ABSTRACT
Clinically relevant members of the Scedosporium/Pseudallescheria species complex and Lomentospora prolificans are generally resistant against currently available systemic antifungal agents in vitro, and infection due to these species is difficult to treat. We studied the in vivo efficacy of a new fungicidal agent, olorofim (formerly F901318), against scedosporiosis and lomentosporiosis in neutropenic animals. Cyclophosphamide-immunosuppressed CD-1 mice infected by Scedosporium apiospermum, Pseudallescheria boydii (Scedosporium boydii), and Lomentospora prolificans were treated by intraperitoneal administration of olorofim (15 mg/kg of body weight every 8 h for 9 days). The efficacy of olorofim treatment was assessed by the survival rate at 10 days postinfection, levels of serum (1-3)-β-d-glucan (BG), histopathology, and fungal burdens of kidneys 3 days postinfection. Olorofim therapy significantly improved survival compared to that of the untreated controls; 80%, 100%, and 100% of treated mice survived infection by Scedosporium apiospermum, Pseudallescheria boydii, and Lomentospora prolificans, respectively, while less than 20% of the control mice (phosphate-buffered saline [PBS] treated) survived at 10 days postinfection. In the olorofim-treated neutropenic CD-1 mice infected with any of the three species, serum BG levels were significantly suppressed and fungal DNA detected in the target organs was significantly lower than in controls. Furthermore, histopathology of kidneys revealed no or only a few lesions with hyphal elements in the olorofim-treated mice, while numerous fungal hyphae were present in control mice. These results indicate olorofim to be a promising therapeutic agent for systemic scedosporiosis/lomentosporiosis, devastating emerging fungal infections that are difficult to treat with currently available antifungals.
KEYWORDS: olorofim, F901318, scedosporiosis, lomentosporiosis, Scedosporium apiospermum, Pseudallescheria boydii, Lomentospora prolificans, neutropenia
TEXT
Among pathogenic fungi, members of the fungal genera Scedosporium/Pseudallescheria and Lomentospora have been increasingly recognized as emerging opportunists affecting immunosuppressed patients and seemingly immunocompetent, previously healthy individuals (1). These are environmental molds commonly found in soil, animal droppings, water sources, and sewage (2).
Currently, Scedosporium and Pseudallescheria are regarded as a composite of species, the Scedosporium/Pseudallescheria species complex (3). This complex includes Scedosporium aurantiacum, Scedosporium minutisporum, Scedosporium desertorum, Scedosporium cereisporum, Scedosporium dehoogii, Scedosporium angustum, Scedosporium apiospermum, Scedosporium ellipsoideum, Scedosporium fusoideum, and Pseudallescheria boydii (Scedosporium boydii) (4, 5). Lomentospora prolificans (formerly Scedosporium prolificans), however, is considered distinct from the genus Scedosporium based on phylogenetic and morphological differences (6).
Overall, the most frequent species among clinical isolates are Scedosporium apiospermum, Pseudallescheria boydii, and Lomentospora prolificans. They cause a wide range of clinical manifestations, from superficial infections, allergic reactions, and colonization of the respiratory tract to eumycetoma, severe invasive localized or disseminated disease, and fungemia (1, 7–11).
Surveillance data showed that, depending on the geographic location and underlying risk factors, systemic scedosporiosis and lomentosporiosis account for 13 to 33% of invasive mold infections, excluding aspergillosis (12). These species generally have low susceptibility to currently available systemic antifungal agents (13), and the diseases they cause are often refractory to treatment (14), yielding high mortality rates of up to 90%, especially when the etiologic agent is L. prolificans (15, 16).
Alternative treatments are needed, and new classes of antifungals with novel mechanisms of action may improve therapeutic outcomes in refractory disease caused by these species. Olorofim is an orotomide, a new class of antifungal agent, that disrupts de novo pyrimidine biosynthesis (17) by preventing the catalytic activity of fungal dihydroorotate dehydrogenase (DHODH) on the inner mitochondrial membrane (17). DHODH is present in both fungi and mammals but with very low sequence identity (17), and that explains why olorofim selectively inhibits fungal DHODH >2,000-fold more potently than human DHODH. In two recent studies, olorofim demonstrated potent in vitro activity against Scedosporium species and L. prolificans (18, 19). The in vivo efficacy of olorofim against these species, however, is yet to be investigated. In the present study, we aimed to assess the in vivo efficacy of olorofim against disseminated scedosporiosis and lomentosporiosis in neutropenic mice.
(A preliminary version of the manuscript was presented at 9th Trends in Medical Mycology, Nice, France, 11 to 14 October 2019 [20]).
RESULTS
In vitro antifungal drug susceptibility.
