Abstract
Fluorinated compounds, while rarely used by nature, are emerging as fundamental ingredients in biomedical research, with applications in drug discovery, metabolomics, biospectroscopy, and, as the focus of this review, peptide/protein engineering. Leveraging the fluorous effect to direct peptide assembly has evolved an entirely new class of organofluorine building blocks from which unique and bioactive materials can be constructed. Here, we discuss three distinct peptide fluorination strategies used to design and induce peptide assembly into nano-, micro-, and macrosupramolecular states that potentiate high-ordered organization into material scaffolds. These fluorine-tailored peptide assemblies employ the unique fluorous environment to boost biofunctionality for a broad range of applications, from drug delivery to antibacterial coatings. This review provides foundational tactics for peptide fluorination and discusses the utility of these fluorous-directed hierarchical structures as material platforms in diverse biomedical applications.
Keywords: biomaterials, fluorine biochemistry, peptides, supramolecular assembly
1 ∣. INTRODUCTION
Self-assembling peptide materials have spurred transformative advances over the last two decades in semiconductor technologies,[1-5] optics,[4,6-11] catalysis,[11-16] and biomedicine.[4,17-23] By definition, self-assembly is the spontaneous intra- and intermolecular organization of components through noncovalent interactions, including hydrogen bonding, electrostatics, π – π stacking, van der Waals forces, and hydrophobic interactions. These cooperative processes pattern molecules into thermodynamically favorable, higher-ordered structural states either driven by microenvironmental cues or triggered upon presentation of a chemical interface or biologic stimulus.[24-27] The resulting three-dimensional hierarchical structures possess unique electro- and bio-chemical properties that often only emerge upon formation of the supramolecular functional state. Examples of such assemblies can be found across all forms of natural life, from microorganisms to mammals. Instances include cellular functions choreographed through phospholipid-peptide assembly at the cell surface or cell motility and vesicular transport coordinated by tubulin monomer formation into dynamic cytoskeletal filaments. In biologic systems, self-assembly processes are often influenced by solvation effects to direct divergent intermolecular interactions to nonequilibrium states or kinetically trapped conformations.[28-29]
Among the repertoire of biomolecules with which to construct biomaterials, peptides have attracted particular interest due to their chemical versatility and bio-reactivity. In many cases, self-assembling peptides can produce diverse materials with physicochemical properties and secondary structural states not easily executed by conventional nonbiologic materials.[30-31] As the pool of commercially available noncanonical amino acids grows, so too does the development of de novo, non-natural peptides. Among these, fluorinated sequences have gained attention as valuable building blocks to tune the assembly mechanism and kinetics of next-generation biomaterials.[32-35] As the most electronegative element, fluorine has a strong inductive effect and imparts C─F bonds with a significant dipole moment and low polarizability. When incorporated into amino acids, or appended to peptide scaffolds, fluorination leads to the emergence of an entirely new class of biopolymers that are now being employed as novel biosensors, molecular probes, and enzyme inhibitors.[36-41] More recently, and as will be focused on in this review, organofluorine self-assembling peptides can be used to readily tune the chemical, electronic, and mechanical properties of nano-, micro-, and macroscale biomaterials. Enhanced proteolytic and thermal stability are additional advantages intrinsic to fluorinated peptide-based scaffolds.[42-44]
Accordingly, organofluorine peptides are defined by unusual bioactivities and structural states compared to their nonfluorinated counterparts. In this review, we summarize the general synthetic methodologies used to generate self-assembling organofluorine peptides and discuss their implications in obtaining diverse material structures and morphologies (Figure 1). Building upon structure-activity relationships derived from these studies, we present recent developments in the application of fluoropeptides as drug delivery vehicles, imaging probes, and biofunctional coatings, or combinations thereof.
FIGURE 1.
De novo peptides (blue) incorporating fluorinated substitutes (green) access diverse supramolecular morphologies, including ribbons, fibrils, tubes and particles (shown in order from left to right). The unique assembly phenomena that underly these higher ordered structural states are driven by fluorine-fluorine interactions that occur both intra- and inter-molecularly
2 ∣. METHODS OF PEPTIDE FLUORINATION AND THEIR EMERGENT PROPERTIES
The availability and diversity of fluorinated ligands and amino acids, in combination with the accessibility of synthetic approaches to obtain noncanonical residues,[45] has broadened interest in developing fluorinated sequences for bio-organic applications. Numerous studies have demonstrated that incorporating electron-withdrawing fluorine substituents into a biopolymer can intramolecularly influence the chemical environment of functional groups within peptide scaffolds and directly potentiate long-range intermolecular organization via fluorine-fluorine interactions.[35,46] In fact, changing the position of just one fluorine atom in a sequence can dramatically alter the hydrophobicity, amphiphilicity, and secondary structure of peptide chains. Although a number of favorable properties result from peptide fluorination, the effects of introducing fluorines into this class of biopolymers are difficult to predict and largely restricted to empirical, formulaic approaches. Recently, Koksch and co-workers have begun to systematically identify structure-property relationships and develop design principles to rationally engineer fluorinated peptide scaffolds.[37,42,46] There are three general strategies to incorporating fluorine atoms into peptides: (a) introduction of non-natural, fluorinated α-amino acids; (b) ligation of fluoroalkyl appendages; and (c) fluorination of the peptide backbone, as summarized in Figure 2 and discussed below.
FIGURE 2.
Left, fluorinated amino acids can be readily incorporated into non-natural sequences via solid-phase techniques. Examples of commonly employed fluorine-containing residues are shown. Middle, fluoroalkyl appendages ligated to the peptide terminus can drive unique assembly phenomena of natural sequences. Right, backbone fluorination of cyclic peptides can improve cyclisation efficiency, allow for tailoring of molecular conformations, and enhance self-assembly via the fluorous effect
Noncanonical, fluoro-amino acids represent valuable tools for de novo engineering of non-natural peptides and proteins. Fluorous variants often show improved stability, enhanced folding, and programmable bioactivities compared to their natural counterparts.[42,46-47] There are now several commercially available alkyl, cyclic, and aromatic amino acids that integrate at least one fluorine atom in their side chain. A comprehensive survey on the stereoselective synthetic methods to produce fluorinated α-amino acids has recently been reviewed by Koksch and others.[45,48] The Koksch group has also developed flow methods for the rapid synthesis of racemic, fluorinated α-amino acids without the need for the protecting groups traditional in solid-phase techniques.[49] Diastereoselective reactions yielding highly fluorinated β-amino acids have recently been reported as well.[50] Incorporation of these fluorine-bearing α- and β-residues has now been widely adopted to control the supramolecular morphology of assembling sequences, leading to the formation of cylindrical nanofibers,[51] ribbons,[51] bundles,[52-53] coiled coils,[54] oligomers,[55] nanoparticles,[56] nanopores,[57] and nano-emulsions.[33-34] Interestingly, these fluorous-templated assembly phenomena, which results from the increased hydrophobicity of fluorinated sequences, has recently been exploited to develop peptide inhibitors of amyloid-β (Aβ) oligomerization involved in Alzheimer's disease.[58-59] Substitution of 19F-labeled amino acids into sequences has also seen use as a spectroscopic handle to interrogate peptide conformational dynamics. This has allowed researchers to exploit fluorine NMR to investigate the interactions of 19F-labeled peptides within their environment, including the study of solvation effects driving antimicrobial peptide activity,[60-62] membrane-bound structural states of cell-penetrating peptides,[63-64] and ligand-protein biophysics for drug discovery.[65-66] Non-natural peptides bearing 19F amino acids have also been developed as quantitative MR imaging probes, as discussed in more detail later in this review.
The second widely adopted method to incorporate fluorine atoms into peptides is the engraftment of fluoro-alkyl tails onto self-assembling sequences (Figure 2, middle). The resulting amphiphiles can be programmed to assemble into an array of structural states, including cylinders and ribbons, mediated by fluorine-fluorine oligomerization. Stupp and Meade have exemplified this approach through the ligation of perfluorinated 7- or 8-C hydrophobic tails onto Val2-Ala2 scaffolds varied by containing C-terminal charged residues (Lys2, Lys3 or Glu2, Glu3).[41,67] These charged peptide amphiphiles are designed to undergo supramolecular assembly in response to varying calcium concentrations[41] and environmental pH[67] to optimize 19F MRI signals. Interestingly, increasing pH from 4.5 to 8.0 led to a transition of the C7-Val2-Ala2-Glu2 assembled state from β-sheet ribbon nanostructures to cylindrical fibers, with a corresponding increase in MR signal.[67] Perfluoroalkyl tails have also been grafted onto peptide dendrimers to promote their assembly into >100 nm oligomeric polyplexes and augment their cellular uptake and endosomal escape properties.[32,68]
As an alternative to fluorinated hydrophobic tails and amino acids, other groups have chosen to incorporate fluorine into the backbone of cyclic peptides to fine tune their conformational states (Figure 2, right).[36,69] Utilizing cyclic-RGD as a template, a commonly employed bioactive ligand for αvβ3 cellular integrins,[70-71] Au and co-workers exploited stereoselective fluorination to influence the secondary structure and bioactive properties of the cyclic peptide.[69] For example, mass spectrometry and NMR studies revealed that incorporation of backbone-fluorinated γ-amino acids enhanced or reduced peptide macrocyclization efficiency, dependent on the fluorine-induced shape, and accessed novel high-energy geometries with unique cis-trans conformations.[69] Accordingly, these fluorine-induced molecular arrangements showed a marked effect on the binding, spreading and angiogenic potential of human microvascular endothelial cells (HMEC-1); with fluorinated analogues generally suppressing biologic responses compared to the nonfluorinated cyclic-RGD control. In separate work, backbone fluorination of cyclized tetra- and hexapeptides led to the formation of self-assembled nanotubes that serve as transmembrane anion-selective transporters.[36] Here, addition of fluorine atoms induced reverse turns that facilitated β-strand conformations and tubular assemblies,[36,72] ultimately imparting α,γ-cyclic hexapeptide nanotubes (EC50 = 2.14 μM) with improved NO3− ion transport efficiency over α,γ-cyclic tetrapeptide analogues (EC50 = 14.75 μM).
Finally, although outside the scope of this review, it is important to note that the impact of perfluorinated solvents on peptide secondary structure and assembly phenomena have long been appreciated. For instance, fluorinated alcohols such as 2,2,2-trifluoroethanol and hexafluoroisopropanol have been used for decades to promote and stabilize α-helical and β-hairpin conformations.[73-76] Similarly, phase-separated thin film electrodes have been realized by templating the assembly of an elastin-like peptide at the surface of a hydrophobic, perfluorinated ionomer.[77]
3 ∣. FLUOROPEPTIDE NANO- AND MICROASSEMBLIES
The varying methods of incorporating fluorinated substituents into peptide sequences, which serve to induce or enhance their self-assembly, can yield a diverse array of nano- and micro-structures with varying morphologies and length scales. The resultant self-assembled state is highly dependent on fluoropeptide design and is influenced by parameters such as: location of fluorinated substitutes in the peptide; the quantity of non-natural amino acids or the length of fluoroalkyl chains incorporated into the sequence; and the presence of hydrophobic or fluorophilic environments. These aspects converge to direct fluoropeptides into three general nano- and micro- hierarchical structures: (a) spherical particles, (b) fibrous assemblies, and (c) cylindrical scaffolds.
