Abstract
Background
Recent advances in esophageal cancer treatment require a reevaluation of the relationship between institutional case‐volume and patient outcome. The aim of this study was to analyze and update the association between surgical case‐volume and both in‐hospital and long‐term mortality after esophagectomy for esophageal cancer.
Methods
Data of all adult patients who received esophageal cancer surgery in Korea between 2004 and 2017 were extracted from the database of the National Health Insurance Service. Hospitals were categorized into three groups according to the average annual number of esophageal cancer surgery: low‐volume (<12 cases/year), medium‐volume (12–48 cases/year), and high‐volume centers (>48 cases/year). Postoperative in‐hospital and 1‐, 3‐, and 5‐year mortality were analyzed according to the categorized groups using logistic regression.
Results
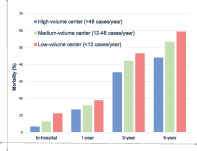
In total, 11, 346 esophageal cancer surgeries in 122 hospitals were analyzed. In‐hospital mortality in the high‐, medium‐, and low‐volume centers were 3.4%, 6.4%, and 11.1%, respectively. In‐hospital mortality was significantly higher in low‐ volume (adjusted odds ratio, 3.91; confidence interval, 3.18–4.80; p < 0.001) and medium volume (adjusted odds ratio, 2.21; confidence interval, 1.80–2.74, p < 0.001) centers compared to high‐volume centers. Patients who received esophageal cancer surgery in a low‐or medium‐volume center also had higher 1‐, 3‐, and 5‐year mortality compared to patients who received the surgery in a high‐volume center.
Conclusions
Centers with lower case‐volume showed higher in‐hospital mortality and long‐term mortality after esophageal cancer surgery.
Keywords: case‐volume effect, esophageal cancer, esophagectomy
This study analyzed the case‐volume effect on postoperative outcomes after esophagectomy using the most recent data retrieved from National Healthcare Insurance Service database of Korea. Centers with lower case‐volume of esophageal cancer surgery showed higher in‐hospital mortality and long‐term mortality after esophageal cancer surgery.
INTRODUCTION
Esophageal cancer is notorious for its poor prognosis and rapid aggressive spread and ranks seventh and eleventh on the list of most frequent male cancer deaths in the United States (US) and in Korea.1, 2, 3 The 5‐year survival rate of 20% in esophageal cancer was the fourth lowest among all cancers in the United States between 2009 and 2015.2 Esophagectomy with neoadjuvant chemoradiotherapy or chemotherapy is the widely accepted standard treatment4 and advances of detection techniques have increased the proportion of patients in operable stages.5 Nevertheless, esophagectomy is still considered as a high‐risk procedure because of significant postoperative morbidity and mortality.6
The amount of individual or institutional clinical experience has been associated with patient outcomes, especially in high‐risk patients or complex procedures.7, 8 Institutions with higher surgical case‐volume in coronary artery bypass,9 liver and heart transplantation,10, 11 or pancreaticoduodenectomy,12 have been shown to outperform institutions with less case‐volume.
Studies regarding esophagectomy have shown that centers with higher case volume tend to show better postoperative outcome.13, 14, 15 Moreover, a previous meta‐analysis suggested that case volume of individual surgeons may also be associated with patient prognosis after esophagectomy for esophageal cancer.16
However, the impact of surgical case‐volume that reflects the recent advances of esophageal cancer management including early detection, surgical techniques, and adjuvant therapies is unclear, because higher standards that many medical institutions stride for may have diluted the previously reported case‐volume effect.
The objective of this study was to analyze the association between the case‐volume of esophagectomy for esophageal cancer and postoperative outcomes including in‐hospital mortality and long‐term mortality. A population‐based retrospective cohort study was performed using the Korean National Healthcare Insurance Service (NHIS) database.
PATIENTS AND METHODS
The study protocol was exempted from review by the institutional review board of Seoul National University Hospital (E‐1905‐098‐1034). Informed consent was waived by the review board because of the anonymous nature of the data.
Data source and study population
Data were obtained from the NHIS database, which contains all healthcare data that are covered by the National Health Insurance (NHI) program in Korea. The NHIS is the single payer of the health insurance system in Korea and covers more than 97% of the Korean population. The database is consisted of claims data for the population covered by the NHI program and the Medical Aid program in Korea.
