Abstract
Background
To investigate the burden of thyroid cancer and its attributable risk factors in 204 countries and territories during 30 years.
Methods
We extracted data from the Global Burden of Disease (GBD) 2019 database, including incidence, mortality, disability‐adjusted life‐years (DALYs), and the attributable risk factors of thyroid cancer from 1990 to 2019. Estimated annual percentage changes (EAPC) were calculated to assess the changes in age‐standardized incidence rate (ASIR), age‐standardized mortality rate (ASMR), and age‐standardized DALYs rate (ASDR). We also examined the associations between cancer burden and the sociodemographic index (SDI).
Results
The global new cases, death, and DALYs of thyroid cancer in 2019 were 233 847 (95% UI: 211 637–252 807), 45 576 (95% UI: 41 290‐48 775), and 1 231 841 (95% UI: 1 113 585–1 327 064), respectively. From 1990 to 2019, the ASIR of thyroid cancer showed an upward trend (EAPC = 1.25), but ASMR (EAPC = −0.15) and ASDR (EAPC = −0.14) decreased. The burden of thyroid cancer varied at regional and national levels, but the association between ASIR and SDI was positive. We found that the burden of thyroid cancer was mainly concentrated in females and that the age of onset tended to be younger. The proportion of DALYs from thyroid cancer attributable to high body‐mass index was higher in high SDI regions, especially in males.
Conclusions
The global incidence of thyroid cancer has continued to increase in the past three decades. The high body‐mass index as an important risk factor for thyroid cancer deserves greater attention, especially in high SDI regions.
Keywords: burden of disease, disability‐adjusted life‐years, incidence, mortality, thyroid cancer
The burden of thyroid cancer varies in 204 countries and territories from 1990 to 2019. The global incidence of thyroid cancer (a) has increased significantly, while the mortality (b) and DALYs (c) have decreased in the past three decades.
INTRODUCTION
Thyroid cancer is a malignant tumor originating from the thyroid follicular epithelium or parafollicular epithelial cells, and it is also the most commonly found malignancy in endocrine tumors.1 According to GLOBOCAN estimates, new cases of thyroid cancer were 586 000 globally with an incidence ranking ninth among all cancers in 2020.2 As the world population ages and expands, with an increase in life expectancy and socioeconomic development, the incidence and mortality of thyroid cancer has undergone significant changes.3 Thus, regular assessment of the burden of thyroid cancer and analysis of the epidemiological trends are important in the formulation of future health policies.
Previous studies have reported the burden of thyroid cancer based on the Global Burden of Disease (GBD) 2017 data.4, 5, 6, 7 The GBD 2019, with its up to date population‐based cancer registry data and methodological refinements, provides the most comprehensive estimates of thyroid cancer burden.3, 8, 9, 10 Compared with previous studies, the added value of this study is that cancer registry data have been added from more countries and the minimum age group has been updated (5–9 years old). Here, we used the data from the GBD 2019 to analyze the temporal and geographical trends of the incidence, mortality, disability‐adjusted life‐years (DALYs) in thyroid cancer by global, regional, national, age, sex, and sociodemographic index (SDI) from 1990 to 2019. In addition, we also analyzed the attributable risk factors for thyroid cancer DALYs among different SDI regions.
METHODS
Data source
Incidence, mortality, DALYs, and its corresponding age‐standardized rates (ASR) of thyroid cancer in 204 countries and territories from 1990 to 2019 were obtained from the Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool). In GBD 2019, thyroid cancer was defined by the International Classification of Diseases (ICD) codes: C73‐C73.9, D09.3, D09.8, D34‐D34.9, D44.0, Z85.850, 193–193.9, and 226–226.9. Annual DALYs of thyroid cancer attributable to risk factors were also extracted from the GBD results tool. The GBD 2019 study divides the world into 21 regions and 204 countries and territories according to geographic location. SDI is a composite index of development status strongly correlated with health outcomes, used to divide regions or countries into five levels (high, high‐middle, middle, low‐middle, and low). The SDI values and reference quintiles for all GBD 2019 locations were available in GBD datasets. In addition, age groups were divided into 19 subgroups based on an interval of 5 years. The methodological details have previously been described in the GBD 2019 study.3, 8, 9
Statistical analysis
The estimation process of thyroid cancer was introduced in GBD 2019 publications. The 95% uncertainty interval (UI) is reported for all estimates. The ASR (per 100 000 population) was calculated by the sum of the products of age‐specific rates (a i, where i denotes the i th age) and the number of population (or weight w i) in the same age group i of the selected reference standard population, divided by the sum of the standard population weights: ASR = × 100 000. The estimated annual percentage changes (EAPC) were used to describe the trend of ASR within a specified time interval. The EAPC were estimated by a linear regression model: y = α + βx + ε, where y is ln(ASR), x is the calendar year, and ε is the error term. The EAPC were calculated as 100 × (exp[(β]) ‐ 1) and its 95% confidence interval (CI) obtained from the linear regression model. When the estimated EAPC value and its lower 95% CI were both >0, ASR is considered as an upward trend. Conversely, if the estimated EAPC value and its upper 95% CI were both< 0, ASR is considered a downward trend. R software (Version 4.0.5) and Microsoft Excel (Version 2019) were used to statistics and visualization.
