ABSTRACT
This work reports the synthesis and characterization by Fourier transform infrared spectroscopy (FTIR), 1H, 13C, and 79Se nuclear magnetic resonance (NMR), mass spectrometry, and elemental analysis techniques as well as the in vitro evaluation of the leishmanicidal activity of 13 new selenophosphoramidate derivatives. Among the new compounds, four of them (compounds 1f, 1g, 2f, and 2g), which exhibited the best profiles, were tested against infected macrophages and were selected for further studies related to their leishmanicidal mechanism. In this regard, trypanothione redox system alteration was determined. Compound 1g, under similar conditions, was more effective than the corresponding references. In addition, theoretical calculations showed that this compound also presents most physicochemical and pharmacokinetic properties within the ranges expected for orally available drugs. It is believed that selenophosphoramidate functionalities may represent a scaffold to be explored toward the development of new agents for leishmania treatment.
KEYWORDS: diselenide, phosphoramidate, selenocyanate, thiols, trypanothione reductase
INTRODUCTION
Leishmaniasis is the most prevalent neglected and poverty-associated tropical disease caused by the different species of protozoa parasites of the genus Leishmania. Even though there are not reliable data available, it is estimated that more than 12 million people are infected and 350 million people are at risk of being infected (1). Although there are three different clinical forms of leishmaniasis, cutaneous (CL), mucocutaneous (ML), and visceral (VL) also known as Kala-azar, this project focuses specifically in VL since it is the most serious form of the disease and is potentially fatal if untreated (2). VL is mainly caused by Leishmania infantum and Leishmania donovani species. Current pharmacological therapy, such as pentavalent antimonials (sodium stibogluconate, meglumine antimoniate), amphotericin B—particularly in a liposomal formulation, pentamidine, miltefosine, and paromomycin (aminosidine) present important limitations, including long-term administration, toxic side effects, high cost in countries where VL is endemic, and a high number of resistance cases (3). These problems confirm the urgent need to develop new effective, affordable, and nontoxic antileishmanial treatments.
During the last two decades, substantial efforts have been invested to discover more efficient therapeutic agents, and new structures have been proposed. However, only limited reports have investigated the antileishmanial effect of selenium, alone or in combination, as a plausible antileishmanial chemotype (4–6). In this context, it is well documented that selenium, as a trace element, plays a pivotal role in boosting immunity against pathogens (7). Previous studies showed that selenium had potent and selective effects against some species of Leishmania (8). More recently (5), the combination of amphotericin B (AmB) with selenium, in a simple or niosomal form, demonstrated a positive interaction between AmB and selenium. Conversely, the antioxidant capacity of selenocompounds has been evidenced since some of them inhibit trypanothione reductase, the major reductase of trypanosomatids that contributes to redox homeostasis (9). Although oxygen, sulfur, and selenium are located in the same group of the periodic table, different behaviors have been observed in redox responsivity along with distinct bond properties among others. These differences might affect drug delivery and present advantages for biological activity as a result of the inclusion of the selenium atom in organic molecules (10).
These facts as well as our wide experience in the development of selenium derivatives (11–18) encouraged our endeavor to extend the chemical space for obtaining new antileishmanial agents. In one of these previous studies, we synthesized and tested in vitro against Leishmania infantum some families of selenocyanate and diselenide compounds (18). From these studies emerged 4-aminophenylselenocyanate (compound 0a) (Fig. 1) and bis(4-aminophenyl)diselenide (compound 0b) (Fig. 1) that exhibited promising leishmanicidal activity, equivalent to the reference drugs edelfosine and miltefosine. Conversely, miltefosine represents the first and only oral drug approved for this disease. It is a compound derived from alkylphospholipids (Fig. 1) that is effective against Leishmania sp. and Trypanosoma cruzi both in vitro and in vivo showing selective antiparasitic activity. This selectivity led to clinical studies of orally administered miltefosine to patients with visceral leishmaniasis, especially those infected with antimony-resistant strains.
FIG 1.
Miltefosine and parent compounds.
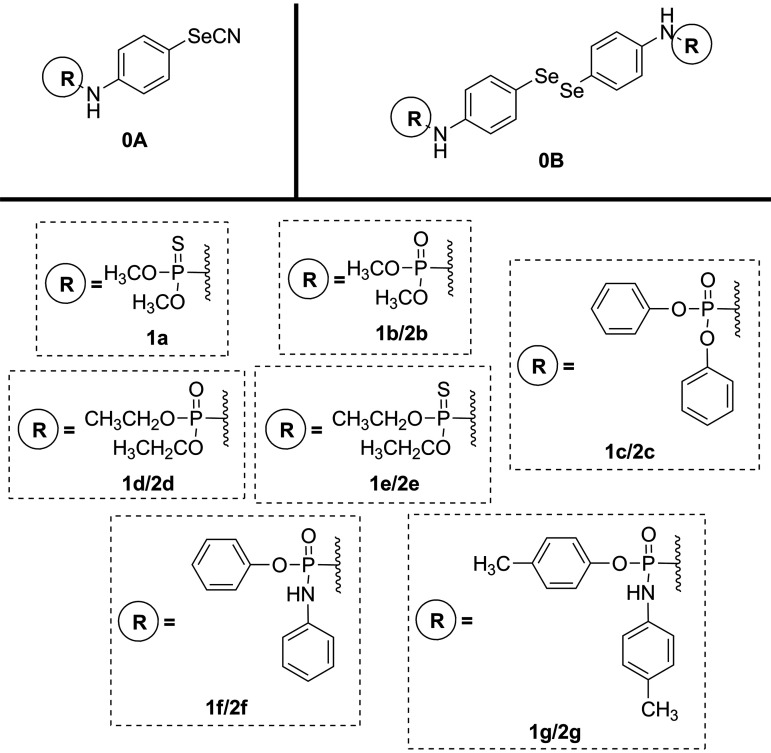
The development of new derivatives based on the hybridization of two bioactive molecules is a novel approach that might result in bioactivity enhancement. In order to gain further insight into the leishmanicidal potential of compounds 0a and 0b, we aimed at designing some novel hybrids of 4-aminophenylselenocyanate and bis(4-aminophenyl)diselenide with alkyl and aryl phosphoramidate entities (Fig. 2). It is remarkable that motifs 0a and 0b are the main backbone of the design strategy. The phosphorus moiety was introduced as a straightforward strategy for the synthesis of new compounds inspired in miltefosine. In addition, the diverse alkyl and aryl substitutional units of the phosphoryl groups provide better bioavailability as well as stability of compounds in biological media.
FIG 2.
Structures of the newly synthesized compounds.
Concerning the possible mechanism of action for the new derivatives and taking into account the importance of Se in redox processes (19), as reported by Tobe et al. (20), we analyzed the alteration of the trypanothione-trypanothione reductase (TryR) system. Trypanothione is a unique peptide whose structure is formed by two glutathione molecules linked by a spermidine in a cyclic structure (21). It is one of the most relevant thiols of leishmania parasites, playing an important role in the defense against oxidative and chemical stress. Together with NADPH-dependent TryR, the tryparedoxin peroxidases metabolic system reduces peroxides using the electrons donated by the reduced trypanothione, T(SH)2, generated (22). Moreover, this redox system substitutes glutathione and glutathione reductase system found in humans (23), making it an attractive drug target.
