Abstract
Brain arteriovenous malformation (bAVM) is the most common cause of intracranial hemorrhage (ICH), particularly in young patients. However, the exact cause of bAVM bleeding and rupture is not yet fully understood. In bAVMs, blood bypasses the entire capillary bed and directly flows from arteries to veins. The vessel walls in bAVMs have structural defects, which impair vascular integrity. Mural cells are essential structural and functional components of blood vessels and play a critical role in maintaining vascular integrity. Changes in mural cell number and coverage have been implicated in bAVMs. In this review, we discussed the roles of mural cells in bAVM pathogenesis. We focused on 1) the recent advances in human and animal studies of bAVMs; 2) the importance of mural cells in vascular integrity; 3) the regulatory signaling pathways that regulate mural cell function. More specifically, the platelet-derived growth factor-B (PDGF-B)/PDGF receptor-β (PDGFR-β), EphrinB2/EphB4, and angiopoietins/tie2 signaling pathways that regulate mural cell-recruitment during vascular remodeling were discussed in detail.
Keywords: Brain arteriovenous malformation, intracranial hemorrhage, mural cells, PDGF-B/PDGFR-β, EphrinB2/EphB4, angiopoietins/tie2
Introduction
Brain arteriovenous malformations (bAVMs) are tangles of abnormal vessels, called Nidus, that connect arteries directly to veins. Arteriovenous shunts are hallmarks of bAVMs (Lawton et al., 2015; Rangel-Castilla et al., 2014; Yun et al., 2012; Zhang et al., 2016). The malformed vessels in bAVMs are tortuous and markedly dilated. The vascular wall structure is abnormal and thus is fragile and prone to rupture, leading to the life-threatening intracranial hemorrhage (ICH) and catastrophic neurological consequences (Gross and Du, 2013). ICH is the first clinical presentation in approximately half of all bAVM patients.
The deep-seated lesions, and venous outflow pattern can alter flow and wall shear stress and caused cerebral venous hypotension. All of these can cause elevation of VEGF and abnormal vascular remodeling (Gao et al., 2009; Lawton et al., 1997; Zhu et al., 2006), leading to a reduction of vascular pericyte and smooth muscle coverage through the pathways discussed in this review. Increasing evidence indicate that abnormal vascular remodeling and vascular instability are associated with bAVM development and its abnormal phenotypes, including dilated perinidal capillaries (Figure 1) (Attia et al., 2003; Sato et al., 2004; Tu et al., 2006), intranidal or feeding artery aneurysms (Gross and Du, 2013), microhemorrhage and rupture (Abla et al., 2015; Guo et al., 2012; Pekmezci et al., 2016). However, the exact mechanisms underlying bAVM bleeding remain unclear.
Figure 1.
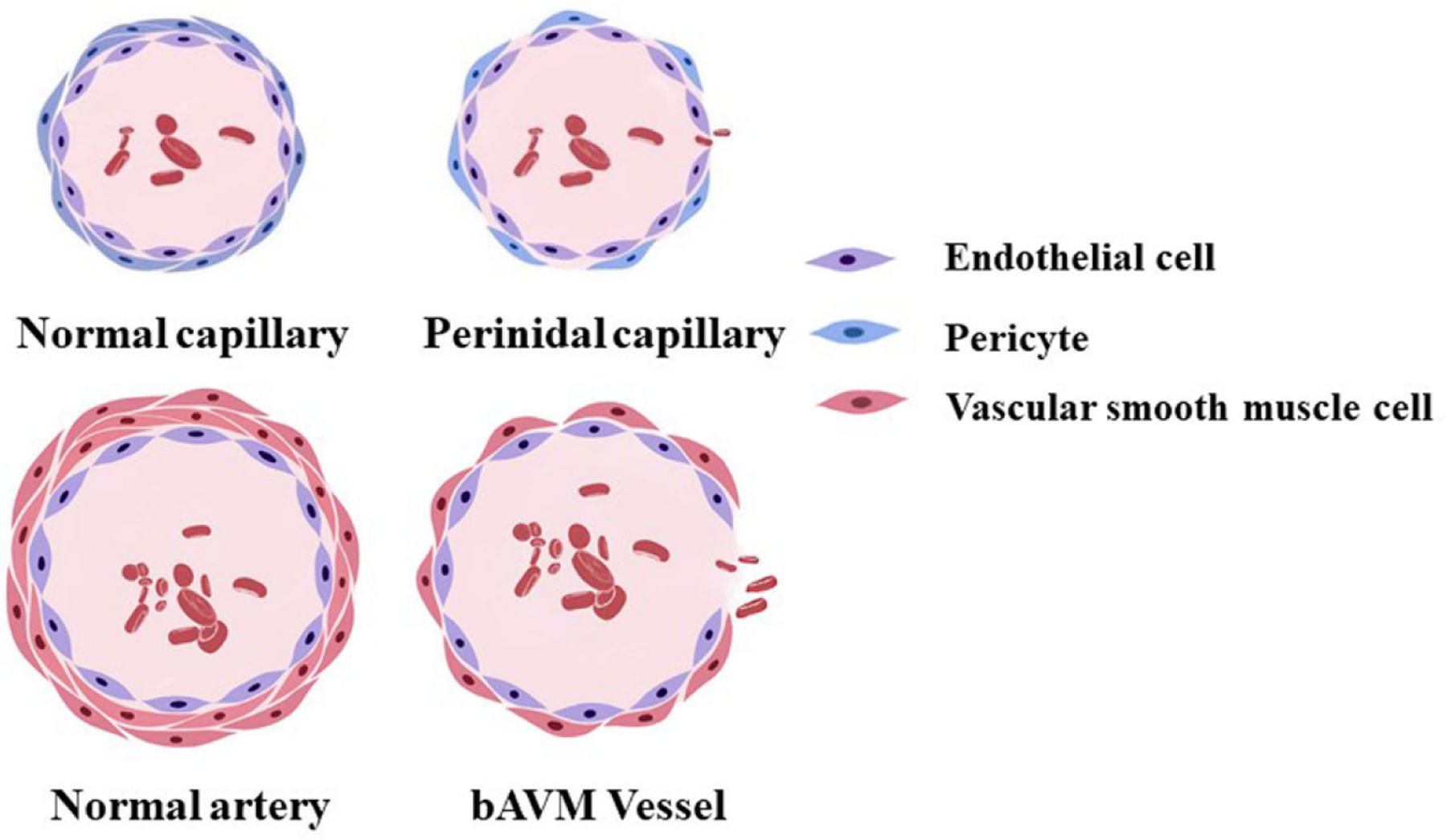
A schematic diagram of a normal capillary, a perinidal capillary, a normal artery and a bAVM vessel. The perinidal capillary has fewer peridytes and the bAVM vessel has fewer vAMCs than normal capillary and artery which render the vessel prone to bleed and rupture.
The cerebrovasculature contains several cell types, including endothelial cells, pericytes and vascular smooth muscle cells (vSMCs, Figure 1) (Pekmezci et al., 2016; Tu et al., 2006). Under normal circumstances, these cells coordinate to maintain the cerebrovascular integrity and function. Mural cells, including pericyptes and vSMCs, are no longer deemed as a passive cellular player, but rather as an integral part of the vessel wall playing various functions to maintain normal homeostasis. In the central nervous system (CNS), the vascular coverage and topographic density of mural cells are exceptionally high (Shepro and Morel, 1993). In fact, mural cells play a critical role in sustaining the blood-brain barrier (BBB) integrity (Daneman and Prat, 2015). The significant loss of mural cells in bAVM vessels predisposes them to vascular leakage and microhemorrhage (Chen et al., 2013b). In a recent review, we have summarized several biological risk factors of bAVM (Shaligram et al., 2019), such as elevated VEGF level, reduced mural cell coverage, and altered hemodynamics. Changes in hemodynamics, including high flow, increased wall shear stress, and venous hypertension, can also trigger inflammation, BBB leakage, and elevation of VEGF levels, which would result in a substantial increase in the risk of hemorrhage (Cheng et al., 2019). In this review, we discussed the role of mural cells and several pathways that regulate mural cell recruitment during angiogenesis and vasculogenesis in bAVM vascular instability and rupture.
