ABSTRACT
Carbapenem-resistant Acinetobacter baumannii and Enterobacterales are identified as urgent threats, and multidrug-resistant (MDR) Pseudomonas aeruginosa and extended-spectrum beta-lactamase (ESBL)-producing pathogens are identified as serious threats by the Centers for Disease Control and Prevention (CDC). SPR206 is a novel polymyxin derivative with potent in vitro and in vivo activity against A. baumannii, P. aeruginosa, and multiple clinically important species of Enterobacterales, including multidrug- and extensively drug-resistant strains. This was a first-in-human (FIH) double-blind, placebo-controlled, single-, and multiple-ascending-dose study of the safety, tolerability, and pharmacokinetics (PK) of SPR206 in 94 healthy subjects. Following intravenous (i.v.) administration (1-h infusion) at single doses of 10 mg to 400 mg and multiple doses of 25 mg to 150 mg every 8 h (q8h) for 7 days and 100 mg q8h for 14 days, SPR206 was generally safe and generally well tolerated. While the incidence of adverse events increased with dose, most were of mild severity. Systemic exposure (maximum concentration of drug in serum [Cmax] and area under the concentration-time curve [AUC]) to SPR206 was approximately dose proportional, time to peak concentrations ranged from 1.1 to 1.3 h, and half-life ranged from 2.4 to 4.1 h. No appreciable accumulation occurred with repeated dosing of SPR206, and trough concentrations suggest that steady state was achieved by day 2. Urinary excretion of unchanged SPR206 was dose dependent across single- (SAD) and multiple-ascending-dose (MAD) cohorts, and the percentage of dose excreted as SPR206 was up to >50%. Importantly, no evidence of nephrotoxicity was observed over 14 days of 100 mg q8h dosing of SPR206; a dosing regimen anticipated to exceed requirements for clinical efficacy. (This study has been registered at ClinicalTrials.gov under identifier NCT03792308.)
KEYWORDS: antimicrobial safety, pharmacokinetics, polymyxins
INTRODUCTION
Among Gram-negative pathogens, antimicrobial resistance is a growing problem worldwide (1). Carbapenem-resistant Acinetobacter baumannii and Enterobacterales have been identified as urgent threats, and multidrug-resistant (MDR) Pseudomonas aeruginosa and extended-spectrum beta-lactamase (ESBL)-producing pathogens are considered a serious threat by the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) (1, 2). Acinetobacter baumannii is associated with serious infections, including bacteremia, hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP), and complicated urinary tract infections (cUTIs) (3, 4), and over 60% of infections due to A. baumannii are MDR (5–8) and are associated with excess morbidity and mortality rates of 50% or greater (3, 9–15). Carbapenem resistance in clinical isolates of P. aeruginosa approaches approximately 20% (16), and infections caused by this pathogen are associated with substantial morbidity and increased rates of mortality (17, 18). The WHO, the CDC, and others have highlighted the urgent need to identify new antimicrobial agents to treat serious infections due to MDR pathogens (1, 2, 19–21).
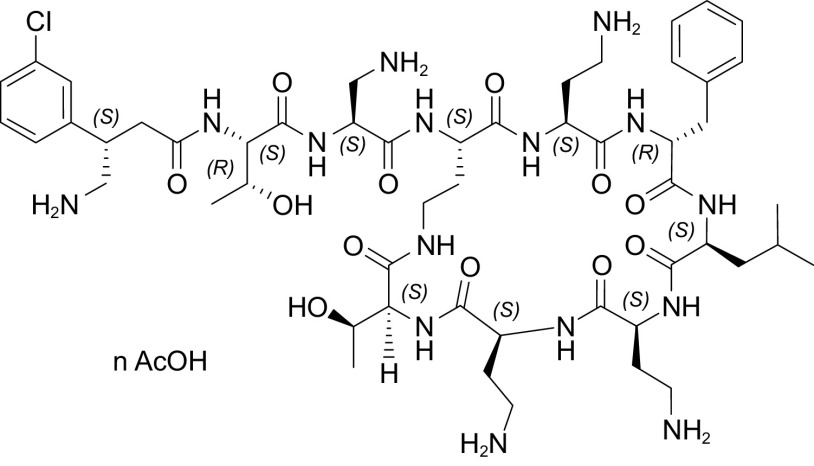
SPR206 is a novel polymyxin derivative (Fig. 1) with potent in vitro and in vivo activity against A. baumannii, P. aeruginosa, and multiple clinically important species of Enterobacterales, including drug-resistant ESBL-producing and Ambler class A, B, C, and D beta-lactamase-producing strains. In vitro studies have shown SPR206 exhibits lower MICs (MIC90 range, 0.12 to 0.5 μg/ml) than colistin and meropenem against A. baumannii, Klebsiella pneumoniae, and P. aeruginosa (22–27), and in vivo studies in thigh, lung, and urinary tract infection models in mice indicate that SPR206 achieves efficacy endpoints (reduction in bacterial burden in CFU/g) at similar or lower required doses (mg/kg) than polymyxin B (PMB) with a change from baseline in Log10 of −4.6 for SPR206 and −2.8 for polymyxin B at 20 mg/kg (28–30). Nonclinical toxicology studies in mice, rats, and nonhuman primates have demonstrated that SPR206 exhibits a lower risk for kidney toxicity (nephrotoxicity) than colistin and polymyxin B, including a mouse model where no histopathological changes in the kidney were noted with SPR206 compared with all animals with polymyxin B (22, 31, 32). A suite of glycolipoprotein (GLP) repeat dose toxicology, safety pharmacology, and absorption, distribution, metabolism, and excretion (ADME) studies have shown SPR206 to be generally safe and generally well tolerated at exposures above those anticipated to be required for efficacy, with low risk for respiratory, central nervous system, or cardiovascular events and low risk for clinical drug-drug interactions. In preclinical studies, SPR206 demonstrated no apparent accumulation after repeat dosing in rats and monkeys and undergoes minimal metabolism in vitro and in vivo, and SPR206 exhibits relatively low protein binding across species, including human (<21%). SPR206 is undergoing clinical development as an intravenous (i.v.) therapy to treat serious Gram-negative infections of the lung, bloodstream, intraabdominal, and urinary tract in the hospital setting caused by MDR pathogens. This first-in-human (FIH) study evaluated the safety, tolerability, and pharmacokinetics (PK) of SPR206 in healthy subjects.
FIG 1.
Chemical structure of SPR206.
RESULTS
Subject disposition and baseline characteristics.
