Abstract
In Thailand, glyphosate is popular herbicide to control pests in the agricultural sector. This study aimed to measure glyphosate exposure concentrations through inhalation, dermal contact, and urinary glyphosate concentrations among 43 vegetable farmers spraying glyphosate in Bungphra Subdistrict, Phitsanulok Province. Four types of spraying equipment were used, manual pump backpack (n = 3), motorized spray backpack (n = 22), battery pump backpack (n = 16), and high pressure pump (n = 2). Breathing zone air samples were collected using glass fiber filters; dermal contact samples were collected using 100 cm2 cotton patches attached on 10 body locations and urine samples were collected at 3 time points: morning void urine the day before spraying, the end of spraying event, and the morning void urine the next day of spraying. The results showed that the geometric mean (GM; geometric standard deviation [GSD]) of breathing zone concentrations of glyphosate exposure were 9.37 (10.17) μg/m3. The GM (GSD) of total dermal patches exposure concentrations were 7.57 (0.01) mg/h. The legs, back, and arms were the most exposed body areas. The GM (GSD) of urinary glyphosate was found highest among vegetable farmers using manual backpack 46.90 (1.35) μg/g creatinine. Farmers should wear masks and boots to reduce glyphosate exposure by inhalation and dermal contact.
Keywords: glyphosate, vegetable farmers, dermal and inhalation exposures, biomonitoring
Introduction
Glyphosate (N-(phosphonomethyl) glycine) is a synthetic broad spectrum, postemergent, nonselective, and universal herbicide (Agostini et al. 2020) widely used to kill unwanted plants in agricultural areas. Glyphosate products have been increasingly used by farmers in preparing fields before planting and in till soil conservation programs (Amrhein et al. 1980; Székács and Darvas 2012). The United States Environmental Protection Agency (US EPA) classified glyphosate as group E-evidence of noncarcinogenicity in humans (United States Environmental Protection Agency 2018) but the International Agency for Research on Cancer (IARC) classified as probably carcinogenic to humans (Group 2A) (International Agency for Research on Cancer 2015). Rats orally exposed to [14C] glyphosate had very low transformation and glyphosate was presented as unchanged parent compound. Aminomethylphosphonic acid (AMPA) was the only metabolite, accounting for only 0.2–0.3% of the applied dose of [14C] glyphosate (International Programme on Chemical Safety 1994). Glyphosate is mostly not metabolized in the human body and thus the parent compound can be measured in urine. Oral ingestion of glyphosate in rats suggested an elimination half-life of 33 h for glyphosate in humans (International Agency for Research on Cancer 2016). A recent human study suggested a rapid phase half-life between 4 and 17 h (Faniband et al. 2017). Connolly et al. (2019) estimated half-life of glyphosate in urine was between 3 and 20 h after exposure. In addition, glyphosate could cause various health symptoms after exposure including gastrointestinal symptoms, altered consciousness (Zouaoui et al. 2013), hypertension, respiratory distress (Tominack et al. 1991; Sapbamrer and Seesen 2020) metabolic acidosis and renal failure (Jayasumana et al. 2013, 2014, 2015; Zouaoui et al. 2013). Moreover, glyphosate exposure increased the risks of chromosomal damage and cytotoxicity in humans (Bolognesi et al. 2009). Further, case–control studies found that glyphosate increased the risk of nonHodgkin’s lymphoma (McDuffie et al. 2001; Hardell et al. 2002; Eriksson et al. 2008).
Pesticide exposure can occur through several pathways and routes, both directly and indirectly. When farmers directly handle pesticides such as mixing and applying and cleaning pesticide equipment; they have opportunity to be exposed to pesticides via spill-age, spraying, splattering, and drifting (Harvey 2014). Exposure generally occurs through the skin, but inhalation and indirect ingestion can occur as well. Several researchers have studied breathing zone air exposure of glyphosate during spraying (Jauhiainen et al. 1991; Lavy et al. 1992; Johnson et al. 2005; Morshed et al. 2011). Some studies have investigated glyphosate biomonitoring exposure of agricultural family members (Acquavella et al. 2004; Curwin et al. 2005; Mesnage et al. 2012). The investigation of glyphosate exposure through inhalation and dermal absorption and urinary excretion of glyphosate among farmers spraying glyphosate would provide useful information of exposure from occupation (Niemann et al. 2015). Spraying glyphosate with different types of spraying equipment was scarcely found in the related literature. This study aimed to assess glyphosate exposure using different spraying equipment through inhalation, dermal contact, and urinary excretion of glyphosate before and after spraying among vegetable farmers. The study will provide a comprehensive assessment of occupational exposures among vegetable farmers and factors affected the exposure through inhalation and dermal absorption which are useful for developing interventions for exposure prevention.
Material and methods
Study population and spraying equipment
This research employed a cross-sectional study design recruiting vegetable farmers in Bungphra Subdistrict, Phitsanulok Province, Thailand. The research protocol was approved by the Ethics Committee for Human Research, Faculty of Public Health, Mahidol University, Bangkok, Thailand (COA No. MUPH 2015–136). A total of 43 vegetable farmers who grew different types of vegetables such as yard long bean, kale, morning glory, coriander, Chinese cabbage, spring onions, and cucumber were recruited in the study. Inclusion criteria comprised male or female vegetable farmers over 18 years who sprayed glyphosate to kill weeds and had worked on a farm at least one year. They were interviewed by a trained assistant researcher concerning characteristics of farmers and farms, pesticide use on farms, personal protective equipment (PPE) used, agricultural activity and their health problems related to pesticide use. The farmers used four types of spraying equipment: manual pump backpack (a), motorized spray backpack (b), battery pump backpack (c), and high-pressure pump (d) (Figure 1).
Figure 1.

Type of spraying equipment: (a) manual pump backpack; (b) motorized spray backpack; (c) battery pump backpack; and (d) high pressure pump.
Data collection
Breathing zone air samples
Personal air samples using glass fiber filters in cassettes were collected from farmers during mixing and spraying glyphosate on their farms following the Occupational Safety and Health Administration (OSHA) method no. PV2067 (Occupational Safety and Health Administration 1989; Figure 2a). The personal sampling pump drew the air at a flow rate of 1 L/min. After sampling, the glass fiber filters were kept in a small zip lock bag at −70 °C until analysis.
Figure 2.

The breathing zone air sampling (a) and patches sampling on bare skin of farmers on 10 locations, right and left upper arm, forearms, upper legs, lower legs, forehead, and back (b).
Dermal contact samples
Cotton cloth (10 × 10 cm) was sewed on top of an aluminum foil pad (11 × 11 cm) at the edge. The aluminum foil pads were attached to the bare skin of sprayers with adhesive tape at 10 locations, including the forehead, upper back, right upper arm, left upper arm, right forearm, left forearm, right upper leg, left upper leg, right lower leg, and left lower leg before mixing and spraying glyphosate as shown in Figure 3 (Mahaboonpeeti et al. 2018; United States Environmental Protection Agency 2009). The cotton patches were placed on the skin under the clothing farmers wore while spraying. Some patches were open to the air if farmers wore short pants and short sleeve shirts. We did not attach a patch on the chest of the subjects because some women felt uncomfortable taking their shirt off. The researcher observed the mixing and spraying process and recorded the type of spraying equipment, PPE used, and clothing worn. At the end of application, the patch samples were kept immediately in polyethylene bottles, covered with a cap and stored at −70 °C until analysis.