RESULTS
Global trends of thyroid cancer
The global new cases of thyroid cancer in 2019 were 233 847 (95% UI: 211637–252 807), representing an increase of 167% over 1990 (Table 1). From 1990 to 2019, the age‐standardized incidence rate (ASIR) of thyroid cancer showed an upward trend (EAPC = 1.25, 95% CI: 1.12–1.37). Worldwide, thyroid cancer caused 45 576 (95% UI: 41290‐48 775) deaths in 2019 with an age‐standardized mortality rate (ASMR) of 0.57 (95% UI: 0.51–0.61) (Table S1). Over the past 30 years, the ASMR showed a downward trend (EAPC = −0.15, 95% CI: −0.19 to −0.11). Compared with 1990, the number of DALYs increased by 84.56% in 2019 (Table S2). The age‐standardized DALYs rate (ASDR) had a consistent trend with ASMR from 1990 to 2019 (EAPC = −0.14, 95% CI: −0.18 to −0.1).
TABLE 1.
Incident cases and age‐standardized incidence rate of thyroid cancer between 1990 and 2019
| All ages, no. ×103 (95% UI) | Change (%) | Age‐standardized rate per 100 000, no. (95% UI) | EAPC (95% CI) | |||
|---|---|---|---|---|---|---|
| Characteristics | 1990 | 2019 | 1990–2019 | 1990 | 2019 | 1990–2019 |
| Global | 87.58 (82.24–92.72) | 233.85 (211.64–252.81) | 167% | 2.01 (1.9–2.12) | 2.83 (2.56–3.06) | 1.25 (1.12–1.37) |
| Male | 23.79 (22.21–25.7) | 76.01 (68.23–82.92) | 219.56% | 1.16 (1.09–1.24) | 1.9 (1.71–2.07) | 1.89 (1.77–2.02) |
| Female | 63.8 (58.66–68.29) | 157.83 (140.4–173.07) | 147.4% | 2.82 (2.61–3.02) | 3.74 (3.32–4.1) | 0.98 (0.85–1.12) |
| Sociodemographic index | ||||||
| High SDI | 32.9 (31.78–33.89) | 68.41 (62.05–74.86) | 107.95% | 3.4 (3.28–3.5) | 4.59 (4.17–5.03) | 1.21 (0.9–1.51) |
| High‐middle SDI | 25.52 (23.78–26.87) | 57.88 (52.33–63.92) | 126.85% | 2.28 (2.13–2.4) | 3.05 (2.76–3.37) | 1.04 (0.94–1.15) |
| Middle SDI | 16.65 (15.14–18.61) | 64.33 (56.64–71.61) | 286.41% | 1.31 (1.2–1.51) | 2.44 (2.15–2.71) | 2.34 (2.25–2.42) |
| Low‐middle SDI | 8.6 (7.22–10.11) | 30.05 (25.92–33.62) | 249.25% | 1.13 (0.97–1.33) | 1.91 (1.65–2.12) | 1.8 (1.74–1.85) |
| Low SDI | 3.87 (2.92–5.04) | 11.43 (9.35–13.45) | 195.15% | 1.23 (0.98–1.57) | 1.59 (1.31–1.86) | 0.93 (0.88–0.99) |
| Region | ||||||
| Andean Latin America | 0.43 (0.37–0.52) | 2.15 (1.66–2.69) | 403.24% | 1.76 (1.53–2.12) | 3.63 (2.79–4.54) | 2.68 (2.43–2.93) |
| Australasia | 0.52 (0.48–0.56) | 1.74 (1.36–2.23) | 234.82% | 2.31 (2.13–2.52) | 4.44(3.47–5.72) | 2.72 (2.37–3.07) |
| Caribbean | 0.49 (0.45–0.52) | 1.25 (1.04–1.48) | 156.53% | 1.7 (1.57–1.82) | 2.43 (2.04–2.89) | 1.42 (1.29–1.55) |
| Central Asia | 0.76 (0.67–0.88) | 1.47 (1.31–1.65) | 93.25% | 1.44 (1.27–1.67) | 1.69 (1.51–1.89) | 0.42 (0.11–0.73) |
| Central Europe | 4.35 (3.97–4.53) | 5.31 (4.62–6.12) | 22.09% | 3.04 (2.77–3.16) | 3.09 (2.67–3.57) | −0.17 (−0.26 to −0.08) |
| Central Latin America | 1.8 (1.7–1.86) | 7.18 (6.14–8.4) | 299.6% | 1.8 (1.69–1.87) | 2.9 (2.49–3.4) | 1.57 (1.49–1.65) |
| Central Sub‐Saharan Africa | 0.19 (0.14–0.26) | 0.52 (0.36–0.73) | 170.83% | 0.72 (0.51–0.98) | 0.8 (0.54–1.14) | 0.34 (0.27–0.4) |
| East Asia | 11.09 (9.4–13.05) | 41.58 (34.75–50.2) | 274.86% | 1.07 (0.92–1.27) | 2.11 (1.77–2.54) | 2.59 (2.45–2.72) |
| Eastern Europe | 6.11 (5.75–6.87) | 12.26 (10.67–14.15) | 100.51% | 2.28 (2.15–2.56) | 4.25 (3.7–4.93) | 2.44 (2.17–2.7) |
| Eastern Sub‐Saharan Africa | 2.06 (1.4–2.89) | 5.34 (4.11–6.79) | 159.02% | 1.94 (1.41–2.65) | 2.19 (1.72–2.72) | 0.3 (0.15–0.45) |
| High‐income Asia Pacific | 6.73 (6.34–7.58) | 15.66 (13.13–18.06) | 132.84% | 3.32 (3.13–3.75) | 4.98 (4.19–5.79) | 2.16 (1.38–2.94) |
| High‐income North America | 12.63 (12.14–13) | 28.3 (24.46–32.79) | 124.11% | 3.96 (3.82–4.08) | 5.4 (4.64–6.27) | 1.04 (0.85–1.23) |
