ABSTRACT
From January 2019 to April 2020, 32 KPC-producing, ceftazidime-avibactam (CZA)-resistant Klebsiella pneumoniae strains were isolated in a university hospital in Rome, Italy. These strains belonged to the sequence type 512 (ST512), ST101, and ST307 high-risk clones. Nine different CZA-resistant KPC-3 protein variants were identified, five of them never previously reported (KPC-66 to KPC-70). Among the nine, KPC-31, KPC-39, KPC-49, KPC-66, KP-68, KPC-69, and KPC-70 showed amino acid substitutions, insertions, and deletions in the Ω loop of the protein. KPC-29 has a duplication, while the novel KPC-67 has a triplication, of the KDD triplet in the 270-loop, a secondary loop of the KPC-3 protein. Genomics performed on contemporary resistant and susceptible clones underlined that these novel mutations emerged in blaKPC-3 genes located on conserved plasmids: in ST512, all blaKPC-3 mutant genes were located in pKpQIL plasmids, while the three novel blaKPC-3 mutants identified in ST101 were on FIIk-FIA(HI1)-R plasmids. Selection also promoted multiplication of the carbapenemase gene copy number by transposition, recombination, and fusion of resident plasmids. When expressed in Escherichia coli recipient cells cloned in the high-copy-number pTOPO vector, the Ω loop mutated variants showed the CZA-resistant phenotype associated with susceptibility to carbapenems, while KPC variants with insertions in the 270-loop showed residual activity on carbapenems. The investigation of CZA resistance mechanisms offered the unique opportunity to study vertical, horizontal, and oblique evolutionary trajectories of K. pneumoniae high-risk clones.
KEYWORDS: Klebsiella pneumoniae, antimicrobial resistance, carbapenemase
INTRODUCTION
Multiple-drug-resistant (MDR) Klebsiella pneumoniae strains emerged globally as an important cause of health care-associated infections in the last decade, and carbapenems, once used as a first-line therapeutic choice, are losing their edge since the emergence of carbapenem-resistant strains (1–5). Epidemiological studies have reported the wide spread of eight major MDR clones: clonal group 15 (CG15), CG20, CG29, CG37, CG147, CG101, CG258, and CG307 (6). Among them, CG258, CG307, CG147, and CG101 were reported as the most common clones causing health care-associated infections in Italy (7, 8). Klebsiella pneumoniae carbapenemase (KPC), encoded by the blaKPC-2 and blaKPC-3 genes, has been described as the most common carbapenemase encountered in K. pneumoniae in Europe (9, 10). Both genes have been mobilized among a limited number of plasmids, pKpQIL being the most common in CG258 (10–13). pKpQIL plasmids usually carry the blaKPC gene located in the Tn4401 transposon, the mer operon, conferring resistance to mercuric ions, and the Tn3 transposon carrying the blaTEM-1 gene (14, 15). The pKpQIL plasmids coevolved in CG258 along with the pKPN and IncX3 plasmids (16). The pKPN plasmids contribute to multidrug resistance, being often associated with the blaCTX-M-15 extended-spectrum beta-lactamase (ESBL) gene and also with ars, pco, and sil clusters encoding resistance to arsenic, copper, and silver, respectively (17). IncX3 plasmids, often coresident with pKpQIL and pKPN in CG258, contribute to cephalosporin resistance by association with the blaSHV-12 ESBL type genes (18).
The emergence of carbapenem resistance complicated the treatment of infections sustained by K. pneumoniae, and second-line antibiotics such as colistin (19, 20), tigecycline (21, 22), aminoglycosides (23, 24), and fosfomycin (25, 26) have been introduced in therapy. In 2016, the European Medicines Agency authorized clinical use of the combination of two substances, ceftazidime and avibactam (https://www.ema.europa.eu/en/medicines/human/EPAR/zavicefta). Ceftazidime is a well-known and characterized third-generation cephalosporin, and avibactam is a new beta-lactamase inhibitor that prevents class A (including KPC), class C, and some class D beta-lactamases from hydrolyzing ceftazidime and restores the activity of this drug in KPC- and OXA-48-producing Enterobacterales but not in metallo-beta-lactamase producers (27–29).
In March 2018, CZA therapy was approved by the Italian authorities (https://www.gazzettaufficiale.it/eli/gu/2018/01/20/16/sg/pdf) and was introduced in Italian hospitals for the treatment of complicated intra-abdominal and urinary tract infections, nosocomial pneumonia, and sepsis sustained by carbapenemase-resistant Enterobacterales. Since CZA usage began, several studies have reported the sporadic occurrence of CZA-resistant strains carrying KPC protein variants. Among them, KPCs with the D179Y amino acid substitution located in the Ω loop of the protein have been the most frequently reported KPC-2 and KPC-3 variants worldwide (30–34). The D179Y substitution in KPC-2 and KPC-3 confers a CZA-resistant phenotype associated with susceptibility or decreased resistance to carbapenems (35).
Here, we report the genetic and phenotypic analysis of 32 CZA-resistant K. pneumoniae strains isolated in a university hospital in Rome, Italy. Multiple evolutionary trajectories of CZA-resistant high-risk clones were observed. Nine different CZA-resistant blaKPC-3 variants were identified in these strains, together with plasmid recombination and multiplication of the blaKPC gene number by transposition.
RESULTS
Ceftazidime-avibactam resistance surveillance.
From January 2019 to April 2020, 3,290 KPC-producing K. pneumoniae strains (blaKPC identified by GeneXpert, Cepheid, CA, USA) were isolated at the Laboratory of Clinical Microbiology of the University Hospital Policlinico Umberto I of Rome, Italy, from 1,316 patients. All strains were tested for ceftazidime (CAZ), cefoxitin (FOX), imipenem (IMI), and meropenem (MEM) MICs by microdilution systems, while CZA MICs were tested using the CZA gradient test (Liofilchem, Roseto degli Abruzzi, Italy). Of the 3,290 strains, 32 blaKPC-positive strains, isolated from 30 patients, showed increased MICs for CZA from an average of 0.25/4 to 2/4 (ceftazidime-avibactam) mg/liter observed in the majority of KPC-producing K. pneumoniae strains to a MIC of ≥8/4 (ceftazidime-avibactam) mg/liter (Table 1). Nineteen of these strains were isolated from patients in intensive care units (ICU), 7 from medical wards (MED), 4 from hematology wards (HEM), and 2 from surgical units (SU).
TABLE 1.
