Abstract
Since the 1960s, the frequency of methicillin-resistant Staphylococcus aureus as a recurrent cause of nosocomial infections has increased. Since multidrug-resistant Staphylococcus has overcome antimicrobial treatment, the development of putative vaccines based on virulence factors could be a great help in controlling the infections caused by bacteria and are actively being pursued in healthcare settings. This mini-review provides an overview of the recent progress in vaccine development, immunogenicity, and therapeutic features of some S. aureus macromolecules as putative vaccine candidates and their implications against human S. aureus-related infections. Based on the reviewed experiments, multivalent vaccines could prevent the promotion of the diseases caused by this bacterium and enhance the prevention chance of S. aureus infections.
Keywords: candidate vaccines, immunoprophylaxis, prophylaxis, Staphylococcus aureus, staphylococcal infections Staphylococcus aureus
Highlights
-
-
Vaccine development against staphylococcal infections is still in its infancy. Irrefutably, more studies on staphylococcal virulence factors and immune evasion are needed to reach a complete understanding of virulence mechanisms.
-
-
Many investigations have put forward a large number of targets for vaccine development against Staphylococcus aureus, which increase the number of putative targets.
-
-
Since numerous changeable infection-related factors exist and are also expressed in staphylococcal species, multivalent vaccines consisting of several antigens related to different infection stages are required.
Introduction
Staphylococcus aureus is a widespread commensal and pathogen bacterium. S. aureus bacteria induce staph food poisoning that leads to gastrointestinal illness through eating foods contaminated with the toxins produced. About 25% of animals and people have staph in their nose and on their skin (Le Loir et al., 2003). It is also one of the most isolated bacteria among both nosocomial and community-acquired infections. It causes many types of human infections and syndromes such as mild skin and soft tissue infections, bacteremia, endocarditis, pneumonia, metastatic infections, sepsis, and toxic shock syndrome (van Belkum, 2006). A hospital environment and medical devices contaminated with S. aureus can affect the health of patients. Over the past decades, staphylococcus nosocomial infections have significantly increased (Kuklin et al., 2006; Hogea et al., 2014). Since the 1960s when the first methicillin-resistant S. aureus (MRSA) was identified, a major challenge has begun (Adhikari et al., 2012). The emergence of antibiotic-resistant strains of staphylococci, mainly MRSA, emphasizes the serious control of S. aureus-related infections (O’Neill et al., 2008)—for example, the outbreak of S. aureus bloodstream infections in the United States in 2017 induced nearly 20,000 deaths (Kourtis et al., 2019). However, there is no current vaccine for S. aureus infection. Several S. aureus virulence factors have been evaluated as vaccine candidates. Infections caused by MRSA in hospital wards have decreased due to increased health assessments and the presentation of effective vaccines. Staphylococcus spp. conserved surface components with a high rate of expression in the bloodstream or biofilm-forming process factors stand as suitable staphylococcal candidate vaccines to decrease the staphylococcal disorders (Van Mellaert et al., 2012; Hogea et al., 2014). Thus, it is essential to know the relevant factors involved in biofilm formation from a molecular pathogenesis perspective and to discover the physiological status of these virulence factors within the body in order to realize whether they have the potency to develop an aggressive behavior.
Discussion
Vaccine Development Based on the Targets
Many investigations have put forward a large number of targets for vaccine development against S. aureus, which increase the number of putative targets. In the classical approach, different targets with certain functions have been studied and evaluated as subunit vaccine. New target candidates have also been suggested by reverse vaccinology and bioinformatics (Zhang et al., 2003; Bowden et al., 2005; Gill et al., 2005). In order to cover the genetic diversity of a pathogen in vaccine development strategies, its pan-genome should be analyzed, and its molecular epidemiology should also be examined (Mora and Telford, 2010).
