Abstract
Development of biomaterials mimicking tumor and its microenvironment has recently emerged for the use of drug discovery, precision medicine, and cancer biology. These biomimetic models have developed by reconstituting tumor and stroma cells within the 3D extracellular matrix. The models are recently extended to recapitulate the in vivo tumor microenvironment, including biological, chemical, and mechanical conditions tailored for specific cancer type and its microenvironment. In spite of the recent emergence of various innovative engineered tumor models, many of these models are still early stage to be adapted for cancer research. In this article, we review the current status of biomaterials engineering for tumor models considering three main aspects - cellular engineering, matrix engineering, and engineering for microenvironmental conditions. Considering cancer-specific variability in these aspects, our discussion is focused on pancreatic cancer, specifically pancreatic ductal adenocarcinoma (PDAC). In addition, we further discussed the current challenges and future opportunities to create reliable and relevant tumor models.
Keywords: Biomaterials, Tumor model, Cancer cells, Extracellular matrix, Tumor microenvironment
1. Introduction
Reliable and predictive tumor models are essential to perform detailed mechanism-based analyses as well as target identification, drug discovery studies, and personalized treatment planning across the full spectrum of cancer research. To meet the diverse needs of cancer research, various models have been developed, ranging from developing in vitro cell lines to establishing ex vivo co-culture models to in vivo animal models. Cell culture models provide convenience and controllability but often inadequately represent the complex tumor microenvironment (TME). Animal models mimic certain aspects of the TME better, but such models are inherently complex and deciphering their study results for mechanistic understanding is difficult.
Biomimetic tumor models have emerged as there have been significant recent advancements in biomaterials and microfabrication technologies. In vitro 3D models provide a more realistic the complex and heterogeneous TME compared to 2D models and enable simplified study of biochemical and biophysical factors which is not possible in animal models. Current 3D models include spheroids, organoids, matrix scaffolds, tumor-on-chips, and printed tissue constructs. These next generation models have attempted to address the limitations of current 2D models while providing mimicry of in vivo tumor models. Tumor spheroid and organoids are being adopted because of the simplicity and convenience in copying the densely packed cells in the 3D context. Matrix scaffolds enable systematic study of microenvironmental cues including cell-cell and cell-matrix interactions via culture of multiple cell types. Microfluidic tumor models are capable of controlling and imposing various chemical and physical conditions on the TME. These biomimetic models show great promise as a complimentary bridging approach between in vitro models and animal models [1–3]. Research efforts have been mainly focused on developing approaches that better recapitulate the in vivo setting. However, current models are still limited in mimicking the complex PDAC microenvironment including the microanatomy and pathophysiology. In addition, biomimetics have started being used and implemented in drug discovery and to identify elements of cancer-type specific modes of pathogenesis. A thorough validation of these models’ accuracy and repeatability is crucial. In addition, development for high throughput, user-friendly, and standardized configurations is warranted.
Here, we review the current status of biomaterials engineering for tumor 3D modeling. To reconstitute the TME, three aspects of tumor tissue are engineered - cellular engineering, matrix engineering, and environmental engineering, as illustrated in Fig. 1. These aspects vary drastically depending on the cancer type, stage, and organs [4]. Even for a given cancer type, heterogeneity at molecular, cellular, and tissue-levels present [5, 6]. Thus, rather than providing a generic description regarding cancerous tissues, this review is focused on pancreatic cancer, more specifically pancreatic ductal adenocarcinoma (PDAC) unless otherwise mentioned. PDAC is the fourth leading cause of cancer death in the United States, with extremely low 5-year survival rate of 9% [7]. Treatment for PDAC remains especially challenging due to the desmoplastic tumor microenvironment (TME) composed of cancer associated fibroblasts (CAFs), extracellular matrix (ECM), and immune cells. Reliable and predictive tumor models are essential to perform detailed mechanism-based analyses as well as target identification, drug discovery studies, and personalized treatment planning across the full spectrum of PDAC research. We will review each aspect of engineering the TME and discuss challenges and opportunities to generate tumor models with more predictive capability.
Fig. 1. Aspects of Cancer Engineering.
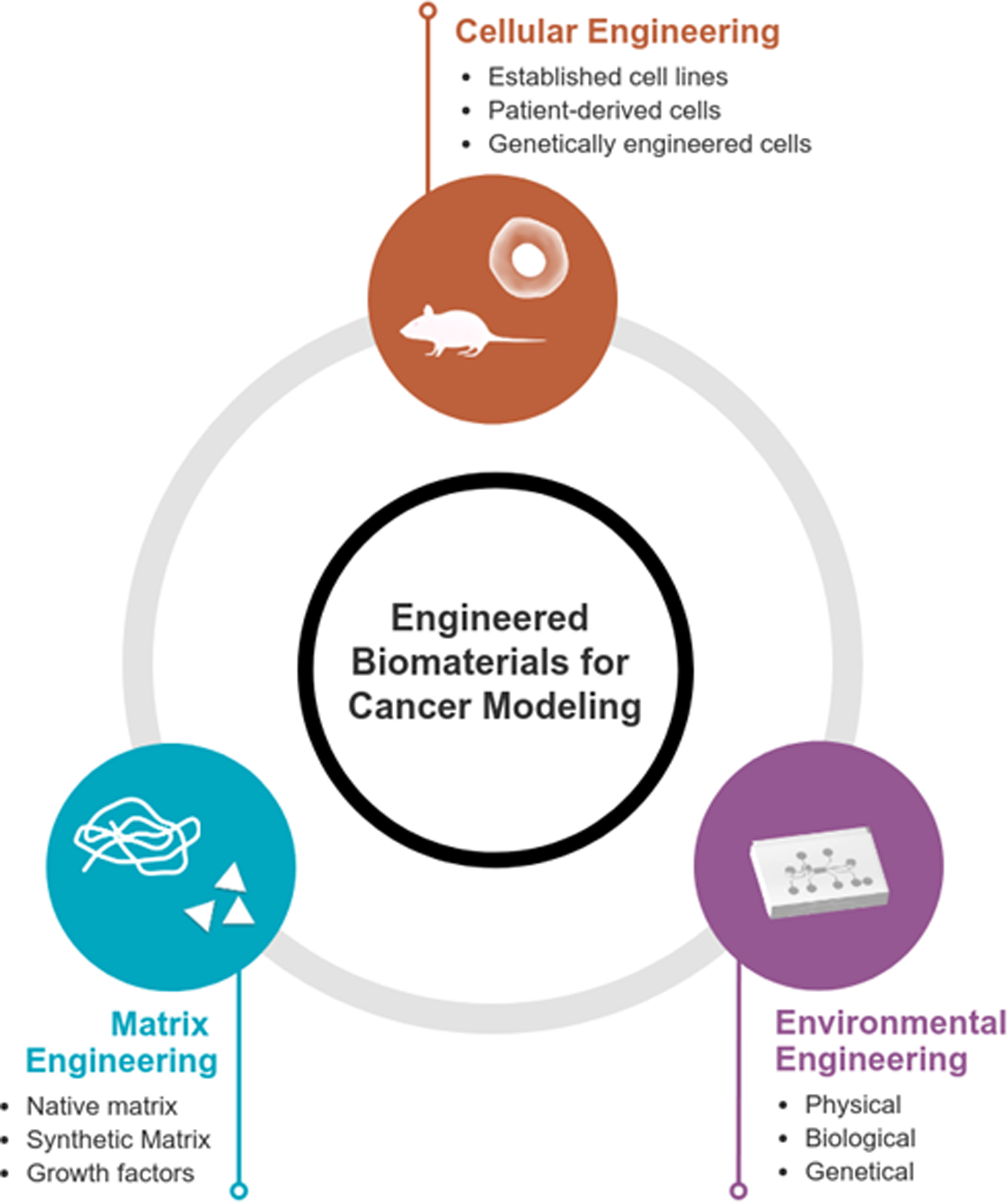
In developing models of cancer, cellular, matrix, and environmental engineering aspects should carefully be considered. Significant research has been performed in each area for accurate drug screening platforms.
2. Cellular Engineering
2.1. Molecular and cellular features of PDAC
PDAC results from accumulated oncogenic mutations in ductal or acinar cells of the exocrine pancreas. Initiating mutations drive downstream cellular, transformation, tumor growth, and complex changes within the TME that results in a highly aggressive and metastatic solid tumor malignancy refractory to therapy. PDAC tumorigenesis arises from either ductal cells themselves or from a transition of exocrine cells termed acinar-to-ductal metaplasia (ADM) in which acinar cells undergo dedifferentiation into ductal- or epithelial-like cells [6]. Primordial lesions are characterized by intraepithelial neoplasias (PanIN; which is known to be the most common), mucinous cystic neoplasia (MCN), and intraductal pancreatic mucinous neoplasia (IPMN). During tumor development, oncogenes are upregulated and the function tumor suppressor genes is lost. The most frequently oncogenic mutations for PDAC activating KRAS mutations and inactivating mutations in tumor suppressor genes such as CDKN2A/p16, SMAD4, and TP53, as illustrated in Fig. 2 [8, 9].
Fig. 2. Pancreatic Cancer Pathogenesis.
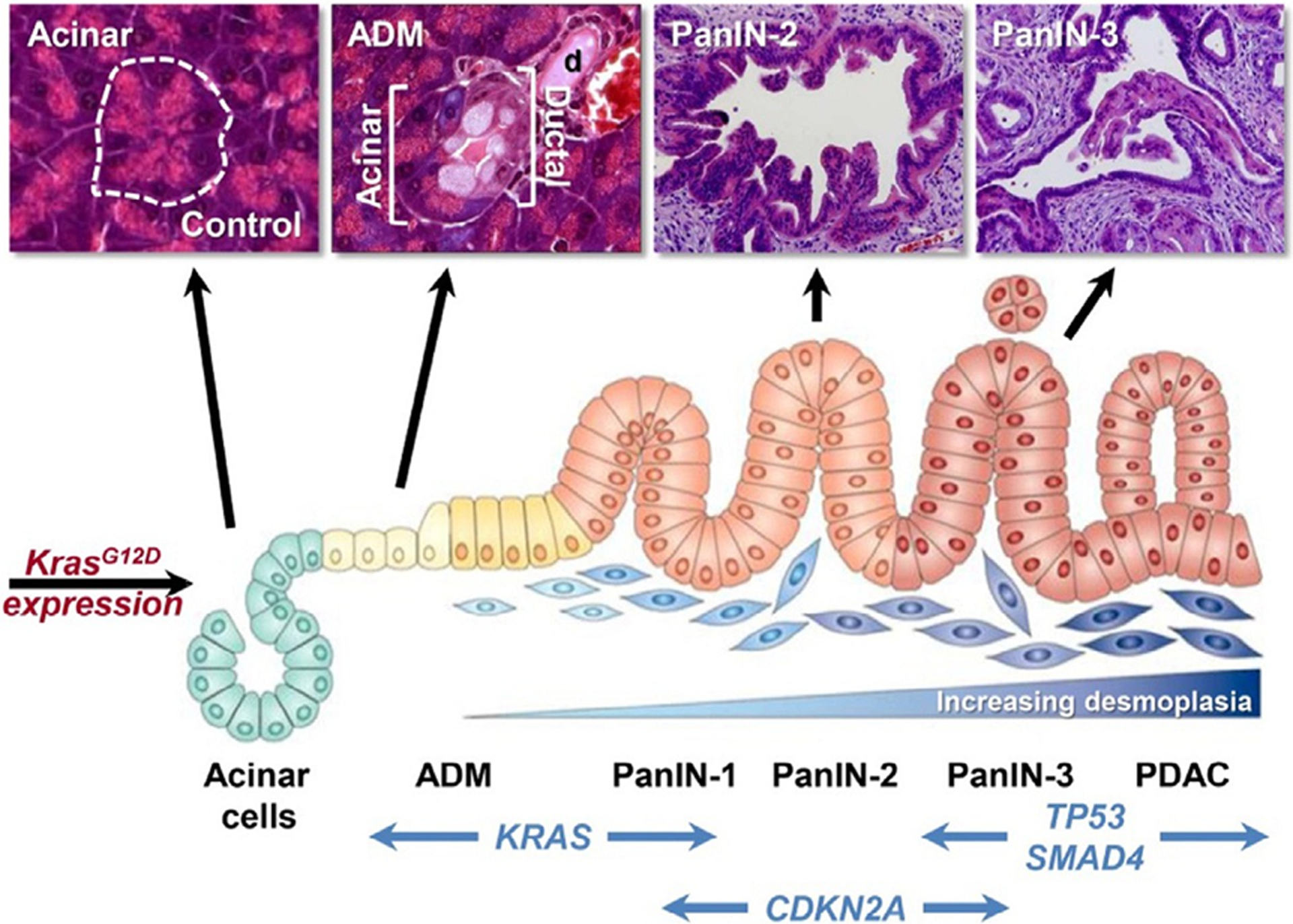
The schematic and mouse histology demonstrate step-wise development of pancreatic cancer. The accumulation of driver mutations such as KRAS, CDKN21, TP53, and SMAD4 induces the transformation of normal acinar cells to acinar-to-ductal metaplasia (ADM), pancreatic intraepithelial neoplasia (PanIN), and eventually pancreatic ductal adenocarcinoma (PDAC). Adapted from reference [1] with permission from Elsevier.
Tumor development is complicated by the interaction of tumor cells with a number of cellular components of the TME, which is predominantly composed of CAFs, immune cells, endothelial cells and epithelial cells. The heterogeneity of PDAC resulting from its epigenetics and cellular characteristics has led to identification of several subtypes. Most classifications are based on the expression of transcription factors and composition of stromal cells that leads to epithelial-like or mesenchymal-like characteristics of PDAC [10]. Some classifications are based on immune cell compositions, especially the presence of B and T cells. Different immune cell compositions has also been associated with variations in mutations rates of transcription factors [11]. In addition, the highly controversial roles of stromal cells also necessitate the need to understand their multifarious nature. For example, some studies report protumorigenic effects of CAFs by reducing cytotoxic T cell response [12, 13]. Moreover, reprogramming CAFs back to the normal fibroblast state with curcumin had suppressed pancreatic cancer migration [14]. Contrarily, depletion of CAFs was shown to result in more invasive tumors resulting in diminished survival of the host [15]. Consequently, identifying genotype-phenotype relationships of pancreatic cancer cells with the various stromal cell components as well as characterization of their subtypes remain crucial elements in recapitulating the tumor heterogeneity in developing PDAC models.
2.2. Established cell lines
Human pancreatic cancer cell lines are derived from patient donors with varying age, sex, origin, presence of metastasis and having received differing treatments [16, 17]. Table 1 provides a summary of commonly adapted human pancreatic cancer cell lines and their genetic characteristics. The genetic diversity of cells results in considerable differences in their phenotypes including metastatic characteristics such as adhesion to extracellular proteins, migration, invasion, and angiogenic potential [16]. Thus, cell lines in tumor models should be carefully selected with consideration of molecular and genetic features of tumors mimicking.
Table 1.
Summary of commonly used human pancreatic cancer cell lines and their driver mutations.
| Cell Line | Origin | Metastasis | Gene Mutation | References |
|---|---|---|---|---|
| AsPC-1 | Ascites | Yes | KRAS, TP 53, CDKN2A/p16, SMAD4/DPC4 | [203–209] |
| BxPC-3 | Primary tumor | No | TP53, CDKN2A/p16, SMAD4/DPC4 | [204, 207–210] |
| Capan-1 | Liver metastasis | Yes | KRAS, TP53, CDKN2A/p16, SMAD4/DPC4 | [204, 205, 207, 208, 211] |
| Capan-2 | Primary tumor | No | KRAS, TP53, CDKN2A/p16 | [204, 205, 207, 212] |
| CFPAC-1 | Liver metastasis | Yes | KRAS, TP53, CDKN2A/p16, SMAD4/DPC4 | [204, 206–208, 213] |
| HPAC | Primary tumor | - | KRAS, CDKN2A/p16 | [204, 214] |
| HPAF-II | Ascites | Yes | KRAS, TP53, CDKN2A/p16 | [204–206, 208, 215] |
| Hs 766 T | Lymph node metastasis | Yes | KRAS, TP53, CDKN2A/p16, SMAD4/DPC4 | [204, 205, 207, 208, 216] |
| MIA PaCa-2 | Primary tumor | - | KRAS, TP53, CDKN2A/p16 | [205–207, 209, 217] |
| PANC-1 | Primary tumor | Yes | KRAS, TP53, CDKN2A/p16 | [204–207, 209, 218] |
| SU.86.86 | Liver metastasis | Yes | KRAS, TP53 | [204, 207–209, 219] |
The adhesive properties of cell lines can vary considerably depending on the expression of cell surface receptors and the precise components comprising the target extracellular matrix. While some studies demonstrate PANC-1 having greatest attachment to collagen IV and fibronectin, others report PANC-1 having the least affinity to laminin [18, 19]. Studies also report contradicting results of PANC-1 attachment to the same extracellular matrix such as fibronectin [19, 20]. The two studies in question measured the effect of β1 integrin and (IL)-1α, respectively, on cell adhesion. The differing relative magnitude of PANC-1 adhesion to fibronectin compared to other PDAC cell lines from these studies suggest that the cell interaction with the environment is quite complex even within the same cancer cell type. Other contributing factors to the differences in phenotypes may be linked to the use of different techniques for quantifying cells, temporal differences, or other elements of individual experimental protocols [16]. Thus, when selecting established cell lines for in vitro cancer models, specific experimental conditions regarding to matrix components should be carefully considered and evaluated. Furthermore, the detailed genotype-phenotype relationship warrants further investigation.