Adult patients (≥19 years) who received esophageal cancer surgery between 2004 and 2017 were included. Patients were identified using NHI procedure codes for simple esophagectomy, esophageal bypass reconstruction, esophageal reconstruction after resection, and curative operation of esophageal malignant tumor. Afterward, patients with the International Classification of Diseases, 10th revision (ICD‐10) code of C15, malignant neoplasm of esophagus, were included for analysis.
Variables and study endpoints
Age, sex, and pre‐existing comorbidities such as hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, chronic liver disease, chronic kidney disease, and cerebrovascular disease were collected using ICD‐10 codes and prescription history to adjust for when analyzing the correlation with the patient outcome. Procedure codes for adjuvant and neoadjuvant therapy were also used for covariable data extraction. Data on mortality were obtained, which were automatically reported to NHI after the healthcare coverage was terminated because of death.
The primary endpoint of this study was in‐hospital mortality. Secondary endpoints included 1‐, 3‐, and 5‐year mortality.
Definition of case volume
Case‐volume was defined as the average annual number of esophageal cancer surgeries. Hospitals were categorized into three groups according to the case‐volume: low‐volume centers (<12 cases/year), medium‐volume centers (12–48 cases/year), and high‐volume centers (>48 cases/year). The cutoff values were determined after visual inspection of case‐volume distribution.
Statistical analyses
Continuous variables were compared and analyzed using the t‐test or Mann–Whitney U‐test and categorical variables were analyzed using Pearson's χ2 test. Postoperative in‐hospital and 1‐, 3‐, and 5‐year mortality after esophageal cancer surgery were analyzed according to the categorized groups based on case‐volume. Relevant risk factors were evaluated using multivariable logistic regression analyses after adjusting for age, sex, comorbidities, adjuvant therapy, and year of the surgery. In‐hospital mortality cases without adjuvant therapy were not included in long‐term survival analysis. The results of the logistic regression were expressed as odds ratio (OR), 95% confidence interval (CI), and p value. All analyses were performed using SAS 9.4 (SAS Institute), and p < 0.05 was considered statistically significant.
RESULTS
In total, 11, 346 esophageal cancer surgeries were performed in 122 hospitals in Korea between 2004 and 2017. Patient and center characteristics are summarized in Table 1. More than 90% of the patients were male and the majority were over 60 years old. (Table 1) Almost half of the surgeries were performed in six highest‐volume centers with median average surgical volume of 95.5 (Table 1).
TABLE 1.
Patient and center characteristics
| Total | Low volume (<12 cases/year) | Medium volume (12–48 cases/year) | High‐volume (>48 cases/year) | p | |
|---|---|---|---|---|---|
| No. of centers | 122 | 96 | 20 | 6 | |
| No. of patients | 11 346 | 2384 | 3344 | 5618 | |