The Search for Vaccine Targets
Poly(glutamic acid) (PGA) stands for a good vaccine candidate against the mentioned bacterium, owing to its protection effects against antimicrobial peptides during biofilm-related infections and neutrophil phagocytosis. The result of an experiment indicated that arisen antibodies to conjugated PGA are able to protect three models of animals, including guinea pig, mouse, and rabbit, against anthrax (Joyce et al., 2006).
Phenol-soluble modulins (PSMs) are considered as another promising group as vaccine target. Recently, a study showed that PSMβ peptides had an inhibitory effect on bacterial dissemination from implants (Rennermalm et al., 2004; Wang et al., 2011). Unlike most mentioned vaccine candidates, PSMβ interferes the dissemination of biofilm-associated infection via preventing detachment mechanisms.
Some Putative Vaccine Candidates to S. aureus
Capsular Polysaccharide
The function of conjugated microencapsulated S. aureus type 8 (the isolate came from bovine mastitis milk) to Pseudomonas aeruginosa exotoxin A (ETA) was assessed in a mouse model. The antibody response was triggered 3 days following the immunization and lasted for 13 days of the observation period after the second injection in some mice. The antibody response and the survival rate were higher in the group of mice immunized with the CP8–ETA conjugates in comparison with those receiving complete Freund’s adjuvant or phosphate-buffered saline. Based on the result of this experiment, the CP8–ETA vaccine is able to protect mice against S. aureus bacteremia (Han et al., 2000).
Iron-Regulated Surface Determinant B
The S. aureus iron-regulated surface determinant B (IsdB), a prophylactic vaccine against S. aureus infection, as an iron-sequestering protein exists in many S. aureus clinical isolates and methicillin-resistant and methicillin-sensitive isolates and is expressed on the surface of all tested isolates. As the mice were immunized with IsdB formulated with amorphous aluminum hydroxyphosphate sulfate, high immunogenicity of IsdB in rhesus macaques was observed. Furthermore, a fivefold increase in antibody titers was seen after a single immunization, which indicates IsdB potency as a vaccine against S. aureus disease in humans (Jones et al., 2001; Kuklin et al., 2006). A randomized study on the preoperative receipt of Merck V710 S. aureus vaccine containing non-adjuvanted IsdB demonstrated that all V710 recipients and only about 8% of the placebo recipients died of postoperative S. aureus infection following a major cardiothoracic surgery. These results may raise the concern of researchers about the immunization itself, which might affect either the safety or the efficacy of the development of staphylococcal vaccines (McNeely et al., 2014; Daly et al., 2017). In another cohort study, in spite of modern perioperative management, postoperative S. aureus infection occurred in 1% of adult patients. The mortality rates were also 3% for methicillin-resistant S. aureus infections and 13% for MRSA infections (Allen et al., 2014).
Virus-Like Particle-Based Vaccines
The coordination of the expression of the required virulence factors in the invasive infection of S. aureus happens using secreted cyclic auto-inducing peptides (AIPs) and the accessory gene regulator (agr) operon. AIPs are small in size and require a thiolactone bond. In order to solve this issue, the virus-like particles were utilized as a vaccine platform (PP7) for a conformationally restricted presentation of a modified AIP1 amino acid sequence (AIP1S). AIP1-specific antibodies inhibited agr activation in vivo; moreover, it reduced pathogenesis and increased bacterial clearance in murine skin and a soft tissue infection model carrying a highly virulent agr type I S. aureus isolate, which all indicated vaccine efficacy and that it might have a great impact on antibiotic resistance (Daly et al., 2017).
Staphylococcus aureus Alpha-Hemolysin
Based on the results of previous studies, a recombinant vaccine for S. aureus alpha-hemolysin should have a heptameric structure for its crystal. HIa, a pore-forming toxin, is expressed by the majority of S. aureus strains. HIa was examined for vaccination with AT-62aa along with a glucopyranosyl lipid adjuvant–stable emulsion. Then, the results indicated that sepsis protection in an experimental model of S. aureus infection was done by utilizing Newman and the pandemic strain USA300 (LAC). This model demonstrated the AT-62aa is a proper vaccine candidate. The identification of AT-62aa protective epitopes may also result in novel immunotherapy for S. aureus infection (Adhikari et al., 2012).
