A classic and highly influential model of visual processing proposes that the role of the retina is to compress visual information for optimal transmission to the brain [1]. Drawing on ideas from information theory, an efficient retinal code could be defined as one that reduces redundancy to communicate as much information as possible, given the optic nerve’s limited capacity. From this redundancy reduction hypothesis, a theory of retinal color coding emerged in which the three most common retinal ganglion cell (RGC) types captured much of the variance in natural spectra [2]. Within this compact code, the ‘Blue-ON’ small bistratified RGC was thought to be the only pathway necessary for comparing short (S) wavelength-sensitive cones to long (L) and medium (M) wavelength-sensitive cones [3,4]. Here, we discovered a new wide-field RGC type receiving the same cone-opponent input as the small bistratified RGC, indicating that there is more redundancy in the retinal color code than previously appreciated.
To explore S-cone circuit diversity, we reconstructed the neurons and synapses of the S-cone connectome from a serial electron microscopy volume of macaque central retina. Building on our previous work [5], we reconstructed 70 S-ON bipolar cell (BC) terminals providing excitatory ribbon synaptic input to 20 small bistratified RGCs (Figure 1A). This extensive network of well-studied S-cone circuits provided a base for confident identification of novel S-cone neurons. The small bistratified RGC’s Blue-ON response is more specifically S-ON/LM-OFF cone opponency [3]. S-ON BC ribbon synaptic input provides the S-ON and some of the LM-OFF response (Figure 1F). Accordingly, other neurons receiving the same S-ON BC input are predicted to have similar spectral tuning [5].
Figure 1. Connectomic reconstructions of two types of ‘Blue-ON’ RGC in the primate retina.
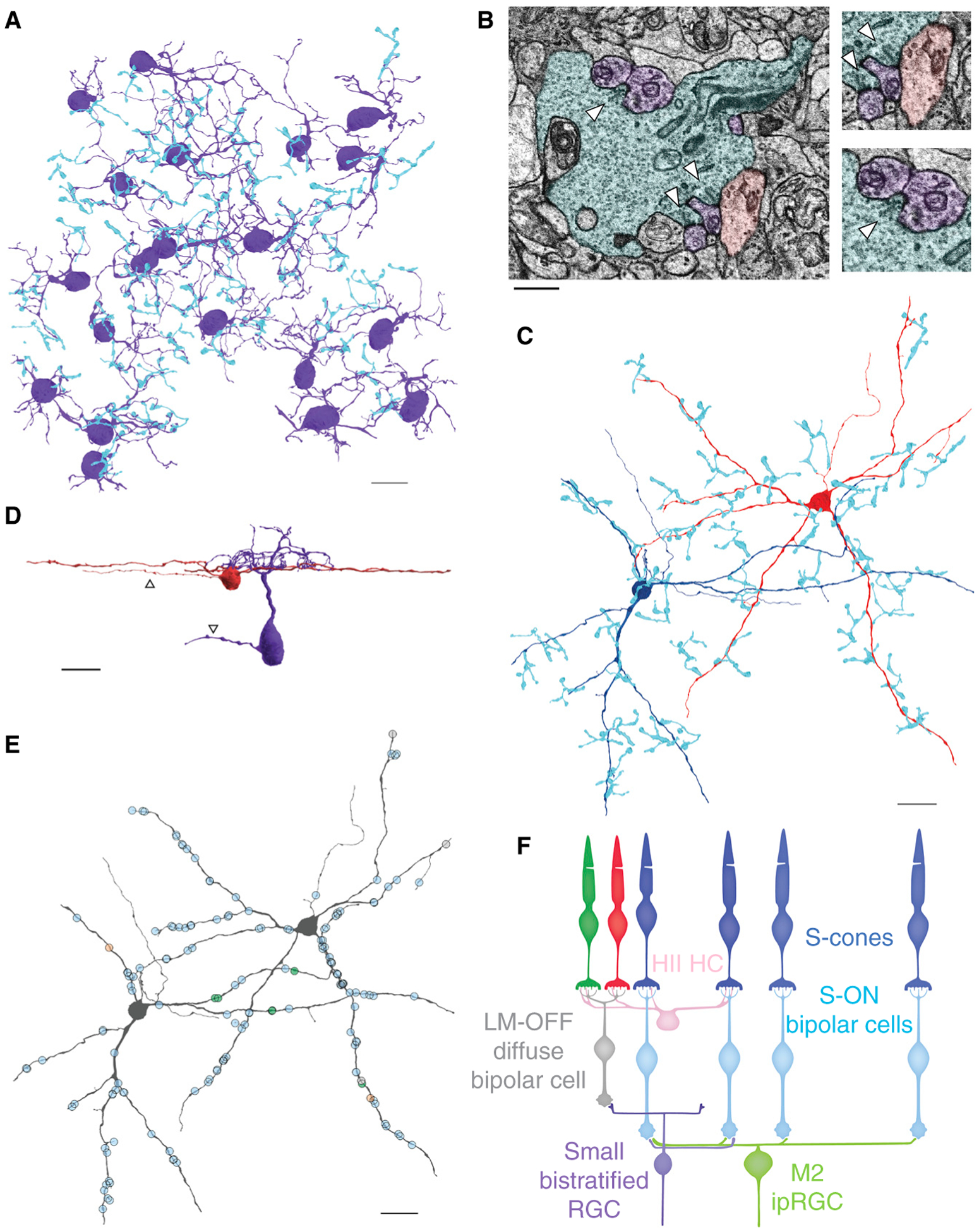
(A) Reconstructions of S-ON BC axon terminals (blue) providing input to small bistratified RGCs (purple). (B) Electron micrograph of an S-ON BC terminal (blue) and post-synaptic dendrites of small bistratified RGCs (purple) and a wide-field RGC (red). Arrowheads mark presynaptic ribbons within the S-ON BC terminal, which are adjacent to a membrane density on the postsynaptic neuron. (C) Reconstructions of two wide-field RGCs (dark blue, red) and presynaptic S-ON BC terminals (blue). (D) 3D reconstructions of a small bistratified RGC (purple) and wide-field RGC (red) viewed from the side. Arrowheads specify the axons of each cell. (E) Locations of ribbon synaptic input to the wide-field RGCs. S-ON BC, ON midget BC and DB6 BC inputs are marked in blue, green and orange, respectively. (F) Circuit diagrams for the wide-field RGCs and small bistratified RGCs. The S-ON bipolar cell carries an LM-OFF response contributed by HII horizontal cell-mediated antagonistic feedback to S-cones from neighboring L/M-cones. The scale bar in 1B is 2 microns and the rest are 20 microns.