Herein, we present the synthesis of 13 novel derivatives of alkyl and aryl phosphoramidates (Fig. 2) from compounds 0a and 0b together with their in vitro evaluation against the amastigote form of L. infantum and the cytotoxicity on one different complementary human cell line (THP-1) in order to select those compounds with high selectivity. The most promising compounds were tested in infected macrophages. In addition, we have studied the potential role of the compounds in the parasites trypanothione redox system alteration as the leishmanicidal action mechanism.
RESULTS
Chemistry.
The procedures to synthesize the final compounds were outlined in Fig. 3.
FIG 3.
Synthetic route of the reported compounds. Reagents and conditions. (i) DMSO, 0.5 h, room temperature (RT). (ii) Aniline, 1 h, RT. (iii) NaBH4, ethanol, 2 h, RT. (iv) Phosphoryl chloride, acetonitrile, 1 to 2 h, under reflux. (v) Phosphoryl chloride, chloroform, 5 to 6 h, under reflux.
Compounds 0a and 0b were prepared as previously reported (18). Compounds 1a to 1g were obtained by reaction of compound 0a solved in acetonitrile with the corresponding commercially available phosphoryl chlorides in a molar ratio (1:1) in the presence of triethylamine and under nitrogen atmosphere. The reaction mixture was stirred for 1 h at 80°C. The products were purified by flash chromatography.
Compounds 2b to 2g were obtained from compound 0b, solved in chloroform with the corresponding phosphoryl chlorides in a molar ratio (1:2), respectively, in the presence of triethylamine and under nitrogen atmosphere. The reaction mixture was stirred during 5 h at 60°C. The products were purified by flash chromatography using hexane/ethyl acetate as eluents.
The new compounds were obtained with moderate yield. All chemical structures of compounds were confirmed by infrared (IR) spectroscopy (Fourier transform infrared spectroscopy [FTIR]), 1H, 13C, and 77Se nuclear magnetic resonance spectroscopy (NMR), elemental analysis, and mass spectrometry.
Some difficulties with respect to the substituents were observed. This method failed for compound 2a with problems during purification, such as precipitation during chromatographies and creating emulsions during work-up procedures. The search for an alternative procedure (others solvents, temperature, different molar ratio) resulted in decomposition of reagents. Since the compounds for biological tests must be of the highest purity, neither flash chromatography purification nor crystallization led to sufficiently pure compound and had to be rejected.
Biological evaluation. (i) Activity in amastigotes and cytotoxic activity in human cells.
New derivatives were tested in vitro against intracellular amastigote forms of L. infantum following the previously described method (18). Miltefosine and edelfosine were used as positive controls. We also examined the biological effects of synthetic precursors 0a and 0b as negative controls to establish the role of the phosphoramidate tail. Dose-response experiments were performed to determine their potencies expressed as 50% effective concentration (EC50). Table 1 summarizes the results. The cytotoxic effects of new derivatives were evaluated against THP-1 mammalian cells in order to determine the respective selectivity index (SI). The selectivity index (SI) was defined as the ratio of the EC50 values of compounds against THP-1 cells relative to those obtained against L. infantum axenic amastigotes (Table 1). As shown in Table 1, 11 derivatives (compounds 1b, 1c, 1d, 1e, 1f, 1g, 2b, 2d, 2e, 2f, and 2g) were similar or more active than miltefosine (EC50 = 2.84 μM), a marketed antileishmanial drug. If we focused in edelfosine, the other reference drug, seven of them (compounds 1d, 1g, 2b, 2d, 2e, 2f, and 2g) displayed higher potency.
TABLE 1.
EC50 ± SEM (μM) values for the compounds on amastigotes and cytotoxic activity in THP-1 cell line after 24 h treatment
| Compound | EC50 (μM ± SD) |
SIa | |
|---|---|---|---|
| L. infantum amastigotes | THP-1 cells | ||
| 1a | 8.47 ± 1.97 | 11.80 ± 1.94 | 1.39 |
| 1b | 2.82 ± 0.23 | 6.06 ± 0.25 | 2.15 |
| 1c | 1.34 ± 0.15 | 4.78 ± 1.56 | 3.58 |
| 1d | 0.36 ± 0.22 | 1.36 ± 0.19 | 3.82 |
| 1e | 2.01 ± 0.43 | 12.42 ± 2.60 | 6.19 |
| 1f | 0.99 ± 0.65 | 12.04 ± 2.33 | 12.12 |
| 1g | 0.56 ± 0.25 | 7.58 ± 0.42 | 13.54 |
| 2b | 0.33 ± 0.14 | 0.89 ± 0.19 | 2.71 |
| 2c | 14.32 ± 0.04 | >25 | >1.74 |
| 2d | 0.17 ± 0.09 | 0.39 ± 0.26 | 2.31 |
| 2e | 0.79 ± 0.70 | 3.42 ± 1.04 | 4.35 |
| 2f | 0.20 ± 0.06 | 1.42 ± 0.10 | 7.10 |
| 2g | 0.14 ± 0.10 | 1.17 ± 0.29 | 8.36 |
| 0ab | 9.29 ± 1.16 | >50 | >5.38 |
| 0bb | 0.65 ± 0.02 | 20.40 ± 2.80 | 24 |
| Miltefosine | 2.84 ± 0.10 | 18.5 ± 0.60 | 6.5 |
| Edelfosine | 0.82 ± 0.13 | 4.96 ± 0.16 | 6.0 |
Selectivity index (SI) is the ratio of IC50 values of compounds against THP-1 cells relative to those against L. infantum amastigotes.
Biological data for parent compounds.
In order to consider the activity relationship related to structure, the results revealed that the majority of the diselenide derivatives, with the exception of compound 2c, were lightly more active than their selenocyanate analogues. This trend was similar to that observed in previous reports of our research group (12, 15, 18). Conversely, the replacement of oxygen by sulfur is detrimental for the activity (compound 1a versus 1b or 1d versus 1e). The increase in the length of the alkyl chain could increase the activity (compound 1d and 2d versus 1b and 2b). In general, no significant differences were observed between aliphatic and aromatic functionalizations.
Taking into account that for antimicrobial drugs selectivity index (SI) is more important than the killing effect in absolute terms, four compounds (1f, 1g, 2f, and 2g) have SI values ranging from 7.10 to 13.54, exceeding the references for miltefosine and edelfosine (SI = 6.5 and 6, respectively).
Based on an interesting SI and their EC50 values between 0.14 and 0.99 μM, the derivatives 1f, 1g, 2f, and 2g were unveiled to be superior in their profile to the rest of the compounds and were selected as promising hits for further testing.
(ii) Leishmanicidal activity in infected macrophages.
To evaluate the efficacy of the newly synthesized compounds on the intracellular form of L. infantum, amastigote-infected THP-1 cell derivatives 1f, 1g, 2f, and 2g were selected. Edelfosine was used as a reference. The data demonstrated (Fig. 4) that compounds 1g, 2f, and 2g presented equal or even better leishmanicidal activity at 3 μM than edelfosine.