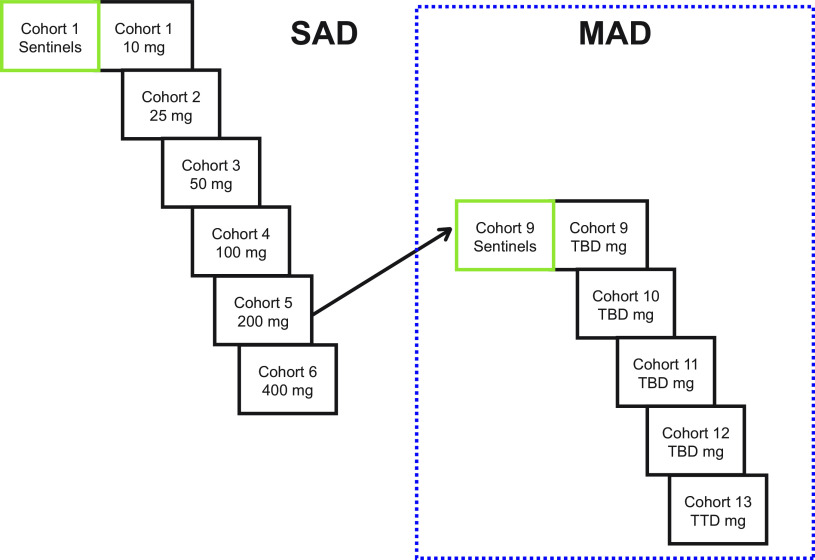
In the single-ascending-dose (SAD) phase, 54 subjects were enrolled; 48 were randomized to one of six ascending-dose cohorts and received SPR206 at 10 mg, 25 mg, 50 mg, 100 mg, 200 mg, and 400 mg or placebo at a ratio of 3:1, respectively (Fig. 2). Due to reversible, paresthesia-like events experienced by subjects at the 400-mg dose, subjects in cohort 7 were administered a deescalated dose of 300 mg, and 6 subjects were dosed (4 SPR206 and 2 placebo) instead of 8 in other cohorts (6 SPR206 and 2 placebo). All 54 subjects completed the study and were included in the safety analysis, and all 40 subjects who received SPR206 were included in the PK analysis.
FIG 2.
Study design.
In the multiple-ascending-dose (MAD) phase, 40 subjects were randomized to one of four ascending-dose cohorts and received SPR206 at 25 mg, 50 mg, 100 mg, and 150 mg or placebo every 8 h (q8h) for 7 days at a ratio of 3 active to 1 placebo (Fig. 2). Cohort 13 (n = 8) received a dose of 100 mg of SPR206 or placebo q8h for 14 days at the same ratio of 3 active to 1 placebo. All 40 (100.0%) subjects completed the study and were included in the safety analysis, and all 30 subjects who received SPR206 were included in the PK analysis.
In the SAD phase, the median age for all subjects was 26.0 years (range, 18.0 to 48.0 years), mean (standard deviation) body weight was 76.4 (9.4) kg, and mean (standard deviation) body mass index (BMI) was 24.3 (2.4) kg/m2. The majority were white (38 [70.4%]), and 14 were Asian (25.9%). In the MAD phase, the median age was 29.5 years (range, 18.0 to 55.0 years), mean body weight was 78.4 (10.2) kg, and mean BMI was 24.9 (2.3) kg/m2. The majority of participants were white (31 [77.5%]), and 9 (22.5%) were Asian.
Safety/tolerability.
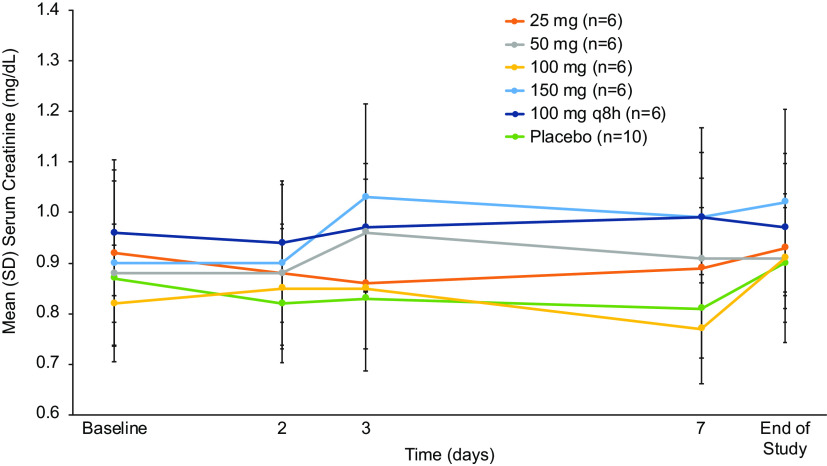
In the SAD phase, the incidence of adverse events (AEs) generally increased at doses of ≥300 mg, and in the MAD phase, the incidence of AEs was dose dependent (Table 1). Across both the SAD and MAD phases, 84% of all AEs were of mild severity, and there were no severe AEs. Two (2.1%) subjects in the highest dose group in the MAD phase (150 mg q8h) discontinued SPR206 for paresthesia and hypoesthesia. In the MAD phase, 2 subjects experienced a mild elevation of alanine aminotransferase levels >2 and <3 times the upper limit of normal (ULN) and aspartate aminotransferase levels >1.5 and <2 times the ULN, which resolved by day 15 without intervention. Alkaline phosphatase, bilirubin, and gamma-glutamyl transferase levels remained normal. One subject in the 150 mg q8h for 7 days cohort experienced mild changes in renal function: serum creatinine and calculated creatinine clearance (CrCl) remained within normal limits during dosing and follow-up, but CrCl decreased by 27% from baseline on day 7 and serum creatinine increased by 0.36 mg/dl on day 6. Of note, this subject experienced approximately 23% higher plasma area under the concentration-time curve from 0 to 8 h (AUC0–8) and maximum concentration of drug in serum (Cmax) on day 1 and day 7 relative to the mean values for the cohort. No other subjects at any dose or duration of dose experienced changes in serum creatinine (Fig. 3) or calculated creatinine clearance and no clinically significant changes in fractional excretion of calcium and magnesium or urine cation/Cr ratios (Ca and Mg) to suggest a change in renal function was observed. No serious AEs were reported, and no clinically significant changes were observed in vital signs, physical examination, or electrocardiogram (ECG) parameters.
TABLE 1.
Incidence of treatment emergent adverse events (safety population)
| Phase and adverse event parameter | No. (%) of subjects according to SPR206 group | |||||||
|---|---|---|---|---|---|---|---|---|
| Single ascending dose group (no. of subjects) | 10 mg (n = 6) | 25 mg (n = 6) | 50 mg (n = 6) | 100 mg (n = 6) | 200 mg (n = 6) | 400 mg (n = 6) | 300 mg (n = 4) | Pooled placebo (n = 14) |
| At least one treatment-emergent adverse event | 1 (16.7) | 0 | 1 (16.7) | 1 (16.7) | 2 (33.3) | 6 (100) | 4 (100) | 4 (28.6) |
| At least one treatment-related adverse event | 1 (16.7) | 0 | 1 (16.7) | 1 (16.7) | 2 (33.3) | 6 (100) | 4 (100) | 1 (7.1) |
| TEAEa occurring in >1 subject | ||||||||
| Dizziness | 0 | 0 | 0 | 0 | 0 | 5 (83.3) | 4 (100) | 1 (7.1) |
| Headache | 0 | 0 | 0 | 0 | 0 | 3 (50.0) | 1 (25.0) | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 2 (33.3) | 0 | 1 (7.1) |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 2 (33.3) | 0 | 0 |
| Vision blurred | 0 | 0 | 0 | 0 | 0 | 4 (66.7) | 0 | 0 |
| Paresthesia | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 3 (75.0) | 0 |
| Paresthesia oral | 0 | 0 | 0 | 0 | 0 | 0 | 3 (75.0) | 0 |
| Multiple ascending dose group (SPR206 doses every 8 h) | 25 mg (n = 6) × 7 days | 50 mg (n = 6) × 7 days | 100 mg (n = 6) × 7 days | 150 mg (n = 6) × 7 days | 100 mg (n = 6) × 14 days | Pooled placebo (n = 10) | ||
| At least one treatment-emergent adverse event | 4 (66.7) | 5 (83.3) | 6 (100) | 6 (100) | 4 (66.7) | 4 (40.0) | ||
| At least one treatment-related adverse event | 1 (16.7) | 4 (66.7) | 5 (83.3) | 4 (66.7) | 4 (66.7) | 2 (20.0) | ||
| TEAE leading to drug withdrawal | 0 | 0 | 0 | 2 (33.3) | 0 | 0 | ||
| TEAE occurring in >1 subject | ||||||||
| Dizziness | 0 | 1 (6.7) | 0 | 0 | 2 (33.3) | 2 (20.0) | ||
| Headache | 1 (6.7) | 2 (33.3) | 2 (33.3) | 0 | 1 (16.7) | 1 (10.0) | ||
| Paresthesia oral | 0 | 2 (33.3) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 1 (10.0) | ||
| Hypoesthesia oral | 0 | 0 | 3 (50.0) | 3 (50.0) | 0 | 0 | ||
| Constipation | 0 | 3 (50.0) | 0 | 1 (16.7) | 0 | 0 | ||
| Dry mouth | 0 | 0 | 0 | 2 (33.3) | 0 | 1 (10.0) | ||
| Infusion site phlebitis | 0 | 1 (16.7) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 0 | ||
TEAE, treatment emergent adverse event.
