Abstract
Cerebral radiation necrosis (RN), a complication of Gamma Knife radiosurgery, is difficult to treat, although bevacizumab seems to be effective. However, clinical data pertaining to bevacizumab treatment for RN are scarce, and its high price is problematic. This study explored the effectiveness of low-dose bevacizumab for RN caused by Gamma Knife. We retrospectively analyzed 22 patients who suffered cerebral RN post-Gamma Knife, and received bevacizumab treatment because of the poor efficacy of glucocorticoids. Low-dose bevacizumab (3 mg/kg) was administered for two cycles at 2-week intervals. T1- and T2-enhanced magnetic resonance imaging (MRI) images were examined for changes in RN status. We also monitored the dose of glucocorticoid, Karnofsky Performance Status (KPS) score, and adverse drug reactions. The mean volume of RN lesions decreased by 45% on T1-weighted images with contrast enhancement, and by 74% on T2-weighted images. All patients discontinued the use of glucocorticoids. According to the KPS scores, all patients showed an improvement in their symptoms and neurological function. No side effects were observed. Low-dosage bevacizumab at a dose of 3 mg/kg every 2 weeks is effective for treating cerebral RN after Gamma knife for brain metastases.
Keywords: low-dosage, bevacizumab, radiation necrosis, gamma knife, brain metastase
Introduction
Gamma Knife, a type of stereotactic radiosurgery (SRS), is effective for local control of brain metastases (1). Unfortunately, Gamma Knife leads to the intractable complication of radiation necrosis (RN) (2). At present, glucocorticoids are the standard treatment for cerebral RN, in spite of their adverse side effects and limited treatment efficacy (3). The efficacy of other non-invasive treatments, including antiplatelet, anticoagulation and hyperbaric oxygenation, is considered controversial (1, 2, 4, 5). Surgical management can relieve clinical symptoms due to removal of the mass and reduced intracranial hypertension; however, surgery carries risks and causes neurological damage (6).
Recently, because of its ability to block vascular endothelial growth factor (VEGF), bevacizumab has proved to be an effective treatment for RN, in large dosage of 5–10 mg/kg every 2 weeks for 2–6 cycles (7–14). However, data on bevacizumab as a therapy for RN are still limited. In addition, bevacizumab is expensive, which creates a large economic burden for patients and society overall. In this study, we evaluated the efficacy of low-dosage bevacizumab treatment for RN following Gamma Knife in patients with brain metastases.
Materials and Methods
Patients' Characteristics
We analyzed 22 patients treated with bevacizumab for cerebral RN, caused by Gamma Knife in our center, between January 2013 and December 2017 (Table 1). All patients had metastatic brain tumors 13 from lung adenocarcinoma, 2 from Small cell lung cancer, 1 from neuroendocrine lung cancer, 2 from breast cancer, 1 from renal clear cell carcinoma, 1 from maxillary sinus carcinoma and 1 from esophageal Cancer, 1 from ovarian cancer, and long-term glucocorticoid treatment yielded unsatisfactory results.
Table 1.
Characteristics of patients with radiation necrosis after Gamma Knife for brain metastases.
