Abstract
Background:
The aim of this meta-analysis was to compare the short-term outcomes surrounding the efficacy and complication rate between different modalities of pyloromyotomy and gastric electrical stimulation (GES) in the treatment of gastroparesis.
Methods:
Comprehensive, computerized research was performed on PubMed, Embase, and the Cochrane Central Register of Controlled Trials. We additionally reviewed relevant articles, without any language limitations, published prior to April 15, 2020. Meta-analysis was conducted using RevMan 5.3 software.
Results:
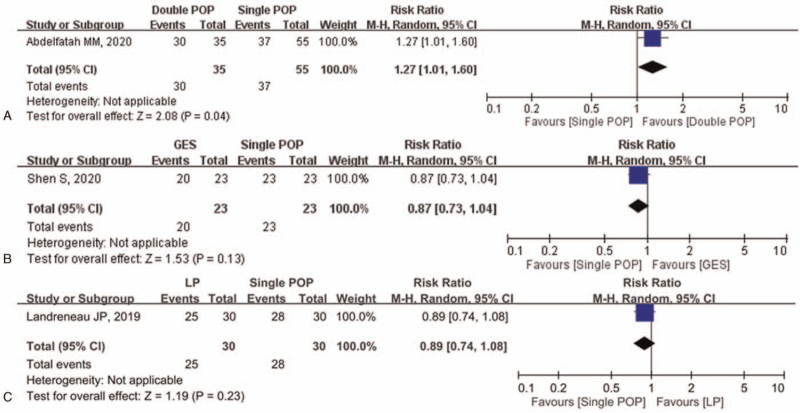
Three studies totaling 196 participants who had received 4 interventions, including single per-oral pyloromyotomy (POP), double POP, laparoscopic pyloromyotomy, and GES, were eligible for analysis. Compared to single POP, double POP achieved a better clinical response with a pooled relative risk (RR) of 1.27 (95% confidence interval [CI], 1.01–1.60, P = .04), while laparoscopic pyloromyotomy and GES showed no difference with a pooled RR of 0.89 (95% CI, 0.74–1.08, P = .23) and 0.87 (95% CI, 0.73–1.04, P = .13), respectively. As for the recurrence and complication rates, only GES showed a borderline significance of recurrence in comparison to single POP (RR 2.17, 95% CI, 1.00–4.71, P = .05), while there were no differences in the remainder of the comparisons.
Conclusions:
We conducted a detailed comparison of 3 modalities of pyloromyotomy and GES in the treatment of gastroparesis, with the results suggesting that double POP demonstrated better clinical success with similar recurrence and complication rates. In addition, GES may result in more recurrence amongst these interventions.
Keywords: gastric electrical stimulation, gastroparesis, meta-analysis, pyloromyotomy, systemic review
1. Introduction
Gastroparesis is a motility disorder defined by the manifestations of chronic upper gastrointestinal symptoms and prolonged gastric emptying in the absence of mechanical obstruction.[1,2] Two large population-based cohort studies have shown that the adjusted prevalence of gastroparesis was 13.8 to 24.2 per 100,000 persons in western countries, with the condition affecting predominantly females.[3,4] The most common etiologies of gastroparesis involve idiopathic, diabetic, and postsurgical disease. After treatment failure following both dietary modification and tailored doses of prokinetic medications, several therapeutic options remain available, including botulinum toxin injection,[5,6] balloon dilations,[7] transpyloric stenting,[8] gastric electrical stimulation (GES), laparoscopic or endoscopic pyloromyotomy, and gastrectomy. Botulinum toxin injection and transpyloric stenting usually offer temporary alleviation, with further concern being had regarding stent migration in transpyloric stenting.[9] Balloon dilations have been reported mostly in retrospective studies and are repeated if symptoms recur. The latest guidelines from the European Society of Gastrointestinal Endoscopy recommend against the use of botulinum toxin injection, balloon dilations, and transpyloric stenting in unselected patients with gastroparesis,[1] while a gastrectomy is seldom currently performed due to the appearance of minimally invasive procedures, including different modalities of pyloromyotomy and GES. However, the optimum intervention option for gastroparesis remains elusive.