FIG 4.
Green fluorescent protein-positive (GFP+) cell percentage values for the compounds in intracellular amastigotes.
(iii) Redox system alteration.
As mentioned in the introduction, selenium compounds can interfere with redox processes, and to further confirm the alteration of the parasite redox system by these molecules, intracellular thiol levels were determined inside the parasites by flow cytometry. It is well-known that thiols play a significant role in redox status, homeostasis, and viability of parasite. They are involved both in the oxidative and reductive pathways, acting as radical scavengers, thus protecting the parasites from oxidative damage (24, 25). Figure 5 shows the results of the treatment of the L. infantum amastigotes for 1 h with the four selected molecules. Compounds 2f and 2g duplicated the levels of intracellular thiols related to control, while derivatives 1f and 1g decreased the thiol levels to a fifth part.
FIG 5.
Levels of intracellular thiols after treatment with menadione, 1f, 1g, 2f, and 2g. Results are expressed as a mean ± standard error of the mean (SEM) of three independent experiments. *, P < 0.1 (with respect the control [DMSO]).
(iv) Alteration of L. infantum trypanothione system.
According to the obtained results and the observed thiol depletion, we decided to analyze the effect of the compounds on the main redox regulation mechanism of these parasites, the trypanothione system. Trypanothione reductase is a flavoenzyme that has a unique role in trypanothione-based redox metabolism and oxidant defense. It is a flavin adenine dinucleotide (FAD)-containing homodimeric protein essential for parasite survival. The flavoenzyme utilizes NADP in the reaction to convert oxidized trypanothione (TS2) to reduced trypanothione T(SH)2, which is further used up by the tryparedoxin/tryparedoxin peroxidase system to neutralize the reactive oxygen species (ROS) generated by the macrophages (26, 27). The activity of the enzyme was measured in the presence of the four selected compounds at different doses.
The obtained data showed that diselenide derivatives 2f and 2g did not affect the TryR reaction. On the contrary, no reaction was detected in the presence of 25 μM compounds 1f and 1g. In the presence of 8 μM compound 1g, an initial latency period could be observed (Fig. 6) in which no reduced 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) was generated. The most plausible explanation for this observation is a direct reduction of compound 1g by the newly synthetized T(SH)2, which is not able to react with DTNB until most of compound 1g has been reduced. This latency period is followed by a progressive increase in the absorbance at 412 nm that is indicative of DTNB reduction by T(SH)2. The much slower rates of DTNB reduction in the presence of compound 1g in comparison with those observed in the presence of dimethyl sulfoxide (DMSO) could be an indication of TryR inhibition by this compound, but because of the already suggested reaction between T(SH)2 and compound 1g, this reduction in enzyme activity could also be due to a decrease in the effective TS2 concentration as a consequence of its direct binding to the Se atom of compound 1g.
FIG 6.
TryR activity during treatment with compound 1g.
A second explanation for the observed latency period could be a direct reduction of compound 1g by TryR using NADPH as electron donor.
Accordingly, NADPH consumption by TryR in the absence of TS2 was analyzed. As shown in Fig. 7, NADPH oxidation rates by TryR are similar in the presence or in the absence of compound 1g (25 μM), which constitutes a strong indication that this molecule does not act as an alternative substrate for TryR.
FIG 7.
NADPH measurement after treatment with compound 1g.
Considering all this data, we hypothesized that selenocyanate 1g may act as a thiol scavenger molecule by forming a temporary hybrid moiety (HSTS-SeR) after its binding with one of the -SH groups of the substrate T(SH)2. Subsequently, the remaining free -SH in trypanothione would react with the new intermediate disulfide/selenide (-S-Se-), thus regenerating the oxidized form of the substrate (TS2) and releasing the compound as a selenol derivative (Fig. 8).
FIG 8.
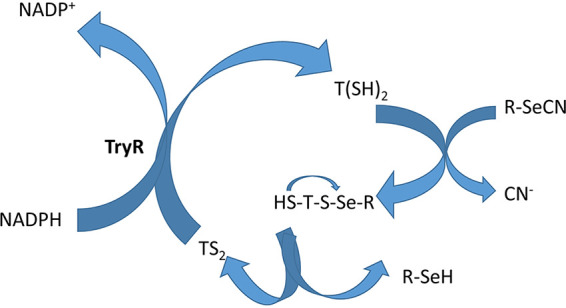
Mechanistic hypothesis for the thiol scavenger activity shown by compound 1g.
The carbon-nitrogen of the cyano bond, directly attached to the selenium atom, produces a very distinctive signal in the infrared spectrum (2,155 cm−1). By analyzing the enzymatic reaction with the compound through this technique after an hour (Fig. 9), the disappearance of this peak can be clearly observed, which reinforces the proposed hypothesis in Fig. 8.
FIG 9.
Infrared spectra of compound 1g before (red line) and after the enzymatic reaction (blue line).
DISCUSSION
It is remarkable that most set phosphoramidate derivatives (with the exception of compounds 1a and 2c) were active against L. infantum amastigotes in a similar manner to miltefosine used as reference. This means that the antiamastigote activity was nearly not affected by the nature of the phosphoryl substituent on the parent compounds. Nevertheless, it is this entity who guides the toxicity against the macrophage cell line THP-1. These compounds were moderately cytotoxic to the THP-1 cell line, and this did not allow these compounds to reach selectivity indexes (SI) comparable or superior to the references in some cases. In spite of these considerations, four compounds (1f, 1g, 2f, and 2g) showed a very promising antileishmanial profile with EC50 values ranging from 0.14 to 0.99 μM and SI ranging from 7.1 to 13.5. In addition, three of the compounds (1g, 2f, and 2g) confirmed these good results in infected macrophages.
To further understand the mechanism of action for these molecules and considering their possible interference with the cellular redox processes, we focused on the possible alteration of the intracellular thiol levels and on the trypanothione system since trypanothione is one of the most abundant biological thiols in these parasites.
In this context, recent studies have shown a favorable binding of the Se atom to the -SH group of two glutathione molecules, forming a hybrid molecule with potent apoptotic activity (20). The formed selenodiglutathione molecule (GSSeSG) induces apoptosis through cellular intake, reductive metabolism, ROS production, and oxidative damage to DNA. Taking this into account, we analyzed the possibility that the studied compounds could form some kind of hybrid molecule with the trypanothione moiety.
First, the intracellular thiol level was quantified after treatment of the parasites with the selected compounds. Diselenides 2f and 2g induced a 2-fold increase of the thiol levels, but they do not affect the trypanothione reductase activity. However, selenocyanates 1f and 1g significantly decreased the thiol levels, disrupting the redox homeostasis. When analyzing the effect of selenocyanate derivatives on the TryR-catalyzed reaction, no reduction of DTNB was observed during the first 15 min. This may be caused by a preferential reaction between the newly generated T(SH)2 and compound 1g. After consumption of most of the compound, DTNB reduction begins but at a much lower rate compared with that of the control reaction (DMSO). Notwithstanding, this effect might be caused by an inhibition of the enzyme or by a decrease in TS2 concentration as a consequence of its binding to the compound.