3.1 ∣. Spherical particles
The immiscibility of fluorine with aqueous environments often drives the organization of fluoropeptides into spherical morphologies, where the fluorinated substituents are buried within the hydrophobic particle core. The size of these constructs can be readily tuned dependent on the identity, placement, and extent of fluorinated amino acid content in the sequence. A common example is the addition of pentafluoro-Lphenylalanine (F5-Phe) residues on the termini of peptides to drive particle assembly. Zhang et al. used aromatic dipeptides containing one natural residue (Tyr or Trp) and one F5-Phe residue to probe the effects of N- vs C-terminal placement of the fluorinated amino acid for nanoparticle assembly.[78] They showed dipeptides Tyr-F5-Phe, Trp-F5-Phe, and F5-Phe-Trp preferentially favor edge-to-face or offcentered intermolecular orientations to yield nanoparticle assemblies with diameters 40.4 ± 4.0, 39.2 ± 4.9, and 33.2 ± 11.6 nm, respectively. F5-Phe residue placement was also found to dictate polar-π intermolecular interactions of dipeptides that influence the assembly pathway toward either particle formation or nano-fiber and -ribbon lamination.[78] For example, Trp-F5-Phe and F5-Phe-Trp were independent of F5-Phe residue termini placement and instead driven through indole-fluorophenyl side chain interactions to form random coil dipeptides. Conversely, tyrosine-containing dipeptide assembly showed high dependency toward F5-Phe residue location, highlighting the importance of intra-molecular interactions in fluorinated sequences. In complementary work, Yu et al. incorporated a single F5-Phe residue at three different locations along the backbone of a self-assembling peptide-fatty acid amphiphile.[51] All three peptide amphiphiles spontaneously formed β-sheet structures in homogenous mix-tures. However, upon co-assembly with dodecanoic acid, these assemblies transformed into tube, ribbon, or spherical architectures depending on placement of the fluorinated F5-Phe residue (Figure 3). For example, when F5-Phe is placed closest to the peptide N-terminus, which is functionalized with the hydrophobic fatty acid tail, co-assemblies with dodecanoic acid yield homogenous vesicles ~1.4 μm in diameter.[51] This is a result of strong anion-π inter-molecular interactions that yield spherical geometries, which can be subsequently altered to form cylindrical and fibrillar structures by positioning the F5-Phe substituent away from the hydrophobic terminus. This work serves to highlight how rational placement of fluorinated residues can dramatically alter fluoropeptide supramolecular architectures when co-assembled with nonpolar hydrocarbons.
FIGURE 3.
Co-assembly of dodecanoic acid and peptide amphiphiles (PA), which contain F5-Phe (Z), residues in different positions adjacent to the assembly domain (Z1-Z3), produce divergent supramolecular structures dependent on fluorine placement and microenvironment. A-C, Cryo-TEM images of mixtures of PA Z1, PA Z2, and PA Z3 with dodecanoic acid [DA] in a molar ratio of 1:0.4, and E-G, conventional TEM images of mixtures in a 1:1 M ratio without staining (schematic illustrations of the morphologies are shown in the insets). DLS data of the vesicles formed by PA Z1 and dodecanoic acid in 1:1 M ratio are shown in the bottom inset in E. D,H, SAXS profiles of the DA-PA aqueous solutions in molar ratios of 1:0.4, D and 1:1, H. The profiles plot scattered intensity vs the scattering vector q (log-log plot); scattering intensities are offset vertically for clarity, and the fitting curves for the scattering data are shown in black in D. Reprinted with permission from J. Am. Chem. Soc. 2017, 139, 7823. Copyright © 2017 American Chemical Society
Conversely, perfluorocarbons can template the assembly of fluoropeptides into unique highly ordered structures via favored fluorine-fluorine interactions. For instance, incorporation of three F5-Phe residues onto the N-terminus of an RGD-functionalized, glycine-rich sequence enabled its interpolation into fluorous liquids.[33-34] As a result, assembly of the peptide could be templated at the surface of fluorinated nanodroplets to form ultrasound-sensitive peptide emulsions. Particle size could be precisely controlled by tuning the feed ratio of the peptide and fluorous solvent to yield emulsions 300 to 1200 nm in diameter.[33] Further, fluorous-driven assembly of the peptide's N-terminus with the acousto-responsive perfluorocarbon template enabled the presentation of C-terminal RGD-ligands at the emulsion surface to facilitate ultrasound-guided particle binding to mammalian cells in vitro and in vivo; the biomedical implications of which are discussed in Section 5.2.[33-34]
Other groups have appended fluoroalkyl tails onto otherwise nonassembling polymeric building blocks, such as functionalized dextran or polypeptide nucleic acids (PNAs), to drive their assembly via strong fluorine-fluorine interactions. Compaction of the highly fluorinated ligands away from the aqueous exterior often results in oligomeric nanoparticles of 100 to 200 nm in size. This has been recently demonstrated through the engraftment of perfluoroalkyl-capped (─C3F7) poly-l-lysine dendrons onto a dextran backbone, leading to the generation of ~150 nm particles.[56] The fluorophilic nature of the modified lysine dendrons resulted in spontaneous self-assembly of the biopolymer scaffold to form core-shell particles in aqueous environments, defined by a fluorous core and a dextran corona. Further, driven by the strong noncovalent packing of the fluorous interior, the self-assembled particles were found to be highly stable, persisting for over 1 week in aqueous solutions without a significant change in hydrodynamic radii. Importantly, this fluorous-mediated compaction often results in a reduction of particle size relative to nonfluorinated counterparts, with significant implications on biologic activity. For instance, Ellipili et al. has demonstrated that fluorinated PNAs can assemble into highly compact cell-permeable nanospheres.[79-81] In one example, perfluoroundecanoyl-functionalized PNAs possessed a diameter of ~100 to 250 nm, which was half the size of their nonfluorinated, undecanoyl conjugated counterparts (~500 nm).[81] Similarly, Ge et al. demonstrated how the degree of fluorination can be exploited to tune the compact spherical architecture of inhalable self-assembled nanoparticles and alter their pulmonary delivery potential.82 Here, the carboxy side chains of polyglutamic acid homo-peptides were functionalized with guanidine and fluoroalkyl self-assembling tags of different tail lengths (─CF3 to ─C3F7) and graft ratios (3-7 tags per peptide) to promote mucus penetration of particle in airway epithelium. Each of the fluorinated polypeptide formulations assembled to form polyplexes with therapeutic oligonucleotides that were 150 to 175 nm in size, which were slightly more compact than the nonfluorinated analog (~200 nm). The fluorinated polypeptides also adopted a higher degree of helicity (50%-70%) than their nonfluorinated counterpart (<50%), which could be carefully titrated depending on the fluorocarbon chain length and degree of fluorination. Together, these examples show how rationally selected placement, chain length, and degree of fluoroalkyl engraftment onto natural peptide scaffolds can enable the precise conformational tuning of designer structures which exploit the fluorous effect for assembly.
Peptoids, a class of peptidomimetics where side chains are displayed from the backbone nitrogen instead of the α-carbon,[83-84] have also been found to form compact spherical particles directed by fluoroalkyl conjugation. Importantly, the lack of hydrogen bonding propensity in the peptoid backbone allows specific and fine-tuned control of intra- and intermolecular assembly that is mediated exclusively through side chain properties. This has been recently exploited to assemble lipid-like peptoids containing N-(2-carboxyethyl)glycine and N-[2-(4-chlorophenyl)ethyl]glycine residues into highly crystalline membrane-mimetic nanomaterials.[85] While these nonfluorinated peptoid precursors adopt 2D supramolecular thin films, integration of 2,2,3,3,4,4,4-Heptafluorobutylamine residues, or perfluoroalkyl chains (─C3F7), within the hydrocarbon and charged peptoid blocks led to the formation of cell-permeable 3D nanoflower particles that were ~130 nm in diameter (Figure 4).[86] X-ray diffraction indicated that these fluorinated peptoid assemblies were highly crystalline and packed into bilayer-like morphologies stabilized by fluorine-fluorine interactions.
FIGURE 4.
Fluoropeptoid assembly yields bioactive crystalline nanoflowers. A, Peptoid structures and the schematic representation showing the self-assembly of these amphiphilic peptoids into crystalline nanoflower-like particles composed of bilayer-like packing of peptoids, in which the peptoid backbone to backbone distance is 4.6 Å along the x-direction and is 1.6 nm along the y-direction; peptoids were highlighted with elements in various colors (nitrogen, blue; oxygen, red; carbon, gray; fluorine, green). B, TEM image of FPC, followed by negative staining. Scale bar: 100 nm. C, AFM image of FPC. D, X-ray diffraction data of FPC. E, Cell incubated for 1 hour with FPC (100 nM). Scale bars: 10 μm. Reprinted with permission from Small 2018, 14, 1803544. Copyright © 2018 Wiley-VCH
3.2 ∣. Fibrillar assemblies
The formation of vesicular vs fibrillar supramolecular morphologies following fluoropeptide self-assembly is often differentiated by only slight changes in side-chain presentation and microenvironment. As an example, while assembly of Tyr-F5-Phe, Trp-F5-Phe, and F5-Phe-Trp dipeptides produced spherical particles, as discussed above, Phe-F5-Phe, F5-Phe-Phe, and F5-Phe-Tyr organization under similar conditions led to the formation of antiparallel, face-centered ribbon-like laminated fibers via polar-π interactions.78 TEM images confirmed the formation of nanoribbons with average widths of 51.2 ± 4.8, 90.5 ± 3.9, and 32.0 ± 6.1 nm for Phe-F5-Phe, F5-Phe-Phe, and F5-Phe-Tyr, respectively. Similarly, while co-assembly of F5-Phe containing peptide amphiphiles with high concentrations of dodecanoic acid produced particles, reducing the molar ratio of fatty acid to peptide yielded persistent monomeric nanofibers (Figure 3A,B) or laminated nanoribbons (Figure 3C).[51] Independent of final morphology, assembly is driven in the presence of dodecanoic acid via anion-π interactions as the fatty acid carboxylate interacts with the aromatic F5-Phe residue. As the F5-Phe residue moves away from the N-terminal assembly domain the cylindrical nanofibers transition into nanoribbons at low concentrations of dodecanoic acid. This exemplary work demonstrates how integration of a single fluorinated residue drives assembly into fibrillar and ribbon-like constructs inaccessible to the nonfluorinated precursor.
