Abstract
Objective:
The primary aim of this study was to evaluate if maternal age at birth of last child is associated with leukocyte telomere length in a nationally representative population of peri- and postmenopausal women.
Methods:
We conducted a cross-sectional analysis of 1,232 women from the National Health and Nutrition Examination Survey to examine maternal age at last birth and telomere length, surveyed between 1999 and 2002. We included peri- and postmenopausal women age 40 years and older. Maternal age at last live birth was self-reported, and leukocyte telomere length was measured using quantitative polymerase chain reaction. We calculated least-squares geometric mean telomere length across categories of maternal age adjusted for age, race/ethnicity, number of live births, survey cycle, and history of hysterectomy or oophorectomy. P-trend < 0.05 was considered statistically significant. For hypothesis-generation, we explored modification by reproductive and sociodemographic factors.
Results:
Maternal age at last birth was positively associated with telomere length: the multivariable-adjusted least-squares geometric mean leukocyte telomere length across categories of age at last birth (<25, 25–29, 30–34, 35–39, ≥40 years) was 0.90, 0.93, 0.93, 0.95, and 0.96, respectively (P-trend = 0.04). There was suggestive evidence this association may be restricted to those women with 1 or 2 live births or women who reported ever using oral contraceptives (P-interaction<0.10 for both).
Conclusions:
Later maternal age was associated with longer telomere length in a nationally representative population of women. These data provide new insight into the biological relationship between reproductive history and long-term health.
Keywords: age at last birth, telomere length, women, National Health and Nutrition Examination Survey, Centers for Disease Control and Prevention
Introduction
An increasing number of women in the United States are choosing to delay childbearing.1–3 This is reflected in a report by the Centers for Disease Control and Prevention that documented an increase in the average maternal age at first birth for rural and metro counties from 2007 to 2017.4 Later maternal age at birth of last child has been associated with increased maternal longevity.5–8 Moreover, later maternal age has been associated with risk of hormone-dependent cancers – including breast (increased), ovarian (decreased), and endometrial (decreased) – independent of number of births.9–11 However, little data are available to understand how maternal age is associated with biological indicators of long-term health and longevity.
We aimed to address this gap by examining the association between maternal age at birth of last child and maternal telomere length. Telomeres are repeating DNA-protein complexes that protect the ends of chromosomes and are critical for maintaining genomic stability.12 Telomeres shorten with each cell division as well as with oxidative stress and inflammation – they are thus often used as a proxy of cumulative cellular aging and damage.12,13 Epidemiologic studies have reported associations between telomere length and chronic conditions, including cardiovascular disease; type 2 diabetes; neurological conditions;14,15 and various cancers,16–19 including hormonally-driven cancers such as breast and ovarian.20–23
In the only study to date of maternal age and telomere length, the Long Life Family Study reported a positive association between late maternal age at last birth and telomere length in 387 women24 – providing compelling evidence that maternal age may be correlated with telomere length. However, this study was restricted to non-Hispanic white women over 70 years of age with a family history of longevity. Moreover, these data did not consider potential modification by sociodemographic factors related to childbearing patterns and reproductive health decisions. For instance, educational attainment is one of the strongest determinants of maternal age at first birth1,2,25 and may therefore modify this association.
To address these knowledge gaps, we investigated the association between maternal age at birth of last child and telomere length in a large, nationally representative population of women in the National Health and Nutrition Examination Survey. Importantly, we used these data to understand if this relationship holds in a more diverse study population than that studied by Fagan et al. (2017). Additionally, for hypothesis generation, we explored modification of the association by sociodemographic and reproductive factors that may influence telomere dynamics or childbearing patterns.
Methods
Study Population
Data were collected from the National Health and Nutrition Examination Survey (NHANES), a nationally-representative, cross-sectional survey of civilian and non-institutionalized persons in the United States conducted by the Centers for Disease Control and Prevention (CDC).26 We included data from the two cycles for which leukocyte telomere length was measured: 1999–2000 and 2001–2002.
We restricted our population to women with measured telomere length (N=4,056). Of these women, only those who met the following criteria were included in the final study population (Figure 1): 1) at least 40 years of age, 2) self-reported peri- or post-menopausal, 3) complete data for number of births and maternal age, 4) at least one live birth, and 5) did not have a partial/full hysterectomy and/or unilateral/bilateral oophorectomy before the age of 40. Most women complete natural childbirth by age 40 in the U.S.1 and women whose menstrual periods cease before age 40 are defined as experiencing premature menopause.27 Thus, our final population included 1,232 women age 40 years and older who had completed natural childbearing.
Figure 1.
