ABSTRACT
Augmented renal clearance (ARC) can cause underexposure to vancomycin, thereby increasing the risk of treatment failure. Our objective was to evaluate population pharmacokinetics and optimize the dosing regimen of vancomycin in a pediatric population with ARC. Sparse pharmacokinetic sampling and therapeutic drug monitoring (TDM) data were collected from pediatric patients with ARC treated with vancomycin. A pharmacokinetic model was developed using NONMEM 7.2. The dosing regimen was optimized using Monte Carlo dose simulations. A total of 242 vancomycin serum concentrations from 113 patients (age range, 0.4 to 14.9 years; 49 females and 64 males) were available. The mean vancomycin dose was 58.8 mg/kg body weight/day (13.6 mg/kg/dose), and the mean vancomycin serum trough concentration was 6.5 mg/liter. A one-compartment pharmacokinetic model with first-order elimination was developed. Body weight and age were the most significant and positive covariates for clearance and volume of distribution. For the pediatric population with ARC, the current recommended vancomycin dose of 60 mg/kg/day was associated with a high risk of underdosing. To reach the target area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC) ratio of 400 to 700 in these pediatric patients, the vancomycin dose should be increased to 75 mg/kg/day for infants and children between 1 month and 12 years of age and 70 mg/kg/day for adolescents between 12 and 18 years of age. In conclusion, a one-compartment pharmacokinetic model with first-order elimination was established with body weight and age as significant covariates. An optimal dosing regimen was developed in pediatric patients with ARC aged 1 month to 18 years.
KEYWORDS: vancomycin, augmented renal clearance, children, pharmacokinetic, dose simulation
TEXT
Vancomycin is a glycopeptide antibiotic with excellent antibacterial activity against Gram-positive bacteria, and it is still the first-line treatment for infections caused by methicillin-resistant Staphylococcus aureus (MRSA) in children (1). Vancomycin is administered by intravenous infusion with a duration of at least 1 h. It is almost entirely eliminated by the renal route.
The Infectious Diseases Society of America (IDSA) guidelines recommend the use of vancomycin at 15 mg/kg body weight/dose every 6 h to treat serious or invasive infectious disease in children, but the recommended doses have not been broken down according to age groups (2). The best index to evaluate the clinical efficacy of vancomycin is the ratio of the concentration-time curve (AUC) to the MIC (AUC/MIC) (3). To achieve the recommended target AUC/MIC of ≥400, the IDSA guidelines recommend that the target vancomycin trough concentrations in adult and pediatric patients should be 15 to 20 mg/liter. However, there is evidence that this recommended dose of 60 mg/kg/day could lead to subtherapeutic trough concentrations in critically ill patients (4–7).
Recently, the concept of augmented renal clearance (ARC) has emerged, which is described as enhanced renal clearing capacity primarily occurring in critically ill patients (8). It has been shown that adult patients with ARC exhibit lower vancomycin concentrations and may have subtherapeutic concentrations with the currently recommended dosing regimen (9, 10). Though a few studies demonstrated that ARC is associated with increased vancomycin clearance in children, the optimal dose of vancomycin has not been established for different age groups of pediatric patients (11). In critically ill children with ARC, inadequate vancomycin treatment doses may lead to increased infection-related morbidity and mortality, necessitating the optimization of the vancomycin dosing regimen. The objective of our study was to develop a population pharmacokinetic model of vancomycin in pediatric patients with ARC in order to clarify the pharmacokinetic characteristics and optimize the dosing regimen for these pediatric patients.
RESULTS
Study population.
A total of 242 vancomycin plasma samples from 113 patients (175 therapeutic drug monitoring [TDM] and 67 sparse pharmacokinetics) were included in this investigation. Among the patients were 40 infants with an age of <2 years, 62 children with age of 2 to <12 years, and 11 adolescents with an age of 12 to 18 years. The median (range) age and bodyweight at the time of study were 4.5 (0.4 to 14.9) years and 15.0 (6.0 to 62.0) kg, respectively. A summary of patient characteristics is presented in Table 1. The concentration range of vancomycin plasma samples was 0.67 to 40.28 mg/liter. The concentration-versus-time profile is shown in Fig. 1.
TABLE 1.