Naturally occurring polypeptide-based nanofibers have also been shown to laterally assembly into bundles upon addition of fluorinated residues. The Nilsson group has shown how substitution of hydrophobic F5-Phe residues in place of alanine in the amphipathic Ac-(Ala-Lys-Ala-Glu)2-NH2 peptide can more rapidly and efficiently drive its β-sheet rich fibrillar assembly.[87] Substituting two of the four alanines in the sequence for F5-Phe residues led to π- π side chain interactions necessary to produce 4 nm width fibrils, which subsequently bundle to form twisted or flattened ribbons that are 11 to 14 nm thick. In contrast, the nonfluorinated residue, cyclohexane alanine, formed unstructured aggregates due to their inability to efficiently stack in consequence of the steric environment nonconducive to self-assembly. In another example, biosynthetic incorporation of 5,5,5-trifluoroleucine (TFL) residues into helical coiled-coil polypeptides expressed in Escherichia coli promoted bundling of the fluorinated proteins into highly thermostable nano- and micro-fibers.[44] At the monomeric level, the fluorinated peptides showed increased α-helical content over nonfluorinated analogs, resulting in more efficient, TFLmediated, head-to-tail pentameric subunit formation into 3.6 nm wide protofibrils. These protofibrils underwent lateral bundling to form mature fibers with diameters ranging from ~40 nm to 2 μm.
3.3 ∣. Nano-rods, -tubes, and -pores
Rational fluorine placement on fluoropeptides can also promote their organization into hollow core assembles that encompass a variety of architectures, from nanotubes to nanopores. Antiparallel, porous cylindrical nanorods have been recently reported by Burade et al. via incorporation of turn-promoting fluorinated residues into tripeptides, enabling their axial organization into high aspect ratio rods.[72] Here, incorporation of a fluorine atom in the sugar containing acyclic αγα-tripeptide induced a reverse-turn and U-shaped molecular conformation. This arrangement allowed for antiparallel stacking of the tripeptides, further stabilized via intermolecular hydrogen bonding, to produce heterogenous helical nanorods 2 to 50 μm in diameter and several hundreds of microns long. Similar nano-rod persistence lengths have been observed for fluorinated dendritic tripeptide amphiphiles.[88] Here, incorporation of trifluoromethylated tyrosine residues into aromatic tripeptide dendrimers sufficiently increased scaffold hydrophobicity to drive its self-assembly into >500 nm length nanorods at physiological pH and salinity.
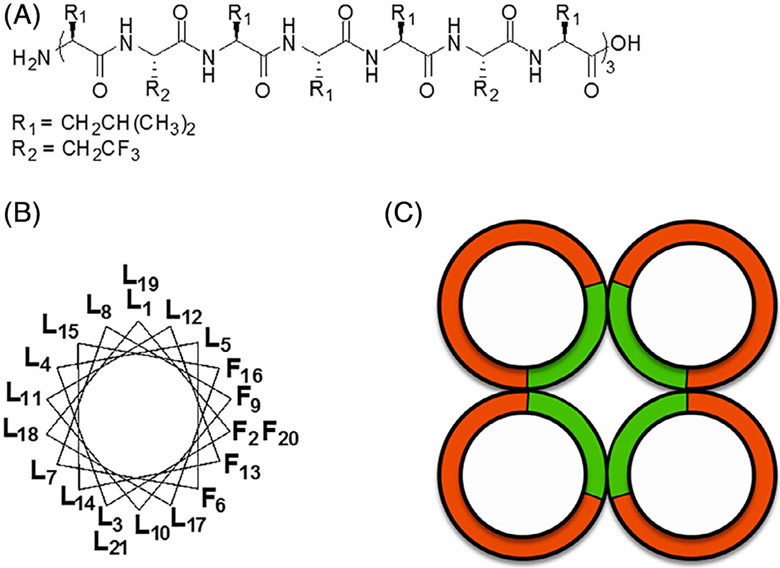
Completely hollow supramolecular architectures, typically produced from silicon carbide or graphene,[89-91] have also been reported via the self-assembly of short fluoropeptides. Building upon their earlier work on αγα-tripeptides, Burade et al. have reported fluorinated sugar amino acid derived α,γ-cyclic tetra- and hexapeptides that adopt symmetric flat oval- and triangular-ring shaped β-strands.[36] Nan-otubes are constructed when these disk-shaped molecules undergo intermolecular backbone hydrogen bonding. Similarly, high aspect ratio nanotubes have been prepared using self-assembling dipeptides of alanine and 3-amino-2-(2-fluorophenyl)-3-phenylpropanoic acid.[92] The small size of these di- and tri-peptides limits their assembly to monomeric nanopores, while a 21-mer leucine-rich peptide (LX2) incorporating six trifluoroethylglycine residues has been shown to produce multimeric assemblies.[57] When presented with a hydrophobic environment, such as a phospholipid bilayer, LX2 self-assembles into well-defined α-helices defined by opposing fluorine- and leucine-rich faces. Aided by in silico modeling, LX2 bundles were suggested to organize into stable tetrameric nanopores via favorable fluorine-fluorine interactions (Figure 5), permitting efficient transmembrane ion transport, as discussed in Section 5.4.
FIGURE 5.
A, Linear structure of the fluorinated 21-residue peptide LX2, with 15L-leucines (L) and 6L-2, 2, 2-trifluoroethylglycine (F); B, Axial wheel projection illustrating the amphiphilic fluorous/hydrophobic faces of LX2 α-helix; C, Schematic representation of a tetrameric arrangement of LX2, showing in green the fluorinated faces of the fourhelix bundle, and in red the hydrophobic contours. Reprinted with permission from PLoS ONE, 2016, 11(11) Copyright © 2016 PLOS ONE
4 ∣. FLUOROPEPTIDE MACROASSEMBLIES
Fluorine substituents incorporated into peptide sequences have remarkable effects on intermolecular assembly and evolution into highly stable nano- and micro- scale structures, as presented in Section 3. Implementing these fluorination approaches in bulk solution propagates long-range entanglement of supramolecular fibrils for the fabrication of macroscopic hydrogels. In this section, we describe how using fluorous gelator subunits improves macro-assembly rate and efficiency in aqueous media via fluorine sequestration, ultimately enhancing the mechanical properties of the pursuant hydrogel constructs (Table 1) and imparting them with unparalleled proteolytic and thermal stability. Two common approaches are employed for fluoropeptide-based hydrogel assemblies: (a) incorporation of noncanonical fluorinated phenylalanine on Fmoc-based peptides and (b) N-terminal modification of natural residues with pentafluorobenzene (PFB).
TABLE 1.
Physical properties of fluorinated Phe-based hydrogleators (G′ = storage modulus; G″ = loss modulus)
| Hydrogelator | Appearancea | G′ (Pa) | G″ (Pa) | Fiber dia. (nm) | Source |
|---|---|---|---|---|---|
| Fmoc-Phe | O | 39 ± 3 | 5 ± 1 | 295 ± 84 | Liyanage (2016) |
| Fmoc-2-F-Phe | T | 2185 ± 59 | 154 ± 20 | 18 ± 2 | Ryan (2010) |
| Fmoc-3-F-Phe | T | 4219 ± 209 | 404 ± 39 | 21 ± 3 | Ryan (2010) |
| Fmoc-4-F-Pheb (1) | T | 443 ± 85 | 35 ± 16 | 26 ± 3 | Ryan (2010) |
| Fmoc-F5-Phe | T | 3056 ± 71 | 320 ± 12 | 9 ± 2 | Ryan (2009) |
| Fmoc-4-NO2-Phe | T | 410 ± 18 | 66 ± 4 | 12 ± 2 | Liyanage (2016) |
| Fmoc-4-CN-Phe | T | 140 ± 21 | 17 ± 6 | 25 ± 4 | Liyanage (2016) |
| Fmoc-4-F-Pheb (2) | T | 102 ± 7 | 9 ± 3 | 9 ± 3 | Liyanage (2016) |
| Fmoc-4-NH2-Phe | T | 527 ± 47 | 61 ± 5 | 11 ± 2 | Liyanage (2016) |
| Fmoc-Tyr (4-OH) | T | 506 ± 55 | 59 ± 13 | 13 ± 2 | Liyanage (2016) |
| Fmoc-4-CH3-Phe | T | 280 ± 26 | 53 ± 11 | 21 ± 2 | Liyanage (2016) |
| Fmoc-3-F-Phe/Fmoc-3-F-Phe-NH2 | N/A | 160 ± 20 | 40 ± 5 | 13 ± 3 | Ryan (2011) |
| Fmoc-F5-Phe/Fmoc-F5-Phe-NH2 | N/A | 110 ± 30 | 6 ± 2 | 12 ± 2 | Ryan (2011) |
| PFB-Phe | ST | ~3000 | ~150 | 31 ± 5 | Hsu (2014) |
| PFB-Phe-Gly | T | ~12 000 | ~1500 | 8 ± 1 | Hsu (2014) |
| PFB-Phe-Ala | T | ~4400 | ~300 | 10 ± 2 | Hsu (2014) |
| PFB-Phe-Val | ST | ~3200 | ~87 | 14 ± 2 | Hsu (2014) |
| PFB-Phe-Phe | T | ~34 000 | ~8000 | 5 ± 1 | Hsu (2014) |
| PFB-Phe:PFB-Phe-Phe | Hsu (2015) | ||||
| 3:1 | T | 2800 | 550 | 14.7 ± 3 | |
| 1:1 | ST | 3000 | 200 | 8.3 ± 2 | |
| 1:3 | T | 26 000 | 4500 | 7.7 ± 2 | |
| 4-fluorobenzyl-capped Phe-Phe | N/A | ~5700 | ~1500 | 8 ± 1 | Wu (2016) |
O, opaque; T, transparent; ST, semi-transparent. N/A indicates gel appearance was not described in original work.
Reports of hydrogelators with the same molecular structure from (1) Ryan (2010) and (2) Liyanage (2016).