GES has been introduced as a therapeutic option for refractory gastroparesis particularly in diabetic patients,[10] but its efficacy may be unsettling due to a 1-year clinical response rate of between 45% and 74%,[11–14] as well as a complication-associated removal rate of 6.3% to 12.8%.[12,15,16] Another emerging alternative therapy, per-oral pyloromyotomy (POP), also known as gastric peroral endoscopic pyloromyotomy, has demonstrated comparable clinical success and adverse events when using surgical pyloromyotomy as a comparison.[17] Similar to laparoscopic pyloromyotomy (LP), POP divides the pylorus without the invasiveness required in other surgeries during the laparoscopic procedure. Furthermore, double POP is performed with 2 circular pyloromyotomies in 1 session when compared to single POP. Recently, 1 systemic review reported improved outcomes in clinical response and gastric emptying scintigraphy regarding POP alone in the treatment of gastroparesis,[18] thus the application of this datum in clinical practice may be of limited value. Therefore, the aim of this systemic review and meta-analysis was to compare the short-term outcomes surrounding the efficacy, as well as the complication rate between different modalities of pyloromyotomy and GES, in the treatment of gastroparesis.
2. Materials and methods
2.1. Search strategy and selection criteria
This systematic review was performed according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) recommendations.[19] Comprehensive, computerized research was performed on the electronic databases of PubMed, Embase, and the Cochrane Central Register of Controlled Trials published prior to April 15, 2020 without any language restrictions. Additionally, we conducted a manual literature search of references in retrieved articles and significant reviews in eligible publications. A detailed description of the search strategies is provided in Table S1, Supplemental Digital Content. Ethical approval and informed consent from the participants were not necessary as there was no individual participant data involved.
We included trials which evaluated the short-term outcomes of 2 comparators, including different types of pyloromyotomy and GES in adults having a confirmed diagnosis of gastroparesis according to the Gastroparesis Cardinal Symptom Index (GCSI),[20] a known validated questionnaire for gastroparesis-related symptoms, and gastric emptying scintigraphy.[2] The index remains a gold standard in the evaluation of gastric motility. Reports that have studied pediatric patients, pregnant women, or patients with histories of active upper gastrointestinal bleeding, surgery, malignancy (including gastric or non-gastric origins), and severe concurrent comorbidities were excluded. We grouped the therapies into 4 interventions: double POP, single POP, LP, and GES.
2.2. Outcome measures
We determined the clinical response of intention to treat analysis 3 to 6 months after completion of therapeutic intervention from the enrolled studies. We also analyzed the recurrence and complication rates during the follow-up period in order to investigate therapeutic safety.
2.3. Data extraction and quality assessment
Two investigators (S-SH and L-HN) independently screened the titles and abstracts for eligibility, with their full texts being assessed in order to clarify the eligibility status of each article. Any disagreements were discussed until a consensus was reached, with a third investigator (S-SI) being consulted when necessary. We calculated the intention to treat or attempted to contact the corresponding authors if it was not indicated in the article. Two reviewers (S-SH and L-HN) extracted data independently, with their data then subsequently checked by a third investigator (S-SI). The following variables were extracted independently by 2 investigators: the first author, year of publication, country of study, study design, sample size, comparison intervention, outcome measurements, participants’ characteristics, and risk of bias in the enrolled studies. We also calculated outcome measurements, including the relative risk (RR) and a 95% confidence interval (CI).
Two authors (S-SH and L-HN) independently evaluated the risk of bias in the enrolled studies, based upon the Newcastle-Ottawa Scale assessment tool.[21] Any disagreements were discussed until a consensus was reached. A third investigator (S-SI) was consulted whenever necessary.
2.4. Data synthesis and statistical analysis
The results were analyzed using Review Manager V.5.3 software (Nordic Cochrane Centre, Copenhagen, Denmark). An RR with a 95% CI was used to present the short-term outcomes of gastroparesis after patients had undergone therapeutic intervention. These were completely produced using a random effect model to allow for the expected heterogeneity amongst the enrolled studies.
3. Results
3.1. Literature search and eligible studies
The detailed searching strategy is summarized in Figure 1. Initially, we identified 1143 abstracts and reviewed 14 full-text articles independently after the exclusion of 1129 studies which were not relevant to our topic. Finally, we included 3 articles involving a total of 196 participants for the purpose of qualitative and quantitative synthesis.[22–24]
Figure 1.
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic reviews and Meta-analyses.
3.2. Characteristics and clinical parameters of included studies
The methodological narrations and characteristics of the study design and outcomes of the 2 retrospective cohort trials, plus 1 case-controlled study, are summarized in Tables 1 and 2. Amongst these studies, the sample sizes ranged from 46 to 90, while the ranges for age and percentage of female gender were 42.0 to 47.6, and 76.7 to 87.0, respectively. All studies were conducted in the United States. The duration of gastroparesis ranged from 2.3 to 5.8 years, with the GCSI score prior to the procedure ranging between 3.75 and 4.00. Regarding etiology, idiopathic origin predominantly prevailed, followed by diabetes and postsurgical consequences.