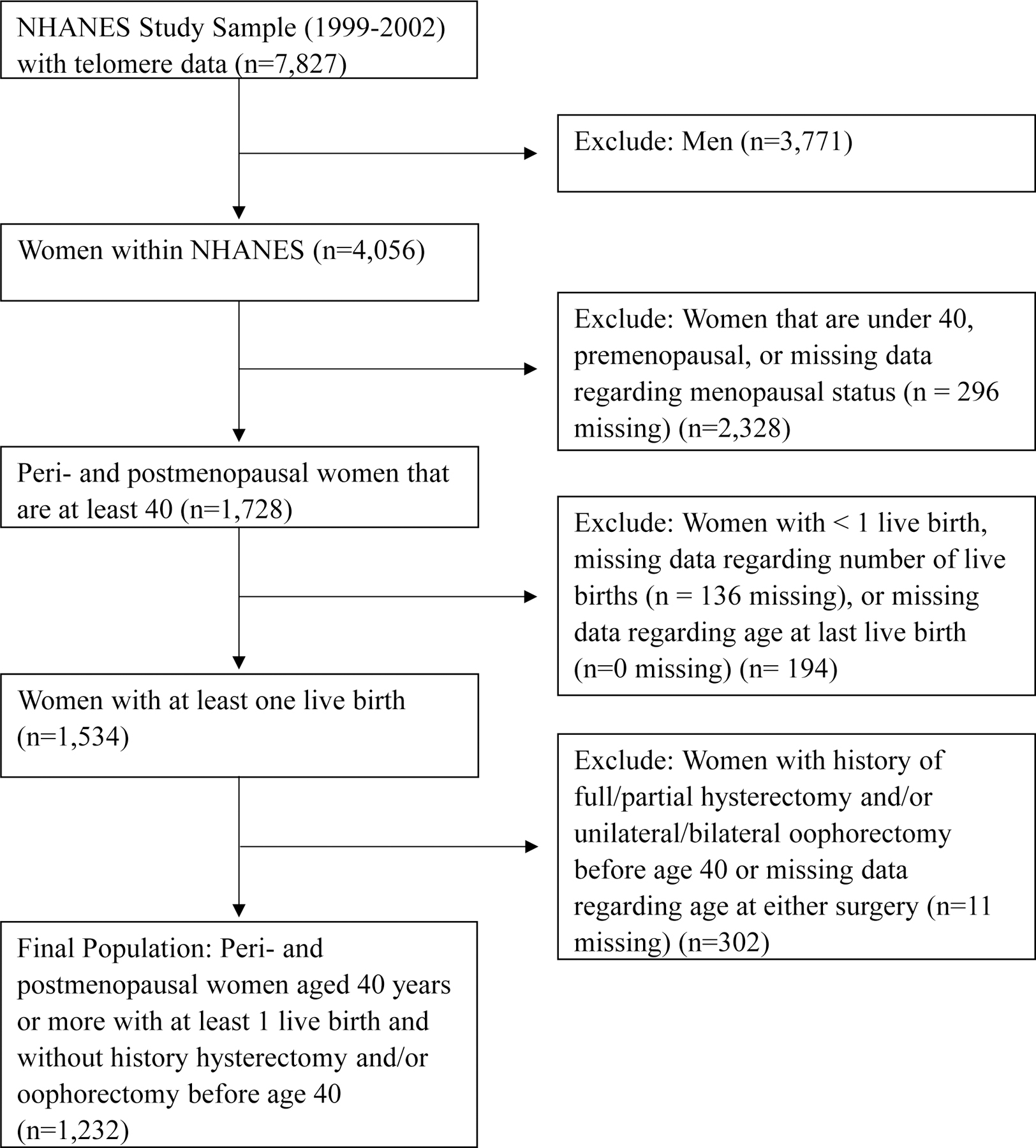
Inclusion/exclusion criteria used to obtain the final study population.
By design, NHANES oversampled older adults, low-income individuals, and persons of certain racial/ethnic minority groups. Thus, to ensure estimates are nationally representative of the non-institutionalized U.S. population, participants were assigned survey weights provided by NHANES, which accounted for unequal sampling probabilities and non-response.26
Ethics Approval
Collection of the NHANES survey data was approved by the NCHS Research Ethics Review Board at the CDC, and all data are publicly available.28 The Memorial Sloan Kettering Cancer Center institutional review board determined this study did not require additional human subjects approval.
Assessment of Reproductive Factors and Other Covariates
Women in NHANES were asked to report information on their reproductive health to a trained interviewer at a mobile examination center.29,30 We defined maternal age at birth of last child, hereafter referred to as age at last birth, using the question “How old were you at the time of your last live birth?” Mean age at last birth was comparable between NHANES cycles (1999–2000: 30.5 years; 2001–2002: 30.9 years). We defined peri- or post-menopausal status by an answer of “Going-gone through menopause” to the question “What is the reason you have not had regular periods in the last 12 months?” There were no additional measures to clearly differentiate between peri- or post-menopausal status; thus, we used this broad definition of menopausal status. Previous literature suggests that self-reported menopausal status is reproducible and accurate.31 Thus, we consider this an adequate definition based on available data. Additional self-reported reproductive factors were similarly assessed in the NHANES reproductive health section. These included pregnancy history (number of live births, age at first live birth), use of exogenous hormones (oral contraceptive use, postmenopausal hormone therapy use), and history of hysterectomy and/or oophorectomy.
In addition to reproductive factors, NHANES collected data for other factors that may be related to age at last birth or telomere length: age at blood collection, race/ethnicity, educational status, poverty-income ratio, body-mass index (BMI, kg/m2), alcohol consumption (over the past 12 months), high cholesterol status, congestive heart disease, stroke, smoking status, physical activity (metabolic equivalent hours of activity/month – MET-hours/month), diabetes, cancer, and general health status at time of interview (Table 1). Height and weight were measured by a trained examiner, whereas all other factors considered in this study were assessed via self-report to a trained interviewer during a computer-assisted personal interview.
Table 1.
