ABSTRACT
Tenofovir use is associated with lower risk of mother-to-infant transmission of the virus, and discontinuation of the treatment is not safe. However, the safety of the drug during pregnancy and breastfeeding is not clear. In this study, we aimed to determine the tenofovir concentration in plasma of mother-infant pairs along with breast milk in chronic hepatitis B patients during the lactation period. A total of 11 mother-infant pairs were enrolled in the study. All the mothers received tenofovir disoproxil fumarate (TDF) 245 mg/day for at least 1 month because of chronic hepatitis B infection. Maternal blood, breast milk, and infant blood samples were obtained concomitantly. Tenofovir concentrations were determined by liquid chromatography-tandem mass spectrometry. The median concentrations of tenofovir in maternal plasma and breast milk samples were 88.44 (interquartile range [IQR], 62.47 to 116.17) ng/ml and 6.69 (IQR, 4.88 to 7.03) ng/ml, respectively. Tenofovir concentrations were undetectable (<4 ng/ml) in all of the infant plasma samples. The ratio of tenofovir concentration in breast milk to that in maternal plasma was 0.07. Tenofovir disoproxil fumarate passes through the breast milk in a small amount. Infants had no detectable tenofovir level in their plasma. Our study suggests that tenofovir disoproxil fumarate treatment is safe during the breastfeeding period in chronic hepatitis B patients.
KEYWORDS: chronic hepatitis B, breast milk, infant plasma, tenofovir
INTRODUCTION
Hepatitis B virus (HBV) infection during pregnancy presents unique management issues for both mother and fetus. One of these is management of antiviral treatment during pregnancy and the postpartum period. Antiviral treatment is recommended in certain situations for pregnant chronic hepatitis B patients such as a high viral load or existence of cirrhosis (1). Tenofovir disoproxil fumarate (TDF) is the preferred antiviral regimen due to its potency, safety profile, and low risk of resistance.
Treatment of chronic hepatitis B (CHB) should be continued during pregnancy and lactation. Immunological changes during the postpartum period, which are most likely related to immune reconstitution, have been associated with hepatitis flares. TDF has the potential to improve maternal alanine aminotransferase elevations, which can occur during pregnancy or soon after delivery in untreated mothers (2). Hence, it seems safer to continue using TDF even after delivery to prevent flares. For the patients with advanced disease, discontinuation of the treatment is further challenging. Furthermore, antiviral use during pregnancy may have a role for prevention of mother-to-infant transmission of the virus. Among pregnant women with high viral load during the third trimester, the rate of mother-to-child transmission was lower among those who received TDF therapy than among those who received usual care without antiviral therapy (3, 4).
After immunoprophylaxis, including hepatitis B immunoglobulin and hepatitis B vaccine, breast feeding does not increase the risk of transmission hepatitis B virus infection in infants (5, 6). The Society for Maternal-Fetal Medicine recommends that women with HBV infection be encouraged to breastfeed as long as the infant receives immunoprophylaxis at birth (7). Furthermore, the American Academy of Pediatrics recommends exclusive breastfeeding for the first 6 months and continuation of breastfeeding for approximately a year or longer (8). Considering the importance of breastfeeding and the risk of flares with discontinuation of antiviral treatment, breastfeeding and continuing antiviral therapy during this period seem to be the most appropriate approach. However, the safety of TDF treatment during lactation is unclear.
The previous studies conducted with HIV-infected patients and animal models showed limited tenofovir transmission via breast milk, which is unlikely to have any biologic effects on nursing infants (9–13). In addition, no short-term adverse effects were found in infants exposed to TDF through breast milk (14–16). This study aimed to determine TDF concentrations in mothers (plasma and breast milk) and breastfed infants (plasma) based on real-world data.
RESULTS
Study population.
The study population included 11 Caucasian nursing mother-infant pairs. TDF was well tolerated by all mothers, without any adverse effect. Monthly screened laboratory studies did not show significant abnormality. The characteristics of the mothers and infants are summarized in Table 1. Seven women stated they were breastfeeding exclusively at the time of the study. None of the patients had a concomitant disease. No correlation was found between drug concentrations and the interval between the last TDF dose and the sample collection as well as duration of therapy.