KPC-producing Klebsiella pneumoniae strains analyzed in this study
| Patienta | Strain | Wardb | ST | KPC variant | KPC amino acid changec | Isolation date (mo-day-yr) | Sourced | CZA treatede | MIC (mg/liter) |
WGSf | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CZA | MEM | ||||||||||
| P2 | 2 | ICU | 512 | KPC-67 | D272insKDDKDD | 01-01-19 | BC | Yes | 48 | 16 | Yes |
| P4 | 4 | ICU | 512 | KPC-49 | R164S | 03-04-19 | BC | Yes | 12 | 8 | Yesg |
| P5 | 5 | ICU | 512 | KPC-66 | L167delEL | 03-11-19 | BC | Yes | 12 | 8 | Yes |
| P6 | 6 | ICU | 512 | KPC-29 | D272insKDD | 04-08-19 | BC | Yes | 24 | 16 | Yesg |
| P7 | 7 | ICU | 512 | KPC-3 | None | 05-02-19 | BC | No | 12 | >32 | Yesg |
| P8 | 8 | ICU | 512 | KPC-67 | D272insKDDKDD | 05-15-19 | BC | No | 24 | 16 | |
| P9 | 9 | ICU | 512 | KPC-31 | D179Y | 06-21-19 | BC | Yes | 32 | 2 | Yes |
| P10 | 10 | ICU | 101 | KPC-68 | S182insSS | 07-20-19 | BC | Yes | 32 | 2 | Yes |
| P11 | 11 | ICU | 512 | KPC-67 | D272insKDDKDD | 07-23-19 | BC | Yes | 16 | 8 | |
| P12 | 12 | MED | 101 | KPC-3 | None | 07-26-19 | RTS | No | 8 | >16 | |
| P13 | 13 | ICU | 512 | KPC-67 | D272insKDDKDD | 08-06-19 | UR | No | 32 | 16 | |
| P14 | 14 | MED | 307 | KPC-31 | D179Y | 08-07-19 | RS | No | 12 | <0.12 | |
| P16 | 16 | ICU | 101 | KPC-39 | A172T | 09-02-19 | BC | Yes | 24 | 16 | Yesg |
| P17 | 17 | MED | 307 | KPC-31 | D179Y | 10-10-19 | BC | No | 12 | <0.12 | Yes |
| P18 | 18 | SU | 512 | KPC-67 | D272insKDDKDD | 10-16-19 | UR | No | 24 | 8 | |
| P19 | 19 | SU | 101 | KPC-66 | L167delEL | 10-17-19 | AF | No | 12 | 2 | Yesg |
| P20 | 20 | ICU | 512 | KPC-67 | D272insKDDKDD | 10-28-19 | BC | No | 12 | 8 | Yesg |
| P21 | 21 | MED | 101 | KPC-31 | D179Y | 11-15-19 | UR | No | >256 | >16 | Yesg |
| P22 | 22 | ICU | 101 | KPC-31 | D179Y | 11-29-19 | RS | No | 24 | 1 | |
| P23 | 23 | HEM | 307 | KPC-31 | D179Y | 12-03-19 | RS | No | 12 | <0.12 | Yesg |
| P24 | 24 | HEM | 512 | KPC-66 | L167delEL | 12-09-19 | RS | No | 12 | <0.12 | Yesg |
| P26 | 26 | HEM | 307 | KPC-31 | D179Y | 12-10-19 | RS | No | 12 | <0.12 | |
| P27 | 27 | ICU | 512 | KPC-67 | D272insKDDKDD | 12-13-19 | UR | Yes | 24 | 8 | |
| P28 | 28 | MED | 101 | KPC-31 | D179Y | 12-17-19 | AF | Yes | 24 | 1 | |
| P29 | 29 | ICU | 512 | KPC-67 | D272insKDDKDD | 12-30-19 | RS | No | 24 | 16 | |
| P30 | 30 | HEM | 307 | KPC-31 | D179Y | 01-13-20 | UR | No | 12 | <0.12 | |
| P31 | 31 | ICU | 101 | KPC-68 | S182insSS | 01-25-20 | BC | Yes | 32 | 1 | Yesg |
| P20 | 20-2 | ICU | 512 | KPC-67 | D272insKDDKDD | 02-10-20 | UR | No | 24 | 32 | Yesg |
| P32 | 32 | MED | 512 | KPC-70 | D179Y; T268A | 02-28-20 | RTS | Yes | >256 | 2 | Yesg |
| P34 | 34 | MED | 111 | KPC-69 | W165insGL | 03-10-20 | RS | Yes* | 8 | <0.12 | Yesg |
| P24 | 24-2 | ICU | 512 | KPC-67 | D272insKDDKDD | 04-06-20 | WS | No | 16 | 16 | Yesg |
| P35 | 35 | ICU | 101 | KPC-31 | D179Y | 04-17-20 | RS | Yes* | 16 | 4 | |
| P1 | 1 | ICU | 512 | KPC-3 | None | 12-03-18 | BC | No | 1 | >32 | REF |
| P3 | 3 | ICU | 307 | KPC-3 | None | 02-18-19 | BC | No | 0.75 | 16 | REF |
| P15 | 15 | ICU | 512 | KPC-3 | None | 08-17-19 | BC | No | 0.25 | >32 | REFg |
| P25 | 25 | HEM | 512 | KPC-3 | None | 10-03-19 | BC | No | 1.5 | >32 | REF |
| P33 | 33 | HEM | 101 | KPC-3 | None | 03-05-20 | BC | No | 2 | >32 | REF |
Strains from patients P1, P3, P15, P24, and P32 were CZA-susceptible, carbapenem-resistant strains collected during the surveillance period for genomic comparison. Strains 20-2 and 24-2 were collected from patients P20 and P24, respectively.
ICU, intensive care unit; MED, medical clinic; HEM, hematology ward; SU, surgical unit.
Amino acid changes detected in comparison with the reference KPC-3 protein (WP_004152396.1). ins, insertion; del, deletion.
BC, blood culture; UR, urine; RS, rectal swab; WS, wound swab; RTS, respiratory tract sample; AF, ascitic fluid.
CZA treatment prior to CZA-resistant K. pneumoniae strain isolation. An asterisk indicates a patient colonized by a CZA-resistant strain who was previously treated with CZA for bloodstream infection sustained by a CZA-susceptible strain.
WGS, whole-genome sequencing; REF, reference strain.
Strain analyzed by long-read sequencing (MinIon, Nanopore Oxford Technologies). Strains 21 and 23 were sequenced by MinIon only.
Fourteen patients positive for KPC-producing K. pneumoniae had undergone treatment with CZA prior to the isolation of a CZA-resistant strain. Nine of the CZA-resistant strains from treated patients were isolated from blood cultures, one from urine samples, and two each from respiratory tract and ascitic fluid samples. Two strains were from rectal swabs of patients who previously had bloodstream infections sustained by CZA-susceptible strains.
Sixteen patients were not previously treated with CZA: in 6 patients CZA-resistant strains were isolated from rectal swabs, in 8 from urine, wound swabs, and respiratory tract samples, and in 4 from blood cultures (P20 and P24 patients had positive isolation of CZA-resistant strains from different sources) (Table 1).
Multiplicity of ceftazidime-avibactam-resistant KPC-3 variants.
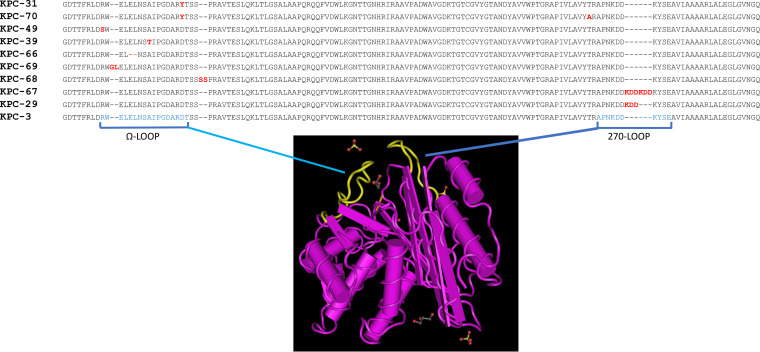
DNA sequencing of the blaKPC genes was obtained for the 32 strains showing resistance or reduced susceptibility to CZA. Nine different KPC-3 protein variants were identified: four previously described, namely, KPC-29 (WP_096807439), KPC-31 (WP_073668892), KPC-39 (WP_128268237), and KPC-49 (WP_197749402) (36), and five never previously reported, assigned by GenBank as KPC-66 to KPC-70 (Fig. 1). KPC-31, KPC-39, and KPC-49 variants showed D179Y, A172T, and R164S single amino acid substitutions in the region of the Ω loop of the protein, respectively. KPC-70 showed the D179Y T268A double substitution. KPC-68 showed the duplication of the SS amino acid residues at position 182, KPC-69 the insertion of GL amino acids at position 165, and KPC-66 a deletion of the EL residues at position 167. The KPC-29 and KPC-67 variants showed a wild-type Ω loop but carried the duplication and triplication of the amino acid 272-to-275 KDD triplet of the protein, respectively (Fig. 1). The KDD triplet maps in a secondary loop of the KPC-3 protein, named the “270-loop” (37, 38) (Fig. 1).
FIG 1.
KPC-3 variants identified in the ceftazidime-resistant Klebsiella pneumoniae strains. Protein sequence alignment of amino acid residue positions 156 to 298 of KPC-3 variants is shown with respect to the KPC-3 reference protein sequence (NCBI reference sequence WP_004152396.1 [59]). Mutation mapping was performed on the MMDB 6QWD crystal structure of KPC-3 downloaded at the NCBI Cn3D macromolecular structure database visualized by the Cn3D 4.3.1 viewer tool (60) at the Cn3D home page (https://www.ncbi.nlm.nih.gov). Amino acid residues involved in the two loops highlighted in yellow in the KPC-3 three-dimensional model are indicated in blue letters. Amino acid substitutions, insertions, and deletions in ceftazidime-avibactam-resistant KPC variants are indicated in red letters.