4.1 ∣. Fmoc-based Hydrogelators
Self-assembly of Fmoc-functionalized di- and tri-peptides have been reported for decades by Nilsson, Ulijn, Gazit, and others,[93-98] where hydrogen bonding and π-π interactions between polycyclic fluorenyl anthracenes drives the formation of nano-fibrillar gels. Using Fmocphenylalanine residues imparts additional intermolecular stabilization via hydrophobic and dipole interactions between benzyl side chains of adjacent phenylalanine residues. Therefore, conjugating substituents to the benzyl ring of phenylalanine alters dipolar effects and impacts supramolecular assembly. This has been shown using Fmoc-Phe-derived hydrogelators with varying electron-donating or electron-withdrawing groups on the benzyl side chains, where the fastest hydrogelation rate occurs when dipoles are complementary in charge between neighboring aromatics.[99] Accordingly, hydrogelation rate is slowed when like charges interact in the positions of these dipoles. Herewith, the degree of fluorine substitution on the Phe side chain and the position on the benzyl ring significantly impacts Fmoc-Phe self-assembly; the Nilsson group have systematically defined several of these important structure-activity relationships. First, there is a strong positional dependence of fluorine on the assembly kinetics of mono-halogenated Fmoc-n-F-Phe derivatives, with gelation rates increasing following ortho < meta < para.[100] Second, this positional dependence alters fibril diameter during gelation (para < meta < ortho). The Nilsson group suggests these behaviors are complementary, where larger fibrils are able to quickly assemble into interfibrillar bundles, but have reduced entanglement density that leads to a less stable and more mechanically compliant gel.[100] Thus, counterbalancing gelation rate and fiber diameter with the mono-halogenated Fmoc-3-F-Phe formed the stiffest hydrogel of the three available formulations. The physical properties of Fmoc-3-F-Phe gels approximate those of the previously established penta-fluorinated Fmoc-F5-Phe hydrogels (Table 1),[101] suggesting a single fluorine substituent is sufficient to take advantage of the fluorous-mediated increase in material mechanical rigidity. However, mono-fluorinated Fmoc-n-F-Phe hydrogelators benefit from reduced gelation time compared with their penta-fluorinated counterpart. For example, mono-fluorinated Fmoc-3-F-Phe forms a competent gel in ~5 minutes, a significant improvement over ~30 minutes for penta-fluorinated analogs. Relatedly, substitution of nonhalogen groups on the benzyl ring affects the structure of Fmoc-Phe hydrogels, where multiple electron-rich and electron-deficient Fmoc-4-X-Phe derivatives were shown to facilitate the production of stable, transparent hydrogels.[99]
In addition to their effects on gelation rate and mechanical rigidity, mono- and penta-fluorinated Fmoc-Phe-derived gels have divergent, biologically relevant structural properties. First, these materials show an optimal gelation pH between 3.5 and 3.9 in water, varying based on monomer pKa, that limits their utility for biologic applications (pH 7.4). To address this, the Nilsson group further investigated C-terminal modifications of the hydrogelator to alter the charge state of the amino acid and thus tune its pH-dependent gelation conditions.[102] Two amidated formulations (Fmoc-3F-Phe-NH2 and Fmoc-F5-Phe-NH2) formed fibrillar assemblies, and Fmoc-F5-Phe-NH2 formed fibril-based hydrogels in water. However, a mixture of amideterminated and carboxy-terminated derivatives were found to be most effective in forming gels in ionic solutions (PBS, pH 7.4). Unfortunately, hydrogels assembled by these mixtures do not approach the structural properties of their parent mono- or penta-fluorinated hydrogelators. Similarly, gels prepared from nonfluorinated Fmoc-Phe derivatives containing a C-terminal cationic amine were stable in ionic solutions.[103] Exploiting these properties, the Nilsson group have encapsulated the anti-inflammatory drug diclofenac in the cationic gel via charge complementation and validated its activity in vivo using a pain mitigation mouse model.[104] Beyond C-terminal modifications, the same group investigated the assembly of dipeptides prepared from Fmoc-Phe- and either -Asp or -Arg residues that form supramolecular fibronectin mimetics.[105] Co-assembly of these hydrogelators creates a fibril stack that imitates the presentation of the cell-adhesion motif RGD. Importantly, Asp and Arg are not directly affecting the assembly of fibrils, which is still reliant on Fmoc-Fmoc interactions, and thus co-assembly of the dipeptides showed fibril formation and gelation properties similar to the uniform assembly of unmodified Fmoc-3-F-Phe.[105]
4.2 ∣. Perfluorobenzene-based hydrogelators
Fmoc-Fmoc interactions have been extensively employed to potentiate fibril formation of low molecular weight hydrogelators, however, Fmoc is a rather bulky residue. For this reason, fluorine-derived gelators without Fmoc have been designed. A non-Fmoc-based hydrogelator system was proposed by the Lin group by exploiting interactions between perfluorobenzene (PFB) and benzene.[106] This system relies on alternative, parallel stacking of PFB and the benzyl side chain of L-Phe for assembly. Placing the PFB capping moiety adjacent to the Phe residue causes intramolecular quadrupole-quadrupole and quadrupole-dipole stabilization with the two aromatic rings stacked on each other to induce fibril formation, a precursor to gelation. Gelation behavior of the PFB-capped L-Phe molecule, or several dipeptide derivatives of Phe and Gly, Ala, or Val, were tested. It should be noted that PFB-L-Phe has the minimum number of atoms (38) found in any amino acid based hydrogelator to date. They observed supramolecular hydrogels were exclusively formed for the dipeptide samples when the L-Phe building block is intact. Conversely, swapping Phe in the dipeptides with other amino acids completely abrogated hydrogelation.[106] Further, the authors found that fibril diameters and mechanical rigidity of gels formed from the PFB-capped dipeptides were dependent on the identity of the C-terminal amino acid to L-Phe. For example, stiffness decreased and fibril diameter increased with increasing molecular weight of the additional amino acid (Gly < Ala < Val). Moreover, these hydrogelators were observed to be generally biocompatible, with >80% viability of CTX TNA2 cells observed up to 500 μM concentrations of either PFB-L-Phe or PFB-L-Phe-L-Gly.[106]
In follow up work, Lin and co-workers investigated the benefits of a PFB-capped L-Phe-L-Phe formulation, noting that co-assembly of PFB-L-Phe and PFB-L-Phe-L-Phe produced a stable hydrogel at physiologic pH.[107] Shifting the molar ratio of the co-assembled constituents to favor PFB-L-Phe-L-Phe decreased fibril diameter, but generally increased the gel's mechanical strength (Table 1). Parallel work investigated the contribution of fluorination on gelator assembly and found that the nonfluorinated analog (benzyl-L-Phe-L-Phe) was unable to form a competent hydrogel.[108] Hydrogelation of diphenylalanine linked to a 4-fluoro-benzyl group (4FB-L-Phe-L-Phe) was structurally comparable to that of the previously reported perfluorinated PFB-capped dipeptides and possessed a similar biocompatibility in PC-3 cells. Again, these results collectively suggest that only a single fluorine substituent is needed to sufficiently alter the dipeptide's physicochemical properties and drive hydrogel formation via fluorine-fluorine interactions.
Outside of pure fluorobenzene-based gels, Montclare and co-workers previously reported protein co-polymers made of two self-assembled blocks derived from elastin (E) and coiled-coil domains of cartilage oligomeric matrix proteins (COMP, C).[109] The Phe residues in these proteins were mutated with fluorinated Phe. While the secondary conformations of F5-ECE and F5-EC were similar to their nonfluorinated counterparts, the fluorine-containing building blocks produced more structured supramolecular assemblies. The F5-CE biopolymer, however, was defined by increased α-helical and β-sheet rich structure compared to the largely random coil wild-type. It is worth noting that increasing temperatures caused further rearrangement of the fluorinated species, with F5-CE and F5-ECE displaying predominantly β-sheet character at elevated temperatures.
5 ∣. BIOLOGIC APPLICATIONS
The fluorous microenvironments created by supramolecular fluoropeptide self-assembly provides a unique setting from which valuable biofunctionality emerges. Here, we describe how the fluorous effect imparts organofluorine peptide-based biomaterials with attractiveproperties for biomedical applications, including (a) circumventing DNA/RNAi delivery barriers, (b) creating diagnostic and therapeutic contrast agents, (c) building biocompatible and antifouling tissue scaffolds, and (d) enabling selective transmembrane ion transportation.
5.1 ∣. Nucleic acid delivery
Systemic delivery of oligonucleotides faces numerous pharmacologic barriers, including nuclease degradation by serum proteases, rapid renal clearance, and cellular membrane impermeability. Polycationic transfection reagents are widely used as nucleic acid carriers due to their dual purpose to complex with the oligonucleotide anionic phosphate backbone and induce cellular internalization via adsorption to the negatively charged phospholipid bilayer. Despite achieving endosomal escape via the proton sponge effect, resulting in high transfection efficiencies, their utility is often counterbalanced by notorious cytotoxic effects.[110-114] Accordingly, development of safe and efficient oligonucleotide delivery vehicles is crucial for translation into clinical use.[115-116] Fluoropeptide interpolation into hydrophobic lipid bilayers has recently been exploited to minimize transfection cytotoxicity while improving oligonucleotide uptake and expression efficiency. Self-assembled fluoropeptide nanoparticles,[81,86] polyplexes,[32,68,82,117] and nanotubes[92] have shown to enhance cellular internalization through supramolecular organization. For instance, the aforementioned perfluoroalkyl-conjugated compact PNA nanospheres improved internalization into NIH3T3 and HeLa cells by 10-fold compared to the nonfluorinated analog.[81]
Following internalization, endosomal escape and trafficking in the cytosol is necessary for the delivered oligonucleotide to interact with cellular machinery for gene expression or knockdown. Here, fluorinated polyplexes also stand out, showing a greater capacity in a variety of cells to transport oligonucleotide cargo to the cytoplasm or perinuclear space compared to both their nonfluorinated analogs, as well as standard polycationic transfection reagents.[32,68,117] As previously mentioned in Section 3.1, Ge et al. demonstrated the impact that fluorination degree has on the helicity and micro-assembly of guanidinylated polyglutamic acid.[82] In turn, the degree of fluorination also impacts the efficiency of cellular internalization for these assemblies, where low fluorine content (≤18% mol graft ratio of fluorocarbon side chains) imparts improved cellular uptake over that of nonfluorinated polyplexes.[82] However, increasing fluorination to >30% graft ratio compromises this improvement, most likely due to negating the counterbalance with the cationic guanidine side groups. As a result, low fluorination level polypeptides (P3F16 and P7F7) were identified as optimal polyplex materials showing gene silencing of ~70% at the protein and mRNA level.[82] In these examples, although the addition of perfluoroalkyl tags to the self-assembled peptide carriers reduced their ability to employ the proton-sponge effect while in endosomes, efficient escape from endosomal vesicles was still observed.[32] This suggests organofluorine peptides can interact with endosomal membranes via hydrophobic interactions to promote efficient oligonucleotide escape, without reliance on the often toxic proton sponge effect. As another example, fluorinated peptoid crystals, or nanoflowers, demonstrated that fluorines grafted onto an amphiphilic peptoid fostered rapid and nondisruptive fusion with the plasma and endosomal membranes to promote carrier uptake and endosomal escape into human nonsmall cell lung carcinoma cells (Figure 4E).[86] Advantageously, these efficient oligonucleotide delivery strategies did not compromise cell viability (>90% viability observed after 24 hour incubation).[68,82,86,117]
Fluorocarbons are defined by an extremely low surface energy in aqueous solutions, which leads to low interaction affinities with hydrophilic proteins.[118] This property has also been leveraged in oligonucleotide delivery to prevent destabilization of the polyplex by serum proteins. For example, work by Cai et al. and Wu et al. have collectively shown that oligonucleotide polyplexes with fluorinated peptide dendrimers reduce serum adsorption and better retain their transfection efficacies relative to both nonfluorinated vehicles and standard transfection reagents, even at high serum content (50%).[32,117] Similarly, perfluoroalkyl tags facilitate transmucosal penetration of oligonucleotide polyplexes across the glycoprotein-rich mucus layer in lung epithelium, a long-standing barrier to the utility of nucleic acid delivery systems to the respiratory tract. This was shown by Ge et al. using fluorinated guanidinated helical polypeptides, which enabled transmucosal delivery of nucleic acids to transfect alveolar macrophages (Figure 6).[82] Again, the optimized fluorinated polyplexes, P3F16 and P7F7, showed excellent permeability (>20-fold) and penetration in the mucus layer (~200-fold) when compared to the nonfluorinated analogs, which adsorbed to mucin proteins and became entrapped in mucus. The authors went on to test this system following intratracheal administration of polypeptide/siTNF-α polyplexes in an acute lung injury mouse model, resulting in potent therapeutic and anti-inflammatory gene silencing.[82] Strategically, fluorinating oligonucleotide delivery systems can help overcome the many barriers nanocarriers face during systemic delivery to enhance their overall therapeutic efficiency.