Olorofim has shown potent and consistent in vitro activity against all 14 isolates of Scedosporium apiospermum, Pseudallescheria boydii, and Lomentospora prolificans that we received from Nathan P. Wiederhold (18). We have confirmed the olorofim MICs against these strains. L. prolificans demonstrated the highest MICs for all antifungals. Table 1 shows the MICs for three isolates that were used in our in vivo study. Olorofim had the lowest MIC range (0.016 to 0.031 μg/ml), followed by voriconazole (0.25 to 8 μg/ml) and amphotericin B (8 μg/ml).
TABLE 1.
In vitro antifungal susceptibility profiles of Scedosporium apiospermum, Pseudallescheria boydii, and Lomentospora prolificans used in animal studies, using CLSI guidelines
| Organism | Source of isolate | MIC (μg/ml) ofa: |
||
|---|---|---|---|---|
| Amphotericin B | Voriconazole | Olorofim | ||
| Scedosporium apiospermum (DI-17-07) | Clinical | 8 | 1 | 0.016 |
| Pseudallescheria boydii (DI-17-11) | Clinical | 8 | 0.25 | 0.016 |
| Lomentospora prolificans (DI-17-14) | Clinical | 8 | 8 | 0.031 |
The data represent the geometric mean MICs for three independent replicates of each strain.
Olorofim therapy was effective in neutropenic CD-1 mice infected with any of the three species.
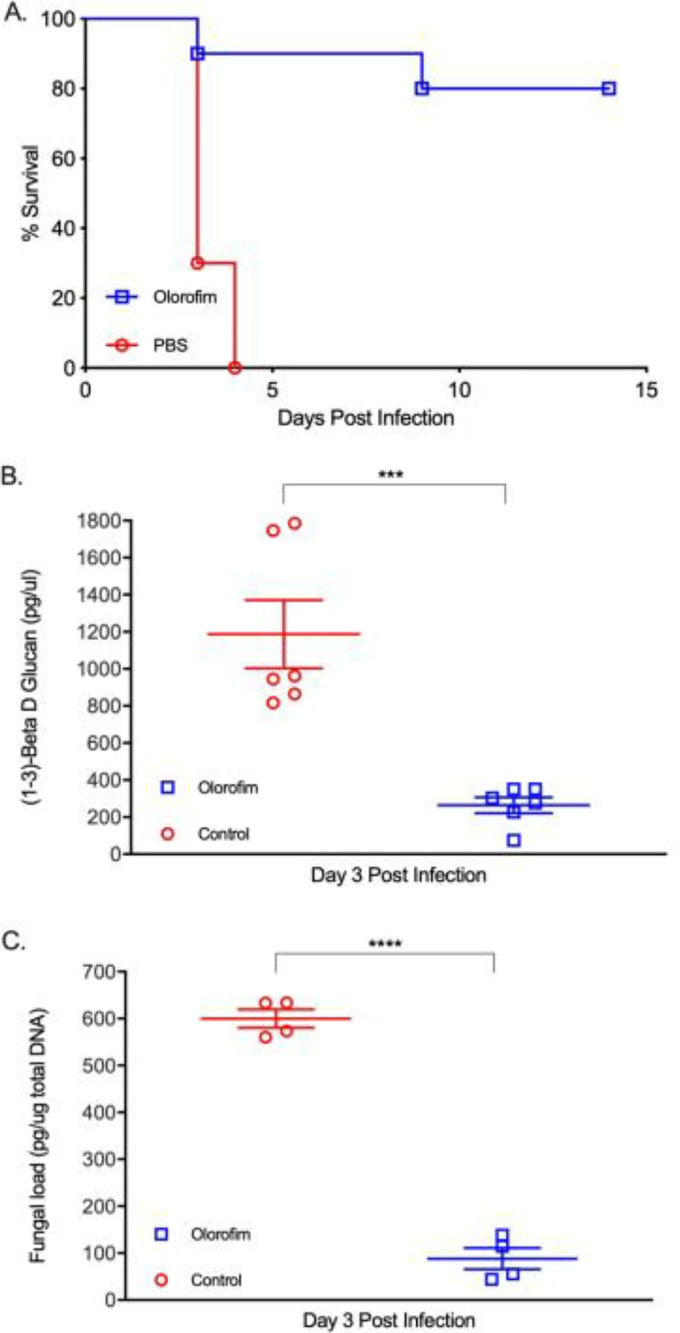
In the neutropenic CD-1 mouse model, olorofim therapy (15 mg/kg of body weight every 8 h [q8h]) significantly improved survival compared to that of the untreated controls: 80%, 100%, and 100% of treated mice survived infection by S. apiospermum (strain DI-17-07), P. boydii (strain DI-17-11), and L. prolificans (strain DI-17-14), respectively (Fig. 1A, 2A, and 3A). In contrast, less than 20% of the mice in the control groups (phosphate-buffered saline [PBS] treated) survived infection by the same isolates within 10 days postinfection. Olorofim therapy suppressed (1-3)-β-d-glucan (BG) levels in sera of infected mice (Fig. 1B, 2B, and 3B) and also significantly reduced the fungal DNA burden in the kidney at day 3 postinfection (Fig. 1C, 2C, and 3C). Histopathology of the kidney sections prepared from the olorofim-treated CD-1 mice infected with any of the three etiological agents at 3 days postinfection showed no or only a few lesions with hyphal elements (Fig. 4, bottom). In contrast, the kidneys of CD-1 PBS control mice showed abundant fungal growth (Fig. 4, top) with severe inflammatory infiltrations and prominent necrosis (data not shown). Thus, olorofim treatment was effective in neutropenic mice infected by any of the three etiologic agents of scedosporiosis/lomentosporiosis.