Staphylococcus aureus LukS-PV-Attenuated Subunit Vaccine
LukS-mut9 is an attenuated mutant of LukS-PV with a high immunogenic response. This mutant has shown significant protection in mouse sepsis model. Recent findings revealed that the protection of the Panton–Valentine leukocidin (PVL) vaccine in mice model is related tocross-protective responses against other homologous toxins, owing to the generated polyclonal antibodies by LukS-mut9, which can neutralize other canonical and non-canonical leukotoxin pairs. There has been a correlation between the arisen antibodies, PVL subunits, and sepsis in patients with high antibody titer against the mentioned subunits (Adhikari et al., 2012).
Four-Component Staphylococcus aureus Vaccine
In a study conducted based on a murine S. aureus infection model, antigen-specific antibodies were accumulated in the pouch, and the infection was mitigated following immunization with 4CStaph and bacterial inoculation in an air pouch generated on the back of the animal. The upregulation of FcR and the presence of antigen-specific antibodies induced by immunization with 4CStaph could increase bacterial opsonophagocytosis. Alternative protection mechanisms may be activated by a proper vaccine, balancing neutropenia, which is a condition often happening to S. aureus-infected patients (Torre et al., 2015).
The Mixture of PBP2a and Autolysin as a Candidate Vaccine Against Methicillin-Resistant S. aureus
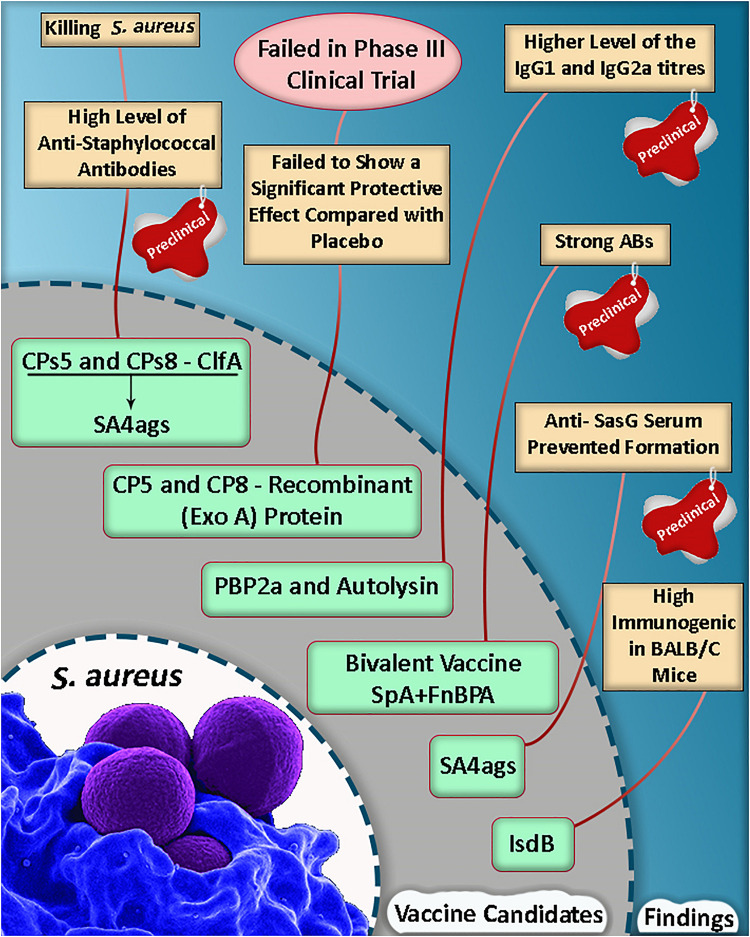
Based on a study, the mortality rate was reduced in mice, and they were protected against lethal MRSA challenge as well as single proteins following an active vaccination with a mixture of r-PBP2a/r-autolysin and a conjugated form of the vaccine (Haghighat et al., 2017). Some of the selective putative vaccine candidates and a summary of the vaccine candidate development in S. aureus are listed in Table 1 and Figure 1.