| No. | Sex | Original tumor | Metastatic site | Prescription dose (Gy) | % Decrease in gadolinium | % Decrease in T2 | KPS change |
|---|---|---|---|---|---|---|---|
| 1 | F | Lung adenocarcinoma | Left temporal and parietal | 22 | 21 | 86 | 50 |
| 2 | F | Lung adenocarcinoma | Left occipital | 20 | 18 | 83 | 50 |
| 3 | F | Lung adenocarcinoma | Right parietal | 18 | 27 | 45 | 40 |
| 4 | M | Lung adenocarcinoma | Left frontal | 15 | 82 | 59 | 50 |
| 5 | F | Lung adenocarcinoma | Right frontal and parietal | 20 | 34 | 52 | 20 |
| 6 | F | Lung adenocarcinoma | Left occipital | 20 | 55 | 72 | 10 |
| 7 | M | Lung adenocarcinoma | Right frontal | 18 | 10 | 64 | 10 |
| 8 | M | Neuroendocrine lung cancer | Right parietal | 24 | 34 | 61 | 40 |
| 9 | M | Small cell lung cancer | Left posteior horn of lateral ventricle | 24 | 82 | 80 | 40 |
| 10 | M | Small cell lung cancer | Right parietal and occipital | 25 | 49 | 79 | 40 |
| 11 | F | Breast cancer | Left frontal | 24 | 38 | 81 | 40 |
| 12 | M | Renal clear cell carcinoma | Left frontal | 22 | 31 | 69 | 40 |
| 13 | M | Maxillary sinus carcinoma | Right temporal | 15 | 42 | 79 | 50 |
| 14 | M | Esophageal cancer | Right occiptal | 24 | 56 | 83 | 10 |
| 15 | M | Lung adenocarcinoma | Right parietal | 20 | 23 | 82 | 40 |
| 16 | M | Lung adenocarcinoma | Left parietal | 18 | 70 | 90 | 30 |
| 17 | F | Lung adenocarcinoma | Right frontal | 22 | 73 | 85 | 40 |
| 18 | M | Lung adenocarcinoma | Right posteior horn of lateral ventricle | 15 | 33 | 85 | 20 |
| 19 | F | Lung adenocarcinoma | Left frontal | 22 | 67 | 66 | 20 |
| 20 | F | Lung adenocarcinoma | Right posteior horn of lateral ventricle | 22 | 81 | 85 | 20 |
| 21 | F | Breast cancer | Right frontal | 15 | 30 | 78 | 30 |
| 22 | F | Ovarian cancer | Left temporal | 20 | 28 | 68 | 20 |
Diagnostic Criteria of RN
Pathology is the gold standard for a diagnosis of RN, although it is difficult to obtain biopsy specimens. Therefore, RN is usually diagnosed with the help of medical imaging (14, 15). In our study, the final diagnosis of RN was based on history of receiving Gamma Knife, clinical symptoms, physical examination, imaging, and biopsy. Low T1-weighted and high T2-weighted magnetic resonance imaging (MRI) images revealed cerebral edema surrounding the tumor and an enhanced irradiated field (Figure 1). Perfusion computed tomography (PCT) demonstrated low cerebral blood volume (CBV), low cerebral blood flow (CBF) and higher mean transit time (MTT) in RN (16).
Figure 1.
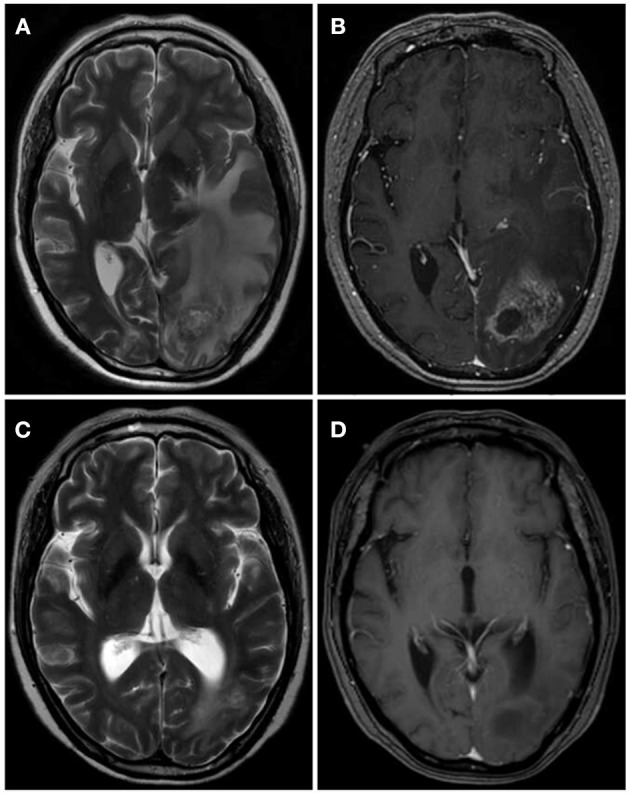
One case of RN after Gamma Knife before and after bevacizumab treatment. (A,B) MRI showed the lesion was located in left occipital with large edema area and obvious enhancement surrounding the tumor. (C,D) After the therapy of bevacizumab (3 mg/kg) for 2 cycles, the zone of lesion reduced dramatically.