FIG 1.
Efficacy of olorofim against S. apiospermum in CD-1 mice. CD-1 mice were treated for 9 days with either 15 mg/kg olorofim or PBS every 8 h via intraperitoneal administration, starting 6 h postinfection (intravenous [i.v.]) with 1 × 104 conidia/mouse S. apiospermum (DI-17-07). Animals were randomized into groups of 17. (A) The survival rate of mice (n = 10) was monitored for 10 days. (B) On day 3 postinfection, blood samples were collected from olorofim-treated and untreated mice (n = 3) to measure (1-3)-β-d-glucan (BG) in serum samples. ***, P ≤ 0.001. (C) In addition, on day 3 postinfection, kidneys were harvested (n = 4) and fungal burdens estimated by quantification of fungal DNA using qPCR. ****, P ≤ 0.0001. Error bars show standard deviations.
FIG 2.
Efficacy of olorofim against P. boydii in CD-1 mice. The CD-1 mice were treated for 9 days with either 15 mg/kg olorofim or PBS every 8 h via intraperitoneal administration, starting 6 h postinfection (i.v.) with 1 × 104 conidia/mouse P. boydii (DI-17-11). Animals were randomized into groups of 17. (A) The survival rate of mice (n = 10) was monitored for 10 days. (B) On day 3 postinfection, blood samples were collected from olorofim-treated and untreated mice (n = 3) to measure (1-3)-β-d-glucan (BG) in serum samples. ***, P ≤ 0.001. (C) In addition, on day 3 postinfection, kidneys (n = 4) were harvested and fungal burdens estimated by quantification of fungal DNA using qPCR. ****, P ≤ 0.0001. Error bars show standard deviations.
FIG 3.
Efficacy of olorofim against L. prolificans in CD-1 mice. The CD-1 mice were treated for 9 days with either 15 mg/kg olorofim or PBS every 8 h via intraperitoneal administration, starting 6 h postinfection (i.v.) with 1 × 104 conidia/mouse L. prolificans (DI-17-14). Animals were randomized into groups of 17. (A) The survival rate of mice (n = 10) was monitored for 10 days. (B) On day 3 postinfection, blood samples were collected from olorofim-treated and untreated mice (n = 3) to measure (1-3)-β-d-glucan (BG) in serum samples. ****, P ≤ 0.0001. (C) In addition, on day 3 postinfection, kidneys (n = 4) were harvested and fungal burdens estimated by quantification of fungal DNA using qPCR. **, P ≤ 0.01. Error bars show standard deviations.
FIG 4.
Representative sections of GMS-stained kidneys of CD-1 mice infected with Lomentospora prolificans. Histopathology sections of kidneys in CD-1 mice at day 3 postinfection showed numerous fungal elements throughout the organ in control group mice infected with any of the three etiological agents of scedosporiosis/lomentosporiosis. However, only a few lesions with hyphal elements were observed in the animals that received olorofim therapy.
DISCUSSION
There are three major classes of systemic antifungals currently licensed for the treatment of invasive fungal infections (IFIs): polyenes (various formulations of amphotericin B), triazoles (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole), and echinocandins (caspofungin, micafungin, and anidulafungin) (21). However, therapeutic options against scedosporiosis and lomentosporiosis are limited. Scedosporium species are typically resistant to amphotericin B in vitro (13) and have reduced susceptibility to echinocandins (22). In vivo studies also demonstrated the lack of efficacy for amphotericin B deoxycholate and liposomal amphotericin B against disseminated infection by Scedosporium spp. in neutropenic mice (23).
Triazoles also demonstrated various degrees of in vitro efficacy against Scedosporium species. Moreover, Lomentospora prolificans is usually resistant to all currently approved systemic antifungal agents (13). The high degree of intrinsic antifungal resistance, as well as reduced susceptibility among the species of the Scedosporium complex and Lomentospora, makes these infections difficult to manage.
Given that treatment options in patients with invasive disease due to these fungi are limited or nonexistent, efforts are ongoing to find alternative therapeutic options. Several investigational antifungals with novel mechanisms of action that may overcome both the low susceptibility and adverse side effects are currently under development (21, 24). Among them, ibrexafungerp (25) and fosmanogepix (26) demonstrated good in vitro activity against Scedosporium species complex and L. prolificans. The new triazole derivative albaconazole (ALBA; UR-9825) also showed potent activity against these pathogens in both in vitro (27) and in vivo studies (28).
