ABSTRACT
Remdesivir is a nucleoside monophosphoramidate prodrug that has been FDA approved for coronavirus disease 2019 (COVID-19). However, the clinical efficacy of remdesivir for COVID-19 remains contentious, as several trials have not found statistically significant differences in either time to clinical improvement or mortality between remdesivir-treated and control groups. Similarly, the inability of remdesivir to provide a clinically significant benefit above other investigational agents in patients with Ebola contrasts with strong, curative preclinical data generated in rhesus macaque models. For both COVID-19 and Ebola, significant discordance between the robust preclinical data and remdesivir’s lackluster clinical performance have left many puzzled. Here, we critically evaluate the assumptions of the models underlying remdesivir’s promising preclinical data and show that such assumptions overpredict efficacy and minimize toxicity of remdesivir in humans. Had the limitations of in vitro drug efficacy testing and species differences in drug metabolism been considered, the underwhelming clinical performance of remdesivir for both COVID-19 and Ebola would have been fully anticipated.
KEYWORDS: GS-441524, GS-5734, in vitro models, in vivo models, nonhuman primates, pharmacokinetics, prodrug, remdesivir
INTRODUCTION
Remdesivir (RDV) is a nucleoside monophosphoramidate prodrug that has been FDA approved for coronavirus disease 2019 (COVID-19) principally on the basis of one double-blind, randomized control trial (RCT) (1), which demonstrated a faster median time to recovery in RDV-treated patients by about 5 days (10 days) than in the placebo group (15 days). However, the clinical efficacy of RDV remains contentious, as a smaller double-blind RCT showed no statistical difference in clinical improvement between RDV- and placebo-treated patients (2); interim results from a larger RCT conducted by the WHO revealed no significant difference in mortality between patients treated with RDV and those treated with the standard of care (SOC) (3, 4), prompting the organization to recommend against using RDV for COVID-19 (4). Such clinical results diametrically oppose the impressive preclinical data, which showcased RDV’s strong, broad-spectrum antiviral activity in cell culture (5–7) and in preclinical species (8–11). Here, we show that overestimations of RDV’s clinical performance arise from several preclinical assumptions made at both the in vitro and in vivo levels, with the principal assumption being that the conventional drug development framework is relevant to prodrugs such as RDV. We show that this preconceived notion fails to account for the significant differences between “soft” drugs (those that are subject to metabolic transformation) such as RDV and “hard” drugs (those that are metabolically inert) which permeate the FDA-approved drug landscape (12). By reassessing key data on RDV at the cell culture and preclinical organismal levels, we elucidate the predictability of RDV’s underwhelming clinical performance and provide insights for future development of phosphate prodrugs.
REMDESIVIR IS A McGUIGAN PRODRUG THAT PREDOMINANTLY DEPOSITS BIOACTIVE TRIPHOSPHATE IN THE LIVER
RDV is a phosphoramidate prodrug of the McGuigan (ProTide) class that was originally designed to deliver a membrane-impermeable nucleoside triphosphate (NTP) analogue (GS-443902) for the treatment of hepatitis C (HCV) (13–16). McGuigan prodrugs such as RDV, which contain a phosphate protected by aryloxy and amino acid ester moieties, are intended for intracellular conversion of the active NTP through bioactivation by carboxylesterase 1 (CES1), cathepsin A (CTSA), and histidine triad nucleotide binding protein 1 (HINT1) (16, 17). During RDV’s development, several other nucleoside and nucleotide analogues were also being investigated for their anti-HCV efficacy, with the most advanced compounds being pyrimidine analogues (NCT00120835, NCT00869661, and NCT01096576). As purine analogues, RDV and its sibling compounds (14) were thus developed against the backdrop of pyrimidine analogues. Application of the McGuigan prodrug strategy onto anti-HCV pyrimidine analogues had two aims: (i) rapid loading of the active NTP in the liver, where HCV is trophic, and (ii) overcoming the rate-limiting initial phosphorylation step observed for some pyrimidine analogues (18). While the former reason is generally applicable to multiple phosphate prodrugs, the latter reasoning has yet to be explicitly demonstrated for purine (adenosine) analogues such as RDV and its parent nucleoside, GS-441524. In fact, the prohibitively slow initial phosphorylation step appears to be limited to some uridine analogues, such as RO2433 (parent nucleoside of sofosbuvir) (18), with many cytidine and thymidine analogues appearing to undergo initial phosphorylation quite readily (19, 20). Multiple studies have already demonstrated the ability of GS-441524 to undergo conversion to the active NTP through direct measurement of GS-443902 levels or through indirect assessment of the low-micromolar 50% effective concentration (EC50) of GS-441524 against virus-infected cells; varying EC50s for GS-441524 and RDV in head-to-head comparisons further suggest that the cellular potency of either compound is cell line and cell type dependent (see Data Set S1) (5, 7, 8, 21, 22), despite the fact that the exquisite specificity of GS-443902 for the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) is undisputed (Km, ∼9 nM) (23–25). Thus, as an artifact of its initial HCV indication, the primary function of the McGuigan promoiety on RDV is preferential hepatic bioactivation (26).
Much of the initial excitement around the potency of RDV in cell culture models of SARS-CoV-2 centered on its low-micromolar potency in a variety of cell lines and cell types, including primary human airway epithelial (HAE) cells, in which the lowest reported EC50 for RDV against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was obtained (5). Against Ebola virus (EBOV)-infected cells in culture, treatment with RDV yielded similarly impressive, nanomolar EC50s (6, 8, 27) (Data Set S1). Among other oversights discussed subsequently, these superficially impressive in vitro efficacy data were assessed in isolation—without considering how such data compare to RDV’s cytotoxicity in hepatocytes. Placed in the context of primary human hepatocytes (PHH) in vitro, the levels of active NTP formed by primary HAE cells are unambiguously dominated by those formed in PHH (Fig. 1). Simply put, even in the cell model where RDV performs best against SARS-CoV-2, levels of active NTP are still approximately 4-fold higher in PHH than in primary HAE cells when both cell types are subjected to identical culturing and treatment conditions (5, 28).
FIG 1.
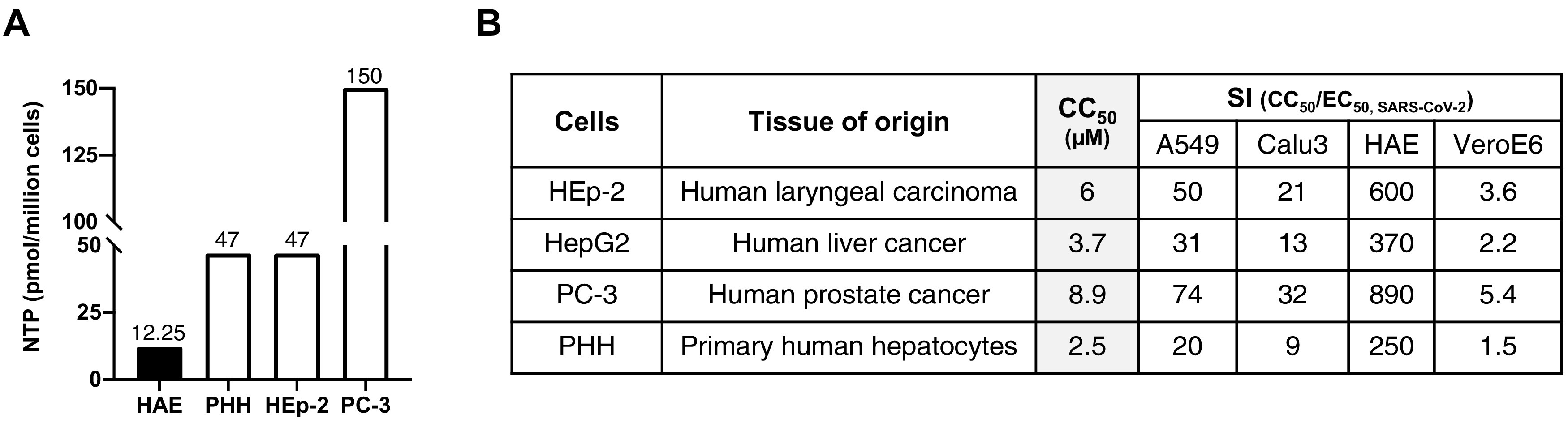
Remdesivir is preferentially bioactivated in hepatocytes. (A) RDV NTP (GS-443902) formation in PHH and HEp-2 cells is about 4-fold higher than in HAE cells and about 12-fold higher in PC-3 cells than in HAE cells. Data are means of data replotted from references 5 (HAE) and 28 (PHH, HEp-2, and PC-3). Cells were seeded at approximately 1 × 106 cells/well and were incubated with 1 μM RDV for 24 h. Levels of NTP in HAE cells represent the average of quadruplicate technical replicates from 2 donors. (B) Comparison of selectivity indices (SIs) for CC50s of RDV in HEp-2, HepG2, PC-3, and PHH and RDV EC50s obtained in A549, Calu3, HAE, and Vero E6 cells infected with SARS-CoV-2. EC50s used were obtained from references 37 (A549) and 5 (Calu3, HAE, and Vero E6).