Therapeutic Regimen Based on Bevacizumab
All patients received two low dosage of bevacizumab (3 mg/kg), 2 weeks apart, for 2–4 courses. Two patients had two separate cycles of bevacizumab because RN recurred during the 6 months follow-up after bevacizumab discontinuation. All patients were hospitalized for observation of adverse drug reactions.
Treatment Assessment
Enhanced MRI was performed before and after bevacizumab treatment, and was then repeated 2 months after the end of bevacizumab therapy in the two patients with repeated cycles. When patients developed neurological symptoms, MRI scans were evaluated immediately. We calculated the volume of RN lesions on T1 and T2 sequences, and compared the findings to those based on images acquired before bevacizumab was started. When patients underwent MRI scans pre- and post-bevacizumab, or to check for the onset of new symptoms, we could analyze the clinical data in terms of changes in neurological symptoms, glucocorticoid dosage, Karnofsky Performance Status (KPS) score, and clinical outcome.
Results
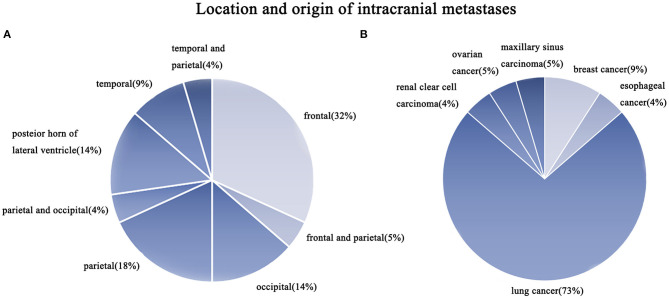
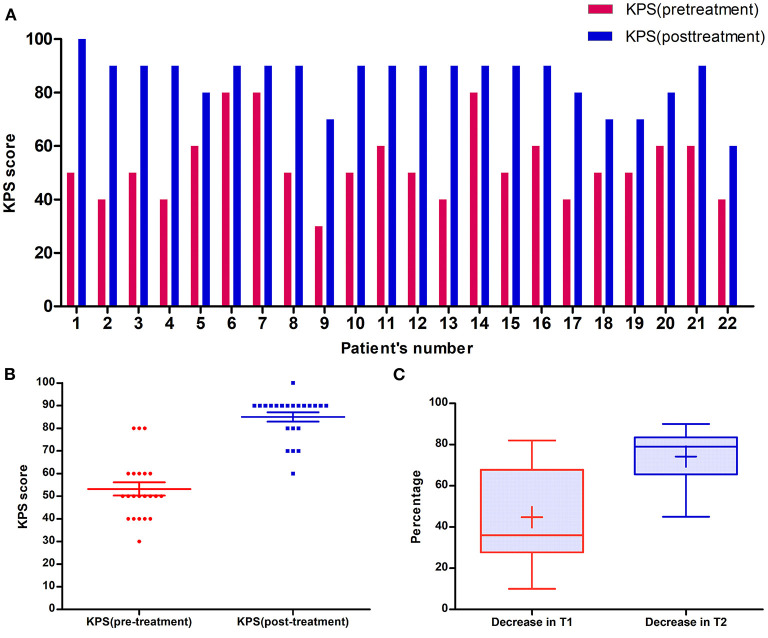
Table 1 shows patients ranged in age from 48 to 79 years (median: 64 years old). Eleven patients were male and others were female; all patients had undergone Gamma Knife with the prescription dosage of 15–25 Gy (median dosage: 20 Gy). In these patients, sixteen (72.7%) patients had brain metastases from lung cancer (Figure 2). Among 22 patients, 7 (31.8%) patients had metastases located in the frontal lobe, and 4 (18.2%) patients had metastases located in the parietal lobe (Figure 2). The mean volume of the RN lesions was 14.5 cm3 (range from 3.1 to 45.9 cm3) on T1 images post-gadolinium, and 109.25 cm3 (range: 26.7–266.8 cm3) on T2 images before bevacizumab treatment. After bevacizumab therapy, the average lesion volume was 6.99 cm3 (range: 0.7–20.6 cm3) on T1 contrast images and 26.1 cm3 (range: 5.6–60.8 cm3) on T2 images. Therefore, the average volume of RN lesions was reduced by 45% on T1-weighted contrast images and 74% on T2-weighted images (Figure 3). All patients discontinued the use of glucocorticoids after receiving two cycles of bevacizumab therapy. KPS scores were increased by an average of 31.8 in all patients' post-therapy (Figure 3). No side effects were observed.
