Summary:
Three-dimensional (3D) printing is used extensively in cranio-maxillo-facial (CMF) surgery, but its usage is limited in the setting of acute trauma specifically, as delays in outsourcing are too great. Therefore, we developed an in-house printing solution. The purpose of this study was to describe this process for surgeons treating acute CMF trauma. This series describes the printing process, time required, and printing material costs involved for in-house printing applied to a variety of acute CMF trauma cases involving the upper, middle, and lower thirds of the face and skull. All consecutive patients requiring in-house 3D printed models in a level 1 trauma center for acute trauma surgery in mid-2019 were identified and analyzed. Nine patients requiring the printing of 12 in-house models were identified. The overall printing time per model ranged from 2 hours, 36 minutes to 26 hours, 54 minutes (mean = 7h 55 min). Filament cost was between $0.20 and $2.65 per model (mean = $0.95). This study demonstrates that in-house 3D printing can be done in a relatively short period of time, therefore allowing 3D printing usage for various acute facial fracture treatments. The rapid improvements in the usability of 3D software and printing technology will likely contribute to further adoption of these technologies by CMF-trauma surgeons.
INTRODUCTION
The usage of three-dimensional (3D) printing in acute craniomaxillofacial (CMF) surgery is promising1 but limited because of the long delays involved in obtaining commercially printed models.2–4 When printed by a manufacturer, models need to be shipped to the hospital, adding unnecessary delays that are incompatible with urgent surgery. When printed in-house (by the customer), models are available immediately in time for surgery. An in-house printing solution (ie, driven by the customer) has been designed and implemented in a level 1 university trauma center, to overcome the long shipping delays involved with outsourcing to a manufacturer. In-house 3D printing is reported to be effective for specific fractures,5–8 but additional information was needed relative to the process and the technical aspects of in-house printing for a diversity of acute facial fractures.
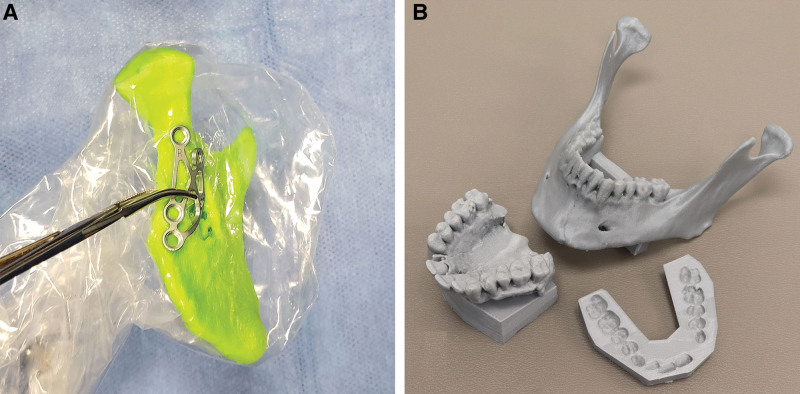
The in-house printing solution for acute trauma was applied to a series of cases in mid-2019. The prints were used for press-fitting plates to anatomically reduced “patient-specific” fracture models, press-fitting orbital implants, press-fitting meshes to the desired vault contour, and fabricating occlusal splints for complex maxilla-mandibular fractures (Fig. 1).
Fig. 1.
In-house printing of 3D models for acute trauma surgery. A, A patient-specific ascending rami and condyle model is used to bend intraoperatively a subcondylar fracture plate before implantation. B, A printed model of a maxilla presenting virtually reduced palatal and maxillary fractures, a mandible presenting a reduced symphysial fracture, and a temporary dental splint to align the models on an articulator. Unfortunately, at this time, outsourced VSP providers cannot provide plans for in-house medical grade occlusal splint printing because of regulation. In this case, a medical grade acrylic occlusal splint was then hand-made on the aligned models and sterilized for surgery.