CONVENTIONAL CELL CULTURE PROTOCOLS FAIL TO ACCOUNT FOR THE COMPLEX PHARMACOKINETICS OF REMDESIVIR IN VIVO
Discrepancies between the in vitro and in vivo conditions are exacerbated for prodrugs that are susceptible to extracellular metabolism, such as RDV. Key considerations that are particularly pertinent for (phosphate) prodrugs but tend to be overlooked under standard cell culturing protocols include magnitude of exposure, exposure time, and distribution patterns incurred by route of administration. One could argue that such shortcomings could be accounted for in subsequent in vivo evaluations. Nevertheless, it appears that decisions to advance or halt the development of certain prodrugs are contingent upon in vitro and ex vivo data without consideration of the assumptions inherent in standard cell culture protocols or without revision to the way in which cell culture assessments are performed even after observing the prodrug’s pharmacokinetic behavior in vivo. For instance, a typical cell-based screening assay involves incubating cells with virus for approximately 30 to 60 min before removing virus-containing medium, washing the cells, and then incubating the cells with drug for 48 to 72 h (5, 6, 29). While appropriate for drugs with long half-lives (t1/2) (i.e., most hard drugs), such conditions skew efficacy results for soft drugs with short t1/2, such as RDV. Under standard screening conditions, 4 assumptions are made (Table 1): (i) viral load is constant, (ii) the target population of cells is constantly exposed to drug for 48 to 72 h at supraphysiological concentrations of drug, (iii) the ratio of drug concentration to the number of target cells is high, (iv) preferential extraction by other organs or cell types is negligible. Using RDV as an example, such assumptions are inconsistent with its clinical pharmacokinetics, given that it has a t1/2 of less than 1 h in humans (30, 31). For the brief time that intact RDV is present in systemic circulation, it is both unclear and unlikely that tissue distribution is uniform, despite RDV’s large apparent volume of distribution during terminal phase (Vz) (32); preferential hepatic extraction (Fig. 1) and competing plasma esterase hydrolysis undermine the ability of RDV to durably reach the primary site of SARS-CoV-2 infection (type II alveolar cells) and exert its antiviral activity (30, 31).
TABLE 1.
Assumptions in standard cell culture procedures do not reflect the in vivo behavior for prodrugs like RDV
| Standard procedure | Incorrect assumption | In vivo reality |
|---|---|---|
| Incubate cells with virus for 30–60 min | Viral load is constant | Viral load is not always constant |
| Incubate cells with drug for 48–72+ h | Target population of cells is constantly exposed to prodrug for 48–72+ hours at supraphysiological [drug] | Prodrug exposure can be short and is influenced by the t1/2 of the prodrug |
| [Drug]:target cell population is high in vivo | [Drug]:target cell population is low in vivo | |
| Preferential extraction by other organs or cell types is negligible | Drug distribution can be uneven due to preferential metabolism by certain tissue over others |
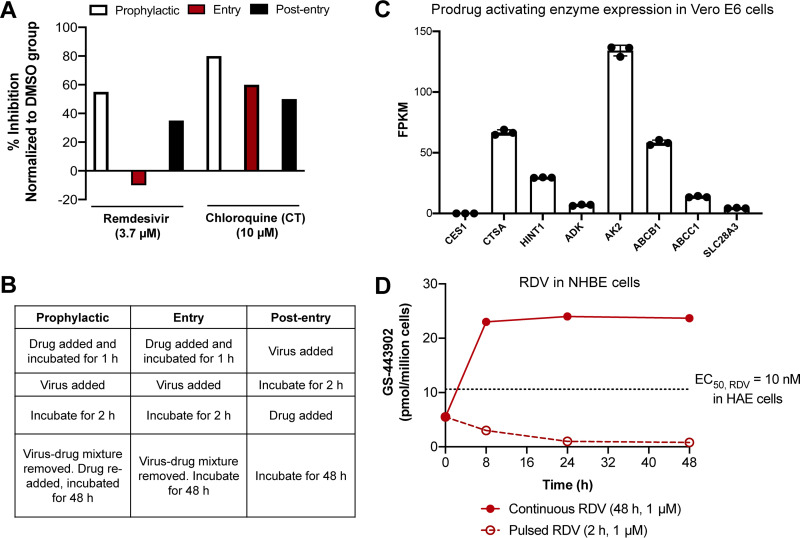
A set of experiments by Wang and colleagues demonstrated that the magnitude of SARS-CoV-2 inhibition by RDV in Vero E6 cells was largely contingent upon the duration of drug exposure (29). Seeking to probe the mechanism of SARS-CoV-2 inhibition by RDV and chloroquine (CQ), Wang et al. performed a series of “prophylactic,” “entry,” and “postentry” experiments (Fig. 2A and B). Of the 3 conditions tested, the entry experiment most closely resembled the in vivo pharmacokinetics of RDV, as cells were pulsed with drug for just 3 h before being washed and allowed to incubate with fresh medium for the duration of the experiment. Like RDV, CQ is subject to metabolism to its main metabolite, N-desethylchloroquine (DCQ). However, unlike RDV, with its short t1/2 (<1), the t1/2 of CQ is approximately 18 to 30 days (33, 34). Thus, the short pulsing experiment more accurately reflects the in vivo pharmacokinetic situation for RDV but not CQ. Noting differences in the tested concentrations of RDV and CQ, RDV demonstrated no inhibition of SARS-CoV-2 after a 3-h drug pulse at 3.7 μM; in contrast, CQ maintained similar levels of SARS-CoV-2 inhibition across all 3 conditions (Fig. 2A, Entry). Discordant inhibitory values for RDV across the 3 conditions suggest that the antiviral efficacy of RDV is considerably diminished when target cells are pulsed rather than continuously exposed to RDV. One potential confounder to this interpretation could be that these experiments were performed in Vero E6 cells, which have rather high expression of p-glycoprotein (p-gp) (Fig. 2C, ABCB1). It has been reported that RDV is a substrate for ABCB1 (32); in contrast, CQ has been shown to be a substrate for ABCC1 (35), which is expressed at lower levels in Vero E6 than ABCB1 (Fig. 2C). Thus, while pulsed experiments in Vero E6 may exaggerate the reduced anti-SARS-CoV-2 activity of RDV, the totality of these data points to the dependency of RDV’s antiviral activity in the target cell population on duration of exposure.
