeling somewhat uncomfortable. It was clear from our healthy subjects data that additional gains in image quality could be obtained by scanning later; however, with only four uptake rooms in the center, imaging at 3 h post-injection would have generated significant logistical challenges. Furthermore, it is also the case that some guidelines for patient management with FDG-PET - specifically, Deauville criteria for lymphoma 9 - are based on imaging at 1 h post-injection. We felt strongly that imaging at 1 h post-injection was a decision based on legacy protocols that would result in significant wasted performance for a scanner such as this, and would be a disservice both to our patients and to the future of the field. To resolve this dilemma, we turned to a number of internationally eminent experts in clinical PET for advice, and, perhaps unsurprisingly, we obtained as many different view-points as people that we asked! In the end we opted for an approach devised by our Nuclear Medicine Medical Director, Dr. Cameron Foster, who suggested that we image all patients at 2 h post-injection, but that lymphoma patients should also be imaged at 1 h post-injection. The early scan could then be used in the context of the Deauville criteria without modification, while still providing the patient with the excellent image quality available from the 2 h scan (Fig 4). For 2 h scanning, we increased the injection to 296 MBq (8 mCi). We have been running this protocol since December 2019.
Figure 3:
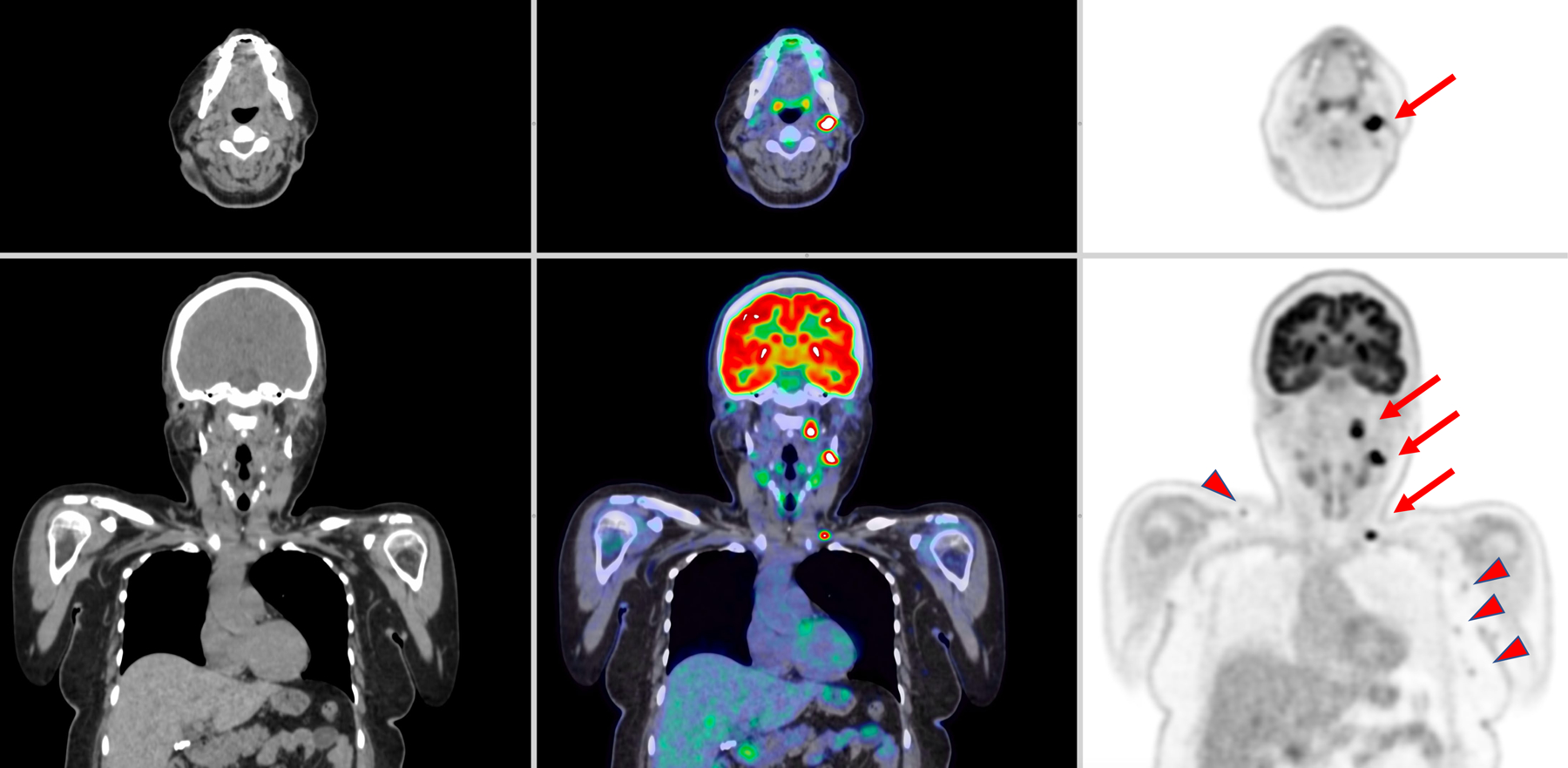
Patient with diffuse large B-cell lymphoma. The scanning protocol consisted of intravenous the injection of 193 MBq of 18FDG, 90-minute uptake time and 20-minute scan. CT images (left), PET/CT fused images (center) and PET images (right) are shown. The three lesions in the left cervical and supraclavicular regions are highlighted by the red arrows represented lymphoma. Several other lower FDG avidity lymph nodes in the left axilla and right supraclavicular region (arrowhead) are noted. These findings were interpreted as benign lymph nodes.