Baseline characteristics of 113 patients
| Characteristica | Value |
||
|---|---|---|---|
| No. | Median | Range | |
| Patients | 113 | ||
| Infants (<2 yrs) | 40 | ||
| Children (2 to <12 yrs) | 62 | ||
| Adolescents (12 to 18 yrs) | 11 | ||
| Samples | 242 | ||
| Sex (no. of male/no. of female) | 64/49 | ||
| Age (yr) | 4.50 | 0.44–14.88 | |
| Current wt (kg) | 15.0 | 6.00–62.00 | |
| Scr (mg/dl) | 0.28 | 0.16–0.56 | |
| eGFR | 199 | 160–332 | |
| BUN (mmol/liter) | 2.90 | 0.71–13.4 | |
| CysC (mg/liter) | 0.75 | 0.52–1.28 | |
| ALB (g/liter) | 35.8 | 21.8–67.3 | |
| AST (U/liter) | 32.0 | 6.10–498 | |
| ALT (U/liter) | 29.1 | 3.50–390 | |
| Primary diseases | |||
| Infection | 62 | ||
| Nonsolid tumor | 41 | ||
| Solid tumor | 10 | ||
| Vancomycin dose (mg/kg/day) | 58.82 | 11.69–133.93 | |
| Vancomycin dose (mg/kg/dose) | 13.64 | 5.00–22.32 | |
Scr, serum creatinine concentration; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; CysC, cystatin C; ALB, serum albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
FIG 1.
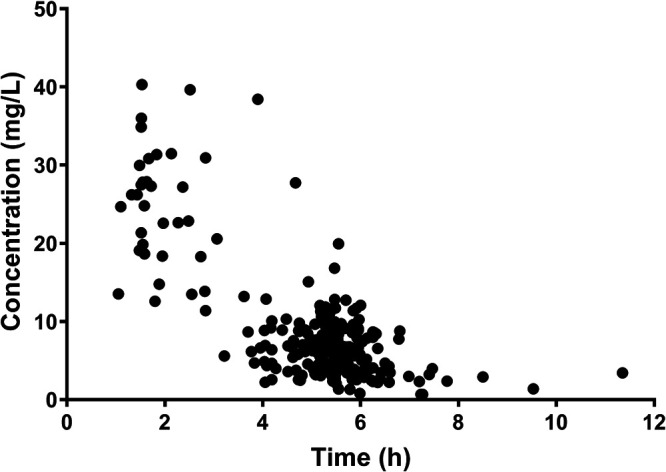
Vancomycin concentration versus time.
Pharmacokinetic model building.
A one-compartment model with first-order elimination provided the best fit of the drug concentration-versus-time data. The model was parameterized in terms of clearance rate (CL) and apparent volume of distribution (V) of vancomycin. Interindividual variability of CL and V and residual variability were best described by an exponential model. The interindividual variabilities of CL and V declined from 55.5% and 63.3% in the base model to 22.6% and 14.7% in the final model, respectively. The residual variability changed slightly from 31.3% in the base model to 31.8% in the final model.
In the forward selection process, a priori, the body weight was incorporated into the base model, which caused a significant drop in the objective function value (OFV) of 150.295 points. Age caused an additional significant drop in OFV of 13.497 points. Although estimated glomerular filtration rate (eGFR) was added to the model in the forward selection process of covariates, it was eliminated in the backward selection process. None of other tested covariates caused an additional significant decline in the OFV. The specific covariate analysis results are shown in Table 2.
TABLE 2.
Covariate analysis results
| Selection process | Covariatea | Pharmacokinetic parameter(s)b |
OFVc | ΔOFVd | Interindividual variability (%) |
|---|---|---|---|---|---|
| Structural model | 959.157 | 55.5 | |||
| Step 1: Allometric model of wt | CL, V | 808.862 | 150.295e | 27.5 | |
| Age | CL | 795.365 | 13.497f | 22.6 | |
| Sex | 808.843 | 0.019f | 27.5 | ||
| Step 2: Forward selection (in base of allometric model of wt) | Age | V | 809.343 | 0.481f | |
| Sex | 808.852 | 0.01f | |||
| eGFR | CL | 801.794 | 7.068f | 25.8 | |
| Scr | 808.512 | 0.35f | 27.3 | ||
| BUN | 806.810 | 2.052f | 27.3 | ||
| CysC | 805.736 | 3.126f | 27.4 | ||
| Full model | Wt | CL, V | 791.732 | 22.3 | |
| Age | CL | ||||
| eGFR | CL | ||||
| Step 3: Backward selection | eGFR | CL | 795.364 | 3.632g | 22.6 |
| Final model | Wt | CL, V | 795.364 | 22.6 | |
| Age | CL |
eGFR, estimated glomerular filtration rate; Scr, serum creatinine concentration; BUN, blood urea nitrogen; CysC, cystatin C.