Figure 3.
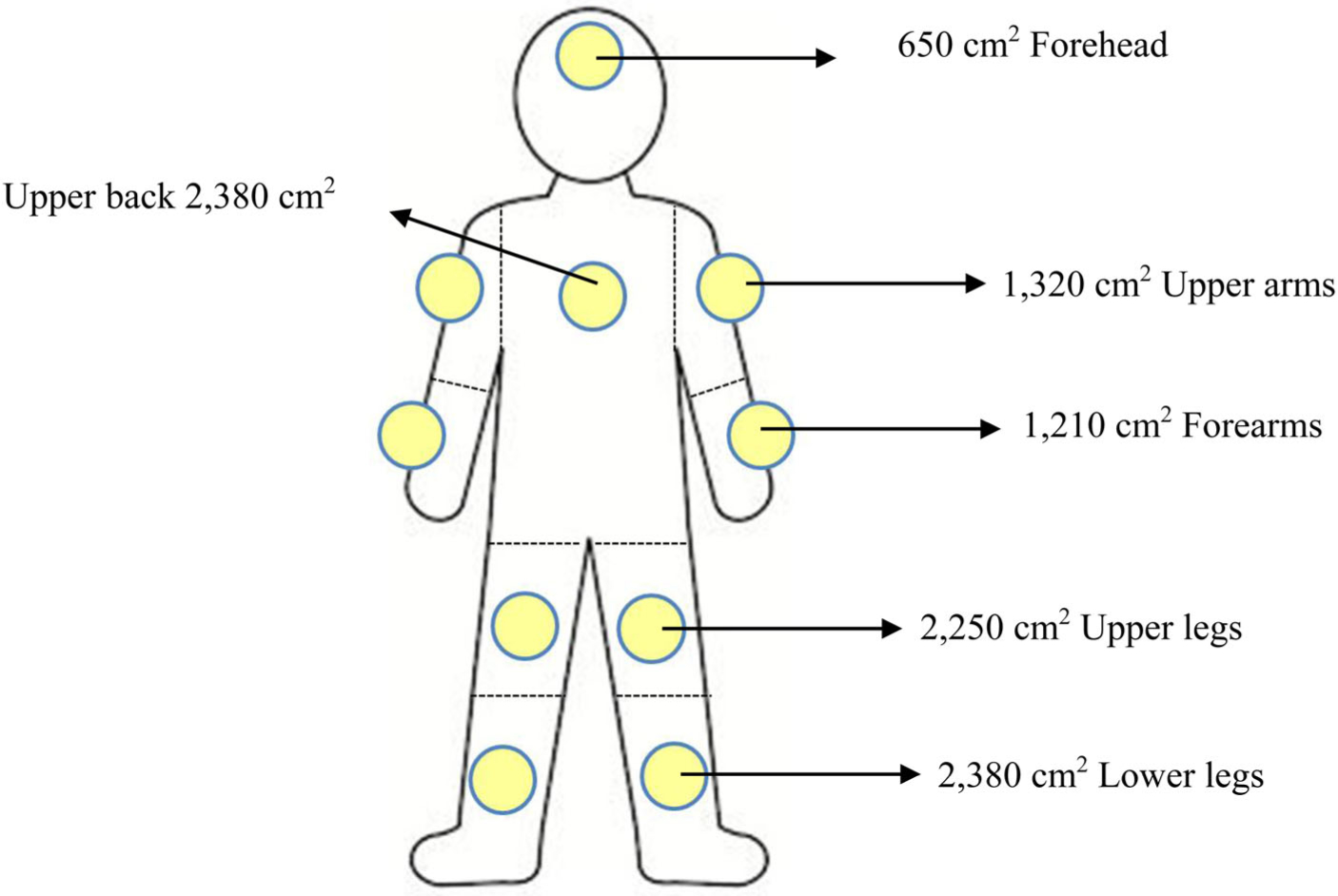
Standard adult body surface areas at forehead, upper back, right and left forearms, upper arms, upper legs, lower legs.
Urine samples
Spot urine samples were collected three times regarding the first morning void urine before glyphosate spraying day, the end of glyphosate spraying event, and at first morning voids in the next morning after glyphosate spraying day. All urine samples were collected in 50 ml polyethylene bottles and stored at −70 °C until analysis.
Working condition
The working condition of sprayer were measured by area heat stress monitors (TSI Inc., model: QT-36, serial number: TSK100005, Wisconsin, USA) for wet bulb globe temperature (WBGT; °C), multi-function ventilation meter for measuring relative humidity (% RH), and wind speed (m/s; TSI Inc., model: 9515, serial number: T95151502007, Minnesota, USA) during spraying.
Analysis of samples
Chemical reagents
Glyphosate (N-(phosphonomethyl)glycine) and DL-2-amino-3-phosphonopropionic acid (APPA; internal standard) and acetonitrile, for high performance liquid chromatography (HPLC), gradient grade, >99.9% were obtained from Sigma-Aldrich (Singapore). In addition, 9-fluorenylmethyl chloroformate (FMOC-Cl), 99.0% for HPLC was purchased from Fluka (Buchs, Switzerland). Ammonium formate (99%) was purchased from Fisher Scientific (Spain). Sodium tetraborate was purchased from Thermo Fisher Scientific (Albany, Auckland, New Zealand). Ultrapure water was obtained from a Milli-Q system (Millipore, Bedford, MA, USA) while other chemicals were analytical reagent grade (AR).
Breathing zone air samples
The analysis method for glyphosate samples from glass fiber filters and field blanks was modified from OSHA Method no. PV2067 (OSHA 1989). The glass fiber filters spiked with 100μl of 25μg/ml APPA (internal standard) were placed in a 50ml polyethylene tube. In all, 5 ml of 0.050M sodium borate buffer (pH of 9) was added and the tube was placed in an ultrasonic bath for 30min at 50°C and centrifuged at 4000rpm for 5min. Then 200μl of extracted solution was derivatized with 200μl of 0.04M 9-fluorenylmethyl chloroformate (FMOC-Cl) and 200 μl of sodium tetraborate. The tube was mixed for 30s and left at room temperature for 30min. The excess FMOC-Cl was removed by extracting with 0.5ml of dichloromethane. The derivatized solution was filtrated by PVDF syringe filter (0.2 μm) and 20μl was injected to HPLC with a fluorescence detector. The calibration curve for glyphosate was prepared at concentrations of 0.025, 2.5, 5, 7.5, 1, and 12.5μg. The average recovery of glyphosate was 90.38, 90.25, and 94.54% at glyphosate concentrations of 0.5, 2.5, and 10μg, respectively, and the detection limit of glyphosate concentration in the filter was 1.5ng. The creatinine in urine was analyzed using enzymatic colorimetric method (Roche Diagnostics, COBAS. 2009).