The primary goal of this study was to describe in-house 3D printing for acute facial trauma, documenting the process, printing time, and printing material costs, occurring before admitting the patient to the operating room. Details provided should help a surgeon determine if there is enough time to undertake 3D planning and printing before surgery to benefit from custom-bent implants, as well as assessing the success rate of the process, critical information often omitted from other reports. It does not include the study of operating room time and costs, or the cost-benefits of virtual-surgical planning to obtain patient-specific prints.
METHODOLOGY
In this retrospective case series, all in-house 3D prints that were prepared for consecutive acute CMF trauma surgeries (≤21 days posttrauma) operated in a level 1 trauma center from March to July 2019 were reviewed.
Virtual surgical planning (VSP) involves virtually reducing fractures seen on a patient’s CT scan or replacing shattered bone with mirrored images from the unaffected contralateral side with a 3D modeling software. The anatomically corrected 3D model obtained by VSP serves as a template for bending custom plates and meshes before or during surgery. Planning may also involve virtually reducing maxillary and mandibular fractures to restore a virtually acceptable occlusion, from which an occlusal splint is printed.
Simple cases were planned locally with Autodesk MeshMixer v3.5.474 (Autodesk, San Rafael, Calif., USA), a free 3D tool. For complex cases, such as extensive facial fractures or cases requiring intraoperative navigation,9,10 VSP was undertaken with a commercial provider via videoconferencing, as is usually done for other elective CMF cases. Instead of being printed by the VSP provider, the resulting STL (stereo-lithography) file of the 3D model was downloaded over the internet and printed in-house.
Autodesk MeshMixer was used to confirm model conformity and printability. Printing resolution, use of supports to prevent model tipping during printing, etc. were determined with a slicer: Ultimaker Cura v3.6.0–v4.1.0 (Utrecht, Utrecht, the Netherlands). A slicer is a software that “slices” the model into printable layers for a specific printer.
In-house model printing was done on an Ultimaker 3 printer (Utrecht, Utrecht, the Netherlands) with a variety of polylactic acid filaments from Ultimaker and Materio3D (Saint-Hubert, QC, Canada) at resolutions ranging from 0.06 mm to 0.2 mm with a standard 0.4 mm print nozzle. (See Video 1 [online], which displays how a patient-specific 3D model is printed. The fractures are first reduced in a 3D software, after which they are exported and printed with a 3D printer. These models are then used to prebend anatomic plates and meshes for surgery.) Water-soluble polyvinyl alcohol (Ultimaker PVA (Utrecht, Utrecht, the Netherlands) was used as a soluble printing support as needed for complex structures.
Video 1. Video 1 from “In-house 3D model printing for acute cranio-maxillo-facial trauma surgery: Process, time, and printing material costs.” This video demonstrates how a patient-specific 3D model is printed. The fractures are first reduced in a 3D software, after which they are exported and printed with a 3D printer. These models are then used to pre-bend anatomic plates and meshes for surgery.
After printing was completed, printing supports were removed, and a visual quality check (for example, searching for defects due to a lack of filament during the print) was performed. The 3D model was then used to prebend plates or meshes, which were sterilized. The material was adapted before surgery if time allowed it. Current Canadian and American regulations prevent sterilization of in-house printed models. Models were therefore wrapped in a sterile plastic bag before being brought into the surgical field if required to bend plates intraoperatively.
RESULTS
Twelve11 models were printed for nine consecutive patients presenting with acute facial fractures and requiring 3D models for treatment (Table 1). Patients were operated on between posttrauma days 3 and 15 (mean = 8 days). The overall in-house printing phase time per model ranged from 2 hours, 36 minutes to 26 hours, 54 minutes (mean = 7h 55 min).
Table 1.