Table 1.
Narrations of enrolled trials.
| Author | Year | Country | Study type | Sample size | Comparison intervention | Outcome measures |
| Abdelfatah et al | 2020 | USA | Retrospective case-controlled study (single center) | 90 | Double POP vs single POP | Clinical response: Decrease of at least 1 point in the average total GCSI score with more than a 25% decrease in at least 2 subscales of cardinal symptoms |
| Shen et al | 2020 | USA | Retrospective cohort study (single center) | 46 | GES vs single POP | Clinical response: No clinical recurrence which was defined as gastroparesis symptoms that were refractory to medical management and required at least 1 gastroparesis-related hospitalization, as well as a persistent GCSI score ≥3 for at least 6 months |
| Landreneau et al | 2019 | USA | Retrospective cohort study (single center) | 60 | LP vs single POP | Clinical response: No readmission within 30 days |
GCSI = Gastroparesis Cardinal Symptom Index, GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy.
Table 2.
Characteristics of enrolled trials.
| Author | Mean age (years) | Female (%) | BMI (kg/m2) | Duration (years) | Diabetic (%) | Idiopathic (%) | Postsurgical (%) | GCSI score before procedure |
| Abdelfatah et al | 47.6 | 81.1 | 27.4 | 5.8 | 42.2 | 50.0 | 7.8 | 3.82 |
| Shen et al | 42.0 | 87.0 | 23.1 | 2.3 | 47.8∗ | 52.2 | NA | 3.75 |
| Landreneau et al | 44.8 | 76.7 | 25.5 | NA | 16.7 | 63.3 | 20.0 | 4.00 |
BMI = body mass index, GCSI = Gastroparesis Cardinal Symptom Index, NA = not available.
Diabetes and others.
The comparison geometry for the efficacy of different modalities of pyloromyotomy and GES is shown in Figure 2. Traditional meta-analyses of the included regimens are shown in Figure 3. When compared with single POP (the reference regimen), only double POP showed significantly greater efficacy (RR 1.27, 95% CI: 1.01–1.60, P = .04), while LP and GES showed no differences with pooled RRs of 0.89 (95% CI, 0.74–1.08, P = .23) and 0.87 (95% CI, 0.73–1.04, P = .13), respectively. In Figure 4 we have calculated the separated data on the recurrence rate and revealed that GES alone had a borderline significance of recurrence in comparison to single POP (RR 2.17, 95% CI, 1.00–4.71, P = .05), while there were no differences in the remainder of the comparisons. Figure 5 shows that there was also no difference found among single POP, LP, and GES regarding the complication rate. We did not perform a funnel plot of this meta-analysis due to there being only 1 trial in each comparator group.
Figure 2.
Comparison geometry amongst different modalities of pyloromyotomy and gastric electrical stimulation. GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy.
Figure 3.
Forest plot of direct comparisons (RR) amongst different modalities of pyloromyotomy and gastric electrical stimulation (efficacy). CI = confidence interval, GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy, RR = relative risk.
Figure 4.
Forest plot of direct comparisons (RR) amongst different modalities of pyloromyotomy and gastric electrical stimulation (recurrence rate). CI = confidence interval, GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy, RR = relative risk.
Figure 5.
Forest plot of direct comparisons (RR) amongst different modalities of pyloromyotomy and gastric electrical stimulation (complication rate). CI = confidence interval, GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy, RR = relative risk.
We conducted a meta-analysis using the Newcastle-Ottawa Scale assessment tool to detect any risk of bias for the retrospective cohort and case-controlled studies (Table 3). The scores in all the trials were more than 7 points, which suggests a high quality.
Table 3.
Assessment of risk of bias in retrospective cohort and case-controlled trials by Newcastle-Ottawa Scale tool.
| Author | Year | Selection | Comparability | Outcome | Score |
| Abdelfatah et al | 2020 | 3 | 2 | 3 | 8 |
| Shen et al | 2020 | 4 | 2 | 2 | 8 |
| Landreneau et al | 2019 | 4 | 1 | 2 | 7 |
4. Discussion
In this meta-analysis, we comprehensively evaluated a detailed comparison of 3 different types of pyloromyotomy and GES in the treatment of gastroparesis, with the results suggesting that double POP demonstrated better clinical success with similar recurrence and complication rates. Additionally, GES may result in more recurrence amongst these interventions.