FIG 2.
Duration of drug exposure impacts the antiviral efficacy of remdesivir. (A) Continuous exposure to RDV leads to durable in vitro inhibition of SARS-CoV-2, but pulsed treatment with RDV results in diminished antiviral activity in Vero E6 cells. Data are means of triplicate data replotted from reference 29. Across the three experiments, RDV was tested at 3.7 μM and CQ was tested at 10 μM. Due to its long in vivo t1/2, CQ serves as a positive control. (B) Corresponding treatment procedures for prophylactic, entry, and postentry experiments described for panel A. Pulsed experiments correspond to the entry trial. (C) Transcriptome sequencing (RNA-seq) data showing expression of relevant prodrug bioactivating enzymes for RDV in Vero E6 cells, presented as means and standard deviations (SD) from 3 experiments (see the supplemental material). ADK, adenosine kinase; AK2, adenylate kinase 2. AK2 and SLC29A3 were found to mediate RDV potency and toxicity in a genome-wide CRISPR screen (55). (D) Formation of active triphosphate (GS-443902) in normal human bronchial epithelial (NHBE) cells following pulsed (open circles) and continuous (filled circles) incubation. Data are adapted from reference 36 and are presented as means for at least 2 independent replicates. The dotted line at 10.6 pmol/million cells corresponds to the mean C24 of triphosphate formed by RDV in SARS-CoV-2-infected HAE cells when the EC50 was determined to be 10 nM, as reported in reference 5.
In a more recent publication, Mackman et al. compared the formation of GS-443902 when normal human bronchial epithelial (NHBE) cells were incubated with either pulsed (2 h) or continuous (48 h) concentrations (1 μM) of drug in an attempt to better recapitulate the transient in vivo t1/2 of RDV (Fig. 2D) (36). Expectedly, concentrations of GS-443902 were considerably diminished under pulsed conditions (Fig. 2D), which supports reduced antiviral activity by RDV observed by Wang et al. (29) under entry conditions (Fig. 2A). In fact, when the levels of GS-443902 generated by RDV under pulsed and continuous conditions are compared to the concentration at 24 h (C24) of GS-443902, corresponding to an EC50 of 10 nM in SARS-CoV-2-infected HAE cells (5), it becomes apparent that short exposure to RDV is insufficient to achieve the desired, low-nanomolar antiviral effects in vitro (Fig. 2D). This supports the dramatically reduced antiviral activity observed by Wang et al. in Vero E6 cells (29).
While the aforementioned experiments address the second assumption (drug exposure), they do not resolve the third (ratio of drug concentration to cell number) and fourth (preferential extraction by other organs) assumptions and thus must be taken with grains of salt rather than as definitive extrapolations of in vivo behavior (Table 1). The pulsed experiments described by Wang et al. (29) and by Mackman et al. (36) assume the ratio of drug concentration to cell number is high (Table 1, third assumption), which opposes the in vivo reality where the ratio of drug concentration to cell number is low and is exacerbated by uneven drug distribution due to preferential metabolism in cell types such as the liver (Table 1, fourth assumption) (26). It is admittedly difficult to account for these in vivo factors in cell culture; however, the challenge of modeling them does not give license to disregard their significance. Indeed, Mackman et al. demonstrated that a 10-mg/kg single intravenous (i.v.) dose of RDV in African green monkeys and cynomolgus macaques resulted in high accumulation of GS-443902 and RDV metabolites in the liver and kidney, suggesting nonuniform distribution of the prodrug (Table 1, fourth assumption; Fig. S1) and reemphasizing the inability of conventional cell culture protocols to account for this important in vivo reality (36). As is apparent in the stark contrast between the in vitro potency of RDV and its modest clinical efficacy (2, 3, 27, 37), results from in vitro experiments offer an incomplete portrayal of the in vivo situation; data obtained from such studies should thus be reconsidered in light of the 4 key assumptions (Table 1) when judgments are made regarding which compounds to advance to the clinic.
PROLONGED TREATMENT WITH REMDESIVIR IN NONHUMAN PRIMATES FAILS TO ANTICIPATE CLINICALLY SIGNIFICANT HEPATOTOXICITY
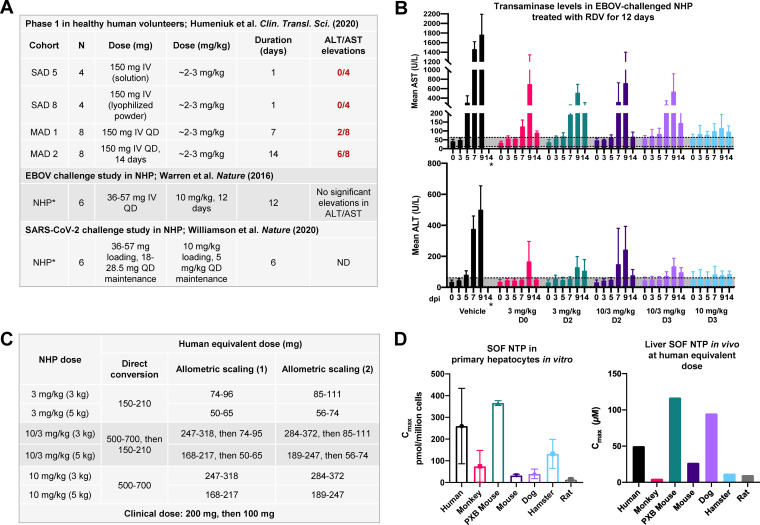
While biochemical and immunohistological data suggest that preferential hepatic metabolism of RDV could result in liver related dose-limiting toxicities (DLTs) in the clinic, such shortcomings were not accounted for in nonhuman primates (NHPs) subjected to repeated dosing of RDV. During a 28-day EBOV challenge study, rhesus macaques were subject to various dosing regimens of RDV for 12 days (8). There were two particularly outstanding findings from this study. First, only the continuous 10-mg/kg dose resulted in “profound suppression of EBOV replication and protected 100% of EBOV-infected animals against lethal disease, ameliorating clinical disease signs and pathophysiological markers, even when treatments were initiated 3 days after virus exposure when systemic viral RNA was detected in two out of six treated animals.” Second, 12-day treatment with RDV at 10 mg/kg did not lead to significant elevations in the ratio of alanine aminotransferase to aspartate transaminase (ALT/AST) (Fig. 3B). The latter is particularly surprising, as a 10-mg/kg dose in a 50- to 70-kg human directly equates to 500 to 700 mg of RDV, which is more than 2 times higher than the amount of RDV used clinically and more than twice the duration of RDV currently recommended for COVID-19 (1–3, 38, 39). Even with allometric scaling (40), 10 mg/kg RDV in NHPs translates to about 168 to 284 mg of RDV in humans (Fig. 3C), which exceeds the recommended loading dose of 200 mg followed by 100 mg for 5 to 10 days. Unlike NHPs, which can tolerate 10 mg/kg RDV for 12 days without significant transaminase elevations, humans would not be able to withstand the allometrically scaled dose for the same duration, given that duration-dependent, low-grade transaminase elevations had already emerged in 25% and 75% of healthy human volunteers administered 150 mg once a day (QD) for 7 and 14 days, respectively (Fig. 3A and B) (30, 41). Allusions to higher hepatic extraction of McGuigan prodrugs in human than in monkey hepatocytes had been demonstrated in another Gilead study with sofosbuvir (SOF), another McGuigan prodrug (42). Following a 2-h pulse with 10 μM SOF in primary hepatocytes across species, human hepatocytes formed about 3-fold-higher levels of active SOF NTP than monkey hepatocytes (Fig. 3D). Likewise, maximum concentrations (Cmax) of SOF NTP in vivo were found to be much higher in human liver (∼50 μM) (43) than in monkey liver (∼5 μM) when SOF was administered at the allometrically scaled dose (400 mg in humans) (Fig. 3D) (42). Coupled with the observation that 12-day dosing with RDV at 10 mg/kg does not yield significant ALT/AST elevations in NHP (8), these data strongly suggest there is preferential hepatic extraction of McGuigan prodrugs in humans compared to NHP.