| Annual case volume | 3.8 [0.8, 11.0] | 2.2 [0.5, 5.6] | 21.4 [14.6, 30.8] | 95.5 [55.6, 160.1] | <0.001 |
| Age | 64.2 (8.5) | 64.6 (8.6) | 64.3 (8.8) | 63.9 (8.3) | 0.001 |
| 19–60 | 3230 (28.5%) | 643 (27.0%) | 966 (28.9%) | 1621 (28.9%) | <0.001 |
| 61–70 | 4870 (42.9%) | 1021 (42.8%) | 1381 (41.3%) | 2468 (43.9%) | |
| 71–80 | 3009 (26.5%) | 653 (27.4%) | 907 (27.1%) | 1449 (25.8%) | |
| ≥81 | 237 (2.1%) | 67 (2.8%) | 90 (2.7%) | 80 (1.4%) | |
| Male | 10 502 (92.6%) | 2209 (92.7%) | 3068 (91.8%) | 5225 (93.0%) | 0.088 |
| Comorbidities | |||||
| Hypertension | 4680 (41.3%) | 991 (41.6%) | 1341 (40.1%) | 2348 (41.8%) | 0.272 |
| Diabetes mellitus | 2965 (26.1%) | 642 (26.9%) | 830 (24.8%) | 1493 (26.6%) | 0.114 |
| Coronary artery disease | 1603 (14.1%) | 295 (12.4%) | 426 (12.7%) | 882 (15.7%) | <0.001 |
| Chronic obstructive pulmonary disease | 991 (8.7%) | 170 (7.1%) | 323 (9.7%) | 498 (8.9%) | 0.003 |
| Chronic liver disease | 4022 (35.5%) | 921 (38.6%) | 1184 (35.4%) | 1917 (34.1%) | 0.001 |
| Chronic kidney disease | 149 (1.3%) | 37 (1.6%) | 44 (1.3%) | 68 (1.2%) | 0.471 |
| Cerebrovascular disease | 980 (8.6%) | 218 (9.1%) | 300 (9.0%) | 462 (8.2%) | 0.291 |
| Adjuvant therapy | <0.001 | ||||
| No neoadjuvant therapy | 3476 (30.6%) | 1115 (46.8%) | 1408 (42.1%) | 953 (17.0%) | |
| Neoadjuvant chemotherapy | 6325 (55.8%) | 1069 (44.8%) | 1751 (52.4%) | 3505 (62.4%) | |
| Neoadjuvant chemoradiotherapy | 1545 (13.6%) | 200 (8.4%) | 185 (5.5%) | 1160 (20.7%) | |
| Surgery year | |||||
| 2004–2010 | 5075 (44.7%) | 1090 (45.7%) | 1514 (45.3%) | 2471 (44.0%) | 0.270 |
| 2011–2017 | 6271 (55.3%) | 1294 (54.3%) | 1830 (54.7%) | 3147 (56.0%) |
Values are expressed as mean (standard deviation), median [interquartile range] or n (%).
The overall in‐hospital mortality was 5.9% (670/11,346) after esophageal cancer surgery. Logistic regression for in‐hospital mortality demonstrated significantly higher mortality in low‐volume (adjusted OR = 3.91; CI = 3.18–4.80; p < 0.001) and medium‐volume (adjusted OR = 2.21; CI = 1.80–2.74, p < 0.001) centers compared to high‐volume centers (Table 2). Patients over 70 years showed higher risk‐adjusted in‐hospital mortality compared to patients <60 years. Male sex and requirement of neoadjuvant chemoradiotherapy were also identified as risk factors for in‐hospital mortality (Table 2). In addition, patients who underwent esophagectomy in the 2004–2010 period showed a significantly higher in‐hospital mortality compared patients who underwent esophagectomy in the 2011–2017 period.
TABLE 2.
Logistic regression analysis for in‐hospital mortality after esophageal cancer surgery
| In‐hospital mortality [n/N (%)] | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Case‐volume | |||||
| High‐volume (>48 cases/year) | 192/5618 (3.4%) | 1 | 1 | ||
| Medium‐volume (12–48 cases/year) | 214/3344 (6.4%) | 1.93 (1.58–2.36) | <0.001 | 2.21 (1.80–2.74) | <0.001 |
| Low‐volume (<12 cases/year) | 264/2384 (11.1%) | 3.52 (2.90–4.27) | <0.001 | 3.91 (3.18–4.80) | <0.001 |
| Age | |||||
| 19–60 | 116/3230 (3.6%) | 1 | 1 | ||