Sociodemographic and reproductive characteristics of 1,232 peri- and postmenopausal women from the National Health and Nutrition Examination Survey.
| Age-Adjusted LTLb |
||||
|---|---|---|---|---|
| Characteristic | N | Weighted %a | Geometric Mean | 95% Confidence Interval |
| Demographic Characteristics | ||||
|
| ||||
| Age at blood collection (years) | ||||
| 40–49 | 128 | 14.6 | 1.05 | 0.98–1.14 |
| 50–59 | 276 | 30.6 | 0.97 | 0.94–1.01 |
| 60–69 | 355 | 22.3 | 0.96 | 0.91–1.01 |
| 70–79 | 277 | 21.4 | 0.88 | 0.84–0.92 |
| 80–85 | 196 | 11.1 | 0.83 | 0.79–0.87 |
| Race/ethnicity | ||||
| Non-Hispanic White | 695 | 79.1 | 0.93 | 0.90–0.96 |
| Non-Hispanic Black | 170 | 6.8 | 0.96 | 0.91–1.01 |
| Mexican American | 264 | 3.7 | 0.88 | 0.84–0.93 |
| Other Hispanic | 68 | 6.6 | 0.96 | 0.84–1.09 |
| Other race/mixed | 35 | 3.9 | 0.88 | 0.79–0.98 |
| Education | ||||
| < High school | 462 | 24.7 | 0.92 | 0.88–0.96 |
| High school grad/GED | 303 | 27.9 | 0.93 | 0.88–0.98 |
| Some college/associate | 287 | 26.9 | 0.93 | 0.89–0.97 |
| ≥ College grad | 179 | 20.5 | 0.94 | 0.90–0.98 |
| Poverty Income Ratio | ||||
| < 1 | 178 | 11.4 | 0.93 | 0.88–0.99 |
| 1 – 1.99 | 301 | 19.9 | 0.90 | 0.85–0.95 |
| 2–3.99 | 306 | 25.9 | 0.94 | 0.90–0.98 |
| ≥ 4 | 301 | 32.6 | 0.93 | 0.89–0.97 |
| BMI at blood collection | ||||
| Underweight/Normal (<25) | 345 | 32.3 | 0.93 | 0.89–0.98 |
| Overweight (25- <30) | 390 | 29.8 | 0.94 | 0.90–0.97 |
| Obese (≥30) | 457 | 34.9 | 0.92 | 0.87–0.96 |
| Smoking status | ||||
| Never | 741 | 57.9 | 0.93 | 0.89–0.97 |
| Former | 324 | 26.8 | 0.94 | 0.90–0.97 |
| Current | 165 | 15.2 | 0.92 | 0.86–0.97 |
| Quartiles of Physical activity (MET-hours/month) | ||||
| Q1 (≤ 24.8) | 151 | 13.31 | 0.92 | 0.86–0.98 |
| Q2 (>24.8 - ≤61.1) | 156 | 13.17 | 0.90 | 0.86–0.95 |
| Q3 (>61.1 – ≤124.1) | 119 | 13.32 | 0.93 | 0.89–0.97 |
| Q4 (>124.1) | 139 | 13.33 | 1.00 | 0.95–1.05 |
| Frequency of alcohol consumption over past 12 months | ||||
| < 1/month | 876 | 65.93 | 0.92 | 0.88–0.95 |
| ≥ 1/month - < 4/week | 238 | 22.46 | 0.96 | 0.90–1.03 |
| ≥ 4/week | 116 | 11.54 | 0.94 | 0.90–0.99 |
| Self-Reported Health Status | ||||
| Excellent | 160 | 16.8 | 0.95 | 0.91–0.99 |
| Very Good | 308 | 29.7 | 0.91 | 0.88–0.95 |
| Good | 405 | 32.6 | 0.93 | 0.89–0.98 |
| Fair | 290 | 17.2 | 0.92 | 0.87–0.97 |
| Poor | 67 | 3.7 | 0.94 | 0.86–1.03 |
|
| ||||
| Reproductive Characteristics | ||||
|
| ||||
| Age at last live birth (years) | ||||
| < 25 | 170 | 17.5 | 0.90 | 0.83–0.97 |
| 25–29 | 300 | 26.6 | 0.92 | 0.88–0.96 |
| 30–34 | 331 | 26.0 | 0.93 | 0.89–0.97 |
| 35–39 | 289 | 21.8 | 0.94 | 0.91–0.98 |
| ≥ 40 | 142 | 8.1 | 0.97 | 0.89–1.05 |
| Age at first live birth (years) | ||||
| ≤ 19 | 336 | 24.0 | 0.91 | 0.88–0.95 |
| 20–22 | 340 | 27.9 | 0.92 | 0.88–0.96 |
| 23–26 | 305 | 26.4 | 0.94 | 0.91–0.97 |
| ≥ 27 | 249 | 21.6 | 0.95 | 0.90–1.00 |
| Age at menopause (years) | ||||
| < 45 | 463 | 39.8 | 0.93 | 0.89–0.98 |
| 45–49 | 298 | 24.8 | 0.92 | 0.88–0.96 |
| 50–54 | 348 | 27.1 | 0.92 | 0.89–0.96 |
| ≥ 55 | 123 | 8.4 | 0.94 | 0.89–0.98 |
| Number of live births | ||||
| 1 | 145 | 14 | 0.95 | 0.89–1.01 |
| 2 | 305 | 30.8 | 0.90 | 0.86–0.95 |
| 3 | 281 | 24.6 | 0.93 | 0.89–0.97 |
| 4 | 166 | 11.9 | 0.94 | 0.88–1.00 |
| ≥ 5 | 335 | 18.8 | 0.95 | 0.91–0.98 |
| Ever used oral contraceptives | ||||
| No | 653 | 43.9 | 0.93 | 0.89–0.98 |
| Yes | 579 | 55.8 | 0.92 | 0.89–0.95 |
| Ever used postmenopausal hormones | ||||
| No | 730 | 53.8 | 0.93 | 0.89–0.97 |
| Yes | 502 | 45.7 | 0.93 | 0.89–0.97 |
| Ever had hysterectomy and/or oophorectomy | ||||
| Yes, either | 334 | 27.0 | 0.95 | 0.90–1.01 |
| No, neither | 895 | 72.9 | 0.92 | 0.89–0.95 |
| Time elapsed since last birth (years) | ||||
| ≤ 22 | 234 | 23.2 | 0.97 | 0.92–1.03 |
| 23–31 | 300 | 25.8 | 0.93 | 0.88–0.98 |
| 32–40 | 344 | 25.7 | 0.93 | 0.89–0.97 |
| ≥ 41 | 354 | 25.3 | 0.91 | 0.84–0.98 |
Percentages may not add up to 100 due to missing data.