FIG 3.
Humans exhibit higher hepatic extraction of McGuigan prodrugs than nonhuman primates. (A) Doses of RDV trialed in the single ascending dose (SAD) and multiple ascending dose (MAD) arms of the phase 1 trial for a 50- to 70-kg human compared to the dose administered in a 3.6- to 5.7-kg NHP. The human and NHP doses have been converted to milligrams per kilogram and milligrams, respectively. Values were obtained from references 8, 30, and 11. (B) ALT and AST levels in NHP (rhesus macaques; 6 per group) challenged with EBOV and treated with RDV for a total of 12 days (adapted from reference 8). Regions shaded in gray correspond to normal ALT/AST ranges in NHP (ALT,5 to 61 U/liter; AST, 12 to 63 U/liter) (56). NHPs treated at 10 mg/kg for 12 days (light blue) did not experience significant elevations in ALT/AST. *, No ALT/AST values were obtained at 14 days postinfection (dpi) in vehicle control animals because 100% of animals had died. (C) Human equivalent dose (HED) of NHP RDV doses tested in reference 8 via direct conversion and allometric scaling as described in reference 40. Allometric scaling (1) uses an exponent of 0.75, while allometric scaling (2) uses an exponent of 0.80. HEDs were calculated for rhesus macaques weighing 3 kg (light gray) and 5 kg (dark gray) using the Clymer allometric scaling calculator. (D) Sofosbuvir (SOF) is another McGuigan prodrug that is more readily metabolized in PHH than primary monkey hepatocytes in vitro (left) and in vivo (right). (Left) Levels of SOF NTP in primary hepatocytes following a 2-h pulse of 10 μM SOF. (Right) Levels of SOF NTP in human explanted livers and in preclinical model species at the allometrically scaled human dose (400 mg). Open bars, Cmax; shaded bars, C24. C24 in primary monkey livers was below the detection threshold. Values were replotted from references 43 and 42. Formation of SOF NTP is higher in PHH than primary monkey hepatocytes in vitro and in vivo, suggesting more efficient metabolism of McGuigan prodrugs in human than monkey livers.
As a result of model-specific disparities in the hepatic extraction of McGuigan prodrugs, the dose escalation that would likely be required to clarify RDV’s antiviral activity in COVID-19 (2) and Ebola (44, 45) patients is prohibited due to on-target hepatoxicity (30). Thus far, the largest clinical study that examined the relationship between RDV treatment and reduction of SARS-CoV-2 titers was published by Wang and colleagues in The Lancet (2). Although it was criticized for its small trial size (n = 237), this study showed that RDV was unable to demonstrate (i) a statistically significant time to improvement compared to placebo controls and (ii) reduction in viral loads in the upper and lower airways compared to controls (2). Since that study was published, some unique case reports have described the ability of RDV to reduce viral loads in the upper airway (46, 47). Still, the bulk of available data suggest that the current recommended dose (200 mg loading, 100 mg maintenance for 5 to 10 days [48]) is insufficient (41) to yield the robust antiviral activity to which many in vitro studies have alluded (5, 37, 49). Insufficient dosing in humans is corroborated by data on RDV in EBOV-infected patients, in which RDV was unable to demonstrate significant reductions in viral loads at the standard dose (200 mg loading, 100 mg maintenance for 9 to 12 days) in hospitalized patients (45) or at a lower dose (100 mg for 5 days) during treatment in patients with viral persistence in semen (44).
Preclinical evaluation of RDV at the allometrically scaled dose (10 mg/kg loading, 5 mg/kg maintenance for 6 days postinfection [dpi]) in rhesus macaque models with mild COVID-19 (11, 50) found that there was no significant reduction in viral titers in the upper airway by RDV compared to untreated animals, with the exception of the trachea (Fig. 4A and B) at 7 dpi. Treatment with RDV yielded a global reduction in viral load in all examined lobes of the lung; however, this dose was unable to exert a more profound reduction in viral titers during the treatment period (Fig. 4A). The underwhelming nature of these data is underscored by the close relationship between viral load and disease severity (51).
FIG 4.
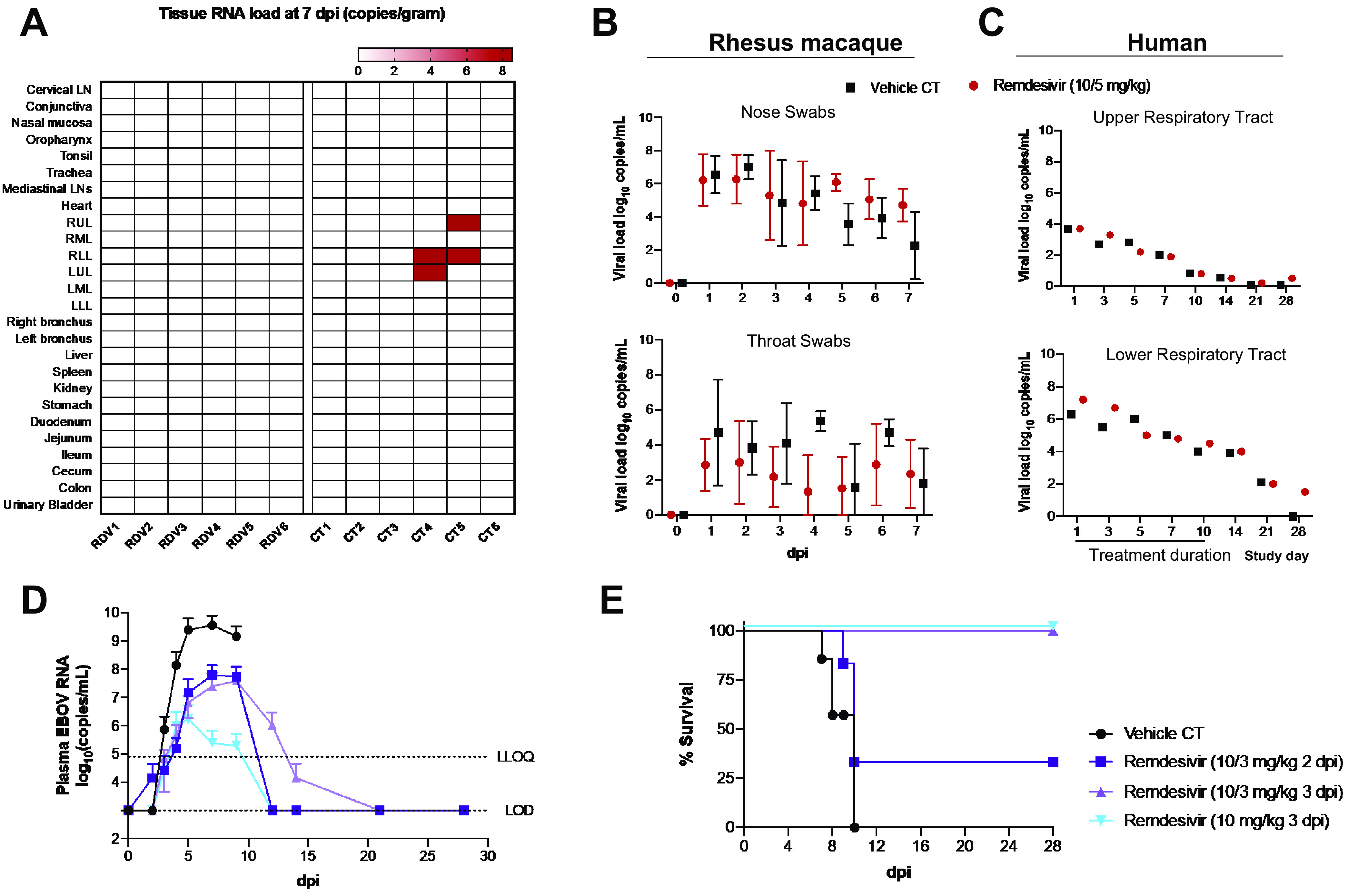
Human equivalent dose of RDV in rhesus macaque models of COVID-19 or EBOV does not yield robust reductions in viral titers. (A and B) SARS-CoV-2-infected rhesus macaques (2.6 × 106 50% tissue culture infective doses [TCID50] of nCoV-WA1-2020) treated with the allometrically scaled dose of 10 mg/kg 1 dpi followed by 5 mg/kg 2 to 6 dpi yields a modest global reduction in SARS-CoV-2 viral titers. Data are adapted from the supplemental information in reference 11. (A) Heat map of organs profiled for the presence of SARS-CoV-2 RNA in RDV-treated (n = 6) and control (CT) (n = 6) animals. LN, lymph node; UL, upper lobe; ML, middle lobe; LL, lower lobe. (B) Viral loads in nose swabs and throat swabs between RDV-treated and CT animals. (C) No significant decrease in viral loads in the upper and lower respiratory tracts in patients treated with RDV (200 mg on day 1, 100 mg on days 2 to 10) was seen compared to placebo. Data are adapted from reference 2 and are presented as means (n = 107 [RDV] and 52 [placebo]). (D) Rhesus macaques were infected with EBOV (1,000 PFU; EBOV H. sapiens-tc/COD/1995/Kikwit) and treated i.v. with either 10 mg/kg RDV at 2 dpi for 12 days (light blue), a 10-mg/kg loading dose at 3 dpi and then 3 mg/kg for 11 days (dark purple), a 10-mg/kg loading dose at 3 dpi and then 3 mg/kg for 11 days (light purple), or vehicle (black). Animals (6 per group) were monitored for a total of 28 days. The most distinct reductions in viral titers occurred for NHPs in the 10-mg/kg treatment group (light blue). (E) Survival curves for NHPs at 28 dpi for treatment groups indicated. Though viral titers dropped to the limit of detection (LOD) for the 10/3-mg/kg D2 group (D, dark purple D) by day 12, only 2/6 NHPs in this group survived (E, dark purple), skewing viral titer data. At the same time, this reinforces the close relationship between stark reductions in viral titers and improved disease outcomes, as the remaining 2/6 animals in the 10/3-mg/kg D2 (2 days postinfection) group survived at 28 dpi. Data are adapted from reference 8.