FIG 3.
Mean (standard deviation) serum creatinine values over time with multiple ascending doses of SPR206 (safety population).
Pharmacokinetics. (i) SAD phase.
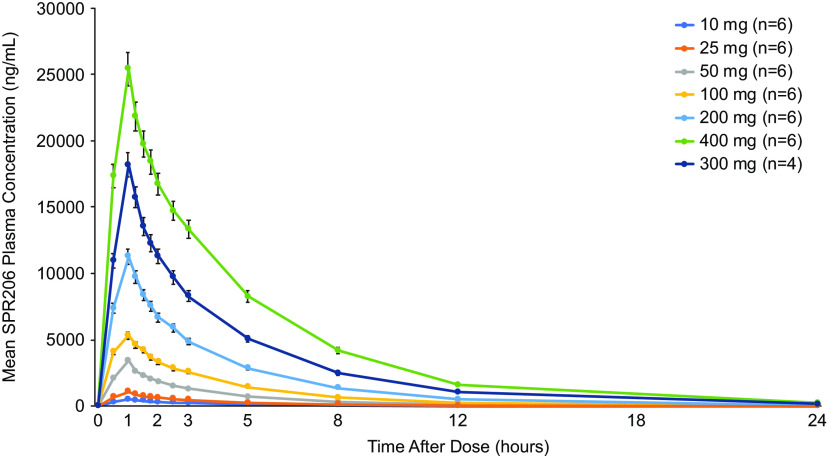
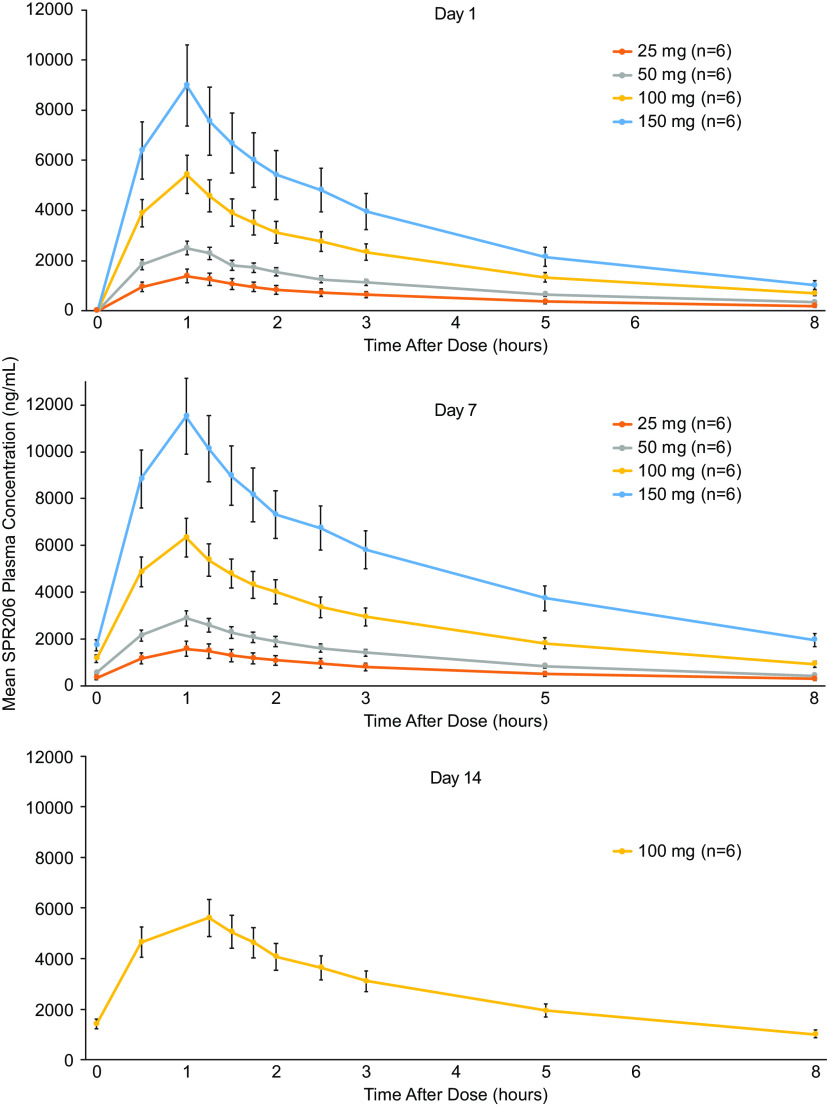
Plasma SPR206 concentrations increased with increasing dose (Fig. 4). Overall, mean SPR206 concentration-time profiles showed an initial peak at the end of infusion (i.e., 1 h following the start of infusion), followed by a biexponential decline. Mean peak plasma concentrations (Cmax) and systemic exposure (AUC) generally increased in a dose-proportional manner with SPR206 dose (Table 2). Interindividual variability in systemic exposure to SPR206 across doses was low with geometric coefficient of variation (CV) for AUC0–last, AUC0-inf, and Cmax ranging from 7.0% to 18.2%, 6.9% to 17.4%, and 6.9% to 13.8%, respectively. Dose proportionality estimates (90% confidence interval [CI]) for Cmax, AUC0–last, and AUC0–inf were 1.05 (1.02, 1.08), 1.14 (1.11, 1.18), and 1.11 (1.07, 1.14), respectively. Although 90% CIs did not include the value of 1, the upper limit of all CIs was <1.2, indicating that SPR206 exposure was generally dose proportional, although strict dose proportionality cannot be concluded.
FIG 4.
Mean (standard deviation) plasma SPR206 concentrations after single ascending doses (PK population).
TABLE 2.