We have demonstrated that TryR does not catalyze the electron transfer between NADPH and compound 1g, so any reduction of the compound must be mediated by T(SH)2 generation.
Altogether, these data suggested the possible formation of a hybrid molecule after the linkage of the Se atom of compound 1g and one of the -SH groups of the reduced trypanothione. In order to confirm this interaction, we analyzed the infrared (IR) spectrum of compound 1g after its incubation in the TryR reaction mixture. As expected, the spectrum confirmed the loss of the characteristic signal for the Se-CN bond (2,155 cm−1).
The evaluation of absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties as well as the check for amenability to the Lipinski rule of five of the top compounds (see the supplemental material) revealed that only compounds 1f and 1g did not violate those criteria. The software SwissADME is a useful tool for this primary level of screening and helps reduce pharmacokinetics-related failure during clinical trials at a later stage.
However, globally considered, the best performance was observed for derivative 1g, which was the most active and selective, effective in infected macrophages in a similar form to reference edelfosine, altered trypanothiones redox system, did not violate Lipinski’s rule, and exhibited a better profile in the bioavailability radar (see the supplemental material) than the reference drugs. Thus, compound 1g can be considered a good prototype for the future development of drugs against leishmaniasis.
A graphical summary of this work is depicted in Fig. 10, and general structures with NMR assignations for compounds 1a to 1g and 2b to 2g can be found in Fig. 11.
FIG 10.
Schematic illustration of the synthesis, leishmanicidal activity, and mechanism of action for the selenocompounds described.
FIG 11.
General structures with NMR assignation for compounds 1a to 1g and 2b to 2g.
MATERIALS AND METHODS
Chemistry.
Melting points (mp) were determined with a Mettler FP82 þ FP80 apparatus (Greifensee, Switzerland). 1H, 13C, and 77Se NMR spectra were recorded on a Bruker 400 Avance Neo spectrometer (Rheinstetten, Germany) using DMSO-d6 as solvent. IR spectra were recorded on a Thermo Nicolet FTIR Nexus spectrophotometer using KBr pellets for solid samples or NaCl plates for oil compounds. Elemental analysis was performed on a LECO CHN-900 elemental analyzer. Purity of all final compounds was 99% or higher. Thin-layer chromatography (TLC) assays were carried out in Alugram SIL G7UV254 sheets (Macherey-Nagel, Düren, Germany). Chemicals were purchased from E. Merck (Darmstadt, Germany), Panreac Química S.A. (Montcada i Reixac, Barcelona, Spain), Sigma-Aldrich Quimica, S.A. (Alcobendas, Madrid, Spain), and Acros Organics (Janssen Pharmaceuticalaan, Geel, Belgium).
General procedure of synthesis of compounds 1a to 1g.
Compounds 1a to 1g were obtained in variable yields (10 to 35%) by reaction of 4-aminophenylselenocyanate, triethylamine, and the corresponding phosphoryl chlorides added dropwise, in acetonitrile, for 30 min with N2 flux, at 80°C. The reaction mixture was kept under stirring and under reflux for 1 h. The solvent was then removed under vacuum. The oily residue was treated with water (2 × 50 ml) and extracted with ethyl ether. The organic phase was dried with Na2SO4, filtered, mixed with silica, and removed under vacuum, obtaining a solid mixture that was purified by automated flash chromatography using hexane/ethyl acetate as eluents.
(i) Dimethyl-N-(4-selenocyanatephenyl)thiophosphoramidate (1a).
From O,O′-dimethyl chlorothiophosphate. Yellow powder; mp: 95.92°C. Yield: 34%. IR (KBr) cm−1.: 3,281 (N-H); 2,151 (CN); 1,589 (P=S). 1H NMR (400 MHz, DMSO-d6) δ 3.66 (d, J31H-31P = 14.0 Hz, 6H; CH3), 7.08–7.14 (m, 2H; H2+H6), 7.60 (d, J2-3= 8.8 Hz, 2H; H3+H5), 8.74 (d, J21H-31P = 14.8 Hz, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ 53.7 (d, J213C-31P = 4.8 Hz; CH3), 105.8 (CN), 114.6 (C1), 119.6 (d, J313C-31P = 7.6 Hz; C3+C5), 136.0 (C2+C6), 142.4 (d, J213C-31P = 2.5 Hz; C4). 77Se NMR (76 MHz, DMSO-d6) δ 317.9. MS (m/z % abundance): 322 (100, M+), 125 (98), 93 (96). Elemental analysis for C9H11N2O2PSSe. Calculated/found (%): C: 33.64/33.56; H: 3.42/3.68; N: 8.72/8.45.
(ii) Dimethyl-N-(4-selenocyanatephenyl)phosphoramidate (1b).
From O,O′-dimethyl chlorophosphate. Yellow powder; mp: 111.1°C. Yield: 24%. IR (KBr) cm−1: 3,185 (N-H); 2,151 (CN); 1,589 (P=O). 1H NMR (400 MHz, DMSO-d6) δ: 3.65 (d, J31H-31P = 11.4 Hz, 6H; CH3), 7.03–7.10 (m, 2H; H3+H5), 7.55–7.61 (m, 2H; H2+H6), 8.41 (d, J21H-31P = 9.2 Hz, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 53.0 (d, J213C-31P = 5.4 Hz; CH3), 105.4 (CN), 113.4 (C1), 118.6 (d, J313C-31P = 7.7 Hz; C3+C5), 135.6 (C2+C6), 142.6 (d, J213C-31P = 1.6 Hz; C4). 77Se NMR (76 MHz, DMSO-d6) δ: 317.0. MS (m/z % abundance): 306 (70, M+), 226 (96), 109 (100). Elemental analysis for C9H11N2O3PSe. Calculated/found (%): C: 35.41/35.63; H: 3.60/3.88; N: 9.18/9.11.
(iii) Diphenyl-N-(4-selenocyanatephenyl)phosphoramidate (1c).
From O,O′-diphenyl chlorophosphate. White powder; mp: 156.55°C. Yield: 28.5%. IR (KBr) cm−1: 3,172 (N-H); 2,145 (CN); 1,586 (P=O). 1H NMR (400 MHz, DMSO-d6) δ: 7.18–7.29 (m, 8H; A, H3+H5; B, H2+H4+H6), 7.41 (t, 4H; B, H3+H5), 7.61–7.69 (m, 2H; A, H2+H6), 9.17 (d, J21H-31P = 10.1 Hz, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 105.4 (CN), 114.7 (A: C1), 119.1 (d, J313C-31P = 8.0 Hz; A, C3+C5), 120.0 (B, C2+C6), 125.4 (B, C4), 130.0 (B, C3+C5), 135.6 (A, C2+C6), 141.5 (A, C4), 149.9 (d, J213C-31P = 6.3 Hz; B, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 318.5. MS (m/z % abundance): 430 (100, M+), 77 (40). Elemental analysis for C19H15N2O3PSe. Calculated/found (%): C: 53.15/52.85; H: 3.49/3.75; N: 6.52/6.19.