TABLE 1.
Characteristics of the nursing mothers with chronic hepatitis B using tenofovir disoproxil fumarate
| Patient characteristicsa | Median value (IQR) |
|---|---|
| Age of mothers (yrs) | 31 (29–36) |
| Wt (kg) | 62 (54–67) |
| Ht (cm) | 164 (156–169) |
| BMI | 22.2 (20.3–28.3) |
| Duration of TDF treatment (mo) | 18 (4–24) |
| Interval between the last dose of TDF and collection of the sample (h) | 12 (10–17) |
| Age of the infants (mo) | 3 (2–6) |
| AST (U/liter) | 27 (22–38) |
| ALT (U/liter) | 29 (17–62) |
| Creatinine (mg/dl) | 0.7 (0.6–0.8) |
Total of 11 patients, all Caucasian. BMI, body mass index; IQR, interquartile range; TDF, tenofovir disoproxil fumarate.
Drug concentrations.
The concentration of tenofovir in the breast milk and blood samples was in the range of 4 to 600 ng/ml, as measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Drug levels are presented in Table 2.
TABLE 2.
Tenofovir concentrations in all samples
| Patient ID no.a | Tenofovir concn (ng/ml) in: |
||
|---|---|---|---|
| Maternal plasma | Breast milk | Infant plasma | |
| 1 | 88.443 | 6.95 | 0 |
| 2 | 79.001 | 4.88 | 0 |
| 3 | 93.431 | 4.44 | 0 |
| 4 | 49.890 | 5.05 | 0 |
| 5 | 115.836 | 8.10 | 0 |
| 6 | 116.175 | 7.03 | 0 |
| 7 | 57.788 | 6.75 | 0 |
| 8 | 62.475 | 5.71 | 0 |
| 9 | 87.719 | 4.86 | 0 |
| 10 | 162.621 | 10.00 | 0 |
| 11 | 133.994 | 6.69 | 0 |
a ID, identification.
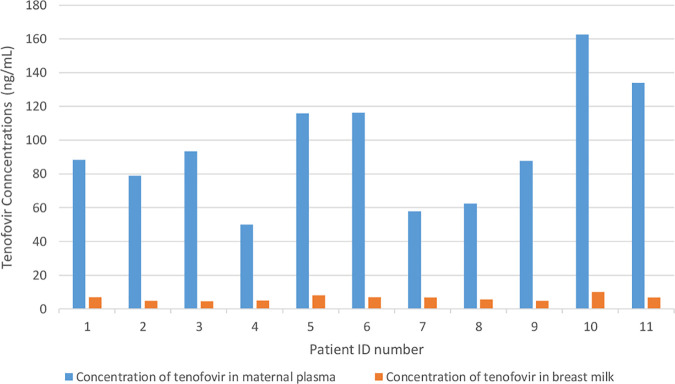
The tenofovir concentrations in maternal plasma was found to be 88.44 (interquartile range [IQR], 62.47 to 116.17) ng/ml, while the tenofovir concentration in breast milk was 6.69 (IQR, 4.88 to 7.03) ng/ml (Fig. 1). Tenofovir was undetectable (<4 ng/ml) in all infants.
FIG 1.
Comparison of tenofovir concentrations in maternal plasma and breast milk.