HPLC condition
The HPLC system (Agilent 1260 Series, Agilent Technologies (Thailand) Co., Ltd., Bangkok, Thailand) was employed to detect fluorescence (excitation 265 nm, emission 315 nm). A column C18 (150 mm × 4.6 mm I.D. × 5 μm) was used at a temperature of 40 °C. Two types of mobile phase were used in this study: one was 10 mM ammonium formate (pH 8.5) in water (A) and the other was acetonitrile (B). The samples were run in gradient mode: 0–8 min (A:B, 85:15 v/v), 9 min (A:B, 80:20 v/v), 10 min (A:B, 50:50 v/v), 12–15 min (A:B, 5:95 v/v), and 16 min (A:B, 85:15 v/v). The injection volume was 20 μl at a flow rate of mobile phase of 0.8 ml/min.
Dermal sample
The extraction of glyphosate from cotton patch samples was modified from Delhomme et al. (2011). The patches spiked with 200 μl of 25 μg/ml APPA (internal standard) was placed in a 50 ml polyethylene tube. About 10 ml of ultrapure water was added and the tube was placed in an ultrasonic bath for 30 min at 50 °C and centrifuged at 4000 rpm for 5 min. Then 200 μl of extracted solution was derivatized in the same manner as the filter and 20 μl was injected to the HPLC. The average recovery of glyphosate was 93.73, 93.13, and 92.03% at glyphosate concentrations of 1, 5, and 20 μg, respectively. The detection limit of glyphosate in dermal samples was 3 ng.
The concentration of glyphosate on patch samples (μg/h) was calculated using the recommended US EPA guidelines (United States Environmental Protection Agency 2009). The glyphosate concentration found on the dermal cotton patch (mg) was divided by the cotton patch area (100 cm2) and total time of the spraying (h). Then the dermal cotton patch concentration (mg/cm2/h) was multiplied by the adult body surface areas shown in Figure 3 to determine dermal contact exposure level as presented in mg/h. Total dermal exposure was calculated by summing the mg/h concentrations for the 10 samples in 10 locations placed on each individual. We did not attach a patch on the chest of the subjects because some were women and felt uncomfortable taking their shirt off.
Urine samples
About 1 mL sample of urine was transferred to a polypropylene tube, 100 μL of 0.5 μg/ml APPA (internal standard) and 1 mL of acetonitrile was added to precipitate protein in urine. The mixture was vortexed for 30 s and centrifuged at 5000 rpm for 5 min. Then 1 mL of acetonitrile was added again to precipitate the protein twice. The 1 mL supernatant was evaporated under a gentle stream of nitrogen (high purity grade) for 15 min. The solution was derivatized with 100 μL 0.1 M borate buffer (pH 9) and 100 μL 0.2 M FMOC-Cl and maintained at room temperature for 2 h. In all, 0.5 mL of dichloromethane was added, and the mixture was vortexed and centrifuged at 5000 rpm for 5 min. Finally, 20 μL was injected in the HPLC–FLD system. The calibration curve of urinary glyphosate concentrations indicated 5, 10, 25, 50, 100, and 150 ng/ml. The accuracy of glyphosate in urine was 76.88 and 98.40% at concentrations of 20 and 100 ng/ml. The quality controlurine sample containing glyphosate (20 and 100 ng/ml) were analyzed together with urine samples. The limit of detection (LOD) of this method was 1 ng/ml.
Data analysis
The data were analyzed using Statistical Package for Social Science Program; SPSS for window, Version 23 (IBM Thailand Co., Bangkok, Thailand). All samples with glyphosate concentrations below the LOD were replaced with the before statistical evaluation. Statistical significance was set at α = 0.05. Descriptive statistics were used to analyze demographic characteristics, while information of cultivation and glyphosate use were expressed in frequency, percentage, standard deviation, mean, maximum, and minimum. The distribution of glyphosate concentrations in urine (μg/g creatinine), breathing zone air samples (μg/m3), and dermal samples (mg/h) did not show normal distribution, so they were transformed to a natural log before statistical analysis. The results were reported in geometric mean (GM) and geometric standard deviation (GSD). The breathing zone air concentration, dermal exposure, and urinary glyphosate concentrations in the morning void after spraying day among vegetable farmers and different spraying equipment were compared using one-way ANOVA. The factors affecting log(e) of the breathing zone air concentration, dermal exposure, and urinary glyphosate concentrations in the morning void after spraying day of vegetable farmers were investigated by using linear regression.
Results
Characteristics of vegetable farmers
Most vegetable farmers were male (81.4%) and had graduated from primary school (65.1%). The average age was 49.5 years (SD = 8.9). They were current smokers (25.6%) and drinkers (58.1%) (Table 1).
Table 1.
Characteristics of vegetable farmers (n = 43).
| General characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 35 (81.4) |
| Female | 8 (18.6) |
| Age (years) | |
| Mean (SD) | 49.5 (8.9) |
| Range (min-max) | 28–69 |
| Education level | |
| Primary school | 28 (65.1) |
| High school/vocational certificate | 13 (30.3) |
| High vocational certificate/bachelor | 2 (4.6) |
| Tobacco consumption | |
| Never | 28 (65.1) |
| Used to smoke | 4 (9.3) |
| Current smoker | 11 (25.6) |
| Alcohol consumption | |
| Never | 13 (30.3) |
| Used to drink | 5 (11.6) |
| Current drinker | 25 (58.1) |
Cultivation of vegetable sprayers
Most (95.3%) vegetable farmers had their own farmlands and 65.1% mixed pesticides near their farms. All vegetable farmers mixed and sprayed pesticides themselves. They performed various activities in vegetable farming such as watering (93.0%), using chemical fertilizer (90.7%), sowing (88.4%), and picking weeds (53.4%). Regarding spraying equipment, they generally used motorized backpack sprayers (51.2%) and battery backpack sprayers (37.2%). They had worked in the agricultural field ranging from 1 to 59 years (average 26.6 years) and had used pesticides from 1 to 47 years (average 24.1 years). The average number of times vegetable farmer cultivated was 14.0 times annually. The average distance from home to farm area was 562.7 m. The average cultivating area totaled 1.3 ha. On spraying day, the average duration for mixing and spraying glyphosate was 41.7 min. The working conditions on spraying day exhibited an average wind speed of 1.4 m/s, relative humidity of 63.5%, and WBGT of 32.1 °C (Table 2).
Table 2.