CAFs are the most abundant cellular component of the PDAC stroma. The origin of CAFs is thought to be resident pancreatic stellate cells (PSCs) that upon activation by a variety of extracellular stimuli differentiate into CAFs that may be identified by the expression of α-smooth muscle actin (α-SMA) and collagen I [6, 21, 22]. In the normal pancreas, PSCs control synthesis and degradation of extracellular matrix. However, with the onset of neoplastic activity, activated PSCs produce large amounts of ECM, leading to a characteristic dense PDAC stroma. CAFs derived from PSCs are known to have a significant role in tumor progression and promote chemoresistance by remodeling the ECM. Among the CAFs, two major subtypes have been identified based on their functions [21]. Inflammatory CAFs, (i.e., iCAFs) reside distant from tumor cells, have lower αSMA levels, and highly express cytokines and chemokines. Myofibroblastic CAFs (i.e., myCAFs) are located near tumor cells and depend on juxtracrine interactions. They have higher αSMA and lower cytokine expression. These myCAFs are associated with tumor stiffness, hypoxia, and avasculation whereas iCAFs have been known to promote aggressive, immunosuppressive and chemotherapy-resistant tumor behavior. Such cell specific characteristics in determining their subtypes have largely been investigated through isolation of primary tissues [23]. Endothelial cell lines are also widely used for studies in angiogenesis, metastasis, and migration studies [24]. Typical human endothelial cell lines utilized include human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMECs). Most endothelial cells are derived from primary tissues, limiting their passage number for culture. Some immortalized cell lines, including CAFs and endothelial cells, have been shown to retain biochemical characteristics of the primary cells [25]. Isolation of cancer specific stromal cell lines and identification of their subtypes could enhance precise engineering of the TME in cancer modeling.
Established cell lines are valued in many studies as they are well characterized, low-cost, and easy to propagate, which enables development of biomarkers and potential drug targets. However, there are limited cell lines available that represent each of the different subtypes and overall heterogeneity of cells even within a single tumor. Moreover, PDAC cell lines have been shown to have acquired mutations and phenotypes ex vivo that favor their growth in vitro. Thus, there are significant differences in cell lines compared to primary tumors and xenografts [22, 26, 27]. Nevertheless, established cell lines still possess imperative advantage in high-throughput preclinical drug screening studies.
2.3. Patient-derived cells: Primary cells isolated from patient tumor tissues
Primary cell lines retain the heterogenous nature of pancreatic cancer cells and closely resemble the original tumor. Particularly with the emerging importance of personalized medicine, successful and efficient culture of primary cells is essential. Thus, different studies have established protocols to develop primary cell lines which also involves mouse and organoid models. Patient-derived xenograft (PDX) and patient-derived orthotopic xenograft (PDOX) models make possible in vivo expansion of primary cells. PDX enables culture of pancreatic cells in native environment, however, is limited by the amount recovered from the xenograft model. This may be overcome by the use of PDOX models [28]. Several studies have proposed facilitated methods to conventional isolation methods. A two-step protocol is developed that does not require serial xenotransplantations, increasing the efficiency [29]. With subsequent in vitro culture, a success rate of 43% was achieved from pancreatic cancer of various pathological types. Recently, accelerated development of PDX models of neuroblastoma is reported in noticeably shortened amount of time [30]. Alternatively, methods separate from the use of mice are also pursued. Tumor-biopsy-derived cultures have been generated across various tumor tissue types within weeks of biopsy. However, the success rate, ranging 9–38% depending on the tissue type, is lower than mouse models [31]. Multi-cell type organoids are also developed with PDAC cells and activated myCAFs, thereby preserving the cellular heterogeneity of the TME [32]. Nevertheless, isolating human primary cells without in vivo expansion is still challenging due to dense tissue fibroblasts that can overgrow cancer cells. Mouse models can increase such isolation efficiency as human fibroblasts are replaced by murine stromal cells. In spite of such advantage, this results in human cancer cells with murine stromal cells which could lead to tumor cells that do not fully recapitulate the original tumor [29]. Widespread establishment of rapid protocols with minimal usage of mouse models may contribute to important advancements in patient-derived cells and personalized treatment.
2.4. Genetically engineered cell lines
The use of genetically-engineered mouse models (GEMM) has provided an outstanding in vivo platform for the study of pancreatic cancer. The current ‘gold standard’ of GEMM for PDAC is the KPC mouse model that harbors oncogenic KrasG12D and dominant negative Trp53R172H, whose concomitant expression is driven by either acinar (e.g., Elastase-cre) or exocrine pancreas progenitor cell (e.g., Pdx-1-cre)-specific transgene. This model has been shown to recapitulate many aspects of human PDAC disease progression, including acinar-to-ductal metaplasia, the full spectrum of PanINs, primary invasive PDAC, and metastatic dissemination of pancreatic cancer [33, 34]. Additionally, the KPC model replicates the human PDAC desmoplastic tumor microenvironment including immune cell composition [35, 36], CAFs heterogeneity [21], and extracellular matrix proteins [5, 37]. As such, the KPC mouse model has been used as the frontline mouse model to test potential PDAC therapeutics [38]. The KPC mouse model mimics many key immune features of human PDACs including poor infiltration of effector T cells, massive deposition of myeloid-derived suppressor cells (MDSCs), cancer associated macrophages and regulatory T cells establishing an immune suppressive tumor microenvironment [39–41]. Thus, comparing to PDX or PDOX models, KPC models provide the advantages of studying immune-related cellular mechanisms and the development of immune-mediated therapeutic strategies.
In addition to analysis of spontaneous in situ PDAC disease, an effective method for detailed in vivo mechanism-based studies has been developed in which pancreatic cancer cells are generated by isolating primary tumors to establish pools or clonal derivatives of cells from GEMMs (e.g., KPC cells). The effectiveness of this approach is attributed in part to the fact these cell lines may be manipulated ex vivo followed by reintroduction into mice of the same genetic strain for analyses in an immunocompetent environment. The functionality of KPC-derived cell lines as a tool for hypothesis-driven investigation has been significantly enhanced through the use of Crispr-Cas9 gene editing to selectively eliminate tumor-derived signaling molecules. In so doing, a precise molecular dissection of factors originating from tumor cells versus those supplied by the tumor microenvironment may be performed [42].
Although GEMMs provide a number of advantages to study PDAC progression, their use should be taken into practical and technical considerations. The routine breeding of GEMMs can be time consuming and expensive, particularly when studies require combining 3 or more different modified alleles or transgenes. The maintenance of GEMMs can also be challenging due to a reduced lifespan of the genetically-modified animal. Indeed, the spontaneous and aggressive development of cancer in which all cells of a given type within the pancreas (e.g., exocrine cells) are susceptible to tumorigenesis can result in significant and rapid morbidity and mortality. The aggressive nature of the disease has the potential to impede the analysis of elements of late stage PDAC (e.g., stromal changes or metastasis). Lastly, GEMMs have much fewer mutations and less genetic complexity compared to human PDAC tumors. Whereas this aspect of the model has the advantage of allowing investigators to focus on specific pathways, it comes with a notable limitation as human PDAC has been shown to be quite heterogeneous in terms of the mutations involved, tumor cell heterogeneity, and stromal make-up [43, 44]. Thus, the studies of PDAC genetics/therapeutics using GEMMs cannot fully reflect treatment response in human PDAC patients.
2.5. Stem cell engineering
The ability to reprogram primary cells to pluripotent stem cell state has created novel opportunities in disease modelling. Induced pluripotent stem cells (iPSCs) brought advantage of exploring early stages of cancer which is often not feasible with patient-derived cell lines or xenografts. iPSCs may be reprogrammed from normal or mutated somatic cells via gene transfer of OCT4, SOX2, KLF4 and c-MYC transcription factors [45]. iPSCs derived from cancer cells could also recapitulate the properties of cancer stem cells (CSCs). CSCs only represent about 5% of the total tumor and are therefore very challenging to isolate [46, 47]. CSCs are resistant to standard therapies and are critical factors responsible for tumor relapse as they may regenerate tumor cells after treatment. These tumor initiating cells can also evade immune detection with decreased levels of T-cell activation molecules and increased T-cell inhibitory molecules [48]. Hence, cancer derived iPSCs may elucidate crucial knowledge in pancreatic cancer molecular pathways and CSCs associated chemoresistance. Despite its applicability, there are only a handful of research on pancreatic cancer reprogrammed iPSCs available. It was reported that PDAC cells were reprogrammed to iPSC by lentiviral transduction of reprogramming factors [49]. Upon injection of iPSC-like cells into immunodeficient mice, the cells were able to recapitulate various stages of pancreatic cancer. Cautious consideration in adapting incompletely reprogrammed “iPSC-like” cells is advised as they may not fully recapitulate the epigenetic properties of primary cells [50]. Nevertheless, iPSCs derived pancreatic cancer organoids and orthotopic models continue to serve as practical tools as alternative to patient-derived primary cells and personalized treatment [51, 52].
3. Matrix engineering
3.1. The role of ECM
The desmoplastic stroma of PDAC consists primarily of matrix proteins secreted by CAFs, such as collagen, fibronectin, hyaluronic acid, and laminin [5, 53]. The dense stroma is a physical obstacle to many drug treatments for pancreatic cancer. Thus, understanding the properties of ECM in tumor stroma formation and its impact on tumor cells is vital for enhancing therapeutic efficacy. ECM functions as a structural support for solid tumors by maintaining tissue integrity. Dysregulation in the ECM also triggers several biochemical and regulatory pathways that promote tumor proliferation, migration, and metastasis.
ECM provides structural support in two forms: basement membrane (BM) and interstitial matrix. The BM is present between the stromal component and the epithelium. The BM is mainly composed of laminin, collagen IV and XV, entactin, heparin sulfate proteoglycans, and nidogen. The interstitial ECM is most abundant with collagen I, III and V as well as fibronectin [53, 54]. Overexpression of BM proteins such as collagen XV is antitumorigenic by reducing the migration of PDAC cells. On the other hand, escalation of circulating collagen IV was observed in patient tissue samples and has been shown to protect PDAC cells against apoptosis in vitro [53]. Tissue disruption associated with pancreatic tumor initiation and growth activates coagulation pathways resulting in extravascular fibrin deposits, which are eventually replaced by collagens secreted by fibroblasts [55]. Resulting deposition of matrix proteins contributes to poorer prognosis of PDAC. Despite substantial matrix as part of a dense desmoplastic stroma in PDAC, the TME of PDAC tumors is poorly vascularized.
The ECM also plays a fundamental role in chemical and mechanical signaling pathways as well as communication via proteins, growth factors, receptors, and adhesion molecules. Commonly encountered growth factors secreted within the PDAC TME include, transforming growth factor-β (TGF-β), fibroblast growth factors (FGFs), hepatocyte growth factors (HGFs) and vascular endothelial growth factors (VEGFs) [1, 53]. For instance, TGF-β promotes ECM production by activation of PSCs and collagen synthesis [53]. Tumor cells sense the mechanical stimulation from the ECM through interaction via transmembrane glycoprotein integrins and actin skeleton, which activates further downstream signaling pathways and modulate cell activity. Different combinations of integrin subunits, such as β1, β2, and α2β, prevents adhesion, proliferation, and migration of pancreatic cancer cells to ECM proteins [56–58]. As such, understanding the functional interplay between ECM components, soluble molecules, and cell surface receptors can lead to additional knowledge of how PDAC interacts with its microenvironment.
3.2. Proteomics, chemical and mechanical properties of PDAC matrix
Proteomics is a technique centered on global protein analysis of tumor tissue. Understanding modification in proteins over the course of pancreatic cancer development can clarify the role of certain proteins in tumor progression and establish novel biomarkers. Beachley et al. accentuates the importance of recognizing proteomics by quantitative analysis of organ-specific protein concentrations, as shown in Fig. 3 [4]. As a result of varying ECM protein content and the cell adhesion properties depending on the tissue type, it is crucial to plan differing matrix protein targeting strategies per cancer type. Sanh et al. specified that some proteins (cathepsin D, integrin 1, and plasminogen) were overexpressed in pancreatitis and PDAC tissues compared to normal tissues; moreover, pancreatitis and PDAC had similar proteomics. Additionally, 31 proteins were differentially expressed comparing PDAC and normal tissues [59]. They also identified that increased levels of serum pancreatic lipase in pancreatitis correlated with the occurrence of malignant tumors, rendering it as a potential biomarker. Similarly, mass spectrometry (MS) based proteomics was used to compare proteins in normal tissue, pancreatitis and PDAC. Collagen was the most abundant protein from tissue at all stages of the cancer [5]. Specifically, fibrillar collagens COL1A1, 1A2 and 3A1 constituted over 90% of all collagen, with 2.6-fold increase in PDAC compared to normal tissue. Moreover, matrisome proteins were highly upregulated in PDAC compared to PanIN and pancreatitis. In addition, 136 of 146 proteins overexpressed in PanIN compared to normal pancreas were also overexpressed in PDAC. Thus, they may be recognized as biomarkers for early PDAC progression. Also, protein composition was compared in the human pancreas before and after decellularization using isobaric demethylated leucine (DiLeu) labeling [60]. Results indicated an expected pattern that while matrisome proteins were preserved, most cellular proteins were removed. Decellularization of human tissues may provide new biomaterial scaffolds for cancer modelling.
Fig. 3. Proteomics of Different Tissue and Organ ECM.
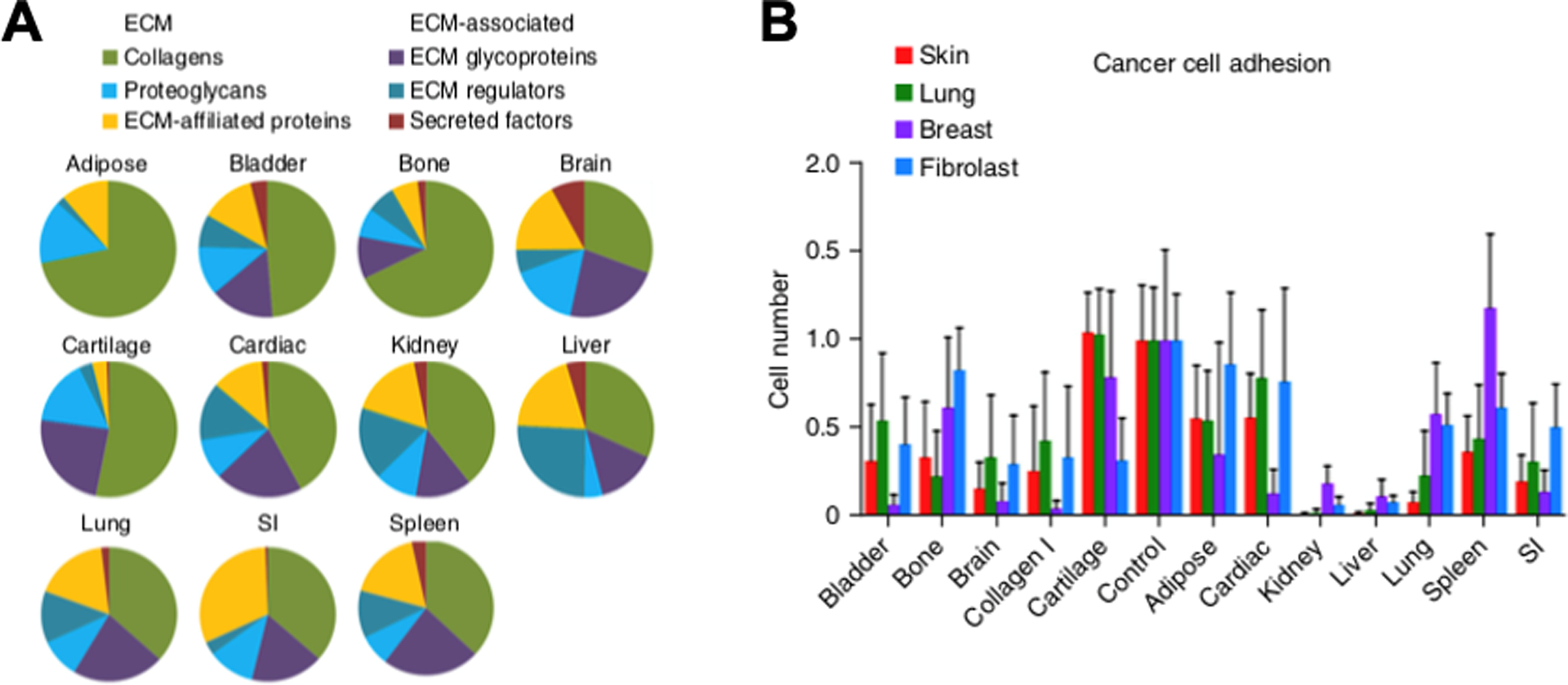
A. Mass spectrometry analysis of porcine tissue samples illustrates varying organ-dependent ECM protein compositions. B. Cell-tissue adhesion interaction vary significantly among organs for human lung, breast, and skin cancer cell lines and control mouse fibroblast line. Adapted from Reference [4] with permission from Springer Nature.
