Abstract
In this study, activation of the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signalling pathway was analyzed in proliferating rat hepatocytes both in vivo after partial hepatectomy and in vitro following epidermal growth factor (EGF)-pyruvate stimulation. First, a biphasic MEK/ERK activation was evidenced in G1 phase of hepatocytes from regenerating liver but not from sham-operated control animals. One occurred in early G1 (30 min to 4 h), and the other occurred in mid-late G1, peaking at around 10.5 h. Interestingly, the mid-late G1 activation peak was located just before cyclin D1 induction in both in vivo and in vitro models. Second, the biological role of the MEK/ERK cascade activation in hepatocyte progression through the G1/S transition was assessed by adding a MEK inhibitor (PD 98059) to EGF-pyruvate-stimulated hepatocytes in primary culture. In the presence of MEK inhibitor, cyclin D1 mRNA accumulation was inhibited, DNA replication was totally abolished, and the MEK1 isoform was preferentially targeted by this inhibition. This effect was dose dependent and completely reversed by removing the MEK inhibitor. Furthermore, transient transfection of hepatocytes with activated MEK1 construct resulted in increased cyclin D1 mRNA accumulation. Third, a correlation between the mid-late G1 MEK/ERK activation in hepatocytes in vivo after partial hepatectomy and the mitogen-independent proliferation capacity of these cells in vitro was established. Among hepatocytes isolated either 5, 7, 9, 12 or 15 h after partial hepatectomy, only those isolated from 12- and 15-h regenerating livers were able to replicate DNA without additional growth stimulation in vitro. In addition, PD 98059 intravenous administration in vivo, before MEK activation, was able to inhibit DNA replication in hepatocytes from regenerating livers. Taken together, these results show that (i) early induction of the MEK/ERK cascade is restricted to hepatocytes from hepatectomized animals, allowing an early distinction of primed hepatocytes from those returning to quiescence, and (ii) mid-late G1 MEK/ERK activation is mainly associated with cyclin D1 accumulation which leads to mitogen-independent progression of hepatocytes to S phase. These results allow us to point to a growth factor dependency in mid-late G1 phase of proliferating hepatocytes in vivo as observed in vitro in proliferating hepatocytes and argue for a crucial role of the MEK/ERK cascade signalling pathway.
In adult rodents, hepatocytes have a long life span and rarely divide under normal conditions. However, under certain physiopathological stress situations such as partial hepatectomy (PHT), viral infection, toxic injury induced by administration of chemicals such as carbon tetrachloride, thioacetamide, and galactosamine, and choline-deficient diet, they are able to divide in response to liver mass loss. Among these situations, liver regeneration triggered by PHT represents a well-established in vivo model to dissect hepatocyte growth control mechanisms (17). Indeed, surgical removal of 70% of the liver mass induces a synchronized growth response in most hepatocytes from the remaining tissue, and the cell cycle is characterized by a quick G0/G1 transition after liver removal, followed by a long G1 phase of about 18 h (16, 23, 40).
Different growth factors regulate this process by providing both stimulatory and inhibitory signals for cell growth. Immediately after PHT, hepatocytes enter in a state of prereplicative competence before they can fully respond to growth factors. This priming step is an initiating event that involves increased DNA binding activity of NF-κB and activation of other transcription factors such as STAT3, C/EBPβ, and AP1, presumably induced by tumor necrosis factor alpha and other cytokines, mainly interleukin-6, which results in cell entry in G1 phase (13, 14, 50, 57, 58, 62). These initiated cells are able to further progress in early G1 phase. However, they require a growth factor stimulation to progress up to late G1 and enter in S phase, thereby defining a growth factor-dependent restriction point which has been precisely localized in vitro in mid-late G1 in primary rat hepatocytes (39). The signal mediators that could be involved at this restriction point are epidermal growth factor (EGF), transforming growth factor alpha, and mainly hepatocyte growth factor (6, 20, 40, 51). However, the mechanisms of regulation that specifically control progression from the priming step in very early G1 to late G1 in hepatocytes remain poorly elucidated. Analysis of the transduction signals to the nucleus could lead to a better understanding of these specific mechanisms of regulation in the liver.