CL, clearance; V, volume of distribution.
OFV, objective function value.
ΔOFV, variation of objective function value.
ΔOFV of the allometric model of weight and the structural model.
ΔOFV of allometric model in forward selection process and the allometric model of weight.
ΔOFV of allometric model in backward selection process and the full model.
The median (range) values of estimated weight-normalized CL and V at steady state were 0.14 (0.07 to 0.24) liters/h/kg body weight and 0.45 (0.37 to 0.48) liters/kg body weight, respectively. Figure 2 indicates the weight-normalized CL of different age groups. The weight-normalized CL declined with age.
FIG 2.
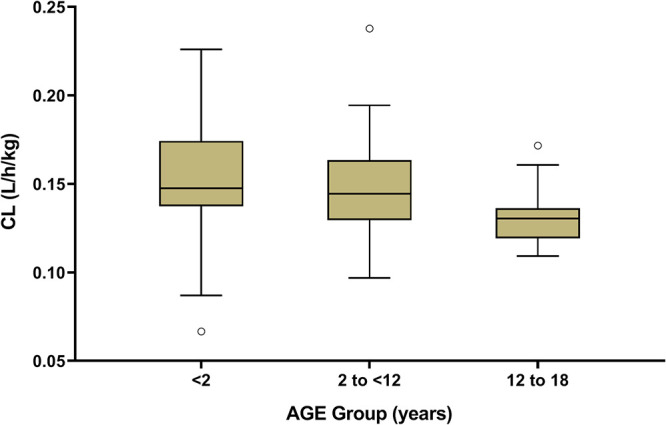
Effect of age on vancomycin clearance.
Model diagnosis.
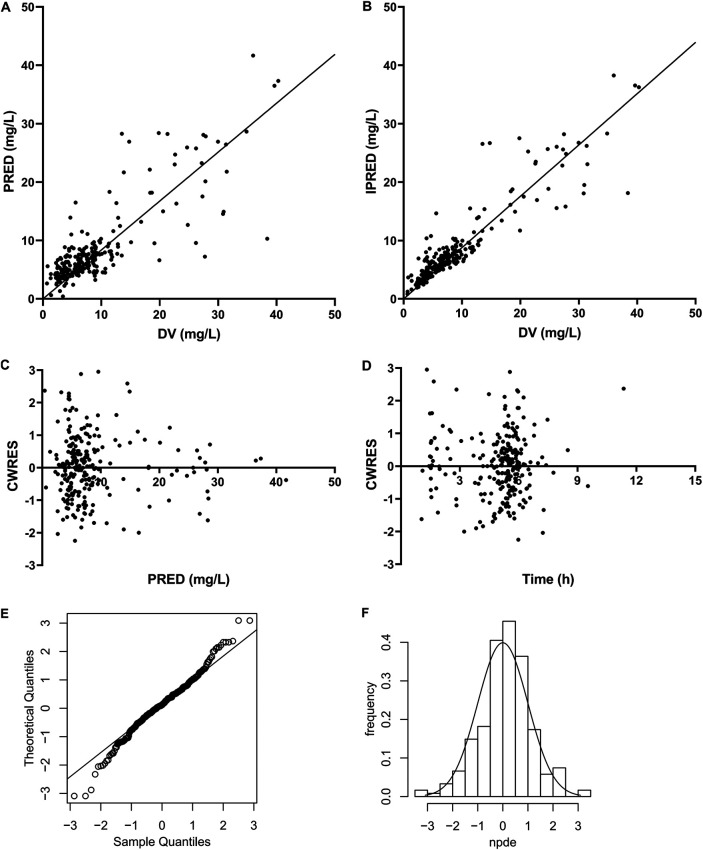
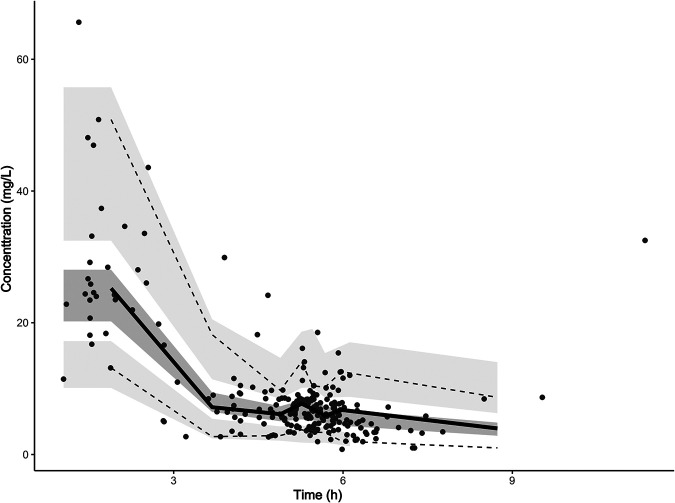
Model diagnostics showed that the final model of vancomycin had an acceptable goodness of fit. Figure 3A and B show no bias in predicting concentration. Figure 3C and D show that there is no trend between population predicted concentration (PRED) and conditional weighted residuals (CWRES) or between time and CWRES. The results of normalized prediction distribution errors (NPDE) are shown in Fig. 3E and F. The average value of NPDE was 0.123, and the variance was 1.02, which indicated that the model was consistent with individual data. In addition, as shown in Table 3, the median values of the parameters estimated by a bootstrap method were consistent with the corresponding values of the final model, indicating that the final model was stable and that the parameters estimated by the population pharmacokinetic model can be reconfirmed. The visual predictive check (VPC) result (Fig. 4) showed that the 5th, 50th, and 95th percentiles of the observed concentrations were within the 95% confidence interval of predicted concentration, which proves the accuracy and adaptability of the model.