| North Africa and Middle East | 3.88 (3.23–4.45) | 19.25 (15.68–22.28) | 396.01% | 1.7 (1.44–2) | 3.46 (2.89–3.96) | 2.62 (2.53–2.71) |
| Oceania | 0.05 (0.04–0.07) | 0.16 (0.11–0.22) | 199.47% | 1.45 (1.15–1.86) | 1.8 (1.3–2.4) | 0.68 (0.57–0.78) |
| South Asia | 7.93 (6.67–9.9) | 31.53 (26.59–36.44) | 297.65% | 1.05 (0.9–1.32) | 1.9 (1.61–2.19) | 2.06 (1.94–2.18) |
| Southeast Asia | 7.2 (5.81–8.28) | 25.58 (20.57–29.89) | 255.3% | 2.25 (1.86–2.55) | 3.72 (3.01–4.32) | 1.76 (1.7–1.82) |
| Southern Latin America | 0.96 (0.88–1.02) | 2 (1.56–2.56) | 108.73% | 2.04 (1.88–2.18) | 2.58 (2–3.31) | 0.67 (0.53–0.81) |
| Southern Sub‐Saharan Africa | 0.34 (0.3–0.38) | 0.74 (0.63–0.86) | 114.4% | 1 (0.85–1.1) | 1.13 (0.97–1.3) | 0.43 (0.35–0.52) |
| Tropical Latin America | 1.64 (1.57–1.73) | 4.53 (4.25–5.03) | 176.67% | 1.53 (1.46–1.61) | 1.82 (1.71–2.02) | 0.67 (0.41–0.93) |
| Western Europe | 18.05 (16.98–18.74) | 26.22 (22.59–30.01) | 45.23% | 3.63 (3.41–3.77) | 3.93 (3.4–4.5) | 0.17 (−0.03 to 0.36) |
| Western Sub‐Saharan Africa | 0.37 (0.3–0.44) | 1.08 (0.86–1.33) | 188.99% | 0.36 (0.28–0.42) | 0.43 (0.34–0.52) | 0.69 (0.64–0.75) |
Abbreviations: CI, confidence interval; EAPC, estimated annual percentage change; SDI, sociodemographic index; UI, uncertainty interval.
Regional trends of thyroid cancer
In 2019, the regions with the most incident cases were East Asia (41 580, 95% UI: 34 751–50 204), South Asia (31 534, 95% UI: 26 591–36 439), and high‐income North America (28 296, 95% UI: 24 461–32 790) (Table 1). Andean Latin America had the most increases in incident cases (403.24%) between 1990 and 2019. The ASIR of thyroid cancer showed a downward trend only in Central Europe (EAPC = −0.17, 95% CI: −0.26 to −0.08). It is worth noting that Andean Latin America also had the most increases in deaths (222.77%) and Central Europe had the most significant downward trend in ASMR (EAPC = −2.02, 95% CI: −2.2 to −1.84) (Table S1). The highest DALYs were observed in South Asia (291 575, 95% UI: 254 403–330 796) and the highest ASDR was observed in Eastern Sub‐Saharan Africa (27.78, 95% UI: 21.85–31.40) in 2019 (Table S2). From 1990 to 2019, Australasia (EAPC = 0.96, 95% CI: 0.78–1.14), Andean Latin America (EAPC = 0.54, 95% CI: 0.39–0.69), and South Asia (EAPC = 0.46, 95% CI: 0.31–0.60) had the greatest increase in ASDR for thyroid cancer.
National trends of thyroid cancer
China (39 079, 95% UI: 32 278–47 658), USA (26 270, 95% UI: 22 444–30 610), and India (23 823, 95% UI: 19 467–28 644) had the most incident cases in 2019 among 204 countries and territories (Table S3). The highest increase in incident cases was found in the United Arab Emirates (1790.38%), Saudi Arabia (1560.17%), and Qatar (1414.95%). Moreover, the ASIR showed a significant increase in Republic of Korea (EAPC = 5.67, 95% CI: 3.81–7.56), Saudi Arabia (EAPC =5.67, 95% CI: 5.37–5.98), and Vietnam (EAPC = 5.05, 95% CI: 4.58–5.52) during the past three decades (Figure 1(a)). Compared with 1990, the United Arab Emirates (1012.23%), Qatar (446.67%), and Djibouti (422.47%) had the most increases in deaths from thyroid cancer in 2019 (Table S4). Honduras (3.17, 95% UI 0.95–4.35) and Ethiopia (1.92, 95% UI 1.4–2.46) showed the highest ASMR in 2019. The EAPC showed the most increase in Armenia (EAPC = 3.03, 95% CI: 2.34–3.72) and the most decrease in Poland (EAPC = −3.15, 95% CI: ‐3.51 to −2.78) (Figure 1(b)). The most DALYs caused by thyroid cancer were found in India (217 465, 95% UI: 181112–254 846), China (187 319, 95% UI: 156236–219 112), and USA (62 493, 95% UI: 56922–67 954) in 2019 (Table S5). Armenia (EAPC = 2.82, 95% CI: 2.16–3.49) and Poland (EAPC = −3.34, 95% CI: −3.76 to −2.92) also had the most significant upward and downward trend in ASDR, respectively (Figure 1(c)).
