Abstract
During the spring 2020 COVID-19 surge, hospitals in Southeast Michigan were overwhelmed, and hospital beds were limited. However, it is unknown whether threshold for hospital admission varied across hospitals or over time.
Using a statewide registry, we performed a retrospective cohort study. We identified adult patients hospitalized with COVID-19 in Southeast Michigan (3/1/2020-6/1/2020). We classified disease severity on admission using the World Health Organization (WHO) ordinal scale. Our primary measure of interest was the proportion of patients admitted on room air. We also determined the proportion without acute organ dysfunction on admission or any point during hospitalization. We quantified variation across hospitals and over time by half-month epochs.
Among 1315 hospitalizations across 22 hospitals, 57.3% (754/1,315) were admitted on room air, and 26.1% (343/1,315) remained on room air for the duration of hospitalization. Across hospitals, the proportion of COVID-19 hospitalizations admitted on room air varied from 32.3% to 80.0%. Across half-month epochs, the proportion ranged from 49.4% to 69.4% and nadired in early April 2020. Among patients admitted on room air, 75.1% (566/754) had no acute organ dysfunction on admission, and 35.3% (266/754) never developed acute organ dysfunction at any point during hospitalization; there was marked variation in both proportions across hospitals. In-hospital mortality was 13.7% for patients admitted on room air vs 26.3% for patients requiring nasal cannula oxygen.
Among patients hospitalized with COVID-19 during the spring 2020 surge in Southeast Michigan, more than half were on room air and a third had no acute organ dysfunction upon admission, but experienced high rates of disease progression and in-hospital mortality.
Keywords: COVID-19, hospitalization, patient admission, pneumonia, severe acute respiratory syndrome coronavirus-2, viral pneumonia
1. Introduction
Pneumonia and respiratory failure are common manifestations of severe acute respiratory syndrome coronavirus-2. However, approximately 25% of patients hospitalized for COVID-19 never require supplemental oxygen.[1,2] The reasons for admitting patients with mild disease are likely multi-factorial, including high burden of comorbid disease and concern that patients are at high risk for further clinical deterioration.
Over the course of the pandemic, regional surges of infections have overwhelmed hospitals and healthcare systems[3–5] and professional societies developed guidelines to triage scarce medical resources.[5,6] There has been great interest in identifying risk factors for COVID-19 disease progression and developing tools to identify patients who are likely to deteriorate using clinical factors (e.g., vital signs, co-morbidities, laboratory values on admission) given the scarcity of inpatient hospital beds[2,7–10] and the association of hospital crowding and workload with worse outcomes.[11–14] However, it is unclear how illness severity on admission varied across hospitals or over time.
In this study, we sought to examine illness severity on admission over time and across hospitals in Southeast Michigan during the spring 2020 surge. Specifically, we measured the proportion of patients hospitalized with COVID-19 who had
-
1.
no oxygen requirement on admission,
-
2.
no acute organ dysfunction on admission, and
-
3.
no acute organ dysfunction at any point during hospitalization.
We hypothesized that the proportion of patients admitted on room air would decline over study time-period as clinicians gained experience treating COVID-19.
2. Methods
This is a multi-hospital observational cohort study of patients hospitalized for COVID-19 in Southeast Michigan and reported to the MI-COVID-19 registry. This project was reviewed and approved by the University of Michigan Institutional Review Board.
2.1. Data source: MI-COVID-19
MI-COVID-19 is a Michigan statewide multi-center collaborative quality initiative (CQI) sponsored by Blue Cross Blue Shield / Blue Care Network of Michigan to improve care for adult patients hospitalized with COVID-19.[15,16] Professional abstractors at participating hospitals collected data (including demographics, medical history, symptoms, laboratory values, treatments, and outcomes) from the medical record of eligible patients using a structured template. For hospitals unable to abstract all eligible hospitalizations, a random sample of eligible patients was selected for abstraction (cases were sorted by day of admission and, for each day, a pseudo-random number (minute of hospital discharge) was used to select patients for detailed abstraction).
2.2. Patient cohort
We identified all patients hospitalized for laboratory-confirmed severe acute respiratory syndrome coronavirus-2 infection (confirmed via reverse-transcriptase polymerase chain reaction testing) admitted between March 1, 2020 to June 1, 2020 and discharged by August 24, 2020 at hospitals in Southeast Michigan (defined as counties within the Southeast Michigan Council of Governments: Livingston County, Macomb County, Monroe County, Oakland County, Saint Clair County, Washtenaw County, Wayne County).[17] We focused on Southeast Michigan since the majority of cases in Michigan during March-May 2020 occurred in this region,[18] and we were interested in admission practices during the regional surge of COVID-19. For our primary analysis, we excluded patients admitted via inpatient-to-inpatient inter-hospital transfer since our primary goal was to examine disease severity on the calendar day of admission. However, in sensitivity analyses we examined all hospitalizations, including those admitted via inter-hospital transfer. For all analyses, we excluded hospitalizations at facilities with fewer than 10 COVID-19 hospitalizations in the MI-COVID-19 registry, in order to have sufficient hospitalizations per facility to support cross-hospital comparisons.