TABLE 1.
Some putative vaccine candidates which could be considered in vaccine development against Staphylococcus aureus.
| Putative macromolecules | Features | Advantage | Disadvantage | References |
| Polysaccharide intercellular adhesion (PIA) | Surface polysaccharide poly-N-acetyl-β-(1-6)-glucosamine also known as PIA | Produced in vitro by either S. aureus or Staphylococcus epidermidis with high levels of acetate substituting for amino groups; generate opsonic and protective antibodies PIA has been extensively evaluated as a putative candidate for vaccine development | Immunization with PIA and other polysaccharides must be boosted or conjugated to a safe protein carrier | Maira-Litrán et al., 2005; Maira-Litrán et al., 2012; Miller et al., 2020 |
| Teichoic acid | (A) Glycerol and ribitol phosphate copolymer by phosphodiester bonds (B) It is assigned as main macromolecule to the primary attachments and accumulation phase in biofilm formation (C) It is chiefly important in inflammation and immune evasion | One of the main Gram-positive bacteria-adhesive macromolecules In a study, the efficacy of mAb was determined as >90% against CoNS clinical isolates. Up to 90% of bacterial killing activity was detected at doses <10 μg/ml as an apt opsonophagocytic result, which prevents related infections in animal models | Immunization with PIA and other polysaccharides must be boosted | Ali et al., 2020; van Dalen et al., 2020 |
| Accumulation-associated protein | Presence in both S. aureus and S. epidermidis that plays an essential role in the attachment and aggregation of biofilm phases | Polyclonal antibodies inhibit biofilm formation Its conjugation to a confirmed protective polysaccharide, such as PIA, could eliminate the biofilm formation process by inducing cellular immunity-related immunoglobulin subtypes (IgG2a and IgG2b) to activate memory cells | Arisen antibodies to AaP have no effect on polysaccharide-dependent biofilm-forming S. aureus and S. epidermidis | Yan et al., 2014 |
| Fibronectin binding protein A | Presence in S. aureus | Specific antipeptide immunoglobulin (Ig) G and IgA antibodies were detected in the serum and respiratory mucosa of vaccinated mice. Responses to the major pilus backbone protein Spy0128 showed robust antibody responses to this antigen both systemically and in the respiratory and intestinal mucosa | The mechanism(s) of protection are unclear | Clow et al., 2020 |
| Virulence factor | Secreted factors α-hemolysin, staphylococcal enterotoxin B, and the three surface proteins staphylococcal protein A, iron surface determinant B N2 domain, and manganese transport protein C | Induce comprehensive cellular and humoral immune responses to reduce bacterial loads, inflammatory cytokine expression, and inflammatory cell infiltration and decrease pathology after challenge with a sub-lethal dose of S. aureus | No significant differences in lymphocyte subset distribution and serous cytokine levels (IL-4, IL-5, TNF-α, IFN-γ, IL-2, and IL-6) between the vaccine and the placebo groups | Creech et al., 2020; Zeng et al., 2020; Alabdullah et al., 2021 |
| Phosphatidylinositol phosphodiesterase | Secreted by extracellular pathogens such as S. aureus | Strong humoral response in the vaccine mice that provided 75% protection against S. aureus | Large-scale in vivo studies are called | Soltan et al., 2020 |
AaP, accumulation-associated protein; TA, teichoic acid; mAB, monoclonal antibody.
FIGURE 1.