Geometric means and corresponding 95% confidence intervals for leukocyte telomere length (LTL) are age-adjusted, with the exception of the variable corresponding to age at blood collection.
Measurement of Relative Leukocyte Telomere Length
The collection of blood samples and measurement of telomere length in NHANES have been detailed elsewhere.32–34 Briefly, leukocyte telomere length (LTL) was measured in individuals aged 20 years or older using genomic DNA extracted from peripheral blood leukocytes (stored at -80°C). Relative LTL was measured using quantitative polymerase chain reaction (qPCR).35 The average relative LTL was calculated as the ratio of the Telomere repeat copy number to standard reference Single-gene (36B4) copy number (T/S ratio, hereafter referred to as “leukocyte telomere length”). Using a different sample and laboratory than that used for NHANES, the relative T/S ratio has been shown to correlate well with absolute telomere length measured by Southern blot (r = 0.82; P < 0.0001).35 LTL was measured three times on three different days, with duplicates, creating six points from which to calculate an average LTL value for each participant. Mean LTL across the population was lower in 1999–2000 (geometric mean LTL: 0.93) vs. 2001–2002 (geometric mean LTL: 1.00). Telomere length was measured at the time of the examination. Thus, measured telomere length for all women in our study represents their cross-sectional, post-childbearing, age-specific LTL.
Statistical Analyses
LTL was right skewed in our data, so we used the natural logarithm of LTL to reduce the influence of extreme values. We calculated the distribution of sociodemographic and reproductive factors in our study population as well as the geometric mean of LTL across these factors (adjusted for age at blood collection).
To assess the overall association between maternal age at last birth and maternal LTL, we estimated the adjusted least-squares geometric mean LTL by category of age at last birth (< 25, 25–29, 30–34, 35–39, ≥ 40 years) using two generalized linear models. (This involved calculating the adjusted least-squares mean for log-transformed LTL within each category of maternal age and then exponentiating the results to obtain the geometric mean.) We first used a “minimally-adjusted” model, which regressed log-transformed LTL on age at last live birth, age at blood collection (continuous), and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/multi-racial). To assess the influence of additional potential confounders related to age at last birth or LTL, we next used a “fully-adjusted” model, which regressed log-transformed LTL onto the factors included in the minimally-adjusted model with the addition of number of live births (1, 2, 3, 4, ≥ 5), history of hysterectomy and/or oophorectomy after age 40 (ever vs. never), and survey cycle (1999–2000, 2001–2002). When building the fully-adjusted model, we first considered inclusion of either age at first live birth or number of live births in addition to factors in the minimally-adjusted model. However, both could not simultaneously be included with age at last birth because of high collinearity between these reproductive variables. We selected number of live births for inclusion because its addition resulted in a greater change (vs. age at first birth) in the regression coefficient for age at last birth. We determined the addition of other covariates using a forward stepwise-selection procedure in which variables were included if they resulted in a 10% change in the regression coefficient for age at last live birth and LTL. The covariates we considered for inclusion were informed by previous literature; these variables are listed in Table 1. After using this procedure, history of hysterectomy and/or oophorectomy after age 40 and survey cycle were added to the final, fully-adjusted model. We tested for a linear trend across age at last birth by including its categories as an ordinal predictor in multivariable linear regression models.
All covariates except for poverty-income ratio had missing data for less than 10% of participants. Because missing data methods perform similarly with a low proportion of missing data,36 we replaced missing values for each of these variables with the median value to preserve statistical power. Because poverty income ratio was missing more than 10% of responses, we created a “missing” category for this variable. No participants were missing data for more than four of the considered covariates. This approach reduced the variance of variable distributions. In sensitivity analysis, we used complete case methods and observed no appreciable differences (data not shown).
We next conducted exploratory analyses to assess whether the association between age at last birth and LTL varied across educational attainment (< high school, high school graduate/GED, some college/associate, ≥ college graduate); age at blood collection (≥70 vs. <70 years); number of live births (≤2 vs. >2); oral contraceptive use (ever vs. never); or postmenopausal hormone therapy use (ever vs. never). We selected these factors a priori based on previous literature that indicated these variables may be related to childbearing decisions or alter telomere length/dynamics. Age, education, and parity are all known to be associated with childbearing patterns.2,3,25 Further, while the association of age with telomere length is well-established, parity has also been found to be associated with telomere length.37 Postmenopausal hormone therapy and oral contraceptives often contain estrogen, which can affect telomere dynamics.38 We used global Wald tests to compare nested models with and without multiplicative interaction terms between age at last birth (modeled ordinally) and each modifier (modeled categorically).
All statistical tests were 2-sided, and α=0.05 for the primary analysis. In exploratory analyses of modification of the association (for which power was limited), we used α=0.10 since these were conducted for hypothesis-generation. Statistical analyses were completed in SAS 9.4 (SAS Institute, Cary, NC). Generalized linear models were run using PROC SURVEYREG in SAS, which allowed us to implement the proper weights based upon NHANES procedures.
Results
Primary Analyses: Maternal age at last birth and LTL
The mean age of women at blood collection was 63 years, and the mean age at last birth was 31 years. As expected, increased age at blood collection was inversely associated with shortened LTL (r = -0.30, P < 0.0001), demonstrating that our telomere data conform to this well-established association.39 After adjustment for age, LTL did not differ appreciably by key sociodemographic or lifestyle characteristics, such as education, BMI, smoking status, or alcohol use (Table 1). There appeared to be some variation in telomere length by race/ethnicity, and telomeres were longest in the most physically active women.