FIGURE 1.
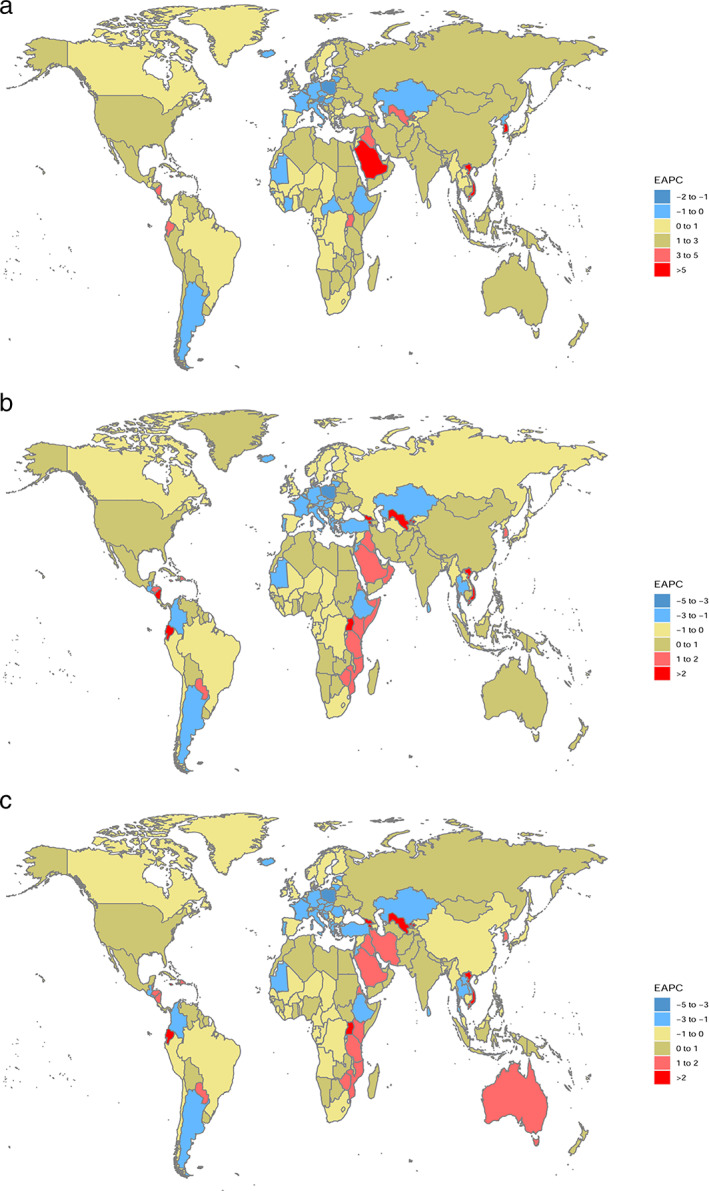
The estimated annual percentage change of thyroid cancer in 204 countries and territories. (a) Age‐standardized incidence rate. (b) Age‐standardized mortality rate. (c) Age‐standardized DALYs rate. EAPC, estimated annual percentage change
Burden of thyroid cancer by SDI
A positive association was found between SDI and ASIR in regional and national level (Figure 2). The high‐level SDI regions and countries had the higher ASIR than the low‐level SDI. However, no associations were observed in ASMR and ASDR of thyroid cancer among different SDI regions and countries (Figure S1 and Figure S2).
FIGURE 2.
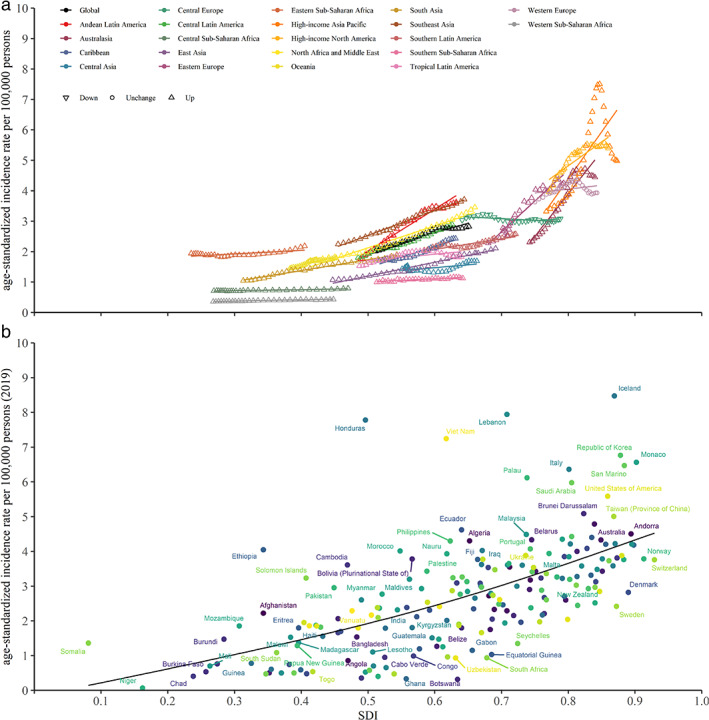
The association between ASIR of thyroid cancer and SDI for 21 regions (a) and 204 countries and territories (b). ASIR, age‐standardized incidence rate; SDI: sociodemographic index
Burden of thyroid cancer by age and sex
The 55–59 years age group (10 257, 95% UI: 9027 to 11 475) and 50–54 years age group (17 547, 95% UI: 15 534 to 19 685) had the most incident cases of thyroid cancer among females and males in 2019, respectively (Figure 3(a)). The incidence rate was highest in the 95 years or older age group both males (10.11, 95% UI: 6.87 to 11.90) and females (16.66, 95% UI: 11.35 to 19.83). The age groups where most deaths were in males and females were those aged 75–79 years (2566, 95% UI: 2217–2823) and 70–74 years old (3546, 95% UI: 3163–3892), respectively (Figure 3(b)). The mortality rate of females was higher than males in most age groups. The number and rate of DALYs in females were highest in 60–64 year olds (83 923, 95% UI: 73 540–92 137) and 75–79 year olds (77.59, 95% UI: 68.64–84.51), respectively (Figure 3(c)).