FIG 3.
Model evaluation for vancomycin. (A) Population predicted (PRED) versus observed (DV) concentrations; (B) individual predicted (IPRED) versus DV concentrations; (C) conditional weighted residuals (CWRES) versus PRED; (D) CWRES versus time; (E) Q-Q plot of the distribution of the normalized prediction distribution errors (NPDE) versus the theoretical N(0,1) distribution; (F) histogram of the distribution of the NPDE, with the density of the standard Gaussian distribution overlaid.
TABLE 3.
Population pharmacokinetic parameters of vancomycin and bootstrap results (n = 1,000)
| Pharmacokinetic parameter | Value | RSEa (%) | Bootstrap median (5% to 95%) |
|---|---|---|---|
| CL (liters/h)b | |||
| θ1 | 2.27 | 5.9 | 2.30 (1.86–2.45) |
| θ2 | 0.11 | 28.7 | 0.11 (0.06–0.34) |
| V (liters)c | |||
| θ3 | 6.82 | 17.2 | 7.15 (6.04–9.35) |
| Interindividual variability (%) | |||
| CL | 22.6 | 14.7 | 22.7 (16.6–30.9) |
| V | 14.7 | 62.7 | 15.6 (5.1–31.7) |
| Residual variability (%) | 31.8 | 7.6 | 31.2 (27.8–34.5) |
RSE, relative standard error of the estimate.
CL, clearance. CL = θ1 × (wt/15)0.75× (age/4.5)θ2, where wt is body weight, and age is measured in years.
V, volume of distribution. V = θ3 × (wt/15).
FIG 4.
Visual predictive check (VPC) of the final model. Black spots represent the observed vancomycin concentrations. Solid and dashed lines represent the 50th percentile and the 5th and 95th percentiles, respectively, of the observed concentrations; the three shaded areas represent the 95% confidence intervals of the 5th, 50th, and 95th percentiles of the simulated concentrations.
Dosing regimen evaluation and optimization.
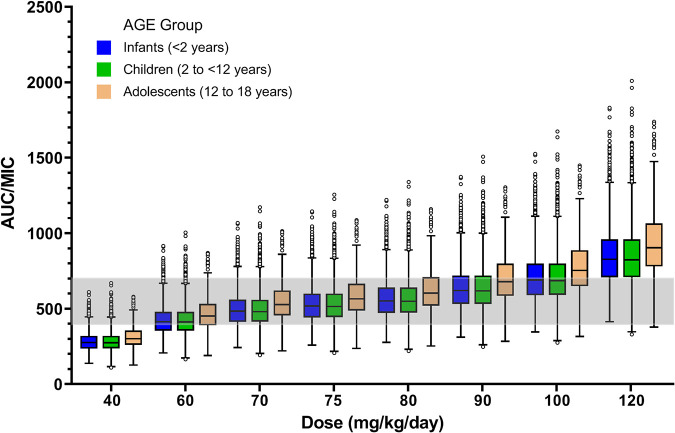
The attainment rates of AUC/MIC with the current dosing regimen were 28.4%, 38.2%, and 33.3% in infants, children, and adolescents, respectively. We simulated the vancomycin dose of 40, 60, 70, 75, 80, 90, 100, and 120 mg/kg body weight/day to optimize the effect and minimize toxicity. Figure 5 illustrates the AUC/MIC of every vancomycin dose for different age groups. It indicates that 75 mg/kg/day was the optimal daily dose for infants and children, and 70 mg/kg/day was the optimal daily dose for adolescents. When using a vancomycin dosing regimen of 75 mg/kg/day in infants and children with ARC, the proportion with AUC/MIC values between 400 and 700 were 78.7% and 77.7%, respectively. For adolescents, using a vancomycin dosing regimen of 70 mg/kg/day, the proportion with AUC/MIC values between 400 and 700 was 78.8%.