Printing Time and Costs
| Case | Diagnosis | Model Number | Model Description | Resolution (mm) | Printing Time (h:mm) | Issues | Cost |
|---|---|---|---|---|---|---|---|
| 1 | Left medial orbital wall and floor | 1.1 | Orbit, left | 0.10 | 2:59 | $0.55 | |
| 2 | Panfacial: frontal sinus, NOE, right LeFort 123, left LeFort 12, right mandibular parasymphysis | 2.1 | Frontal bar | 0.20 | 2:43 | $1.03 | |
| 2.2 | Orbits, bilateral | 0.10 | 7:42 | Failed | $1.06 | ||
| 3 | Left medial orbital wall and floor | 3.1 | Orbit, left | 0.10 | 3:16 | $0.80 | |
| 4 | Right subcondylar fracture | 4.1 | Ramus and condyle, right | 0.15 | 2:36 | $0.41 | |
| 5 | Mandibular right body, left parasymphysis. Bilateral Lefort 1 and palate | 5.1 | Mandible | Design issue | |||
| 6 | Left zygoma, NOE, medial/lateral/floor left orbit | 6.1 | Orbit, left | 0.10 | 2:37 | $0.20 | |
| 7 | Panfacial: base of skull, bilateral orbits, NOE, Lefort 12 bilateral | 7.1 | Orbit, right | 0.10 | 4:39 | $0.35 | |
| 8 | Panfacial: bilateral Lefort 123, 4 walls right orbit, medial/lateral/floor left orbit, right mandibular body | 8.1 | Bilateral orbits, midface | 0.10 | 7:25 | $0.59 | |
| 9 | Panfacial: bilateral NOE, left LeFort 123, right Lefort 12, palate, left parasymphysis, right mandibular body | 9.1 | Mandible (articulator) | 0.10 | 17:34 | $1.55 | |
| 9.2 | Midface | 0.10 | 26:54 | $2.65 | |||
| 9.3 | Maxilla (articulator) | 0.15 | 8:36 | $1.33 | |||
| Mean | 0.12 | 7:55 | $0.95 |
Twelve (12) models were printed for 9 patients. Resolution ranged from 0.10 mm to 0.20 mm. The printing time per model ranged from 2 hours, 36 minutes to 26 hours, 54 minutes (mean 7h 55 min). Costs per model varied from $0.20 to $2.65 (mean $0.95). The printing success rate was 83%.
In total, 83% of models printed successfully, a success rate similar to the 86% reported by the manufacturer.6 One failure (8.5%, n = 1) was because of a printing defect (underextrusion of polylactic acid causing an obviously “spongy” model) and a second (8.5%, n = 1) was because of a software bug which corrupted the 3D file. Both problems could not be corrected in time for surgery.
Filament cost per model was between $0.20 and $2.65USD (mean = $0.95). The cost of the printer was around $3500USD at the time of the study.
DISCUSSION
This article focuses on a key issue of 3D printing for acute CMF trauma: the time to manufacture a model. Despite most aspects of 3D printing1,12 being perfected for elective reconstructions, models cannot currently be received in time for acute surgery. As a result, we elected and designed an in-house printing process that avoids outsourcing production and shipping delays.
The printing time for in-house models was acceptable to allow for timely surgery. Successful prints were all considered of adequate surgical-grade quality (Fig. 1). Additionally, we elected to print only the region of interest to decrease the printing time.11,13
Problems encountered with VSP software bugs and printing failures cannot be prevented at this time. Although always present, these issues are very rarely reported in the surgical literature. As printers and software quickly evolve, we expect increased reliability of both printers and software in the near future.
Successfully printed models were found to be of adequate accuracy, an observation also made by other groups using in-house printing.7,13–16 Polylactic acid filament printing was also found to be reliable.13 All plates bent on a 3D model were used without modification of shape once placed on the patient. There were no operative takebacks due to inadequate implant shape.
For simple facial fractures when visualization of the fracture is good, it is often easier to bend plates in situ as is done usually. However, for more complex cases, such as orbital fractures, frontal bar fractures, mandibular fractures or when a significant degree of comminution is present, bending plates or meshes anatomically on a 3D patient-specific model preoperatively can be beneficial. For orbital fractures, bending a metallic orbital implant is usually impossible to achieve in situ. It is also impossible to compare its shape with the contralateral side to achieve orbital symmetry, a critical step in avoiding globe malposition. Prebending the orbital implant to a patient-specific 3D printed model ensures that the plate has the right shape.