(iv) Diethyl-N-(4-selenocyanatephenyl)phosphoramidate (1d).
From O,O′-diethyl chlorophosphate. Brown powder; mp: 88.07°C. Yield: 31.6%. IR (KBr) cm−1: 3,148 (N-H); 2,149 (CN); 1,588 (P=O). 1H NMR (400 MHz, DMSO-d6) δ: 1.22 (t, J = 7.1 Hz, 6H; CH3), 3.84–4.15 (m, 4H; CH2), 7.05 – 7.11 (m, 2H; H3+H5), 7.53–7.61 (m, 2H, H2+H6), 8.34 (d, J21H-31P = 9.2 Hz, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 16.0 (d, J313C-31P = 6.7 Hz; CH3), 62.2 (d, J213C-31P = 5.3 Hz; CH2), 105.4 (CN), 113.1 (C1), 118.5 (C3+C5), 135.6 (C2+C6), 142.9 (d, J213C-31P = 1.2 Hz; C4). 77Se NMR (76 MHz, DMSO-d6) δ: 316.5. MS (m/z % abundance): 334 (45, M+), 198 (100), 118 (37). Elemental analysis for C11H15N2O3PSe. Calculated/found (%): C: 39.63/39.70; H: 4.50/4.74; N: 8.41/8.31.
(v) Diethyl-N-(4-selenocyanatephenyl)thiophosphoramidate (1e).
From O,O′-diethyl chlorothiophosphate. Brown powder; mp: 60.39°C. Yield: 33.8%. IR (KBr) cm−1: 3,308 (N-H); 2,153 (CN); 1,590 (P=S). 1H NMR (400 MHz, DMSO-d6) δ: 1.22 (t, J = 7.1 Hz, 6H; CH3), 3.87–4.25 (m, 4H; CH2), 7.10–7.18 (m, 2H; H3+H5), 7.59 (d, J2-3 = 8.6 Hz, 2H; H2+H6), 8.67 (d, J21H-31P = 14.9 Hz, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 15.6 (d, J313C-31P = 7.7 Hz; CH3), 62.7 (d, J213C-31P = 4.7 Hz; CH2), 105.4 (CN), 113.8 (C1), 119.1 (d, J313C-31P = 7.7 Hz; C3+C5), 135.5 (C2+C6), 142.2 (d, J213C-31P = 2.4 Hz; C4). 77Se NMR (76 MHz, DMSO-d6) δ: 317.3. MS (m/z % abundance): 350 (100), 118 (80), 97 (62). Elemental analysis for C11H15N2O2PSSe. Calculated/found (%): C: 37.82/38.02; H: 4.30/4.55; N: 8.02/7.69.
(vi) Phenyl-N-phenyl-N′-(4-selenocyanatephenyl)phosphoramidate (1f).
From phenyl-N-phenyl-phosphoramidochloridate. Yellow powder; mp: 98.35°C. Yield: 37.3%. IR (KBr) cm−1: 3,403 (N-H); 3,049 (N-H); 2,156 (CN); 1,596 (P=O). 1H NMR (400 MHz, DMSO-d6) δ: 6.56–6.62 (m, 2H; A, H3+H5), 6.64 (d, J21H-31P = 7.9 Hz, 1H; NH), 6.82 (t, J = 7.3 Hz, 1H; C, H4), 7.03–7.13 (m, 5H; B, H2+H6+H4; C, H2+H6), 7.18 (t, J = 7.8 Hz, 2H; C, H3+H5), 7.30 (dd, J = 8.8, 7.1 Hz, 2H; B, H3+H5), 7.33–7.38 (m, 2H; A, H2+H6), 7.89 (s, 1H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 105.0 (CN), 115.0 (A, C1), 117.1 (d, J313C-31P = 7.6 Hz; A, C3+C5), 120.0 (C, C2+C6), 120.2 (d, J313C-31P = 4.6 Hz; B, C2+C6), 123.9 (C, C4), 128.8 (B, C3+C5), 128.9 (B, C4), 129.4 (C, C3+C5), 136.4 (A, C2+C6), 141.7 (A, C4; B, C1), 150.7 (C, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 311.5. MS (m/z % abundance): 429 (10, M+), 93 (100), 65 (60). Elemental analysis for C19H16N2O2PSe. Calculated/found (%): C: 52.77/52.38; H: 4.63/4.79; N: 9.72/9.48.
(vii) Di-(4-methylphenyl)-N-(4-selenocyanatephenyl)phosphoramidate (1g).
From di-p-tolyl chlorophosphate. Yellow powder; mp: 168.73°C. Yield: 33.7%. IR (KBr) cm−1: 3,141 (N-H); 2,155 (CN); 1,570 (P=O). 1H NMR (400 MHz, DMSO-d6) δ: 2.20 (s, 6H; CH3), 6.71–6.79 (m, 2H; A, H3+H5), 6.98 (d, J = 8.1, d, 4H; B, H3+H5), 7.07 (d, J = 8.1, 4H; B, H2+H6), 7.40–7.46 (m, 2H; A, H2+H6). 13C NMR (100 MHz, DMSO-d6) δ: 20.8 (CH3), 106.2 (CN), 117.8 (A, C3+C5), 120.3 (d, J313C-31P = 4.9 Hz; B, C2+C6), 120.4 (A, C1), 130.4 (B, C3+C5), 133.2 (B, C4), 136.7 (A, C2+C6), 147.3 (A, C4), 150.4 (d, J213C-31P = 6.9 Hz; B, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 318.3. MS (m/z % abundance): 458 (8, M+), 278 (100), 108 (82). Elemental analysis for C21H19N2O3PSe. 1/2 HCl. Calculated/found (%): C: 52.90/52.97; H: 4.11/4.52; N: 5.86/5.36.
General procedure of synthesis of compounds 2b to 2g.
Compounds 2b to 2g were obtained by reaction of a solution of bis(4-aminophenyl)diselenide and triethylamine in chloroform, where the corresponding phosphoryl chlorides were added dropwise for 30 min with N2 flux at 80°C. The reaction mixture was kept under stirring and under reflux for 5 h. The solvent was then removed under vacuum. The oily residue was treated with water (2 × 50 ml) and extracted with ethyl ether. The organic phase was dried with Na2SO4, filtered, mixed with silica, and evaporated under vacuum, obtaining a solid mixture that was purified by automated flash chromatography using hexane/ethyl acetate as eluents.
(i) 4,4′-diselanediylbis(4,1-phenylene) tetramethyldiamidophosphate (2b).