It is well established that mitogen-activated protein kinases (MAPKs) are activated in response to external stimuli in numerous cell types and play a central role in many signal transduction pathways. Extracellular signal-regulated kinases (ERKs) are activated by phosphorylation of threonine and tyrosine residues. Two highly related mammalian MAPKs, p44 and p42, also called ERK1 and ERK2, have been cloned and found to be ubiquitously expressed in vertebrates and highly homologous to yeast kinases (42). Their phosphorylation is catalyzed by protein kinases known as MEKs, which display extremely high substrate selectivity toward p42/p44 MAPKs. MEKs are also rapidly phosphorylated by c-Raf on serine residues that are necessary and sufficient for MEK activation (63). A number of studies have provided strong evidence for a role of MEK/ERK pathway in proliferation of rodent fibroblasts (12, 47, 53). The general assumption that the activated MAPK cascade may influence the expression of some early genes such as c-fos has been assessed (28, 31). In addition, several results support the idea that other MAPK cascades also drive specific cell cycle responses to extracellular stimuli, including P38 and JNK, which may positively or negatively influence DNA synthesis (36, 49).
Few studies have been reported on activation of transduction signals in hepatocytes, and to our knowledge, they have used mainly primary hepatocyte cultures (1, 7, 29, 32, 55, 56). Reports indicate that EGF may stimulate hepatocyte growth through two distinct pathways, either dependent on or independent of Raf, which lead to activation of MAPKs (19). An age-related decline in MAPK activity in EGF-stimulated rat hepatocytes has also been described (38), and the importance of the JNK pathway has been emphasized by Auer et al. (5). Recently, altered expression of p42/p44 MAPK in human and rat hepatocellular carcinoma has been reported (27, 43, 52). There has been much interest in the kinetics of cell cycle-associated proteins, namely, cyclins and Cdks, and their association in complexes and activation in mitogen-stimulated hepatocytes. In that respect, in some studies, increased levels of cyclin D1 were found to accompany hepatocyte entry in G1, while in many others, cyclin D1 induction correlated with cell progression through the mitogen-associated restriction point in mid-late G1 (3, 33, 39, 41). However, the mechanisms involved in this regulation have not been identified in hepatocytes.
In this work, we determined the kinetics of MEK/ERK activation in hepatocytes induced to proliferate in vivo after liver PHT and in vitro by exposure to growth factors. Activation was correlated with cell progression from early to late G1 phase, raising the question of whether this MEK/ERK cascade plays a role in the decision of hepatocytes to progress toward the G1/S boundary.
MATERIALS AND METHODS
Chemicals.
[γ-32P]ATP (3,000 Ci/mmol) and [methyl-3H]thymidine (5 Ci/mmol) were from Amersham Corp. (Buckinghamshire, England); sodium pyruvate and insulin I-5500 were from Sigma (Saint Quentin Fallavier, France); recombinant human EGF was from Promega (Madison, Wis.); PD 98059 in the solvent dimethyl sulfoxide (DMSO) was from Calbiochem (La Jolla, Calif.).
Antibodies.
Anti-MEK1 and MEK2 antibodies are rabbit polyclonal antisera directed against the carboxy terminus of rat MEK1 and the amino terminus of human MEK2 proteins (Santa Cruz Biotechnology). Anti-phospho-MEK1/2, anti-phospho-ERK1/2, and anti-phospho-P38 antibodies were rabbit polyclonal antisera directed to a synthetic phosphoserine 217/221 peptide corresponding to residues 214 to 226 of human MEK1, a synthetic phosphotyrosine peptide corresponding to residues 196 to 209 of human p44 MAPK, and a synthetic phosphotyrosine 180/182 peptide corresponding to residues 341 to 360 of human P38, respectively (New England Biolabs).
Animals.
Female Sprague-Dawley rats (weighing around 200 g) were purchased from Charles River France (Saint Aubon les Elbeuf, France). Animals were offered food and water ad libitum, and experiments were carried out in accordance to the French laws and regulation. Surgical removal of 70% of the liver induces a synchronized growth response involving almost only hepatocytes during the first wave of replication. Remnant livers were collected after PHT according to a predetermined kinetics previously described (25, 40). Laparotomies were performed as controls (sham operations). At different times following PHT, animals were sacrificed; the livers were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until analysis.
Administration of PD 98059.
Intravenous PD 98059 administration via the jugular vein was performed in rats 10 h after PHT as follows. Five milliliters of saline containing 75 μM PD 98059 in 0.37% DMSO was injected (0.5 ml/min); 5 h later (15 h after PHT), hepatocytes were isolated by collagenase perfusion and cultured in the absence of growth factor. In control animals, the same volume of saline–+0.37% DMSO was injected into the jugular vein as in PD 98059 experiments. Cell viability was similar in PD 98059 and control experiments.
Cell cultures.