Figure 4:
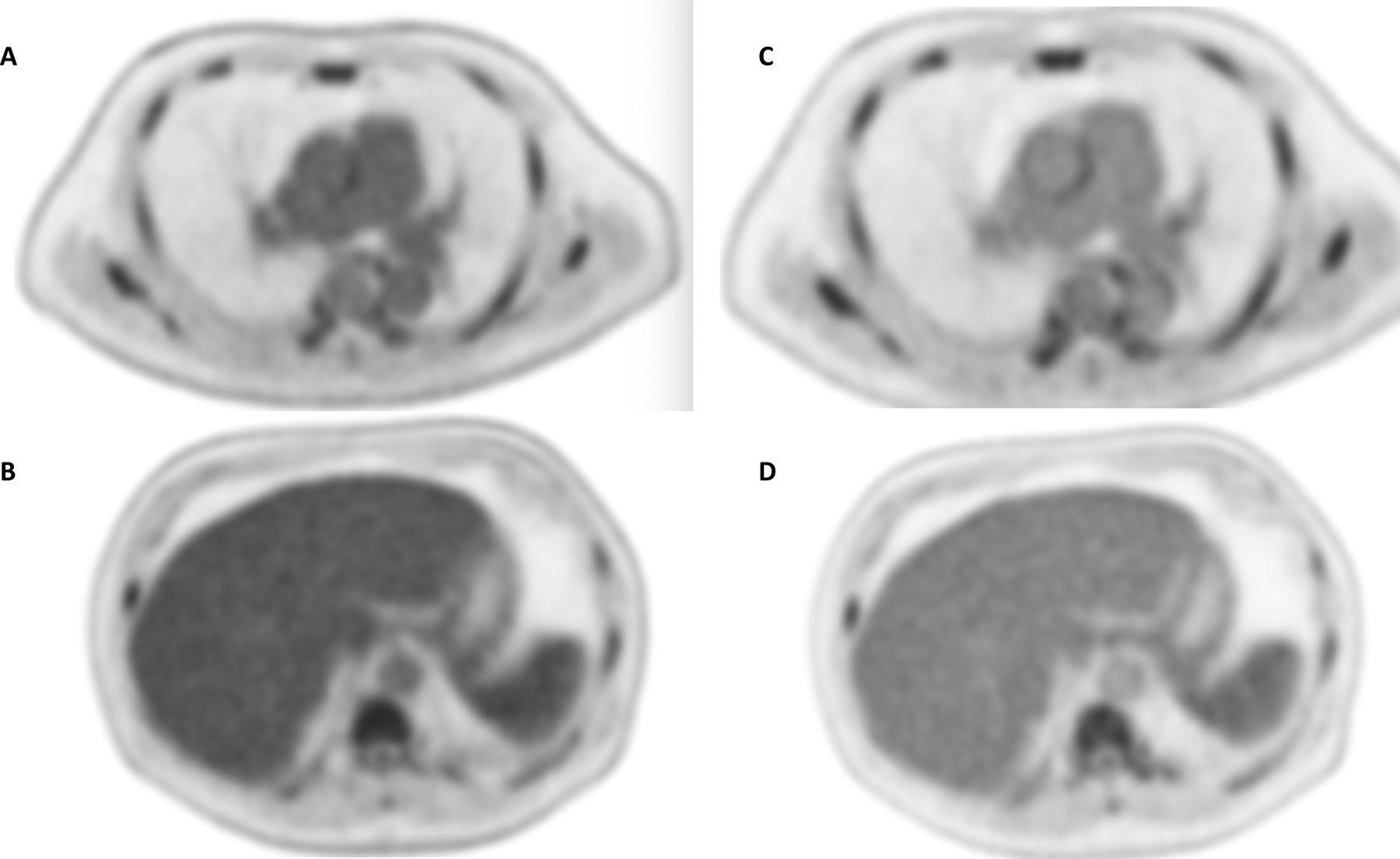
60- year-old study subject suffering from lymphoma. After injection of 296 MBq of 18F-FDG, 20-minute scans were obtained at 60 (A,B) and 120 minutes (C, D). Comparing the mediastinal (top row) and upper abdominal region (bottom row) between 60- and 120-minute scans, both blood pool activity within the aorta and the liver uptake significantly decrease. Both mediastinal blood pool and liver uptake are used to normalize lesions and assess Deauville score.
The scan duration is also a protocol parameter that warrants discussion. Since data are always acquired in list-mode, it is possible to reconstruct shorter scans from the 20-minute-long acquisitions. Using data from our healthy subjects and from our first patients, we reconstructed a number of 5-minute and 10-minute scans and reviewed the resulting image quality. While the shorter scans were of diagnostic quality, it was felt that the 20-minute scans remained noticeably better. Following our philosophy of aiming for the best possible image quality, we have continued with the 20-minute acquisition protocol, reserving shorter scans only for those patients who experience excessive discomfort or who have difficulty in remaining still. On the topic of motion, we have noticed that since the entire patient remains in the field of view for the entire scan, motion is a larger issue for a 20-minute TB PET/CT scan than it would be for a 20-minute conventional PET/CT scan. Motion correction is now an active area of research within our group.
Initial Experience with 18F-Fluciclovine and 68Ga-DOTATATE