2.3. Study definitions
We classified disease severity on calendar day of admission according to the World Health Organization (WHO) ordinal scale[19] (Supplement Table S1), a pragmatic, 8-point ordinal scale that objectively classifies patients’ disease severity into ambulatory (1=asymptomatic; 2=mild limitation of activities), hospitalized with mild disease (3=no supplemental oxygen; 4=oxygen by mask or nasal prongs), hospitalized with severe disease (5=non-invasive ventilation or high-flow oxygen; 6=intubated and mechanically ventilated; 7=mechanically ventilated + additional organ support (vasopressors, renal replacement therapy, or extracorporeal membrane oxygenation)), and deceased (8=dead). Ordinal scales have previously been used to evaluate treatments for influenza.[20,21] The WHO developed this scale to harmonize studies of COVID-19,[19] and it has been used in multiple COVID-19 clinical trials.[22–25]
Among patients admitted on room air, we measured the rate of
-
1.
acute organ dysfunction on the calendar day of admission, and
-
2.
acute organ dysfunction at any point during hospitalization.
Acute organ dysfunction was defined as at least 1 of the following: (a) receipt of any respiratory support (e.g., supplemental oxygen, heated high flow nasal cannula, non-invasive positive-pressure ventilation, or invasive mechanical ventilation); (b) acute renal dysfunction, as defined by new dialysis/renal replacement therapy or creatinine elevation ≥50% above baseline among patients without pre-existing end-stage renal disease; (c) acute hematologic dysfunction, as defined by platelet count <100 cells/μL and ≤50% below baseline; (d) acute liver dysfunction, as defined by total bilirubin elevation of >2.0 mg/dL and ≥50% above baseline; (e) receipt of vasopressor therapy; or (f) in-hospital mortality. Baseline organ function was defined by the best laboratory value during the hospitalization, consistent with the Centers for Disease Control and Prevention's Adult Sepsis Event definition.[26]
2.4. Study outcomes and analysis
The primary measure of interest was the proportion of COVID-19 hospitalizations admitted on room air (WHO-class 3). We completed several analyses to understand admission practices and outcomes for these patients. First, we compared patient characteristics, early treatments (i.e., those received during the first 2 days of hospitalization), and outcomes across subgroups defined by WHO-class on admission, and tested for trends across WHO-classes using Cochran-Armitage Trend, Cochran-Mantel-Haenszel, and Jonckheere-Terpstra tests for ordinal, dichotomous, and continuous variables, respectively. Second, we examined the proportion of COVID-19 hospitalizations admitted on room air across hospitals and over time by half-month epochs. For this second analysis, we also completed sensitivity analyses in which we included patients admitted via inter-hospital transfer. Third, we examined the proportion of patients admitted on room air who
-
1.
had no acute organ dysfunction on admission, and
-
2.
had no acute organ dysfunction at any point during hospitalization, and how these proportions varied across hospitals and over time.
Fourth, among patients admitted on room air, we assessed the extent to which patient characteristics on hospital admission predicted progression to acute organ dysfunction. Specifically, we fit a multivariable logistic regression model predicting the development of acute organ dysfunction, using the following patient characteristics as predictors: age, place of residence prior to hospitalization, 3 co-morbidities (congestive heart failure, diabetes, and kidney disease), shortness of breath on presentation, maximum heart rate and respiratory rate on presentation, and maximum creatinine on admission. A small proportion of patients admitted on room air had non-respiratory acute organ dysfunction present on calendar day of admission; these patients were excluded from the predictive model. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient cohort
Among 2,193 hospitalizations for COVID-19 in the MI-COVID-19 registry, 878 (40.0%) were excluded, leaving 1,315 hospitalizations at 22 hospitals for inclusion in our primary analysis (Supplement Figure S1). Specifically, we excluded 86 hospitalizations admitted after June 1, 2020, 132 hospitalizations admitted via inter-hospital transfer, 651 hospitalizations outside of Southeast Michigan, and 9 hospitalizations from hospitals with fewer than 10 cases in the registry. The final cohort was a median age of 65 years, 50.1% male, and 51.9% Black (Supplement Table S2). In-hospital mortality was 21.1%, and 60-day post discharge mortality was 26.2%.
3.2. Disease severity on hospital admission
On the calendar day of admission, 754 patients (57.3%) were on room air (WHO-class 3 disease) (Table 1), 501 (38.1%) received supplemental oxygen by nasal prongs or mask (WHO-class 4), 13 (1.0%) received heated high flow or non-invasive positive pressure ventilation (WHO-class 5), 12 (0.9%) received invasive mechanical ventilation but no other organ support (WHO-class 6), and 29 (2.2%) received invasive mechanical ventilation plus additional organ support(s) (WHO-class 7). In sensitivity analyses, we included an additional 128 hospitalizations admitted via inter-hospital transfer for a total cohort size of 1443; on the calendar day of admission, 802 (55.8%) had WHO-class 3 disease severity, 546 (38.0%) had WHO-class 4 disease, 18 (1.3%) had WHO-class 5 disease, 22 (1.5%) had WHO-class 6 disease, and 49 (3.4%) had WHO-class 7 disease.
Table 1.