1. Recent advances on bAVM-study: insights from patient cohorts to animal models
More than 95% of bAVM patients are sporadic cases without reported family history (Inoue et al., 2007). About 5% of bAVMs are familial. Hereditary hemorrhagic telangiectasia (HHT; also called Osler-Weber-Rendu syndrome) is one of the major causes of the familial form of bAVM. HHT is an autosomal dominant vascular disorder, affecting approximately 1 in 5,000 people, or 1.2 million people worldwide (Govani and Shovlin, 2009; Shovlin, 2010). It is characterized by recurrent epistaxis, chronic bleeding from telangiectasias in the skin and gastrointestinal tract, and AVMs in various organs, e.g. brain, lungs, and liver (Shovlin, 2010).
It is known that HHT patients carry heterozygous mutations in endoglin (ENG, HHT1), Activin receptor-like kinase 1 (ALK1, also called ACVRL1, HHT2), or SMAD4 (juvenile polyposis-HHT) genes (Johnson et al., 1996; Larsen Haidle and Howe, 1993; McAllister et al., 1994). HHT1 and HHT2 account for about 90% of all HHT cases (Richards-Yutz et al., 2010). Intriguingly, ENG, ALK1, and SMAD4 exert their functions in regulating the development of arteriovenous network, mainly in the endothelial cells (Mahmoud et al., 2010; Ola et al., 2018; Tual-Chalot et al., 2014), through transforming growth factor (TGF)-β and bone morphogenetic protein (BMP) signaling pathways. Genotype-phenotype studies in HHT patients have revealed that brain and pulmonary AVMs are more often associated with HHT1, while liver and gastrointestinal AVMs are more prevalent in HHT2 patients (Bayrak-Toydemir et al., 2006; Karlsson and Cherif, 2018).
The genesis of sporadic bAVM is starting to emerge in recent years. Several studies showed a high prevalence of somatic activating mutations in KRAS/BRAF and MAP2K1/MER in sporadic bAVMs (Hong et al., 2019; Karlsson and Cherif, 2018; Nikolaev et al., 2018; Oka et al., 2019; Priemer et al., 2019), suggesting that the MER/ERK signaling pathways plays an important role in AVM pathogenesis.
Animal models has advanced research in bAVMs, which not only shedding light on the fundamental mechanisms but also providing scientific rationale for identification and test of therapeutic targets. Thus far, several animal models have been successfully established that have reproducible AVM phenotypes in adult mice by conditional knockout of Eng or Alk1 genes, the two known HHT causal genes in combination with brain focal angiogenic stimulation (Chen et al., 2013b; Chen et al., 2014b; Choi et al., 2014; Walker et al., 2011). Recently, Kim et al. have successfully established an inducible endothelial Alk1 overexpression mouse model (Kim et al., 2020). The authors demonstrated that ALK1-overexpression can rescue the AVM phenotypes in both Alk1- and Eng-inducible knockout (iKO) mice through normalizing the expression of SMAD and NOTCH target genes and resorting the effect of BMP9 on suppression of phosphor-AKT levels in ENG-deficient endothelial cells. ENG-overexpression could not inhibit the AVM manifestations in Alk1-iKO models. These findings suggest that the AVM development in HHT is caused by defects in the BMP9/10-ENG-ALK1-SMAD4 signaling pathways. In addition, animal models mimic sporadic bAVM have been established in mouse and zebrafish through endothelial-specific gain of function mutations in Kras gene (Fish et al., 2020). Using these models, the authors demonstrated that activation of MEK instead of PI3K signaling is required for KRAS mediated AVM progression, and inhibition of MEK is a promising therapeutic target for the treatment of bAVM patients.
2. Pericytes and vascular smooth muscle cells (vSMCs) in bAVM hemorrhage
Over the years, scientists in bAVM study field have focused their attention mainly on the endothelial cells. it is known that endothelial cells play important roles in bAVM pathogenesis. Mutations of AVM causative genes in endothelial cells is necessary for bAVM development (Chen et al., 2014a; Chen et al., 2014b; Choi et al., 2014; Fish et al., 2020; Nikolaev et al., 2018). Endothelial cells in bAVM are different from quiescent brain endothelial cells. They express various angiogenic factors, such as VEGF and ET-1, that are not detected in quiescent brain endothelial cells (Jabbour et al., 2009). Other abnormal characteristics of bAVM endothelial cells are impaired endothelial cell specification in bAVM (Walker et al., 2011), and increased migration and abnormal tubule formation culture (Jabbour et al., 2009).
2.1. vSMCs
The function of mural cells, including vSMCs and pericytes in vascular development, homeostasis and vascular malformation has drawn attention lately. Changes in mural cell number, contractility or the attachment of mural cells to the endothelium are associated with diseases such as diabetic retinopathy, vascular malformations, and hereditary stroke (Chen et al., 2013b; Hammes, 2005; Yamamoto et al., 2020). Recent works showed that both human and mouse bAVM vessels have fewer mural cell coverage than normal brain vessels (Winkler et al., 2018; Zhu et al., 2018b).
The vSMCs are circumferentially located in the medium part of the blood vessels, named tunica media, where they provide structural integrity to the vessel wall and regulate blood flow through regulating vessel dilation and contraction (Frosen and Joutel, 2018). In bAVM, vSMCs switch from a quiescent non-proliferative contractile phenotype to an active synthetic phenotype, accompanying with abnormal migration and growth of the cerebral blood vessels (Jaminon et al., 2019). Malformed vessels in bAVM, have reduced or incomplete alpha smooth muscle actin (αSMA) in the vSMC, as well as decreased elastin coverage in the internal elastin lamina (Davis et al., 2018). Our group showed previously that Alk1-deficiency impairs vascular integrity through reduction of both α-SMA positive vSMCs and pericytes (Chen et al., 2013b). ALK1 regulates vSMC differentiation and recruitment during vascular development in the embryonic stage (Oh et al., 2000).
2.2. Pericytes
Pericytes are the predominant mural cell population of the cerebral microvasculature, covering roughly 90% of the abluminal side of the vessel wall (Bell et al., 2010; Winkler et al., 2012; Winkler et al., 2013). They are the components of the smallest diameter blood vessels such as arterioles, capillaries, and venules, and share their basal membrane with the endothelium. Pericytes exert significant modulatory influences on maintaining cerebrovascular integrity and functions, including the control of cerebral neovascularization, endothelial cells proliferation and migration, vascular diameter and cerebral blood flow; as well as maintaining microvascular stability and permeability. Pericytes play multiple roles in regulating angiogenesis, BBB integrity, and vascular stability (Armulik et al., 2011; Sweeney et al., 2016; Zhao et al., 2015). Pericytes have been shown to prevent hypoxia-induced BBB disruption in vitro (Hayashi et al., 2004).
The pericyte number and coverage can be quantified using membrane-bound markers, such as PDGFR-β, CD146, aminopeptidases A and N (CD13), and neuron-glial 2 (NG2) as well as commonly used cytoplasmic markers for pericyte identification, including αSMA, non-muscle myosin, desmin, vimentin, and nestin (Ribatti et al., 2011). Using these method, it has been found that in sporadic AVMs, pericyte number and coverage were reduced (Winkler et al., 2018). Reduction of pericyte’s number and coverage is correlated with microhemorrhage in unruptured bAVM and faster blood flow rate through bAVM nidus, suggesting that loss of pericytes contributes to vascular fragility and hemodynamic changes in bAVMs (Winkler et al., 2018). Moreover, vascular endothelial growth factor (VEGF) stimulation in the Alk1-deficient brain reduces vascular integrity, which is associated with extravasation of intravascular components, such as fibrinogen, red blood cells, and inflammatory cells into the brain parenchyma around the bAVM vessels (Chen et al., 2013a).