Likewise, the relationship between RDV dose and degree of viral reduction was readily observed in a rigorous study designed to investigate the optimal RDV dose for the treatment of EBOV in NHPs. Among the 12-day dosing regimens investigated in EBOV-challenged NHPs, two schedules initiated at 3 dpi yielded a 100% survival rate at 28 dpi: (i) 10 mg/kg and (ii) 10 mg/kg loading followed by 3 mg/kg maintenance for 11 days. Despite the fact that both schedules yielded the same survival efficacy, the authors note that “antiviral effects were consistently greater in animals administered repeated 10 mg/kg GS-5734 [RDV] doses (8)” (Fig. 4D). In fact, the direct relationship between drug dose, reduction in antiviral activity, and improved disease outcomes is most apparent in a comparison of the outcomes between the lowest (3 mg/kg beginning at 2 dpi) and highest (10 mg/kg beginning at 3 dpi) dosing regimens tested (Fig. 4D, light purple versus light blue): whereas the former yielded 66% survival by 28 dpi and moderate reductions in viral titers early in treatment, the latter yielded 100% survival by 28 dpi and robust reductions in viral titers early in treatment. In contrast to the large, unexplained disparities in survival for the 10/3-mg/kg dosing regimen (Fig. 4E, dark purple versus light purple), frank reductions in viral titers and 100% survival were unique to the 10-mg/kg D3 cohort (Fig. 4D and E, light blue). Viewed in totality, these data demonstrate that the antiviral effects of RDV can be augmented by up-dosing. As RDV had already been investigated for EBOV before SARS-CoV-2, knowledge of its safety and tolerability in healthy human volunteers had already been established (30) well before preclinical studies into its in vivo efficacy against SARS-CoV-2 had begun, and 10-mg/kg/day dosing was not evaluated (11). However, the dose-dependent decreases in viral titers with EBOV (8), coupled with the higher affinity of GS-443902 for the SARS-CoV-2 RdRp compared to the EBOV RdRp (23), strongly suggest that 10-mg/kg/day dosing would have demonstrated a more obvious antiviral benefit in NHP models.
Inadequate dosing of RDV in humans likely explains suboptimal antiviral effects for both COVID-19 and EBOV. Though Warren et al. concluded that 10-mg/kg/day dosing offered the best response in EBOV-challenged NHPs (8), liver-related DLTs in healthy human volunteers (Fig. 3) ultimately precluded administration at the human equivalent dose (HED) for the duration required for more dramatic reductions in viral titers in NHPs (30). If the 10-mg/kg RDV dose regimen had been directly translated in humans, an HED of 168 to 284 mg RDV for 12 days would have been administered. Instead, RDV was instead administered at 200 mg on day 1, followed by 100 mg from day 2 and continuing to days 9 to 13 for EBOV or days 2 to 10 for COVID-19 (1, 2, 45). These dosing schedules resemble the 10/3-mg/kg schedule used for NHPs challenged with EBOV or the 10/5-mg/kg schedule used for NHPs challenged with SARS-CoV-2, neither of which resulted in stark reductions in viral titers for their respective virus (Fig. 4B and D) (8, 11).
Viewed together, these data suggest that reductions in viral titers—and the closely related improvements in disease outcomes (Fig. 4D and E)—are dose dependent. Stronger antiviral effects by RDV would likely be observed in humans if it were possible to safely escalate the dose (41, 52). Considering the dose recommendations for RDV, large-scale studies on the relationship between up-dosing RDV and reductions in viral titers have thus not been conducted. However, there exists a single notable case report from 2015 documenting the effects of high-dose RDV in reducing EBOV titers in a Scottish nurse who relapsed with EBOV—the first time RDV had been administered in an EBOV patient (52). While she had received other agents during her hospitalization, there was a period in which only RDV was administered on a compassionate-use basis—before safety in humans had been fully characterized. This patient received a daily i.v. infusion of 150 mg RDV for 3 days, which was then increased to 225 mg for 11 days. In addition to being the highest reported dosing schedule for any human, it most closely resembles the 12-day 10-mg/kg dosing schedule that was found to be most effective in the rhesus model of EBOV (8). While the report indicates that the first reported decline in viral RNA occurred 1 day before RDV treatment began, it is interesting that a sharp, sustained reduction in viral titers in cerebrospinal fluid (CSF) and plasma occurred during RDV treatment. Ultimately, clinical recovery was observed when RDV was supplemented with dexamethasone on day 4 of 14 of RDV treatment (52). Despite the inherent limitations of a case report, these data hint that up-dosing could clarify the magnitude of antiviral activity by RDV and could warrant further investigation into the dose-dependent nature of its antiviral activity in humans. Due to the low-grade transaminase elevations observed in healthy human volunteers treated with RDV (30), constraints on the RDV dose have understandably prevented larger-scale studies from being initiated to support initial observations made in the Scottish nurse case report (52). Both in vitro and phase 1 data indicate that the up-dosing that would be required to boost the presently questionable antiviral effects of RDV (2, 3) is precluded by its nephro- and hepatotoxicity (28, 30).