| 61–70 | 243/4870 (5.0%) | 1.41 (1.12–1.77) | 0.003 | 1.40 (1.11–1.76) | 0.004 |
| 71–80 | 268/3009 (8.9%) | 2.62 (2.10–3.28) | <0.001 | 2.81 (2.23–3.56) | <0.001 |
| ≥81 | 43/237 (18.1%) | 5.95 (4.07–8.69) | <0.001 | 6.30 (4.25–9.36) | <0.001 |
| Sex | |||||
| Female | 27/844 (3.2%) | 1 | 1 | ||
| Male | 643/10502 (6.1%) | 1.97 (1.33–2.92) | <0.001 | 1.85 (1.25–2.76) | <0.001 |
| Comorbidities | |||||
| Hypertension | 301/4680 (6.4%) | 1.17 (1.00–1.37) | 0.046 | 0.94 (0.79–1.12) | 0.489 |
| Diabetes mellitus | 206/2965 (7.0%) | 1.27 (1.08–1.51) | 0.005 | 1.15 (0.96–1.38) | 0.139 |
| Coronary artery disease | 97/1603 (6.1%) | 1.03 (0.83–1.29) | 0.787 | 0.93 (0.73–1.17) | 0.518 |
| Chronic obstructive pulmonary disease | 70/991 (7.1%) | 1.24 (0.96–1.60) | 0.106 | 1.11 (0.85–1.44) | 0.450 |
| Chronic liver disease | 278/4022 (6.9%) | 1.31 (1.12–1.54) | <0.001 | 1.27 (1.07–1.50) | 0.005 |
| Chronic kidney disease | 14/149 (9.4%) | 1.67 (0.96–2.91) | 0.072 | 1.25 (0.70–2.23) | 0.462 |
| Cerebrovascular disease | 77/980 (7.9%) | 1.41 (1.10–1.80) | 0.007 | 1.19 (0.92–1.55) | 0.190 |
| Adjuvant therapy | |||||
| Neoadjuvant chemotherapy | 316/6325 (5.0%) | 1 | 1 | ||
| No neoadjuvant therapy | 228/3476 (6.6%) | 1.33 (1.12–1.59) | 0.001 | 1.03 (0.85–1.24) | 0.783 |
| Neoadjuvant chemoradiotherapy | 126/1545 (8.2%) | 1.69 (1.36–2.09) | <0.001 | 2.56 (2.04–3.22) | <0.001 |
| Surgery year | |||||
| 2004–2010 | 328/5075 (6.5%) | 1.20 (1.03–1.40) | 0.024 | 1.37 (1.16–1.61) | <0.001 |
| 2011–2017 | 342/6271 (5.5%) | 1 | 1 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
After adjusting for age, sex, comorbidities, adjuvant therapy, and surgery year, there was an inverse relationship between annual case‐volume and long‐term mortality. Patients who received esophageal cancer surgery in a low‐ or medium‐volume center showed significantly higher 1‐, 3‐, and 5‐year mortality compared to patients who received the surgery in a high‐volume center (Table 3). All‐cause mortality at 1, 3, and 5 years after esophageal cancer surgery were 15.2% (1625/10,666), 39.5% (3,544/8,968), and 49.8% (3,634/7,301), respectively. Older age and male sex were identified as significant risk factors for long‐term mortality (Table 3).
TABLE 3.
Multivariable logistic regression analysis for 1‐, 3‐, and 5‐year mortality after esophageal cancer surgery
| 1‐year mortality | 3‐year mortality | 5‐year mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | p | n/N (%) | OR (95% CI) | p | n/N (%) | OR (95% CI) | p | |
| Case‐volume | |||||||||
| High‐volume (>48 cases/year) | 729/5421 (13.4%) | 1 | 1614/4569 (35.3%) | 1 | 1643/3730 (44%) | 1 | |||
| Medium‐volume (12–48 cases/year) | 498/3129 (15.9%) | 1.35 (1.19–1.54) | <0.001 | 1114/2649 (42.1%) | 1.43 (1.29–1.59) | <0.001 | 1144/2143 (53.4%) | 1.55 (1.38–1.74) | <0.001 |
| Low‐volume (<12 cases/year) | 398/2116 (18.8%) | 1.63 (1.41–1.88) | <0.001 | 816/1750 (46.6%) | 1.67 (1.48–1.88) | <0.001 | 847/1428 (59.3%) | 1.94 (1.70–2.21) | <0.001 |
| Age | |||||||||
| 19–60 | 416/3109 (13.4%) | 1 | 947/2638 (35.9%) | 1 | 952/2160 (44.1%) | 1 | |||