Pericyte deficiency is not just confined to bAVM, but also present in other neurological diseases that are associated with vascular abnormalities, such as Alzheimer’s disease (Sagare et al., 2013), amyotrophic lateral sclerosis (Winkler et al., 2014), and cavernous malformation (Schulz et al., 2015). Therefore, pericyte deficiency is a common denominator of reduced vascular stability in the brain.
2.3. Interaction of endothelial cells and pericytes
The interaction of endothelial cells and pericytes is tightly controlled and modulated by several molecules, such as PDGF-B, transforming growth factor beta 1 (TGFβ1), VEGF, angiopoietins (Angs), Notch and ephrins. Pericytes maintain BBB function by releasing high levels of Ang-1 and TGFβ1 (Dohgu et al., 2005; Hori et al., 2004). Ang-1 derived from pericytes induces occludin expression via Tie2 receptor expressed by endothelial cells (Hori et al., 2004). Pericyte deficiency can lead to low levels of occludin in endothelial cells, which is associated with reduction of tight junction proteins and an increase of BBB permeability (Persidsky et al., 2006). Understanding the function of the factors involved in pericyte-endothelial cells interaction can help design therapies to prevent vascular permeability and destabilization in bAVM.
3. Signaling pathways: PDGF-B/PDGFR-β, EphrinB2/EphB4, and Angs/tie2
Several signaling pathways are involved in the abnormal phenotypes of bAVMs. In vascular development, a vascular fate, being arterial or venous, is determined by many signaling pathways (Walcott et al., 2016; Winkler et al., 2019). Below, we summarize the function of three major signaling pathways involved in this fate determination process and mural cell-recruitment during angiogenesis: PDGF-B/PDGFR-β, EphrinB2/EphB4, and Angs/tie2 (Figure 2).
Figure 2.
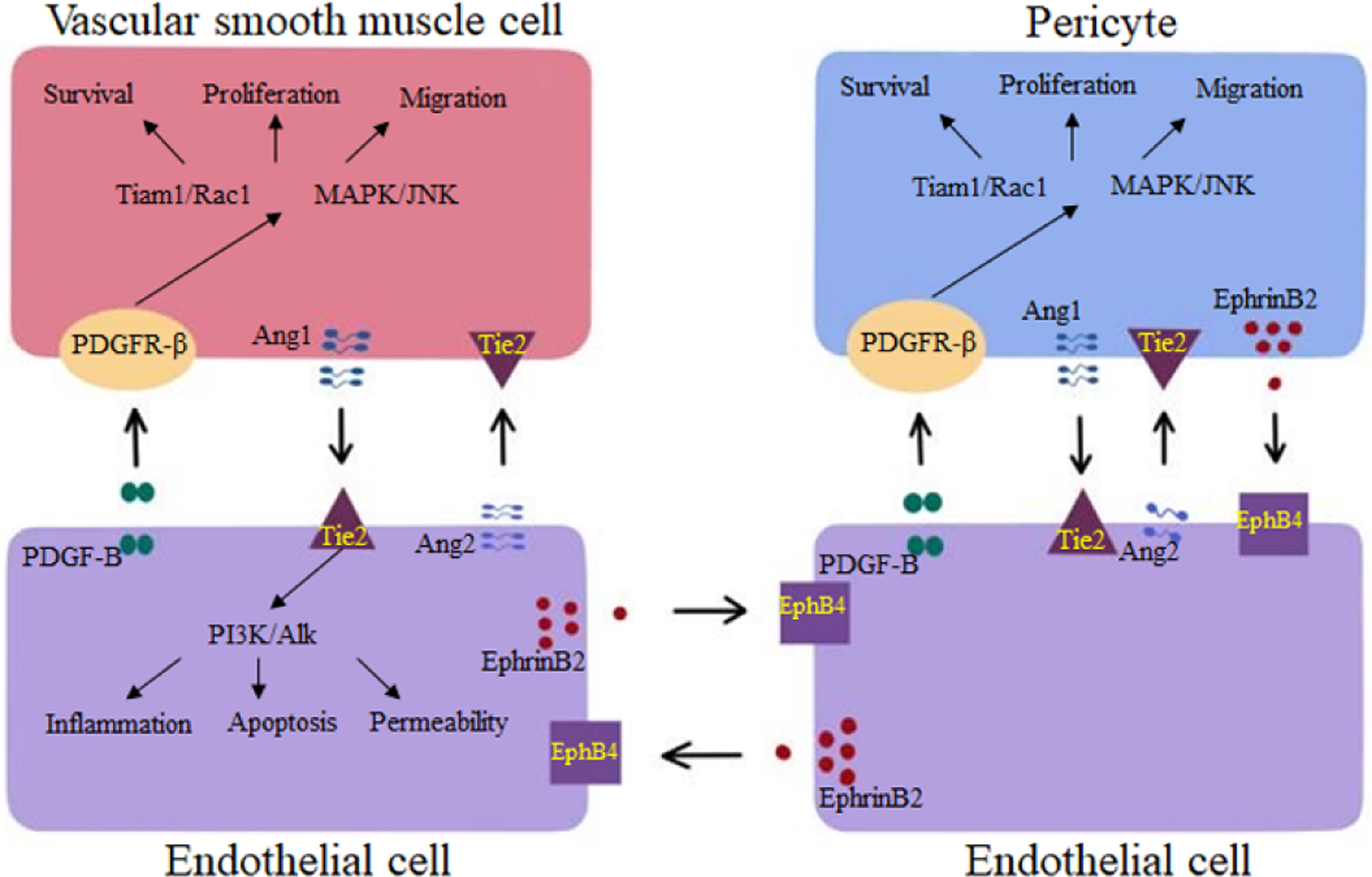
Summary of key signaling pathways involved in the regulation of mural cell recruitment during angiogenesis and vasculogenesis and their association with bAVM phenotypes.
3.1. PDGF-B/PDGFR-β signaling
PDGFR-β is expressed in multiple cell types, including pericytes, vSMCs, and neurons (Fredriksson et al., 2004; Ishii et al., 2006). As a ligand, PDGF-B is secreted from the endothelial cells of angiogenic sprouts where it works as an attractant for pericytes. PDGF-B can also stimulate vSMCs proliferation (Abramsson et al., 2003; Hellstrom et al., 1999). PDGF-B -binds to PDGFR-β triggering receptor dimerization and phosphorylation, leading to activation of multiple downstream signal transduction pathways, which ultimately modulate survival, migration, apoptosis, proliferation, and differentiation of vascular cells. The PDGF-B/PDGFR-β are key elements in regulating pericyte recruitment and are important for an endothelium-to-mural cell paracrine signaling which maintains vascular integrity and stabilization (Gaengel et al., 2009; Shaligram et al., 2019). Homozygous deletion of Pdgf-b or Pdgfr-β in rodents results in high embryonic mortality due to widespread hemorrhage (Hellström et al., 2001). Maintenance of normal capillaries and BBB requires PDGF-B/PDGFR-β signaling. Genetic deletion of Pdgf-b in animals leads to pericyte loss and BBB breakdown (Armulik et al., 2010; Hellstrom et al., 1999; Hirunpattarasilp et al., 2019). Disruption of PDGF-B/PDGFR- signaling can also cause excessive vascular abnormalities and microaneurysms (Enge et al., 2002).