FIG 5.
Box plot for AUC/MIC values of vancomycin with a MIC of 1 mg/liter in children. Outliers are marked as black circles; the gray shaded area is AUC/MICs between 400 and 700, indicating the safety and efficacy of vancomycin doses.
DISCUSSION
Our study evaluated the population pharmacokinetics of vancomycin in pediatric patients with ARC, and we have established an optimal dose regimen of vancomycin for these patients between the ages of 1 month and 18 years. ARC has been observed in patients during the critical stages of their illness. The pediatric patients we studied (62 with infections, 41 with nonsolid tumors, and 10 with solid tumors) represent different diseases that are associated with ARC.
The high incidence of ARC in critically ill children is receiving increasing attention (12). Van Der Heggen et al. (13) reported that 67% of critically ill children develop ARC during their stay at the intensive care unit. Another study has shown that ARC was identified in 1 of 10 critically ill children who were treated with vancomycin (14). In our previous study, the incidence of ARC in children with MRSA in the intensive care unit (ICU) was 31.7% (15). The physiological mechanism of ARC could be associated with systemic inflammatory response syndrome (SIRS) (16–18). The mechanism of SIRS includes not only infective factors but also noninfective factors. A large number of clinical studies have confirmed that ARC can affect the pharmacokinetic characteristics of antibiotics that are primarily cleared by the kidney, which is potentially dangerous for patients with severe infections (19). From these investigations, it is clear that ARC can result in subtherapeutic vancomycin concentrations (20–23). Since vancomycin is a very important antimicrobial agent for use against Gram-positive bacteria, it is essential to have a population pharmacokinetic model of patients with ARC for an effective and accurate dosing regimen.
In our final model, the mean values of estimated CL and V were 0.14 liters/h/kg and 0.45 liters/kg, respectively. The value of CL in our study was obviously higher than those seen in pediatric patients in general (0.066 [0.020 to 0.112] liters/h/kg) (24), indicating that the elimination of vancomycin in pediatric patients with ARC is much higher than in those without ARC. Since high clearance results in lower blood concentrations, in our study, the plasma concentrations of vancomycin in 88% of patients did not reach the desired effective value (trough concentrations at steady state [Css,min] < 10 mg/liter), and 65.3% of patients did not reach the attainment rate (AUC/MIC > 400). This means that the current dose of vancomycin for pediatric patients with ARC is inadequate. Previous studies have shown that significant covariates for vancomycin clearance included weight and age (4, 24). Our covariate analysis showed similar results. The trend of weight-normalized CL with age was similar to that of previous studies (4). Body weight and age were directly related to children’s physical development and drug elimination during childhood. We would like to emphasize that there was no significant relationship between creatinine clearance and vancomycin clearance in our study. This finding is consistent with the previously published study in adult patients with ARC (25). We suspect that drug clearance might reach saturation when renal function is enhanced in an ARC state.
According to the 2020 revised consensus guideline of therapeutic monitoring of vancomycin for serious MRSA infections (26), dose adjustment using the AUC is the most accurate and optimal for vancomycin dosing. It is widely reported that an AUC/MIC ratio of ≥400 is closely associated with the clinical efficacy (27–29). As shown in simulation results, the attainment rates of AUC/MIC using current dosing regimens were 28.4%, 38.2%, and 33.3% in infants, children, and adolescents, respectively. These data are surprising, and it means that it is impossible to reach pharmacokinetic-pharmacodynamic (PK/PD) efficacy targets in pediatric patients with ARC using the current dosing regimens. On the other hand, considering that high-dose exposure of vancomycin could lead to an increased risk of nephrotoxicity, an upper limit AUC value of around 700 mg/liter for 0 to 24 h of exposure has been proposed (30–32). Therefore, we considered both the exposure effect and exposure-toxicity effect of vancomycin to determine the most optimal dose of vancomycin. In our study, the recommended doses were 75 mg/kg/day for use in infants and children at ages between 1 month and 12 years and 70 mg/kg/day for use in adolescents. We are the first to establish a detailed vancomycin dosing schedule for use in pediatric patients with ARC. However, our study has several limitations. Given the limited number of patients, our model was not validated externally, and the optimal dosing regimen derived from modeling and simulation should be evaluated in clinical practice to confirm its clinical benefits.