| 61–70 | 651/4622 (14.1%) | 1.08 (0.94–1.24) | 0.289 | 1516/3903 (38.8%) | 1.16 (1.04–1.29) | 0.007 | 1613/3235 (49.9%) | 1.27 (1.14–1.43) | <0.001 |
| 71–80 | 491/2741 (17.9%) | 1.62 (1.39–1.88) | <0.001 | 988/2281 (43.3%) | 1.55 (1.38–1.76) | <0.001 | 992/1798 (55.2%) | 1.74 (1.52–1.99) | <0.001 |
| ≥81 | 67/194 (34.5%) | 4.31 (3.11–5.96) | <0.001 | 93/146 (63.7%) | 3.89 (2.72–5.56) | <0.001 | 77/108 (71.3%) | 3.68 (2.38–5.69) | <0.001 |
| Sex | |||||||||
| Female | 94/816 (11.5%) | 1 | 191/647 (29.5%) | 1 | 185/506 (36.6%) | 1 | |||
| Male | 1531/9850 (15.5%) | 1.34 (1.07–1.68) | 0.011 | 3353/8321 (40.3%) | 1.58 (1.32–1.89) | <0.001 | 3449/6795 (50.8%) | 1.76 (1.45–2.13) | <0.001 |
| Comorbidities | |||||||||
| Hypertension | 682/4378 (15.6%) | 0.96 (0.84–1.08) | 0.465 | 1420/3589 (39.6%) | 0.94 (0.85–1.04) | 0.213 | 1414/2824 (50.1%) | 0.95 (0.86–1.06) | 0.382 |
| Diabetes mellitus | 461/2757 (16.7%) | 1.13 (0.99–1.28) | 0.064 | 928/2248 (41.3%) | 1.09 (0.98–1.22) | 0.103 | 910/1787 (50.9%) | 1.03 (0.92–1.16) | 0.636 |
| Coronary artery disease | 236/1505 (15.7%) | 0.99 (0.84–1.16) | 0.893 | 507/1268 (40.0%) | 1.02 (0.90–1.16) | 0.769 | 518/1036 (50%) | 0.99 (0.86–1.14) | 0.909 |
| Chronic obstructive pulmonary disease | 168/920 (18.3%) | 1.23 (1.02–1.47) | 0.028 | 323/793 (40.7%) | 1.00 (0.86–1.17) | 0.997 | 343/659 (52%) | 1.05 (0.89–1.24) | 0.555 |
| Chronic liver disease | 574/3741 (15.3%) | 0.98 (0.87–1.10) | 0.737 | 1228/3103 (39.6%) | 0.98 (0.89–1.08) | 0.670 | 1261/2508 (50.3%) | 1.00 (0.90–1.11) | 0.975 |
| Chronic kidney disease | 29/135 (21.5%) | 1.44 (0.93–2.22) | 0.099 | 48/98 (49.0%) | 1.43 (0.94–2.17) | 0.091 | 36/58 (62.1%) | 1.72 (0.99–2.99) | 0.056 |
| Cerebrovascular disease | 149/902 (16.5%) | 1.09 (0.89–1.32) | 0.406 | 301/735 (41.0%) | 1.07 (0.91–1.26) | 0.397 | 291/540 (53.9%) | 1.17 (0.97–1.41) | 0.093 |
| Adjuvant therapy | |||||||||
| Neoadjuvant chemotherapy | 822/6006 (13.7%) | 1 | 1856/5051 (36.7%) | 1 | 1899/4077 (46.6%) | 1 | |||
| No neoadjuvant therapy | 497/3241 (15.3%) | 1.28 (1.12–1.47) | <0.001 | 1195/2870 (41.6%) | 1.33 (1.20–1.48) | <0.001 | 1298/2459 (52.8%) | 1.30 (1.16–1.46) | <0.001 |
| Neoadjuvant chemotherapy with radiotherapy | 306/1419 (21.6%) | 2.94 (2.57–3.36) | <0.001 | 493/1047 (47.1%) | 2.90 (2.58–3.26) | <0.001 | 437/765 (57.1%) | 2.85 (2.50–3.26) | <0.001 |
| Surgery year | |||||||||
| 2004–2010 | 806/4739 (17.0%) | 1.43 (1.28–1.60) | <0.001 | 1997/4739 (42.1%) | 1.35 (1.23–1.47) | <0.001 | 2470/4739 (39.8%) | 1.36 (1.23–1.51) | <0.001 |
| 2011–2017 | 819/5927 (13.8%) | 1 | 1547/4229 (36.6%) | 1 | 1989/3165 (62.8%) | 1 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
DISCUSSION
In this study, institutional case‐volume was shown to be a significant factor for in‐hospital mortality after esophageal cancer surgery. In‐hospital mortality was significantly lower in centers with higher esophageal cancer surgery case‐volume compared to centers with less case‐volume. Long‐term mortality of up to 5 years after the surgery was also significantly lower in high case‐volume centers.