Ceftazidime-avibactam-resistant KPC-3 variants in K. pneumoniae high-risk clones.
To study the genetic environment and characteristics of K. pneumoniae strains carrying the different KPC variants, whole-genome sequencing (WGS) was performed on 19 selected CZA-resistant and 5 CZA-susceptible K. pneumoniae isolates (Table 1; Fig. 2). Selected CZA-resistant strains were 6/7 strains isolated in the first surveillance period (January to June 2019), 8/18 in the second surveillance period (July to December 2019), and 5/7 in the last period of surveillance (January to April 2020).
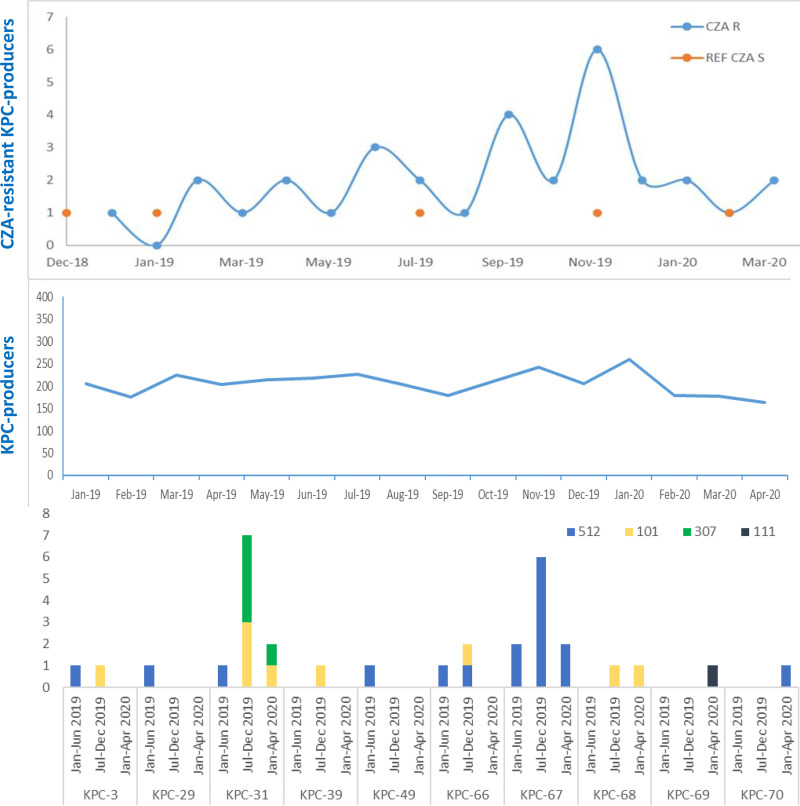
FIG 2.
Epidemiological analysis of isolates along the time of surveillance and distribution of the ceftazidime-avibactam-resistant KPC variants per Klebsiella pneumoniae sequence type. The blue curves represent the number of isolates per month of KPC producers and CZA-resistant KPC producers (blue line with blue dots), respectively, during the January 2019 to April 2020 period. Orange dots represent the CZA-susceptible strains collected during the surveillance and used as controls for genomic comparison. Bars represent the distribution of the variants per ST in the three surveillance periods: January to June 2019, July to December 2019, and January 2020 to April 2020.
In silico multilocus sequence typing (MLST) (39, 40) identified ST512, ST101, ST307, and ST111 among the sequenced strains.
PCR-based assays were used to assign STs in the strains that were not subjected to WGS. Overall, 17/32 (53%) strains were assigned to ST512, 9/32 (29%) to ST101, 5/32 (15%) to ST307, and 1/32 (3%) to ST111 (Table 1).
The distribution of the STs along the study period showed that in the first surveillance period, seven ST512 CZA-resistant strains were isolated from seven patients with bloodstream infections, all from the ICU. In these strains, five different CZA-resistant variants were identified: KPC-29, KPC-31, KPC-49, KPC-66, and KPC-67. Strain 7 produced KPC-3 (Table 1; Fig. 2).
In the second surveillance period, surveillance was not limited to patients in the ICU but was extended to patients hospitalized in medical, surgical, and hematology wards. Eighteen CZA-resistant strains were isolated. KPC-31, KPC-66, KPC-67, KPC-39, and the new KPC-68 variant were identified in ST512, ST101, and ST307 clones.
In the third period of surveillance, seven CZA-resistant strains were isolated, including ST111 carrying the novel KPC-69 variant and one strain of ST512 presenting the novel KPC-70 variant. One patient (P24) colonized by a KPC-66-producing ST512 strain in December 2019 had a wound infection sustained by a KPC-67 ST512 strain in April 2020.
Surveillance was interrupted in April 2020 because the hospital completely rearranged the ICUs to handle the emergence of COVID-19.
Overall, six different CZA-resistant KPC variants were identified in ST512 strains and four were identified in ST101, while only KPC-31 was found in ST307. KPC-31 and KPC-66 were detected in at least two different STs, while KPC-67 was exclusive to ST512 and KPC-39 and KPC-68 were exclusive to ST101 (Fig. 2).
Vertical evolution to ceftazidime-avibactam resistance in Klebsiella pneumoniae ST512 and ST101.
To better define the clonality of CZA-resistant strains that emerged in the hospital, 14 ST512 (11/17 CZA-resistant isolates in the study and 3 CZA-susceptible KPC-3-producing isolates collected in the same period) and 4 ST101 (3/9 CZA-resistant, 1 susceptible) isolates sequenced in this study were compared with 4 and 10 genomes of epidemiologically unrelated ST512 and ST101 strains, respectively. To correlate contemporary CZA-resistant ST512 isolates with historical ST512 strains circulating in the same hospital 10 years ago, three genomes of the KP-1, KP-2, and KP-3 KPC-3-producing K. pneumoniae strains were also included for comparison (41).
Phylogenetic analysis showed that all but one (strain 9) of the CZA-resistant ST512 strains derived from a common ancestor (1 to 41 single-nucleotide polymorphisms [SNPs] in the 4,535 genes of the core genome calculated by the Genealogies Unbiased By recomBinations In Nucleotide Sequences [Gubbins] algorithm) (42) (Fig. 3A; see Table S4 in the supplemental material), which was discerned from unrelated strains circulating in other Italian hospitals and also from those isolated in the same hospital in 2012 (41, 43, 44).
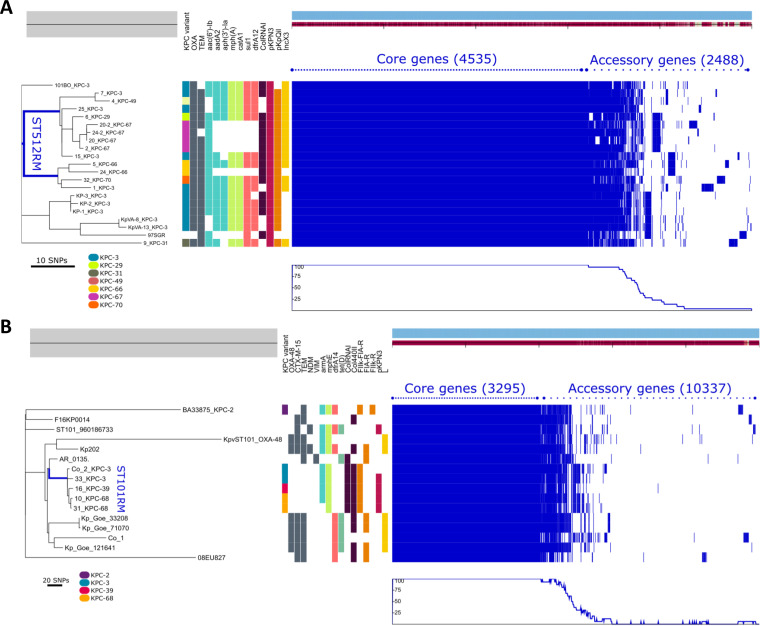
FIG 3.