Patient characteristics by WHO disease severity class∗ on admission (N = 1315).
| WHO3∗ (Room air) | WHO4∗ (Supplemental Oxygen by Nasal Cannula) | WHO5-7∗ (Advanced respiratory support) | P ∗∗ | |
| Number, N (%) | 754 (57.3%) | 501 (38.1%) | 54 (4.1%) | |
| Age, median (IQR) | 63 (50,75) | 67 (56,79) | 70 (59,81) | <.001 |
| Male, N (%) | 48.4% | 50.9% | 59.3% | .13 |
| Race, N (%) | 0.39 | |||
| White | 37.4% | 42.5% | 33.3% | |
| Black | 53.3% | 49.0% | 57.4% | |
| Other/unknown | 9.3% | 8.5% | 9.3% | |
| Place of residence prior to hospitalization, % | <.001 | |||
| Home | 79.6% | 70.5% | 63.0% | |
| Other | 20.4% | 29.5% | 37.0% | |
| Comorbidities, % | ||||
| Diabetes | 37.7% | 37.3% | 42.6% | .75 |
| Moderate or severe kidney disease | 28.0% | 26.0% | 25.9% | .44 |
| Asthma | 12.1% | 14.2% | 14.8% | .26 |
| Congestive Heart failure | 11.7% | 16.8% | 18.5% | <.01 |
| Chronic obstructive pulmonary disease | 8.2% | 18.2% | 18.5% | <.001 |
| Cancer | 8.0% | 7.8% | 7.4% | .87 |
| Receipt of immunosuppressive or steroid prior to hospitalization | 8.5% | 10.4% | 13.0% | .15 |
| Median duration of symptoms prior to presentation (in days) | 5 | 5 | 3 | .09 |
| Symptoms on presentation, % | ||||
| Fever (>100.4) | 34.5% | 35.5% | 27.8% | .78 |
| Generalized malaise | 10.6% | 7.2% | 3.7% | .01 |
| Weakness | 27.7% | 22.4% | 24.1% | .06 |
| Dyspnea/shortness of breath | 62.7% | 80.4% | 88.9% | <.001 |
| Cough (New or Worsening) | 68.4% | 74.7% | 55.6% | .53 |
| Non-pleuritic chest pain | 10.3% | 8.0% | 1.9% | .03 |
| Pleuritic chest pain | 8.8% | 4.6% | 1.9% | <.01 |
| Calendar day of admission laboratory values∗∗∗ | ||||
| None measured, % | 2.4% | 1.0% | 0 | .04 |
| Median lactate, mg/dL | 1.4 | 1.5 | 2.2 | <.01 |
| Median creatinine, mg/DL | 1.1 | 1.1 | 1.5 | <.01 |
| Median white blood cell count, cells/μL | 6.3 | 7.1 | 10.2 | <.001 |
| Median absolute lymphocyte count, cells/μL | 0.9 | 0.9 | 1.1 | .11 |
| Median hemoglobin, g/dL | 13.2 | 12.9 | 13.9 | .32 |
| Median platelet, cells/μL | 197 | 207 | 185 | .07 |
| Median total bilirubin, mg/dL | 0.6 | 0.6 | 0.8 | <.01 |
| Radiology tests performed on calendar day of admission, % | ||||
| Chest x-ray performed | 86.6% | 94.0% | 96.3% | <.001 |
| Chest ct performed | 9.6% | 8.0% | 11.1% | .63 |
| Admission location, % | <.001 | |||
| Observation unit | 13.3% | 4.2% | 0 | |
| Ward (general medicine/surgical) | 67.6% | 66.5% | 9.3% | |
| Step-down | 13.0% | 19.6% | 3.7% | |
| ICU | 6.1% | 9.8% | 87.0% | |
IQR = interquartile range, WHO = World Health Organization.
WHO disease severity classifications: WHO3: no supplemental oxygen; WHO4: oxygen by mask or nasal prongs; WHO5: non-invasive ventilation or high-flow oxygen; WHO6: intubated and mechanically ventilated; WHO7: mechanically ventilated + additional organ support (pressors, renal replacement therapy or extra-corporeal membrane oxygenation). Given the small number of patients with severe disease on admission, WHO-classes 5, 6, and 7 were grouped together.
P value for trend.
For each patient, most abnormal laboratory value was used to calculate median value.
3.3. Variation in patient characteristics, early treatment, and outcomes by WHO disease severity on admission
Patient characteristics and outcomes differed across the ordinal disease severity classes (Table 1). Patients admitted on room air (WHO-class 3) were younger (63 vs 67 vs 70 years) and more likely to be living at home (79.6% vs 70.5% vs 63.0%) than patients with WHO-class 4 or WHO-classes 5 to 7 on admission (P < .001) (Table 1). Patients admitted on room air had similar rates of diabetes (37.7%–42.6%), chronic kidney disease (25.9%–28.0%), and asthma (12.1%–14.8%) as patients with WHO-class 4 or WHO-classes 5 to 7 on admission (P > .05), but lower rates of chronic obstructive pulmonary disease (COPD) (8.2% vs 18.2%–18.5%) and congestive heart failure (11.7% vs 16.8%–18.5%) (P < .001 and P < .01, respectively) (Table 1). Patients admitted on room air had lower median lactate (1.4 mg/dL vs 1.5–2.2 mg/dL, P < .01), lower median creatinine (1.09 vs 1.14–1.54 mg/dL, P < .01), and lower median white blood cell count (6.3 cells/uL vs 7.1–10.2 cells/uL, P < .001) on calendar day of admission than patients with WHO-class 4 or WHO-class 5 to 7 disease, respectively (Table 1).