A summary of vaccine candidate development in Staphylococcus aureus (Shinefield et al., 2002; Kuklin et al., 2006; Fowler et al., 2013; Haghighat et al., 2017; Dupont et al., 2018; Yang et al., 2018).
Limitation
Several vaccine candidates which are of recent progress in vaccine development are only presented in this study. Therefore, more explanation was not mentioned about the general function of the vaccine candidate molecules, particularly with regard to PSM, ETA, IsdB, alpha-hemolysin, LukS-PV, PBP2a, and autolysin.
Conclusion
Vaccine development against staphylococcal infections is still in its infancy. Irrefutably, more studies on staphylococcal virulence factors and immune evasion are required to enable us to reach a complete understanding of the virulence mechanisms. Since numerous changeable infection-related factors exist and also expressed in staphylococcal species, multivalent vaccines consisting of several antigens related to different infection stages are needed. There are few ways to deal with S. aureus infections due to their high antibiotic resistance and also because the infections caused by this microorganism are increasing. However, fortunately, since sufficient research has been done on the effects of various vaccine candidates regarding the S. aureus virulence factor, the capability of biofilm production could be noticed as one of the most important factors in bacterium colonization as well. If a suitable vaccine candidate can be included to (1) inhibit biofilm formation and (2) prevent the effect of bacterial virulence factors, then the possibility of preventing and eliminating infections can be imagined. It is expected that designing a multivalent vaccine with the above-mentioned content will raise the effectiveness of antibodies and lead to the eradication of S. aureus-related infections.
Author Contributions
BM contributed to conceptualization, data collection, data curation, and writing of the manuscript. RB, HZ, and MD contributed to data collection. AS contributed to data collection and writing of the manuscript. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the colleagues in the bacteriology and virology departments at Zanjan University of Medical Sciences for their sincere support. Special thanks go to Dr Mehdi Ghaemi (Department of Anesthesiology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran) for their kind contributions in preparing and final editing of the manuscript.
References
- Adhikari R. P., Karauzum H., Sarwar J., Abaandou L., Mahmoudieh M., Boroun A. R., et al. (2012). Novel structurally designed vaccine for S. aureus α-hemolysin: protection against bacteremia and pneumonia. PLoS One 7:e38567. 10.1371/journal.pone.0038567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabdullah H. A., Overgaard E., Scarbrough D., Williams J. E., Mohammad Mousa O., Dunn G., et al. (2021). Evaluation of the Efficacy of a Cholera-Toxin-Based Staphylococcus aureus Vaccine against Bovine Intramammary Challenge. Vaccines 9:6. 10.3390/vaccines9010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Berni F., Enotarpi J., Van Der Marel G. A., Codée J. D. (2020). Synthetic teichoic acid chemistry for vaccine applications. Recent Trends Carbohydr. Chem. 2020 207–238. 10.1016/b978-0-12-820954-7.00006-2 [DOI] [Google Scholar]
- Allen K. B., Fowler V. G., Jr., Gammie J. S., Hartzel J. S., Onorato M. T., Dinubile M. J., et al. (2014). Staphylococcus aureus infections after elective cardiothoracic surgery: observations from an international randomized placebo-controlled trial of an investigational S aureus vaccine. Open Forum Infect. Dis. 1:ofu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden M. G., Chen W., Singvall J., Xu Y., Peacock S. J., Valtulina V., et al. (2005). Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology 151 1453–1464. 10.1099/mic.0.27534-0 [DOI] [PubMed] [Google Scholar]