There was a positive association between maternal age at last birth and LTL (Figure 2; Table, Supplemental Digital Content 1, which contains geometric means and confidence intervals used to create Figure 2). After adjustment for age, race/ethnicity, number of live births, history of hysterectomy and/or oophorectomy after 40 years of age, and survey cycle, the least-squares geometric mean LTL across categories of age at last birth (<25, 25–29, 30–34, 35–39, ≥40 years) was 0.90, 0.93, 0.93, 0.95, and 0.96, respectively (P-trend=0.04). This difference in telomere length is equivalent to 9 years of aging when comparing women in the oldest (≥40 years) and youngest (<25 years) categories of age at last birth. There was no difference in the association by survey cycle (P-interaction=0.53). Results were unchanged after excluding N=100 women with a history of any cancer except non-melanoma skin cancer (data not shown). Because both maternal age at last birth and LTL may be proxies of overall health, we assessed associations restricted to participants with either good, very good, or excellent general health status (N=875). Similarly, there was no appreciable difference in the association in this sub-population.
Figure 2.
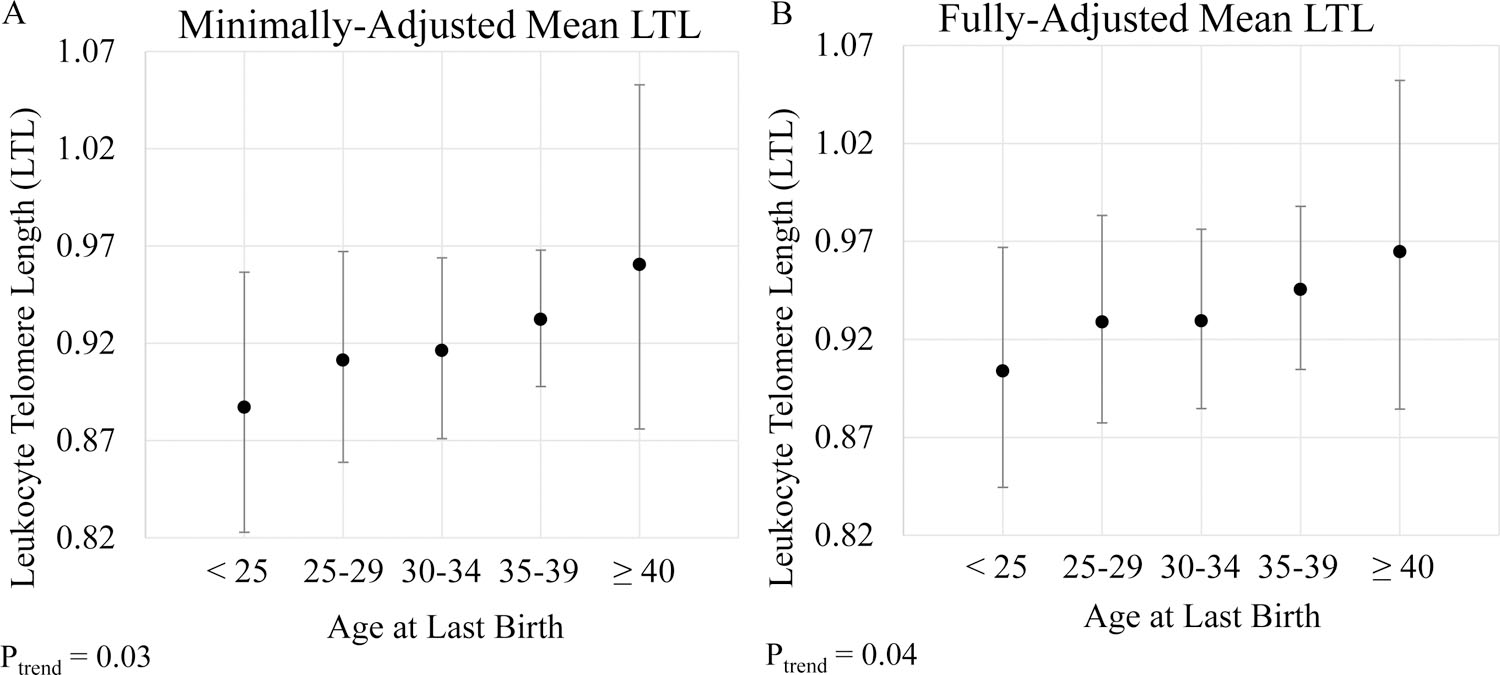
Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth (X-axis) in the entire study population (n = 1,232). (A) Adjusted for age at blood collection (continuous) and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, Other Hispanic, Other race/mixed). (B) Adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, Other Hispanic, Other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2-sided and obtained by modeling age at last birth as an ordinal variable.
Exploratory Analyses: Effect modification by educational attainment and other factors
We explored if the association between age at last birth and telomere length differed by sociodemographic or reproductive factors. We initially hypothesized educational attainment may modify this association due to its relationship with reproductive decision-making; however, we did not observe evidence for this (P-interaction=0.90; Figure, Supplemental Digital Content 2). We did, however, observe suggestive evidence that the association between age at last birth and longer telomeres may be driven by women with 1 or 2 live births (1 or 2: P-trend = 0.01; 3 or more: P-trend = 0.97; P-interaction=0.08; Figure, Supplemental Digital Content 3) or those who reported ever using oral contraceptives (Ever: P-trend = 0.01, Never: P-trend = 0.74, P-interaction = 0.03; Figure, Supplemental Digital Content 4). In contrast, we did not observe evidence of modification by age at blood collection (P-interaction=0.11; Figure, Supplemental Digital Content 5) or postmenopausal hormone therapy use (P-interaction = 0.68; Figure, Supplemental Digital Content 6). It is important to note these subgroup analyses were intended for hypothesis generation and should be interpreted cautiously given limited statistical power for tests of heterogeneity.