Hepatocytes were isolated from Sprague-Dawley male (150 to 200 g) rat livers by the two-step perfusion procedure using 0.025% collagenase (Boehringer) buffered with 0.1 M HEPES (pH 7.4) as previously described (24). They were plated at a density of 105 cells per cm2 in 35-mm-diameter dishes in 2 ml of minimal essential medium 199 (3:1 [vol/vol]) containing penicillin (100 IU/ml), streptomycin (100 μg/ml), insulin (5 μg/ml) and bovine serum albumin (BSA; 1 mg/ml) (basal medium) and supplemented with 10% fetal calf serum. Four hours after plating, the medium was replaced with fresh basal medium without fetal calf serum; 48 h later, hepatocytes were stimulated or not with EGF (50 ng/ml) and sodium pyruvate (10 mM). Control culture was performed in basal medium for 24 h; then the medium was renewed in the absence of insulin, and hepatocytes were stimulated at 48 h with EGF alone (50 ng/ml). Media were renewed every day. At the indicated times, PD 98059 in DMSO was added at defined concentrations. All control features were changed at the same time as that of PD 98059-treated cells.
[3H]thymidine incorporation.
The rate of DNA synthesis was measured in animals following PHT or sham operation as described elsewhere (40). In cell cultures, 2 μCi of [methyl-3H]thymidine (5 Ci/mmol) was added for given periods of time prior to cell harvesting. Cells were washed twice in phosphate-buffered saline, scraped from the petri dish, and aliquoted for protein content determination and [3H]thymidine counting following precipitation and washing in trichloroacetic acid.
EGF receptor phosphorylation and detection.
For every time point, a liver biopsy was homogenized in homogenization buffer and adjusted to a concentration of 5 mg of protein/ml; 50-μg aliquots of proteins were then adjusted to final concentrations of 60 mM β-glycerophosphate, 30 mM nitrophenylphosphate, 25 mM morpholinepropanesulfonic acid (pH 7.0), 5 mM EGTA, 15 mM MgCl2, 1 mM dithiothreitol, and 0.1 mM orthovanadate in a mixture containing 2 μg of oligopeptide EGF receptor substrate and 1 μCi of [γ-32P]ATP and incubated for 20 min at 30°C as recommended by the manufacturer (Amersham). Addition of 60 μl of sample buffer stopped the reaction.
The samples were boiled for 5 min, and phosphoproteins were separated by one-dimension sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 20% gels (35). Labeled phosphoproteins were detected by autoradiography of dried gels. Oligopeptide-specific bands were excised, and radioactivity was quantified by scintillation counting.
Immunoblotting.
After SDS-PAGE, proteins were transferred onto nitrocellulose membranes by using a transblot cell apparatus (Millipore) for 20 min at 2.5 mA/cm2 in transfer buffer as specified by the manufacturer. Subsequently, filters were rinsed in Tris-buffered saline (TBS; pH 7.4), blocked with TBS–3% BSA for 30 min at room temperature, and incubated overnight at 4°C with diluted antiserum in TBS–3% BSA. After three washes in TBS, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. After three washes in TBS, proteins were detected by the Amersham ECL (enhanced chemiluminescence) kit procedure, and membranes were subjected to ECL. Optimal electrophoretic conditions were obtained by performing SDS-PAGE on 10% gels at 45 V for 14 h followed by 2 h at 100 V.
Transfection assays and Northern blotting.
Hepatocytes were maintained in basal medium for 24 h and then transfected by a cationic liposome-mediated method (DOTAP liposomal transfection reagent; Boehringer Mannheim) as described by the manufacturer. The constitutively activated MEK1 phosphorylation site mutant Asp218, Asp222 (MEK1-DDER), and the corresponding empty vector were used as described by Greulich and Erikson (22). Cells were lysed 36, 48, and 60 h after transfection, RNA was extracted with the Qiagen RNeasy kit, and Northern blotting was performed as described previously (40).
RESULTS
Cyclin D1 induction in G1 phase in proliferating hepatocytes after PHT.
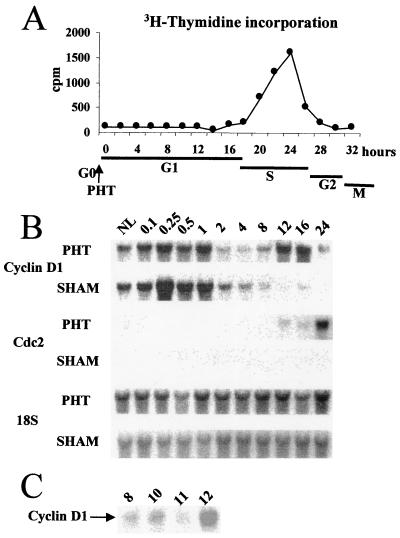
Both DNA synthesis starting at 18 h after surgery and Cdc2 transcripts accumulating from 16 h after PHT allowed us to estimate that the G1 phase of proliferating hepatocytes lasted up to 16 to 18 h in our experimental conditions (Fig. 1A and B). In hepatocytes, Cdc2 accumulation has been associated to S phase progression in both in vivo and in vitro models (39, 40).