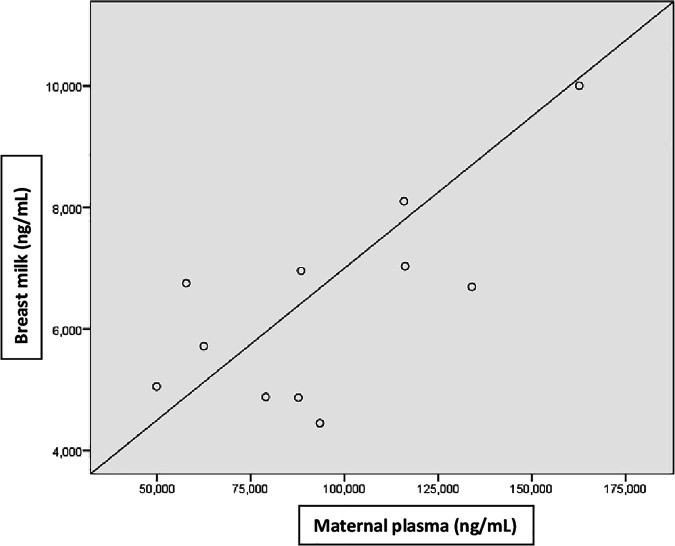
The ratio of tenofovir concentrations in breast milk to that in maternal plasma was 0.07. There was a strong, positive, and statistically significant correlation between the tenofovir concentrations in breast milk and those in maternal plasma (r, 0.7; P < 0.05) (Fig. 2).
FIG 2.
Correlation of tenofovir concentrations in breast milk and maternal plasma.
DISCUSSION
This study is the first report on tenofovir transfer to nursing infants via breast milk in hepatitis B patients. Single dose or short-term data were already available in HIV patients. In a study conducted in Malawi and Brazil, HIV-infected mothers were given a single dose of either 600 mg or 900 mg of tenofovir during labor, and tenofovir was detected in their breastmilk samples at concentrations ranging from 6.3 to 17.8 ng/ml (17). In another study conducted in Malawi, the breast milk-to-maternal plasma ratio was 0.07 during the first month after delivery (18). Similarly, in a study assessing maternal plasma and breast milk tenofovir concentrations at peak and trough levels, the breast milk tenofovir concentrations were considerably lower than those in maternal plasma. The median peak and trough milk/plasma (M/P) ratios were 0.03 (IQR, 0.01 to 0.05) and 0.07 (IQR, 0.05 to 0.08), respectively (19). Waitt et al. studied the tenofovir levels in dried blood and dried breast milk samples from 29 Nigerian and 30 Ugandan HIV-infected women and reported a median milk/plasma ratio of 0.015 (0 to 0.03) (20). In an animal model, tenofovir was detected in the milk, with 2% to 4% detected in maternal serum following a 30-mg/kg single subcutaneous dose (10). As in the previous studies, our results showed that the tenofovir concentration in breast milk is significantly lower than in maternal plasma.
Tenofovir is a dianion at physiological pH and has poor membrane permeability. In addition, oral bioavailability of tenofovir is poor; hence, it is administered as the prodrug tenofovir disoproxil fumarate or tenofovir alafenamide (21). Breast milk is expected to contain tenofovir almost exclusively in an unesterified anionic form, which has low oral bioavailability (19). The undetectable tenofovir concentrations in the infants can be explained by the pharmacokinetic features of tenofovir. Undetectable infant tenofovir concentrations are unlikely to produce toxicity or select for resistant viruses (10).
A pharmacokinetic study assessed tenofovir exposure in HIV-infected women dosed at 600 mg and 300 mg TDF at the start of labor reported simulated peak median infant tenofovir daily doses from breast milk of 4.2 μg/kg, which is 0.03% of the therapeutic oral infant dose. However, infant plasma drug concentrations were not directly measured, and poor bioavailability of tenofovir was not considered in this study (11). Hence, the simulated results overestimated tenofovir concentrations in nursing infants. In another study, tenofovir was undetectable in 46 of 49 (94%) nursing infant plasma samples. The other three detectable plasma concentrations were at 0.9, 0.9, and 17.4 ng/ml (19), two of which are below the threshold value for our study. Waitt et al. reported tenofovir concentrations in dried blood samples of nursing infants, and no infant had measurable tenofovir concentration levels (20). In contrast, the study conducted in Malawi showed that tenofovir (median [IQR], 24 [0 to 51.6] and 0 [0 to 29.9] ng/ml) was detectable in infants at 1 and 12 months after delivery, respectively (18).
However, TDF was used at 300 mg/day among the patients in previous studies, because the primary disease was HIV infection. In experimental studies, data were obtained during short-term and high-dose TDF utilization in the peripartum period. In addition, the use of multiple medications and the metabolic changes caused by HIV infection may affect the pharmacokinetics of tenofovir. Therefore, it should be considered that the available data may not represent accurate drug concentrations in chronic hepatitis B patients.