Olorofim is one of the investigational compounds under clinical development for the treatment of invasive mold infections (21). On the basis of preliminary clinical data, olorofim has received the FDA’s Breakthrough Therapy designation both for “treatment of invasive mold infections in patients with limited or no treatment options, including aspergillosis refractory or intolerant to currently available therapy, and infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species” and for “central nervous system coccidioidomycosis refractory or otherwise unable to be treated with standard of care therapy.” Currently, a phase IIb clinical trial of oral olorofim is recruiting patients with IFIs and those lacking treatment options (ClinicalTrials.gov registration no. NCT03583164). Overall, olorofim has no activity against Candida and Mucorales, while it inhibits the growth of many ascomycetous mold species, such as isolates of Aspergillus spp., including azole-resistant isolates of Aspergillus fumigatus (29), cryptic aspergilli (30), Scedosporium/Pseudallescheria species complex and Lomentospora spp. (18, 19), Madurella mycetomatis (31), certain species of Fusarium and Penicillium spp. (17), and Talaromyces marneffei (32). Potent activity of olorofim has also been demonstrated in experimental animal models of disseminated infections caused by A. fumigatus (34, 35), Aspergillus flavus (36), Aspergillus nidulans (34), and Aspergillus tanneri (34), as well as the dimorphic human pathogen Coccidioides immitis (33). In each case, the efficacy of olorofim has resulted in prolonged survival and reduction in both serum biomarkers and tissue fungal burden.
In the present study, we showed that olorofim controls the growth of S. apiospermum, P. boydii, and L. prolificans in vitro and in vivo. Treatment with olorofim improved the survival of mice infected with any of the three species. Olorofim therapy was also effective in reducing mortality, pathology of tissues, BG levels, and fungal DNA loads in a murine model of systemic scedosporiosis and lomentosporiosis. In addition, the efficacy of olorofim was unrelated to the fungal species tested and their MICs against triazole or amphotericin B.
Disseminated scedosporiosis and lomentosporiosis are mainly seen among immunocompromised patients with hematological malignancies and in solid organ transplant recipients (37). Although the respiratory tract is considered the main route of entry for these pathogens, infection may occur via trauma in the setting of nosocomial infections, such as those caused by contaminated catheters (38). In these patients, if persistent profound neutropenia and T-cell dysfunctions are the main predisposing factors, then infection is associated with poor prognosis (39). Our data presented here indicate that olorofim is efficacious in the treatment of invasive scedosporiosis and lomentosporiosis in a profoundly neutropenic murine model of infection. These results are in agreement with the recent studies that showed successful treatment of a potentially life-threatening case of disseminated osteomyelitis (40) and a case of chest wall infection due to strains of L. prolificans refractory to available therapies (41).
In conclusion, olorofim is predicted to be a promising new drug to treat scedosporiosis and lomentosporiosis, since the etiologic agents of these diseases have uniformly low MICs against olorofim and the mode of action of olorofim is entirely different from those of extant antifungals.
MATERIALS AND METHODS
Fungal strains.
A collection of 14 clinical isolates of S. apiospermum, P. boydii, and L. prolificans originating from different patients were kindly supplied by Nathan P. Wiederhold, University of Texas Health Science Center at San Antonio, TX, USA. The identity of each isolate used for in vivo study was confirmed at the species level via PCR amplification and sequence-based analysis of the internal transcribed spacer (ITS) of the ribosomal DNA (rDNA) region and the calmodulin gene, as described previously (4). The isolates were stored in 10% glycerol at −80°C and were revived on malt extract agar (MEA) at 37°C for 5 to 7 days. To prepare conidial suspensions, all isolates were freshly cultured on MEA for 5 to 7 days at 37°C. The conidia were then harvested in 0.01% Tween 20–phosphate-buffered saline (PBS) by filtering through a sterile BD Falcon 40-μm-pore-size cell strainer (BD Biosciences, San Jose, CA) and washed with sterile distilled water.
In vitro antifungal susceptibility testing.
All 14 isolates of the three species were tested for in vitro susceptibility against currently available systemic antifungals (18) and olorofim by using the Clinical and Laboratory Standards Institute (CLSI) M38-ED3:2017 reference method for broth dilution antifungal susceptibility testing of filamentous fungi (42). Olorofim was provided by F2G, Ltd., and all other drugs were purchased from Sigma (St. Louis, MO). The MIC was defined as the lowest concentration that completely inhibited growth in comparison to the growth in the drug-free well (control) as assessed by visual inspection. All incubations were performed in three biological replicates on different days, and MICs were read after incubation for 48 h at 35 to 37°C.
Animals and husbandry.