Likewise, early treatments differed across disease severity classes (Table 2). However, while use was lower among patients admitted on room air, a substantial proportion were still treated with adjunctive therapies. For example, during the first 2 days of hospitalization, patients admitted on room air were treated with antibacterial agents (64.2%), hydroxychloroquine (44.3%), azithromycin (35.3%), corticosteroids (21.9%), and Vitamin C (6.2%). Among patients admitted on room air, the most common acute organ dysfunctions experienced during hospitalization were pulmonary dysfunction (54.5%), renal dysfunction (32.6%), and shock (11.4%). In-hospital mortality was 13.7%, compared to 26.3% among patients admitted on supplemental oxygen, and 74.1% among patients receiving advanced respiratory support on the day of admission, P < .001.
Table 2.
Early treatment and outcomes by WHO disease severity class∗ on admission.
| WHO3∗ (Room air) | WHO4∗ (Supplemental Oxygen by Nasal Cannula) | WHO5-7∗ (Advanced respiratory support) | P ∗∗ | |
| Early treatment∗∗∗, % | ||||
| Antibiotics | 64.2% | 76.1% | 83.3% | <.001 |
| Steroids | 21.9% | 42.5% | 44.4% | <.001 |
| Azithromycin | 35.3% | 43.7% | 51.9% | <.001 |
| Hydroxychloroquine | 44.3% | 59.3% | 63.0% | <.001 |
| Vitamin C (PO or IV) | 6.2% | 10.4% | 1.9% | .18 |
| Outcomes | ||||
| Ever in ICU, % | 8.5% | 13.4% | 53.7% | <.001 |
| Median days in ICU, (IQR) | 0 (0, 0) | 0 (0, 0) | 1 (0, 6) | <.001 |
| Organ dysfunctions (ever)∗∗∗∗, % | ||||
| Shock | 11.4% | 18.0% | 74.1% | <.001 |
| Respiratory failure | 9.4% | 16.6% | 88.9% | <.001 |
| Acute renal dysfunction | 32.6% | 36.9% | 64.8% | <.001 |
| New dialysis | 3.9% | 4.4% | 16.7% | <.01 |
| Acute hematologic dysfunction | 5.4% | 6.4% | 11.1% | <.001 |
| Acute liver dysfunction | 2.7% | 4.4% | 5.6% | .06 |
| Median number of acute organ dysfunctions | 0 | 0 | 3 | <.001 |
| Median length of stay, days (IQR) | 6 (4, 9) | 6 (4, 10) | 7 (4, 13) | <.01 |
| LOS for hospital survivors, days (IQR) | 5 (3, 8) | 6 (4, 10) | 13 (9, 15) | <.001 |
| In-hospital mortality, % | 13.7% | 26.3% | 74.1% | <.001 |
| 60-day post-discharge mortality, % | 18.0% | 32.7% | 77.8% | <.001 |
| Rehospitalization, % | 14.8% | 19.2% | 21.4% | .06 |
IQR = interquartile range, WHO = World Health Organization.
WHO disease severity classifications: WHO3: no supplemental oxygen; WHO4: oxygen by mask or nasal prongs; WHO5: non-invasive ventilation or high-flow oxygen; WHO6: intubated and mechanically ventilated; WHO7: mechanically ventilated + additional organ support (pressors, renal replacement therapy or extra-corporeal membrane oxygenation). Given the small number of patients with severe disease on admission, WHO-classes 5, 6, and 7 were grouped together.
P value calculated as P value for trend.
Early treatment refers to therapies received within 2 calendar days of admission.
Organ dysfunction defined as: Shock: received vasopressor; Respiratory failure: received invasive mechanical ventilation; Acute renal dysfunction: new dialysis / renal replacement therapy or creatinine elevation ≥50% above baseline; Acute hematologic dysfunction: platelet count <100 and decrease in platelet count by ≥50% compared to baseline; Acute liver dysfunction: total bilirubin >2.0 mg/dL and ≥50% compared to baseline. Baseline renal, hematologic, and liver function determined by lowest creatinine or bilirubin and highest platelet count, respectively, recorded during hospitalization.
3.4. Variation in admission disease severity across hospitals and over time
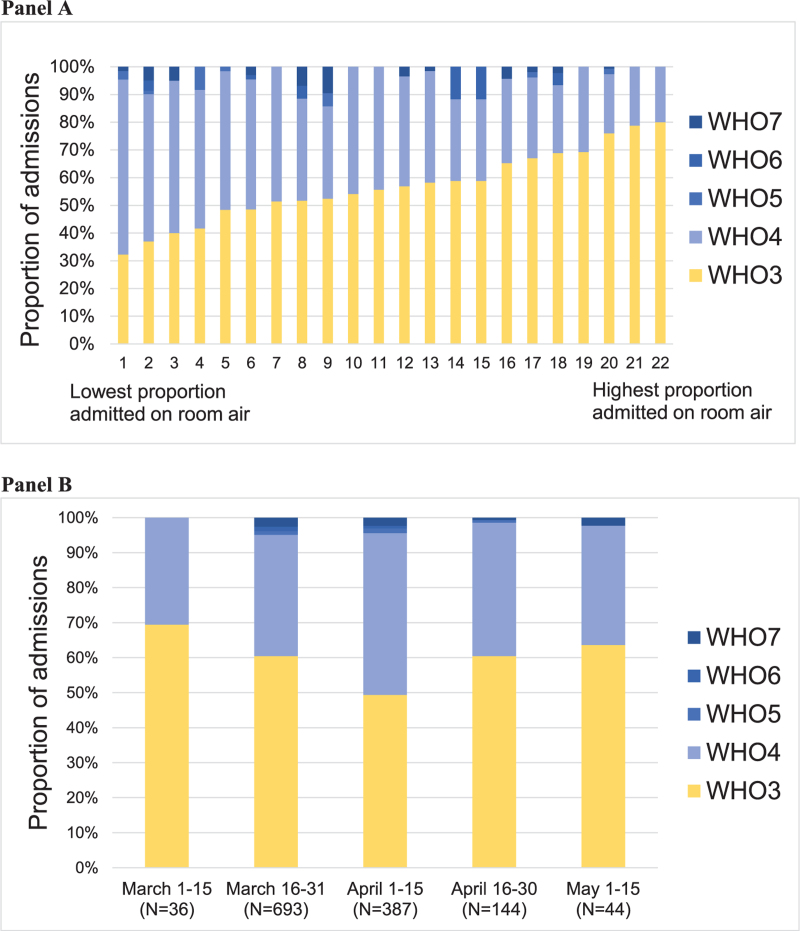
The proportion of COVID-19 hospitalizations admitted on room air varied markedly across hospitals (32.3%–80.0%) and, to a lesser degree, over time (49.3%–69.4%) as measured across half-month epochs (Fig. 1). The proportion of patients admitted on room air nadired in early April, when case counts were highest in Southeast Michigan.