In another scenario, such as with a typical comminutive frontal bar fracture, fragments can be missing and will not hold in place for fixation. The metallic mesh covering the frontal injury therefore provides the shape of the forehead. Achieving anatomical bending of the mesh before fixing remaining bone fragments to it is therefore crucial to achieve an aesthetically pleasing result. Conforming the mesh to a 3D patient-specific model makes this step much easier.
Lastly, having a plate that adapts perfectly to the patient’s anatomy can help with fracture reduction. For a subcondylar fracture, where visualization can be difficult, fixating the anatomically shaped implant on the condylar neck first can help with fracture reduction as it will help joysticking the condyle and help the fracture edge fall into place.
Outsourced printing carries specific transaction-related costs17 such as the expenses incurred to find a service provider, inquire about service costs and bargaining, the time required to write and sign a contract, and shipping delays. When these costs are too high, such as those implied by a very short printing cycle and delivery requirements for acute trauma, the only economical alternative is to print in-house. A detailed cost-benefit analysis would be needed to document this point. However, a small cost variation between outsourced and on-site printing could not justify the fact that at this time, the lengthy delays characterizing outsourced printing would imply receiving the models several days after the surgery.
Currently, Canadian and American regulations do not allow approval of off-the-shelf “biocompatible” and/or “sterilizable” filaments to print sterilizable models for surgery. Thus, in-house models may either be used to prebend implantable materials that are sterilized before surgery, or the models need to be wrapped in sterile plastic bags if required in the surgical field.
LIMITATIONS
With the evolution of 3D software and prosumer 3D printers, we expect a rapid improvement in model printing speed in the near future, possibly leading to a more widespread use of this technology. In terms of future research, an objective assessment of model accuracy, process efficiency, and reconstruction accuracy could be conducted. Furthermore, cost-benefit studies would be beneficial to assess possible cost and operating time reduction.
CONCLUSIONS
3D printing is used extensively in all areas of CMF surgery but is not routinely used in the acute care setting. The long production delays of outsourced printing solutions prevent obtaining models in time for most acute traumas. In this retrospective study of consecutive acute CMF trauma cases requiring 3D printed models, we have demonstrated that in-house 3D printing can be done reliably in a relatively short period of time, therefore allowing 3D printing usage in an acute setting.
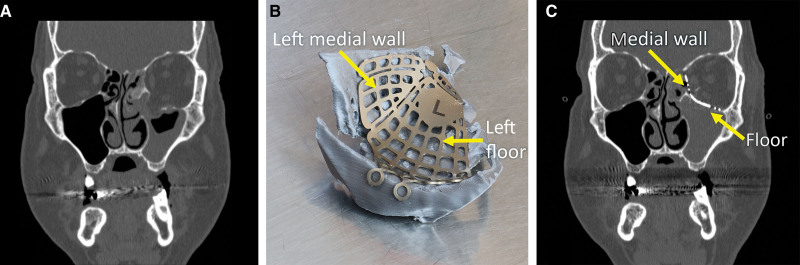
Fig. 2.
Complex fracture involving the left medial wall and floor treated with an orbital implant molded on a 3D model printed in-house. Molding an orbital implant is typically difficult because of poor visibility of the orbit during surgery, and the impossibility to assess symmetry with the contralateral orbit. With VSP, it is possible to model the desired orbital shape and print it. An implant can then be given the desired anatomical shape by contouring the implant to the patient-specific printed model. A, Preoperative coronal section demonstrating the left medial orbital wall and floor fracture. B, The left medial orbital wall and floor implant is contoured on a 3D printed model of the reconstructed orbit before surgery. C, Postoperative coronal section demonstrating the properly shaped left medial orbital wall and floor implant.