Figure 2.
(A) The location of the intracranial metastases in this series of cases, with the frontal lobe having the most lesions, followed by the temporal lobe. (B) The source of metastases in this series of cases, with lung cancer having the most brain metastases (73%).
Figure 3.
(A) The change of each patient's KPS score after bevacizumab treatment. (B) Scatter plot of KPS scores of patients in cohort before and after bevacizumab treatment: the median KPS score of patients before bevacizumab treatment was 50, however, median KPS score increased to 90 after bevacizumab treatment. (C) Boxplot of imageology of patients in cohort before and after bevacizumab treatment. After bevacizumab treatment, the volume of nidus in Gadolinium and T2 sequence decreased by 44.7 and 74.2%, respectively.
Discussion
RN is the most common complication in patients with brain metastases treated by SRS (2). The main factors associated with RN are irradiation dosage, treatment duration, volume of irradiation and fractionation regimen (17, 18). RN usually occurs 3 or more months after radiotherapy (mean: 11.6 months) and 13–17% of patients develop some degree of RN after receiving SRS therapy for 1 year (19, 20). A meta-analysis of RN found that the most common necrotic sites were the frontal lobe (25%), temporal lobe (24%), and parietal lobe (10%). RN causes symptoms including focal or systemic neurological deficits. In severe cases, patients may experience a major decrease in quality of life (17, 21).
The mechanism of RN is unclear. At present, it is believed that RN progresses continuously from endothelial cell dysfunction to tissue hypoxia and necrosis, accompanied by the release of cytokines, such as VEGF (2, 14, 17, 18), which can lead to destruction of the blood-brain barrier permeability and cerebral edema (22, 23). Both animal and human models of RN have shown that high levels of VEGF occur due to blood-brain barrier dysfunction (24, 25). Nordal et al. reported that lab rats without the gene encoding VEGF were more resistant to radiation damage (26). Early blocking of VEGF can lower the risk of RN by reducing vascular permeability. This may help reverse pathological mechanisms, improve symptoms and prevent further disease progression. Bevacizumab seems to be an effective new treatment for RN, which exerts its effects through angiogenesis inhibition.
Data on the efficacy of bevacizumab treatment for RN are limited. However, recent literature has suggested that bevacizumab plays an important role in preventing RN. Gonzalez et al. were the first to report a potential benefit of bevacizumab treatment on RN. After treatment with bevacizumab at a dosage of 5 mg/kg every 2 weeks, or 7.5 mg/kg every 3 weeks, the neurological symptoms of eight patients affected by cerebral RN improved. The average reduction in the abnormal area on T1-weighted post-gadolinium images was 48%, compared to 60% on fluid-attenuated inversion recovery (FLAIR) images (3). Torcuator et al. assessed six biopsy-confirmed RN patients who received low-dosage bevacizumab; follow-up MRI showed an improvement in RN, with an average reduction in the abnormal area on T1-weighted post-gadolinium and FLAIR images of 79 and 49%, respectively (27). In a randomized, double-blind, placebo-controlled study, after bevacizumab treatment (7.5 mg/kg every 3 weeks) 14 patients with biopsy-confirmed RN showed a median reduction in edema volume of 59 and 63% on T2-FLAIR and T1-weighted post-gadolinium images, respectively. In addition, although some complications occurred after bevacizumab treatment, these 14 patients showed improvements in clinical symptoms (15). Delishaj et al. reviewed 125 patients, 114 (91.2%) of whom showed an improvement in neurological symptoms (17). In our study, the mean lesion volume decreased by 41 and 71% on T1-weighted contrast images and T2-weighted images, respectively. The KPS scores of all patients improved by > 30. Previous studies reported that patient dependence on glucocorticoids decreased after bevacizumab treatment, and that the dosage of glucocorticoids could be reduced (7, 23, 28). In our center, all patients gradually tapered off glucocorticoids after two cycles of bevacizumab treatment. Our data are consistent with previous studies, and therefore support a role of bevacizumab in the treatment of RN after Gamma Knife treatment for cerebral metastases.