Phylogenetic analysis of ST512 (A) and ST101 (B) K. pneumoniae lineages. The left side shows the unrooted maximum likelihood phylogenetic trees based on a concatenated core gene alignment. The middle represents metadata, each block indicating the presence or absence of a specific resistance gene and of the plasmid replicon (some of which are not shown for the sake of clarity). A legend shows the colors assigned to each KPC variant in the respective column. The right side shows a gene possession matrix, with each row representing the respective strain gene content. Each of the blue blocks on the right represents the presence of a gene. (A) Phylogenetic comparison of ST512 genomes performed using 10 CZA-resistant and 3 CZA-susceptible strains, isolated in the same surveillance period in the hospital and on 7 genomes of epidemiologically unrelated ST512 strains. Among them, four genomes were downloaded from the collection of ST512 strains at the Klebsiella Pasteur MLST isolate database (https://bigsdb.web.pasteur.fr/klebsiella), and three were KPC-3 ST512 producers isolated at the University Hospital Policlinico Umberto I of Rome in 2012 (KP-1, KP-2, KP-3). The blue line localizes the branches of the tree generated by genomes of the CZA-resistant strains under study (except strain 9 producing KPC-31, which was located in a different branch). (B) Phylogenesis of ST101 strains performed on three CZA-resistant strains (strains 16, 10, and 31) and one CZA-susceptible strain (strain 33) isolated during the surveillance period and for comparison on 11 ST101 genomes downloaded from the NCBI GenBank, including two ST101 strains isolated from patients hospitalized in the ICU ward dedicated to COVID-19 patients and identified in our hospital in April 2020 (strain Co_1, producing OXA-48, and strain Co_2, producing KPC-3).
ST512 strains were separated into two clades. CZA-resistant ST512 strains carrying the KPC-29, KPC-67, and KPC-49 variants were related to each other (1 to 10 SNPs) and to a subset of CZA-susceptible ST512 strains (9 to 13 SNPs; strains 15 and 25) producing KPC-3 and circulating in the hospital in the same period.
In the second ST512 clade, strains with KPC-66 and KPC-70 variants were related to the KPC-3 producer isolated in the hospital in December 2018 (13 to 20 SNPs; strain 1).
The unique ST512 strain producing KPC-31 (strain 9) was unrelated to the other CZA strains and was located on a different branch of the phylogeny tree (42 to 59 SNPs).
Phylogenesis of ST101 showed that CZA-resistant KPC variants arose in a single, endemic ST101 strain circulating in the hospital. In fact, KPC-39 (strain 16) and KPC-68 (strains 10 and 31) producers were highly related to CZA-susceptible ST101 (strains Co-2 and 33) isolated in the same surveillance period (4 to 14 SNPs in the 3,295 genes of the core genome) (Fig. 3B).
Overall, phylogenetic study demonstrated that the multiplicity of KPC variants emerged by vertical evolution of CZA-susceptible, KPC-3-producing high-risk clones contemporarily circulating in the hospital.
Horizontal and oblique evolutionary trajectories of plasmids.
Plasmid analysis was performed to attribute the different blaKPC mutated genes to plasmids. Overall, there was no significant plasmid-mediated transmission of KPC variants among unrelated strains, even when the same KPC variant was identified (i.e., KPC-66 in ST512 and ST101). (i) In ST512 strains, blaKPC-29, blaKPC-49, blaKPC-66, blaKPC-67, and blaKPC-70 genes were located in almost indistinguishable pKpQIL plasmid scaffolds. The blaKPC-69 gene was also located in pKpQIL detected in the ST111 strain (Fig. 4A). (ii) In ST101 strains, blaKPC-39, blaKPC-66, and blaKPC-68 genes were located on a plasmid characterized by the presence of FIA(HI1) and R replicons (Fig. 4B). It carried a region of the pKpQIL plasmid that included the blaKPC transposon. This plasmid also carried the armA gene encoding the 16S rRNA methylase, conferring resistance to all aminoglycosides, and the macrolide resistance mphE gene and was identified only in ST101 strains (Table S1). (iii) In ST307 strains, blaKPC-31 was located on the pKpQIL-pKPN plasmid fusion (studied in strain 23) occurring in the region of the IS15-blaTEM-1-IS26 module of pKpQIL and the IS15-IS26 flanking Tn1721 in the pKPN plasmid. The pKpQIL plasmid was whole in the fusion and identical to the pKpQIL circulating in the other STs, except for the lack of the blaTEM-1a gene. Besides the sil, pco, and ars loci that were common to all pKPN plasmids, the pKPN of this fusion carried a complex pattern of resistance determinants and mobile elements containing the aac(3)-IIa, aph(3″)-Ib, aph(6)-Id, blaCTX-M-15, blaTEM-1B, catB3, dfrA14, qnrB1, sul2, and tet(A) resistance genes, which were not observed in the pKPN plasmids identified in other STs (Fig. 4D; Table S1).
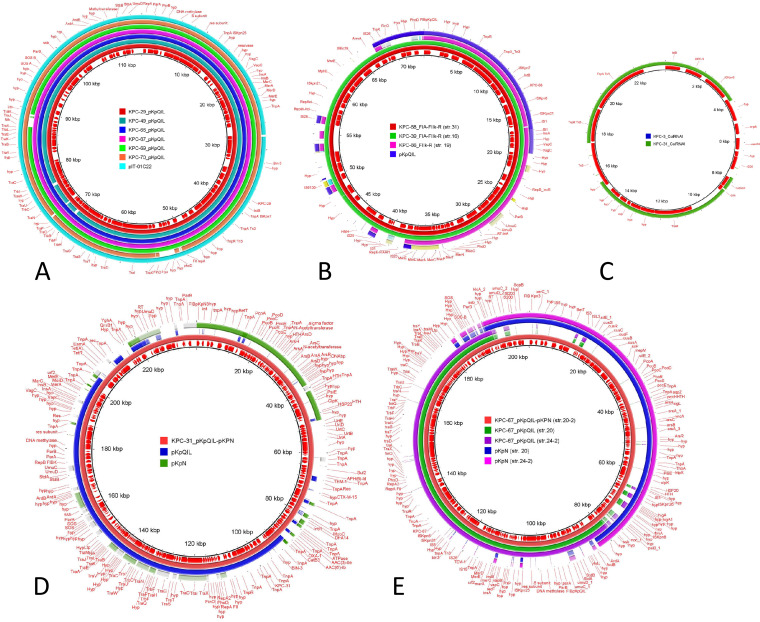
FIG 4.
Visualization by BRIG (BLAST Ring Image Generator) of the major plasmid scaffolds identified in this study. A comparison of plasmid sequences using BRIG v.0.95 is shown. The colored rings represent similarities to the reference sequence. (A) Reference DNA sequence and annotation plasmid pKpQIL carrying blaKPC-29 from strain 6, compared with pKpQIL plasmids carrying blaKPC-49 (strain 4), blaKPC-66.(strain 24), blaKPC-67 (strain 24-2), blaKPC-69 (strain 34), blaKPC-70 (strain 32), and the reference pIT01C22 plasmid (HG969997.1). (B) Reference DNA sequence and annotation plasmid FIA(HI1)-FIIk-R carrying blaKPC-68 from ST101 (strain 31) compared with plasmids carrying blaKPC-39 (strain 16) and blaKPC-66.(strain 19). (C) Reference DNA sequence and annotation of plasmid ColRNAI carrying blaKPC-3 from strain 7 compared with ColRNAI carrying blaKPC-31 (strain 21). (D) Reference DNA sequence and annotation of the plasmid fusion pKpQIL-pKPN carrying blaKPC-31 from ST307 strain 23, compared with plasmid pKpQIL from strain 6 and plasmid pKPN from strain 24-2. (E) Reference DNA sequence and annotation of the plasmid fusion pKpQIL-pKPN carrying blaKPC-67 from ST512 strain 20-2, compared with plasmid pKpQIL (strains 20 and 24-2) and plasmid pKPN (strains 20 and 24-2), respectively. Coding DNA sequences (CDS) are represented by arrows in the inner ring.