Conclusion.
We have established a population pharmacokinetic model of vancomycin in infants, children, and adolescents with ARC. Weight and age significantly influenced vancomycin clearance. The currently recommend dose of vancomycin for pediatric patients with ARC is inadequate. Considering the efficacy and safety of vancomycin, we established a new therapeutic regimen that advises 75 mg/kg/day in infants and children aged between 1 month and 12 years and 70 mg/kg/day in adolescents aged between 12 and 18 years.
MATERIALS AND METHODS
Study design.
Data from sparse pharmacokinetic sampling combined with therapeutic drug monitoring (TDM) data were included in this population pharmacokinetic study. Patients were included if they met the following criteria: (i) age between 1 month and 18 years, (ii) confirmed or suspected Gram-positive infection, (ii) treated with intravenous vancomycin, (iv) having a serum creatinine concentration measured close to the time (<72 h) vancomycin was measured, and (v) an estimated glomerular filtration rate (eGFR) of more than 160 ml/min/1.73 m2 calculated by the Schwartz equation (33). Patients were excluded if they were on renal replacement therapy or if serum creatinine measurements were not available. Samples were taken during treatment with vancomycin, and 2 to 3 samples were taken from each patient. This study was approved by the ethics committee of the Children’s Hospital of Chongqing Medical University.
Assay of serum vancomycin and eGFR.
One milliliter of venous blood was taken into the anticoagulant tube, and the serum was separated for the sample. The serum concentration of vancomycin was determined by a fluorescence polarization immunoassay method (FPIA) using an i100SR system (Abbott, USA). The calibration curve ranges were 3 to 100 mg/liter. The accuracy and coefficient of variation (CVS) of a laboratory control (7.2, 21.4, and 35.6 mg/liter) were 90% to 110% and 3.9%, respectively.
The modified Schwartz formula is as follows:
| (1) |
Where k is 0.45 for infants <1 year, 0.55 for children <12 years and adolescent females, and 0.7 for adolescent males, Scr is serum creatinine concentration (mg/dl), and height is expressed in centimeters (34).
Pharmacokinetic modeling.
The nonlinear mixed effects modeling program NONMEM version 7.2 (Icon Development Solutions, Columbia, MD, USA) and the first-order conditional estimation (FOCE) method with interaction options were used to analyze and estimate the pharmacokinetic parameters and their variability.
(i) Step 1: model building.
An exponential model was used to estimate the interindividual variability of the pharmacokinetic parameters; it was expressed as follows:
| (2) |
where θi and θmean are the parameter values of the ith subject and the typical parameter value in the population, respectively, and ηi is the variability between subjects (assumes a normal distribution with a mean value of 0 and variance of ω2).
The residual variability was estimated using an exponential model, addition model, and mixed model. The best residual model was determined by analyzing the running results and the objective function value (OFV).
Forward and backward selection were used to select covariates. A likelihood ratio was used to test the influence of each covariate on the parameters. Bodyweight, age, gender, Scr, blood urea nitrogen (BUN), cystatin C (CysC), and eGFR were used as potential covariates to influence pharmacokinetic parameters. In the process of forward selection, if the OFV was significantly lower than that of the basic model (reduction > 3.84, P < 0.05), the covariate was added to the model. All statistically significant covariates were included in the full model. Then, in order to reevaluate the importance of these variables, each covariate was removed independently from the full model. Only when the increase of OFV was more than 6.635 (P < 0.01) was the covariate retained in the final model.
(ii) Step 2: model diagnosis.
The final population pharmacokinetic model was validated based on statistical and graphical criteria. Diagnostic scatterplots were used to evaluate the goodness of fit, including the detection value (DV) and population predicted concentration (PRED), DV and individual predicted concentration (IPRED), conditional weighted residuals (CWRES) and PRED, and CWRES and time. The stability of the final model was evaluated by nonparametric bootstrap method. Resampling was repeated 1,000 times, and then the estimated parameters of the bootstrap process were compared with those of the original data set. The final model was evaluated by the normalized prediction distribution errors (NPDE). The final model parameters were used to simulate the data set 1,000 times. NPDE is expected to follow N(0,1) distribution. The results of NPDE were summarized graphically, and the final model was evaluated by comparing whether the deviation between the actual observation and the simulated observation conforms to the normal distribution. NPDE R package (v1.2) was used to draw the following graphs: (i) quantile-quantile (Q-Q) plot of the NPDE and (ii) histogram of the NPDE. A visual predictive check (VPC) was performed to evaluate the final model and parameter estimates. For VPC method evaluation, 1,000 simulation replicates of the original data set were performed with the final model.