Arithmetic mean PK parameters for SPR206 after single ascending doses (PK population)
| Parameter | SPR206 (mean ± SD) according to dose |
||||||
|---|---|---|---|---|---|---|---|
| 10 mg (n = 6) | 25 mg (n = 6) | 50 mg (n = 6) | 100 mg (n = 6) | 200 mg (n = 6) | 400 mg (n = 6) | 300 mg (n = 4) | |
| Cmax (ng/ml) | 495 ± 46.5 | 1,286 ± 184 | 3,422 ± 478 | 5,330 ± 634 | 11,265 ± 1,068 | 25,400 ± 1,747 | 18,175 ± 1,733 |
| Tmax (h)a | 1 (1.0–1.1) | 1 (1.0–1.0) | 1 (1.0–1.0) | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) |
| AUC0–8 (h · ng/ml) | 1,562 ± 203 | 3,806 ± 708 | 9,375 ± 1,098 | 17,324 ± 2,343 | 34,475 ± 2,279 | 89,257 ± 5978 | 57,733 ± 3,754 |
| AUC0–inf (h · ng/ml) | 1,809 ± 258 | 4,443 ± 841 | 10,674 ± 1,223 | 20,402 ± 3,200 | 40,871 ± 3,397 | 10,9757 ± 7,398 | 71,008 ± 5,201 |
| Half-life (h) | 2.6 ± 0.1 | 2.8 ± 0.2 | 2.6 ± 0.3 | 3.0 ± 0.3 | 3.4 ± 0.7 | 3.7 ± 0.5 | 4.1 ± 0.4 |
| CL (liters/h) | 5.6 ± 0.8 | 5.8 ± 0.9 | 4.7 ± 0.5 | 5.0 ± 0.9 | 4.9 ± 0.4 | 3.7 ± 0.3 | 4.2 ± 0.3 |
| Vz (liters) | 21.0 ± 3.4 | 23.5 ± 4.3 | 17.8 ± 2.9 | 21.4 ± 2.8 | 24.0 ± 3.1 | 19.4 ± 3.2 | 25.4 ± 3.3 |
Median (range).
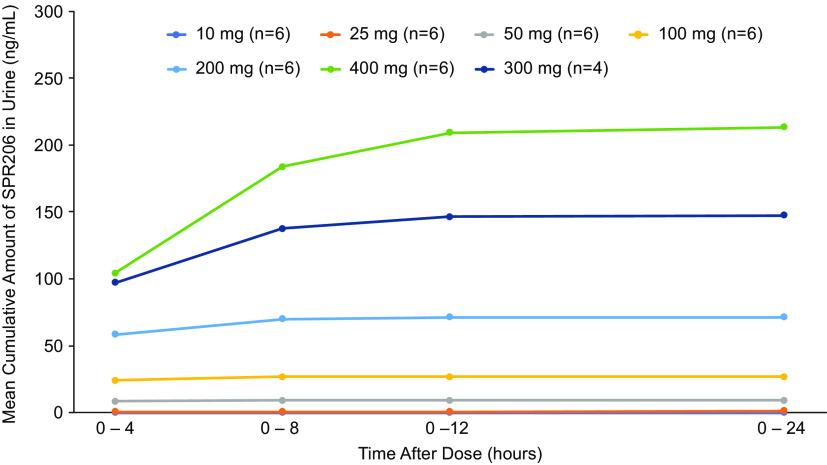
Mean cumulative amount of SPR206 excreted in urine increased with dose following single dose administration (Fig. 5). Generally, SPR206 was mostly excreted during the 0- to 4-h interval following the start of infusion and was low or below quantitation limit (BLQ) for the 4- to 8-h, 8- to 12-h, and 12- to 24-h collection times. Mean (standard deviation [SD]) total amount of SPR206 excreted from 0 to 24 h following the start of infusion on day 1 ranged from 0.08 mg at 10 mg up to 213.4 (24.8) mg at 400 mg (Table 3). Mean percentage excreted of SPR206 ranged from 0.7% to 53.4% after single doses of 10 to 400 mg. Renal clearance increased in a dose-proportional manner.
FIG 5.
Mean cumulative amount of SPR206 excreted in urine after single ascending doses (PK population).
TABLE 3.
SPR206 urine PK parameters in single- and multiple-dose cohorts (PK population)
| SPR206 dose group | Mean ± SDa |
|||
|---|---|---|---|---|
| Ae (mg) | CLR (L/h) | Fe | Fe (%) | |
| SAD | ||||
| 10 mg (n = 1) | 0.75 | 0.034 | 0.007 | 0.70 |
| 25 mg (n = 5) | 1.33 ± 1.09 | 0.32 ± 0.29 | 0.05 ± 0.04 | 5.32 ± 4.38 |
| 50 mg (n = 6) | 9.01 ± 0.95 | 0.85 ± 0.10 | 0.18 ± 0.02 | 18.02 ± 1.93 |
| 100 mg (n = 6) | 26.88 ± 5.06 | 1.35 ± 0.34 | 0.27 ± 0.05 | 26.92 ± 5.06 |
| 200 mg (n = 6) | 71.36 ± 7.68 | 1.77 ± 0.20 | 0.36 ± 0.04 | 35.68 ± 3.85 |
| 400 mg (n = 6) | 213.42 ± 24.78 | 1.97 ± 0.22 | 0.53 ± 0.06 | 53.35 ± 6.21 |
| 300 mg (n = 4) | 147.54 ± 22.49 | 2.13 ± 0.43 | 0.49 ± 0.08 | 49.15 ± 7.50 |
| MAD day 1 | ||||
| 25 mg q8h (n = 5) | 1.04 ± 0.67 | 0.24 ± 0.15 | 0.04 ± 0.03 | 4.14 ± 2.70 |
| 50 mg q8h (n = 6) | 6.51 ± 1.83 | 0.82 ± 0.25 | 0.13 ± 0.04 | 13.03 ± 3.66 |
| 100 mg q8h (n = 6) | 25.28 ± 4.86 | 1.55 ± 0.38 | 0.25 ± 0.05 | 25.28 ± 4.87 |
| 150 mg q8h (n = 6) | 57.66 ± 7.86 | 2.13 ± 0.44 | 0.38 ± 0.05 | 38.42 ± 5.23 |
| 100 mg q8h (14 d) (n = 6) | 24.97 ± 8.08 | 1.58 ± 0.39 | 0.25 ± 0.08 | 24.95 ± 8.08 |
| MAD day 7 | ||||
| 25 mg q8h (n = 6) | 1.76 ± 0.73 | 0.22 ± 0.06 | 0.07 ± 0.03 | 7.03 ± 2.94 |
| 50 mg q8h (n = 6) | 8.51 ± 2.82 | 0.69 ± 0.20 | 0.17 ± 0.06 | 17.03 ± 5.64 |
| 100 mg q8h (n = 6) | 33.09 ± 13.40 | 1.25 ± 0.50 | 0.33 ± 0.13 | 33.08 ± 13.94 |
| 150 mg q8h (n = 4) | 81.17 ± 3.81 | 1.55 ± 0.17 | 0.54 ± 0.03 | 54.10 ± 1.52 |
| 100 mg q8h (14 d) (n = 6) | 36.30 ± 6.72 | 1.29 ± 0.24 | 0.36 ± 0.07 | 36.30 ± 6.73 |
Ae, cumulative amount of drug excreted in successive urine intervals with quantifiable urine concentrations; CLR, renal clearance; Fe, fraction of cumulative fraction of dose recovered in urine as unchanged drug in successive urine intervals with quantifiable urine concentrations; Fe (%), percentage fraction of cumulative fraction of dose recovered in urine as unchanged drug in successive urine intervals with quantifiable urine concentrations.