Plasmid copy number and amplification of blaKPC copies due to transposition of Tn4401 were evaluated with respect to CZA resistance as follows. (i) Two KPC-67-producing strains were isolated from patient P20 at 6 months apart (strains 20 and 20-2). The most recent (strain 20-2) showed significant increases in CZA (from MICs of 12 to 24 mg/liter) and carbapenem (from IMI MICs of 8 to 32 mg/liter) resistance levels. In this strain, the blaKPC-67 gene was located on a pKpQIL-pKPN fusion, occurring between the ardA and ssb-1 genes. Both pKpQIL and pKPN involved in the fusion were identical to those sequenced in the first isolate (strain 20) of this patient (Fig. 4E). The copy number of pKpQIL in strain 20 was estimated at 0.73 copies per genome. The pKpQIL-pKPN fusion in strain 20-2 showed 1.74 copies per genome, a copy number similar to that of the pKpQIL-pKPN fusion described above for strain 23 in ST307 (Table S1). The increased copy number of the plasmid carrying the blaKPC-67 gene may be correlated with the increased CZA MICs observed in strain 20-2. (ii) Triplication of the blaKPC-31 gene was observed in ST101 strain 21, which showed one of the highest CZA MICs of the collection (CZA MIC of >256 mg/liter); two copies of the gene were located on the FIIk-FIA(HI1)-R plasmid (Fig. 4B), and one copy was in a small, high-copy-number ColRNAI (Fig. 4C; Table S1). Duplication of the blaKPC-66 variant in ST101 strain 19 did not have the same impact, resulting in a CZA MIC of 12 mg/liter. In this case, one copy of the gene was located on the FIIk-R plasmid (Fig. 4B), and one copy was in the chromosome (Table S1).
In ST512 strain 7, multiplication of the blaKPC-3 copies increased the CZA MIC to 8 mg/liter; one copy was in pKpQIL, and one copy was transposed in a small multicopy ColRNAI plasmid like that observed in ST101 strain 21 (Fig. 4C).
Phenotypic evaluation of CZA-resistant KPC variants expressed in Escherichia coli.
Some of the CZA-resistant isolates retained carbapenem susceptibility, with meropenem (MEM) MICs of <8 mg/liter. A subset of isolates demonstrated a combination of high CZA MICs and higher MICs for carbapenems (MIC, ≥8 mg/liter) (Table 1). To investigate phenotypic properties of the CZA-resistant KPC variants, a 1,183-bp amplicon containing the entire blaKPC gene with the promoter, including the region from 289 bp upstream of the ATG start codon to 11 bp downstream of the UAA blaKPC stop codon, was cloned in the pCR-Blunt II-TOPO vector, carrying kanamycin and zeocin resistance as markers for selection of the recombinant clones (Invitrogen).
The blaKPC-3, blaKPC-29, blaKPC-31, blaKPC-66, blaKPC-67, blaKPC-68, blaKPC-69, and blaKPC-70 genes were successfully cloned in the TOPO vector and expressed in TOP10 E. coli recipient cells.
Interestingly, all constructs were tested by a multiplex lateral flow immunoassay (NG-CARBA 5; Biotech, Guipry, France). As previously described, constructs carrying KPC variants with amino acid substitutions, insertions, or deletions occurring in the Ω loop gave negative results (45), while both KPC-29 and KPC-67 strains with KPC variants affecting the 270-loop were confirmed to produce KPC protein and can be identified using this method.
pKpQIL transformants were obtained by transformation of purified plasmid DNAs in chemically competent E. coli DH5α from ST512 strains carrying blaKPC-3 (strain 15), blaKPC-29 (strain 6), blaKPC-67 (strain 24), or blaKPC-70 (strain 32), respectively. Transformants of ColRNAI plasmid carrying blaKPC-3 from strain 7 were also obtained.
All recombinants and transformants carrying the blaKPC-3 gene showed high levels of resistance to carbapenems (MIC > 4 mg/liter) and cefoxitin (MIC > 12 mg/liter). CZA MICs changed with respect to the type of plasmid in which the blaKPC-3 gene was located and proportionally to the expected copy number of the plasmids: the MICs were 1 mg/liter for pTOPO, 0.32 mg/liter for ColRNAI, and 0.125 mg/liter for pKpQIL (Table S2).
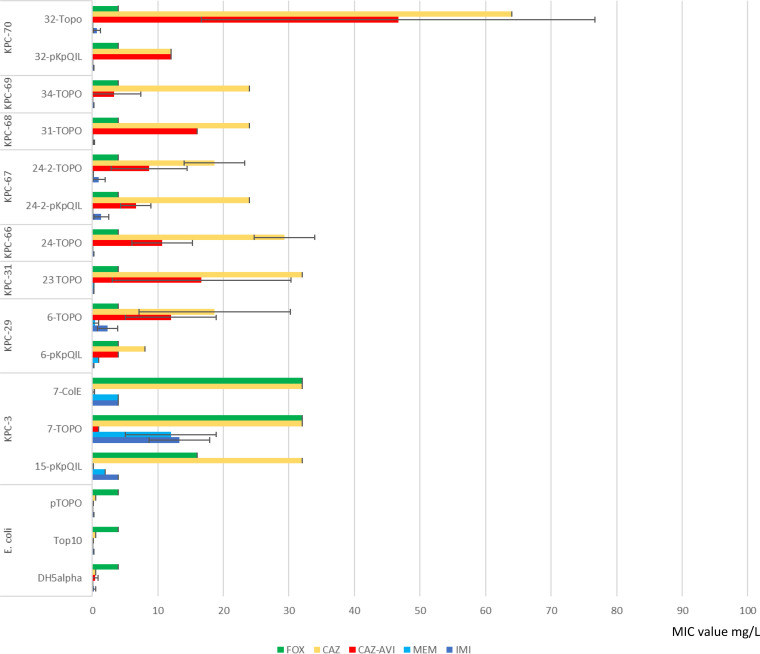
Both KPC-29 and KPC-67 variants that did not harbor Ω loop mutations conferred CZA resistance, which was associated with residual activity on carbapenems when expressed in the high-copy-number pTOPO vector. The other CZA-resistant KPC-31, KPC-66, KPC-68, and KPC-70 variants conferred CZA but not carbapenem resistance (Fig. 5).
FIG 5.
blaKPC gene variant phenotypes expressed in Escherichia coli. MICs were measured by Etest and MicroScan for carbapenems (imipenem [IMI] and meropenem [MEM]; blue bars) and ceftazidime (CAZ; yellow bars), ceftazidime-avibactam (CZA; red bars), and cefoxitin (FOX; green bars) antibiotics. Strains tested were TOP10 Escherichia coli transformed with blaKPC variants cloned in pCR-Blunt II TOPO vector (ThermoFisher). TOP10 cells carrying the empty pTOPO vector were also tested for comparison. Phenotypic tests were also performed on pKpQIL transformants obtained by transformation of E. coli DH5α chemically competent recipient cells, using purified plasmid DNAs from strains 32, 24-2, 6, and 15 and from the multicopy ColRNAI plasmid carrying blaKPC-3 obtained by transformation from strain 7 in DH5α recipient cells.
DISCUSSION
CZA is an important drug; many studies have reported that patients infected by KPC-producing K. pneumoniae and treated with CZA show increased survival compared to patients treated with other antibiotic combinations (46, 47). However, the introduction of the new drug in the hospital produced the selection of beneficial blaKPC mutations in endemic K. pneumoniae high-risk clones, generating the emergence of CZA-resistant strains. All CZA-resistant KPC variants were derived from mutations of the blaKPC-3 gene, the most diffuse carbapenemase gene in these high-risk clones. The first isolation of each of the nine different CZA-resistant KPC variants described in this study occurred in patients undergoing CZA treatment (Table 1), but transmission of CZA-resistant strains to untreated patients was also observed.
Missense, insertion, and deletion mutations occurred in the blaKPC-3 genes located in plasmids. Indistinguishable plasmids characterized each ST, with different plasmid types in the different STs.
It should not be surprising that the use of CZA selected mutations in successful bacterial clones circulating in the hospital. What was surprising was the large number of variants and the vertical evolutionary trajectory of some clones. It was foreseeable that some KPC variants would spread by horizontal transfer of plasmids among different clones, but almost all strains (at least in this initial epidemiological phase) survived to selection by intracellular mutational and recombinational events. Plasmids played a role, not mainly that of conjugation among strains but mostly the promotion of recombination and fusions, whose role in resistance needs to be addressed. Transposition of Tn4401 was also relevant to multiplying the blaKPC gene number and mobilizing the gene on higher-copy-number plasmids, coresident in the same cell or into the chromosome.