Diet modification comprised of low fat foods and low fiber meat, as well as prokinetic medications, are often recommended as first-line therapy in the management of gastroparesis.[25] Although 1 meta-analysis found that cisapride, D2 receptor antagonists, and relamorelin resulted in significant improvements in both gastric emptying and upper gastrointestinal symptoms clinically, as well as offering a positive association between the improvement of symptoms and gastric emptying on meta-regression of the optimal methodology of gastric emptying scintigraphy,[26] the level of evidence has still not yet been well-established. Several minimal invasive interventions[1,25] have been suggested as second-line therapies, including GES and laparoscopic or endoscopic pyloromyotomy. GES was approved as a humanitarian use device and has been given exemption by the US Food and Drug Administration since 2000, owing to large multicenter studies[27,28] which have shown significantly decreased symptoms of vomiting frequency in patients with intractable gastroparesis, particularly those involving diabetic gastroparesis. Another randomized, double-blind, crossover-designed controlled trial[29] showed a reduced frequency in nausea and vomiting in diabetic and non-diabetic patients with gastroparesis-like syndrome[30] or gastroparesis, although GES did not improve either gastric emptying or quality of life. Limited benefits from GES in patients with idiopathic gastroparesis[23] exist due to the requirement of surgical implantation, subsequent pacemaker adjustment or battery replacement, variable clinical response rates,[11–14] and device-associated removal rates,[12,15,16] all of which are factors which may negatively affect the clinical application of GES.
Pyloric spasm is one of the factors in the pathogenesis of gastroparesis, along with abnormal fundic emptying, gastric dysrhythmia, and antral hypomotility.[31] Pyloroplasty has been recently introduced to disrupt barrier function of the pylorus in order to facilitate gastric emptying and improve clinical symptoms. Laparoscopic or endoscopic pyloromyotomy are 2 mainstream interventions for refractory gastroparesis, with one of the largest prospective cohort studies, which had enrolled 177 patients who underwent LP, reporting improvement in gastric emptying in 86% of patients, as well as in the patient's symptom severity scores.[32] Shada et al also showed that the overall morbidity rate for LP was 6.8%, with 10.7% of patients undergoing subsequent surgical interventions. Landreneau et al[24] presented similar clinical improvement data in gastric emptying and GCSI scores in patients receiving LP, although patients experienced a longer hospitalization course, surgery time, and an increasing trend of complicative events when compared to patients receiving single POP. POP, another endoluminal pyloromyotomy, was primarily introduced in 2012, and has become more common in recent years. One meta-analysis demonstrated that the pooled mean differences in GCSI scores and the 4-hour solid-phase gastric emptying scintigraphy following single POP were significant at 1.76 and 26.28, respectively, as well as showing POP as having a 71% clinical success rate.[18] Another recent meta-analysis[17] revealed that pooled clinical success rates based upon GCSI scores and the 4-hour gastric emptying scintigraphy after single POP were not significant at 75.8% and 85.1%, respectively, when compared to surgical pyloromyotomy. In our study we further prove that double POP may offer better clinical success results than both single POP and LP, with similar recurrence and complication rates.
There are several limitations to this meta-analysis. First of all, we enrolled 2 retrospective cohort trials and 1 case-controlled study in our analysis, which may have possibly contributed to selection bias and confounding factors. Although most trials have clarified their patient population using widely validated GCSI scores and 4-hour solid-phase gastric emptying scintigraphy, our GCSI scores lack the ability to discriminate between gastroparesis and functional dyspepsia, while gastric emptying scintigraphy could not optimally distinguish pyloric spasm from other abnormal gastric dysrhythmia or hypomotility, nor accurately predict the severity of symptoms.[33] Even the functional lumen imaging probe still possesses certain technical obstacles to resolve, as well as having standardized normative parameters.[34] Secondly, it is challenging to estimate the clinical outcomes of refractory gastroparesis after a procedure when reporting bias exists due to different outcome definitions, assessments, and follow up periods. Nevertheless, we comprehensively evaluated the short-term outcomes of 3 different types of pyloromyotomy and GES in the treatment of gastroparesis using head-to-head comparisons. Thirdly, we did not perform a funnel plot to explore publication bias, or subgroup analysis to evaluate the effectiveness of different modalities stratified by different etiologies of gastroparesis due to both the small sample size within these studies, and the sparse number of clinical trials. Therefore, further multicenter prospective studies are awaited, particularly in different etiologies of gastroparesis. Finally, we did not include any combination therapies involving pyloromyotomy plus GES simultaneously or other pyloric interventions. Recently, 1 retrospective case-controlled study showed that combining pyloric surgery and GES, or GES alone, appeared to offer greater improvement in nausea/vomiting for patients experiencing predominant refractory gastroparesis, when compared separately to pre-operative status.[35] However, the mechanisms of pyloric interventions and GES[36,37] are completely different, with the indication, feasibility, safety, and long-term efficacy of combination therapies remaining elusive.