The efficacy of olorofim monotherapy was determined in CD-1 mice (4- to 5-week-old females, Charles River Laboratories, USA) as described previously (34, 43). Animals were randomized into groups of 17 [10 mice for monitoring survival rate for 10 days, 3 mice to measure (1-3)-β-d-glucan and to study histopathology of the kidneys, and 4 mice for quantification of fungal DNA by quantitative PCR (qPCR) at day 3 postinfection]. Animals were housed under standard conditions, with drink and food supplied ad libitum. All experiments for each strain were performed using at least two independent replicates. To avoid selection bias, animals were preassigned to the groups for assessment of survival rate, serology, fungal load, and histopathology at the time of infection.
Animal infection models.
The in vivo efficacy of olorofim monotherapy was studied against infection with three species; S. apiospermum (strain DI-17-07), P. boydii (DI-17-11), and L. prolificans (DI-17-14) in neutropenic CD-1 mice using the procedure described previously (34). To render the CD-1 mice neutropenic, cyclophosphamide (150 mg/kg on days −4 and +4 and 100 mg/kg on day −1) was administered. Animals were infected via tail vein with 100 μl freshly prepared conidial suspension corresponding to the 90% lethal dose (LD90) of each species (5 × 104 CFU/mouse for all three species). To ensure the correct inoculum size had been injected, postinfection viability counts of the inocula were assessed. In all survival studies, experienced individuals blinded to the animal treatment monitored the infected mice at least twice daily.
Dosing regimen of olorofim.
For in vivo studies, olorofim intraperitoneal therapy was started 6 h after infection with a total daily dose of 45 mg/kg of body weight (15 mg/kg every 8 h) for 9 days. To prepare working concentrations, the desired amount of drug was weighed, added to dimethyl sulfoxide (DMSO), and vortexed until fully dissolved. Subsequently, polyethylene glycol 400 (PEG 400) was added and the mixture vortexed. A solution of 35.3% hydroxyl propyl cyclodextrin (HPBCD) was prepared in water. The solution of olorofim in DMSO-PEG 400 and the HPBCD solution were mixed to give a clear solution. The final excipient concentrations were 30% HPCD, 5% DMSO, and 10% PEG 400. Appropriate volumes of olorofim solutions were prepared and stored at −20°C for daily use. Prior to use, drug-containing vials were fully thawed and vortexed. The control mice received PBS intraperitoneally. The efficacy of the olorofim treatment was assessed by monitoring survival for 10 days and determining serum BG, tissue histopathology, and fungal burdens in the kidneys (CD-1 mice) 3 days postinfection. Sampling at 3 days postinfection allowed us to obtain accurate and timely data before animals started to die or be euthanized to interrupt animal suffering caused by fungal infection.
Detection of BG.
(1-3)-β-d-glucan (BG) levels were determined from olorofim-treated and untreated mice (n = 3) on day 3 postinfection (Fig. 1 and 3). A protease zymogen-based colorimetric assay (Fungitell; Associates of Cape Cod, Inc.) was used to detect circulating BG, a major cell wall component of fungi, in the serum samples according to the manufacturer’s instructions, and the results are reported as pg/ml.
Determination of fungal burden.
On day 3 postinfection, DNA was extracted from the kidneys using the FastDNA spin kit (MP Biomedicals, Santa Ana, CA) as described previously (44). The concentrations of total DNA isolated from kidneys were measured by using the Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Fungal loads were determined by real-time qPCR using primers and probe targeting the 28S-ITS2 region of the ribosomal subunit gene of each species. The sequences of the primers and probes used for qPCR assays are shown in Table 2. The qPCRs were run with 250 ng of total DNA isolated from kidneys of infected mice. To determine the fungal load in each organ sample (pg/ng of total DNA isolated), the fungal DNA concentration was calculated from standard curves derived from 8-fold dilution series of the genomic DNA of Scedosporium/Pseudallescheria and Lomentospora prolificans.
TABLE 2.
Primer and probe sequences used for qPCR assays
| Organism | Primer/probe | Sequence (5′–3′) |
|---|---|---|
| Scedosporium/Pseudallescheria species complex | Forward | GAGCGTCATTTCAACCCTCG |
| Reverse | ATATGCTTAAGTTCAGCGGGT | |
| Probe | TAAGTCTCTTTTGCAAGCTCGCATTGG-6FAM | |
| Lomentospora prolificans | Forward | TTACAAGCCCAAGGATCGGTGTTGG |
| Reverse | ATATGCTTAAGTTCAGCGGGT | |
| Probe | TTACAAGCCCAAGGATCGGTGTTGG-CY5 |
Histopathological analysis.
The kidneys were harvested from surviving animals for histopathological analysis using hematoxylin and eosin (H&E) and Gomori’s methenamine silver (GMS) staining.
Ethics statement.
The Institutional Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases approved all animal studies (LCIM-5E). Studies were performed in accordance with recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (45).