With 18F-Fluciclovine, we acquire 25 minutes of data starting at the time of injection. Data are reconstructed for clinical interpretation starting at 4 minutes post-injection. Our preference would be to inject no more than 185 MBq (5 mCi), however the label requires a prescription of 370 MBq (10 mCi). Regulations permit a 20% deviation from this value and we aim for the lower end of this range (296 MBq, 8 mCi). There are substantial differences between a 18F-Fluciclovine scan obtained on a TB PET/CT scanner and on a conventional PET/CT scanner. Firstly, the images can be acquired in the entire body at the same time; this allows for early time point scans, especially in the upper part of the body where the radiotracer distribution in the muscles in a conventional PET/CT scanner often interferes with the evaluation 10. Secondly, it delivers better image quality in the pelvic area, which is the site where disease recurrence rate is higher in patients scanned for biochemical recurrence of prostate cancer. Thirdly, it allows for late time point evaluation of the pelvic area, which occasionally reveals lesions that are occult at early time points 10. The high sensitivity of the scanner permits reconstruction of short-duration frames while keeping the noise manageable. In routine clinical examination, we use the analysis of a series of short-duration frames (e.g. 2 minutes) to determine when the bladder starts filling and avoid misinterpreting urinary activity as recurrent disease, especially when motion artefacts are present; this is particularly helpful in individuals with very prompt radiotracer excretion. Together, these advantages are highly significant.
In prior studies, the use of 18F-Fluciclovine has been found to be less sensitive than PSMA-PET for distant metastases. 11 We do not know if TB PET/CT will offer equally significant benefits for PSMA-PET, but our initial experience with 18F-Fluciclovine does suggest that the head-to-head comparison between 18F-Fluciclovine and PSMA should be revisited in this context.