FIGURE 3.
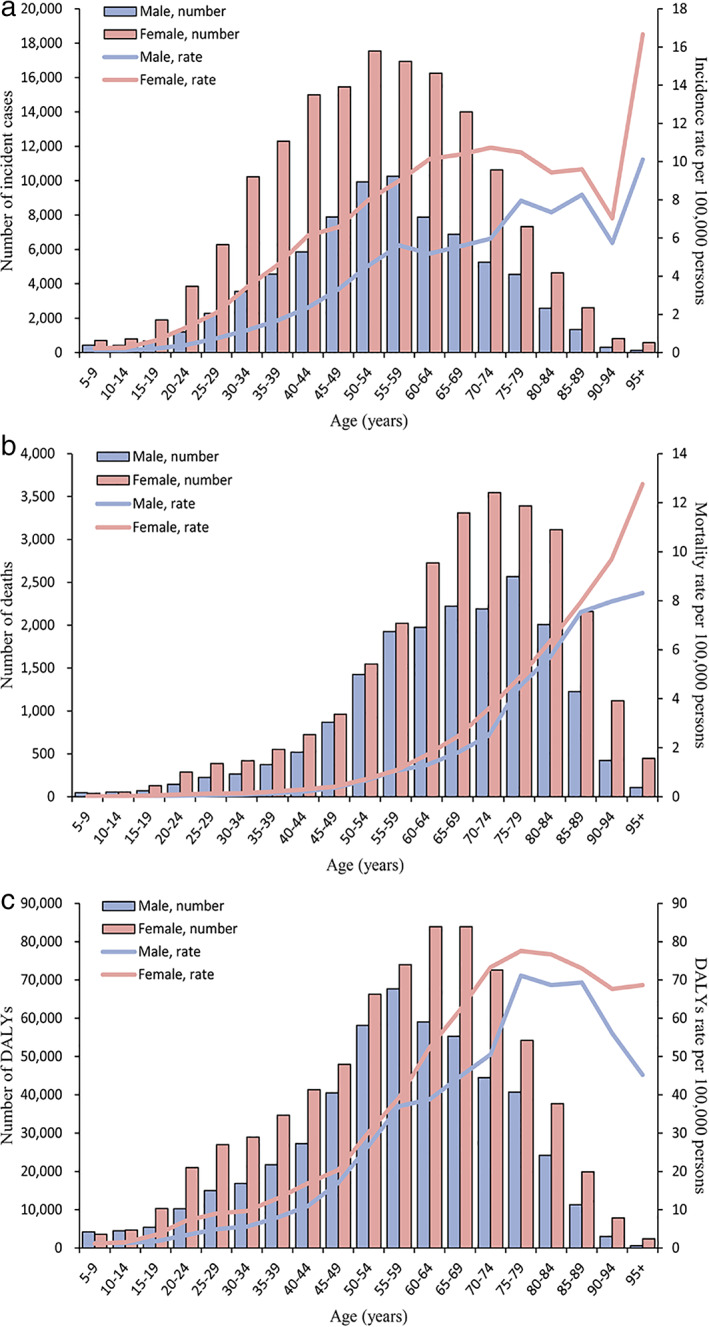
(a) Global incidence, (b) mortality, and (c) DALYs of thyroid cancer by age and sex in 2019. DALYs, disability‐adjusted life‐years
Attributable risk factors
The attributable risk factor for thyroid cancer DALYs was high body‐mass index. The proportion of DALYs from thyroid cancer attributable to high body‐mass index was highest in high SDI regions (14.38%, 95% UI: 7.28–23.00) among both sexes in 2019 (Figure 4(a)). The proportion of DALYs attributable to high body‐mass index was higher in males than females among six GBD regions between 1990 and 2019 (Figure 4(b) and Figure 4(c)).
FIGURE 4.