Cultivation of vegetable sprayers (n = 43).
| Parameter | No. (%) |
|---|---|
| Own farmland | 41(95.3) |
| Mixed pesticide areas | |
| Near farm | 28 (65.1) |
| Near home | 15 (34.9) |
| Activities of vegetable farmers | |
| Tilling | 29 (67.4) |
| Sowing | 38 (88.4) |
| Picking weeds | 23 (53.4) |
| Watering | 40 (93.0) |
| Pesticide mixing | 43 (100.0) |
| Pesticide spraying | 43 (100.0) |
| Harvesting | 36 (83.7) |
| Using natural fertilizer | 25 (58.1) |
| Using chemical fertilizer | 39 (90.7) |
| Driving tractor | 19 (44.2) |
| Spraying equipment | |
| Manual pump backpack sprayers | 3 (7.0) |
| Motorized backpack sprayers | 22 (51.2) |
| Battery backpack sprayers | 16 (37.2) |
| High pressure pump sprayers | 2 (4.7) |
| Working in agricultural field (years) | |
| Mean = 26.6, SD = 14.4, range = 1–59 | |
| Using pesticide (years) | |
| Mean = 24.1, SD = 13.5, range = 1–47 | |
| Cultivation per year (times) | |
| Mean = 14.0, SD = 9.4, range = 3–40 | |
| Distance from home to farm area (m) | |
| Mean = 562.7, SD = 945.1, range = 2–4000 | |
| Cultivating areas (ha) | |
| Mean = 1.3, SD = 0.95, range = 0.3–4.6 | |
| Spraying glyphosate | |
| Duration of spraying glyphosate (min) | |
| Mean = 41.7, SD = 27.7, range = 9–128 min | |
| Number of tanks sprayed (tanks) | |
| Mean = 2.6, SD = 1.9, range = 1–10 tanks | |
| Glyphosate solution used (L) | |
| Mean = 53.2, SD = 41.3, range = 12.2–200.3L | |
| Spraying areas (ha) | |
| Mean = 0.1, SD = 0.1, range = 0.1–0.3 ha | |
| Working condition during sprayed | |
| Wind speed (m/s) | |
| Mean = 1.4, SD = 0.6, range = 0.2–2.6 m/s | |
| Relative humidity (% RH) | |
| Mean = 63.5, SD = 7.2, range = 46.0–78.7% | |
| WBGT (° C) | |
| Mean = 32.1, SD = 1.7, range= 29.0–36.7 °C |
Clothing and PPE used
When mixing and spraying glyphosate, vegetable farmers mostly wore a long sleeve shirt (86.0%), long pants (74.4%), balaclava (55.8%), and boots (51.2%). Only one person wore a disposal mask and goggles (2.3%) (Table 3).
Table 3.
Clothing and personal protective equipment (PPE) used by vegetable sprayers (n = 43).
| Clothing and PPE | No. (%) |
|---|---|
| Cotton mask | 5 (11.6) |
| Disposable Mask | 1 (2.3) |
| Balaclava | 24 (55.8) |
| Long sleeve shirt | 37 (86.0) |
| Short sleeve shirt | 6 (14.0) |
| Long pants | 32 (74.4) |
| Short pants | 11 (25.6) |
| Goggles | 1 (2.3) |
| Latex gloves | 7 (16.3) |
| Boots | 22 (51.2) |
| Plastic apron | 2 (2.7) |
Dermal exposure of glyphosate concentration of vegetable sprayers in different body locations
Total dermal exposure of farmers was 7.57 mg/h; the legs, back, and arms were the most exposed body areas. The highest GM of glyphosate concentration found on the legs was 0.82 mg/h, while the lowest glyphosate concentration found at the forehead was 0.01 mg/h (Table 4).
Table 4.
Glyphosate concentrations in different body locations of vegetable sprayers (n = 43).
| Dermal patches (mg/h) body location | >LOD No. (%) | GM (GSD) (mg/h) | Range (mg/h) |
|---|---|---|---|
| Forehead | 37 (86.05) | 0.01 (0.02) | 0.000001–0.57 |
| Back | 34 (79.07) | 0.06 (0.06) | 0.00009–746.32 |
| Total arm | 39 (90.70) | 0.05 (0.01) | 0.00003–7.54 |
| Total leg | 41 (95.35) | 0.82 (0.02) | 0.003–1204.36 |
| Total dermal exposure | 41 (95.35) | 7.57 (0.01) | 0.046–4828.27 |
Breathing zone glyphosate concentrations, dermal exposure, and urinary glyphosate concentrations of vegetable sprayers
A total of 41 breathing zone air samples (95.3%) were collected revealing the GM of glyphosate concentration in air was 9.37 μg/m3 ranging from 0.01 to 3421.07 μg/m3. Breathing zone air glyphosate concentrations (μg/m3) were compared among four types of spraying equipment using one-way ANOVA; they significantly differed at p = .038. The GM of air concentration of sprayers’ exposure using high pressure pump was significantly higher than that of battery backpack sprayers (134.83 vs. 3.43 μg/m3, p = .029). In addition, the sprayers using motorized backpacks showed higher GM of glyphosate concentration exposure than those using battery backpack sprayers (17.96 vs. 3.43 μg/m3, p = .025). With regards to dermal contact of glyphosate exposure, the farmers using motorized backpack sprayers had the highest glyphosate concentrations on their bodies, but the dermal exposure to glyphosate did not significantly differ among the four types of backpack sprayers (p = .580) (Table 5).
Table 5.
Comparison between spraying equipment among breathing zone glyphosate concentrations (μg/m3), dermal exposure (mg/h), and urinary glyphosate concentrations of vegetable sprayers (μg/g creatinine).
| (1) Manual pump backpack (n = 3) | (2) Motorized spray backpack (n = 22) | (3) Battery pump backpack (n = 16) | (4) High pressure Pump (n = 2) | p value | |
|---|---|---|---|---|---|
| Breathing zone air glyphosate concentrations (μg/m3) | .038* | ||||
| Mean (SD) | 3.94 (3.58) | 221.04 (738.24) | 11.14 (13.86) | 279.68 (346.48) | (2)–(3) |
| GM (GSD) | 2.97 (2.50) | 17.96 (8.18) | 3.43 (10.74) | 134.83 (6.82) | (3)–(4) |
| Range (min-max) | 1.31–8.02 | 0.97–3421.07 | 0.01–56.26 | 34.81–523.21 | |
| Dermal patch glyphosate concentrations (mg/h) | .580 | ||||
| Mean (SD) | 19.40 (32.80) | 269.00 (1022.00) | 123.00 (248.00) | 4.63 (6.46) | |
| GM (GSD) | 2.39 (0.06) | 9.09 (0.01) | 9.69 (0.01) | 0.78 (0.03) | |
| Range (min-max) | 0.45–57.41 | 0.15–4822.89 | 0.01–962.28 | 0.06–9.20 | |
| Urinary glyphosate in next morning after spraying day (mg/g creatinine) | .844 | ||||
| Mean (SD) | 48.32 (14.17) | 42.65 (29.48) | 76.19 (73.25) | 63.63 (75.43) | |
| GM (GSD) | 46.90 (1.35) | 31.11 (2.27) | 43.10 (3.64) | 34.69 (5.58) | |
| Range (min-max) | 34.12–62.56 | 5.87–106.69 | 2.09–239.84 | 10.27–116.74 |
p values were calculated using one-way ANOVA, LSD.
p < .05.