Against liver-derived cell lines and PHH in vitro (Fig. 1b), RDV demonstrated uniquely low 50% cytotoxic concentrations (CC50) (2.5 to 6 μM) compared to >100 μM for its parent nucleoside, GS-441524, which demonstrates similar anti-SARS-CoV-2 activity (28); this concurs with its putative mechanism of bioactivation by enzymes that are highly expressed in the liver (16). These in vitro data explain the dose duration-dependent emergence of low-grade transaminitis observed in the phase 1 dose escalation study in healthy human volunteers: administration of RDV at 150 mg for 7 or 14 days resulted in 25% and 75% of volunteers experiencing grade 1 and 2 ALT/AST elevations, respectively (Fig. 3A) (30). Likewise, the in vitro CC50 against renal proximal tubule epithelial cells (RPTECs), while not as low as that observed in liver cell lines, is considerably lower (12.9 μM) than that observed for its parent nucleoside (>100 μM) (Fig. S1) (28). Coupled with the documented renal elimination of the solubilizing excipient Captisol (53), the lower CC50 of RDV observed in kidney cells in vitro could partly explain observations of exacerbated kidney injury in patients with compromised renal function treated with RDV (41, 54). The latter explanation concurs with tissue distribution studies of the total nucleoside (GS-441524) and nucleotide metabolites (mono- and diphosphates of GS-441524 and the triphosphate, GS-443902) in NHPs following 10-mg/kg single-dose i.v. administration of RDV formulated with the Captisol-containing solution used in the clinic (36). Compared to NHP administered 20 mg/kg i.v. GS-441524 formulated without Captisol, a significantly higher proportion of total nucleoside and nucleotide metabolites were observed in the kidneys of NHPs dosed with RDV (Supplemental Fig. S1) (36). Thus, the unique sensitivity of liver and kidney cells to RDV due to its identity as a hydrophobic McGuigan prodrug (28)—reinforced by the liver- and kidney-related exclusion criteria in its clinical trials (1–3)—indicates that the up-dosing that would be required to boost RDV’s antiviral effects would be likely to broadly result in concomitant hepato- and nephrotoxicity.
CONCLUSION
Preclinical evaluation of compounds should be dynamic. Especially in the case of prodrugs with short in vivo t1/2, such as RDV, cell culture protocols should be revised upon receipt of in vivo data to better reflect the prodrug’s pharmacokinetics in vivo (Table 1). Standard workflows appear to overemphasize low EC50s while underappreciating the contributions of drug exposure, tissue-specific localization, and a drug’s CC50. While it is true that the exigency of the COVID-19 pandemic made RDV amenable to rapid investigation into its utility as a therapeutic, its underwhelming clinical performance ought to have incited a meticulous investigation to identify reasons for RDV’s discordant preclinical strength and clinical weakness. Assumptions inherent in cell-based screening protocols—while perhaps adequate for hard drugs—are poorly suitable for esterase-labile prodrugs such as RDV, in which transient exposure to the target cell population is exacerbated by uneven tissue metabolism (Table 1, assumptions 2 to 4) (26, 30, 31).
At the in vivo level, an overreliance on NHP models when studying McGuigan prodrugs fails to anticipate RDV’s hepatotoxicity in humans at dosing intervals that are well tolerated in NHP, presumably due to species differences in hepatic extraction of McGuigan prodrugs (Fig. 3B and D). Based on studies spearheaded by Wang et al. with SOF (42), it appears that the efficiency of McGuigan prodrug metabolism in human hepatocytes is greater than that in NHPs but less than that in PXB mice and dogs (Fig. 3D). This would suggest that the contribution of dog models for safety and tolerability should not be underappreciated in favor of NHP models when considering the in vivo behavior of McGuigan prodrugs such as RDV. Stated simply, these data together indicate that conventional approaches to in vitro and in vivo studies for hard drugs are poorly suited to complex prodrugs like RDV. If the logic and interpretation of RDV preclinical data were correct, then a more distinct clinical benefit would be anticipated. This is not the case. The gap between the strong preclinical data (6, 8, 27) with RDV across many virus models and its questionable clinical activity (2, 3) suggests that assumptions are being made at the preclinical level that do not reflect the conditions observed in patients. In light of RDV’s clinical performance, we have attempted to explain the causes for such a discordance by parsing the nuances of typical preclinical models used to judge RDV’s efficacy. Our careful analysis offers insight that should be considered when selecting (phosphate) prodrugs for clinical advancement.
ACKNOWLEDGMENTS
V.C.Y. is the CEO of Copycat Sciences, a company developing antiviral nucleoside analogues.
V.C.Y. performed research and analysis and wrote the manuscript. F.L.M. provided critical comments and assisted in the preparation of the manuscript.
Biographies

Victoria C. Yan, M.S. received her undergraduate degree in biochemistry from Mount Holyoke College in 2018 and her M.S. in therapeutics & pharmacology from the University of Texas Health Science Center at Houston/MD Anderson Cancer Center in 2020. As a master’s student in the lab of Florian Muller, she developed novel methods for the synthesis of phosphoramidate prodrugs and synthesized several prodrugs of a phosphonate-containing inhibitor of enolase 2 for the treatment of cancers harboring deletions of enolase 1. During the COVID-19 pandemic, she directed her knowledge of phosph(on)ate prodrug metabolism to remdesivir and has been advocating the use of its parent nucleoside, GS-441524, for COVID-19 treatment. Ms. Yan is the first to report the safety, tolerability, and pharmacokinetics of orally administered GS-441524 in women. As an extension of her graduate work, she founded Copycat Sciences, a phosph(on)ate prodrug company, with foci in virology, precision oncology, and inborn errors of metabolism.

Florian L. Muller, Ph.D., received his undergraduate degree from Washington State University and his Ph.D. from the University of Texas Health Science Center at San Antonio in 2007. His undergraduate and graduate careers were dedicated to the study of mitochondrial reactive oxygen species in aging and neurodegenerative disease. During his postdoctoral training, Dr. Muller joined the lab of Ron DePinho, where he applied his training in basic biochemistry to precision oncology, demonstrating the utility of passenger genomic deletions of metabolic enzymes, as pharmacologically targetable vulnerabilities in cancer. As an assistant professor at M.D. Anderson Cancer Center, he brought this concept closer to the clinic by synthesizing small molecules exploiting such vulnerabilities. This led to the development of novel phosphonate prodrugs, the knowledge of which became relevant during COVID-19 given remdesivir’s identity as a phosphate prodrug. Dr. Muller is currently a senior director at Sporos Bioventures, a venture capital firm specializing in early oncology startups.
Dedicated to Tomas Cihlar, Richard Mackman, and Bill Lee.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC. 2020. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 383:1813–1826. 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. 2020. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395:1569–1578. 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Solidarity Trial Consortium. 2020. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity Trial results. N Engl J Med 384:497–511. 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2020. WHO recommends against the use of remdesivir in COVID-19 patients.