Abnormal expression of PGDF-B and PDGFR-β has been described in bAVMs in human and rodent (Barbosa Do Prado et al., 2019; Winkler et al., 2018; Yildirim et al., 2010). PDGFR-β expression was reduced in the bAVM lesions of Alk1-deficient mice, which was associated with a reduction of mural cell coverage, suggesting a possible crosstalk between ALK1 and PDGF-B/PDGFR-β signaling pathways (Chen et al., 2013b). Winkler et al. (Winkler et al., 2018) has shown that pericyte number and coverage are reduced in sporadic human bAVMs. Importantly, pericyte reductions are greatest in bAVMs with clinical hemorrhage and are associated with a higher microhemorrhage burden in unruptured cases, suggesting that reduction of pericytes contribute to bAVMs hemodynamic changes (Winkler et al., 2018). Upregulation of Pdgf-b expression via a lentiviral vector mediated gene transfer or thalidomide treatment reduced the number of dysplastic vessels and hemorrhage by increasing mural cell coverage in the bAVM lesions in mice. These data demonstrate that PDGF-B/PDGFR-β signaling regulates mural cell plasticity and plays an important role in bAVM pathogenesis (Zhu et al., 2018a).
3.2. EphrinB2/EphB4 signaling
The Eph receptors (EphA1–EphA8, EphA10, EphB1–EphB4, and EphB6) and their ligands, Ephrins (ephrinA1–A5 and ephrinB1–B3) are crucial for multiple events in angiogenesis and vascular maturation, especially in embryonic angiogenesis and the formation of vascular architecture, including axon guidance, lymphatic and endothelial cell specification. Elevated expression of Ephs and Ephrins was first reported in human carcinoma. Subsequent studies evolved their functions in angiogenesis and vasculogenesis (Himanen and Nikolov, 2003; Hirai et al., 1987; Salvucci and Tosato, 2012). Eph/Ephrin signaling allows short-distance endothelial cell-cell communication, which activates signaling pathways, modulates cellular cytoskeleton, and leads cell repulsion or adhesion. Therefore, multiple processes that changes cellular motility and/or morphology depend on Eph/Ephrin signaling (Barquilla and Pasquale, 2015; Kania and Klein, 2016; Vreeken et al., 2020).
Eph receptors are transmembrane proteins with an extracellular domain that contains a ligand-binding domain, a cysteine-rich region, and two fibronectin type-II domains. The intracellular domain of Ephs contains two tyrosine residues, a protein tyrosine kinase domain, a sterile alpha motif (SAM), and a PDZ-binding domain. B-class Ephrins (ephrinB1-ephrinB3) have a transcellular and cytoplasmic domain with a PDZ-binding motif (Boyd et al., 2014; Vreeken et al., 2020). Ephrins binding to Ephs induce forward, reverse, parallel and antiparallel signaling pathways by orchestrating the various functional domains, thereby allowing multiple signaling modes and modulatory mechanisms to be processed with high precision. Ephrins-Ephs forward signaling works as classical ligands and receptors. In reverse signaling, the role of receptors and ligands of ephrin and Eph proteins are switched. When Eph receptors and Ephrins are located on the same cell membrane, they may act as ligands for Ephrins or Eph receptors, respectively, to activate signaling in the same direction (parallel signaling) or they can each function as receptors and ligands to activate signaling in alternative directions (antiparallel signaling) (Holland et al., 1996; Taylor et al., 2017).
Despite the complexity of Ephrin-Eph signaling, the regulation of angiogenesis and vasculogenesis is highly dependent on the specific EphrinB2/EphB4 signaling that has been implicated in the modulation of multiple vascular events, such as sprouting angiogenesis, vascular morphogenesis, and arteriovenous differentiation (Luxan et al., 2019; Pitulescu and Adams, 2010; Yang et al., 2016). In mural cells, EphrinB2 deletion causes embryonical lethality in mice with serious hemorrhage, edema, and vascular deficits in many organs. Cultured ephrinB2-deficient smooth muscle cells are defective in spreading, focal-adhesion formation and polarized migration with increase motility suggesting that EphrinB2 is important for vSMC recruitment and attachment to the vessel wall (Foo et al., 2006). In addition, EphrinB2 is a crucial regulator of PDGFR-β expression and internalization in vSMCs surface, and thereby acts as a molecular switch controlling the downstream signaling activity induced by PDGF-B/PDGFR-β. Ablation of EphrinB2 enhances PDGF-B-induced MAPK and JNK activation, diminishes Tiam1/ Rac1 signaling (Figure 2), a pathway critical for cell migration, proliferation, and spreading (Nakayama et al., 2013).
EphrinB2 and EphB4 have been viewed as the primary molecular markers for endothelial arteriovenous specification. EphrinB2 is expressed exclusively by arterial endothelial cells and EphB4 by venous endothelial cells. Many studies indicate that EphrinB2/EphB4 signaling play roles in AVMs and other cerebrovascular disorders (Bai et al., 2014; Deloison et al., 2012; Gale et al., 2001; Long et al., 1974; Oike et al., 2002). Embryos harboring homozygous mutations in Efnb2 and Ephb4 exhibit vascular defects and vascular malformations (Krebs et al., 2010). A study using an in vitro model of HHT2 showed that loss of Alk1 gene blocked BMP9 signaling, resulting in reduced EphrinB2 expression, enhanced VEGFR2 expression, and dysregulated endothelial sprouting and anastomosis (Kim et al., 2012).
Whole exome sequencing studies in humans have identified mutations in EFNB2, EPHB4, and RASA1 in several congenital cerebrovascular disorders, including Vein of Galen malformation and capillary malformation-arteriovenous malformation (Amyere et al., 2017; Duran et al., 2019; Zeng et al., 2019), which corroborates the findings in model organisms. A recent study showed that dysregulation of the EphrinB2/EphB4 signaling cascade may play a role in AVM development, with potential utility as a diagnostic and therapeutic target (Fehnel et al., 2020).
3.3. Angs/tie2 signaling
Angs are growth factors that signal through Tie receptor. Angs/Tie signaling pathway is essential for vascular maturation and vascular homeostasis (Akwii et al., 2019; Korhonen et al., 2016). The Ang family consists of 4 glycoproteins (Ang1-Ang4). The best-characterized members of the family are Ang1 and Ang2. Ang1 activates the Tie2 receptor, whereas Ang2 is a partial antagonist/agonist ligand for Tie2 receptor (Bilimoria and Singh, 2019; Maisonpierre et al., 1997). Ang1 is a constitutive paracrine agonist ligand for Tie2. It stimulates Akt-dependent phosphorylation and nuclear exclusion of the Forkhead box protein O1 (FOXO1) and its downstream signaling, which contributes to vascular development in the embryonic stage and maintenance of vascular stabilization. Ang2 is an autocrine ligand that functions as a context-dependent agonist or antagonist of Tie2. While Ang1 induces endothelial stabilization, Ang2 can antagonize Ang1 and block Tie2 activation, leading to vessel destabilization and regression (Daly et al., 2004; Kim et al., 2016; Nicolini et al., 2019; Parikh et al., 2006; Sato et al., 1995). The balance between the levels of Ang1 and Ang2 partially determines the levels of Tie receptors and subsequent integrity of blood vessels, which are altered in various diseases, resulting in changes in the magnitude of Ang1 signaling (Iribarren et al., 2011; Ziegler et al., 2013). Besides endothelial cells, Tie2 expression and function have been established in other cell types, including neural cells, macrophages, hematopoietic stem cells, and mural cells (Androutsellis-Theotokis et al., 2009; Park et al., 2003; Teichert et al., 2017; Venneri et al., 2007).