Discussion
In our large nationally representative population, later maternal age at last birth was associated with longer LTL in peri- and postmenopausal women. Our diverse study population offered the opportunity to assess modification of the association by sociodemographic and reproductive factors. Educational attainment did not modify the relationship, but there was suggestive evidence that the association of later age at last birth with longer LTL may be restricted to women with 1 or 2 live births or who reported ever using oral contraceptives. These findings merit further investigation in a large, prospective dataset.
Our data are consistent with the only previous study to date to examine the association between maternal age at last birth and LTL. Fagan et al. (2017) examined this relationship in 387 non-Hispanic white women over age 70 years with a family history of longevity from the U.S. and Denmark in the Long Life Family Study. In this small, restricted population, later maternal age at last birth was associated with longer telomeres: compared with women with earlier age at last birth (<29 years), women with later age at last birth (>33 years) were 2–4 fold more likely to have leukocyte telomere length in the second and third tertiles than in the first tertile. While our findings are consistent, there are important differences between our study populations. The Long Life Family Study was designed to study factors related to longevity.40 They thus included women with (1) a family history of longevity and that either (2a) lived to the oldest 5th percentile of their birth cohort or (2b) lived to at least 70 years of age, despite not satisfying (2a). Thus, this study population over-represented older women (≥70 years) and was enriched for those more likely to experience longevity. Importantly, our study included a >3-fold larger, far more racially and ethnically diverse population than Fagan et al., which included only non-Hispanic white women. Taken together, our data combined with that from Fagan et al. reinforces the rigor and external validity of the observed association between late maternal age at last birth and longer leukocyte telomere length.
There are multiple plausible explanations for this association. Given that our study used cross-sectional data with a single measurement of telomere length, we cannot assess the directionality of this relationship and thus cannot tease apart these possibilities. However, both causal and non-causal explanations exist. From a causal perspective, it is possible that pregnancy affects telomere length. If our results were interpreted strictly from this perspective, they would suggest that later age at last birth leads to having longer telomeres, for example through lessened telomere degradation. However, this conclusion would not align well with previous research: the theory of a physiological tradeoff in reproduction suggests that the “costs” associated with pregnancy would lead to telomere shortening, something that has been supported by experimental studies that had access to both pre- and post-pregnancy telomere lengths.41 Instead, as suggested by a 2019 review by Sudyka, our results would more likely support a “quality” perspective, or the idea that individuals with sets of traits associated with better survival are more likely to have higher reproductive success. Specifically, this suggests that we see this trend because it is individuals with longer telomeres that can give birth at later ages. This could occur, for example, if telomere length itself dictates the age at which a woman can have a child, as proposed by the Telomeric Theory of Reproductive Senescence.42 Briefly, this theory posits that shortened oocyte telomere length can negatively impact egg formation or successful embryo development (for example, through improper chromosome separation in meiosis). Non-causal explanations are also possible: telomere length may serve as a proxy of better general health, corresponding to a woman’s physical ability to become pregnant later in life.
Longitudinal studies will be needed to provide more definitive data for these questions. Moreover, our study is the first to explore if this relationship varies by sociodemographic and reproductive factors; accordingly, larger studies examining modifiers of this relationship are needed. For instance, we examined the relationship by educational attainment because more highly educated women delay childbearing at higher proportions than women with lower educational achievement;1,2,25 however, we did not observe evidence of the association being modified by education. Understanding these relationships are important as it can provide mechanistic insights for the role of telomere dynamics and cellular aging in the associations between reproductive history and long-term health.
Our study has important strengths: it included a large, nationally representative group of women, which increases the generalizability of our results across the U.S. population. Moreover, these women had data collected on a range of sociodemographic, lifestyle, and reproductive factors, enabling us to assess various potential sources of confounding and modification. Nevertheless, our results should be interpreted in the context of some important limitations. We did not have information regarding the use of assisted reproductive technologies (ARTs), which could bias results as these are more often used by older (≥35 years) rather than younger women in the U.S.43 However, we anticipate bias from this source to be minimal. Births from ARTs comprised under 2% of all infants born in the U.S. in 2016, a percent that has been increasing since ART introduction in 1981 and thus was even smaller when our population was completing childbearing. It is likely, then, that infants born through ARTs constitute only a very small percent of the pregnancies in our sample, minimizing the amount of bias introduced. Another weakness is that the sociodemographic covariates (e.g., BMI, poverty-income ratio, smoking status) may not fully capture a woman’s experiences during pregnancy because they were not assessed during her reproductive period. However, these cross-sectional measurements are commonly used throughout epidemiologic studies as proxies for past behaviors (for example, see: Setiawan et al., 2012; Pollack et al., 2018). Further, while there was a small proportion of missing data overall, slightly over 10% of data were missing for poverty-income ratio. We therefore created a missing data category for this variable, which means that, likely, individuals with varying PIRs were combined. Aside from concerns with covariates, as noted above, NHANES is a serial cross-sectional study, and we could not assess a possible temporal relationship between age at last birth and LTL since we did not have both pre- and post-pregnancy telomere lengths. Thus, these data cannot provide an estimate of causal effect. Finally, because there is high intra-individual variation in telomere length,44 large sample sizes are required to understand the true relationships between any exposure and telomere length. Although our population was more than 3-fold larger than that in Fagan et al. – providing adequate power to detect an association between age at last birth and LTL – it was much more diverse, and we had small sample sizes in subgroup analyses. This was particularly true when stratifying by educational attainment, for which only 179 women had a college degree or above. Because we are the first to explore the role of reproductive and sociodemographic factors in modifying this relationship, our stratified results are intended to inform future hypothesis testing in larger studies before any definitive conclusions can be drawn.