From O,O′-dimethyl chlorophosphate. Yellow powder; mp: 162.63°C. Yield: 12.3%. IR (KBr) cm−1: 3,416 (N-H); 2,929 (C-H); 1,591 (P=O); 815 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 3.64 (d, 12H, J331P-1H = 11.4 Hz, CH3), 6.96 (d, 4H, J = 8.6 Hz, H3+H5), 7.40–7.52 (m, 4H; H2+H6), 8.31 (d, J21H-31P = 9.3 Hz, 2H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 52.4 (d, J213C-31P = 5.4 Hz; CH3), 117.5 (d, J313C-31P = 7.7 Hz; C3+C5), 120.6 (C1), 133.7 (C2+C6), 140.9 (C4). MS (m/z % abundance): 198 (75), 93 (100), 65 (98). Elemental analysis for C16H22N2O6P2Se2. Calculated/found (%): C: 34.90/34.98; H: 3.94/4.15; N: 5.01/4.99.
(ii) 4,4′-diselanediylbis(4,1-phenylene) tetraphenyldiamidophosphate (2c).
From O,O′-diphenyl chlorophosphate. Yellow powder, mp: 154.39°C. Yield: 32.5%. IR (KBr) cm−1 3,400 (N-H); 1,591 (P=O); 809 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 7.11–7.16 (m, 4H; A, H3+H5), 7.18–7.25 (m, 12H; B, H2+H4+H6), 7.39 (t, J = 7.9 Hz, 8H; B, H3+H5), 7.49–7.56 (m, 4H; A, H2+H6), 9.05 (d, J21H-31P = 10.2 Hz, 2H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 118.6 (d, J313C-31P = 8.0 Hz; A, C3+C5), 120.1 (d, J313C-31P = 4.8 Hz; B, C2+C6), 122.1 (A, C1), 125.4 (B, C4), 130.0 (B, C3+C5), 134.2 (A, C2+C6), 140.4 (A, C4), 149.9 (d, J213C-31P = 6.3 Hz; B, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 486.2. MS (m/z % abundance): 292 (10), 93 (80), 77 (100), 65 (92). Elemental analysis for C36H30N2O6P2Se2. Calculated/found (%): C: 51.25/51.55; H: 3.67/4.01; N: 3.31/2.98.
(iii) 4,4′-diselanediylbis(4,1-phenylene) tetraethyldiamidophosphate (2d).
From O,O′-diethyl chlorophosphate. Yellow powder, mp: 183.71°C. Yield: 31.1%. IR (KBr) cm−1: 3,185 (N-H); 2,982 (C-H); 1,586 (P=O); 822 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 1.21 (t, J = 7.0 Hz, 12H; CH3), 3.86–4.14 (m, 8H; CH2), 6.97 (d, J = 8.1 Hz, 4H; H3+H5), 7.42 (d, J = 8.1 Hz, 4H; H2+H6), 8.21 (d, J21H-31P = 9.2 Hz, 2H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 16.0 (d, J313C-31P = 6.6 Hz, CH3), 62.1 (d, J213C-31P = 5.1 Hz; CH2), 117.9 (d, J313C-31P = 7.6 Hz; C3+C5), 120.9 (C1), 134.3 (C2+C6), 141.8 (C4). 77Se NMR (76 MHz, DMSO-d6) δ: 491.0. MS (m/z % abundance): 308 (34), 252 (35), 173 (90), 93 (100), 65 (90). Elemental analysis for C20H30N2O6P2Se2. Calculated/found (%): C: 39.08/39.18; H: 4.88/4.80; N: 4.45/4.20.
(iv) 4,4′-diselanediylbis(4,1-phenylene) tetraethyldiamidothiophosphate (2e).
From O,O′-diethyl chlorothiophosphate. Orange oil. Yield: 36.4%. IR (KBr) cm−1: 3,346 (N-H), 2,978 (C-H); 1,589 (P=S); 819 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 1.17–1.26 (m, 12H; CH3), 3.91–4.14 (m, 8H; CH2), 7.00–7.06 (m, 4H; H3+H5), 7.41–7.47 (d, 4H; H2+H6), 8.54 (d, J21H-31P = 14.9 Hz, 2H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 15.6 (d, J313C-31P = 7.7 Hz, CH3), 62.6 (d, J213C-31P = 4.7 Hz; CH2), 118.7 (d, J313C-31P = 7.6 Hz; C3+C5), 121.4 (C1), 133.9 (C2+C6), 141.1 (d, J213C-31P = 2.6 Hz; C4). 77Se NMR (76 MHz, DMSO-d6) δ: 487.2. MS (m/z % abundance): 646 (5, M+), 324 (40), 125 (50), 97 (100). Elemental analysis for C20H30N2O4P2S2Se2. Calculated/found (%): C: 37.25/37.52; H: 4.64/5.05; N: 4.33/4.18.
(v) N-4,4′-diselanediylbis(4,1-phenylene) N’-diphenyl O-diphenyldiamidophosphate (2f).
From phenyl-N-phenyl-phosphoramidochloridate. Yellow powder. Yield: 37.2%. mp: 103.93°C. IR (KBr) cm−1: 3,396 (N-H); 3,203 (N-H); 1,595 (P=O); 805 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 6.47–6.55 (m, 4H; A, H3+H5), 6.67–6.77 (m, 2H; NH), 6.81–6.87 (m, 2H; C, H4), 7.06–7.14 (m, 10H; B, H2+H6+H4; C, H2+H6), 7.17–7.23 (m, 8H; A, H2+H6; C, H3+H5), 7.28–7.35 (m, 4H; B, H3+H5), 7.97 (d, J = 9.5 Hz, 2H; NH). 13C NMR (100 MHz, DMSO-d6) δ: 115.1 (A, C1), 117.6 (d, J313C-31P = 7.7 Hz; A, C3+C5), 120.6 (C, C2+C6), 120.7 (d, J313C-31P = 4.8 Hz; B, C2+C6), 124.6 (B, C4), 129.3 (B, C3+C5), 129.5 (B, C4), 129.9 (C, C3+C5), 136.4 (A, C2+C6), 142.0 (A, C4), 149.8 (B, C1), 151.6 (C, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 521.9. MS (m/z % abundance): 184 (10), 93 (100), 66 (45). Elemental analysis for C36H32N4O4P2Se2. Calculated/found (%): C: 53.74/53.46; H: 4.01/3.94; N: 6.96/6.78.
(vi) 4,4′-diselanediylbis(4,1-phenylene) di-p-tolyl diamidophosphate (2g).
From di-p-tolylchlorophosphate. Yellow powder; mp: 146.21°C. Yield: 25.3%. IR (KBr) cm−1: 3,366 (N-H); 1,568 (P=O); 814 (Se-Se). 1H NMR (400 MHz, DMSO-d6) δ: 2.24 (s, 12H, CH3), 6.55–6.63 (m, 4H; A, H3+H5), 6.99–7.07 (m, 8H; B, H3+H5), 7.09 (d, J = 8.4 Hz, 8H; B, H2+H6), 7.22–7.31 (m, 4H; A, H2+H6). 13C NMR (100 MHz, DMSO-d6) δ: 20.2 (CH3), 115.6 (A, C3+C5), 116.6 (A, C1), 119.7 (d, J313C-31P = 4.9 Hz; B, C2+C6), 129.7 (B, C3+C5), 132.4 (B, C4), 135.6 (A, C2+C6), 147.5 (A, C4), 149.9 (d, J213C-31P = 6.9 Hz; B, C1). 77Se NMR (76 MHz, DMSO-d6) δ: 515.7. MS (m/z % abundance): 344 (5), 184 (55), 108 (100), 97 (80), 65 (50). Elemental analysis for C40H38N2O6P2Se2. Calculated/found (%): C: 55.69/55.74; H: 4.44/4.37; N: 3.25/3.29.