Angs/Tie signaling controls the association of endothelial cells and pericytes (Yuan et al., 2009). Tie2 receptor in pericytes controls sprouting angiogenesis in spheroid assays. Moreover, silencing of Tie2 receptor results in a pro-migratory phenotype by downstream signaling through Calpain, Akt and FOXO3A (Teichert et al., 2017). Mice lacking pericytes presented BBB disruption, increased vascular permeability and higher Ang2 levels, suggesting a possible role of Ang2 in pathological vascular permeability (Daneman et al., 2010).
Several studies have identified Angs/Tie2 signaling pathway as a key element of vascular malformations. Activating somatic Tie2 mutations in endothelial cells was observed in patients’ vascular malformations (VM) lesions (Augustin et al., 2009; Limaye et al., 2009; Soblet et al., 2013; Wouters et al., 2010). Anti-Ang2 antibodies have been shown to alleviate AVM phenotype and normalize blood vessel diameter in preclinical models of HHT (Crist et al., 2019). PI3-Kinase signaling has been shown to be activated downstream of VEGF and Ang2 (Graupera et al., 2013). The therapeutic efficacy of PI3-Kinase inhibitors has been proven in preclinical HHT models (Ola et al., 2016; Robert et al., 2020). Hashimoto et al. (Hashimoto et al., 2001) showed, for the first time, that the presence of abnormal balance in the Angs-Tie2 system is partially associated with the aberrant vascular phenotypes in bAVMs, which is likely due to loosening of cellular adhesion. In addition, next-generation sequencing analyses of human bAVM specimens revealed downregulation of Ang1 level, suggesting a relationship between Ang1 function and the pathophysiology of bAVMs.
Further, the reduction of Ang1 has been correlated with the reduced release of Ang11 by adjacent pericytes. These data are consistent with previous studies (Hashimoto et al., 2001; Hauer et al., 2020; Shenkar et al., 2003). In addition, polymorphisms in Ang4 were associated with a risk of bAVMs (Mikhak et al., 2011). Taken together, Angs/Tie2 pathway may play a key role in regulating mural cell plasticity. Dysregulation of this pathway contributes to the pathogenesis of bAVMs.
Summary
In this review, we discussed normal vascular structure, defects of bAVM vessels, and the association of mural cell-dysfunction with bAVM hemorrhage. We have also discussed the three major signaling pathways that regulate normal angiogenesis, vascular remodeling and endothelial specification, as well as their association with bAVM pathogenesis.
Brain AVM rupture is unpredictable and can cause life-threatening intracranial hemorrhage and long-term disability. Current treatments for bAVMs are all invasive, which include surgery, radiation, endovascular embolization or combinations of two or three of these treatments. All of these treatments are associated with considerable risks, such as stroke, ICH, disability and mortality. The treatment of unruptured bAVMs has become increasingly controversial because the natural history for these patients may be less morbid than invasive therapy (Cockroft et al., 2012; Mohr et al., 2012; Mohr et al., 2010; Mohr et al., 2014; Stapf et al., 2006). Currently, there are no specific and safe medical therapies available for bAVM patients. Due to the excess risks associated with invasive interventions (Mohr et al., 2017), the treatment selection for unruptured bAVM patients are debatable (Cenzato et al., 2017; Nisson et al., 2019). Thus, a safe and effective medical treatment for bAVM patients is urgently needed. Understanding the signaling pathways involved in mural cell recruitment and vascular maturation might shed light on potential new targets.
One example is the identification of the roles of PDGF-B/PDGFR-β signaling pathway in recruiting mural cells to newly formed vessels and bAVM pathogenesis. In HHT patients, thalidomide treatment can reduce the frequency and duration of nosebleed and the need for blood transfusion (Lebrin et al., 2010). Thalidomide treatment also reduced hemorrhage and improve mural cell coverage in mouse bAVMs, most likely through upregulation of Pdgf-b/Pdgfr-β signaling. Increased mural recruitment was associated with reductions in dysplastic vessels (Zhu et al., 2018a). Further, the mechanistic study revealed that the effect of thalidomide was through increasing endothelial Pdgf-b expression. Overexpression of Pdgf-b recapitulated the therapeutic benefit of thalidomide in mice.
Future studies are needed to establish better tools and mouse models to elucidate the role of pericytes in AVM pathogenesis and to develop potential strategies to prevent bAVM hemorrhage through imporoving pericytes/mural cell-caverage of bAVMs vessels.
Acknowledgements
We think Ms. Na Zhao for helping us creating illustrations.
Funding
This study was supported by grants to H.S. from the National Institutes of Health (R01 HL122774, NS027713 and NS112819), from the Michael Ryan Zodda Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
All authors have no financial and personal relationships with other people or organizations that could inappropriately influence our work.
References
- Abla AA, Nelson J, Kim H, Hess CP, Tihan T, Lawton MT, 2015. Silent arteriovenous malformation hemorrhage and the recognition of “unruptured” arteriovenous malformation patients who benefit from surgical intervention. Neurosurgery 76, 592–600; discussion 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C, 2003. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest 112, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwii RG, Sajib MS, Zahra FT, Mikelis CM, 2019. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, Chung W, Dubois J, Lacour JP, Martorell L, Mazereeuw-Hautier J, Pyeritz RE, Amor DJ, Bisdorff A, Blei F, Bombei H, Dompmartin A, Brooks D, Dupont J, Gonzalez-Ensenat MA, Frieden I, Gerard M, Kvarnung M, Hanson-Kahn AK, Hudgins L, Leaute-Labreze C, McCuaig C, Metry D, Parent P, Paul C, Petit F, Phan A, Quere I, Salhi A, Turner A, Vabres P, Vicente A, Wargon O, Watanabe S, Weibel L, Wilson A, Willing M, Mulliken JB, Boon LM, Vikkula M, 2017. Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Circulation 136, 1037–1048. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, Walbridge S, Munasinghe J, Koretsky AP, Lonser RR, McKay RD, 2009. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci U S A 106, 13570–13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C, 2011. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21, 193–215. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, 2010. Pericytes regulate the blood-brain barrier. Nature 468, 557–561. [DOI] [PubMed] [Google Scholar]
- Attia W, Tada T, Hongo K, Nagashima H, Takemae T, Tanaka Y, Kobayashi S, 2003. Microvascular pathological features of immediate perinidal parenchyma in cerebral arteriovenous malformations: giant bed capillaries. J Neurosurg 98, 823–827. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K, 2009. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10, 165–177. [DOI] [PubMed] [Google Scholar]
- Bai J, Wang YJ, Liu L, Zhao YL, 2014. Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J Int Med Res 42, 405–415. [DOI] [PubMed] [Google Scholar]
- Barbosa Do Prado L, Han C, Oh SP, Su H, 2019. Recent Advances in Basic Research for Brain Arteriovenous Malformation. Int J Mol Sci 20, 5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquilla A, Pasquale EB, 2015. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol 55, 465–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou LS, Gedge F, Tang W, Coon H, Mao R, 2006. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A 140, 463–470. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV, 2010. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria J, Singh H, 2019. The Angiopoietin ligands and Tie receptors: potential diagnostic biomarkers of vascular disease. J Recept Signal Transduct Res 39, 187–193. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Bartlett PF, Lackmann M, 2014. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov 13, 39–62. [DOI] [PubMed] [Google Scholar]