FIGURE 6.
Fluorination enhances mucus penetration of polypeptide/siRNA polyplexes. A, Apparent permeability (Papp) of polypeptide/Cy3-siRNA polyplexes across the air-interface culture of Calu-3 cells. B, Representative trajectories of polyplexes in cystic fibrosis (CF) mucus. C, Mean squared displacement (MSD) of polyplexes as a function of the time scale (τ). D, Distribution of the logarithmic MSD of an individual polyplex at τ = 1 second. E, Fluorescence emission spectra of Cy3-siRNA, Cy5-polypeptide, and Cy5-polypeptide/Cy3-siRNA polyplexes after incubation with 5% CF mucus for 0 or 4 hours. F, Fluorescence intensity of the aggregates between mucin (0.3% or 0.5%) and polypeptide/Cy3-siRNA polyplexes following 4-hours incubation. G, Distribution of PG1/Cy3-siRNA, P3F16/Cy3-siRNA, and P7F7/Cy3-siRNA polyplexes in lung epithelial tissues following intratracheal administration (scale bar = 75 μm). Reprinted with permission from Nano Lett. 2020, 20, 1738. Copyright © 2020 American Chemical Society
5.2 ∣. Drug delivery and theranostics
While fluorinated peptide materials have been used sporadically as passive drug delivery vehicles,[119] their unique physicochemical properties have been more widely exploited to construct bioresponsive delivery systems.[56,120-122] For example, Gu and coworkers compared the stability and pH-mediated release of Doxorubicin (DOX) from hydrazine-based dextran nanoparticles functionalized with hydrocarbon or perfluorinated appendages.[120] Here, ligation of the hydrophobic species to the dextran backbone drives particle association in aqueous media, with fluorous carriers showing a >10-fold reduction in critical micelle concentration compared to the nonfluorinated analogs due to the additional fluorine-fluorine interactions. Further, the fluorous carriers showed a greater propensity to penetrate tumor spheroids and undergo degradation and release in acidic media to deliver loaded DOX to tumors in a pH-triggered manner. In similar work, modular addition of 2-(3,5-Bis[trifluoromethyl]-phenyl) acetic acid modified l-lysines into a PEG “comb” copolymer enabled fine control over the lower critical solution temperature necessary to form nanoparticles within the range of 10 to 60 °C.[121] Consequently, thermo-responsive particle degradation and delivery of loaded DOX was shown, facilitating improved pharmacokinetic properties and enhanced anticancer responses in cellular and animal models.[121-122]
The low endogenous abundance of fluorine in biologic environments has also been exploited to improve the therapeutic and diagnostic utility of materials used in photodynamic therapy (PDT), magnetic resonance imaging (MRI), and ultrasound. The electronega-tivity and fluorophilicity of perfluorocarbons, for instance, makes them exceptional passive oxygen carriers across a range of pH and temperature conditions.[123-125] From this, organofluorine peptide-based vehicles have been used to improve tissue oxygenation to aid photodynamic ablation of hypoxic tumor tissues. PDT requires the accumulation of photosensitizers, molecular oxygen, and irradiated light at a tumor site for generation of reactive oxygen species, like singlet oxygen (1O2), to elicit tumor necrosis and apoptosis.[126] Hypoxic regions of tumors lack a sufficient oxygen supply for efficient PDT, and accordingly, molecular oxygen supplementation is imperative for effective antitumor activity.[127-128] For this, Ma et al. developed peptide-dendron 100 nm nanoparticles that assemble to form a fluorous core suitable for co-loading of molecular oxygen and the photosensitizer IR780 (IR780@O2-SFNs).[129] IR780@O2-SFNs demonstrated superior oxygen loading and sustained release in deoxygenated environments compared to hydrocarbon functionalized analogs. The fluorinated constructs were also able to diffuse into the hypoxic core of ex vivo 4T1 spheroid models and accumulated into 4T1-xenografts in tumor-bearing mice in vivo to improve tissue oxygenation. NIR irradiation of tumors in IR780@O2-SFN treated mice resulted in a nearly complete cessation of tumor growth over the course of 12 days.[129]
MRI has also seen improvements in imaging resolution through the use of 19F-MR contrast agents, which take advantage of limited fluorine content in biologic tissues to improve signaling contrast over the typically used 1H nuclei abundantly found in biological systems.[130] Accordingly, nanoscale 19F-fluoropeptides have been designed to switch 19F-MRI signal “off” and “on” via stimuli-responsive assembly controlled by pH,[67] temperature,[131] and calcium,[41] or enzyme concentration.[26,132-133] For instance, Stupp, Meade, and coworkers developed a suite of 19F-peptide amphiphile constructs composed of perfluorinated tails (C7 or C8) and charged residues (lysine, Lys or glutamic acid, Glu) (Figure 7).[41,67] C7-Glu2 self-assembles into ribbon-like structures at pH 4 and cylindrical fibers at pH 9.[67] Interestingly, the morphologic transition point (pH 7) directly correlated to a switch of the off to on state for 19F-NMR/MR signals.[67] Similarly, C7-Glu2 ribbon-like structures were shown to widen in response to increasing Ca2+ concentration, turning 19F-MRI signals off at concentrations >2 mM.[41] This positions the 19F-MRI contrast agent with the potential to spatially track physiological pH changes or abnormal calcium concentrations to probe hypoxic tumor tissue or osteoporosis, respectively. In other work, Yuan and co-workers have developed stimuli-responsive MRI probes in which 19F-labeled 3,5-bis(trifluoromethyl)benzoic acid is conjugated to the side chain of lysine in a 6-mer enzyme-cleavable peptide.[132-133] The functionalized probe self-assembles upon addition of glutathione to form a ~20 nm nanoparticle, which are subsequently cleaved and disassembled in the presence of legumain[132] or caspase 3/7[133] enzymes. This sequential assembly and disassembly allows the probe to effectively turn “off” and “on” 19F MR signal states depending on local enzyme concentrations.
FIGURE 7.
A, 19F-MRI signals of C7-Glu2 and C7-Glu3 (2 mM) upon titration of CaCl2. B, Signal reduction is observed for C7-Glu2 and C7-Glu3 in response to decreasing Ca2+ concentrations. Each image is 1.8 × 1.8 cm2. In the figure C7-Glu2 and C7-Glu3 are labeled as C7E2 and C7E3, respectively. Adapted with permission from ACS Appl. Mater. Interfaces 2017, 9, 46, 39890. Copyright © 2017 American Chemical Society
Finally, the low boiling point of many perfluorocarbons in aqueous media, a consequence of their low surface tensions, leads to liquid-gas phase transitions to occur under clinically relevant acoustic pressures. This has encouraged their use as ultrasound contrast agents and acoustically-activated drug delivery devices.[134-135] For example, our lab has developed templated fluoropeptide nanoparticles for ultrasound-guided cytosolic delivery of biologics.[33-34] Here, a perfluoro-n-pentane fluorous liquid emulsion was prepared using a fluoroamphiphilic peptide stabilizer that self-assembles at the fluorous-liquid interface. This strategy not only elevated the boiling point (bulk bp = 29°C) of the fluorous interior to create stable droplets at physiologic temperatures,[33] but enabled ultrasound to be used to guide and activate the nanoparticles in tissues (Figure 8A). Consequently, vaporization and cavitation of the fluoropeptide vehicle under low ultrasound intensity, when bound to the surfaces of cells, permeabilized the plasma bilayer and enabled direct cytosolic delivery of membrane impermeable cargo with spatial and temporal control (Figure 8B-D).[33-34] In follow up work, Sloand et al. unveiled a fluoro-masking technique that could be used to load high molecular weight proteins, such as immunoglobulin G (IgG), into the fluorous liquid interior to allow for imaging-guided antibody delivery in vivo (Figure 8E and F).[34]
FIGURE 8.
Ultrasound-guided cytosolic protein delivery from fluoropeptide nanoemulsions (NE). A, Doppler ultrasound imaging (5 MHz) of NE vaporization and inertial cavitation of microbubbles as the acoustic pressure is increased. No signal is observed at pressures below the particle's inertial threshold (~0.9 MPa). Cavitation above this pressure threshold is observed as transient Doppler twinkling (1.0 MPa image shown as stacked twinkling events collected over a 40 seconds interval). B, Mean intracellular fluorescence (in relative fluorescence units; RFU) of A549 cells following delivery of labeled phalloidin from NEs at varying US intensity and duty cycle (DC). C, Live-cell image showing delivery of phalloidin (green) from NEs, spatially resolved to a circular area of the A549 cell monolayer subjected to ultrasound. Cell nuclei are stained blue, scale bar = 200 μm. D, Live A549 cells stained with the endosomal marker transferrin (red) following NE mediated delivery of labeled phalloidin. Scale bar = 80 μm. E, Immunofluorescent micrographs of squamous epidermal tumor sections 5 days after administration of antibody (IgG)-loaded NE without (−US) and with (+US; 2 W/cm2) application of the ultrasound trigger (scale bar = 1 mm). ROI magnification (white dashed square) demonstrating intracellular delivery of antibody following US activation of NEIgG particles (scale bar = 10 μm). F, Relative intracellular fluorescence (in relative fluorescence units) of squamous tumor cells isolated from InvtTA × tetORas mice following treatment with NEIgG particles ± the ultrasound trigger. B-D, Adapted with permission from Angew. Chem. Int. Ed. 2017, 56, 11404. Copyright © 2017 Wiley-VCH. A, E-F, Adapted with permission from ACS Nano 2020, 14, 4, 4061. Copyright © 2020 American Chemical Society
5.3 ∣. Tissue engineering and implants
Nilsson and co-workers have extensively studied the utility of low molecular-weight fluoropeptide hydrogelators to prepare implantable gels, including engineered scaffolds that mimic the extracellular matrix.[102-103,105,136] Non-natural, diphenylalanine are particularly attractive and well-studied hydrogelators that construct biomaterials with physiologically relevant viscoelastic properties and biocompatibility.[137] Remarkably, addition of just a single fluorine atom onto the phenylalanine side chain of the dipeptides evokes superior cell viability of the scaffold,[108] while attachment of integrin binding motifs can overcome poor protein adsorption to amplify cellular adhesion.[105,138]] This was shown for an Fmoc-4-F-Phe hydrogelator that was functionalized with a C-terminal RGD integrin binding motif, enabling fibroblast attachment and proliferation on the self-assembled hydrogel surface for 72 hours.[138] Additionally, co-assembly of two dipeptides containing 4-F-Phe and either Asp or Arg formed a supramolecular mimic of the RGD binding ligand that enabled robust fibroblast adhesion and matrix production to create a tissue-like cellular organization.[105]
Moreover, the anti-fouling properties of fluorinated materials have been utilized to generate fluoropeptide scaffolds as functional implant coatings or restorative composites that resist bacterial biofilm formation. In addition to inhibiting bacterial contamination, these fluorous materials must also integrate into the local tissue to prevent implant rejection. To address both of these criteria, fluoropeptide scaffolds comprised of dihydroxyphenylalanine (DOPA) residues have been designed to promote tissue adhesion of the antibacterial biomaterial.[27,139] Dolid et al. demonstrated how antifouling activity of DOPA-based fluoropeptide coatings depend on C-terminal modifications.[27] Here, amide modifications were found to change the C─F bond configuration and alter the surface energy of the titanium implant to which its applied, consequently improving biofouling resistance toward a variety of Gram-positive and -negative pathogens. Moreover, Yuran et al. functionalized DOPA-based fluorous tripeptides with an RGD motif to both impart anti-biofouling properties to titanium implants (>80% E coli growth inhibition) and to promote host cell attachment and spreading (>35% viable cell coverage).[139] Antibacterial resin dental composites have been similarly constructed from fluoropeptides to aid in tooth remineralization that warrants the same design requirements as antifouling coatings. Schnaider et al. created amalgams of Fmoc-F5-Phe self-assembled fibers with dental resin-based composite restoratives, which improved bacterial growth inhibition toward Streptococcus mutans to 95%, while retaining similar biocompatibility and mechanical strength as commercial dental resins (Figure 9).[140]
FIGURE 9.