The other FDA-approved radiotracer that we have been using clinically is 68Ga-DOTATATE. The 68Ga-based radiotracers are in a disadvantageous position when compared to the more imaging-friendly 18F-based radiotracers. The shorter half-life (68 minutes versus 109 minutes) limits the signal while the larger mean positron range (3.66 mm versus 0.66 mm) 12,13 limits the spatial resolution. The exceptional sensitivity of TB PET/CT ameliorates the signal limitation issue, but the larger positron range presents more of a challenge. This is because the uEXPLORER scanner has a spatial resolution of close to 3 mm, which is considerably better than most conventional PET/CT scanners and, due to the exceptional sensitivity, appears to be close to realizable in clinical practice for 18F-FDG imaging. As a result, while in our experience the image quality of 68Ga-DOTATATE remains noticeably better with TB PET/CT than with conventional PET/CT (Fig. 5), the gains are not as stark as we have seen with 18F-based radiotracers.
Figure 5:
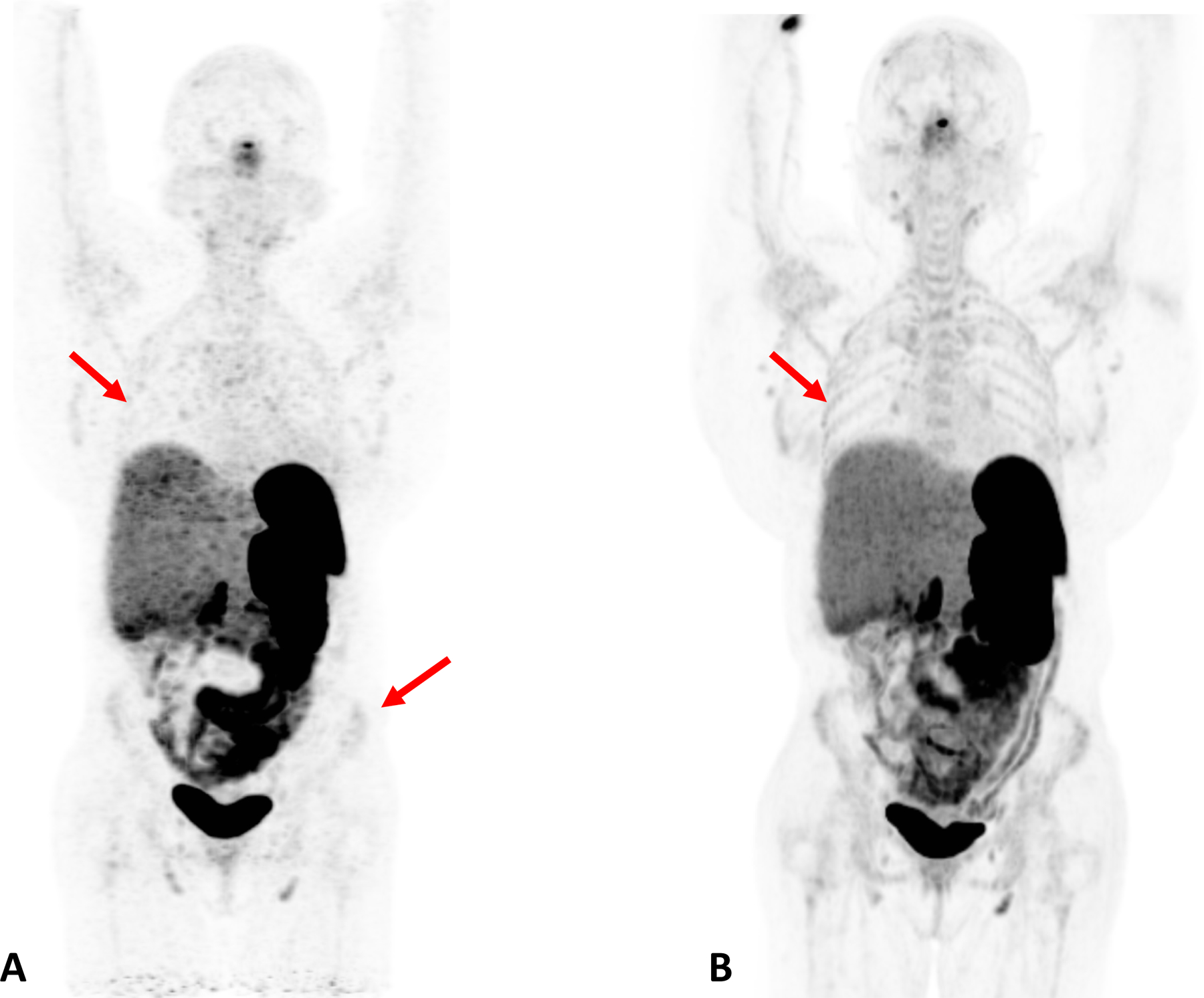
Patient with Cushing Syndrome post bilateral adrenalectomy. A dose of 167 MBq of 68Ga DOTATATE was injected and a 20-minuet scan was performed after 60 minutes of uptake. A) Baseline scan obtained on conventional PET/CT scanner; B) 6 month follow up scan obtained on total body scanner. In the total body PET/CT image, background noise is lower and the signal higher, allowing for clearer visualization of the liver. In addition, the increased signal level on the total body scanner results in new or better visualization of bone details (arrows).