Copy number counts for CZA resistance. Many reports described the increase in blaKPC-2 copies per genome, coupled with porin depletion, as the molecular mechanism for the increasing CZA MICs (48, 49) In the United States, a strain reached both CZA and carbapenem resistance by carrying one copy of a CZA-resistant blaKPC-2 gene mutant and one copy of the wild-type carbapenemase blaKPC-2 (50). Duplication of blaKPC-53 copies has been described in Italy by transposition on a phage (51).
The introduction of the new drug offered the unique opportunity to study evolutionary trajectories of K. pneumoniae clones circulating in the hospital under the introduction of a new antibiotic selective pressure. CZA-resistant KPC variants seem to effortlessly emerge in endemic, successful high-risk clones and could pose a threat to public health. Therefore, clinicians should make an effort to use CZA cautiously.
MATERIALS AND METHODS
Bacterial strain isolation and susceptibility testing.
Bacteria were isolated from samples collected for routine microbiologic processes, specifically wound swabs, rectal swabs, urine samples, ascitic fluid, respiratory tract specimens, and blood cultures.
Isolated colonies were identified by a matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) system (Bruker Daltonik GmbH, Bremen, Germany). Antimicrobial susceptibility was determined by the Vitek2 system (bioMérieux) or MicroScan WalkAway system (Beckman Coulter, Inc., Brea, CA, USA). CZA MICs were determined using the CZA gradient test (Liofilchem, Roseto degli Abruzzi, Italy).
Complete DNA sequencing of the blaKPC gene was obtained by amplification using the KPCFW 5′-ATGTCACTGTATCGCCGTCT-3′ and KPCRV 5′-TTTTCAGAGCCTTACTGCCC-3′ primer pair, with annealing at a melting temperature (Tm) of 58°C, and Sanger sequencing of the 892-bp amplicon determined the blaKPC variants.
WGS.
Whole-genome sequencing (WGS) was obtained by use of the Illumina MiSeq instrument (Illumina, Inc., San Diego, CA, USA). Strains listed in Table S1 in the supplemental material were grown overnight at 37°C on LB agar with ampicillin (50 mg/ml). Genomic DNA was purified from a thin layer collected from the LB plate and resuspension in lysis buffer in accordance with the Macherey Nagel (Düren, Germany) DNA extraction kit procedures. Paired-end libraries were generated using the Nextera XT DNA sample preparation kit with the 2×300PE protocol (Illumina, Inc.). De novo assembly of Illumina reads was performed using Galaxy version 3.14.1 of the SPAdes pipeline through the ARIES public Galaxy server (https://w3.iss.it/site/aries/).
Antimicrobial resistance genes and replicons were detected by ResFinder and PlasmidFinder tools, respectively (https://cge.cbs.dtu.dk/services/). Outer membrane protein-encoding genes (ompK35, ompK36) were studied by BLASTN, BLASTP, and ClustalW, using NCBI references OmpK35 (WP_025711611) and OmpK36 (WP_004201406.1) for comparison (Table S4).
Nanopore sequencing was performed on overnight 10-ml LB liquid broth cultures. The bacterial pellet was resuspended in Tris-EDTA (TE) with 2% SDS and 25 mg/ml proteinase K. Protein digestion was performed for 4 h at 55°C, followed by two phenol (pH 8.0) extractions. High-molecular-weight DNA was obtained by precipitation in 1 volume of isopropanol, picking up the DNA cocoon with a loop. After washing with 70% ethyl alcohol (EtOH), DNA was resuspended overnight in water at +4°C. Libraries were prepared using 400 ng of DNA per strain and barcoded by the rapid barcoding sequencing kit (SQK-RBK004). Pooled libraries were cleaned up using AMPure XP beads and loaded into a MinION flow cell (R9.4.1) in accordance with SQK-RBK004 sequencing procedures. Sequencing was performed on an Mk1C MinION platform. The package pomoxis (https://github.com/nanoporetech/pomoxis) was used to assemble bacterial genomes. Complete plasmid assembly was obtained by resolution of short and long reads using the Unicycler tool available at the https://usegalaxy.eu/ server.
Plasmid copy numbers were estimated by the Unicycler assembler (https://usegalaxy.eu/ server V.0.4.8.0). This tool assigns coverage to the complete circular assembled plasmids proportionally to the coverage of the chromosomal complete circular sequence considered to be 1×. This value was obtained only for genomes for which both Illumina short-read sequencing and MinIon long-read sequencing were available. Multiple blaKPC gene copies in the genome and their respective locations were determined by MinIon Nanopore sequencing assembled by the package pomoxis.
Species identification, in silico multilocus sequence typing (MLST), and wzi typing (K-type) were performed using Kleborate (40). BRIG (BLAST Ring Image Generator) software (52) was used to compare plasmid sequences.
PCR-based multilocus sequence typing.
MLST of the strains that were not subjected to WGS was determined by PCR using previously described primer pairs (53, 54): ST258-512 pilV (pilV-FW, 5′-CGATGGCGCTGGCGACGATTAT-3′; pilV-RV, 5′-CCCGATGGGCAAGAACATGCGT-3′) and ST307 tssJ (T6SS_FW, 5′-CAAGTACGCTGACTGCATGT-3′; T6SS_RV, 5′-CGGCACAGTTAGGAAATCAG-3′). New primers were devised on the fimI gene of the fimbrial cluster that was identified as unique in CG101 genomes (FimI_101FW, 5′-TGTCTGCTCTATCTTTAAGCTC-3′; FimI_101RV, 5′-GTACCGGAGAAAGCAACAGAC-3′). Three ST101 strains detected by the new fimI gene PCR were reconfirmed by Sanger sequencing of the seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) according to the Institut Pasteur MLST scheme (https://bigsdb.pasteur.fr/) (39).
Phylogenetic analysis of the ST512 and ST101 clones.
Genome sequences (obtained in this study or downloaded from GenBank and from the database of the Institut Pasteur [https://bigsdb.pasteur.fr/]) were annotated using Prokka (55). The resulting general feature formats (GFFs) were analyzed using Roary v3.11.3 (56) to identify the core and the accessory genes and to obtain a pangenome alignment.
Removal of recombining regions was carried out by the Gubbins algorithm (42) at the public EU galaxy server (https://usegalaxy.eu/), generating a maximum likelihood phylogenetic tree with RAxML using default parameters (57). Visualization of the tree, metadata, and pangenome was performed with Phandango (58).
KPC-plasmid transformation.
Purified plasmid DNA was obtained from overnight 10-ml LB liquid broth cultures of selected K. pneumoniae strains. Plasmid extraction was performed with the PureYield plasmid midiprep system (Promega Italia SrL, Milan, Italy). Plasmid DNAs were transformed in chemically competent Escherichia coli DH5α cells (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA), with selection for transformants on LB agar plates containing ampicillin (10 mg/liter). After 24 h, colonies were screened for blaKPC using the KPCFW and KPCRV primer pair as described above.
CAZ, FOX, IMI, and MEM MICs of KPC-positive E. coli DH5α transformants were tested in triplicate by microdilution (MicroScan system; Beckman Coulter, Inc.). CZA MICs were tested in triplicate by the CZA gradient test (Liofilchem).
blaKPC gene cloning in pCR-Blunt II TOPO vector.
Each blaKPC gene variant was amplified with KPC_PromFW 5′-GATCCAGGTGGGTCAGTATTACT-3′ KPCRV (as above) with AccuPrime Pfx DNA polymerase and ligated into the pCR-Blunt II TOPO vector (ThermoFisher) by following the manufacturer’s instructions. Ligation mixtures were introduced by transformation in chemically competent TOP10 cells (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA). Transformants were screened on LB agar plates containing kanamycin (ImMedia Kan agar; Invitrogen, Thermo Fisher Scientific). Recombinant clones were tested by PCR and Sanger sequencing of the amplicons using KPC_PromFW, KPCFW, and KPCRV primers on purified TOPO-plasmid DNAs (FastGene plasmid mini prep kit; Nippon Genetics Europe, Dueren, Germany) as the templates.
CAZ, FOX, IMI, and MEM MICs of KPC-positive E. coli TOP10 cells carrying pCR-Blunt II TOPO constructs were tested in triplicate by microdilution (MicroScan system; Beckman Coulter, Inc.) CZA MICs were tested in triplicate by the CZA gradient test (Liofilchem).