Antibacterial capabilities and biocompatibility of the enhanced resin composite restoratives. A, Bacterial growth inhibition kinetics evaluated by turbidity analysis following direct contact of S mutans bacteria with the Fmoc-F5-Phe-incorporated restoratives for 1 hour. B, Bacterial viability evaluation following direct contact analysis with either the control composite restorative (Filtek resin) or Fmoc-F5-Phe nanoassembly-incorporated resin composite restorative using the Live/Dead backlight bacterial viability kit. C,D, 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) cell viability analysis. The cytotoxicity of the Fmoc-F5-Phe-incorporated restoratives toward (C) 3 T3 fibroblast and D, HeLa cells was evaluated by the MTT assay at 2 w/w %, as well as control restoratives treated in the same manner that were not incorporated with nanoassemblies, E,F, Mammalian cell viability utilizing a fluorescent Live/Dead staining assay containing fluorescein diacetate (live cells) and propidium iodide (dead cells). E, 3T3 fibroblasts and F, HeLa cells. The scale bar is 500 μm. Reprinted with permission from ACS Appl. Mater. Interfaces 2019, 11, 24, 21 334. Copyright © 2019 American Chemical Society
5.4 ∣. Ion transport
Synthetic nano-assemblies that enable controlled transmembrane ion transport have long been sought to create hybrid filtration materials and lipid membranes with high permeability and solute selectivity.[141] Fluoropeptide-based nanostructures have been synthesized for such a purpose and rationally designed to provide highly selective transport of cations or anions across lipid membranes. For example, Burade et al. developed two distinct nanopore scaffolds that selectively transport anions through the use of acyclic αγα-tripeptides or fluorinated sugar amino acid derived α,γ-cyclic hexapeptides.[36,72] Both constructs demonstrated dose-dependent translocation of either SCN− or NO3− ions across a mimetic phospholipid bilayer without apparent permeability for cations (Figure 10). Complementary proton NMR and MS analyses revealed that transport is largely mediated by hydrogen bonding of the anions to the amide peptide backbone (NH-A−). Conversely, Godbout et al. showed cation-specific transportation using leucine-rich 21-mer fluoropeptide (LX2) tetrameric assemblies.[57] Here, LX2 self-assembles into tetrameric nanopores, where the fluorine-rich faces within the pore serve to relay the alkali metal ions through the channel for membrane translocation. Interestingly, this system showed size-dependent cation transport, favoring sodium ions over the larger cations, potassium, and cesium. As expected, smaller counterions (Cl− or Br−) bound to Na+ led to faster transport of the ion compared to larger counterions (I−).
FIGURE 10.
Ion transport of fluorinated sugar amino acid derived α,γ-cyclic hexapeptides (2.5 μM) across model membranes (EYPCLUVs⊃HPTS) determined for varying cations, A, and anions, B, in the extravesicular buffer. Reprinted with permission from Org. Lett. 2017, 19, 21, 5948. Copyright © 2017 American Chemical Society
6 ∣. CONCLUSION AND OUTLOOK
Organofluorine peptides scaffolds have emerged as next generation biomaterials designed to exploit the fluorous effect for enhanced supramolecular self-assembly and biofunctionality. Fabrication of fluoropeptides with either fluorinated amino acids, fluoroalkyl appendages, or even a single fluorine-substitution on the backbone, can dramatically alter intra- and intermolecular interactions to tune self-assembly propensity into hierarchical architectures. While fluorinated derivatives of canonical amino acids are now commonplace, continued development of noncanonical fluorine containing residues will serve to expand the synthetic tool kit from which fluoropeptides with unique properties can be rationally designed. However, synthesis of fluoropeptides remains a challenge, particularly adapting solid-phase techniques and purification methods to obtain these non-natural sequences at scales necessary for preclinical studies. Achieving fine control over the degree of fluorination on these sequences is an additional barrier to their widespread adoption. Accordingly, modified solid-phase synthetic methods and resins, as well as a wider variety of fluorinated chromatography media for purification, are needed to broaden the de novo fluoropeptide repertoire.
ACKNOWLEDGMENT
Funding for this work was provided by the NSF Faculty Early Career Development Program (CAREER) to Scott H. Medina under award number DMR-1845053.
Funding information
National Science Foundation Faculty Early Career Development Program (CAREER), Grant/Award Number: DMR-1845053
Biography
Janna Sloand is a fifth year PhD candidate in the Precision Therapeutics and Bioresponsive Materials Laboratory at Penn State University (USA). Her research focuses on developing fluorinated peptide nanomaterials for cytosolic protein delivery, biomedical imaging, and membrane filtration. Prior to her doctoral studies, Janna received a BA in Genetics from Rutgers University (USA) in 2013 and graduated with an MSE in Bioengineering from the University of Pennsylvania in 2016 (USA).

Michael Miller is a first year PhD candidate in the Precision Therapeutics and Bioresponsive Materials Laboratory at Penn State University (USA). His research focuses on developing fluorine-based delivery vectors that can be used for ultrasoundprogrammable gene editing in complex three-dimensional tissues. Prior to joining the Biomedical Engineering graduate program at Penn State, Michael graduated from West Virginia University (USA) with a BS in Biomedical Engineering in 2019.

Scott Medina is an Assistant Professor of Biomedical Engineering at Penn State University (USA), and director of Precision Therapeutics and Bioresponsive Materials Laboratory. His research interfaces chemical biology and nanotechnology to create biomimetic tools for precision medicine. As of June 2020, Dr. Medina has co-authored twenty-four publications and is a recipient of the NIH Fellows Award for Research Excellence (2014 and 2016), NCI Director's Innovation Award (2015), Penn State Outstanding Teaching Award (2019), and a NSF CAREER Award (2019).

Footnotes
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
REFERENCES
- [1].Tao K, Makam P, Aizen R, Gazit E, Science 2017, 358(6365), eaam9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nguyen V, Zhu R, Jenkins K, Yang R, Nat. Commun 2016, 7(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee JS, Yoon I, Kim J, Ihee H, Kim B, Park CB, Angew. Chem. Int. Ed 2011, 50(5), 1164. [DOI] [PubMed] [Google Scholar]
- [4].Makam P, Gazit E, Chem. Soc. Rev 2018, 47(10), 3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee J-H, Heo K, Schulz-Schönhagen K, Lee JH, Desai MS, Jin H-E, Lee S-W, ACS Nano. 2018, 12(8), 8138. [DOI] [PubMed] [Google Scholar]
- [6].Berger O, Adler-Abramovich L, Levy-Sakin M, Grunwald A, Liebes-Peer Y, Bachar M, Buzhansky L, Mossou E, Forsyth VT, Schwartz T, Nat. Nanotechnol 2015, 10(4), 353. [DOI] [PubMed] [Google Scholar]
- [7].Gazit E, Nat. Nanotechnol 2016, 11(4), 309. [DOI] [PubMed] [Google Scholar]
- [8].Lampel A, McPhee SA, Park H-A, Scott GG, Humagain S, Hekstra DR, Yoo B, Frederix PW, Abzalimov RR, Greenbaum SG, et al. , Science. 2017, 356(6342), 1064. [DOI] [PubMed] [Google Scholar]
- [9].Sun B, Li Q, Riegler H, Eickelmann S, Dai L, Yang Y, Perez-Garcia R, Jia Y, Chen G, Fei J, ACS Nano. 2017, 11(10), 10489. [DOI] [PubMed] [Google Scholar]
- [10].Tao K, Fan Z, Sun L, Makam P, Tian Z, Ruegsegger M, Shaham-Niv S, Hansford D, Aizen R, Pan Z, Nat. Commun 2018, 9(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tao K, Xue B, Frere S, Slutsky I, Cao Y, Wang W, Gazit E, Chem. Mater 2017, 29(10), 4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zozulia O, Dolan M, Korendovych I, Chem. Soc. Rev 2018, 47(10), 3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tena-Solsona M, Nanda J, Diaz-Oltra S, Chotera A, Ashkenasy G, Escuder B, Chemistry. 2016, 22(19), 6687. [DOI] [PubMed] [Google Scholar]
- [14].Liu Q, Wang H, Shi X, Wang Z-G, Ding B, ACS Nano. 2017, 11(7), 7251. [DOI] [PubMed] [Google Scholar]
- [15].Dolan MA, Basa PN, Zozulia O, Lengyel ZF, Lebl R, Kohn EM, Bhattacharya S, Korendovych IV, ACS Nano. 2019, 13(8), 9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Omosun TO, Hsieh M-C, Childers WS, Das D, Mehta AK, Anthony NR, Pan T, Grover MA, Berland KM, Lynn DG, Nat. Chem 2017, 9(8), 805. [DOI] [PubMed] [Google Scholar]
- [17].Eskandari S, Guerin T, Toth I, Stephenson RJ, Adv. Drug Delivery Rev 2017, 110, 169. [DOI] [PubMed] [Google Scholar]
- [18].Habibi N, Kamaly N, Memic A, Shafiee H, Nano Today. 2016, 11 (1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fan T, Yu X, Shen B, Sun L, J. Nanomater 2017, 2017, 1. [Google Scholar]
- [20].Rad-Malekshahi M, Lempsink L, Amidi M, Hennink WE, Mastrobattista E, Bioconjugate Chem. 2016, 27(1), 3. [DOI] [PubMed] [Google Scholar]
- [21].Shi J, Fichman G, Schneider JP, Angew. Chem. Int. Ed 2018, 57 (35), 11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Smith DJ, Brat GA, Medina SH, Tong D, Huang Y, Grahammer J, Furtmuller GJ, Oh BC, Nagy-Smith KJ, Walczak P, Nat. Nanotechnol 2016, 11(1), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miller SE, Yamada Y, Patel N, Suarez E, Andrews C, Tau S, Luke BT, Cachau RE, Schneider JP, ACS Cent. Sci 2019, 5(11), 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang J, Liu K, Xing R, Yan X, Chem. Soc. Rev 2016, 45(20), 5589. [DOI] [PubMed] [Google Scholar]
- [25].Mason TO, Buell AK, Biological and Bio-inspired Nanomaterials, Springer, Singapore: 2019, p. 61. [Google Scholar]
- [26].Yuan C, Li S, Zou Q, Ren Y, Yan X, Phys. Chem. Chem. Phys 2017, 19(35), 23614. [DOI] [PubMed] [Google Scholar]
- [27].Dolid A, Reches M, J. Pept. Sci 2019, 25(10), e3212. [DOI] [PubMed] [Google Scholar]
- [28].Lin Y, Penna M, Thomas MR, Wojciechowski JP, Leonardo V, Wang Y, Pashuck ET, Yarovsky I, Stevens MM, ACS Nano. 2019, 13(2), 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grotsch RK, Boekhoven J, Unique properties of supramolecular biomaterials through nonequilibrium self-assembly. in Self-assembling Biomaterials, Elsevier, Sawston, Cambridge, UK: 2018, p. 235. [Google Scholar]
- [30].Berger O, Gazit E, J. Pept. Sci 2017, 108(1), e22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mikhalevich V, Craciun I, Kyropoulou M, Palivan CG, Meier W, Biomacromolecules. 2017, 18(11), 3471. [DOI] [PubMed] [Google Scholar]
- [32].Cai X, Jin R, Wang J, Yue D, Jiang Q, Wu Y, Gu Z, ACS Appl. Mater. Interfaces 2016, 8(9), 5821. [DOI] [PubMed] [Google Scholar]
- [33].Medina SH, Michie MS, Miller SE, Schnermann MJ, Schneider JP, Angew. Chem. Int. Ed 2017, 56(38), 11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sloand JN, Nguyen TT, Zinck SA, Cook EC, Zimudzi TJ, Showalter SA, Glick AB, Simon JC, Medina SH, ACS Nano. 2020, 14(4), 4061. [DOI] [PubMed] [Google Scholar]
- [35].Montclare JK, Frezzo JA, Xu C, Wadghiri YZ U.S. Patent No. 10,376,603. 2019.