FIG. 1.
(A) Time course of [methyl-3H]thymidine incorporation into DNA of regenerating rat liver tissues following PHT. (B) Kinetics of cyclin D1 and Cdc2 mRNA expression in livers from rats after PHT and in sham-operated control animals at the indicated times (hours after surgery). NL, normal liver. (C) Cyclin D1 mRNA expression at times around the mitogen-dependent restriction point (8 to 12 h).
We studied cyclin D1 expression at different time points during the G1 phase. Two peaks of mRNAs were evidenced: an early one at around 10 min after PHT, lasting 1 h, and a second one 12 h later (Fig. 1B). A detailed kinetic analysis allowed us to pinpoint the second induction in mid-late G1 phase between 11 and 12 h (Fig. 1C). This latter peak of cyclin D1 could be compared to that occurring in mid-late G1 and associated with a mitogen-dependent restriction point in EGF-stimulated hepatocytes in vitro (39). The presence of cyclin D1 protein was also demonstrated by Western blotting (data not shown).
In contrast, in sham-operated control animals, in which both DNA synthesis and Cdc2 mRNAs were undetectable, cyclin D1 transcripts transiently accumulated only once, early after laparotomy, and then returned to a very low level after 2 h, remaining low thereafter; this result indicated that liver parenchymal cells in sham-operated animals entered G1 but did not further progress in the cell cycle or reverted to G0. These nonproliferating hepatocytes were used as controls for all further experiments.
Activation of MEK/ERK in early and mid-late G1 of hepatocytes in regenerating liver.
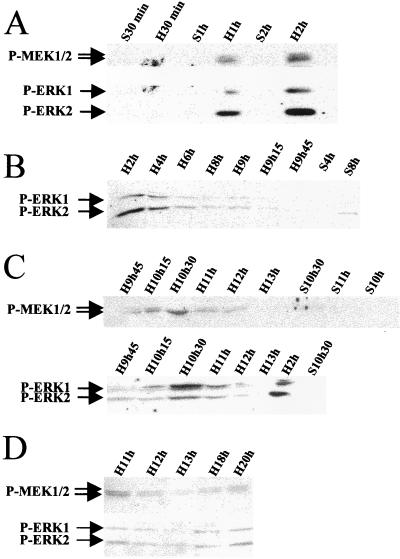
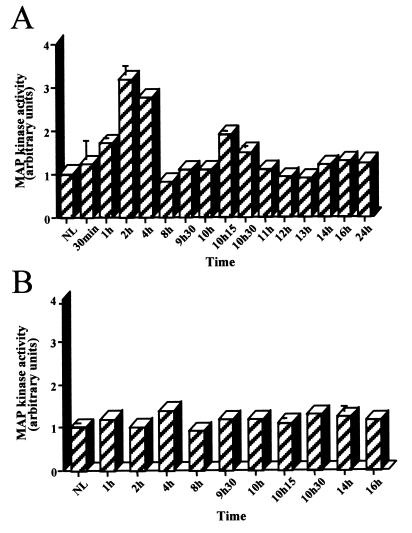
The kinetics of MEK/ERK protein activation were assessed by using antibodies against the phosphorylated forms of MEK1/2 and ERK1/2, at different times from early (Fig. 2A and B) through mid-late (Fig. 2C) G1 up to the G1/S phase transition (Fig. 2D) after PHT. Phospho-MEK and phospho-ERK followed the same kinetics: two peaks of phosphorylation were observed. One occurred early in G1 at 1 h, reaching a maximum at 2 h and decreasing after 4 h to near to zero; the second peak at 10 h 15 min was maximum at 10.5 h and returned to basal level after 11 h. This latter peak was close to but preceded cyclin D1 mRNA induction in mid-late G1, as shown in Fig. 1B and C. A slight increase of phosphorylation was observed around 20 h after PHT, at the G1/S phase transition. No activation was detected in sham-operated animals. In addition, we looked for MEK/ERK cascade kinase activity. ERK kinase activity was defined by measuring its ability to phosphorylate EGF receptor oligopeptide, a specific substrate of both ERK1 and ERK2 (10). ERK substrate appeared phosphorylated both early after PHT with a maximum at 2 h and in mid-late G1 with a maximum at 10.5 h (Fig. 3A). Moreover, no phosphorylation was found in sham-operated control animals (Fig. 3B).