TABLE 4.
Arithmetic mean for PK parameters for SPR206 after multiple ascending doses on day 1 and day 7 or day 14 (PK population)
| Day and parameter | SPR206 (mean ± SD) | ||||
|---|---|---|---|---|---|
| Day 1 | 25 mg q8h × 7 days (n = 6) | 50 mg q8h × 7 days (n = 6) | 100 mg q8h × 7 days (n = 6) | 150 mg q8h × 7 days (n = 6) | 100 mg q8h × 14 days (n = 6) |
| Cmax (ng/ml) | 1,410 ± 241 | 2,525 ± 413 | 5,433 ± 678 | 8,990 ± 1,056 | 5,065 ± 1,003 |
| Tmax (h)a | 1.1 (1.0, 1.3) | 1.1 (1.1, 1.3) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.3) |
| AUC0–8 (h · ng/ml) | 4,391 ± 740 | 7,955 ± 951 | 16,544 ± 2,337 | 27,492 ± 3,452 | 15,573 ± 1,765 |
| AUC0–inf (h · ng/ml) | 5,197 ± 945 | 9,243 ± 1,417 | 19,171 ± 2,874 | 30,981 ± 4,189 | 18,184 ± 1,244 |
| Half-life (h) | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.7 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.2 |
| CL (liters/h) | 4.9 ± 0.9 | 5.5 ± 0.8 | 5.3 ± 0.8 | 4.9 ± 0.6 | 5.5 ± 0.4 |
| Vz (liters) | 18.9 ± 2.6 | 21.5 ± 1.8 | 20.8 ± 2.9 | 17.1 ± 2.1 | 19.9 ± 3.0 |
| Day 7 or day 14 | 25 mg q8h × 7 days (n = 6) | 50 mg q8h × 7 days (n = 6) | 100 mg q8h × 7 days (n = 6) | 150 mg q8h × 7 days (n = 6) | 100 mg q8h × 14 days (n = 6) |
| Cmax (ng/ml) | 1,607 ± 300 | 2,897 ± 270 | 6,345 ± 1099 | 11,518 ± 1,901 | 6,423 ± 841 |
| Tmax (h)a | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.1) | 1.1 (1.1, 1.1) | 1.1 (1.0, 1.1) |
| AUC0–8 (h · ng/ml) | 5,881 ± 1,166 | 10,050 ± 1,050 | 21,340 ± 2,857 | 41,475 ± 6,083 | 22,277 ± 2,574 |
| AUC0–48 (h · ng/ml) | 7,681 ± 1,726 | 12,435 ± 1,796 | 27,531 ± 4,610 | 55,505 ± 7,838 | 29,828 ± 5,112 |
| AUC0–inf (h · ng/ml) | 7,690 ± 1,732 | 12,443 ± 1,802 | 27,671 ± 4,762 | 56,007 ± 7,874 | 30,412 ± 5,699 |
| AUC%ext (h · ng/ml) | 10.0 ± 4.5 | 6.9 ± 2.5 | 1.9 ± 0.4 | 0.9 ± 0.1 | 3.3 ± 1.4 |
| Half-life (h) | 4.0 ± 0.6 | 3.5 ± 0.9 | 5.3 ± 1.4 | 5.9 ± 0.3 | 9.6 ± 8.1 |
| CL (liters/h) | 4.4 ± 0.9 | 5.0 ± 0.5 | 4.8 ± 0.6 | 3.7 ± 0.5 | 4.5 ± 0.5 |
| Vz (liters) | 21.3 ± 3.6 | 21.5 ± 2.2 | 22.5 ± 3.5 | 19.6 ± 3.2 | 23.9 ± 4.0 |
Median (range).
(ii) MAD phase.
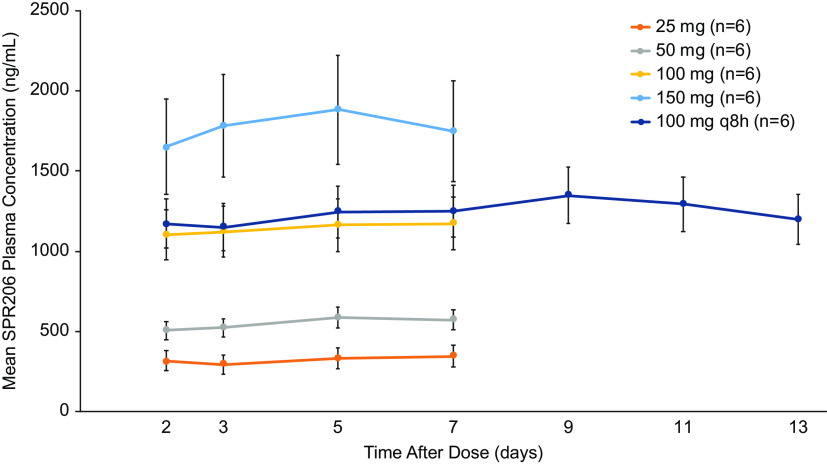
For each cohort, mean plasma SPR206 levels increased with increasing dose levels following q8h dosing for 7 or 14 days (Fig. 6). Systemic exposure (AUC) to SPR206 increased in a dose-proportional manner (Table 4). Time to maximum concentration of drug in serum (Tmax) was 1.1 h across all dose regimens. SPR206 steady state was achieved by day 2 with q8h dosing based on Ctrough levels. Similar to SAD, interindividual variability in systemic exposure to SPR206 was generally low with geometric CVs for AUC0–last, AUC0–inf, and Cmax ranging from 11.8% to 16.8%, 6.8% to 18.7%, and 10.9% to 21.7% on day 1 and 13.8% to 24.3%, 13.7% to 24.6%, and 9.5% to 17.7% on day 7, respectively, across all doses. Based on estimates of the exponent from the power model, estimates (90% CI) for Cmax, AUC0–last, and AUC0–inf were 1.03 (0.96, 1.10), 1.07 (0.98, 1.16), and 1.03 (0.94, 1.12), respectively, and for Cmax, AUC0–last, and AUC0–inf, the 90% CIs included the value of 1 for all three parameters. Trough concentrations of SPR206 remained constant during the 7- and 14-day dosing periods (Fig. 7).
FIG 6.
Mean (standard deviation) plasma SPR206 plasma concentrations following multiple ascending doses on day 1, day 7, and day 14 (PK population).
FIG 7.
Mean (standard deviation) SPR206 trough concentrations following 7-day and 14-day administration.