Dosing regimen optimization.
When vancomycin is used for MRSA with a MIC of 1 mg/liter, the AUC should reach 400 mg · h/liter. The desired pharmacokinetic-pharmacodynamic (PK/PD) relationship of vancomycin is AUC/MIC of ≥400, while and AUC/MIC of >700 may cause renal toxicity (30–32). Therefore, we took AUC/MIC values between 400 and 700 as a safety and effectiveness indicator and calculated the proportion of patients with AUC/MIC values between 400 and 700. The basic information of patients in the original data set was used to build a virtual data set. Monte Carlo simulation was used to simulate the current dose 1,000 times. If the currently used dose is insufficient, the dosing regimen will be adjusted by increasing the daily dose. The calculation of the AUC was as follows:
| (3) |
where the AUC is at 0 to 24 h (steady-state) after vancomycin administration, “dose” is daily dose of vancomycin, and CL is the clearance rate for each individual obtained from the above-described pharmacokinetic model of vancomycin.
Organ function changes greatly in infants and children. According to ICH guideline E11 (R1) (35), we divided all patients into 3 groups by age: infants (age of <2 years), children (age of 2 to <12 years), and adolescents (age of 12 to 18 years).
ACKNOWLEDGMENTS
This study was supported by the Chongqing Clinical Pharmacy Key Specialty Construction Project, Young Taishan Scholars Program of Shandong Province, and Qilu Young Scholars Program of Shandong University.
We declare no conflicts of interest.
REFERENCES
- 1.Sharma R, Hammerschlag MR. 2019. Treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in children: a reappraisal of vancomycin. Curr Infect Dis Rep 21:37. 10.1007/s11908-019-0695-4. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Men P, Li HB, Zhai SD, Zhao RS. 2016. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One 11:e0146224. 10.1371/journal.pone.0146224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, Cho S, Natale S, Bui I, Tran TM, Capparelli EV. 2013. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 32:e155–e163. 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eiland LS, English TM, EilandEH, III.. 2011. Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients. Ann Pharmacother 45:582–589. 10.1345/aph.1P588. [DOI] [PubMed] [Google Scholar]
- 6.Sridharan K, Al-Daylami A, Ajjawi R, Ajooz HAA. 2019. Vancomycin use in a paediatric intensive care unit of a tertiary care hospital. Paediatr Drugs 21:303–312. 10.1007/s40272-019-00343-9. [DOI] [PubMed] [Google Scholar]
- 7.Sosnin N, Curtis N, Cranswick N, Chiletti R, Gwee A. 2019. Vancomycin is commonly under-dosed in critically ill children and neonates. Br J Clin Pharmacol 85:2591–2598. 10.1111/bcp.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sime FB, Udy AA, Roberts JA. 2015. Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol 24:1–6. 10.1016/j.coph.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Baptista JP, Sousa E, Martins PJ, Pimentel JM. 2012. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents 39:420–423. 10.1016/j.ijantimicag.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Udy AA, Roberts JA, Lipman J. 2011. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol 7:539–543. 10.1038/nrneph.2011.92. [DOI] [PubMed] [Google Scholar]
- 11.Lv CL, Lu JJ, Chen M, Zhang R, Li QC, Chen YY, Liu TT. 2020. Vancomycin population pharmacokinetics and dosing recommendations in haematologic malignancy with augmented renal clearance children. J Clin Pharm Ther 45:1278–1287. 10.1111/jcpt.13206. [DOI] [PubMed] [Google Scholar]
- 12.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. 2020. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 13.Van Der Heggen T, Dhont E, Peperstraete H, Delanghe JR, Vande Walle J, De Paepe P, De Cock PA. 2019. Augmented renal clearance: a common condition in critically ill children. Pediatr Nephrol 34:1099–1106. 10.1007/s00467-019-04205-x. [DOI] [PubMed] [Google Scholar]