Limitations.
Our study has some limitations, which includes small sample size. Furthermore, the samples were collected from each mother-infant pair only once. Only 7 of the participants were breastfeeding exclusively, and this might cause variability in the results. Although the ages of the infants were in a broad range (3 to 24 weeks), it is unlikely to have impacted the maternal plasma and breast milk pharmacokinetic profiles. The plasma samples of nursing mothers were not taken before the next dose was administered. This may affect accurate interpretation of the current data and the comparison of the current data with previously published data.
Conclusion.
In conclusion, in this prospective study of mothers with chronic hepatitis B, tenofovir was found in breast milk but at lower levels than in maternal blood. Only negligible amounts of tenofovir may be absorbed by nursing infants due to tenofovir’s poor bioavailability. Hence, infants do not have detectable tenofovir concentrations in their plasma, and tenofovir-related adverse effects are unlikely to be seen in infants. Although our results suggest that TDF is safe during the breastfeeding period, there is still a need for large-scale prospective cohort studies.
MATERIALS AND METHODS
A total of 11 mother-infant pairs were included in the study. These participants were recruited from the Department of Infectious Diseases and Clinical Microbiology, Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa. All the mothers received TDF 245 mg/day for at least 1 month during their breastfeeding period because of chronic hepatitis B infection. Patients coinfected with either HIV or hepatitis C virus (HCV) were excluded from the study. A written informed consent form was signed by all participating mothers. The study was approved by the Cerrahpasa Medical Faculty Ethics Committee (approval number 89125526-605.02).
Maternal blood samples and infant blood samples were obtained from the antecubital vein and dorsal metacarpal vein, respectively. Samples of maternal blood (7 ml), milk (3 ml), and infant blood (3 ml) were collected concomitantly. Blood samples were collected in a K2 EDTA tube. The milk samples were stored immediately after collection, with no procedures performed on them. The blood samples were centrifuged at 4,000 rpm for 10 min within 0.5 h of collection to separate the plasma. All milk and plasma samples were stored at −80°C, while samples that could not be centrifuged within the 0.5 h of collection were temporarily stored at 4°C. Only one set of samples was collected from each mother-infant pair.
Analyses were performed on a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system comprising a Waters Acquity quaternary solvent manager, Waters Acquity sample manager, a thermostated column compartment, and a Waters Acquity H-class ultraperformance liquid chromatograph (UPLC) coupled to a tandem quadrupole (TQ) detector with an electrospray ionization (ESI) interface (Waters, USA). Data acquisition, data processing, and data reporting were performed using a Micromass MassLynx v.4.1 SCN714 with MassLynx Security Manager software running the QuanLynx program (Waters, USA).
Chromatographic separations were achieved using a Shiseido CapCell Pak SG C18 (4.6 by 150 mm, 5 μm) column at 35°C, and the mobile phase was composed of methanol, water, and formic acid (300:700:2 [vol/vol/vol]). The chromatographic run was performed under isocratic conditions at a flow rate of 0.7 ml/min and a run time of 4.5 min, with a 20-μl sample injected into the system via an autosampler conditioned at 10°C.
Multiple reaction monitoring (MRM) transitions were performed at m/z 288.1 to 176.10 for tenofovir and m/z 295.10 to 183.20 for tenofovir-d7. Mass spectrometric detection was performed using the ESI positive ion mode with a 35-V cone voltage, 25-V collision energy, and a 1.5-kV capillary voltage. Desolvation gas flow and cone gas flow were set to 650 liters/h and 50 liters/h, respectively. The source block temperature was 125°C, and the desolvation temperature was 500°C.
The method was validated in a range of 4 to 600 ng/ml for tenofovir. To assess the accuracy and precision of the assay, six replicates of quality control (QC) samples at five different concentration levels (4 ng/ml, 12 ng/ml, 240 ng/ml, 450 ng/ml, and 600 ng/ml) in plasma were analyzed in three analytical runs.