Statistical analysis.
All data analyses were performed using GraphPad Prism, version 9, (GraphPad Software, San Diego, CA). Mortality data were analyzed by the log rank test. Student’s t test was used to define whether there were significant differences between the mean values of two groups. Statistical significance for comparisons was defined as a P value of ≤0.05 (two-tailed) and is reported as follows: nonsignificant (NS), P > 0.05; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
ACKNOWLEDGMENTS
This work was supported by a research fund from the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and partly by the Intramural Research Program of the National Institutes of Health, Clinical Center, Department of Laboratory Medicine. Olorofim (F901318) powder was provided by F2G, Ltd.
We thank Nathan P. Wiederhold (Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center at San Antonio, TX, USA), for his gift of the clinical isolates used in this study.
S.S., Y.C.C. and K.J.K.-C. declare no conflict of interests related to this publication. D.L., M.B., and J.H.R. are employed by and own stock in F2G, Limited.
REFERENCES
- 1.Ramirez-Garcia A, Pellon A, Rementeria A, Buldain I, Barreto-Bergter E, Rollin-Pinheiro R, de Meirelles JV, Xisto M, Ranque S, Havlicek V, Vandeputte P, Govic YL, Bouchara JP, Giraud S, Chen S, Rainer J, Alastruey-Izquierdo A, Martin-Gomez MT, Lopez-Soria LM, Peman J, Schwarz C, Bernhardt A, Tintelnot K, Capilla J, Martin-Vicente A, Cano-Lira J, Nagl M, Lackner M, Irinyi L, Meyer W, de Hoog S, Hernando FL. 2018. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol 56:102–125. 10.1093/mmy/myx113. [DOI] [PubMed] [Google Scholar]
- 2.Rougeron A, Schuliar G, Leto J, Sitterle E, Landry D, Bougnoux ME, Kobi A, Bouchara JP, Giraud S. 2015. Human-impacted areas of France are environmental reservoirs of the Pseudallescheria boydii/Scedosporium apiospermum species complex. Environ Microbiol 17:1039–1048. 10.1111/1462-2920.12472. [DOI] [PubMed] [Google Scholar]
- 3.Luplertlop N. 2018. Pseudallescheria/Scedosporium complex species: from saprobic to pathogenic fungus. J Mycol Med 28:249–256. 10.1016/j.mycmed.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Gilgado F, Cano J, Gene J, Guarro J. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol 43:4930–4942. 10.1128/JCM.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gams W. 2016. Recent changes in fungal nomenclature and their impact on naming of microfungi, p 7–23. In Li D-W, Biology of microfungi, Springer, Cham, Switzerland. 10.1007/978-3-319-29137-6_2. [DOI] [Google Scholar]
- 6.Lackner M, de Hoog GS, Yang LY, Moreno LF, Ahmed SA, Andreas F, Kaltseis J, Nagl M, Lass-Florl C, Risslegger B, Rambach G, Speth C, Robert V, Buzina W, Chen S, Bouchara JP, Cano-Lira JF, Guarro J, Gene J, Silva FF, Haido R, Haase G, Havlicek V, Garcia-Hermoso D, Meis JF, Hagen F, Kirchmair M, Rainer J, Schwabenbauer K, Zoderer M, Meyer W, Gilgado F, Schwabenbauer K, Vicente VA, Pieckova E, Regenermel M, Rath PM, Steinmann J, de Alencar XW, Symoens F, Tintelnot K, Ulfig K, Velegraki A, Tortorano AM, Giraud S, Mina S, Rigler-Hohenwarter K, Hernando FL, Ramirez-Garcia A, Pellon A, et al. 2014. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 67:1–10. 10.1007/s13225-014-0295-4. [DOI] [Google Scholar]
- 7.Bernhardt A, Sedlacek L, Wagner S, Schwarz C, Wurstl B, Tintelnot K. 2013. Multilocus sequence typing of Scedosporium apiospermum and Pseudallescheria boydii isolates from cystic fibrosis patients. J Cyst Fibros 12:592–598. 10.1016/j.jcf.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, Buchanan W, Milanovich J, Sutton DA, Fothergill A, Rinaldi MG, Shea YR, Zaoutis T, Kottilil S, Walsh TJ. 2008. Infections caused by Scedosporium spp. Clin Microbiol Rev 21:157–197. 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo M, Goto H, Yamanaka K. 2018. Case of Scedosporium aurantiacum infection detected in a subcutaneous abscess. Med Mycol Case Rep 20:26–27. 10.1016/j.mmcr.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedayati MT, Tavakoli M, Maleki M, Heidari S, Mortezaee V, Gheisari M, Hassanzad M, Mirenayat MS, Mahdaviani SA, Pourabdollah M, Velayati AA, Vakili M, Abastabar M, Haghani I, Jafarzadeh J, Hedayati N, Seyedmousavi S, Alastruey-Izquierdo A. 2019. Fungal epidemiology in cystic fibrosis patients with a special focus on Scedosporium species complex. Microb Pathog 129:168–175. 10.1016/j.micpath.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Lionakis MS, Bodey GP, Tarrand JJ, Raad II, Kontoyiannis DP. 2004. The significance of blood cultures positive for emerging saprophytic moulds in cancer patients. Clin Microbiol Infect 10:922–925. 10.1111/j.1469-0691.2004.00933.x. [DOI] [PubMed] [Google Scholar]