For cohorts 9 through 12 (q8h dosing for 7 days), the mean (SD) total amount of SPR206 excreted in urine on day 7 (0 to 24 h) following the start of infusion increased with increasing dose ranging from 1.8 mg (0.7) for the 25-mg dose up to 81.2 mg (3.8) for the 150-mg dose. For cohort 13 (100 mg q8h dosing for 14 days), the mean total amount of SPR206 excreted in urine on day 14 was 36.3 mg (6.7). Similar to the SAD phase, the majority of SPR206 excreted in urine was observed during the 0- to 4-h collection period on day 7 or day 14 (cohort 13), and the amount excreted generally decreased for each subsequent collection interval. The mean percentage of dose excreted in urine increased with increasing dose level (Table 3). On day 1, mean (SD) percentage dose excreted was 4.1 (2.7), 13.0 (3.7), 25.3 (4.9), 25.0 (8.1), and 38.4 (5.2) with 25-mg, 50-mg, 100-mg (7 days), 100-mg (14 days), and 150-mg doses, respectively. On day 7, mean (SD) percentage excreted was 7.0 (2.9), 17.0 (5.6), 33.1 (13.4), 36.3 (6.7), and 54.1 (2.5) with 25-mg, 50-mg, 100-mg (7 days), 100-mg (14 days), and 150-mg doses, respectively. Renal clearance increased in a dose-proportional manner. No clinically significant changes in urine cation/Cr ratios (Ca and Mg) were observed.
DISCUSSION
Results from this FIH study demonstrated that SPR206 was generally well tolerated after single and multiple doses with no serious AEs. The most common AEs were mild paresthesia, dizziness, and headache with a greater frequency at higher doses. Five paresthesia events considered nervous system disorders occurred in 4 (7.4%) subjects at 300- or 400-mg doses in the SAD cohort. Eight events of oral hypoesthesia in 6 subjects and 6 events of oral paresthesia occurred in 5 subjects in the MAD portion. All paresthesia events were of mild severity, and none led to study discontinuation. Mild, reversible paresthesia-like events are well described with the polymyxin class of antibiotics (33). In this study, there was only 1 report of mild, self-resolved paresthesia over 14 days of dosing at 100 mg every 8 h. SPR206 had no clinically significant effect on renal function as assessed from serum creatinine and creatinine clearance at doses up to 100 mg administered every 8 h for 14 days. Although remaining well within normal limits, slight changes in a single subject in serum creatinine and calculated creatinine clearance on the last day of dosing (day 7) in the highest dose group of 150 mg q8h may indicate a mild decline in renal function caused by SPR206. Further study in critically ill patients with severe infections due to resistant bacteria are needed to fully assess the impact of SPR206 on renal function. In addition, SPR206 had no effect on the ECG and no clinically significant effect on liver function.
SPR206 exhibited a dose-proportional PK profile over the range of doses studied in both SAD and MAD phases. Minimal accumulation of SPR206 occurred during the MAD phase as evidenced by low accumulation ratios for Cmax (1.1 to 1.3) and AUC0–inf (1.3 to 1.8). Intersubject variability in exposure was low, ranging from 10% to 25% across dose cohorts. Half-life ranged from 2.4 to 4.1 h, which supports q8h dosing. Trough concentrations (Ctrough) of SPR206 indicated that the steady state was achieved by day 2 with repeat dosing. Urinary excretion of unchanged SPR206 was dose dependent across single- and multiple-ascending-dose cohorts, and approximately 50% of the dose was excreted as SPR206. These data will be useful for determining dose and regimen for future studies of SPR206.
Aminoglycosides and polymyxins often are reserved for treating patients with serious infections due to MDR Gram-negative bacteria; however, a major limitation of these drugs is the increased risk for renal toxicity (34–36). The use of aminoglycosides requires regular therapeutic drug monitoring to assess drug concentrations and to adjust dosages as necessary to maintain plasma concentrations within a narrow therapeutic window and to avoid toxicity (37, 38). SPR206 is a novel polymyxin B (PMB) analogue with a β-branched aminobutyrate N terminus, bearing an aryl substituent that has been shown to possess lower kidney cell cytotoxicity and lower exposure in the kidney of rats than PMB (31, 32). The discovery of SPR206 was the culmination of an extensive medicinal chemistry effort focused on significantly improving the nephrotoxicity profile compared to current polymyxins (31), while retaining potent in vitro and in vivo activity against MDR Gram-negative pathogens. The first-in-human phase 1 study of SPR206 presented here demonstrates SPR206 to be devoid of nephrotoxicity at doses up to and including 100 mg q8h for 14 days in healthy subjects, whereas it is documented that colistin use at the recommended dosing regimen is associated with up to 60% incidence of acute kidney injury (38). As such, SPR206 has the potential to offer a spectrum of activity similar or superior to current polymyxins against MDR pathogens causing serious infections but with a meaningful improvement in safety profile and clinical outcome.
SPR206 is being developed for i.v. administration in the hospital settings to treat serious infections of the lung, blood, and urinary tract caused by resistant Gram-negative pathogens. SPR206 may offer an alternative to polymyxin- and aminoglycoside-based therapy in hospitalized patients with serious infections.
MATERIALS AND METHODS
This study was conducted according to the principles of the Declaration of Helsinki and Guidance on Good Clinical Practice. The study protocol, amendments, and informed consent forms were reviewed and approved by an independent ethics committee. All subjects provided written informed consent prior to participating in any study activities. This study was registered at ClinicalTrials.gov under identifier NCT03792308.
Study design.
This was a single-center, phase 1, randomized, double-blind, placebo-controlled, first-in-human study to assess the safety, tolerability, and PK of SPR206 following SAD and MAD administrations (Fig. 7). In the SAD phase, subjects received a single i.v. dose of SPR206, and in the MAD phase, subjects received i.v. doses of SPR206 every 8 h (q8h) over a period of 7 to 14 days. SPR206 was administered as a 1 h i.v. infusion.
In the SAD phase, healthy subjects were screened within 28 days prior to dosing and admitted to the clinical facility on day −1. A single i.v. dose of SPR206 or placebo was administered on day 1. Following completion of all safety assessments and sampling for PK analyses, subjects were discharged on day 2. A follow-up visit occurred 5 to 7 days after day 1 dosing.
In the SAD phase, subjects were randomized to one of seven cohorts consisting of 10-, 25-, 50-, 100-, 200-, 300-, and 400-mg doses of SPR206. Within each cohort, two subjects received placebo and six subjects received SPR206. Two subjects (sentinels) were dosed with SPR206 or placebo 48 h prior to the remaining subjects. The remaining six subjects were only dosed after no safety concerns were identified in the sentinel subjects. After each dose cohort had completed study drug dosing and safety evaluations, a safety monitoring group (SMG) reviewed blinded cumulative safety data (including day 5 to 7 follow-up data) to confirm the safety and tolerability of SPR206. Blood and urine samples were collected for assessment of PK parameters.
In the MAD phase, all subjects were admitted to the clinical facility on day −1. Dosing commenced on the morning of day 1. Three doses were administered daily at approximately 8 (±0.5)-hour intervals for a total of 7 consecutive days (cohorts 9 through 12) and 14 consecutive days (cohort 13). The last dose was administered on the morning of day 14. Subjects were discharged on day 16 following completion of all PK sample collection and safety assessments, and a follow-up visit occurred 12 to 14 days after the last dose.
The MAD phase began after completion of the SAD phase, and the appropriate starting dose level was established. Two subjects received placebo, and six subjects received SPR206 doses of 25, 50, 100, and 150 mg q8h for 7 days and 100 mg q8h for 14 days. In each cohort of the MAD, two subjects (sentinels) began dosing with SPR206 or placebo 72 h prior to the remaining subjects in this cohort. The remaining six subjects were only dosed after no safety concerns were identified by the principal investigator. After each MAD dose cohort had completed administration of study drug and all evaluations, the safety monitoring group reviewed blinded cumulative safety data (including the day 19 to 21 follow-up data) to confirm the safety and tolerability of the study drug. Blood and urine samples were collected to measure SPR206 drug levels to determine SPR206 PK parameters.