- Cenzato M, Boccardi E, Beghi E, Vajkoczy P, Szikora I, Motti E, Regli L, Raabe A, Eliava S, Gruber A, Meling TR, Niemela M, Pasqualin A, Golanov A, Karlsson B, Kemeny A, Liscak R, Lippitz B, Radatz M, La Camera A, Chapot R, Islak C, Spelle L, Debernardi A, Agostoni E, Revay M, Morgan MK, 2017. European consensus conference on unruptured brain AVMs treatment (Supported by EANS, ESMINT, EGKS, and SINCH). Acta Neurochir (Wien) 159, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Chen W, Choi EJ, McDougall CM, Su H, 2014a. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl Stroke Res 5, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Guo Y, Jun K, Wankhede M, Su H, Young WL, 2013a. Alk1 deficiency impairs mural cell recruitment during brain angiogenesis [Abstract]. Stroke 44, ATMP118. [Google Scholar]
- Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, Degos V, Lawton MT, Tihan T, Davalos D, Akassoglou K, Nelson J, Pile-Spellman J, Su H, Young WL, 2013b. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 33, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, Mao L, Arnold T, Young WL, Su H, 2014b. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45, 900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Ma L, Shaligram S, Walker EJ, Yang ST, Tang C, Zhu W, Zhan L, Li Q, Zhu X, Lawton MT, Su H, 2019. Effect of elevation of vascular endothelial growth factor level on exacerbation of hemorrhage in mouse brain arteriovenous malformation. J Neurosurg, 1–8. [DOI] [PMC free article] [PubMed]
- Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H, 2014. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One 9, e88511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockroft KM, Jayaraman MV, Amin-Hanjani S, Derdeyn CP, McDougall CG, Wilson JA, 2012. A perfect storm: how a randomized trial of unruptured brain arteriovenous malformations’ (ARUBA’s) trial design challenges notions of external validity. Stroke 43, 1979–1981. [DOI] [PubMed] [Google Scholar]
- Crist AM, Zhou X, Garai J, Lee AR, Thoele J, Ullmer C, Klein C, Zabaleta J, Meadows SM, 2019. Angiopoietin-2 Inhibition Rescues Arteriovenous Malformation in a Smad4 Hereditary Hemorrhagic Telangiectasia Mouse Model. Circulation 139, 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS, 2004. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 18, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Prat A, 2015. The blood-brain barrier. Cold Spring Harb Perspect Biol 7, a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA, 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RB, Pahl K, Datto NC, Smith SV, Shawber C, Caron KM, Blatt J, 2018. Notch signaling pathway is a potential therapeutic target for extracranial vascular malformations. Sci Rep 8, 17987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloison B, Chalouhi GE, Sonigo P, Zerah M, Millischer AE, Dumez Y, Brunelle F, Ville Y, Salomon LJ, 2012. Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrasound Obstet Gynecol 40, 652–658. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y, 2005. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res 1038, 208–215. [DOI] [PubMed] [Google Scholar]
- Duran D, Zeng X, Jin SC, Choi J, Nelson-Williams C, Yatsula B, Gaillard J, Furey CG, Lu Q, Timberlake AT, Dong W, Sorscher MA, Loring E, Klein J, Allocco A, Hunt A, Conine S, Karimy JK, Youngblood MW, Zhang J, DiLuna ML, Matouk CC, Mane S, Tikhonova IR, Castaldi C, Lopez-Giraldez F, Knight J, Haider S, Soban M, Alper SL, Komiyama M, Ducruet AF, Zabramski JM, Dardik A, Walcott BP, Stapleton CJ, Aagaard-Kienitz B, Rodesch G, Jackson E, Smith ER, Orbach DB, Berenstein A, Bilguvar K, Vikkula M, Gunel M, Lifton RP, Kahle KT, 2019. Mutations in Chromatin Modifier and Ephrin Signaling Genes in Vein of Galen Malformation. Neuron 101, 429–443 e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C, 2002. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. Embo J 21, 4307–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehnel KP, Penn DL, Duggins-Warf M, Gruber M, Pineda S, Sesen J, Moses-Gardner A, Shah N, Driscoll J, Zurakowski D, Orbach DB, Smith ER, 2020. Dysregulation of the EphrinB2-EphB4 ratio in pediatric cerebral arteriovenous malformations is associated with endothelial cell dysfunction in vitro and functions as a novel noninvasive biomarker in patients. Exp Mol Med 52, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Flores Suarez CP, Boudreau E, Herman AM, Gutierrez MC, Gustafson D, DiStefano PV, Cui M, Chen Z, De Ruiz KB, Schexnayder TS, Ward CS, Radovanovic I, Wythe JD, 2020. Somatic Gain of KRAS Function in the Endothelium Is Sufficient to Cause Vascular Malformations That Require MEK but Not PI3K Signaling. Circ Res 127, 727–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH, 2006. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U, 2004. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 15, 197–204. [DOI] [PubMed] [Google Scholar]
- Frosen J, Joutel A, 2018. Smooth muscle cells of intracranial vessels: from development to disease. Cardiovasc Res 114, 501–512. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C, 2009. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29, 630–638. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD, 2001. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol 230, 151–160. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhu Y, Ling F, Shen F, Lee B, Gabriel RA, Hao Q, Yang GY, Su H, Young WL, 2009. Nonischemic cerebral venous hypertension promotes a pro-angiogenic stage through HIF-1 downstream genes and leukocyte-derived MMP-9. J Cereb Blood Flow Metab 29, 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govani FS, Shovlin CL, 2009. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet 17, 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BA, Du R, 2013. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg 118, 437–443. [DOI] [PubMed] [Google Scholar]
- Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, Hetts SW, Hess C, Lawton MT, Bollen AW, Pourmohamad T, McCulloch CE, Tihan T, Young WL, University of California, S.F.B.A.M.S.P., 2012. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke 43, 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, 2005. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 37Suppl 1, 39–43. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL, 2001. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res 89, 111–113. [DOI] [PubMed] [Google Scholar]
- Hauer AJ, Kleinloog R, Giuliani F, Rinkel GJE, de Kort GA, Berkelbach van der Sprenkel JW, van der Zwan A, Gosselaar PH, van Rijen PC, de Boer-Bergsma JJ, Deelen P, Swertz MA, De Muynck L, Van Damme P, Veldink JH, Ruigrok YM, Klijn CJM, 2020. RNA-Sequencing Highlights Inflammation and Impaired Integrity of the Vascular Wall in Brain Arteriovenous Malformations. Stroke 51, 268–274. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C, 1999. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB, 2003. Eph signaling: a structural view. Trends Neurosci 26, 46–51. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F, 1987. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 238, 1717–1720. [DOI] [PubMed] [Google Scholar]
- Hirunpattarasilp C, Attwell D, Freitas F, 2019. The role of pericytes in brain disorders: from the periphery to the brain. J Neurochem 150, 648–665. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T, 1996. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722–725. [DOI] [PubMed] [Google Scholar]
- Hong T, Yan Y, Li J, Radovanovic I, Ma X, Shao YW, Yu J, Ma Y, Zhang P, Ling F, Huang S, Zhang H, Wang Y, 2019. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 142, 23–34. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T, 2004. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 89, 503–513. [DOI] [PubMed] [Google Scholar]
- Inoue S, Liu W, Inoue K, Mineharu Y, Takenaka K, Yamakawa H, Abe M, Jafar JJ, Herzig R, Koizumi A, 2007. Combination of linkage and association studies for brain arteriovenous malformation. Stroke 38, 1368–1370. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Phelps BH, Darbinian JA, McCluskey ER, Quesenberry CP, Hytopoulos E, Vogelman JH, Orentreich N, 2011. Circulating angiopoietins-1 and −2, angiopoietin receptor Tie-2 and vascular endothelial growth factor-A as biomarkers of acute myocardial infarction: a prospective nested case-control study. BMC Cardiovasc Disord 11, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Oya T, Zheng L, Gao Z, Kawaguchi M, Sabit H, Matsushima T, Tokunaga A, Ishizawa S, Hori E, Nabeshima Y, Sasaoka T, Fujimori T, Mori H, Sasahara M, 2006. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem 98, 588–600. [DOI] [PubMed] [Google Scholar]