Present and Future Challenges
The unpreceded signal gain, total-body coverage and exceptional spatial resolution of the TB PET/CT scanner provide the physical explanation of its enhanced, and potentially transformative clinical potential; however, they also present a number of challenges for which the hosting institution should be prepared. Some of these challenges may be found in the logistics and workflows around the scanner. The number of uptake rooms is a critical consideration. If long uptake time or if high throughput is desired, more uptake rooms - potentially, considerably more - will be required than would be needed for a conventional PET/CT scanner. In high throughput environments, attention will need to be paid to dosimetry issues for the technologists. Other challenges arise from integration into patient care. During the initial years of adoption of this technology, it will not be possible to find radiologists and oncologists with prior experience of the modality and oncologists and radiologists will need to engage in a mutual learning/teaching process, much as we have done. Even once experience has been gained, a major clinical challenge is the comparison of images obtained with TB PET/CT scanner and prior or subsequent images obtained with a conventional PET/CT scanner (Fig. 5). Radiologists are already familiar with the difficulties of comparing scans obtained on different devices; however, when a TB PET/CT scan is compared to others, this issue becomes more critical due to the substantial different image quality and potentially (and certainly for 18F-FDG in our hands) different protocols that lead to different radiotracer biodistribution.
Conclusion/Future Direction
We have successfully integrated TB PET/CT into our clinical practice. At our center, the smooth transition was made possible due to the strong research environment, the diverse imaging team and the productive collaboration between imaging physicians and oncologists who all worked together to improve patient care. Our next step is to rethink ways to image with TB PET/CT, improving old clinical protocols, implementing the use of different radiotracers, and establishing a new clinical routine where both research and clinical work can further thrive.
Key Points.
Human imaging on the total-body PET/CT at U.C. Davis has demonstrated superb image quality using both 18F- and 68Ga-based radiotracers.
The increased sensitivity and spatial resolution of Total-body PET/CT creates new challenges in imaging interpretation that must be overcome for optimal integration into the clinical workflow.
The large gain in sensitivity and total-body coverage enables a range of different acquisition protocols; work on understanding how best to use these for maximal clinical benefit is ongoing.
Synopsis:
This work outlines the clinical implementation of Total-body PET/CT from the time of installation at UC Davis EXPLORER Molecular Imaging Center in May 2019 to date (September 2020). This process has not been without challenges: logistical, technical and medical problems were all encountered and are discussed below. The mutual learning/teaching engagement between nuclear medicine technologists, research staff, radiologists and referring physicians has been the key to the past year’s achievements; these include development of ground-breaking clinical protocols for a range of FDA approved radiotracers, and the attainment of a measure of expertise in clinical Total-body PET/CT.