We began by mapping the different RGC types post-synaptic to S-ON BC ribbon synapses. The majority were small bistratified RGCs; however, we also encountered other RGC types (Figure 1B). By reconstructing these unknown neurons, we identified a wide-field monostratified RGC type that receives nearly exclusive excitatory input from S-ON BCs. These wide-field RGCs had large dendrites that branched sparsely in sublamina 5, directly adjacent to the ganglion cell layer, and extended beyond the edges of the 220 × 220 micron volume (Figure 1C,D). Occasionally, smaller processes were observed extending to S-ON BC terminals, then stopping abruptly after receiving ribbon synaptic input. We exhaustively reconstructed the axon terminals of all presynaptic BCs to the two most complete wide-field RGCs and confirmed the identity of all but one presynaptic BC. Each wide-field RGC received just 3.28 ± 2.64 ribbon synapses from each S-ON BC (mean ± s.d., n = 43), yet the total S-cone input was substantial as each received input from 23 and 26 S-ON BCs, respectively (Figure 1E). In total, S-ON BCs provided 95.97% of the identified BC input to the two wide-field RGCs (143 of 149 synapses), with the remaining input from ON midget and DB6 BCs.
Interestingly, there is a previous description of S-ON BC input to a wide-field monostratified RGC in primate; the first report of S-ON BCs also mentioned contacts with an RGC resembling those in Figure 1C [6]. Based on the morphology and stratification of known primate RGC types, the wide-field RGCs in Figure 1C correspond to the M2 intrinsically photosensitive retinal ganglion cells (ipRGCs) [5]. Compared to M1 ipRGCs, M2s have more BC input, lower melanopsin expression and are primarily driven by their synaptic inputs [7]. Still, the S-ON BC input identified here is surprising as it confers the opposite spectral tuning as that reported for M1 ipRGCs [5]. However, this result is consistent with the different spectral tunings reported functionally for human ipRGC subtypes [8]. S-ON BC input to M1 ipRGCs was not observed and the S-cone amacrine cells mediating the M1 ipRGC’s S-OFF responses rarely contacted M2 ipRGCs (Figure S1 in Supplemental Information, published with this article online) [5].