There have been conflicting results regarding the impact of case‐volume on surgical outcome in esophagectomy.13, 15, 17, 18 Although most studies reporting a relationship between higher case‐volume and lower postoperative mortality13, 15 and also with shorter hospital length of stay and lower cost,17 the correlation could not be found in other studies.18 Recently, a retrospective analysis of US Nationwide Inpatient Sample (NIS) data from 1998 to 2011 showed that when centers were classified into low‐ (<6 cases/year), intermediate‐ (6–20 cases/year), and high‐ (>20 cases/year) volume centers according to their annual case‐volume of esophagectomies, centers with higher‐volume showed lower mortality.13 A preceding meta‐analysis of 13 studies between 1990 and 2003 also found a significant reduction in postoperative mortality and improved long‐term prognosis with increasing case‐volume.15 In this study, 20 cases per year was suggested as the cutoff value to reduce postoperative mortality defined as the 30‐day mortality rate or in‐hospital mortality to under 5% after comparing four levels of case‐volume. Another meta‐analysis of articles from 2000–2016 showed an association between higher surgical case‐volume and shorter postoperative length of stay with an implied cutoff value of 17 cases per year.17 Moreover, there is a retrospective study using NIS database of the United States, which demonstrated no correlation between hospital surgical volume and in‐hospital mortality after analysis with three techniques; a continuous linear model, a restricted cubic spline, and a categorical model with quintiles of volume.18 However, there was a limitation in relatively small sample size because only the 2007 NIS data of 6248 patients were included in this analysis.
Case‐volume effect on postoperative outcome seems to be apparent only in high risk surgical procedures. The relationship has been demonstrated in solid organ transplantation, cardiac surgery, or pancreaticoduodenectomy,9, 10, 11, 12 all of which require not only sophisticated skills and techniques of the surgeon but also rigorous preoperative patient evaluation and optimization as well as meticulous postoperative management. Patient outcomes after relatively straightforward surgical procedures such as laparoscopic cholecystectomy showed no significant correlation with surgical case‐volume.19, 20 It may be speculated that the case‐volume effect stems not only from the accumulated experience of the surgeon or the surgical team but also from the multidisciplinary team that provides comprehensive perioperative patient management and the protocols that have evolved over the years of accumulated experience.21 Similarly in esophageal cancer surgery, a recent study reported that low‐volume centers with at least three of five key system characteristics (high nurse ratios, lung transplantation services, complex medical oncology, bariatric surgery services, and positron emission tomography scanners) have comparable mortality rate to medium‐ or high‐volume centers by analyzing national data of 4 years in United States and dividing hospitals into tertiles based on esophagectomy volume.22 Surgical case‐volume and system capacity to support complex procedures were found to be factors associated with favorable esophagectomy outcomes in the aforementioned study.22
Our study found that in‐hospital mortality varied from 3.4%–11.1% depending on the case‐volume category, which is similar to previous reports.13, 23 The cutoff values of 12 and 48 cases/year were determined after visual inspection of the distribution of the annual case‐volume and mortality. There were no cutoff values that were consistently used in prior studies. A recent study adopted 6 and 20 cases per year as cutoff values,13 and the most recent Leapfrog guidelines recommended 20 cases/year as the minimum requirement of annual esophagectomy to demonstrate acceptable mortality rates.24 In a recent report, 4 and 17 cases/year were implicated as the thresholds based on their findings between case‐volume and hospital length of stay.17 It was deemed reasonable to divide the groups with the cutoffs at which the outcome seemed distinctly different.
Concordant with prior studies, old age was identified as a significant risk factor for mortality at all time points.13, 18, 25 In terms of adjuvant therapies, neoadjuvant chemotherapy clearly showed improved long‐term survival in our analysis, which is consistent with previous reports.26, 27 Poor prognosis in patients with additional radiotherapy may reflect advanced cancer stage at diagnosis. Minimal impact of comorbidities on postoperative mortality after esophageal surgery that survival after esophageal cancer surgery seems to be largely dependent on cancer factors than other comorbidities. In addition, a significant difference in mortality was found when comparing the first and second half of the study period. Esophagectomy performed in 2011 and thereafter, showed lower in‐hospital and long‐term mortality.