Urinary glyphosate concentrations were adjusted for urinary creatinine correction and expressed as μg/g creatinine. The GM of glyphosate concentration ranged from 2.09 to 239.84 μg/g creatinine; the lowest in the first morning void urine before glyphosate spraying day (28.21 μg/g creatinine), then the level increased at the end of glyphosate spraying event (38.66 μg/g creatinine), and slightly declined to the highest level at first morning voids the next morning after glyphosate spraying day (37.27 μg/g creatinine). Urinary glyphosate concentrations of farmers, the first morning void urine before glyphosate spraying day, were significantly lower than those at the end of glyphosate spraying event and the next morning void urine after spraying day (p = .02 and .05, respectively). However, urinary glyphosate concentration between end of spraying day and the next day after spraying did not significantly differ (p = .63). The frequency of detecting urinary glyphosate concentrations, in first morning voids the next morning after glyphosate spraying day, was 97.6%. The GM of urinary glyphosate of sprayers was highest among manual pump backpack sprayers (46.90 μg/g creatinine) followed by battery backpack sprayers (43.10 μg/g creatinine), high pressure pump (34.69 μg/g creatinine), and motorized backpack sprayers (31.11 μg/g creatinine).
A multiple linear regression model was performed to predict the log(e) of breathing zone glyphosate concentration (μg/m3), dermal exposure(mg/h) and urinary glyphosate concentrations of vegetable sprayers (μg/g creatinine) using stepwise method based on covariates that were significant in univariate analyses (Table 6). In breathing zone glyphosate concentrations (μg/m3), potential determinants were investigated including age, type of spraying equipment, years of working in agricultural field, relative humidity (% RH) during spraying. The model showed that sprayers using the battery pump, motorized pump and high pressure pump increased exposure by a factor of 4.07 in breathing zone glyphosate concentration (μg/m3) compared with using a manual backpack sprayer. Age (years) reduced exposure by a factor of 0.92, whereas the relative humidity (% RH) during spraying increased exposure by a factor of 1.09. For dermal exposure, boots and long-sleeved shirts were significant in univariate models; only wearing long-sleeved shirts vs. short-sleeved shirt (1/0) were significant predictor of total dermal exposure concentration in vegetable farmers in multivariate models. The model showed that wearing long-sleeved shirts reduced exposure by a factor of 0.03 in dermal exposure (mg/h) when compared with short-sleeved shirt. For urinary glyphosate in vegetable farmers, WBGT (°C), picking weeds, wearing long-sleeved shirts were significant in the univariate model. The increased working WBGT (°C) reduced urinary glyphosate concentrations among vegetable sprayers by a factor of 0.81 in multivariate models. The sprayer who picked weeds vs. not picking the weed (1/0) were significant predictors of urinary glyphosate concentrations; the picking weeds had increased urinary glyphosate concentrations among sprayers by a factor 1.88.
Table 6.
Multiple linear regression models using stepwise method for exposure determinants of log(e) breathing zone glyphosate concentration (μg/m3), dermal exposure (mg/h) and urinary glyphosate concentrations of vegetable sprayers (μg/g creatinine) among vegetable sprayers (N = 43).
| Variables | B | β | Standard error | Exp (B) | p value |
|---|---|---|---|---|---|
| Breathing zone glyphosate concentration (μg/m3) | |||||
| Constant | −1.42 | 2.95 | 0.24 | .631 | |
| Battery pump sprayer (1) vs. motorized pump sprayer (2) vs. | 1.40 | 0.42 | 0.41 | 4.07 | .001* |
| High pressure pump sprayer (3) vs. Manual pump sprayer (0) | |||||
| Age (years) | −0.08 | −0.32 | 0.03 | 0.92 | .009* |
| Relative humidity (% RH) | 0.09 | 0.27 | 0.04 | 1.09 | .025* |
| Dermal exposure (mg/h) | |||||
| Constant | 11.98 | 1.01 | 159,891.58 | <.001* | |
| Long-sleeve shirt vs. Short-sleeve shirt (1/0) | −3.55 | −0.44 | 1.09 | 0.03 | .001* |
| Urinary glyphosate concentrations of vegetable sprayers (μg/g creatinine) | |||||
| Constant | 10.25 | 2.64 | 28,185.64 | <.001* | |
| WBGT (°C) | −0.22 | −0.35 | 0.08 | 0.81 | .008* |
| Picking weeds vs. did not picking weeds (1/0) | 0.63 | 0.31 | 0.27 | 1.88 | .019* |
p values were calculated using linear regression.
p < .05.
Discussion
This study examined glyphosate exposure of 43 vegetable farmers with four types of spraying equipment, manual pump backpack, motorized spray backpack, battery pump backpack and high pressure pump by collecting breathing zone air samples, dermal contact patch samples, and urinary glyphosate concentrations the day before and after spraying and the next day after spraying glyphosate. Approximately 81% of vegetable farmers were male. The males were usually stronger than females; therefore, male farmers had a variety of workloads and high-risk activities such as heavy manual handling, driving tractors to plant and till and mixing and spraying pesticide (Hanchenlaksh et al. 2011). The average age of sprayers was 43 years ranging from 28 to 69 years and most of their education was at primary school, similar to one related study conducted in Chiang Mai Province, Thailand, studying urinary pesticide metabolite concentration among farmers. Their ages ranged from 21 to 50 years, most having primary school education; 78% mixed and sprayed pesticides by themselves (Panuwet et al. 2009). Generally, most farmers grew vegetables in an open field all year round. The main crops produced on the farms included morning glory, Chinese cabbage, and kale depending on market price. The life cycle of their products was short; therefore, they usually cultivated more than one area at the same time. Farmers reported farm area ranged from 0.3 to 4.6 ha and most resided near the farm (2–4000 m). Residing near farms posed a greater potential for exposure to pesticides (Sapbamrer and Seesen 2020).
During glyphosate spraying, the applicators usually wore clothing and PPE to protect themselves such as long-sleeved shirts (86.0%), long pants (74.4%), balaclava (55.8%), and boots (51.2%). They rarely used masks (13.9%), goggles (2.3%), and latex gloves (16.3%). The sprayers did not use PPE because of many reasons such as discomfort while working, poverty, and hot tropical climate conditions. Panuwet et al. (2009) reported that northern farm workers wore PPE such as gloves (75.6%), plastic boots (84.2%), masks (71.4%), and rarely wore plastic suits (10.5%). Wongwichit et al. (2012) reported that farmers in the northern part of Thailand did not wear gloves, masks, or goggles when applying herbicides. Some preferred using a cloth wrapped around their heads as replacement for masks and goggles (MacFarlane et al. 2008; Wongwichit et al. 2012). Kongtip et al. (2018) reported agricultural workers in Thailand preferred wearing long sleeve shirts (75%), boots (68%), cloth wrapped around their face (74%), and rubber gloves (55%) (Kongtip et al. 2018). Hardly ever did farmers report wearing cotton gloves (34%), balaclava (39%), disposable masks (35%), or goggles (17%) because of discomfort, expense and difficulty accessing them. They thought that long sleeve shirts and long pants were sufficient to protect themselves while working with pesticides. Farmers lacked education on the use of PPE (Sapbamrer and Seesan 2020). The weather in the field was very hot. Using cloth masks could allow pesticides to accumulate on the mask and serve as a source of pesticide exposure (Panuwet et al. 2009).