- Jabbour MN, Elder JB, Samuelson CG, Khashabi S, Hofman FM, Giannotta SL, Liu CY, 2009. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery 64, 139–146; discussion 146–138. [DOI] [PubMed] [Google Scholar]
- Jaminon A, Reesink K, Kroon A, Schurgers L, 2019. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA, 1996. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13, 189–195. [DOI] [PubMed] [Google Scholar]
- Kania A, Klein R, 2016. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol 17, 240–256. [DOI] [PubMed] [Google Scholar]
- Karlsson T, Cherif H, 2018. Mutations in the ENG, ACVRL1, and SMAD4 genes and clinical manifestations of hereditary haemorrhagic telangiectasia: experience from the Center for Osler’s Disease, Uppsala University Hospital. Ups J Med Sci 123, 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Pawlikowska L, Su H, Young WL, 2016. Genetics and vascular biology of brain vascular malformations (Chapter 12), in: Grotta JC, Albers GW, Broderick JP, Kasner SE, Lo EH, Mendelow AD, Sacco RL, Wong LK (Eds.), Stroke: Pathophysiology, Diagnosis, and Management, 6th ed. Churchill Livingstone Elsevier, pp. 149–162. [Google Scholar]
- Kim JH, Peacock MR, George SC, Hughes CC, 2012. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis 15, 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Phuong NV, Choe SW, Jeon CJ, Arthur HM, Vary CP, Lee YJ, Oh SP, 2020. Overexpression of Activin Receptor-Like Kinase 1 in Endothelial Cells Suppresses Development of Arteriovenous Malformations in Mouse Models Of Hereditary Hemorrhagic Telangiectasia. Circ Res. [DOI] [PMC free article] [PubMed]
- Korhonen EA, Lampinen A, Giri H, Anisimov A, Kim M, Allen B, Fang S, D’Amico G, Sipila TJ, Lohela M, Strandin T, Vaheri A, Yla-Herttuala S, Koh GY, McDonald DM, Alitalo K, Saharinen P, 2016. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest 126, 3495–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Starling C, Chervonsky AV, Gridley T, 2010. Notch 1 activation in mice causes arteriovenous malformations phenocopied by EphrinB2 and EphB4 mutants. Genesis 48, 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen Haidle J, Howe JR, 1993. Juvenile Polyposis Syndrome, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), Seattle (WA). [PubMed] [Google Scholar]
- Lawton MT, Jacobowitz R, Spetzler RF, 1997. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 87, 267–274. [DOI] [PubMed] [Google Scholar]
- Lawton MT, Rutledge WC, Kim H, Stapf C, Whitehead KJ, Li DY, Krings T, terBrugge K, Kondziolka D, Morgan MK, Moon K, Spetzler RF, 2015. Brain arteriovenous malformations. Nat Rev Dis Primers 1, 15008. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, Arthur HM, Westermann CJ, Disch F, Mager JJ, Snijder RJ, Eichmann A, Mummery CL, 2010. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 16, 420–428. [DOI] [PubMed] [Google Scholar]
- Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M, 2009. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 41, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DM, Seljeskog EL, Chou SN, French LA, 1974. Giant arteriovenous malformations of infancy and childhood. J Neurosurg 40, 304–312. [DOI] [PubMed] [Google Scholar]
- Luxan G, Stewen J, Diaz N, Kato K, Maney SK, Aravamudhan A, Berkenfeld F, Nagelmann N, Drexler HC, Zeuschner D, Faber C, Schillers H, Hermann S, Wiseman J, Vaquerizas JM, Pitulescu ME, Adams RH, 2019. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM, 2010. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res 106, 1425–1433. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD, 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. , 1994. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8, 345–351. [DOI] [PubMed] [Google Scholar]
- Mikhak B, Weinsheimer S, Pawlikowska L, Poon A, Kwok PY, Lawton MT, Chen Y, Zaroff JG, Sidney S, McCulloch CE, Young WL, Kim H, 2011. Angiopoietin-like 4 (ANGPTL4) gene polymorphisms and risk of brain arteriovenous malformations. Cerebrovasc Dis 31, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP, Moskowitz AJ, Parides M, Stapf C, Young WL, 2012. Hull down on the horizon: A Randomized trial of Unruptured Brain Arteriovenous malformations (ARUBA) trial. Stroke 43, 1744–1745. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Moskowitz AJ, Stapf C, Hartmann A, Lord K, Marshall SM, Mast H, Moquete E, Moy CS, Parides M, Pile-Spellman J, Al-Shahi Salman R, Weinberg A, Young WL, Estevez A, Kureshi I, Brisman JL, 2010. The ARUBA trial: current status, future hopes. Stroke 41, e537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP, Overbey JR, von Kummer R, Stefani MA, Libman R, Stapf C, Parides MK, Pile-Spellman J, Moquete E, Moy CS, Vicaut E, Moskowitz AJ, Harkness K, Cordonnier C, Biondi A, Houdart E, Berkefeld J, Klijn CJM, Barreau X, Kim H, Hartmann A, International AI, 2017. Functional impairments for outcomes in a randomized trial of unruptured brain AVMs. Neurology 89, 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ, international A.i., 2014. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383, 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A, Nakayama M, Turner CJ, Hoing S, Lepore JJ, Adams RH, 2013. Ephrin-B2 controls PDGFRbeta internalization and signaling. Genes Dev 27, 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini G, Forini F, Kusmic C, Iervasi G, Balzan S, 2019. Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci 239, 117080. [DOI] [PubMed] [Google Scholar]
- Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, Khyzha N, DiStefano PV, Suutarinen S, Kiehl TR, Mendes Pereira V, Herman AM, Krings T, Andrade-Barazarte H, Tung T, Valiante T, Zadeh G, Tymianski M, Rauramaa T, Yla-Herttuala S, Wythe JD, Antonarakis SE, Frosen J, Fish JE, Radovanovic I, 2018. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med 378, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisson PL, Fard SA, Walter CM, Johnstone CM, Mooney MA, Tayebi Meybodi A, Lang M, Kim H, Jahnke H, Roe DJ, Dumont TM, Lemole GM, Spetzler RF, Lawton MT, 2019. A novel proposed grading system for cerebellar arteriovenous malformations. J Neurosurg, 1–11. [DOI] [PMC free article] [PubMed]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E, 2000. Activin receptor-like kinase 1 modulates transforming growth factor- beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 97, 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike Y, Ito Y, Hamada K, Zhang XQ, Miyata K, Arai F, Inada T, Araki K, Nakagata N, Takeya M, Kisanuki YY, Yanagisawa M, Gale NW, Suda T, 2002. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood 100, 1326–1333. [PubMed] [Google Scholar]
- Oka M, Kushamae M, Aoki T, Yamaguchi T, Kitazato K, Abekura Y, Kawamata T, Mizutani T, Miyamoto S, Takagi Y, 2019. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg 126, e1365–e1373. [DOI] [PubMed] [Google Scholar]
- Ola R, Dubrac A, Han J, Zhang F, Fang JS, Larrivee B, Lee M, Urarte AA, Kraehling JR, Genet G, Hirschi KK, Sessa WC, Canals FV, Graupera M, Yan M, Young LH, Oh PS, Eichmann A, 2016. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat Commun 7, 13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola R, Kunzel SH, Zhang F, Genet G, Chakraborty R, Pibouin-Fragner L, Martin K, Sessa W, Dubrac A, Eichmann A, 2018. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation 138, 2379–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP, 2006. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Kim NH, Jo I, 2003. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res 65, 125–131. [DOI] [PubMed] [Google Scholar]
- Pekmezci M, Nelson J, Su H, Hess C, Lawton MT, Sonmez M, Young WL, Kim H, Tihan T, 2016. Morphometric characterization of brain arteriovenous malformations for clinical and radiological studies to identify silent intralesional microhemorrhages. Clin Neuropathol 35, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD, 2006. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1, 223–236. [DOI] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH, 2010. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev 24, 2480–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priemer DS, Vortmeyer AO, Zhang S, Chang HY, Curless KL, Cheng L, 2019. Activating KRAS mutations in arteriovenous malformations of the brain: frequency and clinicopathologic correlation. Hum Pathol 89, 33–39. [DOI] [PubMed] [Google Scholar]