Subject selection.
Healthy adult subjects aged 18 to 55 years with a body mass index (BMI) of 18.5 to 29.9 kg/m2 inclusive and weight between 55 and 100 kg were eligible. Subjects were medically healthy with no clinically significant abnormalities based on physical examination, vital signs, ECG, and clinical laboratory testing. Subjects were excluded for any clinically significant medical condition or laboratory abnormality; presence or history of any clinically significant cardiac abnormalities including clinically significant ECG abnormalities; history of seizure disorders; history of Clostridium difficile infection; positive human immunodeficiency virus (HIV) antibody, hepatitis B surface antigen (HBsAg), or hepatitis C antibody; positive urine drug/alcohol test or history of substance or alcohol abuse; documented hypersensitivity or anaphylaxis to any medication; use of tobacco or nicotine-containing products within 30 days; receipt of any investigational drug or participation in a clinical trial within 30 days; or use of any prescription or over-the-counter medications within 7 days of randomization.
Study assessments.
Safety assessments included clinical laboratory testing (hematology, coagulation, serum chemistry, urinalysis), vital signs (blood pressure, heart rate, body temperature, respiratory rate), physical examination, and triplicate 12-lead ECG to assess corrected QT interval by Fredericia (QTcF). In the SAD phase, continuous cardiac monitoring was performed from 1 h predose through 24 h postdose. Adverse events were recorded at each study visit.
In the MAD phase, 24-h creatinine clearance (CrCl) based on plasma and urine creatinine concentrations was determined prior to any dosing and following the last dose on day 14. Serum creatinine concentrations were measured from the clinical laboratory tests performed on days −1 and 15. Urine creatinine concentration were measured using 24 h collections prior to the first dose on day 1 and over 24 to 48 h following the start of infusion of the last dose (day 14).
Pharmacokinetic analysis.
Maximum plasma concentration (Cmax), area under the concentration-time curve from time zero to last measurable time point (AUC0–t), area under the concentration-time curve from time zero to infinity (AUC0–inf), area under the concentration-time curve from time zero to tau (AUC0–tau), time to maximum concentration (Tmax), terminal elimination rate constant (kel), terminal half-life (t1/2), terminal clearance (CL), and volume of distribution (Vz) were determined. In addition, area under the concentration-time curve from 0 to 24 h after the start of infusion (AUC0–24) was determined for the SAD phase, and area under the concentration-time curve from time zero to 8 h from start of infusion (AUC0–8) on day 1 and 0 to 48 h (AUC0–48) following the last dose on day 14 as well as predose trough concentrations were determined for the MAD phase. Urine parameters included cumulative amount of drug excreted in successive urine intervals (Ae), renal clearance (CLR), fraction of cumulative fraction of dose recovered in urine as unchanged drug (Fe), and percentage fraction of cumulative fraction of dose recovered in urine as unchanged drug (Fe%). PK parameters were determined by noncompartmental analysis using Phoenix WinNonlin 8.1.
For the SAD phase, blood samples were obtained predose and at 30, 60, 75, 90, 105, 120, and 150 min and at 3, 5, 8, 12, and 24 h following the start of infusion. Urine was collected for PK assessment predose and over the intervals of 0 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h after the start of infusion.
In the MAD phase, plasma samples for PK analysis were collected (i) on day 1—pre-dose and at 30, 60, 75, 90, 105, 120, and 150 min and at 3, 5, and 8 h following start of the first infusion; (ii) prior to the morning dose (within 10 min) on days 2, 3, 5, 7, 9, 11, and 13; and (iii) predose and 30, 75, 90, 105, 120, and 150 min and 3, 5, 8, and 12 h (day 14), 24, 36 (day 15), and 48 h (day 16) following start of the last infusion. Urine was collected for PK assessment on day 1 predose, over the intervals 0 to 4 h and 4 to 8 h after start of first infusion, and on days 14 and 15 over the intervals 0 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h after start of day 14 infusion. Total 24-h urine for calculating CrCl was collected from the morning of day −1 to predose on day 1 and on days 15 and 16 over the interval from 24 to 48 h after the start of the day 14 infusion. Urine creatinine concentrations were measured using 24-h collections prior to the first dose on day 1 and over the 24 to 48 h following start of infusion of the last dose (day 14). Plasma and urine samples for SPR206 levels were analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The assay range for SPR206 was 50 to 5,000 ng/ml in plasma and 100 to 50,000 ng/ml in urine. In plasma, assay precision was 1.3 to 7.7%, accuracy was 1.2 to 9.5%, stability was 194 h at 4°C, and reproducibility was 98.4%. In urine, assay precision was 1.7 to 7.5%, accuracy was −4.8 to 7.3%, stability was 147 h at 4°C, and reproducibility was 97.8%.
Statistical analysis.
Plasma and urine concentrations and PK parameters for SPR206 were summarized for each treatment using descriptive statistics. In the SAD/MAD study, geometric means were calculated for AUC and Cmax. Analyses using linear models were performed to assess dose proportionality (both single dose and multiple dose), time dependence, accumulation, and attainment of steady state after multiple doses. Dose-linearity of Cmax and AUC across the dose range was assessed by fitting the power model and testing for β = 1 using a generalized linear model. Point estimates and 90% confidence intervals (CI) using the residual mean square error obtained from the analysis of variance (ANOVA) were constructed for the comparisons between treatments. The point and CI estimates were back-transformed to give estimates of the ratios (%) of the geometric least square means (LSmeans) and corresponding 90% CI.
ACKNOWLEDGMENTS
We acknowledge the editorial assistance of Richard S. Perry in the development of this manuscript, which was supported by Spero Therapeutics, Cambridge, MA. The study was conducted by James Kuo and Charolette Lemech from Scientia Clinical Research, Ltd., Randwick, Australia.
Research for the SPR206 program is funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201500014C.
All authors performed data analysis and interpretation as well as manuscript review and approval.
L.M. and J.B. were consultants to Spero Therapeutics. All other authors were paid employees of Spero Therapeutics, Cambridge, MA.
This study was supported by Spero Therapeutics, Cambridge, MA.