- [36].Burade SS, Saha T, Bhuma N, Kumbhar N, Kotmale A, Rajamohanan PR, Gonnade RG, Talukdar P, Dhavale DD, Org. Lett 2017, 19(21), 5948. [DOI] [PubMed] [Google Scholar]
- [37].Salwiczek M, Nyakatura EK, Gerling UI, Ye S, Koksch B, Chem. Soc. Rev 2012, 41(6), 2135. [DOI] [PubMed] [Google Scholar]
- [38].Giroud M, Harder M, Kuhn B, Haap W, Trapp N, Schweizer WB, Schirmeister T, Diederich F, ChemMedChem. 2016, 11(10), 1042. [DOI] [PubMed] [Google Scholar]
- [39].Kirberger SE, Maltseva SD, Manulik JC, Einstein SA, Weegman BP, Garwood M, Pomerantz WC, Angew. Chem. Int. Ed 2017, 56(23), 6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hingorani DV, Chapelin F, Stares E, Adams SR, Okada H, Ahrens ET, Magn. Reson. Med 2020, 83(3), 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Preslar AT, Lilley LM, Sato K, Zhang S, Chia ZK, Stupp SI, Meade TJ, ACS Appl. Mater. Interfaces 2017, 9(46), 39890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huhmann S, Koksch B, Eur. J. Org. Chem 2018, 2018(27-28), 3667. [Google Scholar]
- [43].Huhmann S, Stegemann A-K, Folmert K, Klemczak D, Moschner J, Kube M, Koksch B, Beilstein J. Org. Chem 2017, 13(1), 2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].More HT, Zhang KS, Srivastava N, Frezzo JA, Montclare JK, Biomacromolecules. 2015, 16(4), 1210. [DOI] [PubMed] [Google Scholar]
- [45].Moschner J, Stulberg V, Fernandes R, Huhmann S, Leppkes J, Koksch B, Chem. Rev 2019, 119(18), 10718. [DOI] [PubMed] [Google Scholar]
- [46].Berger AA, Völler J-S, Budisa N, Koksch B, Acc. Chem. Res 2017, 50(9), 2093. [DOI] [PubMed] [Google Scholar]
- [47].Odar C, Winkler M, Wiltschi B, Biotechnol. J 2015, 10(3), 427. [DOI] [PubMed] [Google Scholar]
- [48].Qiu XL, Qing FL, Eur. J. Org. Chem 2011, 2011(18), 3261. [Google Scholar]
- [49].Vukelić S, Ushakov DB, Gilmore K, Koksch B, Seeberger PH, Eur. J. Org. Chem 2015, 2015(14), 3036. [Google Scholar]
- [50].Li X, Li Y, Shang H, Org. Biomol. Chem 2016, 14(27), 6457. [DOI] [PubMed] [Google Scholar]
- [51].Yu Z, Erbas A, Tantakitti F, Palmer LC, Jackman JA, Olvera de la Cruz M, Cho N-J, Stupp SI, J. Am. Chem. Soc 2017, 139(23), 7823. [DOI] [PubMed] [Google Scholar]
- [52].Lee K-H, Lee H-Y, Slutsky MM, Anderson JT, Marsh ENG, Biochem. 2004, 43(51), 16277. [DOI] [PubMed] [Google Scholar]
- [53].Molski MA, Goodman JL, Craig CJ, Meng H, Kumar K, Schepartz A, J. Am. Chem. Soc 2010, 132(11), 3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bilgiçer B, Fichera A, Kumar K, J. Am. Chem. Soc 2001, 123(19), 4393. [DOI] [PubMed] [Google Scholar]
- [55].Molski MA, Goodman JL, Chou F-C, Baker D, Das R, Schepartz A, Chem. Sci 2013, 4(1), 319. [Google Scholar]
- [56].Ma S, Zhou J, Wali ARM, He Y, Xu X, Tang JZ, Gu Z, J. Mater. Sci.: Mater. Med 2015, 26(8), 219. [DOI] [PubMed] [Google Scholar]
- [57].Godbout R, Legare S, Auger M, Carpentier C, Otis F, Auger M, Lague P, Voyer N, PLoS One. 2016, 11(11), e0166587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Botz A, Gasparik V, Devillers E, Hoffmann AR, Caillon L, Chelain E, Lequin O, Brigaud T, Khemtemourian L, J. Pept. Sci 2015, 104(5), 601. [DOI] [PubMed] [Google Scholar]
- [59].Brahmachari S, Paul A, Segal D, Gazit E, Future Med. Chem 2017, 9(8), 797. [DOI] [PubMed] [Google Scholar]
- [60].Arias M, Aramini JM, Riopel ND, Vogel HJ, Biochim. Biophys. Acta Biomembr 2020, 1862(6), 183260. [DOI] [PubMed] [Google Scholar]
- [61].Arias M, Hoffarth ER, Ishida H, Aramini JM, Vogel HJ, Biochim. Biophys. Acta Biomembr 2016, 1858(5), 1012. [DOI] [PubMed] [Google Scholar]
- [62].Kubyshkin V, Afonin S, Kara S, Budisa N, Mykhailiuk PK, Ulrich AS, Org. Biomol. Chem 2015, 13(11), 3171. [DOI] [PubMed] [Google Scholar]
- [63].Afonin S, Kubyshkin V, Mykhailiuk PK, Komarov IV, Ulrich AS, J. Phys. Chem. B 2017, 121(27), 6479. [DOI] [PubMed] [Google Scholar]
- [64].Komarov IV, Afonin S, Ulrich AS, Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals, Elsevier, Cambridge, MA: 2019, p. 349. [Google Scholar]
- [65].Richards KL, Rowe ML, Hudson PB, Williamson RA, Howard MJ, Sci. Rep 2016, 6, 19518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang F, Yu X, Liu C, Qu C-X, Gong Z, Liu H-D, Li F-H, Wang H-M, He D-F, Yi F, Nat. Commun 2015, 6, 8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Preslar AT, Tantakitti F, Park K, Zhang S, Stupp SI, Meade TJ, ACS Nano. 2016, 10(8), 7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cai X, Zhu H, Zhang Y, Gu Z, ACS Appl. Mater. Interfaces 2017, 9 (11), 9402. [DOI] [PubMed] [Google Scholar]
- [69].Au C, Gonzalez C, Leung YC, Mansour F, Trinh J, Wang Z, Hu X-G, Griffith R, Pasquier E, Hunter L, Org. Biomol. Chem 2019, 17 (3), 664. [DOI] [PubMed] [Google Scholar]
- [70].Marelli UK, Rechenmacher F, Sobahi TRA, Mas-Moruno C, Kessler H, Front. Oncol 2013, 3, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Roxin À, Zheng G, Future Med. Chem 2012, 4(12), 1601. [DOI] [PubMed] [Google Scholar]
- [72].Burade SS, Shinde SV, Bhuma N, Kumbhar N, Kotmale A, Rajamohanan PR, Gonnade RG, Talukdar P, Dhavale DD, J. Org. Chem 2017, 82(11), 5826. [DOI] [PubMed] [Google Scholar]
- [73].Datta D, Kumar V, Kumar S, Nagaraj R, Chaudhary N, ACS Omega 2019, 4(1), 620. [Google Scholar]
- [74].Pachahara SK, Adicherla H, Nagaraj R, PLoS One 2015, 10(8), e0136567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pachahara SK, Nagaraj R, Biochem. Biophys. Rep 2015, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vincenzi M, Mercurio FA, Leone M, Curr. Protein Pept. Sci 2019, 20(5), 425. [DOI] [PubMed] [Google Scholar]
- [77].Su Z, Pramounmat N, Watson ST, Renner JN, Soft Matter. 2018, 14(18), 3528. [DOI] [PubMed] [Google Scholar]
- [78].Zhang H, Lou S, Yu Z, Langmuir 2019, 35(13), 4710. [DOI] [PubMed] [Google Scholar]
- [79].Ellipilli S, Ganesh KN, J. Org. Chem 2015, 80(18), 9185. [DOI] [PubMed] [Google Scholar]
- [80].Ellipilli S, Palvai S, Ganesh KN, J. Org. Chem 2016, 81(15), 6364. [DOI] [PubMed] [Google Scholar]
- [81].Ellipilli S, vasudeva Murthy R, Ganesh KN, Chem. Commun 2016, 52(3), 521. [DOI] [PubMed] [Google Scholar]
- [82].Ge C, Yang J, Duan S, Liu Y, Meng F, Yin L, Nano Lett. 2020, 20 (3), 1738. [DOI] [PubMed] [Google Scholar]
- [83].Angell YL, Burgess K, Chem. Soc. Rev 2007, 36(10), 1674. [DOI] [PubMed] [Google Scholar]
- [84].Avan I, Hall CD, Katritzky AR, Chem. Soc. Rev 2014, 43(10), 3575. [DOI] [PubMed] [Google Scholar]
- [85].Jin H, Jiao F, Daily MD, Chen Y, Yan F, Ding Y-H, Zhang X, Robertson EJ, Baer MD, Chen C-L, Nat. Commun 2016, 7(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Song Y, Wang M, Li S, Jin H, Cai X, Du D, Li H, Chen CL, Lin Y, Small. 2018, 14(52), 1803544. [DOI] [PubMed] [Google Scholar]