Figure 1.
Variation in COVID-19 admission disease severity across hospitals (Panel A) and over time (Panel B). Panel A shows the proportion of admissions with World Health Organization (WHO) disease severity classes 3-7 on admission, by hospital. Panel B shows the proportion of admissions with WHO disease severity classes 3-7 on admission, by half-month epoch. There were a total of 1309 admissions during the study period; admissions by hospital ranged from 12 to 209; admission by time-period ranged from 36 to 693. May 16 to 30 is not shown as only 5 admissions for COVID-19 occurred during this time-period. WHO disease severity classifications: WHO3: no supplemental oxygen; WHO4: oxygen by mask or nasal prongs; WHO5: non-invasive ventilation or high-flow oxygen; WHO6: intubated and mechanically ventilated; WHO7: mechanically ventilated + additional organ support (pressors, renal replacement therapy or extra-corporeal membrane oxygenation).
In sensitivity analyses including patients admitted via inter-hospital transfer, findings were similar to the primary analysis; the proportion of COVID-19 hospitalizations admitted on room air varied from 30.8% to 80.9% across hospitals (Supplement Figure S2), and from 47.8% to 68.4% across half-month epochs (Supplement Figure S2), again nadiring in early April.
3.5. Disease progression among patients with WHO-class 3 disease on admission
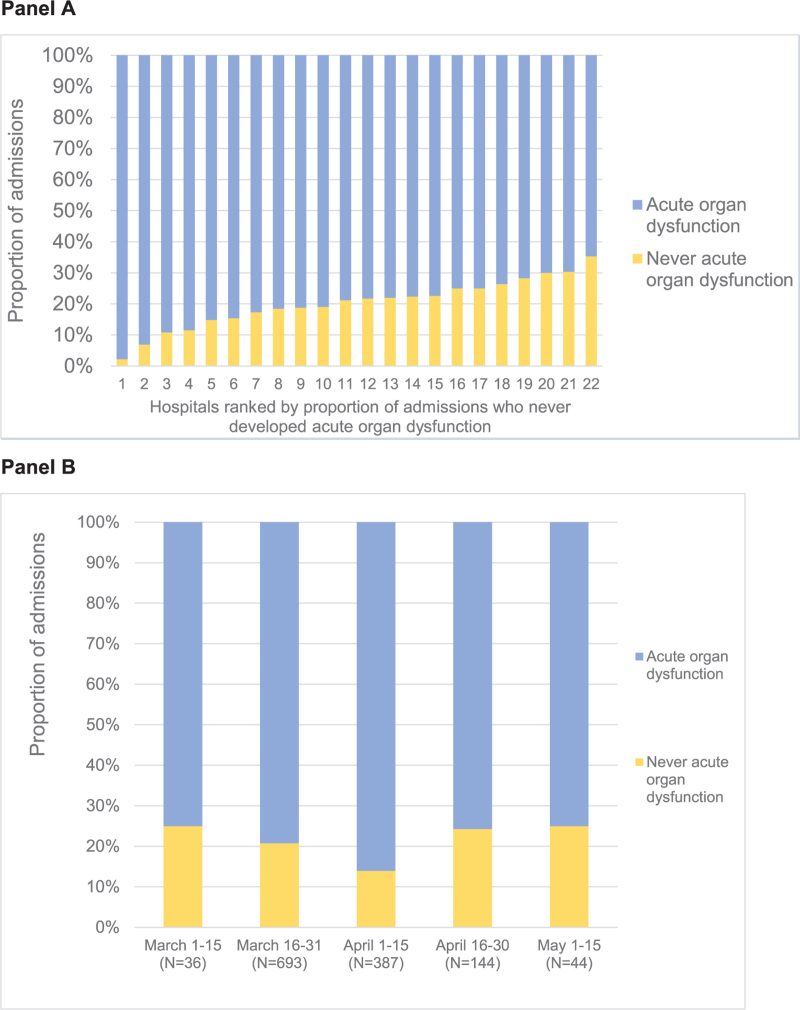
Among the 754 patients admitted on room air, 188 (24.9%) had non-pulmonary acute organ dysfunction on admission. 498 (66.0%) had acute organ dysfunction (pulmonary or non-pulmonary) at some point during hospitalization. Only 256 (19.5% of hospitalizations for COVID-19, and 34.0% of patients admitted on room air) had no acute organ dysfunction at any point during hospitalization. The proportion of COVID-19 hospitalizations with no acute organ dysfunction at any point during hospitalization ranged from 2.2% to 35.3% across hospitals, and from 14.0% to 25.0% across half-month epochs, with a nadir in early April (Fig. 2).