- Clow F., Peterken K., Pearson V., Proft T., Radcliff F. J. (2020). PilVax, a novel Lactococcus lactis-based mucosal vaccine platform, stimulates systemic and mucosal immune responses to Staphylococcus aureus. Immunol. Cell Biol. 98 369–381. 10.1111/imcb.12325 [DOI] [PubMed] [Google Scholar]
- Creech C. B., Frenck R. W., Fiquet A., Feldman R., Kankam M. K., Pathirana S., et al. (2020). Persistence of immune responses through 36 months in healthy adults after vaccination with a novel Staphylococcus aureus 4-antigen vaccine (SA4Ag). Open Forum Infect. Dis. 7:ofz532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly S. M., Joyner J. A., Triplett K. D., Elmore B. O., Pokhrel S., Frietze K. M., et al. (2017). VLP-based vaccine induces immune control of Staphylococcus aureus virulence regulation. Sci. Rep. 7:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C. D., Scully I. L., Zimnisky R. M., Monian B., Rossitto C. P., O’connell E. B., et al. (2018). Two Vaccines for Staphylococcus aureus Induce a B-Cell-Mediated Immune Response. mSphere 3 e00217–e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler V. G., Allen K. B., Moreira E. D., Moustafa M., Isgro F., Boucher H. W., et al. (2013). Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309 1368–1378. 10.1001/jama.2013.3010 [DOI] [PubMed] [Google Scholar]
- Gill S. R., Fouts D. E., Archer G. L., Mongodin E. F., Deboy R. T., Ravel J., et al. (2005). Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat S., Siadat S. D., Sorkhabadi S. M. R., Sepahi A. A., Mahdavi M. (2017). A novel recombinant vaccine candidate comprising PBP2a and autolysin against Methicillin Resistant Staphylococcus aureus confers protection in the experimental mice. Mol. Immunol. 91 1–7. 10.1016/j.molimm.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Han H., Pak S. I., Guidry A. (2000). Prevalence of capsular polysaccharide (CP) types of Staphylococcus aureus isolated from bovine mastitic milk and protection of S. aureus infection in mice with CP vaccine. J. Vet. Med. Sci. 62 1331–1333. 10.1292/jvms.62.1331 [DOI] [PubMed] [Google Scholar]
- Hogea C., Van Effelterre T., Cassidy A. (2014). A model-based analysis: what potential could there be for a S. aureus vaccine in a hospital setting on top of other preventative measures? BMC Infect. Dis. 14:291. 10.1186/1471-2334-14-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M., Morgan M., Humphrey T. J., Lappin-Scott H. (2001). Effect of vancomycin and rifampicin on meticillin-resistant Staphylococcus aureus biofilms. Lancet 357 40–41. 10.1016/s0140-6736(00)03572-8 [DOI] [PubMed] [Google Scholar]
- Joyce J., Cook J., Chabot D., Hepler R., Shoop W., Xu Q., et al. (2006). Immunogenicity and protective efficacy of Bacillus anthracis poly-γ-D-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J. Biol. Chem. 281 4831–4843. 10.1074/jbc.m509432200 [DOI] [PubMed] [Google Scholar]
- Kourtis A. P., Hatfield K., Baggs J., Mu Y., See I., Epson E., et al. (2019). Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. Morb. Mortal. Wkly Rep. 68 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklin N. A., Clark D. J., Secore S., Cook J., Cope L. D., McNeely T., et al. (2006). A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74 2215–2223. 10.1128/iai.74.4.2215-2223.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Loir Y., Baron F., Gautier M. (2003). [i] Staphylococcus aureus [/i] and food poisoning. Genet. Mol. Res. 2 63–76. [PubMed] [Google Scholar]