In a prospective phase II clinical study, bevacizumab at a dosage of 1 mg/kg was used to treat cerebral RN. The regimen included three treatment cycles and one infusion every 3 weeks. Preliminary results showed that the severity of the symptoms decreased after bevacizumab treatment in 90% of patients (29). This suggests that ultra-low dosage bevacizumab could be a valid alternative to the standard dosage. At present, bevacizumab is regarded as a treatment that can be applied subsequent to RN diagnosis; whether it could also be used as a preventive treatment is rarely mentioned. A study including 54 adults male Wistar rats reported less severe RN in animals receiving prophylactic bevacizumab treatment before a 100-Gy radiation dosage (30). If further clinical research confirms these findings, it may be possible to consider bevacizumab as a preventive rather than purely treatment modality.
Bevacizumab has few side effects; however, patients who receive this agent are at risk of gastrointestinal perforation, delayed wound healing, thromboembolic events, asymptomatic ischemic changes, and even symptomatic ischemic changes such as hemiplegia (5, 31). The rate of adverse events (9.5%) reported by Bodensohn et al. (23) was not replicated in our study; in fact, no adverse events were observed in any of the 14 patients treated with low-doage bevacizumab. However, this finding is likely related to the small number of patients and relatively short follow-up time.
Our study had several limitations, including the small sample, which was recruited from a single-center with a descriptive analysis. This may have biased the analysis and conclusions. Larger studies are needed to confirm our findings and determine the optimal bevacizumab treatment schedule for RN.
Conclusions
In conclusion, low-dosage bevacizumab at a dosage of 3 mg/kg every 2 weeks is effective for the treatment of cerebral RN after Gamma Knife for brain metastases.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethics approval has been obtained from the ethics committee of the First Affiliated Hospital of Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QX is guarantor of integrity of the entire study. YW, JS, and LZ designed the study and prepare manuscript. ZFang and CZ collected the patient's information. FX, ZFan, and KH are response to statistical analysis. BH, LW, and TZ contributed to literature research. All authors agreed to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Frazier JL, Batra S, Kapor S, Vellimana A, Gandhi R, Carson KA, et al. Stereotactic radiosurgery in the management of brain metastases: an institutional retrospective analysis of survival. Int J Radiat Oncol Biol Phys. (2010) 76:1486–92. 10.1016/j.ijrobp.2009.03.028 [DOI] [PubMed] [Google Scholar]
- 2.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. (2016) 16:903–14. 10.1080/14737175.2016.1184572 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. (2007) 67:323–6. 10.1016/j.ijrobp.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. (2007) 67:248–55. 10.1016/j.ijrobp.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 5.Lubelski D, Abdullah KG, Weil RJ, Marko NF. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neuro-Oncol. (2013) 115:317–22. 10.1007/s11060-013-1233-0 [DOI] [PubMed] [Google Scholar]
- 6.McPherson C, Warnick R. Results of contemporary surgical management of RN using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol. (2004) 68:41–7. 10.1023/B:NEON.0000024744.16031.e9 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang E, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. (2012) 17:25. 10.1186/2047-783X-17-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. (2013) 15:1257–63. 10.1093/neuonc/not085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuse M, Nonoguchi N, Kawabata S, Yoritsune E, Takahashi M, Inomata T, et al. Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn J Clin Oncol. (2013) 43:337–41. 10.1093/jjco/hys231 [DOI] [PubMed] [Google Scholar]
- 10.Bostrom JP, Seifert M, Greschus S, Schafer N, Glas M, Lammering G, et al. Bevacizumab treatment in malignant meningioma with additional radiation necrosis. an MRI diffusion and perfusion case study. Strahlenther Onkol. (2014) 190:416–21. 10.1007/s00066-013-0505-0 [DOI] [PubMed] [Google Scholar]