The average glyphosate concentration in breathing zone air of 43 sprayers was 9.37 μg/m3, ranging from 0.01 to 3421.07 μg/m3. High pressure pump was associated with the highest glyphosate concentrations among farmers’ breathing zone (134.83 μg/m3), followed by those of motorized spray backpack (17.96 μg/m3), battery backpack sprayers (3.43 μg/m3), and manual sprayed backpack sprayers (2.97 μg/m3). Normally, the high-pressure pump was used on a big farm because the pressure generated was high, around 40–1000 psi (The Center for Agriculture, Food and the Environment. 2020). The applicator may be exposed to the glyphosate spray drift more than any other types of sprayers. The motorized backpack sprayer had the horizontal spray ranging from 12 to 15 m and vertical spray range up to 10 m (Bayer CropScience 2015), whereas the battery backpack sprayer produced spray at a short distance (1–2 m; Bayer CropScience 2015). Manual pump backpack sprayer or hand pump was low weight compared with motorized backpack sprayers (3.5 vs. 10 kg) and suitable for small farm areas. This manual pump required less power than motorized backpack sprayers. The multivariate analysis showed that the high pressure pump, motorized spray backpack, and battery backpack sprayers had higher exposure than the manual sprayed backpack sprayers. The higher percent of relative humidity was significantly resulted in higher inhalation exposure of glyphosate among the farmers which was similar to the review of Damalas and Eleftherohorinos (2011) said that the low relative humidity and high temperature would cause rapid evaporation of spray droplets resulted in lower exposure of sprayer.
Morshed et al. (2011) collected breathing zone air samples of glyphosate for 12 h using motorized knapsack sprayers in Malaysia (Morshed et al. 2011). Air sampling was conducted in 12 h, 4 h prespray, 25 min spraying, and postspray periods (0–4 and 4–8 h). They found the highest glyphosate concentration during 25-min spraying of 42.96 μg/m3 and declined after spraying (0–4 h) of 0.1 μg/m3 and (4–8 h) of 0.051 μg/m3. The exposure of this current study with motorized knapsack sprayer was 17.96 μg/m3, lower than the Malaysian study. Intensive use of glyphosate has resulted in serious contamination of the environment because a substantial amount of applied pesticide has been shown to become airborne during and after application (Seiber et al. 1980). The difference of environmental factors such as temperature, relative humidity, and wind speed could affect inhalation exposure. Johnson et al. (2005) collected glyphosate fluid while spraying with 12 knapsack sprayers using personal air sample pumps; the glyphosate in breathing zone air was detected in 33% of the samples, ranging from 20 to 610 μg/m3 with a median of 120 μg/m3. This current study found lower glyphosate concentrations in breathing zone air than those of Johnson et al. (2005) because their knapsacks were equipped with a lance handle to trigger flow control and increase the speed of spray.
Concerning dermal exposure, battery backpack sprayers had the highest GM of glyphosate in dermal exposure (9.69 mg/h), followed by motorized backpack sprayers (9.09 mg/h), high-pressure pump sprayers (2.39 mg/h), and manual pump backpack sprayers (0.78 mg/h). In this current study, the manual pump backpack sprayers and high-pressure pump sprayers were rarely used, so they should be examined in a further study. The results of this study were similar to those of the study of Delhomme et al (2011) reporting that higher glyphosate concentration on skin of manual backpack sprayers (52.5–2958 ng/cm2) compared with motorized sprayers (0.7–507 ng/cm2; Delhomme et al. 2011). They also indicated that backpack sprayers had severe contamination at the left hand and right foot because the sprayers carried the pesticide hose and applied from right to left. The GM of total dermal exposure of glyphosate was 7.57 mg/h, the highest dermal exposure was found on the legs (0.82 mg/h) followed by back and arms (0.06 and 0.05 mg/h). Johnson et al. (2005) found glyphosate deposited on the lower legs (70.0%) in the applicators because they pointed the nozzle to the ground. The highest exposure was at the lower legs and some sprayers did not wear shoes (23.3%) or slippers (18.6%) (Johnson et al. 2005). In this current study, the sprayers wearing boots had lower GM of glyphosate on the legs than those not wearing (2.87 vs. 0.25 mg/h). Sprayers wearing long sleeves shirt (0.03 mg/h) had significantly lower glyphosate exposure than those wearing short sleeves shirt (0.75 mg/h). The multivariate model also gave the similar results to the study Konthonbut et al. (2018) showing a higher median paraquat exposure of sprayers wearing short sleeves shirt (75.59 μg/h) compared to those wearing long sleeves shirt (5.63 μg/h). Back exposure may have resulted from leakage of the knapsack sprayer. Our results were similar to Mahaboonpeeti et al.’s study (2018) that the legs, back, and arms were the most exposed body areas. Arm exposure could be due to sprayers using their hands to manage the spray nozzle and splashing during glyphosate solution preparation or when filling the spray tank. Appropriate clothing could reduce glyphosate exposure among pesticide applicators. Those wearing long sleeve shirts and boots had a lower dermal exposure to the arms and legs over a 90% difference compared with those wearing short sleeve shirts without boots.
In this study, the GM of urinary glyphosate next morning after spraying day (μg/g creatinine) did not significantly differ from that at the end of spraying task. Connolly et al. (2018) estimated half-life of glyphosate in urine was between 3 and 20 h after exposure; sampling times of less than 24 h would not allow sufficient time for pesticide absorption and excretion, particularly when skin is the dominant route of exposure (Flack et al. 2008; Vitali et al. 2009). The urinary glyphosate on the next morning was used for comparison among different spraying equipment. The GM of urinary glyphosate was highest among sprayers using manual pump backpack sprayers (46.90 μg/g creatinine) followed by battery backpack sprayers (43.10 μg/g creatinine), high pressure pump (34.69 μg/g creatinine), and motorized backpack sprayers (31.11 μg/g creatinine). These results differed from the study of Connolly et al. (2019) reporting that the manual backpack sprayers received lower GM of urinary glyphosate concentration at the end of spraying task (0.93 μg/L) and the next morning (0.95 μg/L) compared with pressurized lance at the end of spraying task (1.82 μg/L) and the next morning (1.54 μg/L). It would be difficult to compare the result of exposure studies with the related publication because we used different analytical methods, sampling strategies and glyphosate concentrations in the spray tank and uncertainties regarding half-life of glyphosate in humans may have created ambiguity with the sampling strategy appropriate for occupational exposure assessment (Connolly et al. 2019). The current study revealed a higher detection frequency of urinary glyphosate among farmers (97.6%) compared with sprayers in northern Thailand (48.0%) (Polyiem et al. 2017). Multivariate analysis showed that WBGT during spraying and picking weeds were factors influencing the urinary glyphosate concentrations in sprayers. The increased WBGT significantly reduced urinary glyphosate concentrations among vegetable farmers. It is similar to the study of Damalas and Eleftherohorinos (2011) reported that air temperature may affect the chemical volatility of the chemicals and the perspiration rate of the human body which would reduce the chemical exposure and Calumpang (Calumpang 1996) reported that the on the warm day (29–38 °C), the exposure of farmers were reduced due to the evaporation of water droplet on shirts or pants of farmers. Whereas, we hypothesize that sprayers who go their agricultural land every day to take care of their crops may be exposed to glyphosate through farm activities with soil during picking weeds, since the typical half-life of glyphosate in soil and surface water are in the ranges of 2–215 days and 2–91 days, respectively (Berman et al. 2018).