REFERENCES
- 1.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 3.Cai B, Echols R, Magee G, Arjona Ferreira JC, Morgan G, Ariyasu M, Sawada T, Nagata TD. 2017. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 4:ofx176. 10.1093/ofid/ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 5.Boral B, Unaldi Ö, Ergin A, Durmaz R, Eser ÖK, Acinetobacter Study Group. 2019. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann Clin Microbiol Antimicrob 18:19. 10.1186/s12941-019-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulens SN, Yi SH, Walters MS, Jacob JT, Bower C, Reno J, Wilson L, Vaeth E, Bamberg W, Janelle SJ, Lynfield R, Vagnone PS, Shaw K, Kainer M, Muleta D, Mounsey J, Dumyati G, Concannon C, Beldavs Z, Cassidy PM, Phipps EC, Kenslow N, Hancock EB, Kallen AJ. 2018. Carbapenem-nonsusceptible Acinetobacter baumannii, 8 US Metropolitan areas, 2012–2015. Emerg Infect Dis 24:727–734. 10.3201/eid2404.171461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis 6:S34–S46. 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LynchJP, III, Zhanel GG, Clark NM. 2017. Infections due to Acinetobacter baumannii in the ICU: treatment options. Semin Respir Crit Care Med 38:311–325. 10.1055/s-0037-1599225. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A, Chuang Y-C, Sun H-Y, Sheng W-H, Yang C-J, Liao C-H, Hsueh P-R, Yang J-L, Shen N-J, Wang J-T, Hung C-C, Chen Y-C, Chang S-C. 2015. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med 43:1194–1204. 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 10.Clark NM, Zhanel GG, Lynch JP. 2016. Emergence of antimicrobial resistance among Acinetobacter species: a global threat. Curr Opin Crit Care 22:491–499. 10.1097/MCC.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 11.Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, Kawai K. 2014. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 20:416–423. 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Bonomo RA. 2015. Combination therapy for extreme drug-resistant (XDR) Acinetobacter baumannii: ready for prime-time? Crit Care Med 43:1332–1334. 10.1097/CCM.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilberberg MD, Kollef MH, Shorr AF. 2016. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 11:21–26. 10.1002/jhm.2477. [DOI] [PubMed] [Google Scholar]
- 15.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. 2016. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care 20:221. 10.1186/s13054-016-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters MS, Grass JE, Bulens SN, Hancock EB, Phipps EC, Muleta D, Mounsey J, Kainer MA, Concannon C, Dumyati G, Bower C, Jacob J, Cassidy PM, Beldavs Z, Culbreath K, PhillipsWE, Jr, Hardy DJ, Vargas RL, Oethinger M, Ansari U, Stanton R, Albrecht V, Halpin AL, Karlsson M, Rasheed JK, Kallen A. 2019. Carbapenem-resistant Pseudomonas aeruginosa at US emerging infections program sites, 2015. Emerg Infect Dis 25:1281–1288. 10.3201/eid2507.181200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaden JT, Park LP, Maskarinec SA, Ruffin F, FowlerVG, Jr, van Duin D. 2017. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother 61:e02671-16. 10.1128/AAC.02671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Chen XL, Huang AW, Liu SL, Liu WJ, Zhang N, Lu XZ. 2016. Mortality attributable to carbapenem-resistant Pseudomonas aeruginosa bacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect 5:e27. 10.1038/emi.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaya-Villar R, Garnacho-Montero J. 2019. How should we treat Acinetobacter pneumonia? Curr Opin Crit Care 25:465–472. 10.1097/MCC.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 20.Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. 2019. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect 25:951–957. 10.1016/j.cmi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. 2019. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol 10:80. 10.3389/fmicb.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul-Mutakabbir JC, Nguyen L, Stamper K, Maassen P, Lev K, Morrisette T, Kebriaei R, Rybak MJ. 2020. Activity of SPR206, a polymyxin derivative, compared to colistin alone and in combination against multidrug-resistant Pseudomonas aeruginosa strains. Open Forum Infect Dis 7:S662–S663. 10.1093/ofid/ofaa439.1478. [DOI] [Google Scholar]
- 23.Arends SJR, Rhomberg PR, Lister T, Cotroneo N, Rubio A, Flamm RK, Mendes RE. 2018. Activity of an investigational polymyxin-b-like compound (SPR206) against a set of Enterobacteriaceae organisms responsible for human infections, poster 81. Abstr ESCMID-ASM.
- 24.Arends SJR, Rhomberg PR, Lister T, Cotroneo N, Rubio A, Flamm RK, Mendes RE. 2019. Activity of an investigational polymyxin-b-like compound (SPR206) against a set of gram-negative bacilli responsible for human infections, poster AAR-796. Abstr ASM Microbe.
- 25.Arends SJR, Rhomberg PR, Lister T, Cotroneo N, Rubio A, Flamm RK, Mendes RE. 2018. In vitro activity evaluation of a next-generation polymyxin, SPR206, against non-fermentative gram-negative bacilli responsible for human infections, poster 80. Abstr ESCMID-ASM.
- 26.Cotroneo N, Tomich A, Zou Y, Lister T, Brown P, Dawson M. 2019. In vitro bactericidal activity of next-generation polymyxin SPR206 against susceptible and multidrug-resistant (MDR) Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae as compared to levofloxacin and meropenem, poster AAR-797. Abstr ASM Microbe.
- 27.Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Wang H. 2020. Evaluation of the in vitro activity of new polymyxin B analogue SPR206 against clinical MDR, colistin-resistant and tigecycline-resistant Gram-negative bacilli. J Antimicrob Chemother 75:2609–2615. 10.1093/jac/dkaa217. [DOI] [PubMed] [Google Scholar]
- 28.Grosser L, Heang K, Teague J, Warn P, Corbett D, Dawson MJ, Rubio A. 2018. In vivo efficacy of SPR206 in murine lung and thigh infection models caused by multidrug resistant pathogens Pseudomonas aeruginosa and Acinetobacter baumannii, poster 139. Abstr ESCMID-ASM.
- 29.Grosser L, Lister T, Heang K, Brown P, Dawson M, Rubio A. 2019. In vivo efficacy of next-generation polymyxin SPR206 in an immunocompetent murine ascending UTI infection model caused by Escherichia coli, poster AAR-798. Abstr ASM Microbe.
- 30.Grosser L, Lister T, Heang K, Warn P, Corbett D, Dawson MJ, Rubio A. 2019. In vivo efficacy of SPR206 in murine lung and thigh infection models caused by multidrug resistant pathogens Pseudomonas aeruginosa and Acinetobacter baumannii, poster AAR-799. Abstr ASM Microbe.
- 31.Brown P, Abbott E, Abdulle O, Boakes S, Coleman S, Divall N, Duperchy E, Moss S, Rivers D, Simonovic M, Singh J, Stanway S, Wilson A, Dawson MJ. 2019. Design of next generation polymyxins with lower toxicity: the discovery of SPR206. ACS Infect Dis 5:1645–1656. 10.1021/acsinfecdis.9b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown P, Boakes S, Duperchy E, Rivers D, Singh J, Dawson MJ. 2018. Understanding the SAR interplay for kidney exposure and cytotoxicity facilitates the design of improved polymyxin derivatives—identification of SPR206 as a development candidate, poster 145. Abstr ESCMID-ASM.
- 33.Falagas ME, Kasiakou SK. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27. 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. 2010. The future of aminoglycosides: the end or renaissance? Chembiochem 11:880–902. 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 35.Nation RL, Rigatto MHP, Falci DR, Zavascki AP. 2019. Polymyxin acute kidney injury: dosing and other strategies to reduce toxicity. Antibiotics (Basel) 8:E24. 10.3390/antibiotics8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zavascki AP, Nation RL. 2017. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 61:e02319-16. 10.1128/AAC.02319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins A, Thomson AH, Brown NM, Semple Y, Sluman C, MacGowan A, Lovering AM, Wiffen PJ, BSAC Working Party on Therapeutic Drug Monitoring. 2016. Amikacin use and therapeutic drug monitoring in adults: do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J Antimicrob Chemother 71:2754–2759. 10.1093/jac/dkw250. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]