Conclusions
This study found that later maternal age at last birth was associated with longer LTL in peri- and postmenopausal women. There was suggestive evidence that this association may be modified by number of live births or oral contraceptive use, such that the association holds only among women with 1 or 2 live births and women with a history of ever using oral contraceptives. These findings, in combination with results from a previous observational study, suggest maternal age, in combination with other key reproductive factors, is correlated with telomere length among peri- and postmenopausal women. This could have implications for our understanding of long-term health in this population. However, before definitive conclusions can be drawn, prospective studies must evaluate the intra-individual changes in telomere length before and after pregnancy as well as the long-term consequences of those changes. That data is necessary to estimate the causal effect of maternal age on telomere length.
Supplementary Material
Supplemental Digital Content 1. Estimated geometric mean leukocyte telomere length and confidence intervals for minimally- and fully-adjusted models. P for trend are 2 sided.
Supplemental Digital Content 2. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by educational attainment. (A) Less than high school, (B) High school graduate or GED, (C) Some college or associate’s degree, and (D) At least 4-year college graduate. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and education (categorical).
Supplemental Digital Content 3. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by number of live births. (A) 1 or 2 live births and (B) 3 or more live births. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and number of live births (categorical).
Supplemental Digital Content 4. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of oral contraceptive use. (A) Ever use of oral contraceptives (B) Never use of oral contraceptives. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and oral contraceptive use (categorical).
Supplemental Digital Content 5. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by age at blood collection. (A) Less than 70 years of age and (B) At least 70 years of age. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and age at blood collection (categorical).
Supplemental Digital Content 6. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by postmenopausal hormone therapy use. (A) Ever used postmenopausal hormone therapy and (B) Never used postmenopausal hormone therapy. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and postmenopausal hormone therapy use (categorical).
Acknowledgements
We are grateful to the support from the Quantitative Sciences Summer Undergraduate Research Experience (QSURE) Fellowship through Memorial Sloan Kettering Cancer Center, without which this project would not be possible.
Funding Sources: The Quantitative Sciences Undergraduate Research Experience (QSURE) is funded by a grant from the National Cancer Institute (R25 CA214255). Additional grant funding was provided through the National Cancer Institute grants P30 CA008748 and P30 CA023108 and National Institute of General Medical Sciences grant P20GM104416.
Footnotes
Financial Disclosures/Conflicts of Interest: None declared.
Presentations: This manuscript was not presented at any national meetings nor as an abstract.
References
- 1.Bui Q, Miller CC. The Age That Women Have Babies: How a Gap Divides America New York Times. 2018. Available at: https://www.nytimes.com/interactive/2018/08/04/upshot/up-birth-age-gap.html.AccessedJan 3, 2019. [Google Scholar]
- 2.Heck KE, Schoendorf KC, Ventura SJ, Kiely JL. Delayed childbearing by education level in the United States, 1969–1994. Matern Child Health J 1997;1:81–8. [DOI] [PubMed] [Google Scholar]
- 3.Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief 2009;21:1–8. [PubMed] [Google Scholar]
- 4.Ely DM, Hamilton BE. Trends in fertility and mother’s age at first birth among rural and metropolitan counties: United States, 2007 – 2017. NCHS Data Brief 2018;323:1–8. [PubMed] [Google Scholar]
- 5.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature 1997;389:133. [DOI] [PubMed] [Google Scholar]
- 6.Grundy E, Kravdal Ø. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol 2008;167:271–279. [DOI] [PubMed] [Google Scholar]
- 7.Muller HG, Chiou JM, Carey JR, Wang JL. Fertility and life span: late children enhance female longevity. J Gerontol A Biol Sci Med Sci 2002;57:B202–B206. [DOI] [PubMed] [Google Scholar]
- 8.Sun F, Sebastiani P, Schupf N, et al. Extended Maternal Age at Birth of Last Child and Women’s Longevity in the Long Life Family Study. Menopause 2015;22:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: A population-based case-control study. Fertil Steril 2004;82:186–195. [DOI] [PubMed] [Google Scholar]
- 10.Lambe M, Hsieh C, Chan H, Ekbom A, Trichopoulos D, Adami H-O. Parity, age at first and last birth, and risk of breast cancer: A population-based study in Sweden. Breast Cancer Res Treat 1996;38:305–311. [DOI] [PubMed] [Google Scholar]
- 11.Setiawan VW, Pike MC, Karageorgi S, et al. Age at last birth in relation to risk of endometrial cancer: Pooled analysis in the epidemiology of endometrial cancer consortium. Am J Epidemiol 2012;176:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 2004;2004:pe43. [DOI] [PubMed] [Google Scholar]
- 13.Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002;27:339–344. [DOI] [PubMed] [Google Scholar]
- 14.Calado RT, Young NS. Telomere Diseases. N Engl J Med 2009;361:2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005;6:611–622. [DOI] [PubMed] [Google Scholar]
- 16.The Telomeres Mendelian Randomization Collaboration. Association between telomere length and risk of cancer and non-neoplastic diseases a mendelian randomization study. JAMA Oncol 2017;3:636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willeit P, Willeit J, Kloss-Brandstatter A, Kronenberg F, Kiechl S. Fifteen-Year Follow-up of Association Between Telomere Length and Incident Cancer and Cancer Mortality. JAMA 2011;306:42–44. [DOI] [PubMed] [Google Scholar]
- 18.Willeit P, Willeit J, Mayr A, et al. Telomere Length and Risk of Incident Cancer and Cancer Mortality. JAMA 2010;304:69–75. [DOI] [PubMed] [Google Scholar]
- 19.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J, Gammon MD, Terry MB, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer 2009;124:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svenson U, Nordfjäll K, Stegmayr B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res 2008; 68: 3618–3623. [DOI] [PubMed] [Google Scholar]
- 22.Mirabello L, Garcia-Closas M, Cawthon R, et al. Leukocyte telomere length in a population-based case–control study of ovarian cancer: a pilot study. Cancer Causes Control 2009;21:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, et al. Shorter telomere length is associated with increased: Ovarian cancer risk in both familial and sporadic cases. J Med Genet 2012;49:341–344. [DOI] [PubMed] [Google Scholar]
- 24.Fagan E, Sun F, Bae H, et al. Telomere Length Is Longer In Women with Late Maternal Age. Menopause 2017;24:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin SP. Diverging Fertility among U . S . Women Who Delay Childbearing Past Age 30. Demography 2000;37:523–533. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. NHANES 2003–2004 Public Data General Release File Documentation, 2005. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf.Accessed Sept 7, 2019.