Ethical approval.
Procedures performed in the study were in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data availability.
Data have been deposited in NCBI GenBank under BioProject accession no. PRJNA648857.
Plasmid nucleotide sequences have been deposited in NCBI GenBank under accession numbers MT809692 (9_pFIA-R), MT809694 (1B_pKpQIL), MT809695 (2B_pKpQIL), MT809697 (17B_pKpQIL), MT809698 (20B_pFIA-R), MT809699 (26B_pKpQIL), MT809700 (40B_pKpQIL), MT809701 (42B_pKpQIL), MT809702 (3B_ColEKPC) MW650887 (27B_pKpQIL_pKPN), MW650888 (8_pKPN), MW650889 (10_pKPN), MW650890 (10_pKpQIL), MW650891 (13_pKPN_pKpQIL), MW650892 (17B_pKpN), MT809688 (3_pKpQIL), MT809693 (18_pKpQIL), MT809689 (19_FIIk-FIA-R), MT809691 (21_pKpQIL), MT809690 (6_pKpQIL), and MT809696 (4_pKpQIL).
ACKNOWLEDGMENTS
We acknowledge the staff of the University Hospital Policlinico Umberto I of Rome, Italy, for excellent technical support.
This work was supported by Sapienza University of Rome Top Scientist 1% Funds 2019 to Alessandra Carattoli and Ateneo Funds 2019 no. RM11916B6A8B5F8D to Giammarco Raponi.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitout JDD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 4.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G, Hellenic Society of Chemotherapy (HSC) and Società Italiana di Terapia Antinfettiva (SITA) . 2018. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 24:133–144. 10.1016/j.cmi.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Wyres KL, Lam MMC, Holt KE. 2020. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol 18:344–359. 10.1038/s41579-019-0315-1. [DOI] [PubMed] [Google Scholar]
- 7.Di Pilato V, Errico G, Monaco M, Giani T, Del Grosso M, Antonelli A, David S, Lindh E, Camilli R, Aanensen DM, Rossolini GM, Pantosti A, AR-ISS Laboratory Study Group on carbapenemase-producing Klebsiella pneumoniae . 2021. The changing epidemiology of carbapenemase-producing Klebsiella pneumoniae in Italy: toward polyclonal evolution with emergence of high-risk lineages. J Antimicrob Chemother 76:355–361. 10.1093/jac/dkaa431. [DOI] [PubMed] [Google Scholar]
- 8.Tavoschi L, Forni S, Porretta A, Righi L, Pieralli F, Menichetti F, Falcone M, Gemignani G, Sani S, Vivani P, Bellandi T, Tacconi D, Turini L, Toccafondi G, Privitera G, Lopalco P, Baggiani A, Gemmi F, Luchini G, Petrillo M, Roti L, Pezzotti P, Pantosti A, Iannazzo S, Mechi MT, Rossolini GM, the Tuscan Clinical Microbiology Laboratory Network . 2020. Prolonged outbreak of New Delhi metallo-beta-lactamase-producing carbapenem-resistant Enterobacterales (NDM-CRE), Tuscany, Italy, 2018 to 2019. Euro Surveill 25:2000085. 10.2807/1560-7917.ES.2020.25.6.2000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, Abudahab K, Goater R, Giani T, Errico G, Aspbury M, Sjunnebo S, Koraqi A, Lacej D, Apfalter P, Hartl R, Glupczynski Y, Huang TD, Strateva T, Marteva-Proevska Y, Andrasevic AT, Butic I, Pieridou-Bagatzouni D, Maikanti-Charalampous P, Hrabak J, Zemlickova H, Hammerum A, Jakobsen L, Ivanova M, Pavelkovich A, Jalava J, Österblad M, Dortet L, Vaux S, Kaase M, Gatermann SG, Vatopoulos A, Tryfinopoulou K, Tóth Á, Jánvári L, Boo TW, McGrath E, Carmeli Y, Adler A, Pantosti A, Monaco M, Raka L, Kurti A, Balode A, Saule M, ESGEM Study Group , et al. 2019. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol 4:1919–1929. 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navon-Venezia S, Kondratyeva K, Carattoli A. 2017. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 41:252–275. 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Chavda KD, Melano RG, Jacobs MR, Koll B, Hong T, Rojtman AD, Levi MH, Bonomo RA, Kreiswirth BN. 2014. Comparative genomic analysis of KPC-encoding pKpQIL-like plasmids and their distribution in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2871–2877. 10.1128/AAC.00120-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papagiannitsis CC, Di Pilato V, Giani T, Giakkoupi P, Riccobono E, Landini G, Miriagou V, Vatopoulos AC, Rossolini GM. 2016. Characterization of KPC-encoding plasmids from two endemic settings, Greece and Italy. J Antimicrob Chemother 71:2824–2830. 10.1093/jac/dkw227. [DOI] [PubMed] [Google Scholar]
- 13.Doumith M, Findlay J, Hirani H, Hopkins KL, Livermore DM, Dodgson A, Woodford N. 2017. Major role of pKpQIL-like plasmids in the early dissemination of KPC-type carbapenemases in the UK. J Antimicrob Chemother 72:2241–2248. 10.1093/jac/dkx141. [DOI] [PubMed] [Google Scholar]
- 14.Cuzon G, Naas T, Nordmann P. 2011. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother 55:5370–5373. 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob Agents Chemother 54:4493–4496. 10.1128/AAC.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David S, Cohen V, Reuter S, Sheppard AE, Giani T, Parkhill J, Rossolini GM, Feil EJ, Grundmann H, Aanensen DM, ESCMID Study Group for Epidemi–ological Markers (ESGEM) . 2020. Integrated chromosomal and plasmid sequence analyses reveal diverse modes of carbapenemase gene spread among Klebsiella pneumoniae. Proc Natl Acad Sci USA 117:25043–25054. 10.1073/pnas.2003407117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyres KL, Hawkey J, Hetland MAK, Fostervold A, Wick RR, Judd LM, Hamidian M, Howden BP, Löhr IH, Holt KE. 2019. Emergence and rapid global dissemination of CTX-M-15-associated Klebsiella pneumoniae strain ST307. J Antimicrob Chemother 74:577–581. 10.1093/jac/dky492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liakopoulos A, Mevius D, Ceccarelli D. 2016. A review of SHV extended-spectrum β-lactamases: neglected yet ubiquitous. Front Microbiol 7:1374. 10.3389/fmicb.2016.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, Network EuSCAPE-Italy c, Grundmann H, Pantosti A, Rossolini GM. 2014. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill 19:14–18. 10.2807/1560-7917.ES2014.19.42.20939. [DOI] [PubMed] [Google Scholar]
- 20.Karaiskos I, Souli M, Galani I, Giamarellou H. 2017. Colistin: still a lifesaver for the 21st century? Expert Opin Drug Metab Toxicol 13:59–71. 10.1080/17425255.2017.1230200. [DOI] [PubMed] [Google Scholar]
- 21.Bassetti M, Poulakou G, Giamarellou H. 2014. Is there a future for tigecycline? Intensive Care Med 40:1039–1045. 10.1007/s00134-014-3343-3. [DOI] [PubMed] [Google Scholar]
- 22.Montravers P, Dupont H, Bedos JP, Bret P, The Tigecycline Group . 2014. Tigecycline use in critically ill patients: a multicentre prospective observational study in the intensive care setting. Intensive Care Med 40:988–997. 10.1007/s00134-014-3323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, Natera C, Rodríguez M, Salcedo I, Rodríguez-López F, Rivero A, Rodríguez-Baño J. 2015. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:905–913. 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 24.Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger I, Rivera-Espinar F, Cano A, Guzmán-Puche J, Pérez-Nadales E, Natera C, Rodrìguez M, Leòn R, Castòn JJ, Rodriguez-Lopez F, Rodríguez-Baño J, Torre-Cisneros J. 2017. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 61:1–11. 10.1128/AAC.00406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. 2014. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 43:52–59. 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Grabein B, Graninger W, Rodríguez Baño J, Dinh A, Liesenfeld DB. 2017. Intravenous fosfomycin—back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect 23:363–372. 10.1016/j.cmi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 27.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirley M. 2018. Ceftazidime-avibactam: a review in the treatment of serious Gram-negative bacterial infections. Drugs 78:675–692. 10.1007/s40265-018-0902-x. [DOI] [PubMed] [Google Scholar]