Because of the nature of the administrative data, clinical data such as cancer staging, surgical technique (minimally invasive surgery or open surgery), and emergency factor were not able to be analyzed. Cancer staging is a potential confounder that may affect the case‐volume effect. However, accessibility to healthcare is very high in Korea and patients with advanced disease are more likely to choose a large center with higher case‐volume, which may attenuate the case‐volume effect. With regards to surgical technique, minimally invasive esophagectomy has contributed to improved survival and decreased complications.28, 29 Although it may be assumed that improved treatment options such as neoadjuvant chemotherapy or minimally invasive surgery would attenuate the case‐volume effect, the impact was still noticeable in our results. An alternative view would be the possibility for consolidation of the case‐volume effect, because of the learning curve in the new paradigm of the surgery. With regard to the urgency aspect of the surgical procedure, the effect of emergent esophagectomy seems to be minimal because most esophageal cancer surgeries are performed on an elective basis.
There are several limitations of our study. First, the analysis was performed using a database that primarily has an administrative function. However, the NHIS database covers all patients who underwent cancer surgeries in Korea and offers a strong explanatory power within the scope of the data provided. Second, despite adjustment with comorbidities, individual clinically relevant variables such as laboratory findings, histological diagnoses, extents of surgical resection, surgical technique (open surgery, laparoscopic or thoracoscopic surgery, or robot‐assisted surgery), and cancer staging were lacking because of the nature of the administrative data. Had there been clinical data, the relationship between case‐volume and outcome would have been easier to explain. Nonetheless, it could not be obtained because of the nature of administrative data. Third, the case‐volume of hospitals could not be broken down to the surgeon level. A previous meta‐analysis showed that the surgeon factor is a major factor influencing the institutional case‐volume effect.16 However, only one to three thoracic surgeons performed esophageal cancer surgeries, even in high‐volume centers. A more granular data with information regarding individual surgeons should be constructed and analyzed in future studies. Fourth, a number of esophageal cancer cases may have been misclassified as gastric cancer, or vice versa,30 which requires consideration in interpreting the results.
In conclusion, analysis of the most recent esophageal cancer surgery data that reflects recent advances in management showed that lower institutional case‐volume was independently associated with higher in‐hospital mortality. In addition, centers with lower case‐volume showed higher long‐term mortality at up to 5 years.
CONFLICT OF INTEREST
All authors have nothing to disclose with regard to commercial support.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors. Data acquisition: E.J.J. and J.J. Data analysis: E.J.J. and J.J. Manuscript drafting: B.R.K., D.Y.J., and H.L. Manuscript revision: B.R.K., H.L., and H.G.R. Final approval: all authors.
ACKNOWLEDGMENTS
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University. (Grant number: 800‐20180443).