In summary, we have reviewed and demonstrated that double POP offers greater clinical success with similar recurrence and complication rates. Additionally, GES alone displayed a borderline significance of recurrence in comparison to single POP, while there were no differences in the remainder of the comparisons.
Acknowledgment
The authors acknowledge the contribution of the Evidence-based Practice and Policymaking Committee, Taichung Veterans General Hospital, Taichung, Taiwan.
Author contributions
S-SI, S-SH, and L-HN designed the meta-analysis, with input from all listed authors. S-SI, S-SH, and L-HN contributed to data acquisition and drafted the article. S-SI, S-SH, and L-HN contributed to data analysis and interpretation. All authors performed critical revision of the manuscript and approved the final draft of the article.
Conceptualization: Sz-Iuan Shiu, Shih-Hsiung Shen, Hua-Nong Luo.
Data curation: Sz-Iuan Shiu, Shih-Hsiung Shen, Hua-Nong Luo.
Formal analysis: Shih-Hsiung Shen, Hua-Nong Luo.
Methodology: Sz-Iuan Shiu, Shih-Hsiung Shen, Hua-Nong Luo.
Software: Sz-Iuan Shiu, Shih-Hsiung Shen, Hua-Nong Luo.
Supervision: Sz-Iuan Shiu.
Writing – original draft: Sz-Iuan Shiu, Shih-Hsiung Shen, Hua-Nong Luo.
Writing – review & editing: Sz-Iuan Shiu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GCSI = Gastroparesis Cardinal Symptom Index, GES = gastric electrical stimulation, LP = laparoscopic pyloromyotomy, POP = per-oral pyloromyotomy, RR = relative risk.
How to cite this article: Shiu SI, Shen SH, Luo HN. Short-term outcomes of different modalities of pyloromyotomy versus gastric electrical stimulation in the treatment of gastroparesis: a systemic review and meta-analysis. Medicine. 2021;100:37(e27291).
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patient consent for publication is not required.
Provenance and peer review is not commissioned; externally peer reviewed.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Weusten BLAM, Barret M, Bredenoord AJ, et al. Endoscopic management of gastrointestinal motility disorders - part 1: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020;52:498–515. [DOI] [PubMed] [Google Scholar]
- [2].Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol 2008;36:44–54. [DOI] [PubMed] [Google Scholar]
- [3].Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ye Y, Jiang B, Manne S, et al. Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut 2020;70:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol 2008;103:416–23. [DOI] [PubMed] [Google Scholar]
- [6].Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 2007;26:1251–8. [DOI] [PubMed] [Google Scholar]
- [7].Gourcerol G, Tissier F, Melchior C, et al. Impaired fasting pyloric compliance in gastroparesis and the therapeutic response to pyloric dilatation. Aliment Pharmacol Ther 2015;41:360–7. [DOI] [PubMed] [Google Scholar]
- [8].Khashab MA, Besharati S, Ngamruengphong S, et al. Refractory gastroparesis can be successfully managed with endoscopic transpyloric stent placement and fixation (with video). Gastrointest Endosc 2015;82:1106–9. [DOI] [PubMed] [Google Scholar]
- [9].Usai-Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: new insights into an old disease. World J Gastroenterol 2020;26:2333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Atassi H, Abell TL. Gastric electrical stimulator for treatment of gastroparesis. Gastrointest Endosc Clin N Am 2019;29:71–83. [DOI] [PubMed] [Google Scholar]
- [11].Heckert J, Sankineni A, Hughes WB, Harbison S, Parkman H. Gastric electric stimulation for refractory gastroparesis: a prospective analysis of 151 patients at a single center. Dig Dis Sci 2016;61:168–75. [DOI] [PubMed] [Google Scholar]
- [12].McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol 2011;9:314–9.e1. [DOI] [PubMed] [Google Scholar]
- [13].McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol 2010;8:947–54. [DOI] [PubMed] [Google Scholar]