- 29.Mosley JF, Smith LL, Parke CK, Brown JA, Wilson AL, Gibbs LV. 2016. Ceftazidime-avibactam (Avycaz): for the treatment of complicated intra-abdominal and urinary tract infections. Drug Forecast 41:479–483. [PMC free article] [PubMed] [Google Scholar]
- 30.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaibani P, Re MC, Campoli C, Viale PL, Ambretti S. 2020. Bloodstream infection caused by KPC-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam: epidemiology and genomic characterization. Clin Microbiol Infect 26:516.e1–516.e4. 10.1016/j.cmi.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhang P, Shi Q, Hu H, Hong B, Wu X, Du X, Akova M, Yu Y. 2020. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin Microbiol Infect 26:124.e1–124.e4. 10.1016/j.cmi.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro AB, Moussa SH, Carter NM, Gao N, Miller AA. 2021. Ceftazidime-avibactam resistance mutations V240G, D179Y, and D179Y/T243M in KPC-3 β-lactamase do not alter cefpodoxime-ETX1317 susceptibility. ACS Infect Dis 7:79–87. 10.1021/acsinfecdis.0c00575. [DOI] [PubMed] [Google Scholar]
- 35.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agents Chemother 61:e02534-16. 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández-García M, Sánchez-López J, Martínez-García L, Becerra-Aparicio F, Morosini MI, Ruiz-Garbajosa P, Cantón R. 2021. Emergence of the new KPC-49 variant conferring an ESBL phenotype with resistance to ceftazidime-avibactam in the ST131-H30R1 Escherichia coli high-risk clone. Pathogens 10:67. 10.3390/pathogens10010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobson CA, Bonacorsi S, Jacquier H, Choudhury A, Magnan M, Cointe A, Bercot B, Tenaillon O, Birgy A. 2020. KPC beta-lactamases are permissive to insertions and deletions conferring substrate spectrum modifications and resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01175-20. 10.1128/AAC.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tooke CL, Hinchliffe P, Bonomo RA, Scho CJ, Mulholland AJ, Spencer J. 2021. Natural variants modify Klebsiella pneumoniae carbapenemase (KPC) acyl-enzyme conformational dynamics to extend antibiotic resistance. J Biol Chem 296:100126. 10.1074/jbc.RA120.016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. 2021. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun 12:4188. 10.1038/s41467-021-24448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villa L, Feudi C, Fortini D, Garciá-Fernández A, Carattoli A. 2014. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob Agents Chemother 58:1707–1712. 10.1128/AAC.01803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 43:e15. 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaiarsa S, Comandatore F, Gaibani P, Corbella M, Dalla Valle C, Epis S, Scaltriti E, Carretto E, Farina C, Labonia M, Landini MP, Pongolini S, Sambri V, Bandi C, Marone P, Sassera D. 2015. Genomic epidemiology of Klebsiella pneumoniae in Italy and novel insights into the origin and global evolution of its resistance to carbapenem antibiotics. Antimicrob Agents Chemother 59:389–396. 10.1128/AAC.04224-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onori R, Gaiarsa S, Comandatore F, Pongolini S, Brisse S, Colombo A, Cassani G, Marone P, Grossi P, Minoja G, Bandi C, Sassera D, Toniolo A. 2015. Tracking nosocomial Klebsiella pneumoniae infections and outbreaks by whole-genome analysis: small-scale Italian scenario within a single hospital. J Clin Microbiol 53:2861–2868. 10.1128/JCM.00545-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonelli A, Giani T, Di Pilato V, Riccobono E, Perriello G, Mencacci A, Rossolini GM. 2019. KPC-31 expressed in a ceftazidime/avibactam-resistant Klebsiella pneumoniae is associated with relevant detection issues. J Antimicrob Chemother 74:2464–2466. 10.1093/jac/dkz156. [DOI] [PubMed] [Google Scholar]
- 46.Tsolaki V, Mantzarlis K, Mpakalis A, Malli E, Tsimpoukas F, Tsirogianni A, Papagiannitsis K, Zygoulis P, Papadonta M-E, Petinaki E, Makris D, Zakynthinos E. 2019. Ceftazidime-avibactam to treat life-threatening infections from carbapenem-resistant pathogens in critically ill mechanically ventilated patients. Antimicrob Agents Chemother 10.1128/AAC.02320-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields R, Nguyen M, Chen L, Press E, Potoski B, Marini R, Doy Y. 2017. Ceftazidime-avibactam is superior to other treatment regimens against Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphries RM, Hemarajata P. 2017. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 61:e00537-17. 10.1128/AAC.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coppi M, Di Pilato V, Monaco F, Giani T, Conaldi PG, Rossolini GM. 2020. Ceftazidime-avibactam resistance associated with increased blaKPC-3 gene copy number mediated by pKpQIL plasmid derivatives in sequence type 258 Klebsiella pneumoniae. Antimicrob Agents Chemother 64:e01816-19. 10.1128/AAC.01816-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, Mehta M, Gomez-Simmonds A, Uhlemann AC. 2018. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 62:e02101-17. 10.1128/AAC.02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Pilato V, Aiezza N, Viaggi V, Antonelli A, Principe L, Giani T, Luzzaro F, Rossolini GM. 2021. KPC-53, a KPC-3 variant of clinical origin associated with reduced susceptibility to ceftazidime-avibactam. Antimicrob Agents Chemother 65:e01429-20. 10.1128/AAC.01429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu F, Lv J, Niu S, Tang Y-W, Pitout JDD, Bonomo RA, Kreiswirtha BN, Chen L. 2018. Multiplex PCR analysis for rapid detection of Klebsiella pneumoniae carbapenem-resistant (sequence type 258 [ST258] and ST11) and hypervirulent (ST23, ST65, ST86, and ST375) strains. J Clin Microbiol 56:e00731-18. 10.1128/JCM.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villa L, Feudi C, Fortini D, Brisse S, Passet V, Bonura C, Endimiani A, Mammina C, Ocampo AM, Jimenez JN, Doumith M, Woodford N, Hopkins K, Carattoli A. 2017. Diversity, virulence, and antimicrobial resistance of the KPC-producing Klebsiella pneumoniae ST307 clone. Microb Genom 3:e000110. 10.1099/mgen.0.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seemann T. 2014. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 56.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hadfield J, Croucher NJ, Goater RJ, Abudahab K, Aanensen DM, Harris SR. 2018. Phandango: an interactive viewer for bacterial population genomics. Bioinformatics 34:292–293. 10.1093/bioinformatics/btx610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tooke CL, Hinchliffe P, Lang PA, Schofield CJ. 2019. Molecular basis of class A beta-lactamase inhibition by relebactam. Antimicrob Agents Chemother 63:e00564-19. 10.1128/AAC.00564-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madej T, Lanczycki CJ, Zhang D, Thiessen PA, Geer RC, Marchler-Bauer A, Bryant SH. 2014. MMDB and VAST+ : tracking structural similarities between macromolecular complexes. Nucleic Acids Res 42:D297–D303. 10.1093/nar/gkt1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.00574-21-s0001.pdf, PDF file, 0.3 MB (333.7KB, pdf)
Data Availability Statement
Data have been deposited in NCBI GenBank under BioProject accession no. PRJNA648857.
Plasmid nucleotide sequences have been deposited in NCBI GenBank under accession numbers MT809692 (9_pFIA-R), MT809694 (1B_pKpQIL), MT809695 (2B_pKpQIL), MT809697 (17B_pKpQIL), MT809698 (20B_pFIA-R), MT809699 (26B_pKpQIL), MT809700 (40B_pKpQIL), MT809701 (42B_pKpQIL), MT809702 (3B_ColEKPC) MW650887 (27B_pKpQIL_pKPN), MW650888 (8_pKPN), MW650889 (10_pKPN), MW650890 (10_pKpQIL), MW650891 (13_pKPN_pKpQIL), MW650892 (17B_pKpN), MT809688 (3_pKpQIL), MT809693 (18_pKpQIL), MT809689 (19_FIIk-FIA-R), MT809691 (21_pKpQIL), MT809690 (6_pKpQIL), and MT809696 (4_pKpQIL).