The intraday precision (percent coefficient of variation [CV%]) for the low, medium 1, medium 2 and high-quality control samples ranged from 0.524% to 2.867%. The interday precision ranged from 1.616% to 3.384%, which was within the acceptance criteria of ≤15%. The intraday precision (CV%) for the limit of quantification quality control samples was 6.937% to 10.226%. The interday precision was 11.165%, which was within the acceptance criteria of ≤20%.
The intraday accuracy for the low, medium 1, medium 2, and high-quality control samples ranged from 100.075% to 110.290%. The interday accuracy ranged from 101.316% to 106.580%, which was within the acceptance criteria of 85% to 115% of nominal concentration. The intraday accuracy for the limit of quantification quality control samples was 98.004% to 117.858%. The interday accuracy was 107.290%, which was within the acceptance criteria of 80% to 120% of nominal concentration.
The results demonstrated that the values were within the acceptable range according to FDA guidance (22) and the EMA guideline (23), and the method was accurate and precise.
The patients were followed up at outpatient settings monthly. At every visit, the patients were questioned about any symptoms and side effects of the drugs. They were thoroughly examined. Complete blood count, alanine aminotransferase (ALT), aspartate transaminase (AST), bilirubins, blood urea nitrogen, calcium, phosphorus, prothrombin time, alkaline phosphatase, and gamma glutamyl transferase were checked monthly.
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 21. Variables were investigated using visual methods (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov test or Shapiro-Wilk test) to determine whether the variables were normally distributed. The parameters were normally distributed, and the correlation coefficients and their significance levels were calculated using the Pearson test. A 5% type I error level was used to infer statistical significance.
ACKNOWLEDGMENTS
We thank registered nurse Hacer Pulat Tekin for her significant contribution in collecting the samples.
We declare no potential conflicts of interest.
This study was funded by the Viral Hepatitis Society and the Istanbul University Scientific Research Projects Coordination Unit (project no TTU-2017-24758).
REFERENCES
- 1.Lee H, Lok AS. 2020. Hepatitis B and pregnancy. InPost TW (ed), UpToDate. UpToDate, Waltham, MA. [Google Scholar]
- 2.Liu J, Wang J, Jin D, Qi C, Yan TT, Cao F, Jin L, Tian Z, Guo D, Yuan N, Feng W, Zhang S, Zhao Y, Chen T. 2017. Hepatic flare after telbivudine withdrawal and efficacy of postpartum antiviral therapy for pregnancies with chronic hepatitis B virus. J Gastroenterol Hepatol 32:177–183. 10.1111/jgh.13436. [DOI] [PubMed] [Google Scholar]
- 3.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, Zhang H, Zou H, Zhu B, Zhao W, Jiang H. 2016. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 374:2324–2334. 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 4.Brown RS, Mcmahon BJ, Lok ASF, Wong JB, Ahmed AT, Mouchli MA, Wang Z, Prokop LJ, Murad MH, Mohammed K. 2016. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology 63:319–333. 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 5.De Martino M, Appendino C, Resti M, Rossi ME, Muccioli AT, Vierucci A. 1985. Should hepatitis B surface antigen positive mothers breast feed? Arch Dis Child 60:972–974. 10.1136/adc.60.10.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. 2002. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol 99:1049–1052. 10.1016/s0029-7844(02)02000-8. [DOI] [PubMed] [Google Scholar]
- 7.Society for Maternal-Fetal Medicine, Dionne-Odom J, Tita ATN, Silverman NS. 2016. Hepatitis B in pregnancy- screening, treatment and prevention of vertical transmission. Am J Obstet Gynecol 214:6–14. 10.1016/j.ajog.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 8.Eidelman AI, Schanler RJ. 2012. Breastfeeding and the use of human milk. Pediatrics 129:496–506. [DOI] [PubMed] [Google Scholar]