As proteomics studies have revealed, ECM protein components are remodeled over the course of cancer progression. ECM is organized by remodeling enzymes matrix metalloproteases (MMP). Twenty six MMPs have been identified with different functions. For example, MMP-1 degrades fibrillar collagens whereas MMP-2 and MMP-9 degrade basement membrane collagen IV [54]. In PDAC patients, the expression of MMP-1, MMP-2, MMP-7, MMP-8, MMP-9, and MMP-13 were elevated [61, 62]. The expression of specific MMPs have been associated with the morphological features of PDAC. For instance, in PDAC, the presence of necrosis has been positively correlated with MMP-7 [61]. MT1-MMP is one of the most extensively studied proteases and is known to cleave growth factors and collagen. MT1-MMP activates growth factor TGF-β1 that prompts collagen production by PSCs. MT1-MMP inhibition in collagen gels increased chemotherapy drug delivery, whereas upregulation of MT1-MMP led to increased impairment of drug delivery [63]. In PDAC, MT1-MMP promotes invasiveness and the inhibition of MT1-MMP expression by cytoplasmic tail binding protein 1 (MTCBP-1) decreased the invasive and metastatic properties in vitro and in mouse models [64]. However, targeting MMP is remains contradictory. For example, suppressing MMP13 expression inhibited PDAC invasion but also reduced chemotherapy efficacy [62]. Moreover, in vivo MMP studies in PDAC is still lacking and more evidence is required in order to draw the conclusion between MMP expression levels and PDAC prognosis. In addition to MMP, there exists other remodeling enzymes such as fibroblasts activation protein (FAP) and lysyl oxidases (LOX). In general, overexpression of remodeling enzymes correlates with high levels of desmoplastic stroma [54].
Mechanical properties of the ECM change and evolve during pancreatic carcinogenesis to render the TME to favor tumor cell proliferation. Robinson et al. observed increased collagen concentration as well as matrix contraction when PSCs were present compared to acellular samples [65]. Moreover, there was increase in collagen fiber alignment with increasing PSC number. The increase in collagen fiber thickness and spacing between fibers suggested that collagen fibers were being drawn together forming bundles. All together led to increase in stiffness for samples with PSCs [66]. They were able to recapitulate the PDAC stiffness when PDAC was cultured with tumor-associated stromal cells in collagen hydrogel and was exposed to conditioned media from PDAC cells. Other noticeable mechanical properties involve fiber alignment of proteins on matrix stiffness as well as migration patterns. High collagen fiber alignment has shown correlation with epithelial expression of EMT, α-SMA expression and overall reduced survival of patients [67].
3.3. Native Matrix: Collagen Type I, IV, fibronectin, hyaluronic acid
Understanding native biomaterial function is crucial not only for means of cell culture but also for development of a 3D model drug screening platform. Drug efficacy has demonstrated significant differences in 2D and 3D environments due to desmoplastic stroma. Therefore, the ability to mimic natural ECM has been a fundamental research area. The functional characteristics and applications in PDAC modeling for common natural ECM proteins is summarized in Table 2.
Table 2.
Applications of native ECM proteins in PDAC modeling
| Native Matrix | Functional Characteristics | Applications in PDAC Modeling | References |
|---|---|---|---|
| Collagen I |
|
MMP expression, cell adhesion, matrix stiffness, fibrillar structure, EMT, cell migration, drug resistance | [73] [74], [75] [65, 76], [77, 78], [221] |
| Fibronectin |
|
Spheroid culture, cell migration | [78, 223, 224] |
| Hyaluronic acid |
|
Spheroid culture, matrix stiffness, drug efficacy | [126, 223, 225] |
| Laminin |
|
Spheroid culture, cell growth | [223, 227] |
| Fibrin(ogen) |
|
Spheroid culture, PSC culture and functions | [224, 229] |
Matrigel is a protein mixture including collagen IV, laminin, entactin, perlecan, cytokines and growth factors which is derived from Engelbreth-Holm-Swarm mouse tumor basement membrane [68]. Matrigel has been widely applied in culturing cancer cells for identifying therapeutic targets and studying drug efficacy. For instance, culturing pancreatic cancer cells in Matrigel with growth factors TGF-β and epidermal growth factor (EGF) was used to assess cancer cell growth and chemoresistance [69]. Another study investigated targeting hypoxia induced drug resistance in PDAC [70]. However, due to high batch-to-batch variability and limitations in identifying protein components, Matrigel may be inappropriate in studying the role of specific ECM proteins in pancreatic cancer cell growth and producing consistent results. Collagen type I and IV are the most abundant protein in interstitial stroma and basement membrane respectively. Bovine and rat-tail collagen matrix are widely applied in many cancer models as they closely mimic tumor ECM, however, they must be prepared at acidic conditions [71]. During preparation, collagen material properties may be determined by altering different parameters including concentration, polymerization temperature, and polymerization pH [72]. Common applications studies utilizing collagen in PDAC modelling include investigation for enzyme activities such as MMP [73], adhesion factors such as E-cadherin [74], matrix stiffness [75], fibrillar structure [65, 76], and EMT and migration [77, 78]. Moreover, development of oligomer collagen, compared with conventional monomer collagen, enabled standardization of collagen preparation which led to direct comparison of collagen microstructure properties on PDAC behavior [79]. Studying various integrin receptors of matrix could also uncover the spatial distribution of ECM around tumor cells [80], which allows potential engineering of models that recapitulate the 3D architecture of PDAC tissue.
Fibronectin is a cell surface glycoprotein secreted by fibroblasts. Binding to the major receptor integrin α5β1, fibronectin induces pro-tumorigenic effects in PDAC through increasing chemoresistance, cell invasion and metastasis [81]. Targeting fibronectin also promotes matricellular protein SPARC function to inhibit PDAC growth and induce apoptosis [82]. However, the role of fibronectin is paradoxical as both tumor suppressor and metastatic promoter. Lin et al. provides extensive review on various hypothesis on the interplay between fibronectin and the TME that leads to the controversial nature [83]. Nonetheless, targeting fibronectin remains unsettled. Regardless of the controversial nature, fibronectin may serve as a practical cell culture medium. Jordahl et al. engineered a novel hydrodynamically induced fibrillar fibronectin (fFN) for 3D cell culture [84]. The hydrogel was supported by a tessellated porous scaffold that closely mimics the fibrillar networks of natural ECM. Notably, this fFN model was in line with the pro-tumorigenic effects of fibronectin when cultured with cancer cells isolated from 14 different patient biopsy samples. The adaptability of this platform to other cancer types requires further examination.
Hyaluronic acid (HA) is a major glycosaminoglycan (GAG). HA matrix provides a stable platform for cell culture, albeit the hydrogel does not have ligands for integrin signaling. HA binds to cell surface receptors CD44 and receptor for hyaluronan-mediated motility (RHAMM) that regulates pancreatic cancer proliferation and drug resistance [71]. HA also increases interstitial pressure by retaining large water volume and resisting compression [85]. Targeting HA synthesis by introducing competitive substrate 4-methylumbelliferone (4-MU) or HA signaling via cell surface receptors, and degrading HA with an enzyme pegylated hyaluronidase (PEGPH20), are potential therapeutics for PDAC [86]. PEGPH20 has demonstrated efficacy in decreasing IFP in mouse models via combination therapies with gemcitabine in preclinical studies [85]. However, clinical trials administering the drug was ultimately halted in phase III due to failure in increasing overall patient survival, leaving HA targeting a highly unknown research area [87].
3.4. Synthetic matrix: Polymer-based matrices
Synthetic polymer matrices are present in porous scaffold or hydrogel form. They possess controllable biochemical and biomechanical properties over a broad range of length scales, rendering them an important advantage over native matrices [88]. Likewise, synthetic matrices can present ECM proteins and soluble bioactive molecules that are present in the TME using various chemical synthesis and modification techniques [89]. The resulting matrices have been used to examine the effects of 3D microenvironment on cancer growth, invasion, and metastasis and to screen therapeutic drugs [90].
Synthetic polymer hydrogels mimicking in vivo tumor tissues can be synthesized by cross-linking various synthetic water-soluble polymers chemically or physically. These polymers include polyethylene glycol (PEG) [91–93], poly(vinyl alcohol) (PVA) [94], and polyacrylamide (PAAm) [95–97]. Various methods are employed to reconfigure synthetic hydrogels to provide a stable material for 3D cell culture. For instance, synthetic hydrogels can be conjugated with ECM protein-mimicking oligopeptides containing Arg-Gly-Asp (RGD), which is an integrin-binding domain of fibronectin [98]. Furthermore, hydrogels can cross-link with MMP, which is overexpressed in tumor tissue [99, 100]. Raza et al. used the PEG hydrogel tethered with RGD peptides and cross-linked with MMP-sensitive linkers (sequence: KCGPQG*IWGQCK, *protease cleavage site) or non-cleavable linker, dithiothreitol, MMPscrm (sequence: KCGPQGPAGQCK). This gel system was used to evaluate the effects of matrix composition and stiffness on growth and morphogenesis of pancreatic ductal epithelial cells (PDEC) (Fig. 4.A) [101]. Cells showed viability higher than 92% even after 10 days, regardless of cross-linkers. However, the gel formed with the MMP-sensitive linker led cells to form larger cell clusters and present higher metabolic activity than the gel formed with non-cleavable linker.
Fig. 4. Synthetic Matrix Models.
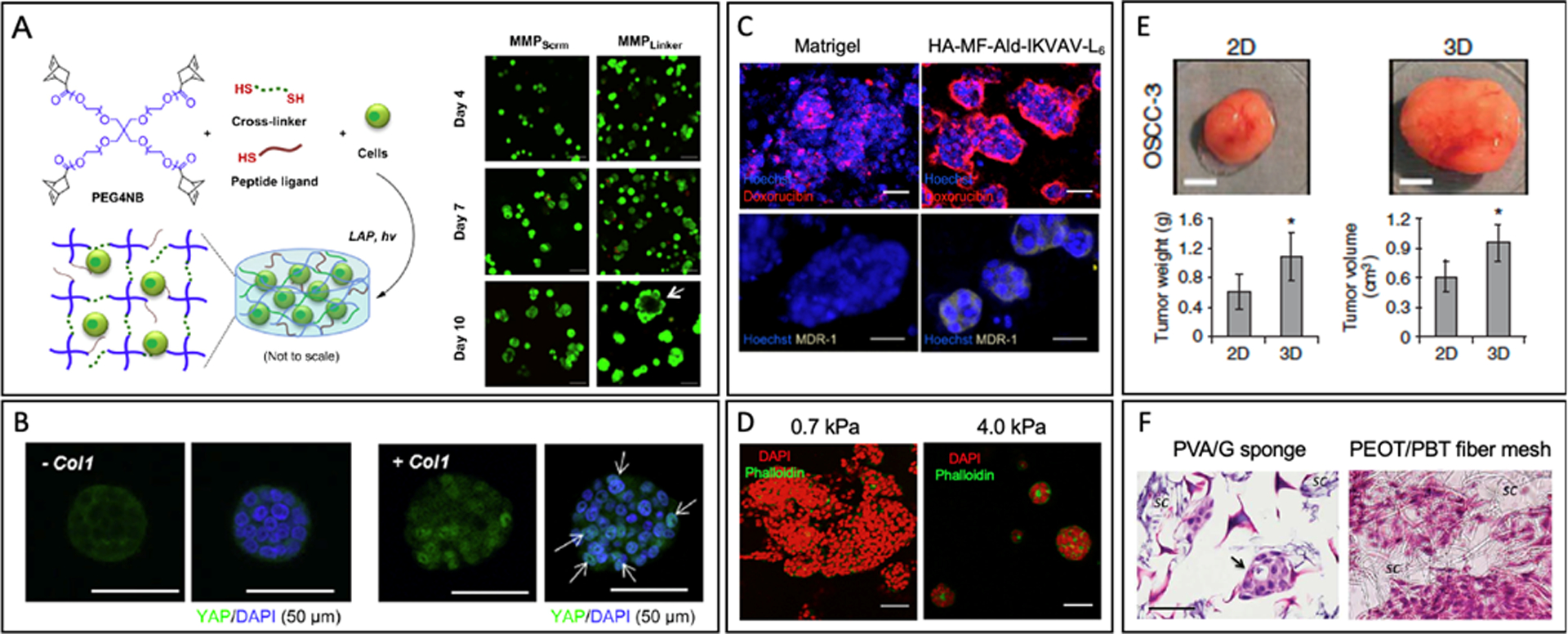
(A) Schematic of 4-arm PEG-norbornene (PEG4NG) undergoes thiol-ene photo-click reaction with multi-functional cross-linkers to form PANC-1 cell-laden PEG-based hydrogels and PANC-1 cell morphology in PEG4NB hydrogels cross-linked with MMP sensitive and non-cleavable linkers after Live/Dead staining. (Scale bar: 100 μm) [101]. (B) Immunofluorescence images of COLO-357 cells showed YAP translocation was more active in hydrogels formed with Col1 at day 10 (YAP: green, nuclei: blue). Arrows indicate co-localization of YAP and cell nuclei [102]. (C) Distribution of doxorubicin (red) in T47D breast cancer cells (nuclei, blue), and expression of multidrug resistance (MDR1) efflux pump (yellowish gray) cultured on Matrigel or HA-MF-Ald-IKVAV-L6 hydrogels. (Scale bar: 50 μm) [116]. (D) HepG2 cells spheroids formed within the pure collagen gels (E0 = 0.7 kPa) and within collagen-PEG gels (E0 = 4.0 kPa) (DAPI: red, phalloidin: green) (Scale bar: 50 μm) [117]. (E) OSCC-3 tumor growth in SCID mice after preculture in 3D PLG scaffolds or on 2D plate. (Scale bar: 50 μm) [123]. (F) Morphology of PDAC cells encapsulated in PVA/G sponge-like scaffold and in PEOT/PBT fiber mesh with hematoxylin and eosin (H&E) staining. (Scale bar: 50 μm) [125].
Another study used multi-arm PEG cross-linked by photopolymerization between norbornene- and thiol-terminated protease-sensitive peptides (sequence: KCGPLG*LYAGCK, *protease cleavage site). The gel physically entrapped type 1 collagen (Col1) during light-induced gelation to study the growth, morphogenesis, and drug resistance of PDAC cells [102]. Elastic modulus of the gel was approximately 2 kPa, which is similar to the stiffness of solid pancreatic tumor tissue. The gel with collagen directed cells to form larger clusters and undergo a more active nuclear translocation of Yes-associated protein (YAP) likely due to the inhibition of Hippo pathway (Fig. 4.B). After a 10-day culture, the stiffness of the gel containing collagen decreased significantly while the one without collagen still had the similar stiffness. PDAC cell clusters formed within the hydrogels had higher chemo-resistance to Gemcitabine than those cultured on a polystyrene tissue culture substrate. Additionally, other PEG-based hydrogels engineered with MMP-sensitive linkers and integrin-binding RGD peptides are used to study growth and phenotypic activities of cancer spheroids formed with ovarian [99], lung [100], and brain [103] cancer cell lines.
Oligopeptides with desired amino acid sequence can form hydrogels via intermolecular self-assembly. These self-assembled peptide gels can present versatile architectural, chemical and bioactive cues by substituting amino acids, tuning the peptide sequence, and adding specific integrin or receptor-binding ligands [104–108]. Peptides self-assemble to form nanostructure via hydrogen bond, π–π stacking, or electrostatic interaction to recapitulate the fibrous architecture of the ECM [109]. Some examples of oligopeptide gels used for 3D cancer tissue engineering are RADA16 hydrogel (COCH3-(RADA)4-CONH2) [110], EAK16 hydrogel (CONH3-(AEAEAKAK)2-CONH2) [111], H9e peptide hydrogel (FLIVIGSIIGPGGDGPGGD) [112], and EFK8 hydrogel (FEFEFKFK) [113]. RADA16-I peptides can self-assemble into an antiparallel β-sheet and form a fiber network with 10nm diameter and 50–200 nm pore diameter. The resulting gel was used to assess the effect of anti-cancer drug on PANC-1 co-cultured with human normal dermal fibroblasts (hNDF) [110]. The RADA16-I peptides contain arginine-alanine-aspartate-alanine (RADA) sequence that can bind with cells. PANC-1 cells grew with a doubling time of 3 days while hNDF showed no proliferation. PANC-1 cells migrated in the gel more actively when co-cultured with hNDF than mono-cultured. The efficacy of four anti-cancer drugs, including Gemcitabine, 5-Fluorouracil (5-FU), and 4-Methylumbelliferone (4-MU), was evaluated in the 3D co-culture system. Gemcitabine prevented PANC-1 proliferation most effectively but also had high cytotoxicity on fibroblasts. 4-MU had a faint effect on PANC-1 while it showed high toxicity on fibroblasts as well. Overall, the gel provided a microenvironment that mitigated the efficacy of drugs with PDAC cells co-cultured with fibroblasts. Likewise, another study used d-form EAK16 peptides that form a nanofirous scaffold [111]. Each fiber width varied from 10 to 500 nm. Liver cancer cells cultured in the d-EAK16 hydrogel remained viable and proliferated during 12-day culture. The gel facilitated sequestration of enzymes and growth factors. More interestingly, the d-EAK16 gel showed a higher resistance to proteases degradation than I-form. l-form EAK16 peptide gel degraded by natural enzymes including pronase, trypsin, and pepsin, while the d-form EAK16 gel remains stable in the enzymatic solution.