Conclusion
The GM of glyphosate concentration in breathing zone air was 9.37 μg/m3 ranging from 0.01 to 3421.91 μg/m3. The GM of total dermal patches samples concentrations was 7.57 mg/h. The legs, back, and arms were the most exposed body areas. The frequency of detection of urinary glyphosate concentrations the first morning void and the next morning after glyphosate spraying day was 97.6% among farmers and the GM of urinary glyphosate was found to be the highest among vegetable farmers spraying by manual knapsack (46.90 μg/g creatinine) followed by battery pump backpack (43.10 μg/g creatinine), high pressure pump (34.69 μg/g creatinine), and motorized spray backpack (31.11 μg/g creatinine). Farmers should wear masks and boots to reduce glyphosate exposure through inhalation and dermal contact during glyphosate spraying.
Acknowledgments
We are appreciative of the Center of Excellence on Environmental Health and Toxicology (EHT), for supporting laboratory facilities and equipment. We thank all participants and all research assistants in Bungphra Health Promoting Hospital in Phitsanulok Province, Thailand.
Funding
This research was financially supported by the University of Phayao and the CWEND GEOHealthHub supported by the NIH Fogarty International Center, National Institutes of EnvironmentalHealth Science and the Center for Disease Control under Award Numbers U01 TW010091.
References
- Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. 2004. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 112(3):321–326. doi: 10.1289/ehp.6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini LP, Dettogni RS, dos Reis RS, Stur E, dos Santos EV, Ventorim DP, Garcia FM, Cardoso RC, Graceli JB, Louro ID. 2020. Effects of glyphosate exposure on human health: insights from epidemiological and in vitro studies. Sci Total Environ. 705:135808. doi: 10.1016/j.scitotenv.2019.135808 [DOI] [PubMed] [Google Scholar]
- Amrhein N, Deus B, Gehrke P, Steinrücken HC. 1980. The site of the inhibition of the shikimate pathway by glyphosate: II. Interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiol. 66(5):830–834. doi: 10.1104/pp.66.5.830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer CropScience. 2015. Agricultural backpack spraying manual: A Bayer CropScience Guide for Applicators in Agricultural Crop Protection.
- Berman MC, Marino DJ, Quiroga MV, Zagarese H. 2018. Occurrence and levels of glyphosate and AMPA in shallow lakes from the Pampean and Patagonian regions of Argentina. Chemosphere. 200:513–522. doi: 10.1016/j.chemosphere.2018.02.103 [DOI] [PubMed] [Google Scholar]
- Bolognesi C, Carrasquilla G, Volpi S, Solomon KR, Marshall EJ. 2009. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: association to occupational exposure to glyphosate. J Toxicol Environ Health. 72(15–16):986–997. doi: 10.1080/15287390902929741 [DOI] [PubMed] [Google Scholar]
- Calumpang SMF. 1996. Exposure of four Filipino farmers to parathion-methyl while spraying string beans. Pestic Sci. 46(1):93–102. doi:. [DOI] [Google Scholar]
- Connolly A, Basinas I, Jones K, Galea KS, Kenny L, McGowan P, Coggins MA. 2018. Characterising glyphosate exposures among amenity horticulturists using multiple spot urine samples. Int J Hyg Environ Health. 221(7):1012–1022. doi: 10.1016/j.ijheh.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Connolly A, Coggins MA, Galea KS, Jones K, Kenny L, McGowan P, Basinas I. 2019. Evaluating glyphosate exposure routes and their contribution to total body burden: a study among amenity horticulturalists. Ann Work Expo Health. 63(2):133–147. doi: 10.1093/annweh/wxy104 [DOI] [PubMed] [Google Scholar]
- Connolly A, Jones K, Basinas I, Galea KS, Kenny L, McGowan P, Coggins MA. 2019. Exploring the half-life of glyphosate in human urine samples. Int J Hyg Environ Health. 222(2):205–210. doi: 10.1016/j.ijheh.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Barr DB, Heederik D, Reynolds SJ, Ward EM, Alavanja MC. 2005. Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa. J Expo Sci Environ Epidemiol. 15(6):500–508. doi: 10.1038/sj.jea.7500428 [DOI] [PubMed] [Google Scholar]
- Damalas CA, Eleftherohorinos IG. 2011. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 8(5):1402–1419. doi: 10.3390/ijerph8051402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhomme O, Raeppel C, Teigné D, Briand O, Millet M. 2011. Analytical method for assessing potential dermal exposure to pesticides of a non-agricultural occupationally exposed population. Anal Bioanal Chem. 399(3):1325–1334. doi: 10.1007/s00216-010-4434-9 [DOI] [PubMed] [Google Scholar]
- Eriksson M, Hardell L, Carlberg M, Akerman M. 2008. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 123(7): 1657–1663. doi: 10.1002/ijc.23589 [DOI] [PubMed] [Google Scholar]
- Faniband MH, Littorin M, Mora AM, Winkler M, Fuhrimann S, Lindh CH. 2017. Biomonitoring of the herbicide glyphosate in a population from Zarcero, Costa Rica, 10th International Symposium on Biological Monitoring in Occupational and Environmental Health (ISBM-10). http://www.jeangilder.it/icoam2017/wp-content/uploads/2017/09/ISBM_AbstractBook.pdf. [Google Scholar]
- Flack S, Goktepe I, Ball LM, Nylander-French LA. 2008. Development and application of quantitative methods for monitoring dermal and inhalation exposure to propiconazole. J Environ Monit. 10(3):336–344. doi: 10.1039/b714882h [DOI] [PubMed] [Google Scholar]
- Hanchenlaksh C, Povey A, O’Brien S, de Vocht F. 2011. Urinary DAP metabolite levels in Thai farmers and their families and exposure to pesticides from agricultural pesticide spraying. Occup Environ Med. 68(8):625–627. doi: 10.1136/oem.2010.060897 [DOI] [PubMed] [Google Scholar]
- Hardell L, Eriksson M, Nordstrom M. 2002. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-control studies. Leuk Lymphoma. 43(5):1043–1049. doi: 10.1080/10428190290021560 [DOI] [PubMed] [Google Scholar]
- Harvey B 2014. Protecting farming families & field-workers. Professional Safety. 59(3):68–70. [Google Scholar]
- International Agency for Research on Cancer. 2015. IARC monographs volume 112: evaluation of five organophosphate insecticides and herbicides. http://www.iarc.fr/en/media-centre/iarc-news/pdf/MonographVolume112.pdf. [PubMed]
- International Agency for Research on Cancer. 2016. IARC monographs on the evaluation of carcinogenic risks to humans—glyphosate. http://monographs.iarc.fr/ENG/Monographs/vol112/mono112-10.pdf.
- International Programme on Chemical Safety. 1994. Glyphosate, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 159). https://apps.who.int/iris/bitstream/handle/10665/40044/9241571594-eng.pdf?sequence=1&isAllowed=y.