- 5.Pruijssers AJ, George AS, Schäfer A, Leist SR, Gralinksi LE, Dinnon KH, Yount BL, Agostini ML, Stevens LJ, Chappell JD, Lu X, Hughes TM, Gully K, Martinez DR, Brown AJ, Graham RL, Perry JK, Du Pont V, Pitts J, Ma B, Babusis D, Murakami E, Feng JY, Bilello JP, Porter DP, Cihlar T, Baric RS, Denison MR, Sheahan TP. 2020. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep 32:107940. 10.1016/j.celrep.2020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO, Mackman RL, Hui HC, Perron M, Ray AS, Cihlar T, Nichol ST, Spiropoulou CF. 2017. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci Rep 7:43395. 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Clinton Smith E, Brett Case J, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR, Agostini CM, Gallagher T. 2018. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9:e00221-18. 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, et al. 2016. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531:381–385. 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. 2017. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9:eaal3653. 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 117:6771–6776. 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, Van Doremalen N, Leighton I, Yinda CK, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, De Wit E. 2020. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585:273–276. 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rautio J, Meanwell NA, Di L, Hageman MJ. 2018. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov 17:559–587. 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- 13.Butler T, Cho A, Kim C, Saunders OL, Zhang L. October2009. 1.′-substituted carba-nucleoside analogues for antiviral treatment. Patent WO2009132135. World Intellectual Property Organization.
- 14.Lawitz E, Hill J, Marbury T, Hazan D, Gruener D, Webster L, Majauskas R, Morrison R, DeMicco M, German P, Stefanidis D, Svaroskaia E, Arterburn S, Ray A, Rossi S, McHutchinson J, Rodriguez-Torres M. 2012. GS-6620, a liver-targeted nucleotide prodrug, exhibits antiviral activity and favorable safety profile over 5 days in treatment naïve chronic HCV genotype 1 subjects. EASL 47th Annual Meeting, Barcelona, Spain.
- 15.Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, Aktoudianakis V, Lew W, Ye H, Clarke M, Doerffler E, Byun D, Wang T, Babusis D, Carey AC, German P, Sauer D, Zhong W, Rossi S, Fenaux M, McHutchison JG, Perry J, Feng J, Ray AS, Kim CU. 2014. Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients. J Med Chem 57:1812–1825. 10.1021/jm400201a. [DOI] [PubMed] [Google Scholar]
- 16.Murakami E, Wang T, Babusis D, Lepist E-I, Sauer D, Park Y, Vela JE, Shih R, Birkus G, Stefanidis D, Kim CU, Cho A, Ray AS. 2014. Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620. Antimicrob Agents Chemother 58:1943–1951. 10.1128/AAC.02350-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehellou Y, Rattan HS, Balzarini J. 2018. The ProTide prodrug technology: from the concept to the clinic. J Med Chem 61:2211–2226. 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H, Jiang WR, Robledo N, Leveque V, Ali S, Lara-Jaime T, Masjedizadeh M, Smith DB, Cammack N, Klumpp K, Symons J. 2007. Characterization of the metabolic activation of hepatitis C virus nucleoside inhibitor β-D-2′-deoxy-2′-fluoro-2′-C- methylcytidine (PSI-6130) and identification of a novel active 5′-triphosphate species. J Biol Chem 282:29812–29820. 10.1074/jbc.M705274200. [DOI] [PubMed] [Google Scholar]
- 19.Perrone P, Daverio F, Valente R, Rajyaguru S, Martin JA, Lévêque V, Le Pogam S, Najera I, Klumpp K, Smith DB, Mcguigan C. 2007. First example of phosphoramidate approach applied to a 4′-substituted purine nucleoside (4′-azidoadenosine): conversion of an inactive nucleoside to a submicromolar compound versus hepatitis C virus. J Med Chem 50:5463–5470. 10.1021/jm070362i. [DOI] [PubMed] [Google Scholar]
- 20.Heinemann V, Hertel LW, Grindey GB, Plunkett W. 1988. Comparison of the cellular pharmacokinetics and toxicity of 2’,2’-difluorodeoxycytidine and 1-β-D-arabinofuranosylcytosine. Cancer Res 48:4024–4031. [PubMed] [Google Scholar]
- 21.Li Y, Cao L, Li G, Cong F, Li Y, Sun J, Luo Y, Chen G. 2021. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mouse models. J Med Chem 10.1021/acs.jmedchem.0c01929 [DOI] [PubMed] [Google Scholar]
- 22.Murphy BG, Perron M, Murakami E, Bauer K, Park Y, Eckstrand C, Liepnieks M, Pedersen NC. 2018. The nucleoside analog GS-441524 strongly inhibits feline infectiousperitonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet Microbiol 219:226–233. 10.1016/j.vetmic.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. 2020. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295:6785–6797. 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchesnokov EP, Gordon CJ, Woolner E, Kocincova D, Perry JK, Feng JY, Porter DP, Gotte M. 2020. Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action. J Biol Chem 295:16156–16165. 10.1074/jbc.AC120.015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin W, Mao C, Luan X, Shen DD, Shen Q, Su H, Wang X, Zhou F, Zhao W, Gao M, Chang S, Xie YC, Tian G, Jiang HW, Tao SC, Shen J, Jiang Y, Jiang H, Xu Y, Zhang S, Zhang Y, Xu HE. 2020. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 368:1499–1504. 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan VC, Muller FL. 2020. Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment. ACS Med Chem Lett 11:1361–1366. 10.1021/acsmedchemlett.0c00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL. 2017. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1- f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem 60:1648–1661. 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Barauskas O, Kim C, Babusis D, Murakami E, Kornyeyev D, Lee G, Stepan G, Perron M, Bannister R, Schultz BE, Sakowicz R, Porter D, Cihlar T, Feng JY. 2021. Off-target in vitro profiling demonstrates that remdesivir is a highly selective antiviral Agent. Antimicrob Agents Chemother 65:e02237-20. 10.1128/AAC.02237-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humeniuk R, Mathias A, Cao H, Osinusi A, Shen G, Chng E, Ling J, Vu A, German P. 2020. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci 13:896–906. 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tempestilli M, Caputi P, Avataneo V, Notari S, Forini O, Scorzolini L, Marchioni L, Bartoli TA, Castilletti C, Lalle E, Capobianchi MR, Nicastri E, D’Avolio A, Ippolito G, Agrati C. 2020. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother 75:2977–2980. 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Medicines Agency. 2020. Remdesivir: summary on compassionate use EMA/178637/2020 - Rev. 1.