- 9.Cundy KC, Sueoka C, Lynch GR, Griffin L, Lee WA, Shaw JP. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob Agents Chemother 42:687–690. 10.1128/AAC.42.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Rompay KKA, Hamilton M, Kearney B, Bischofberger N. 2005. Pharmacokinetics of tenofovir in breast milk of lactating rhesus macaques. Antimicrob Agents Chemother 49:2093–2094. 10.1128/AAC.49.5.2093-2094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, Arrivé E, Blanche S, Théodoro F, Avit D, Dabis F, Tréluyer JM, Hirt D. 2011. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Côte d’Ivoire, in the ANRS 12109 TEmAA Study, step 2. Antimicrob Agents Chemother 55:1315–1317. 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrhardt S, Xie C, Guo N, Nelson K, Thio CL. 2015. Breastfeeding while taking lamivudine or tenofovir disoproxil fumarate: a review of the evidence. Clin Infect Dis 60:275–278. 10.1093/cid/ciu798. [DOI] [PubMed] [Google Scholar]
- 13.Waitt C, Diliiy Penchala S, Olagunju A, Amara A, Else L, Lamorde M, Khoo S. 2017. Development, validation and clinical application of a method for the simultaneous quantification of lamivudine, emtricitabine and tenofovir in dried blood and dried breast milk spots using LC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 1060:300–307. 10.1016/j.jchromb.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouraud A, Millaret A, Bernard N, Bruel M, Paret N, Descotes J, Vial T. 2012. Tenofovir exposure through breast feeding: serum concentrations in neonates and clinical follow-up. Fundam Clin Pharmacol 26:9. [Google Scholar]
- 15.Pan CQ, Mi LJ, Bunchorntavakul C, Karsdon J, Huang WM, Singhvi G, Ghany MG, Reddy KR. 2012. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Dig Dis Sci 57:2423–2429. 10.1007/s10620-012-2187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganne-Carrie N, Causse X, Zarski JPH, Riachi G, Roche B, Zoulim F, Desmorat H, Constant T, Fouchard-Hubert I, Cadranel JFD, Ouzan D, Libert OP, Terrier M, Stern C, Marcellin P. 2013. Efficacy and safety results of tenofovir DF (TDF) treatment from the first trimester in HBV pregnant women in real-life clinical practice, abstr 664A–665A. AASLD LiverLearning 2013.
- 17.Mirochnick M, Taha T, Kreitchmann R, Nielsen-Saines K, Kumwenda N, Joao E, Pinto J, Santos B, Parsons T, Kearney B, Emel L, Herron C, Richardson P, Hudelson SE, Eshleman SH, George K, Fowler MG, Sato P, Mofenson L, HPTN 057 Protocol Team. 2014. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr 65:33–41. 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palombi L, Pirillo MF, Marchei E, Jere H, Sagno JB, Luhanga R, Floridia M, Andreotti M, Galluzzo CM, Pichini S, Mwenda R, Mancinelli S, Marazzi MC, Vella S, Liotta G, Giuliano M. 2016. Concentrations of tenofovir, lamivudine and efavirenz in mothers and children enrolled under the Option B-Plus approach in Malawi. J Antimicrob Chemother 71:1027–1030. 10.1093/jac/dkv435. [DOI] [PubMed] [Google Scholar]
- 19.Mugwanya KK, Hendrix CW, Mugo NR, Marzinke M, Katabira ET, Ngure K, Semiyaga NB, John-Stewart G, Muwonge TR, Muthuri G, Stergachis A, Celum CL, Baeten JM. 2016. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: a prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med 13:e1002132. 10.1371/journal.pmed.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waitt C, Olagunju A, Nakalema S, Kyohaire I, Owen A, Lamorde M, Khoo S. 2018. Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. J Antimicrob Chemother 73:1013–1019. 10.1093/jac/dkx507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Library of Medicine (US). 2006. Drugs and Lactation Database (LactMed). Tenofovir. https://www.ncbi.nlm.nih.gov/books/NBK501549/. Accessed 17 March 2021.
- 22.U.S. Food and Drug Administration. 2001. Guidance for Industry. Bioanalytical method validation. U.S. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 23.European Medicines Agency. 2011. Guideline on bioanalytical method validation. European Medicines Agency, Amsterdam, Netherlands. [Google Scholar]