- Jauhiainen A, Räsänen K, Sarantila R, Nuutinen J, Kangas J. 1991. Occupational exposure of forest workers to glyphosate during brush saw spraying work. Am Ind Hyg Assoc J. 52(2):61–64. doi: 10.1080/15298669191364334 [DOI] [PubMed] [Google Scholar]
- Jayasumana C, Gajanayake R, Siribaddana S. 2014. Importance of arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 15(1):124. doi: 10.1186/1471-2369-15-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasumana C, Gunatilake S, Siribaddana S. 2015. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 16(1):103. doi: 10.1186/s12882-015-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasumana M, Paranagama P, Amarasinghe M, Wijewardane K, Fonseka S, Rajakaruna K, Dahanayake K, Mahamithawa A, Samarasinghe U, Senanayake V. 2013. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J Nat Sci Res. 3(1):64–73. [Google Scholar]
- Johnson PD, Rimmer DA, Garrod AN, Helps JE, Mawdsley C. 2005. Operator exposure when applying amenity herbicides by all-terrain vehicles and controlled droplet applicators. Ann Occup Hyg. 49(1):25–32. doi: 10.1093/annhyg/meh073 [DOI] [PubMed] [Google Scholar]
- Kongtip P, Nankongnab N, Mahaboonpeeti R, Bootsikeaw S, Batsungnoen K, Hanchenlaksh C, Tipayamongkholgul M, Woskie S. 2018. Differences among Thai agricultural workers’ health, working conditions, and pesticide use by farm type. Ann Work Expo Health. 62(2):167–181. doi: 10.1093/annweh/wxx099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konthonbut P, Kongtip P, Nankongnab N, Tipayamongkholgul M, Yoosook W, Woskie W. 2018. Paraquat Exposure of Pregnant Women and Neonates in Agricultural Areas in Thailand. IJERPH. 15(6):1163. doi: 10.3390/ijerph15061163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy TL, Cowell JE, Steinmetz JR, Massey JH. 1992. Conifer seedling nursery worker exposure to glyphosate. Arch Environ Contam Toxicol. 22(1):6–13. doi: 10.1007/bf00213295 [DOI] [PubMed] [Google Scholar]
- MacFarlane E, Chapman A, Benke G, Meaklim J, Sim M, McNeil J. 2008. Training and other predictors of personal protective equipment use in Australian grain farmers using pesticides. Occup Environ Med. 65(2):141–146. doi: 10.1136/oem.2007.034843 [DOI] [PubMed] [Google Scholar]
- Mahaboonpeeti R, Kongtip P, Nankongnab N, Tipayamongkholgul M, Bunngamchairat A, Yoosook W, Woskie S. 2018. Evaluation of dermal exposure to the herbicide alachlor among vegetable farmers in Thailand. Ann Work Expo Health. 62(9):1147–1158. doi: 10.1093/annweh/wxy081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. 2001. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Prev Biomarkers. 10(11): 1155–1163. [PubMed] [Google Scholar]
- Mesnage R, Moesch C, Grand RL, Lauthier G, Vendômois S, Gress S, Séralin GE. 2012. Glyphosate exposure in a farmer’s family. J Environ Prot. 3(9):1001–1003. doi: 10.4236/jep.2012.39115 [DOI] [Google Scholar]
- Morshed MM, Omar D, Mohamad RB, Wahed SB. 2011. Determination of glyphosate through passive and active sampling methods in a treated field atmosphere. Afr J Agric Res. 6(17): 4010–4018. doi: 10.5897/AJAR11.533 [DOI] [Google Scholar]
- Niemann L, Sieke C, Pfeil R, Solecki R. 2015. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J Verbr Lebensm. 10(1):3–12. doi: 10.1007/s00003-014-0927-3 [DOI] [Google Scholar]
- Occupational Safety and Health Administration. 1989. OSHA Method Number PV2067: Glyphosate. https://www.osha.gov/dts/sltc/methods/partial/t-pv2067-01-8911-ch/t-pv2067-01-8911-ch.pdf.
- Panuwet P, Prapamontol T, Chantara S, Barr DB. 2009. Urinary pesticide metabolites in school students from northern Thailand. Int J Hyg Environ Health. 212(3):288–297. doi: 10.1016/j.ijheh.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Polyiem W, Hongsibson S, Chantara S, Kerdnoi T, Patarasiri V, Prapamonto T, Sapbamrer R. 2017. Determination and assessment of glyphosate exposure among farmers from northern part of Thailand. J Pharmacol Toxicol. 12(2):97–102. doi: 10.3923/jpt.2017.97.102 [DOI] [Google Scholar]
- Roche Diagnostics, COBAS. 2009. Creatinine plus ver.2 (CREP2) https://w2.med.cmu.ac.th/lab/files/PDF/Chem_imm_method_sheet/method_sheet_cobas/Creatinine_P2.pdf.
- Sapbamrer R, Seesen M. 2020. A systematic review of association between pesticide exposure and respiratory outcomes among farmers and farmworkers. Mal J Med Health Sci. 6(1):312–324. [Google Scholar]
- Seiber JN, Ferreira GA, Hermann B, Woodrow JE. 1980. Analysis of pesticidal residues in the air near agricultural treatment sites (pp. 177–208). doi: 10.1021/bk-1980-0136.ch010 [DOI] [Google Scholar]
- Székács A, Darvas B. 2012. Herbicides—properties, synthesis and control of weeds: forty years with glyphosate. Rijeka: Croatia. [Google Scholar]
- The Center for Agriculture, Food and the Environment. 2020. Sprayers and Spray Application Techniques. https://ag.umass.edu/greenhouse-floriculture/fact-sheets/sprayers-spray-application-techniques.
- Tominack RL, Yang GY, Tsai WJ, Chung HM, Deng JF. 1991. Taiwan National Poison Center survey of glyphosate-surfactant herbicide ingestions. J Toxicol Clin Toxicol. 29(1):91–109. doi: 10.3109/15563659109038601 [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. 2009. Occupational and residential exposure test guidelines:OPPTS 875.2400 dermal exposure https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0157-0012.
- United States Environmental Protection Agency. 2018. Chemical evaluated for carcinogenic potential. http://npic.orst.edu/chemicals_evaluated.pdf.
- Vitali M, Protano C, Del Monte A, Ensabella F, Guidotti M. 2009. Operative modalities and exposure to pesticides during open field treatments among a group of agricultural subcontractors. Arch Environ Contam Toxicol. 57(1):193–202. doi: 10.1007/s00244-008-9225-3 [DOI] [PubMed] [Google Scholar]
- Wongwichit D, Siriwong W, Robson MG. 2012. Herbicide exposure to maize farmers in Northern Thailand: knowledge, attitude, and practices. J Med Med Sci. 3(1):034–038. [Google Scholar]
- Zouaoui K, Dulaurent S, Gaulier J, Moesch C, Lachâtre G. 2013. Determination of glyphosate and AMPA in blood and urine from humans: about 13 cases of acute intoxication. Forensic Sci Int. 226(1–3):e20–e25. doi: 10.1016/j.forsciint.2012.12.010 [DOI] [PubMed] [Google Scholar]