Figure 2.
Variation in the proportion of COVID-19 hospitalizations without acute organ dysfunction during hospitalization, by hospital (panel A) and over time (panel B). Panel A shows the proportion of admissions who never developed any acute organ dysfunction during hospitalization by hospital. Panel B shows the proportion of admissions who never developed acute organ dysfunction over time. There were a total of 1309 admissions during the study period; admissions by hospital ranged from 12 to 209; admission by time-period ranged from 36 to 693. May 16 to 30 is not shown as only 5 admissions for COVID-19 occurred during this time-period. ∗Acute organ dysfunction defined as at least 1 of the following: (A) receipt of any respiratory support (e.g., supplemental oxygen, heated high flow nasal cannula, non-invasive positive-pressure ventilation, or invasive mechanical ventilation); (B) acute renal dysfunction, as defined by new dialysis / renal replacement therapy or creatinine elevation ≥50% above baseline among patients without pre-existing end-stage renal disease; (C) acute hematologic dysfunction, as defined by platelet count <100 cells/μL and ≤50% or more from baseline; (D) acute liver dysfunction, as defined by total bilirubin elevation of >2.0 mg/dL and ≥50% above baseline; (E) receipt of vasopressor therapy; or (F) in-hospital mortality. Baseline renal, hematologic, and liver function were defined by lowest creatinine, highest platelet count, and lowest total bilirubin, respectively, during the hospitalization.
Our multivariable model predicting the development of acute organ dysfunction was moderately predictive, with a c-statistic of 0.75. Among patients admitted on room air, older age and abnormal vital signs on presentation were strongly predictive of development of acute organ dysfunction (Supplement Tables S3 and S4).
4. Discussion
In this multi-institutional cohort of patients hospitalized for COVID-19 in Southeast Michigan during the Spring 2020, the majority (57%) of patients required no supplemental oxygen on the calendar day of admission, and more than a third had no acute organ dysfunction (pulmonary or non-pulmonary) on presentation. However, these figures bely the illness severity of these patients. Among patients admitted on room air, there was a high rate of progression to more severe disease (2/3rd developed acute organ dysfunction during hospitalization), and in-hospital mortality was nearly 14%—markedly higher than in-hospital mortality among all inpatient hospitalizations in the United States in 2017 (∼2%)[27] or inpatient hospitalizations for pneumonia (3%–7%).[28–30]
Beyond quantifying the overall proportion of hospitalizations for COVID admitted on room air or without any acute organ dysfunction, we found marked variation across hospitals and, to a lesser extent, over time. The proportion of patients admitted on room air varied nearly 3-fold across hospitals, ranging from 32% to 80%, and from 50% to 70% over time. The proportion of hospitalizations without acute organ dysfunction varied more than 10-fold across hospitals, from 2% to 35%, and from 14% to 25% over time.
The proportion of patients admitted on room air nadired in early April when case counts peaked in Southeastern Michigan, suggesting a variable threshold for hospital admission during surge or contingency settings. We hypothesized that hospitalizations for lower disease severity would decrease over time as clinicians developed greater understanding of the natural history of COVID-19, and may therefore be better able to triage patients with low disease severity away from hospitalization. However, we found that the proportion of patients admitted on room air instead correlated with case volume in the region, and increased once case volume dropped at the end of the surge. These trends were similar across all our analyses, including analyses examining the proportion of patients admitted without any acute organ dysfunction, the proportion without acute organ dysfunction at any point during hospitalization, and analyses incorporating inter-hospital transfers.
The findings of our study are consistent with cohorts of hospitalized COVID-19 patients in the United States (US). For example, 26% of our cohort never required supplemental oxygen during hospitalization, which is similar to the rate reported across 8 hospitals in Georgia in March 2020, where 24% of patients hospitalized with COVID-19 never required supplemental oxygen.[1] Likewise, in a cohort of patients hospitalized in the Washington DC metropolitan area in March and April 2020, nearly 60% of patients did not require supplemental oxygen on admission, as compared to our cohort where 57% of patients did not require supplemental oxygen on admision.[2] Among our cohort, 35% developed acute kidney injury during hospitalization, 5% received new renal replacement therapy, 16% received invasive mechanical ventilation, and 17% received a vasopressor. These rates of acute organ dysfunction are similar to the rates reported in other US cohorts including in New York, the VA healthcare system, and New Orleans where rates of acute kidney injury ranged from 28% to 37%[31–35], new renal replacement therapy ranged from 4% to 15%,[31–34] use of invasive mechanical ventilation ranged from 13% to 30%,[2,31–34,36] and rates of vasopressor use ranged from 14% to 28%.[31–33,36] Likewise, overall mortality in our cohort (21%) was comparable to other cohorts of hospitalized COVID-19 patients in the US, including the nationwide VA healthcare system (19%),[36] 8 hospitals in Georgia primarily located in the Atlanta metropolitan area (17%),[1] hospitals in the Washington DC metropolitan region (16%),[2] 13 academic and community hospitals in metropolitan New York (16%),[31] and at a single large academic medical center in New York City (21%).[32]
The high rate of disease progression and mortality among patients admitted on room air underscores the importance of admission decisions, and also of monitoring and counseling patients who are not admitted. Given the large number of COVID-19 cases, limited number of hospital beds, and challenges of increasing hospital capacity, many hospitals have provided patients with pulse oximeters and instructions to present to the hospital if hypoxemic.[37–39] However, for the patients in our cohort who required supplemental oxygen on the day of admission, in-hospital mortality was nearly 31%. Moreover, pulse oximeters are prone to under-detect hypoxemia, especially in Black patients,[40] potentially leading to delayed care. Overall, our findings suggest that abnormal pulse oximetry may be an insufficiently sensitive threshold for hospitalization.