Biological evaluation.
(i) Cells and culture conditions. THP-1 cells were kindly provided by G. Michel (Université Nice Sophia Antipolis, Nice, France) and were grown in RPMI 1640 medium (Gibco, Lieden, Netherlands) supplemented with 10% heat inactivated fetal bovine serum (FBS), 5% penicillin/streptomycin, 1 nM HEPES, 2 mM glutamine, and 1 mM sodium pyruvate, pH 7.2, at 37°C and 5% CO2 atmosphere. L. infantum promastigotes (MCAN/ES/89/IPZ229/1/89) was kindly provided by M. Colmenares (Centro de Investigaciones Biológicas, CIB, Madrid, Spain) and grown in RPMI 1640 medium (Gibco, Lieden, Netherlands) supplemented with 10% heat inactivated FBS, 5% penicillin/streptomycin, and 25 mM HEPES, pH 7.2, at 26°C. L. infantum axenic amastigotes were grown in M199 medium (Invitrogen, Leiden, Netherlands) supplemented with 10% heat inactivated FBS, 1 g/liter β-alanine, 100 mg/liter l-asparagine, 200 mg/liter sucrose, 50 mg/liter sodium pyruvate, 320 mg/liter malic acid, 40 mg/liter fumaric acid, 70 mg/liter succinic acid, 200 mg/liter α-ketoglutaric acid, 300 mg/liter citric acid, 1.1 g/liter sodium bicarbonate, 5 g/liter morpholineethanesulfonic acid (MES), 0.4 mg/liter hemin, 10 mg/liter gentamicin, pH 5.4, at 37°C and 5% CO2.
(ii) Leishmanicidal activity and cytotoxicity in vitro assays.
Drug treatment of amastigotes and THP-1 cells was performed during the logarithmic growth phase at a concentration of 2 × 106 parasites/ml and 4 × 105 cells/ml, respectively, at 37°C and 5% CO2 for 24 h. The number and percentage of living parasites/cells was figured out by flow cytometry by the propidium iodide (PI) exclusion method (28).
(iii) Leishmania infection assay.
A total of 120,000 cells/ml THP-1 cells were seeded in 24 multidish plates (Nunc, Roskilde, Denmark) and differentiated to macrophages in 1 ml of RPMI 1640 medium containing 10 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA). After 24 h, medium was removed and 1.2 × 106 Leishmania infantum eGFP-amastigotes in 1 ml of THP-1 medium were added to half of the plate wells. Four hours later, all medium with noninfecting amastigotes was removed, washed 3 times with 1× phosphate-buffered saline (PBS) and replaced with the corresponding treatment dissolved in 1 ml THP-1 medium. Forty-eight hours after the treatment, medium was removed; Cells were washed 3 times with 1× PBS and detached with TrypLE Express (Invitrogen, Leiden, Netherlands) following manufacturer’s protocol. Infection quantization was measured by flow cytometry.
(iv) Measurement of intracellular thiols.
The disturbance of the intracellular thiol levels is evidence of an oxidative stress. For its measurement, 0.5 ml/plate amastigotes at a concentration of 1 × 106/ml was seeded in 6 multidish plates and treated with DMSO (5 μl) as the negative control, menadione (25 μM) as the positive control, and compounds (25 μM). Amastigotes were incubated at 37°C and 5% CO2 for 1 h. Thirty minutes after the treatment, CMFDA (5-chloromethylfluorescein diacetate) was added (20 μM) and incubated for 30 min. After this time, the parasite pellet was washed with PBS 1×, and intracellular thiols quantization was measured by flow cytometry.
(v) Activity against TryR.
The oxidoreductase activity against trypanothione reductase enzyme was determined following the method described by Toro et al. (29). The reaction was done at room temperature in 250 μl of HEPES, pH 8.0 (40 mM), containing EDTA (1 mM), NADPH (150 μM), NADP+ (30 μM), DTNB (25 μM), TS2 (1 μM), glycerol (0.02%), DMSO (1.5%), and recombinant Li-TryR (7 nM). For 50% inhibitory concentration (IC50) value determinations, the enzyme was preincubated with concentrations ranging from 75 μM to 0.29 μM for 10 min prior the addition of TS2 and NADPH. Reaction was monitored by the increase in absorbance at 412 nm for 1 h at 26°C in a VERSAmax microplate reader (Molecular Devices, CA, USA). All of the assays were conducted in triplicate in at least three independent experiments. Data were analyzed using a nonlinear regression model with the GraFit6 software (Erithacus, Horley, Surrey, UK). Mepacrine was used as positive control.
(vi) ADME and Lipinski properties.
Absorption, distribution, metabolism, and excretion (ADME) properties were calculated using FAF filters. In brief, substructure searches within ligands using previous knowledge of pan-assay interference compounds (PAINS) (nonspecific assay confounding compounds), reactive groups, solubility, Lipinski, Veber, and EGAN rules, among others, provide the filters for chemical compounds to flag possible nondesirable properties. Absorption properties were also calculated using SwissADME program (http://www.swissadme.ch).
ACKNOWLEDGMENTS
We wish to express our gratitude to the Institute of Tropical Health of University of Navarre (ISTUN), Caixa Foundation, Roviralta and Ubesol, Spain.