- [14].Velanovich V. Quality of life and symptomatic response to gastric neurostimulation for gastroparesis. J Gastrointest Surg 2008;12:1656–63. [DOI] [PubMed] [Google Scholar]
- [15].Shada A, Nielsen A, Marowski S, et al. Wisconsin's Enterra Therapy Experience: a multi-institutional review of gastric electrical stimulation for medically refractory gastroparesis. Surgery 2018;164:760–5. [DOI] [PubMed] [Google Scholar]
- [16].Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care 2004;27:1071–6. [DOI] [PubMed] [Google Scholar]
- [17].Mohan BP, Chandan S, Jha LK, et al. Clinical efficacy of gastric per-oral endoscopic myotomy (G-POEM) in the treatment of refractory gastroparesis and predictors of outcomes: a systematic review and meta-analysis using surgical pyloroplasty as a comparator group. Surg Endosc 2020;34:3352–67. [DOI] [PubMed] [Google Scholar]
- [18].Uemura KL, Chaves D, Bernardo WM, Uemura RS, de Moura DTH, de Moura EGH. Peroral endoscopic pyloromyotomy for gastroparesis: a systematic review and meta-analysis. Endosc Int Open 2020;8:E911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther 2003;18:141–50. [DOI] [PubMed] [Google Scholar]
- [21].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [22].Abdelfatah MM, Li B, Kapil N, et al. Short-term outcomes of double versus single pyloromyotomy at peroral endoscopic pyloromyotomy in the treatment of gastroparesis (with video). Gastrointest Endosc 2020;92:603–9. [DOI] [PubMed] [Google Scholar]
- [23].Shen S, Luo H, Vachaparambil C, et al. Gastric peroral endoscopic pyloromyotomy versus gastric electrical stimulation in the treatment of refractory gastroparesis: a propensity score-matched analysis of long-term outcomes. Endoscopy 2020;52:349–58. [DOI] [PubMed] [Google Scholar]
- [24].Landreneau JP, Strong AT, El-Hayek K, et al. Laparoscopic pyloroplasty versus endoscopic per-oral pyloromyotomy for the treatment of gastroparesis. Surg Endosc 2019;33:773–81. [DOI] [PubMed] [Google Scholar]
- [25].Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013;108:18–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 2019;156:1650–60. [DOI] [PubMed] [Google Scholar]
- [27].Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion 2002;66:204–12. [DOI] [PubMed] [Google Scholar]
- [28].Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:421–8. [DOI] [PubMed] [Google Scholar]
- [29].Ducrotte P, Coffin B, Bonaz B, et al. Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Gastroenterology 2020;158:506–14.e2. [DOI] [PubMed] [Google Scholar]
- [30].Liu N, Abell T. Gastroparesis updates on pathogenesis and management. Gut Liver 2017;11:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Parsi MA, Jirapinyo P, Abu Dayyeh BK, et al. Techniques and devices for the endoscopic treatment of gastroparesis (with video). Gastrointest Endosc 2020;92:483–91. [DOI] [PubMed] [Google Scholar]
- [32].Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc 2016;30:1326–32. [DOI] [PubMed] [Google Scholar]
- [33].Cangemi DJ, Lacy BE. Gastroparesis and functional dyspepsia: different diseases or different ends of the spectrum? Curr Opin Gastroenterol 2020;36:509–17. [DOI] [PubMed] [Google Scholar]
- [34].Clarke JO, Ahuja NK, Fernandez-Becker NQ, et al. The functional lumen imaging probe in gastrointestinal disorders: the past, present, and future. Ann N Y Acad Sci 2020;1482:16–25. [DOI] [PubMed] [Google Scholar]
- [35].Zoll B, Jehangir A, Edwards MA, et al. Surgical treatment for refractory gastroparesis: stimulator, pyloric surgery, or both? J Gastrointest Surg 2020;24:2204–11. [DOI] [PubMed] [Google Scholar]
- [36].Lin Z, Hou Q, Sarosiek I, Forster J, McCallum RW. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil 2008;20:464–70. [DOI] [PubMed] [Google Scholar]
- [37].Moraveji S, Bashashati M, Elhanafi S, et al. Depleted interstitial cells of Cajal and fibrosis in the pylorus: novel features of gastroparesis. Neurogastroenterol Motil 2016;28:1048–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.