Our study has some limitations. First, we used the WHO ordinal scale to classify disease severity on admission, as it is a commonly-used and objective set of criteria. However, this scale focuses heavily on respiratory manifestations of disease, so does not fully capture the myriad potential indications for hospitalization. We therefore also measured acute organ dysfunction on presentation, as this is a more sensitive indicator of potential indications for hospitalization. Second, we are unable to assess the appropriateness of hospitalization for an individual patient. Thus, while the proportion of patients admitted on room air or without any organ dysfunction varied markedly across hospitals, some of this variation may be appropriate. However, the magnitude of variation observed and correlation with case counts suggests that there is a variable threshold for hospitalization by context. Third, we defined acute organ dysfunction based on absolute and relative changes in laboratory values. Some may prefer different thresholds to define organ dysfunction, but our focus was on understanding variation across hospitals and over time, which was facilitated by our objective definition.
5. Conclusions
Among patients hospitalized with COVID-19 during the spring 2020 surge in Southeast Michigan, more than half were on room air and a third had no acute organ dysfunction upon admission, but nonetheless experienced high rates of disease progression and in-hospital mortality. There was marked variation across hospitals and over time in the proportion of patients admitted on room air. The proportion nadired in early April when case counts peaked, suggesting that admission thresholds varied according to hospital demand and not as a function of experience with treating COVID-19.
Acknowledgments
We would like to acknowledge all participating CQIs and their members. Participating CQIs include: Hospital Medicine Safety (HMS) Consortium, Michigan Bariatric Surgery Collaborative (MBSC), Michigan Surgical Quality Collaborative (MSQC), Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI), Michigan Value Collaborative (MVC), Michigan Emergency Department Improvement Collaborative (MEDIC), Michigan Radiation Oncology Quality Collaborative (MROQC), Michigan Anticoagulation Quality Improvement Initiative (MAQI2), Michigan Spine Surgery Improvement Collaborative (MSSIC), Integrated Michigan Patient-Centered Alliance in Care Transitions (IMPACT), and the Michigan Trauma Quality Improvement Program (MTQIP).
Author contributions
Conceptualization: Max T. Wayne, Wenjing Weng, Megan O’Malley, Paul Bozyk, Mona M. Doshi, Scott A. Flanders, Jakob I. McSparron, Pratima Sharma, Lakshmi Swaminathan, Hallie C. Prescott.
Data curation: Max T. Wayne, Wenjing Weng, Megan O’Malley, Hallie C. Prescott.
Formal analysis: Max T. Wayne, Wenjing Weng, Megan O’Malley, Paul Bozyk, Mona M. Doshi, Scott A. Flanders, Jakob I. McSparron, Pratima Sharma, Lakshmi Swaminathan, Hallie C. Prescott.
Investigation: Max T. Wayne, Megan O’Malley, Paul Bozyk, Mona M. Doshi, Scott A. Flanders, Jakob I. McSparron, Pratima Sharma, Lakshmi Swaminathan, Hallie C. Prescott.
Methodology: Max T. Wayne, Megan O’Malley, Paul Bozyk, Mona M. Doshi, Scott A. Flanders, Jakob I. McSparron, Pratima Sharma, Lakshmi Swaminathan, Hallie C. Prescott.
Project administration: Max T. Wayne, Megan O’Malley, Hallie C. Prescott.
Resources: Hallie C. Prescott.
Software: Megan O’Malley, Hallie C. Prescott.
Supervision: Jakob I. McSparron, Hallie C. Prescott.
Validation: Max T. Wayne, Wenjing Weng, Megan O’Malley, Mona M. Doshi, Hallie C. Prescott.
Visualization: Max T. Wayne, Wenjing Weng, Hallie C. Prescott.
Writing – original draft: Max T. Wayne, Hallie C. Prescott.
Writing – review & editing: Max T. Wayne, Wenjing Weng, Megan O’Malley, Paul Bozyk, Mona M. Doshi, Scott A. Flanders, Jakob I. McSparron, Pratima Sharma, Lakshmi Swaminathan, Hallie C. Prescott.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CQI = collaborative quality initiative, WHO = World Health Organization.
How to cite this article: Wayne MT, Weng W, O’Malley M, Bozyk P, Doshi MM, Flanders SA, McSparron JI, Sharma P, Swaminathan L, Prescott HC. Variation in COVID-19 disease severity at hospital admission over time and across hospitals: a multi-institution cohort of Michigan hospitals. Medicine. 2021;100:37(e27265).
This work was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network, as part of their Value Partnerships program. This material is the result of work supported with resources and use of facilities at the Ann Arbor VA Medical Center. The views in this manuscript do not reflect the position or policy of AHRQ, the views of the Department of Veterans Affairs, or the US government.