Primate ipRGCs project to the suprachiasmatic nucleus, olivary pretectal nucleus (OPN), and, like the small bistratified RGCs, the lateral geniculate nucleus [7]. Their projections to the OPN drive the pupillary light reflex, which responds at both the onset and offset of S-cone isolating lights [9]. M1 ipRGCs are proposed to contribute to the OFF response, and S-ON BC input to M2 ipRGCs could explain the ON response. More generally, this circuit provides an additional candidate neural substrate for the wide-ranging effects of short-wavelength light on ipRGC-mediated non-image-forming visual functions essential to human health, which were originally attributed solely to melanopsin.
Small bistratified RGCs were long thought to be the only pathway carrying S-cone-specific ON signals from the primate retina, but here we identified another RGC type with the same S-ON BC input. Accordingly, the new wide-field RGC is predicted to share the small bistratified RGC’s S-ON/LM-OFF spectral tuning (Figure 1F). In theory, the wide-field RGCs’ large spatial receptive field and spectral tuning could have been obtained downstream by pooling the outputs of the small bistratified RGCs. Why then have multiple pathways carrying ‘redundant’ spectral signals? Importantly, the spectral tuning of the two Blue-ON RGCs is only redundant if considered independently of each RGC’s other response properties, such as their spatial scale, intrinsic photosensitivity and OFF BC input (Figure 1D,F). In reality, these properties must be considered together as they operate simultaneously and in concert to shape each RGC type’s final spike output. A simple interpretation of the differing upstream circuitry and downstream projections of the two Blue-ON RGCs is that they reflect different roles in vision. However, this interpretation does not fit into canonical models of primate vision built on the hypothesis that the sole goal of retinal coding is to reduce redundancy [2].
The redundancy reduction hypothesis predicts that an efficient retinal code maximizes information transmission through a small number of RGCs with decorrelated responses. This inability to account for the diversity of RGC types and their correlated responses has driven the development of new hypotheses [10]. However, redundancy reduction ideas remain the foundation of the leading primate color vision models [4], which may need to be critically revisited. While spectral opponency undoubtedly reduces redundancy, this alone produces an impoverished and inaccurate view of retinal color coding. Accounting for both Blue-ON RGC types may also require the context of the visual functions each RGC evolved to mediate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Beia Q. Geibel and Isabelle Rieke-Wey for annotation assistance. We thank James Kuchenbecker for IT support. We are grateful to James Anderson and Bryan Jones for their Viking annotation software. We also thank Ed Parker for excellent technical assistance. Tissue was provided by the Tissue Distribution Program at the Washington National Primate Research Center (WaNPRC) with the help of Chris English. This work was supported by NIH grants R01-EY027859 (J.N.), T32-NS099578 (S.S.P), T32-EY007031 (S.S.P.), P30-EY001730 (Core Grant for Vision Research), P51-OD010425/ORID, R01-EY028927, P30-EY014800 and Research to Prevent Blindness.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information contains one figure and experimental procedures, and can be found with this article online at https://doi.org/10.1016/j.cub.2020.10.010.
DECLARATION OF INTERESTS
The University of Washington has submitted a provisional patent application (628504893) disclosing Systems, Methods, and Devices for Stimulating Circadian Rhythms (authors: S.S.P., M.N., J.N.).
REFERENCES
- 1.Barlow H (1961). Possible principles underlying the transformations of sensory messages. In Sensory Communication, Rosenbith WA, ed. (Cambridge, MA: MIT Press; ), pp. 217–234. [Google Scholar]
- 2.Atick JJ, Li Z, and Redlich AN (1992). Understanding retinal color coding from first principles. Neural Comput. 4, 559–572. [Google Scholar]
- 3.Dacey DM, and Lee BB (1994). The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 367, 731–735. [DOI] [PubMed] [Google Scholar]
- 4.De Valois RL, and De Valois KK (1993). A multi-stage color model. Vision Res. 33, 1053–1065. [DOI] [PubMed] [Google Scholar]
- 5.Patterson SS, Kuchenbecker JA, Anderson JR, Neitz M, and Neitz J (2020). A color vision circuit for non-image-forming vision in the primate retina. Curr. Biol 30, 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariani AP (1984). Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature 308, 184–186. [DOI] [PubMed] [Google Scholar]
- 7.Sondereker KB, Stabio ME, and Renna JM (2020). The diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J. Comp. Neurol 528, 2044–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mure LS, Vinberg F, Hanneken A, and Panda S (2019). Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 366, 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zele AJ, Adhikari P, Cao D, and Feigl B (2019). Melanopsin and cone photoreceptor inputs to the afferent pupil light response. Front. Neurol 10, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow H (2001). Redundancy reduction revisited. Netw. Comput. Neural Syst 12, 241–253. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