Hybrid hydrogels are formulated by combining natural polymers with synthetic polymers [114]. Natural polymers can act as bioactive epitopes that regulate cell adhesion and phenotype, while synthetic polymers can regulate gel softness. Adding acrylate, thiol, vinyl ether, and norbornene enables fine tuning of natural matrices via photopolymerization. For instance, norbornene-modified gelatin (GelNB) and thiolated HA (THA) were photopolymerized with Eosin-Y as initiator under visible light [115]. These materials have provided stable platform for pancreatic cancer cell culture. Moreover, its stiffness could be modified (shear modulus1,2, and 5 kPa) by varying PEG-tetra-thiol (PEG4SH) content to study the effects of stiffness on the pancreatic cancer cell line COLO-357 growth and spheroid formation. Cells grown in stiffer matrix expressed markers that suggests greater progression and metastasis. Hybrid hydrogels has also been utilized to enhance orthotopic implantation. Orthotopic models with pancreatic cancer cells encapsulated in thiolated HA or thiolated gelatin had greater growth and metastasis compared to injected cells. Another study modified HA to present aldehyde group and methyl furan group [116]. The modified HA can be cross-linked with bis(oxyamine)-PEG and further be conjugated with bioactive peptides GRKQAAS-IKVAV-SG4SRL6R2KG (Mal-IKVAVL6). The hydrogel was used to examine the effects of mechanical stiffness and biochemical properties on growth of breast cancer spheroids. The gel stiffness was controlled with the number of cross-links (resulting elastic modulus of 0.6 kPa) while biochemical properties were controlled by bioactive peptide structurer. The tumor spheroids cultured in the bifunctional HA hydrogel showed a lower doxorubicin susceptibility due to limited transport into the spheroid core and a higher expression of the multidrug resistance protein (MDR1) than those cultured within Matrigel (Fig. 4.C).
In addition, an effort was made to decouple or lower the dependency between matrix stiffness and permeability while keeping the number of cell adhesion sites constant. Physically associated collagen fibrils were chemically linked with PEG di-(succinic acid N-hydroxysuccinimidyl ester) (PEG-diNHS) [117]. By doing so, the individual collagen fiber can stiffen without altering the spacing between collagen fibers. With this approach, the elastic modulus of the collagen gel can be varied from 0.7 to 4.0 kPa, which simulates the softness of fat and normal liver tissue, respectively. The diffusion coefficient was kept within the order of 10−7 cm2/s, which was similar to that in the native ECM. The collagen-PEG hybrid gel was used to study the effects of matrix stiffness on the hepatocellular carcinoma phenotype. Decreasing the elastic modulus from 4.0 to 0.7 kPa led the cancer cells to form a larger cell mass (Fig. 4.D) with reduced detoxification activities of hepatocytes and increased secretion activities of proangiogenic vascular endothelial growth factors.
Polyurethane (PU) is a promising polymeric material for various tissue engineering applications and medical implant applications due to it stability, biocompatibility, and highly tunable mechanical and physical properties. However, its application in pancreatic cancer is not yet explored to a great extent. Recently, one study developed PU scaffold culture of pancreatic cancer cell lines AsPC-1, PANC-1 and BxPC-3 [118]. Surface modification with fibronectin greatly enhanced cell adhesion and proliferation. Moreover, the culture was successfully maintained for 29 days and recapitulated in vivo stress gradients and collagen-I production. Overall, the study demonstrated a novel hybrid platform with features that represents the pancreatic cancer microenvironment. Additionally, there are other novel hybrid materials utilizing natural polymers such as Chitosan and poly(γ-glutamic acid) (γ-PGA) that offer diverse scaffolds for pancreatic cancer [119].
Microporous polymeric scaffolds are widely utilized to study cancer cell growth due to their highly interconnected structure and low degradation rate [90]. Polymeric scaffolds are fabricated with polylactide (PLA) [120, 121], polystyrene [122], poly(lactide-co-glycolide) (PLG) [123], and poly(lactic-co-glycolic) acid (PLGA) [124]. Fischbach et al. used PLG scaffolds with pore diameter around 200 μm to study the behavior of oral squamous cell carcinoma cells and lung carcinoma in a 3D environment [123]. After 3 weeks of culture in the PLG scaffold, oral squamous cell carcinoma cells (OSCC-3) formed a larger tumor mass and produced more angiogenic factors than cells cultured on 2D plates (Fig. 4.E). Also, cells retrieved from 3D system had a higher invasive potential with elevated epithelial and mesenchymal markers. Moreover, they were less responsive to anti-cancer drug, the PI3-kinase inhibitor LY294002, than cells cultured on 2D plates. Ricci et al. examined PDAC culture in scaffolds synthesized by poly(vinyl alcohol)/gelatin (PVA/G) mixture and poly(ethylene oxide terephthalate)/poly(butylene terephthalate) (PEOT/PBT) copolymer [125]. These scaffolds were designed into either sponge-like structures or nanofiber meshes. PDAC cultured in the sponge-like scaffolds were able to form clusters with morphology similar to that of primary cancer (Fig 4.F). Also, cells cultured in the sponge-like structure excreted higher tumor-specific MMPs than those in fiber meshes. Overall, this study suggested that sponge-like structure scaffolds are more advantageous to recreating an in vivo-like aggressive pancreatic tumor models.
4. Environmental engineering
Previous sections have elucidated the complex pancreatic cancer TME that consists of heterogeneous stromal cells as well as diverse matrix protein compositions that not only provide structural support for also chemical and mechanical cues that are essential for various cell behaviors. It is not only important to understand the consequences of individual factors in the TME, but also to engineer environments that mechanically and biofunctionally recreate the TME to represent a realistic microenvironment for discovering novel therapeutic targets and high-throughput drug screening in vitro. This section introduces recent studies that recapitulates the 3D microanatomy, cellular interactions, and mechanical conditions for tumor modeling.
4.1. 3D microenvironment with microanatomy
The ability to engineer carefully selected cell lines and ECM into the microanatomy of pancreatic cancer tissue adds a unique dimension to traditional in vitro cell modeling. Advancements in tissue engineering have enabled representations of various spatiotemporal natures of the TME, allowing more relevant conditions for high-throughput drug screening. Multiple studies have developed compartmentalized models with spatially distributed ECM and cell types, most commonly PDACs and PSCs [126, 127]. These models are able to examine the fiber arrangements surrounding PDAC tumors and cellular migration between different layers, which can elucidate important stroma-pancreatic cancer interactions.
Emergence of microfluidic platforms, known as “tumor-on-chip” or “tumor-microenvironment-on-chip”, has opened up new methods 3D microenvironment engineering. Specifically, most cancers have epithelial origin, where cancer cells grow from epithelial ducts. Thus, the capability to reconstruct the microanatomy of difference stages of disease is crucial. Moreover, the ability to recapitulate the anatomy possesses valuable advantage of being able to simulate in vivo cell-ECM mechanical cues. So far, only a handful number of microfluidic platforms that mimic the pancreatic acinar to ductal progression is available. A ductal tumor-microenvironment-on-chip (dT-MOC) is recently reported in which the ductal epithelium surrounded by collagen solution formed through viscous fingering technique, as seen in Fig. 5.A [128]. The structure allows invasion studies of both mesenchymal-like and epithelial-like murine pancreatic cancer cells, thereby mimicking the heterogeneous PDAC TME.
Fig. 5. Environmental Engineering Models.
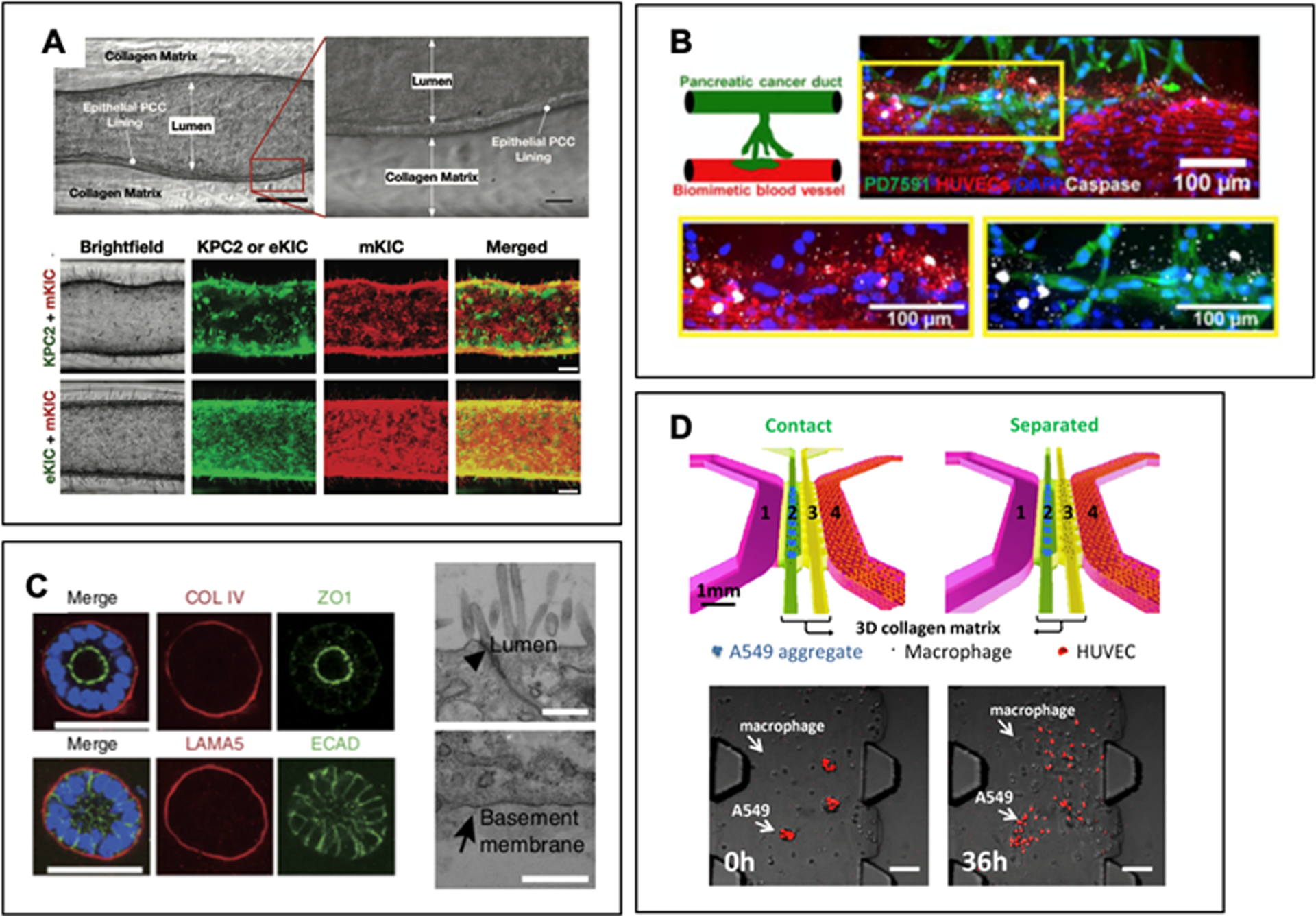
A. Ductal tumor-microenvironment-on-chip (dT-MOC) mimicking PDAC microanatomy. Invasion assays of various PDAC cell lines can reveal cell-stroma interactions. Adapted from Reference [128]. B. Cancer-on-chip model mimicking primary mouse PDAC cell intravasation into blood vessel. PDAC cells wrap around the blood vessel and subsequently invade. Apoptosis (stained white) was observed among endothelial cells (stained red) invaded with PDAC cells. Adapted from Reference [134] with permission from AAAS. C. Patient-derived organoid model of human pancreatic stellate cells (PSCs). The basal matrix proteins secreted by PSCs as well as tight junctions (arrowhead) and apical micro-villi are observed in the organoids. Adapted from Reference [52] with permission from Springer Nature. D. Cell-cell interaction in microfluidic model. Human lung adenocarcinoma aggregates are disseminated when cultured in contact with M2a macrophages. Adapted from Reference [152].
Engineering microvasculature of PDAC has been overlooked, a consequence of the misconception that PDAC has few blood vessels based on its hypovascular nature. Contrary to belief, pancreas is a highly vascularized organ and PDAC specific conditions including hypoxia, high interstitial pressure, and leaky blood vessels underline the significance of incorporating microvasculature into PDAC models [129, 130]. During metastasis, tumor cells and the endothelium interact during migration and invasion within TME into the vasculature to extravasation and colonization of the secondary organs. Integrin, growth factor, and other cellular transduction pathways play distinct roles along the individual processes [131]. For breast cancer models, microfluidic vascular structure has been developed to analyze transendothelial migration of cancer cells as well as immune cells [132, 133]. For pancreatic cancer, the number of studies for such models is significantly limited. A cancer-on-chip model, shown in Fig. 5.B, is developed with both pancreatic cancer duct and blood vessel structures [134]. In response to various chemotactic agents, PDAC cells are able to invade into the collagen matrix forming branched structures, wrap around the blood vessel, and subsequently invade into the vessel. Upon knockout of ALK7, a tumor-suppressive receptor, the invasive property of PDAC cells was diminished. Reproducing in vivo drug treatment results was also reported through co-culture of PDAC, human microvascular endothelial cells, and PSCs [129]. The endothelial cells were seeded above a synthetic membrane and physiologically relevant shear stress was applied to mimic blood flow induced endothelial cell morphology. Despite previously discussed models, mechanisms of pancreatic cancer cell invasion, angiogenesis, and interaction with the endothelium remains a critical knowledge gap.
4.2. Cell-cell and cell-matrix interactions
One of the fundamental elements in studying the PDAC heterogeneity is capturing the interaction between different cell types and matrix components. Organoids are emerged as the simplest model of mimicking multicellularity of solid tumors regardless of the cancer type [2]. Organoids can be synthesized via self-organization of primary cells or differentiated stem cells. Thus, the starting cell type and physical environment that allows for endogenous and/or exogenous signals leading to self-organization is crucial [135]. Organoid models are beginning to be broadly adopted including studying tumor cell growth, angiogenesis, metastasis, and drug screening. They can also be used to investigate tumor carcinogenesis by establishing patient-derived or stem cell-derived organoids from different stages of pancreatic cancer followed by orthotopic transplantation [136, 137]. Alternatively, novel gene-editing technologies such as CRISPR-Cas9 can be combined to induce specific mutations of the molecular pathway to normal cells in pancreatic [138] and colorectal [139] cells. Although this method is still in its early development, it offers valuable insights regarding applications of different technologies in studying organoids. Patient-derived organoids that conserve patient-specific traits also allow an effective drug screening method for personalized treatment as seen in Fig. 5.C [52, 140].
In addition to tumor-tumor cell relationship, multicell type organoids can be generated [2]. For example, co-culture of PDAC cells and PSCs in organoids is a useful tool in understanding tumor-stroma interaction while maintaining a solid tumor aggregate structure [141]. Moreover, with increasing attention on immunotherapy for cancer [142], immune cell infiltration studies in organoids are performed to reveal tumor-immune cell relationship [32]. Organoids can be further utilized to study cell-matrix interactions by culturing in different natural and synthetic hydrogels [143]. Studying cell-matrix interaction in pancreatic cancer is crucial especially due to the dense stroma and the resulting hypoxic microenvironment. Hypoxia alters molecular activities in the ECM by activating hypoxia inducible factor-1 (HIF-1) transcription factor that in turns recruits macrophages by promoting CCL2 secretion [144]. Macrophages can activate PSCs which in response accumulates more ECM proteins. Thus, hypoxia promotes immunosuppressive environment through various mechanisms involving fibroblasts and signaling pathways. For example, PSCs secrete cytokines such as IL-6 and M-CSF under hypoxia which recruits T cells and prevent activation of effector T cells [145]. Despite some studies being able to develop multi cell type organoids that is able to express inflammatory and hypoxic markers [146], more efforts are necessary to recapitulate the gradient of hypoxia in pancreatic cancer within the organoid models.
Organoid-on-a-chip integrates the benefits of organoid and microfluidic platforms. Recently, 3D vascular network representation with organoid culture in an organ-on-a-chip model allows investigation of various cell types while mimicking the PDAC microanatomy [147]. Moreover, co-culture with PDAC spheroids and stromal cells in microfluidic device offer clinically relevant results regarding EMT and drug resistance [148]. However, PDAC organoid-on-a-chip models are highly underdeveloped. Other microfluidic models, not limited to pancreatic cancer, investigate tumor-endothelial and tumor-immune cell interactions [3, 149–151]. Devices with immune cell applications are noticeably rare, with one example shown in Fig. 5.D demonstrating the interaction between lung cancer cells and macrophages [152].
4.3. Mechanical conditions: fluid shear stress, hydraulic pressure, solid stress
Desmoplastic stroma including fibroblasts and matrix proteins, fluid flow, and elevated interstitial pressure act in concert to exert various mechanical forces on pancreatic cancer. These conditions have been associated with promoting tumor migration and metastasis as well as decreased drug distribution to the tumor population [153]. For example, deposition of matrix proteins has been directly correlated with total tissue pressure, limiting drug delivery [154]. 2D cell monolayer models in cell culture disk or microfluidic device with shear stress created by shakers or fluid flow offer simple platforms for studying the effect of stress in cancer and endothelial cells [155, 156]. Especially for endothelial cells, various shear patterns have induced differential cell morphology, orientation, and gene expression [129]. In addition, deformability of cancer cells is an important factor in cancer metastasis to migrate through the dense stroma and convoluted vascular structure. In pancreatic cancer, transwell essays and solid stress application with piston with adjustable weight or AFM resulted stiffer human cell lines having more invasive behavior [157, 158]. These studies allow identification of potential mechanoregulating integrins or proteins, such as laminA [157]. Interestingly, neuropsychological stress has been shown to promote tumorigenesis in KPC mice [159]. Future in vitro models incorporating nerve networks and pancreatic cancer could elucidate compelling relationships.