The authors have no conflicts of interests to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Supplemental digital content is available for this article.
References
- [1].Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Garibaldi BT, Fiksel J, Muschelli J, et al. Patient Trajectories Among Persons Hospitalized for COVID-19. Ann Intern Med Published online September 22, 2020. doi:10.7326/m20-3905. [Google Scholar]
- [3].Institute for Health Metrics and Evaluation (IHME). COVID-19 Projections. Seattle, WA: IHME, University of Washington, 2020. Available from https://covid19.healthdata.org/projections. Accessed October 23, 2020. [Google Scholar]
- [4].Keeley C, Jimenez J, Jackson H, et al. Staffing up for the surge: expanding the New York City Public Hospital Workforce During The COVID-19 Pandemic. Health Aff (Millwood) 2020;39:1426–30. [DOI] [PubMed] [Google Scholar]
- [5].Maves RC, Downar J, Dichter JR, et al. Triage of scarce critical care resources in COVID-19 an implementation guide for regional allocation: an expert panel report of the task force for mass critical care and the American College of Chest Physicians. Chest 2020;158:212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Supady A, Curtis JR, Abrams D, et al. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med Published online 2021. doi:10.1016/S2213-2600(20)30580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin Microbiol Infect 2020;26:1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID-19 patients by risk factors: results from a United States Hospital Claims Database. J Heal Econ Outcomes Res 2020;7:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020;180:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haimovich AD, Ravindra NG, Stoytchev S, et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med 2020;76:442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Morley C, Unwin M, Peterson GM, Stankovich J, Kinsman L. Emergency department crowding: a systematic review of causes, consequences and solutions. PLoS One 2018;13: doi:10.1371/journal.pone.0203316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med 2013;61: doi:10.1016/j.annemergmed.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fagerström L, Kinnunen M, Saarela J. Nursing workload, patient safety incidents and mortality: an observational study from Finland. BMJ Open 2018;8: doi:10.1136/bmjopen-2017-016367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the First 6 Months of the Pandemic. JAMA Intern Med Published online 2020. doi:10.1001/jamainternmed.2020.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Michigan Hospital Medicine Safety Consortium. Mi-COVID19 Initiative. Ann Arbor, MI: HMS, Michigan Hospital Medicine Safety Consortium. Available from https://www.mi-hms.org/qualityinitiatives/mi-covid19-initiative. Accessed October 21, 2020. [Google Scholar]
- [16].Vaughn VM, Gandhi T, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a Multi-Hospital Cohort Study. Clin Infect Dis Published online August 21, 2020. doi:10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Southeast Michigan Council of Governments (SECOM). Detroit, MI. Available from https://semcog.org/. Accessed October 22, 2020 [Google Scholar]
- [18]. Michigan Government. Coronavirus - 18. Michigan Data. Lansing, MI, 2020. Available from https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173—,00.html. Accessed October 22, 2020. [Google Scholar]
- [19].World Health Organization WHO R&D Blueprint: Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed October 22, 2020 [Google Scholar]
- [20].Wang Y, Fan G, Salam A, et al. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically Ill patients with influenza virus infection. J Infect Dis 2020;221:1688–98. [DOI] [PubMed] [Google Scholar]
- [21].Davey RT, Fernández-Cruz E, Markowitz N, et al. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med 2019;7:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Desai A, Gyawali B. Endpoints used in phase III randomized controlled trials of treatment options for COVID-19. EClinicalMedicine 2020;23.doi:10.1016/j.eclinm.2020.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vanassche T, Engelen MM, Van Thillo Q, et al. A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study. Trials 2020;21:1005.doi:10.1186/s13063-020-04878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Centers for Disease Control and Prevention. Hospital toolkit for adult sepsis surveillance. Atlanta, GA: Centers for Disease Control and Prevention; 2018. Available from: https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf. Accessed 2020 October 23. [Google Scholar]
- [27].HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. https://hcupnet.ahrq.gov/. [PubMed] [Google Scholar]
- [28].Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017;65:1806–12. [DOI] [PubMed] [Google Scholar]
- [29].Williams S, Gousen S, DeFrances C. National hospital care survey demonstration projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report 2018;01–11. [PubMed] [Google Scholar]
- [30].AHRQ Publishing and Communications Guidelines. Content last reviewed March 2021. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/research/publications/pubcomguide/index.html. Accessed April 2, 2021. [Google Scholar]
- [31].Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020;98:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Argenzian MG, Bruc SL, Slate CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020;369.doi:10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mohamed MMB, Lukitsch I, Torres-Ortiz AE, et al. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360 2020;1:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol 2021;16:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siew ED, Alp Ikizler T, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012;7:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA - J Am Med Assoc 325:304–6. doi:10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home potential pitfalls and practical guidance. Ann Am Thorac Soc 2020;17:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med 2020;27:681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Read JS. Health Update December 15, 2020. Vermont Department of Health. Update regarding use of pulse oximeters to monitor novel coronavirus 2019 (COVID-19) among individuals with laboratory-confirmed SARS-CoV-2 infection. Available from: https://www.healthvermont.gov/sites/default/files/documents/pdf/COVID-19-HAN-PulseOximetryProgramUpdate.pdf Accessed March 31, 2021. [Google Scholar]
- [40].Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med 2020;383:2477–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.