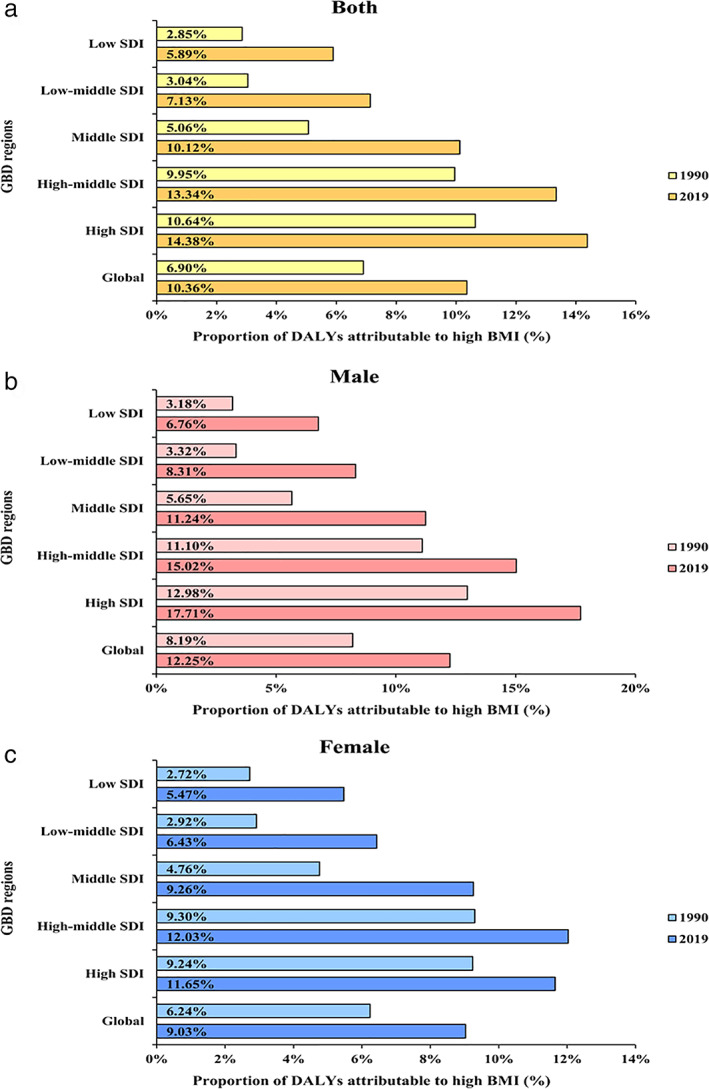
The proportion of DALYs from thyroid cancer attributable to high BMI by sex and SDI between 1990 and 2019. (a) Both. (b) Male. (c) Female. SDI, sociodemographic index; DALYs, disability‐adjusted life‐years; BMI, body‐mass index
DISCUSSION
We found that the global incidence of thyroid cancer has increased significantly, while the mortality and DALYs have decreased during the 30 year study period. We also found the incidence of thyroid cancer had a positive association with the SDI level. However, the associations were not found in mortality and DALYs of thyroid cancer. The incidence, mortality, and DALYs of thyroid cancer in females were higher than males among most age groups. Furthermore, deaths due to thyroid cancer were mainly distributed among the 65–79 year old age group (both males and females). For attributable risk factors, the impact of high body‐mass index on DALYs from thyroid cancer was greater in males than in females in different regions. Compared with previous GBD studies,4, 5, 6, 7 we used up‐to‐date epidemiological data to investigate the burden of thyroid cancer, examined the association between the SDI and incidence, mortality, and DALYs of thyroid cancer, and clarified the disparity of attributable risk factors for DALYs due to thyroid cancer in the GBD region. In addition, this study covered more locations and data sources, including 204 countries and territories. Hence, timely analysis of the epidemiological data is an effective way to provide evidence to support the medical care planning and resource allocation for thyroid cancer.
From 1990 to 2019, the global ASIR of thyroid cancer showed an upward trend, while the ASMR and ASDR showed the opposite trend. This trend in thyroid cancer might have the following reasons. First, with the popularization and application of advanced diagnostic techniques, more occult thyroid carcinoma is discovered accidentally.11, 12 Second, socioeconomic development and increased health awareness have increased the early detection rate of thyroid cancer. Third, overdiagnosis has also contributed to a significant increase in the incidence of thyroid cancer. It has been estimated that the proportion of female thyroid cancer caused by overdiagnosis was about 93% in South Korea, 91% in Belarus, 87% in China, 84% in Italy and Croatia, and 83% in Slovakia and France between 2008 and 2012.13 Finally, there are still other factors which might affect the incidence of thyroid cancer, such as family history, obesity, red meat, processed food consumption, iodine intake, mental factors and environmental pollutants.14 In addition, we found that women's incidence, mortality, and DALYs were higher than men in most age groups. Abnormal levels of female estrogen and unhealthy eating habits are thought to be one of the causes of thyroid cancer.15, 16 Women are generally more susceptible to health care than men (for example, due to reproductive and perimenopausal factors), which might lead to additional opportunities for thyroid examination.13 This might partly explain why the incidence of thyroid cancer in women is increasing, while the incidence in men remains generally stable.