- 27.Okeke T, Anyaehie U, Ezenyeaku C. Premature Menopause. Ann Med Health Sci Res 2013;3:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health and Nutrition Examination Survey. Informed Consent Available at: https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm. Accessed Sept 7, 2019.
- 29.NHANES-2001–2002a. 2001–2002 Data Documentation, Codebook, and Frequencies: Reproductive Health (RHQ_B) Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/RHQ_B.htm. Accessed Jan 3, 2019.
- 30.NHANES-1999–2000a. 1999–2000 Data Documentation, Codebook, and Frequencies: Reproductive Health (RHQ) Available at: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RHQ.htm. Accessed Jan 3, 2019.
- 31.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and Validity of Self-Reported Menopausal Status in a Prospective Cohort Study. Am J Epidemiol 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- 32.NHANES-1999–2000b. Telomere Mean and Standard Deviation (Surplus) Data Documentation, Codebook, and Frequencies Available at: https://wwwn.cdc.gov/Nchs/Nhanes/1999-%0A2000/TELO_A.htm%0A. Accessed Sept 23, 2018.
- 33.NHANES-2001–2002b. Telomere Mean and Standard Deviation (Surplus) Data Documentation, Codebook, and Frequencies Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2001-%0A2002/TELO_B.htm%0A. Accessed Sept 23, 2018.
- 34.Needham BL, Adler N, Gregorich S, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med 2013;85:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y, Peng CYJ. Principled missing data methods for researchers. Springerplus 2013;2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollack AZ, Rivers K, Ahrens KA. Parity associated with telomere length among US reproductive age women. Hum Reprod 2018;33:736–744. [DOI] [PubMed] [Google Scholar]
- 38.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med 2002;80:689–695. [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg DTA. Telomere length measurement validity: The coefficient of variation is invalid and cannot be used to compare quantitative polymerase chain reaction and Southern blot telomere length measurement techniques. Int J Epidemiol 2016;45:1295–1298. [DOI] [PubMed] [Google Scholar]
- 40.National Institute on Aging. National Institute on Aging (NIA) Long Life Family Study (LLFS) Natioinal Institutes Heal. Available at: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000397.v1.p1.AccessedSept 29, 2019. [Google Scholar]
- 41.Sudyka J. Does Reproduction Shorten Telomeres? Towards Integrating Individual Quality with Life-History Strategies in Telomere Biology. BioEssays 2019;41:1900095. [DOI] [PubMed] [Google Scholar]
- 42.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol 2006;18:280–285. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC), American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2016 Assisted Reproductive Technology National Summary Report Atlanta, GA: US Dept of Helath and Human Services, 2018. [Google Scholar]
- 44.Sanders JL, Newman AB. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 2013;35:112–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Estimated geometric mean leukocyte telomere length and confidence intervals for minimally- and fully-adjusted models. P for trend are 2 sided.
Supplemental Digital Content 2. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by educational attainment. (A) Less than high school, (B) High school graduate or GED, (C) Some college or associate’s degree, and (D) At least 4-year college graduate. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and education (categorical).
Supplemental Digital Content 3. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by number of live births. (A) 1 or 2 live births and (B) 3 or more live births. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and number of live births (categorical).
Supplemental Digital Content 4. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of oral contraceptive use. (A) Ever use of oral contraceptives (B) Never use of oral contraceptives. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and oral contraceptive use (categorical).
Supplemental Digital Content 5. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by age at blood collection. (A) Less than 70 years of age and (B) At least 70 years of age. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and age at blood collection (categorical).
Supplemental Digital Content 6. Estimated geometric mean leukocyte telomere length (Y-axis) with 95% confidence intervals across categories of age at last live birth by postmenopausal hormone therapy use. (A) Ever used postmenopausal hormone therapy and (B) Never used postmenopausal hormone therapy. All estimates adjusted for age at blood collection (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/mixed); number of live births (1, 2, 3, 4, ≥ 5); history of hysterectomy and/or oophorectomy after 40 years of age (ever, never); and survey cycle (1999–2000, 2001–2002). P for trend are 2 sided and obtained by modeling age at last birth as an ordinal variable. P for heterogeneity is 2 sided and obtained using a multiplicative interaction term between age at last birth (ordinal) and postmenopausal hormone therapy use (categorical).