- 33.Frisk-Holmberg M, Bergqvist Y, Termond E, Domeij-Nyberg B. 1984. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol 26:521–530. 10.1007/BF00542151. [DOI] [PubMed] [Google Scholar]
- 34.Vries PJ, Oosterhuis B, Boxtel CJ. 1994. Single-dose pharmacokinetics of chloroquine and its main metabolite in healthy volunteers. Drug Invest 8:143–149. 10.1007/BF03259430. [DOI] [Google Scholar]
- 35.Oerlemans R, Van Der Heijden J, Vink J, Dijkmans BAC, Kaspers GJL, Lems WF, Scheffer GL, Ifergan I, Scheper RJ, Cloos J, Assaraf YG, Jansen G. 2006. Acquired resistance to chloroquine in human CEM T cells is mediated by multidrug resistance-associated protein 1 and provokes high levels of cross-resistance to glucocorticoids. Arthritis Rheum 54:557–568. 10.1002/art.21569. [DOI] [PubMed] [Google Scholar]
- 36.Mackman RL, Hui HC, Perron M, Murakami E, Palmiotti C, Lee G, Stray K, Zhang L, Goyal B, Chun K, Byun D, Siegel D, Simonovich S, Du Pont V, Pitts J, Babusis D, Vijjapurapu A, Lu X, Kim C, Zhao X, Chan J, Ma B, Lye D, Vandersteen A, Wortman S, Barrett KT, Toteva M, Jordan R, Subramanian R, Bilello JP, Cihlar T. 2021. Prodrugs of a 1′-CN-4-aza-7,9-dideazaadenosine C-nucleoside leading to the discovery of remdesivir (GS-5734) as a potent inhibitor of respiratory syncytial virus with efficacy in the African green monkey model of RSV. J Med Chem 64:5001–5017. 10.1021/acs.jmedchem.1c00071. [DOI] [PubMed] [Google Scholar]
- 37.Xie X, Muruato AE, Zhang X, Lokugamage KG, Fontes-Garfias CR, Zou J, Liu J, Ren P, Balakrishnan M, Cihlar T, Tseng CTK, Makino S, Menachery VD, Bilello JP, Shi PY. 2020. A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19. Nat Commun 11:5214. 10.1038/s41467-020-19055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn M-Y, Nahass RG, Chen Y-S, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A, GS-US-540–5773 Investigators. 2020. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 383:1827–1837. 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang S-C, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM, GS-US-540–5774 Investigators. 2020. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. JAMA 324:1048–1057. 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West GB, Brown JH. 2005. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol 208:1575–1592. 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- 41.Yan VC, Muller FL. 2021. Remdesivir for COVID-19: why not dose higher? Antimicrob Agents Chemother 65:e2713-20. 10.1128/AAC.02713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, Babusis D, Park Y, Niu C, Kim C, Zhao X, Lu B, Ma B, Muench RC, Sperger D, Ray AS, Murakami E. 2020. Species differences in liver accumulation and metabolism of nucleotide prodrug sofosbuvir. Drug Metab Pharmacokinet 35:334–340. 10.1016/j.dmpk.2020.04.333. [DOI] [PubMed] [Google Scholar]
- 43.Babusis D, Curry MP, Kirby B, Park Y, Murakami E, Wang T, Mathias A, Afdhal N, McHutchison JG, Ray AS. 2018. Sofosbuvir and ribavirin liver pharmacokinetics in patients infected with hepatitis C virus. Antimicrob Agents Chemother 62:e02587-17. 10.1128/AAC.02587-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgs ES, Gayedyu-Dennis D, Fisher W, Nason M, Reilly C, Beavogui AH, Aboulhab J, Nordwall J, Lobbo P, Wachekwa I, Cao H, Cihlar T, Hensley L, Lane HC. 2021. PREVAIL IV: a randomized, double-blind, two-phase, phase 2 trial of remdesivir versus placebo for reduction of Ebola virus RNA in the semen of male survivors. Clin Infect Dis 10.1093/cid/ciab215. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulangu S, Dodd LE, Davey RT, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum J-J, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, the PALM Writing Group, et al. 2019. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 381:2293–2303. 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckland MS, Galloway JB, Fhogartaigh CN, Meredith L, Provine NM, Bloor S, Ogbe A, Zelek WM, Smielewska A, Yakovleva A, Mann T, Bergamaschi L, Turner L, Mescia F, Toonen EJM, Hackstein CP, Akther HD, Vieira VA, Ceron-Gutierrez L, Periselneris J, Kiani-Alikhan S, Grigoriadou S, Vaghela D, Lear SE, Török ME, Hamilton WL, Stockton J, Quick J, Nelson P, Hunter M, Coulter TI, Devlin L, Bradley JR, Smith KGC, Ouwehand WH, Estcourt L, Harvala H, Roberts DJ, Wilkinson IB, Screaton N, Loman N, Doffinger R, Lyons PA, Morgan BP, Goodfellow IG, Klenerman P, Lehner PJ, Matheson NJ, Thaventhiran JED, MRC-Toxicology Unit COVID-19 Consortium. 2020. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun 11:6385. 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boshier FAT, Pang J, Penner J, Hughes J, Parker M, Shepherd J, Alders N, Bamford A, Grandjean L, Grunewald S, Hatcher J, Best T, Dalton C, Bynoe PD, Frauenfelder C, Köeglmeier J, Myerson P, Roy S, Williams R, Thomson EC, de Silva TI, Goldstein RA, Breuer J. 2020. Remdesivir induced viral RNA and subgenomic RNA suppression, and evolution of viral variants in SARS-CoV-2 infected patients. medRxiv 10.1101/2020.11.18.20230599. [DOI] [Google Scholar]
- 48.Food and Drug Administration. 2020. VEKLURY (remdesivir). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf.
- 49.Fiege JK, Thiede JM, Nanda HA, Matchett WE, Moore PJ, Montanari NR, Thielen BK, Daniel J, Stanley E, Hunter RC, Menachery VD, Shen SS, Bold TD, Langlois RA. 2021. Single cell resolution of SARS-CoV-2 tropism, antiviral responses, and susceptibility to therapies in primary human airway epithelium. PLoS Pathog 17:e1009292. 10.1371/journal.ppat.1009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. 2020. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272. 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, Worrall D, Giguel F, Piechocka-Trocha A, Atyeo C, Fischinger S, Chan A, Flaherty KT, Hall K, Dougan M, Ryan ET, Gillespie E, Chishti R, Li Y, Jilg N, Hanidziar D, Baron RM, Baden L, Tsibris AM, Armstrong KA, Kuritzkes DR, Alter G, Walker BD, Yu X, Li JZ, Abayneh B(B), Allen P, Antille D, Balazs A, Bals J, Barbash M, Bartsch Y, Boucau J, Boyce S, Braley J, Branch K, Broderick K, Carney J, Chevalier J, Choudhary MC, Chowdhury N, Cordwell T, Daley G, Davidson S, Desjardins M, The Massachusetts Consortium for Pathogen Readiness, et al. 2020. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 11:5493. 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A, Gifford RJ, Hopkins S, Hughes J, Jabeen F, Johannessen I, Karageorgopoulos D, Lackenby A, Lester R, Liu RSN, MacConnachie A, Mahungu T, Martin D, Marshall N, Mepham S, Orton R, Palmarini M, Patel M, Perry C, Peters SE, Porter D, Ritchie D, Ritchie ND, Seaton RA, Sreenu VB, Templeton K, Warren S, Wilkie GS, Zambon M, Gopal R, Thomson EC. 2016. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388:498–503. 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stella V, He Q. 2008. Cyclodextrins. Toxicol Pathol 36:30–42. 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 54.Lê MP, Le Hingrat Q, Jaquet P, Wicky PH, Bunel V, Massias L, Visseaux B, Messika J, Descamps D, Mal H, Timsit JF, Peytavin G. 2020. Removal of remdesivir’s metabolite GS-441524 by hemodialysis in a double lung transplant recipient with COVID-19. Antimicrob Agents Chemother 64:e01521-20. 10.1128/AAC.01521-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akinci E, Cha M, Lin L, Yeo G, Hamilton MC, Donahue CJ, Bermudez-Cabrera HC, Zanetti LC, Chen M, Barkal SA, Khowpinitchai B, Chu N, Velimirovic M, Jodhani R, Fife JD, Sovrovic M, Cole PA, Davey RA, Cassa CA, Sherwood RI. 2020. Elucidation of remdesivir cytotoxicity pathways through genome-wide CRISPR-Cas9 screening and transcriptomics. bioRxiv 2020.08.27.270819.
- 56.Brunetti-Pierri N, Liou A, Patel P, Palmer D, Grove N, Finegold M, Piccolo P, Donnachie E, Rice K, Beaudet A, Mullins C, Ng P. 2012. Balloon catheter delivery of helper-dependent adenoviral vector results in sustained, therapeutic hFIX expression in rhesus macaques. Mol Ther 20:1863–1870. 10.1038/mt.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information and Figure S1. Download AAC.01117-21-s0001.pdf, PDF file, 0.1 MB (133.8KB, pdf)
Supplemental Data Set S1. Download AAC.01117-21-s0002.xlsx, XLSX file, 0.04 MB (37.6KB, xlsx)