Acknowledgments:
We would like to thank the EXPLORER team at UC Davis for their hard work and strong support. In particular we would like to thank the technologists: Denise Caudle, Heather Hunt, Kristin McBride, Mike Nguyen, Michael Rusnak; the radiologists: Marwah Helmy, Elizabeth Moore, Fatma Sen, Cameron Foster, Rosalie Hagge, Thomas Loehfelm, Matthew Bobinski, Nancy Pham; the physics team: Benjamin Spencer, Eric Berg, Edwin K. Leung, Negar Omidvari, Emilie Roncali, Anthony Siebert, Linda Kroger; the supporting and supervisory staff: Stephen Wetzel, Dana Little, Ellie Nash, Lynda Painting, Ofilio Vigil, Elizabeth Lincoln, Michelle Verdier-Fontes, Kathy Cruz Rodriguez, Jeanee Cooper, Carla Andalis; the ethics experts in the IRB team: John Tupin, Amanda Carioggia, Royce Yokoi; WeiPing Liu and Jeffrey Schmall and United Imaging Engineer team. The oncologist experts: Ken Yoneda, Merin Stephen, Tianhong Li, Primo Lara, Karen Kelly, Aaron Rosenberg, May Cho, Joseph Tuscano, Mamta Parikh, Edward Kim, Amanda Kirane, Marcio Malogolowkin, David Cooke, Lisa Brown, and Johnathan Riess and the United Imaging engineering team.
Funding
NIH R01 CA249422; NIH R01 CA206187
Footnotes
Disclosures:
Ramsey D. Badawi has research support from United Imaging Healthcare.
Lorenzo Nardo has is principle investigator of a service agreement with United Imaging Healthcare
UC Davis has a revenue-sharing agreement with United Imaging Healthcare that is based on uEXPLORER sales.
References:
- 1.Badawi RD, Shi H, Hu P, et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J Nucl Med 2019;60(3):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai SR. Unsuspected pulmonary embolism on CT scanning: yet another headache for clinicians? Thorax 2007;62(6):470–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisey M. The Introduction and Development of Clinical PET in the United Kingdom. In: McCready R, Gnanasegaran G, Bomanji JB, eds. A History of Radionuclide Studies in the UK: 50th Anniversary of the British Nuclear Medicine Society Cham (CH)2016:103–110. [PubMed] [Google Scholar]
- 4.Rusnak M, McBride K. Total-body PET Patient Positioning for Optimal Diagnostic and Research Scans. J Nucl Med 2020;61(supplement 1):3122. [Google Scholar]
- 5.McBride K, Hunt H, Rusnak M, Nguyen M. Bolus Injection Technique for uEXPLORER 18F-FDG PET/CT Dynamic Scans. J Nucl Med 2020;61(supplement 1):3084. [Google Scholar]
- 6.Leung E, Zhang X, Berg E, et al. Relationships between noise-equivalent count rates for extended NEMA NU 2-like scatter phantoms and a human subject scanned using the EXPLORER total-body PET scanner. J Nucl Med 2019;60(supplement 1):1385. [Google Scholar]
- 7.Nahmias C, Wahl LM. Reproducibility of Standardized Uptake Value Measurements Determined by 18F-FDG PET in Malignant Tumors. J Nucl Med 2008;49(11):1804–1808. [DOI] [PubMed] [Google Scholar]
- 8.Davies A, Tan C, Paschalides C, et al. FDG-PET maximum standardised uptake value is associated with variation in survival: Analysis of 498 lung cancer patients. Lung cancer (Amsterdam, Netherlands) 2007;55(1):75–78. [DOI] [PubMed] [Google Scholar]
- 9.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. Leukemia & Lymphoma 2009;50(8):1257–1260. [DOI] [PubMed] [Google Scholar]
- 10.Abdelhafez Y, Nardo L, Leung E, et al. Initial evaluation of 18F-fluciclovine in prostate cancer using dynamic EXPLORER total-body PET/CT. J Nucl Med 2020;61(supplement 1):1244. [Google Scholar]
- 11.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J Nucl Med 2018;59(5):789–794. [DOI] [PubMed] [Google Scholar]
- 12.Jodal L, Le Loirec C, Champion C. Positron range in PET imaging: an alternative approach for assessing and correcting the blurring. Phys Med Biol 2012;57(12):3931–3943. [DOI] [PubMed] [Google Scholar]
- 13.Jødal L, Le Loirec C, Champion C. Positron range in PET imaging: an alternative approach for assessing and correcting the blurring. Physics in Medicine and Biology 2012;57(12):3931–3943. [DOI] [PubMed] [Google Scholar]