- 12.Slavin M, van Hal S, Sorrell TC, Lee A, Marriott DJ, Daveson K, Kennedy K, Hajkowicz K, Halliday C, Athan E, Bak N, Cheong E, Heath CH, Orla Morrissey C, Kidd S, Beresford R, Blyth C, Korman TM, Owen Robinson J, Meyer W, Chen SC, Australia and New Zealand Mycoses Interest Group. 2015. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect 21:490.e1–10. 10.1016/j.cmi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Lackner M, de Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, Klaassen CH, Meis JF. 2012. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 56:2635–2642. 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy MW, Katragkou A, Iosifidis E, Roilides E, Walsh TJ. 2018. Recent advances in the treatment of Scedosporiosis and Fusariosis. J Fungi (Basel) 4:73. 10.3390/jof4020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronnimann D, Garcia-Hermoso D, Dromer F, Lanternier F, French Mycoses Study Group. 2020. Scedosporiosis/lomentosporiosis observational study (SOS): clinical significance of Scedosporium species identification. Med Mycol 59:486–497. 10.1093/mmy/myaa086. [DOI] [PubMed] [Google Scholar]
- 16.Seidel D, Meißner A, Lackner M, Piepenbrock E, Salmanton-García J, Stecher M, Mellinghoff S, Hamprecht A, Durán Graeff L, Köhler P, Cheng MP, Denis J, Chedotal I, Chander J, Pakstis DL, Los-Arcos I, Slavin M, Montagna MT, Caggiano G, Mares M, Trauth J, Aurbach U, Vehreschild MJGT, Vehreschild JJ, Duarte RF, Herbrecht R, Wisplinghoff H, Cornely OA. 2019. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit Rev Microbiol 45:1–21. 10.1080/1040841X.2018.1514366. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, du Pre S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 113:12809–12814. 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold NP, Law D, Birch M. 2017. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 72:1977–1980. 10.1093/jac/dkx065. [DOI] [PubMed] [Google Scholar]
- 19.Biswas C, Law D, Birch M, Halliday C, Sorrell TC, Rex J, Slavin M, Chen SC. 2018. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 56:1050–1054. [DOI] [PubMed] [Google Scholar]
- 20.Seyedmousavi S, Chang YC, Law D, Birch M, Rex J, Kwon-Chung KJ. 2019. In vivo efficacy of olorofim against systemic infection caused by Scedosporium apiospermum, Pseudallescheria boydii, and Lomentospora prolificans in neutropenic CD-1 mice, poster 415. Abstr 9th Trends Med Mycol, Nice, France, 11 to 14 October 2019.
- 21.Seyedmousavi S. 2021. Antifungal drugs. InAbraham DJ, Myers M (ed), Burger’s medicinal chemistry, drug discovery and development, 8th ed, vol 7, chapter 7, p 1-62. John Wiley & Sons, Inc., Hoboken, NJ. 10.1002/0471266949.bmc295. [DOI] [Google Scholar]
- 22.Eschenauer G, Depestel DD, Carver PL. 2007. Comparison of echinocandin antifungals. Ther Clin Risk Manag 3:71–97. 10.2147/tcrm.2007.3.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capilla J, Mayayo E, Serena C, Pastor FJ, Guarro J. 2004. A novel murine model of cerebral scedosporiosis: lack of efficacy of amphotericin B. J Antimicrob Chemother 54:1092–1095. 10.1093/jac/dkh468. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Albrecht K, Groll J, Beilhack A. 2020. Innovative therapies for invasive fungal infections in preclinical and clinical development. Expert Opin Invest Drugs 29:961–971. 10.1080/13543784.2020.1791819. [DOI] [PubMed] [Google Scholar]
- 25.Lamoth F, Alexander BD. 2015. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 59:4308–4311. 10.1128/AAC.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. 2019. In vitro activity of APX001A (manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother 63:e00840-19. 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrillo AJ, Guarro J. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob Agents Chemother 45:2151–2153. 10.1128/AAC.45.7.2151-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capilla J, Yustes C, Mayayo E, Fernández B, Ortoneda M, Javier Pastor F, Guarro J. 2003. Efficacy of albaconazole (UR-9825) in treatment of disseminated Scedosporium prolificans infection in rabbits. Antimicrob Agents Chemother 47:1948–1951. 10.1128/AAC.47.6.1948-1951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buil JB, Rijs A, Meis JF, Birch M, Law D, Melchers WJG, Verweij PE. 2017. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 72:2548–2552. 10.1093/jac/dkx177. [DOI] [PubMed] [Google Scholar]