We also thank the Spanish Government (MICINN project PID2019-104070RBC22) and the Comunidad de Madrid (project PLATESA2-CM ref S-2018/BAA-4370).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Calderon-Anyosa R, Galvez-Petzoldt C, Garcia PJ, Carcamo CP. 2018. Housing characteristics and leishmaniasis: a systematic review. Am J Trop Med Hyg 99:1547–1554. 10.4269/ajtmh.18-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunyoto T, Adam GK, Atia AM, Hamid Y, Babiker RA, Abdelrahman N, Vander Kelen C, Ritmeijer K, Alcoba G, den Boer M, Picado A, Boelaert M. 2018. “Kala-Azar is a dishonest disease”: community perspectives on access barriers to visceral leishmaniasis (Kala-Azar) diagnosis and care in Southern Gadarif, Sudan. Am J Trop Med Hyg 98:1091–1101. 10.4269/ajtmh.17-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvapandiyan A, Croft SL, Rijal S, Nakhasi HL, Ganguly NK. 2019. Innovations for the elimination and control of visceral leishmaniasis. PLoS Negl Trop Dis 13:e0007616. 10.1371/journal.pntd.0007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoudvand H, Shakibaie M, Tavakoli R, Jahanbakhsh S, Sharifi I. 2014. In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucantime-resistant Leishmania tropica. Iran J Parasitol 9:452–460. [PMC free article] [PubMed] [Google Scholar]
- 5.Mostafavi M, Farajzadeh S, Sharifi I, Khazaeli P, Sharifi H. 2019. Leishmanicidal effects of amphotericin B in combination with selenium loaded on niosome against Leishmania tropica. J Parasit Dis 43:176–185. 10.1007/s12639-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostafavi M, Khazaeli P, Sharifi I, Farajzadeh S, Sharifi H, Keyhani A, Parizi MH, Kakooei S. 2019. A novel niosomal combination of selenium coupled with glucantime against Leishmania tropica. Korean J Parasitol 57:1–8. 10.3347/kjp.2019.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taghipour A, Abdoli A, Ramezani A, Abolghazi A, Mofazzal Jahromi MA, Maani S, Heidar Nejadi SM, Rasti S, Shams M, Ghasemi E. 6January2021. Leishmaniasis and trace element alterations: a systematic review. Biol Trace Elem Res 10.1007/s12011-020-02505-0. [DOI] [PubMed] [Google Scholar]
- 8.Lobanov AV, Gromer S, Salinas G, Gladyshev VN. 2006. Selenium metabolism in Trypanosoma: characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucleic Acids Res 34:4012–4024. 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco J, Sardi F, Szilágyi L, Kövér KE, Fehér K, Comini MA. 2017. Diglycosyl diselenides alter redox homeostasis and glucose consumption of infective African trypanosomes. Int J Parasitol Drugs Drug Resist 7:303–313. 10.1016/j.ijpddr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun B, Luo C, Zhang X, Guo M, Sun M, Yu H, Chen Q, Yang W, Wang M, Zuo S, Chen P, Kan Q, Zhang H, Wang Y, He Z, Sun J. 2019. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat Commun 10:3211. 10.1038/s41467-019-11193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno D, Plano D, Baquedano Y, Jimenez-Ruiz A, Palop JA, Sanmartin C. 2011. Antileishmanial activity of imidothiocarbamates and imidoselenocarbamates. Parasitol Res 108:233–239. 10.1007/s00436-010-2073-x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tamimi AS, Etxebeste-Mitxeltorena M, Sanmartin C, Jimenez-Ruiz A, Syrjanen L, Parkkila S, Selleri S, Carta F, Angeli A, Supuran CT. 2019. Discovery of new organoselenium compounds as antileishmanial agents. Bioorg Chem 86:339–345. 10.1016/j.bioorg.2019.01.069. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Rubio C, Campbell D, Vacas A, Ibanez E, Moreno E, Espuelas S, Calvo A, Palop JA, Plano D, Sanmartin C, Nguewa PA. 2015. Leishmanicidal activities of novel methylseleno-imidocarbamates. Antimicrob Agents Chemother 59:5705–5713. 10.1128/AAC.00997-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Montes A, Plano D, Martin-Escolano R, Alcolea V, Diaz M, Perez-Silanes S, Espuelas S, Moreno E, Marin C, Gutierrez-Sanchez R, Sanmartin C, Sanchez-Moreno M. 2017. Library of seleno-compounds as novel agents against Leishmania species. Antimicrob Agents Chemother 61:e02546-16. 10.1128/AAC.02546-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baquedano Y, Alcolea V, Toro MA, Gutierrez KJ, Nguewa P, Font M, Moreno E, Espuelas S, Jimenez-Ruiz A, Palop JA, Plano D, Sanmartin C. 2016. Novel heteroaryl selenocyanates and diselenides as potent antileishmanial agents. Antimicrob Agents Chemother 60:3802–3812. 10.1128/AAC.02529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baquedano Y, Moreno E, Espuelas S, Nguewa P, Font M, Gutierrez KJ, Jimenez-Ruiz A, Palop JA, Sanmartin C. 2014. Novel hybrid selenosulfonamides as potent antileishmanial agents. Eur J Med Chem 74:116–123. 10.1016/j.ejmech.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Plano D, Ibanez E, Calvo A, Palop JA, Sanmartin C. 2011. Novel library of selenocompounds as kinase modulators. Molecules 16:6349–6364. 10.3390/molecules16086349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jimenez-Ruiz A, Palop JA, Sanmartin C. 2011. Selenocyanates and diselenides: a new class of potent antileishmanial agents. Eur J Med Chem 46:3315–3323. 10.1016/j.ejmech.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Kursvietiene L, Mongirdiene A, Bernatoniene J, Sulinskiene J, Staneviciene I. 2020. Selenium anticancer properties and impact on cellular redox status. Antioxidants (Basel) 9:80. 10.3390/antiox9010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobe T, Ueda K, Ando M, Okamoto Y, Kojima N. 2015. Thiol-mediated multiple mechanisms centered on selenodiglutathione determine selenium cytotoxicity against MCF-7 cancer cells. J Biol Inorg Chem 20:687–694. 10.1007/s00775-015-1254-6. [DOI] [PubMed] [Google Scholar]
- 21.Fairlamb AH, Cerami A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol 46:695–729. 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 22.Ilari A, Fiorillo A, Genovese I, Colotti G. 2017. Polyamine-trypanothione pathway: an update. Future Med Chem 9:61–77. 10.4155/fmc-2016-0180. [DOI] [PubMed] [Google Scholar]
- 23.Bin Dukhyil AA. 2019. Targeting trypanothione reductase of Leishmanial major to fight against cutaneous leishmaniasis. Infect Disord Drug Targets 19:388–393. 10.2174/1871526518666180502141849. [DOI] [PubMed] [Google Scholar]
- 24.Ghezzi P, Bonetto V, Fratelli M. 2005. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal 7:964–972. 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 25.Jensen KS, Hansen RE, Winther JR. 2009. Kinetic and thermodynamic aspects of cellular thiol-disulfide redox regulation. Antioxid Redox Signal 11:1047–1058. 10.1089/ars.2008.2297. [DOI] [PubMed] [Google Scholar]
- 26.Matadamas-Martinez F, Hernandez-Campos A, Tellez-Valencia A, Vazquez-Raygoza A, Comparan-Alarcon S, Yepez-Mulia L, Castillo R. 2019. Leishmania mexicana trypanothione reductase inhibitors: computational and biological studies. Molecules 24:3216. 10.3390/molecules24183216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuldeep J, R K, Kaur P, Goyal N, Siddiqi MI. 2021. Identification of potential anti-leishmanial agents using computational investigation and biological evaluation against trypanothione reductase. J Biomol Struct Dyn 39:960–969. 10.1080/07391102.2020.1721330. [DOI] [PubMed] [Google Scholar]
- 28.Alzate JF, Arias AA, Moreno-Mateos D, Alvarez-Barrientos A, Jimenez-Ruiz A. 2007. Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol Biochem Parasitol 152:192–202. 10.1016/j.molbiopara.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Toro MA, Sanchez-Murcia PA, Moreno D, Ruiz-Santaquiteria M, Alzate JF, Negri A, Camarasa MJ, Gago F, Velazquez S, Jimenez-Ruiz A. 2013. Probing the dimerization interface of Leishmania infantum trypanothione reductase with site-directed mutagenesis and short peptides. Chembiochem 14:1212–1217. 10.1002/cbic.201200744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.00590-21-s0001.pdf, PDF file, 1.6 MB (1.6MB, pdf)