Increased ECM stiffness in tumor compared to normal tissue can have a significant influence on cell phenotype, growth, and metastatic potential. Stiffness alters cancer progression through cell-ECM and cell-cell interactions which can also modify the surrounding TME to favor tumor growth [160]. In one study, the shear moduli of synthetic thiol-ene hydrogel PEG4NB crosslinked with dithiolthreitol (DTT) or MMP sensitive linkers was controlled between 6 kPa and 3 kPa [161]. PANC-1 cells cultured in softer gel formed larger clusters and higher metabolic activity compared to stiffer gels. Moreover, cells in the stiffer gel retained mesenchymal phenotype whereas in the softer gel cells exhibited ductal phenotype. Another study investigated PSC behavior in a different synthetic hydrogel model with PAAm gel with varying stiffness (1 kPa and 25 kPa) [162]. PSCs grown in both softer matrix and Matrigel maintained resting-like state whereas those in stiffer matrix had two-fold increase in mRNA levels of αSMA and vimentin, indicating activated state. Moreover, PSCs favored durotactic migration towards stiffer matrix. In future models, co-culture studies with cancer and stromal cells could greatly enhance our understanding on cell-cell interactions in varying stiffness. Additionally, models that can recapitulate progressive stiffness change could more closely mimic the change in protein composition over the course of the disease, which could elucidate novel therapeutic targets for different stages pancreatic cancer.
Advanced 3D models are able to recapitulate more intricate interstitial fluid pressure, cell migration behaviors and drug response under various mechanical conditions. Application of native matrices can model gel fluid and free mobile fluid present in the ECM, such as HA swelling, which consequently elevate tissue pressure and inhibit drug delivery [163]. Fig. 6.A shows a T-MOC platform that can mimic various transport processes [164]. This device is an assemblage of two PDMS layers with endothelial cell aligned porous membrane in between, simulating the drug delivery through the top capillary channel and the bottom tumor interstitium and drainage through the lymphatic vessels. The cancer cells are cultured in 3D collagen matrix and may be co-cultured with other stromal cells to depict PDAC heterogeneity. The combination of previous factors captures the elevated interstitial fluid flow and realistic obstacles to drug delivery in the TME. The T-MOC device has been adapted to study the nanoparticle delivered doxorubicin, which showed a physiologically relevant accumulation and penetration of drug in the interstitial channel, as shown in Fig. 6.B [165]. Microfluidic technology is a versatile model that grants highly controllable variables including biotransport and mechanical conditions for systemic study of diverse cancer phenomena.
Fig. 6. Microfluidic platform recapitulating mechanical condition.
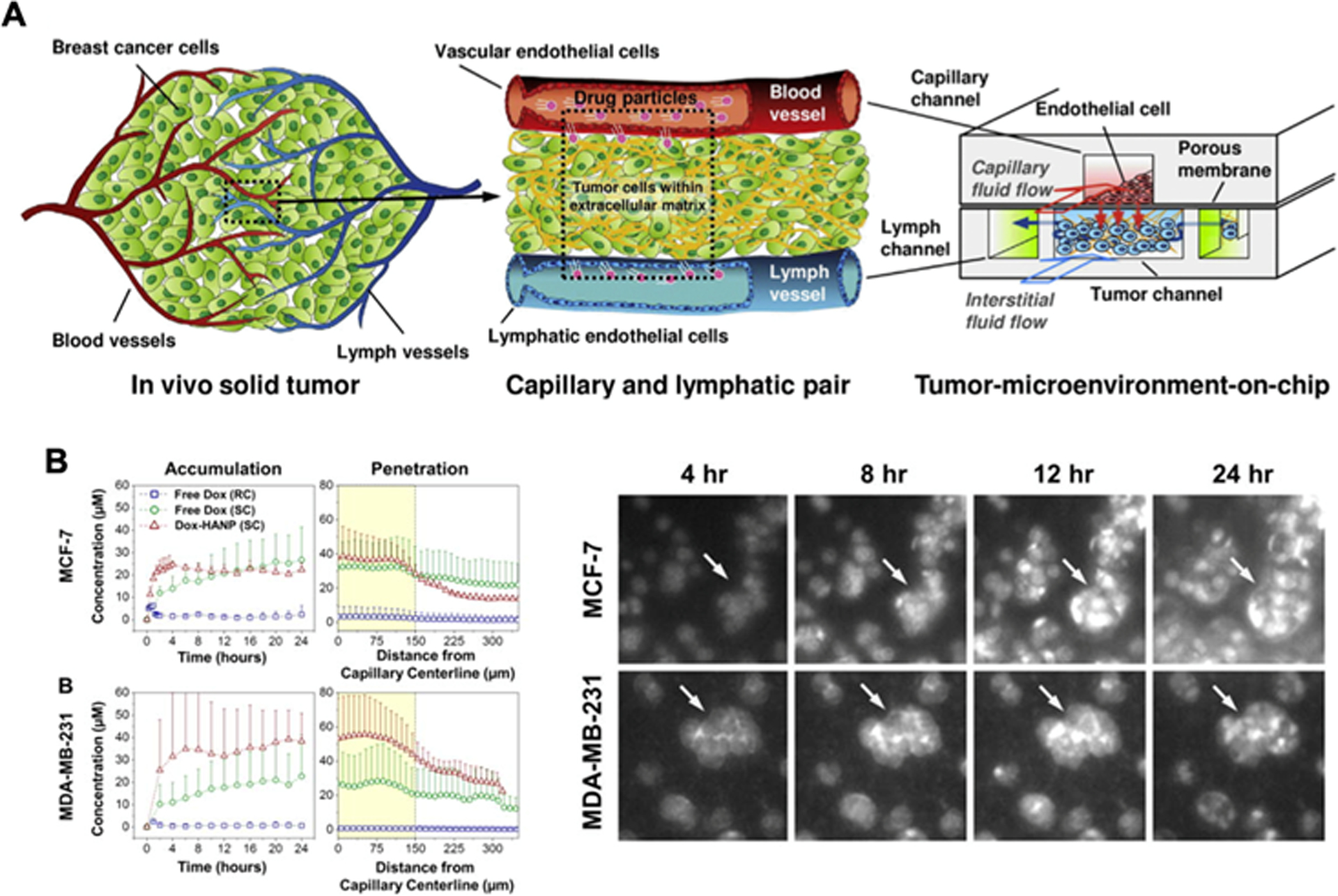
A. Tumor-microenvironment-on-chip (TMOC) platform to mimic elevated interstitial pressure during drug transport from the capillary to the interstitium. Adapted from Reference [164]. B. Drug accumulation and penetration of Doxorubicin in TMOC for breast cancer cells. Fluorescent images show drug uptake by cancer cells. Adapted from Reference [165] with permission from Elsevier.
5. Challenges and opportunities
Engineered tumor models have demonstrated the feasibility of creating the 3D cell culture environment, which is getting closer to the tumor microenvironment in vivo than conventional cell culture platforms. As noted in Fig. 7., the number of publications regarding 3D tumor models has been rapidly increasing and is anticipated to increase. However, as the initial development phase has matured, the limitations of these engineered models has become better appreciated. Numerous challenges of current engineering tumor models must be met to open up opportunities to advance to next-generation engineered tumor models. Although many engineered tumor models reconstitute the key components of tumor tissues, it is still significantly limited in recapitulating the full spectrum of the pathophysiology of the TME. Next-generation tumor models should provide recapitulation of molecular signaling pathways in tumorigenesis and drug resistance, which is essential to identify promising molecular targets and test innovative compounds capable of effectively inhibiting these targets.
Fig. 7. Trend in 3D modeling. Trend in 3D modeling.
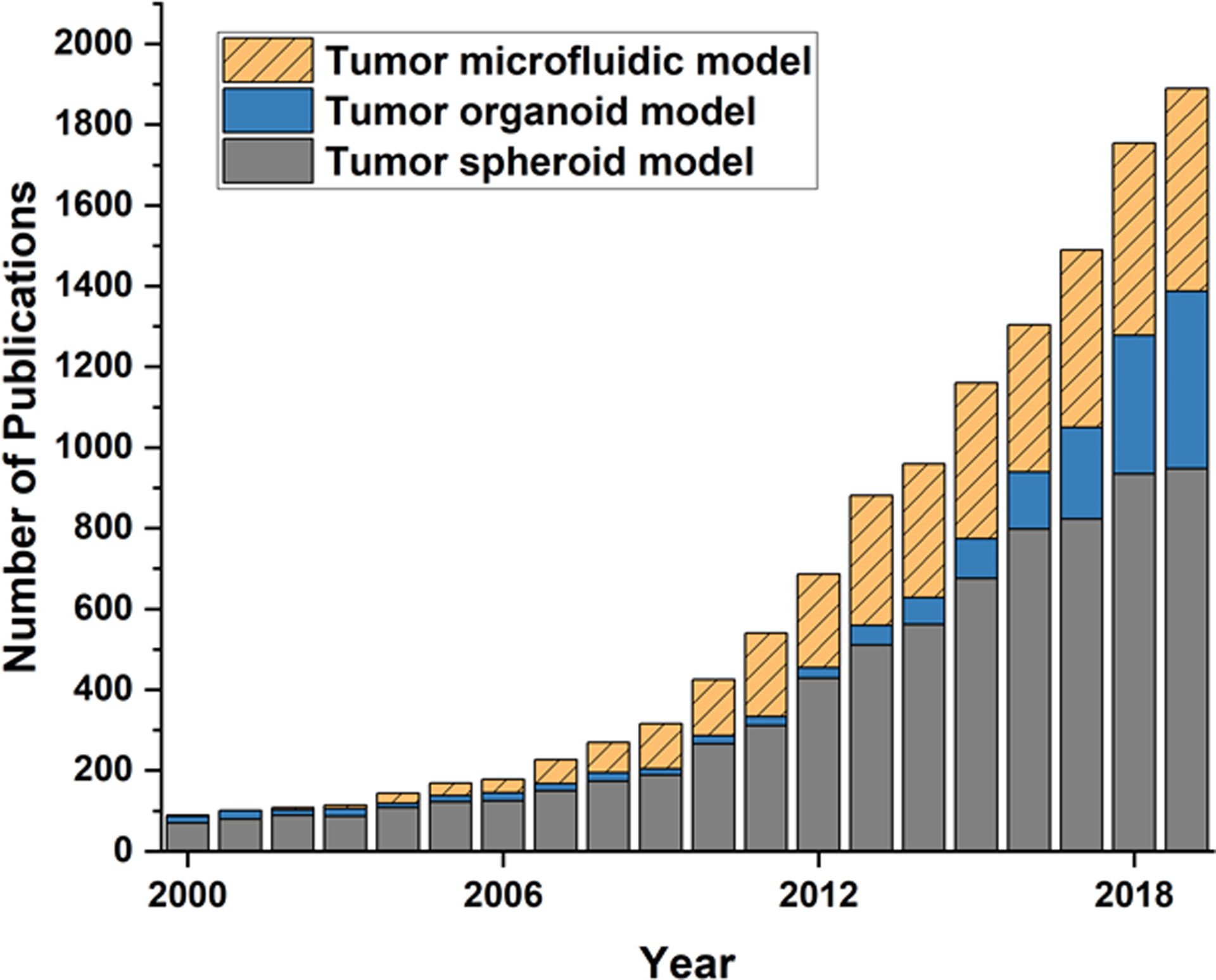
Scientific publication trend for 3D tumor models in the years between 2000 and 2019. Source: Pubmed search of original research publications with keywords “tumor spheroid model”, “tumor organoid model”, “tumor microfluidic model”.
5.1. Mimicry of pathophysiology: Coagulation and fibrosis
The complexity of recapitulating the full-spectrum of tumor cell- stroma interactions in a 3D model system is further complicated by the number of biological systems (e.g., immune, hemostatic, endocrine) that are activated in the PDAC TME. For example, PDAC is significantly associated with venous thromboembolism (VTE), has the highest rate of cancer-associated thrombosis compared to other cancer types [166, 167], and VTE is highly correlated with disease aggressiveness [168, 169]. Moreover, recent studies have shown a reciprocal relationship whereby local activation of the coagulation and fibrinolytic systems within the TME is a significant feature of the pathophysiology of PDAC that has consequences on disease progression and severity. In PDAC, tumor cell derived tissue factor (TF) expression is the major source of the activated coagulation cascade. To make engineering PDAC TME more difficult, stromal TF expression by monocytes and macrophages also contributes to the activation of coagulation cascade. However, studies have shown that the tumor cell is a dominant source of TF in promoting activation of the cascade, blood clot formation and ultimately PDAC pathogenesis [170, 171]. Tumor cells express TF and release extracellular vesicles or microparticles (MPs) of 0.1–1um in diameter size, which has been shown to contribute to the hypercoagulable state observed in cancer patients [170, 172]. In PDAC, increased MP TF activity has been shown to be significantly correlated with cancer stage, VTE complications, and diminished survival of PDAC patients [173–175].
TF exposure and resulting activity culminates with the conversion of the zymogen serine protease prothrombin to the active protease thrombin. Thrombin is the central hemostatic protease with over a dozen downstream targets. Most notably, thrombin cleaves soluble fibrinogen and activates the coagulation transglutaminase Factor XIII. These components produce the stable fibrin clot either within vessels or within extracellular milieu such as the TME. Fibrin microstructure properties are dependent on thrombin concentration [176]. Although fibrin is a widely used scaffold in tissue engineering and culturing iPSCs [177, 178], its application in 3D culture of pancreatic tumor cells is still lacking. Fibrin matrices can influence tumor growth and metastasis through a number of mechanisms. With tumor vasculature, occlusive fibrin thrombi can result in local areas of ischemia and hypoxia that drive reactive within and around a growing tumor. Given that the vasculature of forming tumors is disrupted and becomes “leaky”, extravascular fibrin deposits are a common pathological feature of the TME. Fibrin in the extravascular space can support tumor growth through a number of possible mechanisms, including by serving as a scaffold for cell culture [179]. Additionally, a variety of cell types specifically bind to and migrate on fibrin(ogen) matrices, including, neutrophils, macrophages, fibroblasts, endothelial cells and other cells [180–182]. Direct binding to fibrin(ogen) through both integrin (e.g., αMβ2, αvβ3, α1β5) and non-integrin receptors (e.g., ICAM-1, cadherins) mediates these cell-fibrin(ogen) interactions. In so doing, fibrin(ogen) provides an important spatially-defined signal to initiate specialized cellular functions. In particular for tumor biology and specifically PDAC, the ability of fibrin to engage αMβ2 on macrophages to stimulate macrophage differentiation, polarization, and activation has wide ranging implications given the importance of tumor-associated macrophages in driving cancer progression [181].
A second major class of downstream thrombin effectors are protease-activated receptors (PARs). Thrombin activates PAR-1,−3, −4 that are expressed on a variety of cell types relevant to PDAC tumor biology, including endothelial cells, fibroblasts, immune cells, and tumor cells themselves [183, 184]. Thrombin initiates cell signaling by PARs through cleaving an N-terminal encrypted ligand. Following cleavage, the new N-terminal peptide engages the receptor to activate cell signaling [185]. For PDAC, recent studies demonstrated that TF-thrombin-PAR1 signaling in PDAC tumor cells is a significant driver of tumor growth [42]. Intriguingly, the impact of this signaling axis was by suppressing anti-tumor immunity in the in the TME. Separate studies indicated that PAR1 signaling by stromal cells supported tumor growth and resistance to chemotherapy [186]. Use of a 3D model system represents a significant opportunity for being able to dissect individual contributions of PAR signaling in various cell types to better define the cell-type specific roles of these pathways in tumor biology.
On the opposite side of clot formation is the process of fibrinolysis, which is the dissolution of fibrin clots and matrices by the protease plasmin. Generation of plasmin from the zymogen plasminogen is mediated by one of two plasminogen activators, tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA). For fibrin clots, tPA is the primary plasminogen activator. On cell surfaces, uPA bound to its receptor uPAR mediates plasminogen activation. The activity of tPA and uPA is inhibited by plasminogen activator inhibitors-1 (PAI-1). Following activation, plasmin can degrade fibrin resulting in the release of soluble fibrin degradation products, degrade other ECM proteins, activate MMPs, or activate latent growth factors [187–190]. Notably, uPA/uPAR can also exert plasminogen-independent effects on cellular activities [189]. uPA and uPAR are overexpressed by PDAC cells as well as certain stromal cell types (e.g., cancer-associated fibroblasts) and suppression of uPA or uPAR decreases cancer cell invasion, angiogenesis and tumor development [191–193]. Tumor cell derived PAI-1 has been shown to promote cancer invasion and elevated levels of PAI-1 are potential risk factors for colorectal and breast cancer [194–196]. In PDAC, the results of PAI-1 expression are quite variable. Some studies suggesting it is downregulated and overexpression of PAI-1 in PDAC cell lines inhibited cell invasion, limiting liver metastasis [197, 198]. However, others suggest it is upregulated and functions to activate pancreatic stellate cells to promote PDAC fibrosis and progression [199, 200]. To resolve these conflicts, systematic studies on different cell lines or patients with distinct oncogenic alternations are needed to tease out the correlation between gene mutation, PAI-1 expression and its contribution to PDAC prognosis. Taken together, determining the contribution of the plasminogen activation system can be complex with multiple cellular sources of factors and alternative pathways. Better defining the contribution of uPA, uPAR, plasminogen, PAI-1, and other fibrinolytic system components remains a significant knowledge gap in PDAC pathogenies.