- 14.Avedissian SN, Bradley E, Zhang D, Bradley JS, Nazer LH, Tran TM, Nguyen A, Le J. 2017. Augmented renal clearance using population-based pharmacokinetic modeling in critically ill pediatric patients. Pediatr Crit Care Med 18:e388–e394. 10.1097/PCC.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 15.He CY, Qin YR, Liu CJ, Ren J, Fan JS. 2019. Effect of augmented renal clearance on plasma concentration of vancomycin and treatment outcome in children with methicillin-resistant Staphylococcus aureus infection. Zhongguo Dang Dai Er Ke Za Zhi 21:904–909. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. 2018. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet 57:1107–1121. 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 17.Udy AA, Jarrett P, Stuart J, Lassig-Smith M, Starr T, Dunlop R, Wallis SC, Roberts JA, Lipman J. 2014. Determining the mechanisms underlying augmented renal drug clearance in the critically ill: use of exogenous marker compounds. Crit Care 18:657. 10.1186/s13054-014-0657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minkutė R, Briedis V, Steponavičiūtė R, Vitkauskienė A, Mačiulaitis R. 2013. Augmented renal clearance–an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther 38:462–467. 10.1111/jcpt.12088. [DOI] [PubMed] [Google Scholar]
- 19.Chen IH, Nicolau DP. 2020. Augmented renal clearance and how to augment antibiotic dosing. Antibiotics (Basel) 9:393. 10.3390/antibiotics9070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu Y, Luo Y, Qu L, Zhao C, Jiang M. 2016. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharm Biol 54:2802–2806. 10.1080/13880209.2016.1183684. [DOI] [PubMed] [Google Scholar]
- 21.Molina KC, Hall ST, Barletta JF, Mangram AJ, Dzandu JK, Huang V. 2020. Utilization of augmented renal clearance in trauma intensive care scoring system to improve vancomycin dosing in trauma patients at risk for augmented renal clearance. Surg Infect (Larchmt) 21:43–47. 10.1089/sur.2019.026. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva RD, Talledo O, Neely S, White B, Celii A, Cross A, Kennedy R. 2019. Vancomycin dosing in critically ill trauma patients: the VANCTIC Study. J Trauma Acute Care Surg 87:1164–1171. 10.1097/TA.0000000000002492. [DOI] [PubMed] [Google Scholar]
- 23.Morbitzer KA, Rhoney DH, Dehne KA, Jordan JD. 2019. Enhanced renal clearance and impact on vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. J Intensive Care 7:51. 10.1186/s40560-019-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 51:1–13. 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Chu Y, Luo Y, Ji S, Jiang M, Zhou B. 2020. Population pharmacokinetics of vancomycin in Chinese patients with augmented renal clearance. J Infect Public Health 13:68–74. 10.1016/j.jiph.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77:835–864. 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 27.Álvarez R, López Cortés LE, Molina J, Cisneros JM, Pachón J. 2016. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother 60:2601–2609. 10.1128/AAC.03147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issaranggoon Na Ayuthaya S, Katip W, Oberdorfer P, Lucksiri A. 2020. Correlation of the vancomycin 24-h area under the concentration-time curve (AUC24) and trough serum concentration in children with severe infection: a clinical pharmacokinetic study. Int J Infect Dis 92:151–159. 10.1016/j.ijid.2019.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Drennan PG, Begg EJ, Gardiner SJ, Kirkpatrick CMJ, Chambers ST. 2019. The dosing and monitoring of vancomycin: what is the best way forward? Int J Antimicrob Agents 53:401–407. 10.1016/j.ijantimicag.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, Rybak MJ. 2018. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 62:e01684-17. 10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu JJ, Chen M, Lv CL, Zhang R, Lu H, Cheng DH, Tang SY, Liu TT. 2020. A population pharmacokinetics model for vancomycin dosage optimization based on serum cystatin C. Eur J Drug Metab Pharmacokinet 45:535–546. 10.1007/s13318-020-00621-9. [DOI] [PubMed] [Google Scholar]
- 32.Dao K, Guidi M, André P, Giannoni E, Basterrechea S, Zhao W, Fuchs A, Pfister M, Buclin T, Csajka C. 2020. Optimisation of vancomycin exposure in neonates based on the best level of evidence. Pharmacol Res 154:104278. 10.1016/j.phrs.2019.104278. [DOI] [PubMed] [Google Scholar]
- 33.Hirai K, Ishii H, Shimoshikiryo T, Shimomura T, Tsuji D, Inoue K, Kadoiri T, Itoh K. 2016. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit 38:706–710. 10.1097/FTD.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. 2009. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ICH. 2017. ICH harmonised guideline. Addendum to ICH E11: clinical investigation of medicinal products in the pediatric population, E11 (R1). https://database.ich.org/sites/default/files/E11_R1_Addendum.pdf.