- 11.Delishaj D, Ursino S, Pasqualetti F, Pesaresi I, Desideri I, Cosottini M, et al. The effectiveness of bevacizumab in radionecrosis after radiosurgery of a single brain metastasis. Rare Tumors. (2015) 7:6018. 10.4081/rt.2015.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, et al. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. (2015) 38:304–310. 10.1097/COC.0b013e31829c3139 [DOI] [PubMed] [Google Scholar]
- 13.Xiang-Pan L, Yuxin C, Xiao-Fei W, Na L, Tang-Peng X, Xiao-Tao X, et al. Bevacizumab alleviates radiation-induced brain necrosis: a report of four cases. J Cancer Res Ther. (2015) 11:485–7. 10.4103/0973-1482.140782 [DOI] [PubMed] [Google Scholar]
- 14.Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. (2016) 6:24364. 10.1038/srep24364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. (2011) 79:1487–1495. 10.1016/j.ijrobp.2009.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chernov M, Ono Y, Abe K, Usukura M, Hayashi M, Izawa M, et al. Differentiation of tumor progression and radiation-induced effects after intracranial radiosurgery. Acta Neurochir Suppl. (2013) 116:193–210. 10.1007/978-3-7091-1376-9_29 [DOI] [PubMed] [Google Scholar]
- 17.Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. (2017) 9:273–80. 10.14740/jocmr2936e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali FS, Arevalo O, Zorofchian S, Patrizz A, Riascos R, Tandon N, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. (2019) 21:66. 10.1007/s11912-019-0818-y [DOI] [PubMed] [Google Scholar]
- 19.Sneed PK, Mendez J, Vemer-van den Hoek JGM, Seymour ZA, Ma L, Molinaro AM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. (2015) 123:373–86. 10.3171/2014.10.JNS141610 [DOI] [PubMed] [Google Scholar]
- 20.Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. (2015) 125:149–56. 10.1007/s11060-015-1881-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matuschek C, Bolke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. (2011) 187:135–9. 10.1007/s00066-010-2184-4 [DOI] [PubMed] [Google Scholar]
- 22.Coderre JA, Morris GM, Micca PL, Hopewell JW, Verhagen I, Kleiboer BJ, et al. Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res. (2006) 166:495–503. 10.1667/RR3597.1 [DOI] [PubMed] [Google Scholar]
- 23.Bodensohn R, Hadi I, Fleischmann DF, Corradini S, Thon N, Rauch J, et al. Bevacizumab as a treatment option for radiation necrosis after cranial radiation therapy: a retrospective monocentric analysis. Strahlenther Onkol. (2019) 196:70–6. 10.1007/s00066-019-01521-x [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Chung Y, Kim C, Kim H, Lee H. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. (2004) 19:879–86. 10.3346/jkms.2004.19.6.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstner ER, Duda DG, di Tomaso E, Ryg PA, Loeffler JS, Sorensen AG, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Onco. (2009) 6:229–36. 10.1038/nrclinonc.2009.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordal R, Nagy A, Pintilie M, Wong C. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. (2004) 10:3342–53. 10.1158/1078-0432.CCR-03-0426 [DOI] [PubMed] [Google Scholar]
- 27.Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. (2009) 94:63–8. 10.1007/s11060-009-9801-z [DOI] [PubMed] [Google Scholar]
- 28.Jeyaretna D, Curry WT J, Batchelor T, Stemmer-Rachamimov A, Plotkin S. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. (2011) 29:E159–E62. 10.1200/JCO.2010.31.4815 [DOI] [PubMed] [Google Scholar]
- 29.Zhuang H, Zhuang H, Shi S, Wang Y. Ultra-low-dose bevacizumab for cerebral radiation necrosis: a prospective phase II clinical study. OncoTargets Therap. (2019) 12:8447–53. 10.2147/OTT.S223258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aslan A, Kaya ZB, Bulduk EB, Ocal O, Ucar M, Erpolat OP, et al. Prophylactic bevacizumab may mitigate radiation injury: an experimental study. World Neurosurg. (2018) 116:e791–800. 10.1016/j.wneu.2018.05.094 [DOI] [PubMed] [Google Scholar]
- 31.Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. (2014) 117:321–7. 10.1007/s11060-014-1391-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.