5.2. Immune cells and subpopulation-specific models
Another major challenge is associated with recreating immunosuppressive and inflammatory aspects of the TME [6]. As immunotherapy of cancers emerge, reliable testbed to postulate and test novel hypothesis to improve anti-tumor immunity at TME is highly demanded [142]. However, recapitulating immunosuppressive TME is extremely challenging [3]. This is because the immunosuppressive condition requires delicate balance of immune cell populations in the model. Several attempts to incorporate immune cells have been reported [201], but it is still early stage for broader use. Nevertheless, given the clear contribution of the immune system to tumor progression and the emphasis on developing immune targeted therapies for cancer treatment, incorporating immune components to next generation 3D tumor models should be a point emphasis for development.
The limited availability of cancer cell lines poses another major challenge. Most studies in this area still mainly use established cell lines at public cell repositories, such as ATCC. Although these cell lines are relatively well-characterized, most of them have been cultured in vitro for an extended period of time and may have lost the original molecular and genetic characteristics [22, 26, 27]. Patient-derived cells and organoids can be an alternative, but the characterization of these cells’ molecular and genetic features typically needs additional effort. Moreover, the availability is significantly limited. As co-culture of stromal fibroblasts, endothelial and immune cells have tried, challenge associated with limited cell source become worse [27]. In addition to the cellular components, the composition and concentration of ECM proteins are largely unknown. Although the overexpression of certain proteins at the TME, including type I collagen, hyaluronan, fibronectin, and fibrin, has been studied extensively, quantitative information remains rare. Limitations of available cells and matrix materials make it difficult to create organ-specific and subpopulation-specific tumor models. As discussed previously, the composition of tumor ECM is anticipated to vary significantly depending on the organ and progression of tumors. For example, liver cancers can be primary tumors, but a large percentage of tumors may also arise from metastasis [28, 202]. It is still little known whether the matrix composition of primary and metastatic tumors are the same or different. Moreover, the effects of age, sex, and race on tumor model development are mostly unanswered. Temporal aspects of tumor progression remain another gap since current models are mostly focused on the late stage of tumors.
All these challenges are associated with advancing engineered tumor models toward more reliable and truly recapitulating the TME. Research efforts to address these challenges will lead to next-generation engineered tumor models. These models will ultimately be used to identify novel targets, and discover and test new and effective drugs.
Acknowledgement
This work is partially supported by grants from National Institutes of Health (U01 HL143403, UL1 TR002529 to BH, and R01 CA211098, U01 HL143403, R01 DK112778 to MJF), National Science Foundation (STC-EBICS, CBET-0939511, and CBET-1932192 to HJK), a Challenge Award from the Purdue Center for Cancer Research (P30 CA023168), and the Walther Embedding Program in Physical Sciences in Oncology. SC is supported by Laura Winkelman Davidson Fellowship.
References
- [1].Han B, Qu C, Park K, Konieczny SF, Korc M, Recapitulation of complex transport and action of drugs at the tumor microenvironment using tumor-microenvironment-on-chip, Cancer Lett 380(1) (2016) 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan H, Demirci U, Chen P, Emerging organoid models: leaping forward in cancer research, J Hematol Oncol 12(1) (2019) 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boussommier-Calleja A, Li R, Chen MB, Wong SC, Kamm RD, Microfluidics: A new tool for modeling cancer-immune interactions, Trends Cancer 2(1) (2016) 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH, Tissue matrix arrays for high-throughput screening and systems analysis of cell function, Nat Methods 12(12) (2015) 1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tian C, Clauser KR, Ohlund D, Rickelt S, Huang Y, Gupta M, Mani DR, Carr SA, Tuveson DA, Hynes RO, Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells, Proc Natl Acad Sci U S A 116(39) (2019) 19609–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, Schnurr M, Lauber K, Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches, Radiat Oncol 14(1) (2019) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J Clin 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [8].Ottenhof NA, de Wilde RF, Maitra A, Hruban RH, Offerhaus GJ, Molecular characteristics of pancreatic ductal adenocarcinoma, Patholog Res Int 2011 (2011) 620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, DePinho RA, Genetics and biology of pancreatic ductal adenocarcinoma, Genes Dev 30(4) (2016) 355–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grutzmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, I. Australian Pancreatic Cancer Genome, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM, Genomic analyses identify molecular subtypes of pancreatic cancer, Nature 531(7592) (2016) 47–52. [DOI] [PubMed] [Google Scholar]
- [11].Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, Eichmann MD, Worni M, Gloor B, Perren A, Karamitopoulou E, Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance, Clin Cancer Res 24(18) (2018) 4444–4454. [DOI] [PubMed] [Google Scholar]
- [12].Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, Ban Y, Lavania S, Dawra R, Banerjee S, Vickers S, Merchant NB, Chen SX, Gilboa E, Ramakrishnan S, Saluja A, Dudeja V, NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12, Gastroenterology 155(3) (2018) 880–891 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gorchs L, Fernandez Moro C, Bankhead P, Kern KP, Sadeak I, Meng Q, Rangelova E, Kaipe H, Human Pancreatic Carcinoma-Associated Fibroblasts Promote Expression of Co-inhibitory Markers on CD4(+) and CD8(+) T-Cells, Front Immunol 10 (2019) 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qu C, Wang Q, Meng Z, Wang P, Cancer-Associated Fibroblasts in Pancreatic Cancer: Should They Be Deleted or Reeducated?, Integr Cancer Ther 17(4) (2018) 1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R, Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival, Cancer Cell 25(6) (2014) 719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ, Phenotype and genotype of pancreatic cancer cell lines, Pancreas 39(4) (2010) 425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kong K, Guo M, Liu Y, Zheng J, Progress in Animal Models of Pancreatic Ductal Adenocarcinoma, J Cancer 11(6) (2020) 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McIntyre LJ, Kim YS, Effects of sodium butyrate and dimethylsulfoxide on human pancreatic tumor cell lines, Eur J Cancer Clin Oncol 20(2) (1984) 265–71. [DOI] [PubMed] [Google Scholar]
- [19].Arao S, Masumoto A, Otsuki M, Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells, Pancreas 20(2) (2000) 129–37. [DOI] [PubMed] [Google Scholar]
- [20].Sawai H, Yamamoto M, Okada Y, Sato M, Akamo Y, Takeyama H, Manabe T, Alteration of integrins by interleukin-1alpha in human pancreatic cancer cells, Pancreas 23(4) (2001) 399–405. [DOI] [PubMed] [Google Scholar]
- [21].Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J Exp Med 214(3) (2017) 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krempley BD, Yu KH, Preclinical models of pancreatic ductal adenocarcinoma, Chin Clin Oncol 6(3) (2017) 25. [DOI] [PubMed] [Google Scholar]
- [23].Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, Huang T, Chan MW, Marini FC, Rosol TJ, Bonafe M, Hall BM, Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner, Cancer Res 68(21) (2008) 9087–95. [DOI] [PubMed] [Google Scholar]
- [24].Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC, In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform, Front Bioeng Biotechnol 4 (2016) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jesnowski R, Furst D, Ringel J, Chen Y, Schrodel A, Kleeff J, Kolb A, Schareck WD, Lohr M, Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine, Lab Invest 85(10) (2005) 1276–91. [DOI] [PubMed] [Google Scholar]
- [26].Suklabaidya S, Dash P, Das B, Suresh V, Sasmal PK, Senapati S, Experimental models of pancreatic cancer desmoplasia, Lab Invest 98(1) (2018) 27–40. [DOI] [PubMed] [Google Scholar]
- [27].Lenggenhager D, Amrutkar M, Santha P, Aasrum M, Lohr JM, Gladhaug IP, Verbeke CS, Commonly Used Pancreatic Stellate Cell Cultures Differ Phenotypically and in Their Interactions with Pancreatic Cancer Cells, Cells 8(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choi SI, Jeon AR, Kim MK, Lee YS, Im JE, Koh JW, Han SS, Kong SY, Yoon KA, Koh YH, Lee JH, Lee WJ, Park SJ, Hong EK, Woo SM, Kim YH, Development of Patient-Derived Preclinical Platform for Metastatic Pancreatic Cancer: PDOX and a Subsequent Organoid Model System Using Percutaneous Biopsy Samples, Front Oncol 9 (2019) 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ehrenberg KR, Gao J, Oppel F, Frank S, Kang N, Dieter SM, Herbst F, Mohrmann L, Dubash TD, Schulz ER, Strakerjahn H, Giessler KM, Weber S, Oberlack A, Rief EM, Strobel O, Bergmann F, Lasitschka F, Weitz J, Glimm H, Ball CR, Systematic Generation of Patient-Derived Tumor Models in Pancreatic Cancer, Cells 8(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kamili A, Gifford AJ, Li N, Mayoh C, Chow SO, Failes TW, Eden GL, Cadiz R, Xie J, Lukeis RE, Norris MD, Haber M, McCowage GB, Arndt GM, Trahair TN, Fletcher JI, Accelerating development of high-risk neuroblastoma patient-derived xenograft models for preclinical testing and personalised therapy, Br J Cancer 122(5) (2020) 680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kodack DP, Farago AF, Dastur A, Held MA, Dardaei L, Friboulet L, von Flotow F, Damon LJ, Lee D, Parks M, Dicecca R, Greenberg M, Kattermann KE, Riley AK, Fintelmann FJ, Rizzo C, Piotrowska Z, Shaw AT, Gainor JF, Sequist LV, Niederst MJ, Engelman JA, Benes CH, Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care, Cell Rep 21(11) (2017) 3298–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsai S, McOlash L, Palen K, Johnson B, Duris C, Yang Q, Dwinell MB, Hunt B, Evans DB, Gershan J, James MA, Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models, BMC Cancer 18(1) (2018) 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA, Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse, Cancer Cell 4(6) (2003) 437–50. [DOI] [PubMed] [Google Scholar]
- [34].Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA, Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice, Cancer Cell 7(5) (2005) 469–83. [DOI] [PubMed] [Google Scholar]
- [35].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH, CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans, Science 331(6024) (2011) 1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, Hingorani SR, T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma, Cancer Cell 28(5) (2015) 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR, Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma, Cancer Cell 21(3) (2012) 418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gopinathan A, Morton JP, Jodrell DI, Sansom OJ, GEMMs as preclinical models for testing pancreatic cancer therapies, Dis Model Mech 8(10) (2015) 1185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH, Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer, Cancer Cell 21(6) (2012) 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH, Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages, Gastroenterology 149(1) (2015) 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH, Dynamics of the immune reaction to pancreatic cancer from inception to invasion, Cancer Res 67(19) (2007) 9518–27. [DOI] [PubMed] [Google Scholar]
- [42].Yang Y, Stang A, Schweickert PG, Lanman NA, Paul EN, Monia BP, Revenko AS, Palumbo JS, Mullins ES, Elzey BD, Janssen EM, Konieczny SF, Flick MJ, Thrombin Signaling Promotes Pancreatic Adenocarcinoma through PAR-1-Dependent Immune Evasion, Cancer Res 79(13) (2019) 3417–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, Liu L, Huang D, Jiang J, Cui GS, Yang Y, Wang W, Guo D, Dai M, Guo J, Zhang T, Liao Q, Liu Y, Zhao YL, Han DL, Zhao Y, Yang YG, Wu W, Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma, Cell Res 29(9) (2019) 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nicolle R, Blum Y, Marisa L, Loncle C, Gayet O, Moutardier V, Turrini O, Giovannini M, Bian B, Bigonnet M, Rubis M, Elarouci N, Armenoult L, Ayadi M, Duconseil P, Gasmi M, Ouaissi M, Maignan A, Lomberk G, Boher JM, Ewald J, Bories E, Garnier J, Goncalves A, Poizat F, Raoul JL, Secq V, Garcia S, Grandval P, Barraud-Blanc M, Norguet E, Gilabert M, Delpero JR, Roques J, Calvo E, Guillaumond F, Vasseur S, Urrutia R, de Reynies A, Dusetti N, Iovanna J, Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts, Cell Rep 21(9) (2017) 2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Papapetrou EP, Induced pluripotent stem cells, past and future, Science 353(6303) (2016) 991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chao HM, Chern E, Patient-derived induced pluripotent stem cells for models of cancer and cancer stem cell research, J Formos Med Assoc 117(12) (2018) 1046–1057. [DOI] [PubMed] [Google Scholar]
- [47].Gharibi A, Adamian Y, Kelber JA, Cellular and molecular aspects of pancreatic cancer, Acta Histochem 118(3) (2016) 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lai E, Puzzoni M, Ziranu P, Pretta A, Impera V, Mariani S, Liscia N, Soro P, Musio F, Persano M, Donisi C, Tolu S, Balconi F, Pireddu A, Demurtas L, Pusceddu V, Camera S, Sclafani F, Scartozzi M, New therapeutic targets in pancreatic cancer, Cancer Treat Rev 81 (2019) 101926. [DOI] [PubMed] [Google Scholar]
- [49].Kim J, Zaret KS, Generation of Induced Pluripotent Stem Cell-Like Lines from Human Pancreatic Ductal Adenocarcinoma, Methods Mol Biol 1882 (2019) 33–53. [DOI] [PubMed] [Google Scholar]
- [50].Papapetrou EP, Patient-derived induced pluripotent stem cells in cancer research and precision oncology, Nat Med 22(12) (2016) 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Calle AS, Nair N, Oo AK, Prieto-Vila M, Koga M, Khayrani AC, Hussein M, Hurley L, Vaidyanath A, Seno A, Iwasaki Y, Calle M, Kasai T, Seno M, A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm), Am J Cancer Res 6(12) (2016) 2799–2815. [PMC free article] [PubMed] [Google Scholar]
- [52].Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, Arrowsmith C, Kalloger SE, Renouf DJ, Connor AA, Cleary S, Schaeffer DF, Roehrl M, Tsao MS, Gallinger S, Keller G, Muthuswamy SK, Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids, Nat Med 21(11) (2015) 1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weniger M, Honselmann KC, Liss AS, The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship, Cancers (Basel) 10(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Malik R, Lelkes PI, Cukierman E, Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer, Trends Biotechnol 33(4) (2015) 230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Thomas D, Radhakrishnan P, Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis, Mol Cancer 18(1) (2019) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Grzesiak JJ, Tran Cao HS, Burton DW, Kaushal S, Vargas F, Clopton P, Snyder CS, Deftos LJ, Hoffman RM, Bouvet M, Knockdown of the beta(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis, Int J Cancer 129(12) (2011) 2905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Grzesiak JJ, Bouvet M, The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines, Br J Cancer 94(9) (2006) 1311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pan B, Guo J, Liao Q, Zhao Y, beta1 and beta3 integrins in breast, prostate and pancreatic cancer: A novel implication, Oncol Lett 15(4) (2018) 5412–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sanh N, Fadul H, Hussein N, Lyn-Cook BD, Hammons G, Ramos-Cardona XE, Mohamed K, Mohammed SI, Proteomics Profiling of Pancreatic Cancer and Pancreatitis for Biomarkers Discovery, J Cell Sci Ther 9(4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ma F, Tremmel DM, Li Z, Lietz CB, Sackett SD, Odorico JS, Li L, In Depth Quantification of Extracellular Matrix Proteins from Human Pancreas, J Proteome Res 18(8) (2019) 3156–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jakubowska K, Pryczynicz A, Januszewska J, Sidorkiewicz I, Kemona A, Niewinski A, Lewczuk L, Kedra B, Guzinska-Ustymowicz K, Expressions of Matrix Metalloproteinases 2, 7, and 9 in Carcinogenesis of Pancreatic Ductal Adenocarcinoma, Dis Markers 2016 (2016) 9895721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Slapak EJ, Duitman J, Tekin C, Bijlsma MF, Spek CA, Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression?, Biology (Basel) 9(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Knapinska AM, Estrada CA, Fields GB, The Roles of Matrix Metalloproteinases in Pancreatic Cancer, Prog Mol Biol Transl Sci 148 (2017) 339–354. [DOI] [PubMed] [Google Scholar]