- 30.Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. 2019. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother 74:1586–1590. 10.1093/jac/dkz078. [DOI] [PubMed] [Google Scholar]
- 31.Lim W, Eadie K, Konings M, Rijnders B, Fahal AH, Oliver JD, Birch M, Verbon A, van de Sande W. 2020. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother 75:936–941. 10.1093/jac/dkz529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Liu H, Xi L, Chang YC, Kwon-Chung KJ, Seyedmousavi S. 2020. Antifungal susceptibility profiles of olorofim (formerly F901318), and currently available systemic antifungals against mold and yeast phases of Talaromyces marneffei. Abstr 9th Adv Against Aspergillosis Mucormycosis, Lugano, Switzerland, 27 to 29 February 2020. [DOI] [PMC free article] [PubMed]
- 33.Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Birch M, Law D, Rex JH, Catano G, Patterson TF. 2018. The orotomide olorofim is efficacious in an experimental model of central nervous system coccidioidomycosis. Antimicrob Agents Chemother 62:e00999-18. 10.1128/AAC.00999-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyedmousavi S, Chang YC, Law D, Birch M, Rex JH, Kwon-Chung KJ. 2019. Efficacy of olorofim (F901318) against Aspergillus fumigatus, A. nidulans, and A. tanneri in murine models of profound neutropenia and chronic granulomatous disease. Antimicrob Agents Chemother 63:e00129-19. 10.1128/AAC.00129-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hope WW, McEntee L, Livermore J, Whalley S, Johnson A, Farrington N, Kolamunnage-Dona R, Schwartz J, Kennedy A, Law D, Birch M, Rex JH. 2017. Pharmacodynamics of the orotomides against Aspergillus fumigatus: new opportunities for treatment of multidrug-resistant fungal disease. mBio 8:e01157-17. 10.1128/mBio.01157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negri CE, Johnson A, McEntee L, Box H, Whalley S, Schwartz JA, Ramos-Martin V, Livermore J, Kolamunnage-Dona R, Colombo AL, Hope WW. 2018. Pharmacodynamics of the novel antifungal agent F901318 for acute sinopulmonary aspergillosis caused by Aspergillus flavus. J Infect Dis 217:1118–1127. 10.1093/infdis/jix479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leek R, Aldag E, Nadeem I, Gunabushanam V, Sahajpal A, Kramer DJ, Walsh TJ. 2016. Scedosporiosis in a combined kidney and liver transplant recipient: a case report of possible transmission from a near-drowning donor. Case Rep Transplant 2016:1879529. 10.1155/2016/1879529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs SE, Wengenack NL, Walsh TJ. 2020. Non-Aspergillus hyaline molds: emerging causes of sino-pulmonary fungal infections and other invasive mycoses. Semin Respir Crit Care Med 41:115–130. 10.1055/s-0039-3401989. [DOI] [PubMed] [Google Scholar]
- 39.Cobo F, Lara-Oya A, Rodriguez-Granger J, Sampedro A, Aliaga-Martinez L, Navarro-Mari JM. 2018. Infections caused by Scedosporium/Lomentospora species: clinical and microbiological findings in 21 cases. Med Mycol 56:917–925. 10.1093/mmy/myx147. [DOI] [PubMed] [Google Scholar]
- 40.Tio SY, Thursky K, Ng G, Rex JH, Carney D, Slavin M. 2020. Olorofim for a case of severe disseminated Lomentospora prolificans infection, abstr 118. Abstr 30th Eur Congr Clin Microbiol Infect Dis (ECCMID), Paris, France, 18 to 21 April 2020.
- 41.Chen SAC, Rai NJ, Cunneen S, Cornelissen K, Rex JH, Heath CH, Harvey E. 2000. A case of Lomentospora prolificans (Lo Pro) treated with the novel antifungal olorofim, abstr 2585. Abstr 30th Eur Congr Clin Microbiol Infect Dis (ECCMID), Paris, France, 18 to 21 April 2020.
- 42.CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed. CLSI M38. Clinical and laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Seyedmousavi S, Mouton JW, Melchers WJ, Verweij PE. 2015. Posaconazole prophylaxis in experimental azole-resistant invasive pulmonary aspergillosis. Antimicrob Agents Chemother 59:1487–1494. 10.1128/AAC.03850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seyedmousavi S, Davis MJ, Sugui JA, Pinkhasov T, Moyer S, Salazar AM, Chang YC, Kwon-Chung KJ. 2018. Exogenous stimulation of type I interferon protects mice with chronic granulomatous disease from aspergillosis through early recruitment of host-protective neutrophils into the lung. mBio 9:e00422-18. 10.1128/mBio.00422-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]