- Maira-Litrán T., Bentancor L. V., Bozkurt-Guzel C., O’malley J. M., Cywes-Bentley C., Pier G. B. (2012). Synthesis and evaluation of a conjugate vaccine composed of Staphylococcus aureus poly-N-acetyl-glucosamine and clumping factor A. PLoS One 7:e43813. 10.1371/journal.pone.0043813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira-Litrán T., Kropec A., Goldmann D. A., Pier G. B. (2005). Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 73 6752–6762. 10.1128/iai.73.10.6752-6762.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely T. B., Shah N. A., Fridman A., Joshi A., Hartzel J. S., Keshari R. S., et al. (2014). Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum. Vaccin. Immunother. 10 3513–3516. 10.4161/hv.34407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. S., Fowler V. G., Jr., Shukla S. K., Rose W. E., Proctor R. A. (2020). Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 44 123–153. 10.1093/femsre/fuz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M., Telford J. L. (2010). Genome-based approaches to vaccine development. J. Mol. Med. 88 143–147. 10.1007/s00109-009-0574-9 [DOI] [PubMed] [Google Scholar]
- O’Neill E., Pozzi C., Houston P., Humphreys H., Robinson D. A., Loughman A., et al. (2008). A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190 3835–3850. 10.1128/jb.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennermalm A., Nilsson M., Flock J.-I. (2004). The fibrinogen binding protein of Staphylococcus epidermidis is a target for opsonic antibodies. Infect. Immun. 72 3081–3083. 10.1128/iai.72.5.3081-3083.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinefield H., Black S., Fattom A., Horwith G., Rasgon S., Ordonez J., et al. (2002). Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346 491–496. [DOI] [PubMed] [Google Scholar]
- Soltan M. A., Magdy D., Solyman S. M., Hanora A. (2020). Design of Staphylococcus aureus New Vaccine Candidates with B and T Cell Epitope Mapping, Reverse Vaccinology, and Immunoinformatics. OMICS 24 195–204. 10.1089/omi.2019.0183 [DOI] [PubMed] [Google Scholar]
- Torre A., Bacconi M., Sammicheli C., Galletti B., Laera D., Fontana M. R., et al. (2015). Four-component Staphylococcus aureus vaccine 4C-staph enhances Fcγ receptor expression in neutrophils and monocytes and mitigates S. aureus infection in neutropenic mice. Infect. Immun. 83 3157–3163. 10.1128/iai.00258-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A. (2006). Staphylococcal colonization and infection: homeostasis versus disbalance of human (innate) immunity and bacterial virulence. Curr. Opin. Infect. Dis. 19 339–344. 10.1097/01.qco.0000235159.40184.61 [DOI] [PubMed] [Google Scholar]
- van Dalen R., Peschel A., Van Sorge N. M. (2020). Wall Teichoic Acid in Staphylococcus aureus Host Interaction. Trends Microbiol. 28 985–998. 10.1016/j.tim.2020.05.017 [DOI] [PubMed] [Google Scholar]
- Van Mellaert L., Shahrooei M., Hofmans D., Eldere J. V. (2012). Immunoprophylaxis and immunotherapy of Staphylococcus epidermidis infections: challenges and prospects. Expert Rev. Vaccin. 11 319–334. 10.1586/erv.11.190 [DOI] [PubMed] [Google Scholar]
- Wang R., Khan B. A., Cheung G. Y., Bach T. H., Jameson-Lee M., Kong K. F., et al. (2011). Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 121 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhang L., Ma H., Chiu D., Bryers J. D. (2014). A single B-repeat of Staphylococcus epidermidis accumulation-associated protein induces protective immune responses in an experimental biomaterial-associated infection mouse model. Clin. Vaccin. Immunol. 21 1206–1214. 10.1128/cvi.00306-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhou H., Cheng P., Yang Y., Tong Y., Zuo Q., et al. (2018). A novel bivalent fusion vaccine induces broad immunoprotection against Staphylococcus aureus infection in different murine models. Clin. Immunol. 188 85–93. 10.1016/j.clim.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Zeng H., Yang F., Feng Q., Zhang J., Gu J., Jing H., et al. (2020). Rapid and Broad Immune Efficacy of a Recombinant Five-Antigen Vaccine against Staphylococcus aureus Infection in Animal Models. Vaccines 8:134. 10.3390/vaccines8010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Q., Ren S. X., Li H. L., Wang Y. X., Fu G., Yang J., et al. (2003). Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49 1577–1593. 10.1046/j.1365-2958.2003.03671.x [DOI] [PubMed] [Google Scholar]