- [64].Qiang L, Cao H, Chen J, Weller SG, Krueger EW, Zhang L, Razidlo GL, McNiven MA, Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1, J Cell Biol 218(1) (2019) 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Robinson BK, Cortes E, Rice AJ, Sarper M, Del Rio Hernandez A, Quantitative analysis of 3D extracellular matrix remodelling by pancreatic stellate cells, Biol Open 5(6) (2016) 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rubiano A, Delitto D, Han S, Gerber M, Galitz C, Trevino J, Thomas RM, Hughes SJ, Simmons CS, Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties, Acta Biomater 67 (2018) 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Drifka CR, Loeffler AG, Mathewson K, Keikhosravi A, Eickhoff JC, Liu Y, Weber SM, Kao WJ, Eliceiri KW, Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection, Oncotarget 7(46) (2016) 76197–76213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lv D, Hu Z, Lu L, Lu H, Xu X, Three-dimensional cell culture: A powerful tool in tumor research and drug discovery, Oncol Lett 14(6) (2017) 6999–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sempere LF, Gunn JR, Korc M, A novel 3-dimensional culture system uncovers growth stimulatory actions by TGFbeta in pancreatic cancer cells, Cancer Biol Ther 12(3) (2011) 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Logsdon DP, Grimard M, Luo M, Shahda S, Jiang Y, Tong Y, Yu Z, Zyromski N, Schipani E, Carta F, Supuran CT, Korc M, Ivan M, Kelley MR, Fishel ML, Regulation of HIF1alpha under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual Targeting in Patient-Derived 3D Pancreatic Cancer Models, Mol Cancer Ther 15(11) (2016) 2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lin CC, Korc M, Designer hydrogels: Shedding light on the physical chemistry of the pancreatic cancer microenvironment, Cancer Lett 436 (2018) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Antoine EE, Vlachos PP, Rylander MN, Tunable collagen I hydrogels for engineered physiological tissue micro-environments, PLoS One 10(3) (2015) e0122500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Haage A, Nam DH, Ge X, Schneider IC, Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion, Biochem Biophys Res Commun 450(1) (2014) 213–8. [DOI] [PubMed] [Google Scholar]
- [74].Imamichi Y, Konig A, Gress T, Menke A, Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer, Oncogene 26(16) (2007) 2381–5. [DOI] [PubMed] [Google Scholar]
- [75].Haage A, Schneider IC, Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells, FASEB J 28(8) (2014) 3589–99. [DOI] [PubMed] [Google Scholar]
- [76].Puls TJ, Tan X, Whittington CF, Voytik-Harbin SL, 3D collagen fibrillar microstructure guides pancreatic cancer cell phenotype and serves as a critical design parameter for phenotypic models of EMT, PLoS One 12(11) (2017) e0188870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Huang H, Svoboda RA, Lazenby AJ, Saowapa J, Chaika N, Ding K, Wheelock MJ, Johnson KR, Up-regulation of N-cadherin by Collagen I-activated Discoidin Domain Receptor 1 in Pancreatic Cancer Requires the Adaptor Molecule Shc1, J Biol Chem 291(44) (2016) 23208–23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Procacci P, Moscheni C, Sartori P, Sommariva M, Gagliano N, Tumor(−)Stroma Cross-Talk in Human Pancreatic Ductal Adenocarcinoma: A Focus on the Effect of the Extracellular Matrix on Tumor Cell Phenotype and Invasive Potential, Cells 7(10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kreger ST, Bell BJ, Bailey J, Stites E, Kuske J, Waisner B, Voytik-Harbin SL, Polymerization and matrix physical properties as important design considerations for soluble collagen formulations, Biopolymers 93(8) (2010) 690–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ohlund D, Franklin O, Lundberg E, Lundin C, Sund M, Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop, BMC Cancer 13 (2013) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Topalovski M, Brekken RA, Matrix control of pancreatic cancer: New insights into fibronectin signaling, Cancer Lett 381(1) (2016) 252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Munasinghe A, Malik K, Mohamedi F, Moaraf S, Kocher H, Jones L, Hill NJ, Fibronectin acts as a molecular switch to determine SPARC function in pancreatic cancer, Cancer Lett 477 (2020) 88–96. [DOI] [PubMed] [Google Scholar]
- [83].Lin TC, Yang CH, Cheng LH, Chang WT, Lin YR, Cheng HC, Fibronectin in Cancer: Friend or Foe, Cells 9(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jordahl S, Solorio L, Neale DB, McDermott S, Jordahl JH, Fox A, Dunlay C, Xiao A, Brown M, Wicha M, Luker GD, Lahann J, Engineered Fibrillar Fibronectin Networks as Three-Dimensional Tissue Scaffolds, Adv Mater 31(46) (2019) e1904580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Stromnes IM, DelGiorno KE, Greenberg PD, Hingorani SR, Stromal reengineering to treat pancreas cancer, Carcinogenesis 35(7) (2014) 1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sato N, Cheng XB, Kohi S, Koga A, Hirata K, Targeting hyaluronan for the treatment of pancreatic ductal adenocarcinoma, Acta Pharm Sin B 6(2) (2016) 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Doherty GJ, Tempero M, Corrie PG, HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer, Future Oncol 14(1) (2018) 13–22. [DOI] [PubMed] [Google Scholar]
- [88].Li Y, Kumacheva E, Hydrogel microenvironments for cancer spheroid growth and drug screening, Science Advances 4(4) (2018) eaas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tam RY, Smith LJ, Shoichet MS, Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models, Accounts of Chemical Research 50(4) (2017) 703–713. [DOI] [PubMed] [Google Scholar]
- [90].Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U, Engineering cancer microenvironments for in vitro 3-D tumor models, Materials Today 18(10) (2015) 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology, Advanced Materials 18(11) (2006) 1345–1360. [Google Scholar]
- [92].Lin C-C, Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels, RSC Advances 5(50) (2015) 39844–39853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lin C-C, Raza A, Shih H, PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids, Biomaterials 32(36) (2011) 9685–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gao B, Konno T, Ishihara K, Fabrication of a live cell-containing multilayered polymer hydrogel membrane with micrometer-scale thickness to evaluate pharmaceutical activity, Journal of Biomaterials Science, Polymer Edition 26(18) (2015) 1372–1385. [DOI] [PubMed] [Google Scholar]
- [95].Dai J, Qin L, Chen Y, Wang H, Lin G, Li X, Liao H, Fang H, Matrix stiffness regulates epithelial-mesenchymal transition via cytoskeletal remodeling and MRTF-A translocation in osteosarcoma cells, Journal of the Mechanical Behavior of Biomedical Materials 90 (2019) 226–238. [DOI] [PubMed] [Google Scholar]
- [96].Leight JL, Drain AP, Weaver VM, Extracellular Matrix Remodeling and Stiffening Modulate Tumor Phenotype and Treatment Response, Annual Review of Cancer Biology 1(1) (2017) 313–334. [Google Scholar]
- [97].Gill BJ, West JL, Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology, Journal of Biomechanics 47(9) (2014) 1969–1978. [DOI] [PubMed] [Google Scholar]
- [98].Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ, Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement, Proceedings of the National Academy of Sciences 106(2) (2009) 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC, Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells, Biomaterials 31(32) (2010) 8494–8506. [DOI] [PubMed] [Google Scholar]
- [100].Gill BJ, Gibbons DL, Roudsari LC, Saik JE, Rizvi ZH, Roybal JD, Kurie JM, West JL, A Synthetic Matrix with Independently Tunable Biochemistry and Mechanical Properties to Study Epithelial Morphogenesis and EMT in a Lung Adenocarcinoma Model, Cancer Research 72(22) (2012) 6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Raza A, Ki CS, Lin C-C, The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D, Biomaterials 34(21) (2013) 5117–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ki CS, Lin T-Y, Korc M, Lin C-C, Thiol-ene hydrogels as desmoplasia-mimetic matrices for modeling pancreatic cancer cell growth, invasion, and drug resistance, Biomaterials 35(36) (2014) 9668–9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang C, Tong X, Yang F, Bioengineered 3D Brain Tumor Model To Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using PEG-Based Hydrogels, Molecular Pharmaceutics 11(7) (2014) 2115–2125. [DOI] [PubMed] [Google Scholar]
- [104].Hartgerink JD, Beniash E, Stupp SI, Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials, Proceedings of the National Academy of Sciences 99(8) (2002) 5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ulijn RV, Smith AM, Designing peptide based nanomaterials, Chemical Society Reviews 37(4) (2008) 664–675. [DOI] [PubMed] [Google Scholar]
- [106].Li J, Gao Y, Kuang Y, Shi J, Du X, Zhou J, Wang H, Yang Z, Xu B, Dephosphorylation of d-Peptide Derivatives to Form Biofunctional, Supramolecular Nanofibers/Hydrogels and Their Potential Applications for Intracellular Imaging and Intratumoral Chemotherapy, Journal of the American Chemical Society 135(26) (2013) 9907–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Worthington P, Pochan DJ, Langhans SA, Peptide Hydrogels – Versatile Matrices for 3D Cell Culture in Cancer Medicine, Frontiers in Oncology 5(92) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yang Z, Xu H, Zhao X, Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer, Advanced Science 7(9) (2020) 1903718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rivas M, Del Valle LJ, Alemán C, Puiggalí J, Peptide Self-Assembly into Hydrogels for Biomedical Applications Related to Hydroxyapatite, Gels (Basel, Switzerland) 5(1) (2019) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Betriu N, Semino CE, Development of a 3D Co-Culture System as a Cancer Model Using a Self-Assembling Peptide Scaffold, Gels 4(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Luo Z, Yue Y, Zhang Y, Yuan X, Gong J, Wang L, He B, Liu Z, Sun Y, Liu J, Hu M, Zheng J, Designer D-form self-assembling peptide nanofiber scaffolds for 3-dimensional cell cultures, Biomaterials 34(21) (2013) 4902–4913. [DOI] [PubMed] [Google Scholar]
- [112].Huang H, Ding Y, Sun XS, Nguyen TA, Peptide Hydrogelation and Cell Encapsulation for 3D Culture of MCF-7 Breast Cancer Cells, PLOS ONE 8(3) (2013) e59482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ashworth JC, Thompson JL, James JR, Slater CE, Pijuan-Galitó S, Lis-Slimak K, Holley RJ, Meade KA, Thompson A, Arkill KP, Tassieri M, Wright AJ, Farnie G, Merry CLR, Peptide gels of fully-defined composition and mechanics for probing cell-cell and cell-matrix interactions in vitro, Matrix Biology 85–86 (2020) 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Carvalho MP, Costa EC, Miguel SP, Correia IJ, Tumor spheroid assembly on hyaluronic acid-based structures: A review, Carbohydrate Polymers 150 (2016) 139–148. [DOI] [PubMed] [Google Scholar]
- [115].Shih H, Greene T, Korc M, Lin CC, Modular and Adaptable Tumor Niche Prepared from Visible Light Initiated Thiol-Norbornene Photopolymerization, Biomacromolecules 17(12) (2016) 3872–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Baker AEG, Tam RY, Shoichet MS, Independently Tuning the Biochemical and Mechanical Properties of 3D Hyaluronan-Based Hydrogels with Oxime and Diels–Alder Chemistry to Culture Breast Cancer Spheroids, Biomacromolecules 18(12) (2017) 4373–4384. [DOI] [PubMed] [Google Scholar]
- [117].Liang Y, Jeong J, DeVolder RJ, Cha C, Wang F, Tong YW, Kong H, A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity, Biomaterials 32(35) (2011) 9308–9315. [DOI] [PubMed] [Google Scholar]
- [118].Totti S, Allenby MC, Dos Santos SB, Mantalaris A, Velliou EG, A 3D bioinspired highly porous polymeric scaffolding system for in vitro simulation of pancreatic ductal adenocarcinoma, RSC Advances 8(37) (2018) 20928–20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chiellini F, Puppi D, Piras AM, Morelli A, Bartoli C, Migone C, Modelling of pancreatic ductal adenocarcinoma in vitro with three-dimensional microstructured hydrogels, RSC Advances 6(59) (2016) 54226–54235. [Google Scholar]
- [120].Navarro M, Aparicio C, Charles-Harris M, Ginebra MP, Engel E, Planell JA, Development of a Biodegradable Composite Scaffold for Bone Tissue Engineering: Physicochemical, Topographical, Mechanical, Degradation, and Biological Properties, in: Vancso GJ(Ed.), Ordered Polymeric Nanostructures at Surfaces, Springer Berlin Heidelberg, Berlin, Heidelberg, 2006, pp. 209–231. [Google Scholar]
- [121].Sahoo SK, Panda AK, Labhasetwar V, Characterization of Porous PLGA/PLA Microparticles as a Scaffold for Three Dimensional Growth of Breast Cancer Cells, Biomacromolecules 6(2) (2005) 1132–1139. [DOI] [PubMed] [Google Scholar]
- [122].Gadegaard N, Martines E, Riehle MO, Seunarine K, Wilkinson CDW, Applications of nano-patterning to tissue engineering, Microelectronic Engineering 83(4) (2006) 1577–1581. [Google Scholar]
- [123].Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ, Engineering tumors with 3D scaffolds, Nature Methods 4(10) (2007) 855–860. [DOI] [PubMed] [Google Scholar]
- [124].Ilyas A, Islam M, Asghar W, Menon J, Wadajkar A, Nguyen K, Iqbal S, Salt-Leaching Synthesis of Porous PLGA Nanoparticles, Nanotechnology, IEEE Transactions on 12 (2013) 1082–1088. [Google Scholar]
- [125].Ricci C, Mota C, Moscato S, D’Alessandro D, Ugel S, Sartoris S, Bronte V, Boggi U, Campani D, Funel N, Moroni L, Danti S, Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models, Biomatter 4 (2014) e955386–e955386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Drifka CR, Eliceiri KW, Weber SM, Kao WJ, A bioengineered heterotypic stroma-cancer microenvironment model to study pancreatic ductal adenocarcinoma, Lab Chip 13(19) (2013) 3965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gupta P, Perez-Mancera PA, Kocher H, Nisbet A, Schettino G, Velliou EG, A Novel Scaffold-Based Hybrid Multicellular Model for Pancreatic Ductal Adenocarcinoma-Toward a Better Mimicry of the in vivo Tumor Microenvironment, Front Bioeng Biotechnol 8 (2020) 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bradney MJ, Venis SM, Yang Y, Konieczny SF, Han B, A Biomimetic Tumor Model of Heterogeneous Invasion in Pancreatic Ductal Adenocarcinoma, Small 16(10) (2020) e1905500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Gioeli D, Snow CJ, Simmers MB, Hoang SA, Figler RA, Allende JA, Roller DG, Parsons JT, Wulfkuhle JD, Petricoin EF, Bauer TW, Wamhoff BR, Development of a multicellular pancreatic tumor microenvironment system using patient-derived tumor cells, Lab Chip 19(7) (2019) 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Moon H.-r., Han B, 15 - Engineered tumor models for cancer biology and treatment, in: Park K (Ed.), Biomaterials for Cancer Therapeutics (Second Edition), Woodhead Publishing; 2020, pp. 423–443. [Google Scholar]
- [131].Hamidi H, Ivaska J, Every step of the way: integrins in cancer progression and metastasis, Nat Rev Cancer 18(9) (2018) 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chen MB, Lamar JM, Li R, Hynes RO, Kamm RD, Elucidation of the Roles of Tumor Integrin beta1 in the Extravasation Stage of the Metastasis Cascade, Cancer Res 76(9) (2016) 2513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD, Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function, Proc Natl Acad Sci U S A 109(34) (2012) 13515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nguyen DT, Lee E, Alimperti S, Norgard RJ, Wong A, Lee JJ, Eyckmans J, Stanger BZ, Chen CS, A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling, Sci Adv 5(8) (2019) eaav6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Rossi G, Manfrin A, Lutolf MP, Progress and potential in organoid research, Nat Rev Genet 19(11) (2018) 671–687. [DOI] [PubMed] [Google Scholar]
- [136].Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA, Organoid models of human and mouse ductal pancreatic cancer, Cell 160(1–2) (2015) 324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Muller M, Renz S, Kuo CC, Lin Q, Sendler M, Breunig M, Kleiderman SM, Lechel A, Zenker M, Leichsenring M, Rosendahl J, Zenke M, Sainz B Jr., Mayerle J, Costa IG, Seufferlein T, Kormann M, Wagner M, Liebau S, Kleger A, Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling, Gut 66(3) (2017) 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Moon AOH, Yang Y, Elzey BD, Konieczny SF and Han B, An engineered pancreatic cancer model with intra-tumoral heterogeneity of driver mutations, Lab on a Chip (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T, Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids, Nat Med 21(3) (2015) 256–62. [DOI] [PubMed] [Google Scholar]
- [140].Moreira L, Bakir B, Chatterji P, Dantes Z, Reichert M, Rustgi AK, Pancreas 3D Organoids: Current and Future Aspects as a Research Platform for Personalized Medicine in Pancreatic Cancer, Cell Mol Gastroenterol Hepatol 5(3) (2018) 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hwang HJ, Oh MS, Lee DW, Kuh HJ, Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells, J Exp Clin Cancer Res 38(1) (2019) 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gun SY, Lee SWL, Sieow JL, Wong SC, Targeting immune cells for cancer therapy, Redox Biol 25 (2019) 101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Li Y, Kumacheva E, Hydrogel microenvironments for cancer spheroid growth and drug screening, Sci Adv 4(4) (2018) eaas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Li N, Li Y, Li Z, Huang C, Yang Y, Lang M, Cao J, Jiang W, Xu Y, Dong J, Ren H, Hypoxia Inducible Factor 1 (HIF-1) Recruits Macrophage to Activate Pancreatic Stellate Cells in Pancreatic Ductal Adenocarcinoma, Int J Mol Sci 17(6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG, Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma, Clin Transl Med 8(1) (2019) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Suresh M, Mattheolabakis G, Singh A, Amiji M, <em>In vitro</em> model of inflammatory, hypoxia, and cancer stem cell signaling in pancreatic cancer using heterocellular 3-dimensional spheroids, bioRxiv (2018) 454397. [Google Scholar]