We have observed the heterogeneity in the burden of thyroid cancer at regional and national levels. In 2019, the most incident cases of thyroid cancer were in East Asia, South Asia, and North America. This might be partly related to the huge population base of these areas. South Korea is currently the country with the highest ASIR of thyroid cancer in the world, which might be related to the cancer screening project started in 1999.17 In South Korea, the increased detection rate of thyroid cancer is the result of opportunistic thyroid testing provided as an additional service within the scope of the national screening program,12 while in other countries, the detection rate may depend on the intensity of monitoring and the use of ultrasound or other diagnostic techniques.18 Moreover, we found a positive association between SDI and ASIR of thyroid cancer at the regional and national levels, which means countries and regions with a high SDI have a high incidence of thyroid cancer. Countries with a high SDI had higher levels of medical care and people's health awareness, which is conducive to the early detection of thyroid cancer. Similarly, people with higher socioeconomic status were more likely to receive health care services and had increased contact with the health care system.19 Economic level may be an important factor affecting the burden level of thyroid cancer.
High body mass index is the only attributable risk factor for thyroid cancer in the GBD 2019. In this study, we found that the middle‐to‐high SDI regions had the highest proportion of thyroid cancer DALYs due to high body mass index. Previous studies have proven a positive correlation between high body mass index and the incidence of thyroid cancer, especially in women.20, 21 A cross‐sectional study also proved that the aggressive clinical features of thyroid cancer were related to overweight and obesity.22 Therefore, strengthening the regulation of diet and reducing bodyweight are beneficial for the prevention of thyroid cancer. Furthermore, there are many other risk factors related to the onset and progression of thyroid cancer. Exposure to radiation is the most widely known environmental risk for the development of thyroid cancer.23 The thyroid is particularly susceptible to the carcinogenic effects of radiation, especially in childhood or adolescence.24 Since the introduction of computed tomography (CT) in the 1970s, radiation exposure from medical imaging had become an important risk factor for thyroid cancer.25, 26 In Fukushima, Japan, the results of thyroid ultrasound examination programs might be affected by radiation exposure.27 Both iodine deficiency and iodine supplementation may have long‐term effects on thyroid cancer.23 A retrospective study showed that the rapid increase in the incidence of thyroid cancer in sub‐Saharan Africa is mainly related to iodine deficiency.28 Because of this environmental difference, some African populations had a higher incidence of follicular thyroid cancer compared with high‐income countries with sufficient iodine.28, 29 The incidence of thyroid cancer in Shanghai, China has steadily increased, especially after the implementation of iodine supplementation.30 A 14–65 year follow‐up study showed that iodine supplementation in countries such as Austria, Norway, Denmark and Colombia led to an increase in the incidence of thyroid cancer.31 Smoking is a risk factor for most cancers, but it has been previously reported that it can reduce the risk in the development process of thyroid cancer.32 A meta‐analysis that included 14 case–control studies from the United States, Europe and Asia found that the risk of thyroid cancer among current smokers was significantly reduced (OR = 0.6, 95% CI: 0.6–0.7), while it was not reduced for former smokers (OR = 0.9, 95% CI: 0.8–1.1).33 Other studies had hypothesized that elevated TSH levels could increase the risk of thyroid cancer, but it was interesting that compared with previous smokers and never smokers, current smokers had lower serum TSH levels, which might explain why smoking could reduce the risk of thyroid cancer.34, 35
Although our research was based on the data and methods of GBD 2019, it still had certain limitations. First, the primary data of GBD 2019 cannot cover all countries and regions in the world, which has been reported in the GBD study. Second, the pathological subtypes of thyroid cancer have not been distinguished, and the contribution of different pathological subtypes to the burden of disease may be quite different. Third, because the GBD data was mainly for various countries and regions, it was impossible to evaluate the difference in race and ethnicity. Finally, in this study we only reported the thyroid cancer DALYs due to high body mass index. Since other attributable risk factors for thyroid cancer have not yet been included in GBD 2019, we suggest that relevant studies be conducted in the future for a more comprehensive evaluation. Despite the limitations of this study, the results are still indispensable and could assist in providing evidence to enable the formulation of clinical guidelines and public health policies for thyroid cancer.
In conclusion, the global incidence of thyroid cancer has continued to increase in the past three decades. The high body‐mass index as an important risk factor for thyroid cancer deserves greater attention, especially in high SDI regions. Based on our analysis of the epidemiological trends in thyroid cancer, decision makers could take these into account in order to allocate limited resources more reasonably and formulate relevant policies.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Figure S1 The association between ASMR of thyroid cancer and SDI for 21 regions (a) and 204 countries and territories (b). ASMR, age‐standardized mortality rate; SDI: sociodemographic index.
Figure S2 The association between ASDR of thyroid cancer and SDI for 21 regions (a) and 204 countries and territories (b). ASDR, age‐standardized DALYs rate; SDI: sociodemographic index; DALYs, disability‐adjusted life‐years.
Table S1 Deaths and age‐standardized mortality rate of thyroid cancer between 1990 and 2019.
Table S2 DALYs and age‐standardized DALYs rate of thyroid cancer between 1990 and 2019.
Table S3 Incidence cases and age‐standardized incidence rate of thyroid cancer in 204 countries and territories between 1990 and 2019.
Table S4 Deaths and age‐standardized mortality rate of thyroid cancer in 204 countries and territories between 1990 and 2019.
Table S5 DALYs and age‐standardized DALYs rate of thyroid cancer in 204 countries and territories between 1990 and 2019.