Kim BR, Jang EJ, Jo J, Lee H, Jang DY, Ryu HG. The association between hospital case‐volume and postoperative outcomes after esophageal cancer surgery: A population‐based retrospective cohort study. Thorac Cancer. 2021;12:2487–2493. 10.1111/1759-7714.14096
Funding information Seoul National University, Grant/Award Number: 800‐20180443
REFERENCES
- 1.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Jung K‐W, Won Y‐J, Hong S, Kong H‐J, Lee ES. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020;52:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaur P, Kim MP, Dunkin BJ. Esophageal cancer: recent advances in screening, targeted therapy, and management. J Carcinog. 2014;13:11–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gockel I, Exner C, Junginger T. Morbidity and mortality after esophagectomy for esophageal carcinoma: a risk analysis. World J Surg Oncol. 2005;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. [DOI] [PubMed] [Google Scholar]
- 9.Kim LK, Looser P, Swaminathan RV, Minutello RM, Wong C, Girardi L, et al. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thorac Cardiovasc Surg. 2016;151:1686–92. [DOI] [PubMed] [Google Scholar]
- 10.Yoo S, Jang EJ, Yi NJ, Kim GH, Kim DH, Lee H, et al. Effect of institutional case volume on in‐hospital mortality after living donor liver transplantation: analysis of 7073 cases between 2007 and 2016 in Korea. Transplantation. 2019;103:952–8. [DOI] [PubMed] [Google Scholar]
- 11.Nam K, Jang EJ, Kim GH, Lee H, Kim DH, Ryu HG. Institutional case‐volume and mortality after heart transplantation. Int Heart J. 2019;60:695–700. [DOI] [PubMed] [Google Scholar]
- 12.Hata T, Motoi F, Ishida M, Naitoh T, Katayose Y, Egawa S, et al. Effect of hospital volume on surgical outcomes after Pancreaticoduodenectomy: a systematic review and meta‐analysis. Ann Surg. 2016;263:664–72. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs HF, Harnsberger CR, Broderick RC, Chang DC, Sandler BJ, Jacobsen GR, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide inpatient sample. Surg Endosc. 2017;31:2491–7. [DOI] [PubMed] [Google Scholar]
- 14.van Lanschot JJ, Hulscher JB, Buskens CJ, Tilanus HW, ten Kate FJ, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer. 2001;91:1574–8. [DOI] [PubMed] [Google Scholar]
- 15.Metzger R, Bollschweiler E, Vallbohmer D, Maish M, DeMeester TR, Holscher AH. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310–4. [DOI] [PubMed] [Google Scholar]
- 16.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long‐term survival after oesophagectomy: systematic review and meta‐analysis. Gut. 2014;63:1393–400. [DOI] [PubMed] [Google Scholar]
- 17.Giwa F, Salami A, Abioye AI. Hospital esophagectomy volume and postoperative length of stay: a systematic review and meta‐analysis. Am J Surg. 2018;215:155–62. [DOI] [PubMed] [Google Scholar]
- 18.Kozower BD, Stukenborg GJ. Hospital esophageal cancer resection volume does not predict patient mortality risk. Ann Thorac Surg. 2012;93:1690–6. discussion 96‐8. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MM, Ng SC, Simons JP, Csikesz NG, Shah SA, Tseng JF. Predictors of major complications after laparoscopic cholecystectomy: surgeon, hospital, or patient? J Am Coll Surg. 2010;211:73–80. [DOI] [PubMed] [Google Scholar]
- 20.Donkervoort SC, Dijksman LM, Versluis PG, Clous EA, Vahl AC. Surgeon's volume is not associated with complication outcome after laparoscopic cholecystectomy. Dig Dis Sci. 2014;59:39–45. [DOI] [PubMed] [Google Scholar]
- 21.Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume‐outcome relationship. Arch Surg. 2007;142:23–31. discussion 32. [DOI] [PubMed] [Google Scholar]
- 22.Funk LM, Gawande AA, Semel ME, Lipsitz SR, Berry WR, Zinner MJ, et al. Esophagectomy outcomes at low‐volume hospitals: the association between systems characteristics and mortality. Ann Surg. 2011;253:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seder CW, Magee MJ, Broderick SR, Brown LM, Blasberg JD, Kozower BD, et al. The Society of Thoracic Surgeons general thoracic surgery database 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:1302–6. [DOI] [PubMed] [Google Scholar]
- 24.The Leapfrog Group Complex Adult and Pediatric Surgery. The Leapfrog Group; 2021. Accessed March 27, 2021. https://ratings.leapfroggroup.org/measure/hospital/complex-adult-surgery
- 25.Raymond DP, Seder CW, Wright CD, Magee MJ, Kosinski AS, Cassivi SD, et al. Predictors of major morbidity or mortality after resection for esophageal cancer: a Society of Thoracic Surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg. 2016;102:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. [DOI] [PubMed] [Google Scholar]
- 27.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. Lancet Oncol. 2011;12:681–92. [DOI] [PubMed] [Google Scholar]
- 28.Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta‐analysis. World J Surg Oncol. 2016;14:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C, Zhang L, Wang H, et al. Superiority of minimally invasive Oesophagectomy in reducing in‐hospital mortality of patients with Resectable Oesophageal cancer: a meta‐analysis. PLoS One. 2015;10:e0132889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblad M, Ye W, Lindgren A, Lagergren J. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg. 2006;243:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