- [147].Lai BFL, Lu RXZ, Hu Y, Davenport Huyer L, Dou W, Wang EY, Radulovich N, Tsao MS, Sun Y, Radisic M, Recapitulating Pancreatic Tumor Microenvironment through Synergistic Use of Patient Organoids and Organ-on-a-Chip Vasculature, Advanced Functional Materials n/a(n/a) (2020) 2000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lee JH, Kim SK, Khawar IA, Jeong SY, Chung S, Kuh HJ, Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance, J Exp Clin Cancer Res 37(1) (2018) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Thompson TJ, Han B, Analysis of adhesion kinetics of cancer cells on inflamed endothelium using a microfluidic platform, Biomicrofluidics 12(4) (2018) 042215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Algarni A, Greenman J, Madden LA, Procoagulant tumor microvesicles attach to endothelial cells on biochips under microfluidic flow, Biomicrofluidics 13(6) (2019) 064124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ayuso JM, Truttschel R, Gong MM, Humayun M, Virumbrales-Munoz M, Vitek R, Felder M, Gillies SD, Sondel P, Wisinski KB, Patankar M, Beebe DJ, Skala MC, Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model, Oncoimmunology 8(3) (2019) 1553477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bai J, Adriani G, Dang TM, Tu TY, Penny HX, Wong SC, Kamm RD, Thiery JP, Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and beta2 integrin interactions, Oncotarget 6(28) (2015) 25295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, Timpson P, Goetz JG, Fluids and their mechanics in tumour transit: shaping metastasis, Nature Reviews Cancer 20(2) (2020) 107–124. [DOI] [PubMed] [Google Scholar]
- [154].Nieskoski MD, Marra K, Gunn JR, Kanick SC, Doyley MM, Hasan T, Pereira SP, Stuart Trembly B, Pogue BW, Separation of Solid Stress From Interstitial Fluid Pressure in Pancreas Cancer Correlates With Collagen Area Fraction, J Biomech Eng 139(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Song JW, Gu W, Futai N, Warner KA, Nor JE, Takayama S, Computer-controlled microcirculatory support system for endothelial cell culture and shearing, Anal Chem 77(13) (2005) 3993–9. [DOI] [PubMed] [Google Scholar]
- [156].Choi HY, Yang GM, Dayem AA, Saha SK, Kim K, Yoo Y, Hong K, Kim JH, Yee C, Lee KM, Cho SG, Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3beta activities, Breast Cancer Res 21(1) (2019) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Nguyen AV, Nyberg KD, Scott MB, Welsh AM, Nguyen AH, Wu N, Hohlbauch SV, Geisse NA, Gibb EA, Robertson AG, Donahue TR, Rowat AC, Stiffness of pancreatic cancer cells is associated with increased invasive potential, Integr Biol (Camb) 8(12) (2016) 1232–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kalli M, Papageorgis P, Gkretsi V, Stylianopoulos T, Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration, Ann Biomed Eng 46(5) (2018) 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC, beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer, Cancer Cell 33(1) (2018) 75–90 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gkretsi V, Stylianopoulos T, Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis, Front Oncol 8 (2018) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Raza A, Ki CS, Lin CC, The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D, Biomaterials 34(21) (2013) 5117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lachowski D, Cortes E, Pink D, Chronopoulos A, Karim SA, P.M. J, A.E. Del Rio Hernandez, Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells, Sci Rep 7(1) (2017) 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, Thompson CB, Connor RJ, Thanos CD, Scott Brockenbrough J, Provenzano PP, Frost GI, Michael Shepard H, Hingorani SR, Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase, Biophys J 110(9) (2016) 2106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kwak B, Ozcelikkale A, Shin CS, Park K, Han B, Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip, J Control Release 194 (2014) 157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ozcelikkale A, Shin K, Noe-Kim V, Elzey BD, Dong Z, Zhang JT, Kim K, Kwon IC, Park K, Han B, Differential response to doxorubicin in breast cancer subtypes simulated by a microfluidic tumor model, J Control Release 266 (2017) 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Horsted F, West J, Grainge MJ, Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis, PLoS Med 9(7) (2012) e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Lyman GH, Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis, Cancer 117(7) (2011) 1334–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Menapace LA, Peterson DR, Berry A, Sousou T, Khorana AA, Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer, Thromb Haemost 106(2) (2011) 371–8. [DOI] [PubMed] [Google Scholar]
- [169].Mandala M, Reni M, Cascinu S, Barni S, Floriani I, Cereda S, Berardi R, Mosconi S, Torri V, Labianca R, Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients, Ann Oncol 18(10) (2007) 1660–5. [DOI] [PubMed] [Google Scholar]
- [170].Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL, Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation, J Thromb Haemost 6(9) (2008) 1517–24. [DOI] [PubMed] [Google Scholar]
- [171].Haas SL, Jesnowski R, Steiner M, Hummel F, Ringel J, Burstein C, Nizze H, Liebe S, Lohr JM, Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation, World J Gastroenterol 12(30) (2006) 4843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B, Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy, Clin Cancer Res 15(22) (2009) 6830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I, Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients, J Thromb Haemost 10(7) (2012) 1363–70. [DOI] [PubMed] [Google Scholar]
- [174].Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, Mullauer L, Gnant M, Scheithauer W, Pabinger I, Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features, Eur J Clin Invest 43(3) (2013) 277–85. [DOI] [PubMed] [Google Scholar]
- [175].Geddings JE, Hisada Y, Boulaftali Y, Getz TM, Whelihan M, Fuentes R, Dee R, Cooley BC, Key NS, Wolberg AS, Bergmeier W, Mackman N, Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice, J Thromb Haemost 14(1) (2016) 153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Rowe SL, Lee S, Stegemann JP, Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels, Acta Biomater 3(1) (2007) 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Janmey PA, Winer JP, Weisel JW, Fibrin gels and their clinical and bioengineering applications, J R Soc Interface 6(30) (2009) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Robinson M, Douglas S, Michelle Willerth S, Mechanically stable fibrin scaffolds promote viability and induce neurite outgrowth in neural aggregates derived from human induced pluripotent stem cells, Sci Rep 7(1) (2017) 6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].de la Puente P, Ludena D, Cell culture in autologous fibrin scaffolds for applications in tissue engineering, Exp Cell Res 322(1) (2014) 1–11. [DOI] [PubMed] [Google Scholar]
- [180].Yakovlev S, Medved L, Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: localization of the fibrin-binding site within the third extracellular VE-cadherin domain, Biochemistry 48(23) (2009) 5171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL, Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo, J Clin Invest 113(11) (2004) 1596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gailit J, Clarke C, Newman D, Tonnesen MG, Mosesson MW, Clark RA, Human fibroblasts bind directly to fibrinogen at RGD sites through integrin alpha(v)beta3, Exp Cell Res 232(1) (1997) 118–26. [DOI] [PubMed] [Google Scholar]
- [183].Posma JJ, Grover SP, Hisada Y, Owens AP 3rd, Antoniak S, Spronk HM, Mackman N, Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases, Arterioscler Thromb Vasc Biol 39(1) (2019) 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Palta S, Saroa R, Palta A, Overview of the coagulation system, Indian J Anaesth 58(5) (2014) 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Nieman MT, Schmaier AH, Interaction of thrombin with PAR1 and PAR4 at the thrombin cleavage site, Biochemistry 46(29) (2007) 8603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Tekin C, Shi K, Daalhuisen JB, Ten Brink MS, Bijlsma MF, Spek CA, PAR1 signaling on tumor cells limits tumor growth by maintaining a mesenchymal phenotype in pancreatic cancer, Oncotarget 9(62) (2018) 32010–32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Cesarman-Maus G, Hajjar KA, Molecular mechanisms of fibrinolysis, Br J Haematol 129(3) (2005) 307–21. [DOI] [PubMed] [Google Scholar]
- [188].Collen D, Lijnen HR, Molecular basis of fibrinolysis, as relevant for thrombolytic therapy, Thromb Haemost 74(1) (1995) 167–71. [PubMed] [Google Scholar]
- [189].Mahmood N, Mihalcioiu C, Rabbani SA, Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications, Front Oncol 8 (2018) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH, Evolving role of uPA/uPAR system in human cancers, Cancer Treat Rev 34(2) (2008) 122–36. [DOI] [PubMed] [Google Scholar]
- [191].Gorantla B, Asuthkar S, Rao JS, Patel J, Gondi CS, Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells, Mol Cancer Res 9(4) (2011) 377–89. [DOI] [PubMed] [Google Scholar]
- [192].Cantero D, Friess H, Deflorin J, Zimmermann A, Brundler MA, Riesle E, Korc M, Buchler MW, Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma, Br J Cancer 75(3) (1997) 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, Bucana CD, Callahan J, Parry GC, Evans DB, Boyd DD, Mazar AP, Ellis LM, Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice, Cancer Res 65(17) (2005) 7775–81. [DOI] [PubMed] [Google Scholar]
- [194].Iacoviello L, Agnoli C, De Curtis A, di Castelnuovo A, Giurdanella MC, Krogh V, Mattiello A, Matullo G, Sacerdote C, Tumino R, Vineis P, de Gaetano G, Panico S, Donati MB, Type 1 plasminogen activator inhibitor as a common risk factor for cancer and ischaemic vascular disease: the EPICOR study, BMJ Open 3(11) (2013) e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Humphries BA, Buschhaus JM, Chen YC, Haley HR, Qyli T, Chiang B, Shen N, Rajendran S, Cutter A, Cheng YH, Chen YT, Cong J, Spinosa PC, Yoon E, Luker KE, Luker GD, Plasminogen Activator Inhibitor 1 (PAI1) Promotes Actin Cytoskeleton Reorganization and Glycolytic Metabolism in Triple-Negative Breast Cancer, Mol Cancer Res 17(5) (2019) 1142–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Sawai H, Liu J, Reber HA, Hines OJ, Eibl G, Activation of peroxisome proliferator-activated receptor-gamma decreases pancreatic cancer cell invasion through modulation of the plasminogen activator system, Mol Cancer Res 4(3) (2006) 159–67. [DOI] [PubMed] [Google Scholar]
- [197].Warnecke-Eberz U, Prenzel KL, Baldus SE, Metzger R, Dienes HP, Bollschweiler E, Hoelscher AH, Schneider PM, Significant down-regulation of the plasminogen activator inhibitor 1 mRNA in pancreatic cancer, Pancreas 36(2) (2008) 173–7. [DOI] [PubMed] [Google Scholar]
- [198].Inoue M, Sawada T, Uchima Y, Kimura K, Nishihara T, Tanaka H, Yashiro M, Yamada N, Ohira M, Hirakawa K, Plasminogen activator inhibitor-1 (PAI-1) gene transfection inhibits the liver metastasis of pancreatic cancer by preventing angiogenesis, Oncol Rep 14(6) (2005) 1445–51. [PubMed] [Google Scholar]
- [199].Lupu-Meiri M, Geras-Raaka E, Lupu R, Shapira H, Sandbank J, Segal L, Gershengorn MC, Oron Y, Knock-down of plasminogen-activator inhibitor-1 enhances expression of E-cadherin and promotes epithelial differentiation of human pancreatic adenocarcinoma cells, J Cell Physiol 227(11) (2012) 3621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wang HC, Lin YL, Hsu CC, Chao YJ, Hou YC, Chiu TJ, Huang PH, Tang MJ, Chen LT, Shan YS, Pancreatic stellate cells activated by mutant KRAS-mediated PAI-1 upregulation foster pancreatic cancer progression via IL-8, Theranostics 9(24) (2019) 7168–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Chang JH, Jiang Y, Pillarisetty VG, Role of immune cells in pancreatic cancer from bench to clinical application: An updated review, Medicine (Baltimore) 95(49) (2016) e5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Obenauf AC, Massague J, Surviving at a Distance: Organ-Specific Metastasis, Trends Cancer 1(1) (2015) 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH, Berjian R, Douglass HO, Martin EW, Chu TM, Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites, In Vitro 18(1) (1982) 24–34. [DOI] [PubMed] [Google Scholar]
- [204].Loukopoulos P, Kanetaka K, Takamura M, Shibata T, Sakamoto M, Hirohashi S, Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity, Pancreas 29(3) (2004) 193–203. [DOI] [PubMed] [Google Scholar]
- [205].Berrozpe G, Schaeffer J, Peinado MA, Real FX, Perucho M, Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer, Int J Cancer 58(2) (1994) 185–91. [DOI] [PubMed] [Google Scholar]
- [206].Moore PS, Beghelli S, Zamboni G, Scarpa A, Genetic abnormalities in pancreatic cancer, Mol Cancer 2 (2003) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE, Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma, Nat Genet 8(1) (1994) 27–32. [DOI] [PubMed] [Google Scholar]
- [208].Huang L, Goodrow TL, Zhang SY, Klein-Szanto AJ, Chang H, Ruggeri BA, Deletion and mutation analyses of the P16/MTS-1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor-derived cell lines than in primary ductal adenocarcinomas, Cancer Res 56(5) (1996) 1137–41. [PubMed] [Google Scholar]
- [209].Sun C, Yamato T, Furukawa T, Ohnishi Y, Kijima H, Horii A, Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines, Oncol Rep 8(1) (2001) 89–92. [DOI] [PubMed] [Google Scholar]
- [210].Tan MH, Nowak NJ, Loor R, Ochi H, Sandberg AA, Lopez C, Pickren JW, Berjian R, Douglass HO Jr., Chu TM, Characterization of a new primary human pancreatic tumor line, Cancer Invest 4(1) (1986) 15–23. [DOI] [PubMed] [Google Scholar]
- [211].Kyriazis AP, Kyriazis AA, Scarpelli DG, Fogh J, Rao MS, Lepera R, Human pancreatic adenocarcinoma line Capan-1 in tissue culture and the nude mouse: morphologic, biologic, and biochemical characteristics, Am J Pathol 106(2) (1982) 250–60. [PMC free article] [PubMed] [Google Scholar]
- [212].Kyriazis AA, Kyriazis AP, Sternberg CN, Sloane NH, Loveless JD, Morphological, biological, biochemical, and karyotypic characteristics of human pancreatic ductal adenocarcinoma Capan-2 in tissue culture and the nude mouse, Cancer Res 46(11) (1986) 5810–5. [PubMed] [Google Scholar]
- [213].Schoumacher RA, Ram J, Iannuzzi MC, Bradbury NA, Wallace RW, Hon CT, Kelly DR, Schmid SM, Gelder FB, Rado TA, et al. , A cystic fibrosis pancreatic adenocarcinoma cell line, Proc Natl Acad Sci U S A 87(10) (1990) 4012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Gower WR Jr., Risch RM, Godellas CV, Fabri PJ, HPAC, a new human glucocorticoid-sensitive pancreatic ductal adenocarcinoma cell line, In Vitro Cell Dev Biol Anim 30A(3) (1994) 151–61. [DOI] [PubMed] [Google Scholar]
- [215].Metzgar RS, Gaillard MT, Levine SJ, Tuck FL, Bossen EH, Borowitz MJ, Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies, Cancer Res 42(2) (1982) 601–8. [PubMed] [Google Scholar]
- [216].Smith HS, In vitro properties of epithelial cell lines established from human carcinomas and nonmalignant tissue, J Natl Cancer Inst 62(2) (1979) 225–30. [PubMed] [Google Scholar]
- [217].Yunis AA, Arimura GK, Russin DJ, Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase, Int J Cancer 19(1) (1977) 128–35. [DOI] [PubMed] [Google Scholar]
- [218].Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G, Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas, Int J Cancer 15(5) (1975) 741–7. [DOI] [PubMed] [Google Scholar]
- [219].Drucker BJ, Marincola FM, Siao DY, Donlon TA, Bangs CD, Holder WD Jr., A new human pancreatic carcinoma cell line developed for adoptive immunotherapy studies with lymphokine-activated killer cells in nude mice, In Vitro Cell Dev Biol 24(12) (1988) 1179–87. [DOI] [PubMed] [Google Scholar]
- [220].Henke E, Nandigama R, Ergun S, Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy, Front Mol Biosci 6 (2019) 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Dangi-Garimella S, Sahai V, Ebine K, Kumar K, Munshi HG, Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression, PLoS One 8(5) (2013) e64566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Egeblad M, Nakasone ES, Werb Z, Tumors as organs: complex tissues that interface with the entire organism, Dev Cell 18(6) (2010) 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Durymanov M, Kroll C, Permyakova A, O’Neill E, Sulaiman R, Person M, Reineke J, Subcutaneous Inoculation of 3D Pancreatic Cancer Spheroids Results in Development of Reproducible Stroma-Rich Tumors, Transl Oncol 12(1) (2019) 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Lazzari G, Nicolas V, Matsusaki M, Akashi M, Couvreur P, Mura S, Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity, Acta Biomater 78 (2018) 296–307. [DOI] [PubMed] [Google Scholar]
- [225].Liu HY, Korc M, Lin CC, Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma, Biomaterials 160 (2018) 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Rousselle P, Scoazec JY, Laminin 332 in cancer: When the extracellular matrix turns signals from cell anchorage to cell movement, Semin Cancer Biol 62 (2020) 149–165. [DOI] [PubMed] [Google Scholar]
- [227].Begum A, Ewachiw T, Jung C, Huang A, Norberg KJ, Marchionni L, McMillan R, Penchev V, Rajeshkumar NV, Maitra A, Wood L, Wang C, Wolfgang C, DeJesus-Acosta A, Laheru D, Shapiro IM, Padval M, Pachter JA, Weaver DT, Rasheed ZA, Matsui W, The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma, PLoS One 12(7) (2017) e0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Rowe SL, Stegemann JP, Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios, Biomacromolecules 7(11) (2006) 2942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Hamada S, Shimosegawa T, Fibrinogen induces cytokine and collagen production in pancreatic stellate cells, Gut 58(4) (2009) 550–9. [DOI] [PubMed] [Google Scholar]