- Rangel-Castilla L, Russin JJ, Martinez-Del-Campo E, Soriano-Baron H, Spetzler RF, Nakaji P, 2014. Molecular and cellular biology of cerebral arteriovenous malformations: a review of current concepts and future trends in treatment. Neurosurg Focus 37, E1. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, 2011. The role of pericytes in angiogenesis. Int J Dev Biol 55, 261–268. [DOI] [PubMed] [Google Scholar]
- Richards-Yutz J, Grant K, Chao EC, Walther SE, Ganguly A, 2010. Update on molecular diagnosis of hereditary hemorrhagic telangiectasia. Hum Genet 128, 61–77. [DOI] [PubMed] [Google Scholar]
- Robert F, Desroches-Castan A, Bailly S, Dupuis-Girod S, Feige JJ, 2020. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis 15, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV, 2013. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4, 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salvucci O, Tosato G, 2012. Essential roles of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv Cancer Res 114, 21–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Kodama N, Sasaki T, Matsumoto M, Ishikawa T, 2004. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery 54, 163–168; discussion 168–170. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y, 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376, 70–74. [DOI] [PubMed] [Google Scholar]
- Schulz GB, Wieland E, Wustehube-Lausch J, Boulday G, Moll I, Tournier-Lasserve E, Fischer A, 2015. Cerebral Cavernous Malformation-1 Protein Controls DLL4-Notch3 Signaling Between the Endothelium and Pericytes. Stroke 46, 1337–1343. [DOI] [PubMed] [Google Scholar]
- Shaligram SS, Winkler E, Cooke D, Su H, 2019. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neurosci Ther 25, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkar R, Elliott JP, Diener K, Gault J, Hu LJ, Cohrs RJ, Phang T, Hunter L, Breeze RE, Awad IA, 2003. Differential gene expression in human cerebrovascular malformations. Neurosurgery 52, 465–477; discussion 477–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepro D, Morel NM, 1993. Pericyte physiology. FASEB J 7, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Shovlin CL, 2010. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev 24, 203–219. [DOI] [PubMed] [Google Scholar]
- Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M, 2013. Variable Somatic TIE2 Mutations in Half of Sporadic Venous Malformations. Mol Syndromol 4, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H, 2006. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol 19, 63–68. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV, 2016. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH, McCullough LD, Kasner SE, Mullen MT, Hafler DA, Love JC, Sansing LH, 2017. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 127, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG, 2017. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun 8, 16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Stoodley MA, Morgan MK, Storer KP, 2006. Ultrastructure of perinidal capillaries in cerebral arteriovenous malformations. Neurosurgery 58, 961–970; discussion 961–970. [DOI] [PubMed] [Google Scholar]
- Tual-Chalot S, Mahmoud M, Allinson KR, Redgrave RE, Zhai Z, Oh SP, Fruttiger M, Arthur HM, 2014. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PloS One 9, e98646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L, 2007. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109, 5276–5285. [DOI] [PubMed] [Google Scholar]
- Vreeken D, Zhang H, van Zonneveld AJ, van Gils JM, 2020. Ephs and Ephrins in Adult Endothelial Biology. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott BP, Winkler EA, Rouleau GA, Lawton MT, 2016. Molecular, Cellular, and Genetic Determinants of Sporadic Brain Arteriovenous Malformations. Neurosurgery 63Suppl 1, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL, 2011. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 69, 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Birk H, Burkhardt JK, Chen X, Yue JK, Guo D, Rutledge WC, Lasker GF, Partow C, Tihan T, Chang EF, Su H, Kim H, Walcott BP, Lawton MT, 2018. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 129, 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Lu AY, Raygor KP, Linzey JR, Jonzzon S, Lien BV, Rutledge WC, Abla AA, 2019. Defective vascular signaling & prospective therapeutic targets in brain arteriovenous malformations. Neurochem Int 126, 126–138. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Sagare AP, Zlokovic BV, 2014. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol 24, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV, 2012. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 32, 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV, 2013. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters V, Limaye N, Uebelhoer M, Irrthum A, Boon LM, Mulliken JB, Enjolras O, Baselga E, Berg J, Dompmartin A, Ivarsson SA, Kangesu L, Lacassie Y, Murphy J, Teebi AS, Penington A, Rieu P, Vikkula M, 2010. Hereditary cutaneomucosal venous malformations are caused by TIE2 mutations with widely variable hyper-phosphorylating effects. Eur J Hum Genet 18, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kojima K, Taura D, Sone M, Washida K, Egawa N, Kondo T, Minakawa EN, Tsukita K, Enami T, Tomimoto H, Mizuno T, Kalaria RN, Inagaki N, Takahashi R, Harada-Shiba M, Ihara M, Inoue H, 2020. Human iPS cell-derived mural cells as an in vitro model of hereditary cerebral small vessel disease. Mol Brain 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Jin C, Ma H, Huang M, Shi GP, Wang J, Xiang M, 2016. EphrinB2/EphB4 pathway in postnatal angiogenesis: a potential therapeutic target for ischemic cardiovascular disease. Angiogenesis 19, 297–309. [DOI] [PubMed] [Google Scholar]
- Yildirim O, Bicer A, Ozkan A, Kurtkaya O, Cirakoglu B, Kilic T, 2010. Expression of platelet-derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J Clin Neurosci 17, 1557–1562. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B, 2009. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JH, Kwon do H, Lee EJ, Lee do H, Ahn JS, Kwun BD, 2012. New nidus formation adjacent to the target site of an arteriovenous malformation treated by Gamma Knife surgery. J Neurosurg 117Suppl, 120–125. [DOI] [PubMed] [Google Scholar]
- Zeng X, Hunt A, Jin SC, Duran D, Gaillard J, Kahle KT, 2019. EphrinB2-EphB4-RASA1 Signaling in Human Cerebrovascular Development and Disease. Trends Mol Med 25, 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhu W, Su H, 2016. Vascular Integrity in the Pathogenesis of Brain Arteriovenous Malformation. Acta Neurochir Suppl 121, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Huang H, Ologunde R, Lloyd DG, Watts H, Vizcaychipi MP, Lian Q, George AJ, Ma D, 2015. Xenon treatment protects against remote lung injury after kidney transplantation in rats. Anesthesiology 122, 1312–1326. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen W, Zou D, Wang L, Bao C, Zhan L, Saw D, Wang S, Winkler E, Li Z, Zhang M, Shen F, Shaligram S, Lawton M, Su H, 2018a. Thalidomide Reduces Hemorrhage of Brain Arteriovenous Malformations in a Mouse Model. Stroke 49, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang R, Ma L, Su H, 2018b. Animal Models and Prospective Therapeutic Targets for Brain Arteriovenous Malformation, in: Su H, Lawton M (Eds.), Molecular, Genetic, and Cellular Advances in Cerebrovascular Diseases. World Scientific Publishing Co., pp. 83–126. [Google Scholar]
- Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y, Shen F, Young WL, Yang GY, 2006. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery 59, 687–696; discussion 687–696. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, Hu J, Soehnlein O, Franz WM, Sperandio M, Pohl U, Thomas M, Weber C, Augustin HG, Fassler R, Deutsch U, Kupatt C, 2013. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. [DOI] [PMC free article] [PubMed]