ACKNOWLEDGMENTS
The study was conducted in compliance with the principles of the Declaration of Helsinki and the institutional review board. Final board approval was granted on January 7, 2021.
Footnotes
Published online 17 September 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study did not receive any funding.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Hollier LH, Jr. The confluence of technique and technology in craniofacial surgery. Plast Reconstr Surg. 2021;147:1027–1028. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs CA, Lin AY. A new classification of three-dimensional printing technologies: systematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast Reconstr Surg. 2017;139:1211–1220. [DOI] [PubMed] [Google Scholar]
- 3.D’Urso PS, Atkinson RL, Lanigan MW, et al. Stereolithographic (SL) biomodelling in craniofacial surgery. Br J Plast Surg. 1998;51:522–530. [DOI] [PubMed] [Google Scholar]
- 4.Antony AK, Chen WF, Kolokythas A, et al. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast Reconstr Surg. 2011;128:1080–1084. [DOI] [PubMed] [Google Scholar]
- 5.King BJ, Park EP, Christensen BJ, et al. On-site 3-dimensional printing and preoperative adaptation decrease operative time for mandibular fracture repair. J Oral Maxillofac Surg. 2018;76:1950.e1–1950.e8. [DOI] [PubMed] [Google Scholar]
- 6.Marschall JS, Dutra V, Flint RL, et al. In-house digital workflow for the management of acute mandible fractures. J Oral Maxillofac Surg. 2019;77:2084.e1–2084.e9. [DOI] [PubMed] [Google Scholar]
- 7.Dvoracek LA, Lee JY, Unadkat JV, et al. Low-cost, three-dimensionally-printed, anatomical models for optimization of orbital wall reconstruction. Plast Reconstr Surg. 2021;147:162–166. [DOI] [PubMed] [Google Scholar]
- 8.Elegbede A, Diaconu SC, McNichols CHL, et al. Office-based three-dimensional printing workflow for craniomaxillofacial fracture repair. J Craniofac Surg. 2018;29:e440–e444. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron L, Bouchard S, Bonapace-Potvin M, et al. Intraoperative surgical navigation reduces the surgical time required to treat acute major facial fractures. Plast Reconstr Surg. 2019;144:923–931. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron L, Bouchard S, Bonapace-Potvin M, et al. Reply: intraoperative surgical navigation reduces the surgical time required to treat acute major facial fractures. Plast Reconstr Surg. 2020;146:509e–510e. [DOI] [PubMed] [Google Scholar]
- 11.Msallem B, Beiglboeck F, Honigmann P, et al. Craniofacial reconstruction by a cost-efficient template-based process using 3D printing. Plast Reconstr Surg Glob Open. 2017;5:e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn AQ, Pflibsen LR, Smith AA, et al. Three-dimensional printing in plastic surgery: current applications, future directions, and ethical implications. Plast Reconstr Surg Glob Open. 2021;9:e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naftulin JS, Kimchi EY, Cash SS. Streamlined, Inexpensive 3D Printing of the Brain and Skull. PLoS One. 2015;10:e0136198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim CG, Campbell DI, Clucas DM. Rapid prototyping technology in orbital floor reconstruction: application in three patients. Craniomaxillofac Trauma Reconstr. 2014;7:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W, Sun Y, Tian K, et al. Novel arch bar fabricated with a computer-aided design and three-dimensional printing: a feasibility study. J Oral Maxillofac Surg. 2015;73:2162–2168. [DOI] [PubMed] [Google Scholar]
- 16.Rogers-Vizena CR, Weinstock P, Livingston K, et al. The current role of three-dimensional printing in plastic surgery. Plast Reconstr Surg. 2017;139:811e–812e. [DOI] [PubMed] [Google Scholar]
- 17.Williamson OE. The theory of the firm as governance structure: from choice to contract. J Econ Persp. 2002;16:171–195. [Google Scholar]