Bao W‐Q, Zi H, Yuan Q‐Q, Li L‐Y, Deng T. Global burden of thyroid cancer and its attributable risk factors in 204 countries and territories from 1990 to 2019. Thorac Cancer. 2021;12:2494–2503. 10.1111/1759-7714.14099
REFERENCES
- 1.National Health Commission of the People's Republic of China . Guidelines for diagnosis and treatment of thyroid cancer (2018 edition). Chinese J Gener Surg. 2019;13:1–15. [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai M, Zhang D, Long J, Gong Y, Ye F, Liu S, et al. The global burden of thyroid cancer and its attributable risk factor in 195 countries and territories: a systematic analysis for the global burden of disease study. Cancer Med. 2021;10:4542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y, Mubarik S, Li R, Nawsherwan YC. Trend dynamics of thyroid cancer incidence among China and the U.S. adult population from 1990 to 2017: a joinpoint and age‐period‐cohort analysis. BMC Public Health. 2021;21:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azadnajafabad S, Saeedi Moghaddam S, Mohammadi E, Rezaei N, Ghasemi E, Fattahi N, et al. Global, regional, and national burden and quality of care index (QCI) of thyroid cancer: a systematic analysis of the global burden of disease study 1990‐2017. Cancer Med. 2021;10:2496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open. 2020;3:e208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2019 Demographics Collaborators . Global age‐sex‐specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. 2020;396:1160–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brito JP, Davies L. Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. 2014;21:405–8. [DOI] [PubMed] [Google Scholar]
- 12.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal ML. Worldwide thyroid‐cancer epidemic? The increasing impact of Overdiagnosis. N Engl J Med. 2016;375:614–7. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8:468–70. [DOI] [PubMed] [Google Scholar]
- 14.Peterson E, De P, Nuttall R. BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One. 2012;7:e29177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age‐specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhri E, Fathi W, Hussain F, Hashmi SK. The increasing trends in cases of the Most common cancers in Saudi Arabia. J Epidemiol Glob Health. 2020;10:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet. 2014;384:1848. [DOI] [PubMed] [Google Scholar]
- 18.Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitzel LR, Nguyen N, Li N, Xu L, Regan SD, Sturgis EM. Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity. Thyroid. 2014;24:556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation risk factors for thyroid cancer in the US radiologic technologists study. Am J Epidemiol. 2010;171:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case‐control studies. Thyroid. 2014;24:966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tresallet C, Seman M, Tissier F, Buffet C, Lupinacci RM, Vuarnesson H, et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery. 2014;156:1145–52. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16:17–29. [DOI] [PubMed] [Google Scholar]
- 24.Refetoff S, Harrison J, Karanfilski BT, Kaplan EL, De Groot LJ, Bekerman C. Continuing occurrence of thyroid carcinoma after irradiation to the neck in infancy and childhood. N Engl J Med. 1975;292:171–5. [DOI] [PubMed] [Google Scholar]
- 25.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya K, Gilmour S, Oshima A. Time to reconsider thyroid cancer screening in Fukushima. Lancet. 2014;383:1883–4. [DOI] [PubMed] [Google Scholar]
- 28.Woodruff SL, Arowolo OA, Akute OO, Afolabi AO, Nwariaku F. Global variation in the pattern of differentiated thyroid cancer. Am J Surg. 2010;200:462–6. [DOI] [PubMed] [Google Scholar]
- 29.Ukekwe FI, Olusina DB, Okere PCN. Patterns of thyroid cancers in southeastern Nigeria: a 15 year histopathologic review (2000‐2014). J Clin Diagn Res. 2017;11:EC16–EC9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983‐2007. Asia Pac J Public Health. 2015;27:NP223–9. [DOI] [PubMed] [Google Scholar]
- 31.Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26:1541–52. [DOI] [PubMed] [Google Scholar]
- 32.Burgess JR, Dwyer T, McArdle K, Tucker P, Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978‐1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab. 2000;85:1513–7. [DOI] [PubMed] [Google Scholar]
- 33.Mack WJ, Preston‐Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, et al. A pooled analysis of case‐control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003;14:773–85. [DOI] [PubMed] [Google Scholar]
- 34.Williams ED. TSH and thyroid cancer. Horm Metab Res Suppl. 1990;23:72–5. [PubMed] [Google Scholar]
- 35.Soldin OP, Goughenour BE, Gilbert SZ, Landy HJ, Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The association between ASMR of thyroid cancer and SDI for 21 regions (a) and 204 countries and territories (b). ASMR, age‐standardized mortality rate; SDI: sociodemographic index.
Figure S2 The association between ASDR of thyroid cancer and SDI for 21 regions (a) and 204 countries and territories (b). ASDR, age‐standardized DALYs rate; SDI: sociodemographic index; DALYs, disability‐adjusted life‐years.
Table S1 Deaths and age‐standardized mortality rate of thyroid cancer between 1990 and 2019.
Table S2 DALYs and age‐standardized DALYs rate of thyroid cancer between 1990 and 2019.
Table S3 Incidence cases and age‐standardized incidence rate of thyroid cancer in 204 countries and territories between 1990 and 2019.
Table S4 Deaths and age‐standardized mortality rate of thyroid cancer in 204 countries and territories between 1990 and 2019.
Table S5 DALYs and age‐standardized DALYs rate of thyroid cancer in 204 countries and territories between 1990 and 2019.