- [87].Betush RJ, Urban JM, Nilsson BL, J. Pept. Sci 2018, 110(1), e23099. [Google Scholar]
- [88].Appel R, Tacke S, Klingauf J, Besenius P, Org. Biomol. Chem 2015, 13(4), 1030. [DOI] [PubMed] [Google Scholar]
- [89].Zhao Y, Li X.-g., Zhou X, Zhang Y.-n., Sens. Actuators, B 2016, 231, 324. [Google Scholar]
- [90].Han D, Mei H, Xiao S, Dassios KG, Cheng L, J. Eur. Ceram. Soc 2018, 38(11), 3695. [Google Scholar]
- [91].Liu Y, Hou H, He X, Yang W, Sci. Rep 2017, 7(1), 1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bonetti A, Pellegrino S, Das P, Yuran S, Bucci R, Ferri N, Meneghetti F, Castellano C, Reches M, Gelmi ML, Org. Lett 2015, 17(18), 4468. [DOI] [PubMed] [Google Scholar]
- [93].Jayawarna V, Richardson SM, Hirst AR, Hodson NW, Saiani A, Gough JE, Ulijn RV, Acta Biomater. 2009, 5(3), 934. [DOI] [PubMed] [Google Scholar]
- [94].Fleming S, Debnath S, Frederix PW, Tuttle T, Ulijn RV, Chem. Commun 2013, 49(90), 10587. [DOI] [PubMed] [Google Scholar]
- [95].Fleming S, Ulijn RV, Chem. Soc. Rev 2014, 43(23), 8150. [DOI] [PubMed] [Google Scholar]
- [96].Fichman G, Gazit E, Acta Biomater. 2014, 10(4), 1671. [DOI] [PubMed] [Google Scholar]
- [97].Orbach R, Adler-Abramovich L, Zigerson S, Mironi-Harpaz I, Seliktar D, Gazit E, Biomacromolecules. 2009, 10(9), 2646. [DOI] [PubMed] [Google Scholar]
- [98].Tao K, Levin A, Adler-Abramovich L, Gazit E, Chem. Soc. Rev 2016, 45(14), 3935. [DOI] [PubMed] [Google Scholar]
- [99].Liyanage W, Nilsson BL, Langmuir. 2016, 32(3), 787. [DOI] [PubMed] [Google Scholar]
- [100].Ryan DM, Anderson SB, Nilsson BL, Soft Matter. 2010, 6(14), 3220. [Google Scholar]
- [101].Ryan DM, Anderson SB, Senguen FT, Youngman RE, Nilsson BL, Soft Matter. 2010, 6(3), 475. [Google Scholar]
- [102].Ryan DM, Doran TM, Anderson SB, Nilsson BL, Langmuir. 2011, 27(7), 4029. [DOI] [PubMed] [Google Scholar]
- [103].Rajbhandary A, Raymond DM, Nilsson BL, Langmuir. 2017, 33 (23), 5803. [DOI] [PubMed] [Google Scholar]
- [104].Raymond DM, Abraham BL, Fujita T, Watrous MJ, Toriki ES, Takano T, Nilsson BL, ACS Appl. Bio Mater 2019, 2(5), 2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liyanage W, Vats K, Rajbhandary A, Benoit DS, Nilsson BL, Chem. Commun 2015, 51(56), 11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hsu SM, Lin YC, Chang JW, Liu YH, Lin HC, Angew. Chem. Int. Ed 2014, 53(7), 1921. [DOI] [PubMed] [Google Scholar]
- [107].Hsu SM, Wu FY, Lai TS, Lin YC, Lin HC, RSC Adv. 2015, 5 (29), 22943. [Google Scholar]
- [108].Wu F-Y, Hsu S-M, Cheng H, Hsu L-H, Lin H-C, New J. Chem 2015, 39(6), 4240. [Google Scholar]
- [109].Yuvienco C, More HT, Haghpanah JS, Tu RS, Montclare JK, Biomacromolecules. 2012, 13(8), 2273. [DOI] [PubMed] [Google Scholar]
- [110].Chernousova S, Epple M, Gene Ther. 2017, 24(5), 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, Mol. Ther 2005, 11(6), 990. [DOI] [PubMed] [Google Scholar]
- [112].Godbey W, Wu KK, Mikos AG, J. Controlled Release 1999, 60 (2-3), 149. [DOI] [PubMed] [Google Scholar]
- [113].Amin ZR, Rahimizadeh M, Eshghi H, Dehshahri A, Ramezani M, Iran. J. Basic Med. Sci 2013, 16(2), 150. [PMC free article] [PubMed] [Google Scholar]
- [114].Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL, Mol. Ther 2013, 21(1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kanasty R, Dorkin JR, Vegas A, Anderson D, Nat. Mater 2013, 12(11), 967. [DOI] [PubMed] [Google Scholar]
- [116].Luo D, Saltzman WM, Nat. Biotechnol 2000, 18(1), 33. [DOI] [PubMed] [Google Scholar]
- [117].Wu T, Wang L, Ding S, You Y, Macromol. Biosci 2017, 17(8), 1700114. [DOI] [PubMed] [Google Scholar]
- [118].Turner SR, Litt M, Lynn WS, J. Colloid Interface Sci 1974, 48(1), 100. [Google Scholar]
- [119].Piccionello AP, Pitarresi G, Pace A, Triolo D, Picone P, Buscemi S, Giammona G, J. Drug Targeting 2012, 20(5), 433. [DOI] [PubMed] [Google Scholar]
- [120].Ma S, Zhou J, Zhang Y, He Y, Jiang Q, Yue D, Xu X, Gu Z, ACS Appl. Mater. Interfaces 2016, 8(42), 28468. [DOI] [PubMed] [Google Scholar]
- [121].Zhu J, Xiao Y, Zhang H, Li Y, Yuan Y, Yang Z, Chen S, Zheng X, Zhou X, Jiang Z-X, Biomacromolecules. 2019, 20(3), 1281. [DOI] [PubMed] [Google Scholar]
- [122].Zhu J, Zhang H, Chen K, Li Y, Yang Z, Chen S, Zheng X, Zhou X, Jiang Z-X, Adv. Healthcare Mater 2020, 9(3), 1901331. [DOI] [PubMed] [Google Scholar]
- [123].Castro CI, Briceno JC, Artif. Organs 2010, 34(8), 622. [DOI] [PubMed] [Google Scholar]
- [124].Kim HW, Greenburg AG, Artif. Organs 2004, 28(9), 813. [DOI] [PubMed] [Google Scholar]
- [125].Riess JG, Chem. Rev 2001, 101(9), 2797. [DOI] [PubMed] [Google Scholar]
- [126].Dolmans DE, Fukumura D, Jain RK, Nat. Rev. Cancer 2003, 3 (5), 380. [DOI] [PubMed] [Google Scholar]
- [127].Freitas I, Tumori J. 1985, 71(3), 251. [DOI] [PubMed] [Google Scholar]
- [128].Brown JM, Wilson WR, Nat. Rev. Cancer 2004, 4(6), 437. [DOI] [PubMed] [Google Scholar]
- [129].Ma S, Zhou J, Zhang Y, Yang B, He Y, Tian C, Xu X, Gu Z, ACS Appl. Mater. Interfaces 2019, 11(8), 7731. [DOI] [PubMed] [Google Scholar]
- [130].Major JL, Meade TJ, Acc. Chem. Res 2009, 42(7), 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hill LK, Frezzo JA, Katyal P, Hoang DM, Ben Youss Gironda Z, Xu C, Xie X, Delgado-Fukushima E, Wadghiri YZ, Montclare JK, ACS Nano. 2019, 13(3), 2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yuan Y, Ge S, Sun H, Dong X, Zhao H, An L, Zhang J, Wang J, Hu B, Liang G, ACS Nano. 2015, 9(5), 5117. [DOI] [PubMed] [Google Scholar]
- [133].Yuan Y, Sun H, Ge S, Wang M, Zhao H, Wang L, An L, Zhang J, Zhang H, Hu B, ACS Nano. 2015, 9(1), 761. [DOI] [PubMed] [Google Scholar]
- [134].Fabiilli ML, Haworth KJ, Fakhri NH, Kripfgans OD, Carson PL, Fowlkes JB, IEEE Trans. Sonics Ultrason 2009, 56(5), 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kripfgans OD, Fowlkes JB, Miller DL, Eldevik OP, Carson PL, Ultrasound Biol. Med 2000, 26(7), 1177. [DOI] [PubMed] [Google Scholar]
- [136].Bowerman CJ, Liyanage W, Federation AJ, Nilsson BL, Biomacromolecules. 2011, 12(7), 2735. [DOI] [PubMed] [Google Scholar]
- [137].Ryan DM, Doran TM, Nilsson BL, Chem. Commun 2011, 47 (1), 475. [DOI] [PubMed] [Google Scholar]
- [138].Wang Y, Zhang Z, Xu L, Li X, Chen H, Colloids Surf., B 2013, 104, 163. [DOI] [PubMed] [Google Scholar]
- [139].Yuran S, Dolid A, Reches M, ACS Biomater. Sci. Eng 2018, 4(12), 4051. [DOI] [PubMed] [Google Scholar]
- [140].Schnaider L, Ghosh M, Bychenko D, Grigoriants I, Ya'ari S, Shalev Antsel T, Matalon S, Sarig R, Brosh T, Pilo R, ACS Appl. Mater. Interfaces 2019, 11(24), 21334. [DOI] [PubMed] [Google Scholar]
- [141].Auger M, Lefèvre T, Otis F, Voyer N, Auger M, J. Pept. Sci 2019, 111(1), e24051. [Google Scholar]