FIG. 2.
Kinetics of MEK and ERK protein phosphorylation during G1 phase in regenerating liver. Western blot analysis using specific antibodies directed against the phosphorylated forms of MEK1/2 and ERK1/2 proteins was performed at the indicated times after PHT (H) or sham surgery (S) used as control. (A) In early G1 (30 min to 2 h); (B) in mid-G1 (2 h to 9 h 45 min); (C) in mid-late G1 (9 h 45 min to 13 h); (D) in late G1 (11 h to 20 h).
FIG. 3.
ERK activity in regenerating (A) and nonregenerating sham-operated (B) rat livers. 32P incorporation into EGF receptor oligopeptide specific for ERK kinase from liver tissues extracted at the indicated times (30 min to 24 h) was analyzed. For each sample, phosphorylation was quantified by scintillation counting of the amount of phosphorylated EGF receptor oligopeptide separated by SDS-PAGE on 20% gels. The same 32P incorporation profiles were obtained in three independent experiments. Standard errors above 3% are shown.
MEK/ERK activation is associated with mitogen stimulation in primary hepatocyte cultures.
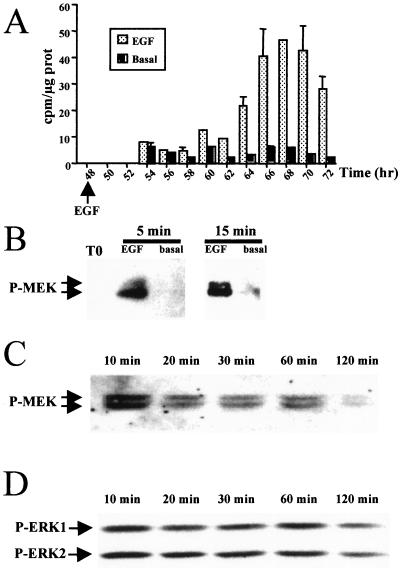
To correlate MEK activation to growth factor stimulation and DNA replication, we compared MEK behavior in hepatocyte cultures stimulated or not in vitro by EGF. It was previously shown that hepatocytes in primary culture progress in G1 independently of growth factor stimulation until the restriction point located in mid-late G1 phase (42 h), where they remain arrested in the absence of mitogenic signal (15, 26, 39). We used 48-h-old hepatocyte cultures maintained in the absence of mitogen and consequently arrested at the mitogen restriction point, providing a high synchrony of hepatocyte population. They were stimulated or not by EGF addition. They underwent DNA synthesis from 16 to 18 h after mitogenic stimulation and then progressed until mitosis (Fig. 4A). In parallel, phosphorylated MEK1/2 and ERK1/2 forms were clearly evidenced within 5 min after stimulation at the restriction point by using anti-phospho-MEK1/2 and -ERK1/2 antibodies. MEK phosphorylation was maximum at 10 to 15 min, while no phosphorylation was detected in unstimulated hepatocytes (Fig. 4B). The highest level of ERK activation was also observed at around 10 min. Then, the amount of phosphorylated forms of the two MAPKs slightly decreased but remained still detectable 2 h after growth factor stimulation (Fig. 4C and D).
FIG. 4.
Kinetics of MEK and ERK protein phosphorylation in EGF-stimulated and unstimulated hepatocyte primary cultures. (A) Time course of [methyl-3H]thymidine incorporation into DNA in unstimulated (Basal) and EGF-stimulated (EGF) hepatocyte cultures. (B to D) Western blotting with anti-phospho-MEK1/2 (P-MEK) (B and C) and anti-phospho-ERK1/2 (D) antibodies. Primary cultures were maintained for 48 h in basal medium, then stimulated with the growth factor (EGF plus pyruvate), and analyzed at the indicated times.
MEK inhibition by PD 98059 resulted in hepatocyte growth arrest.
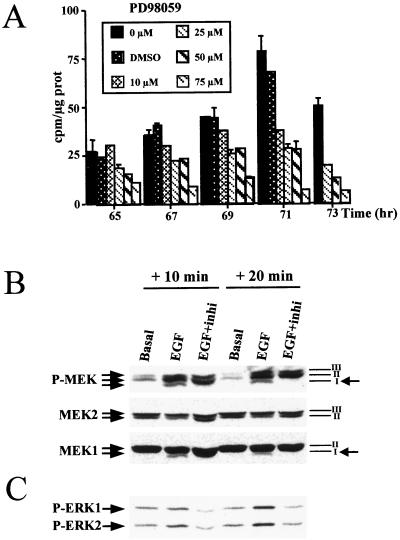
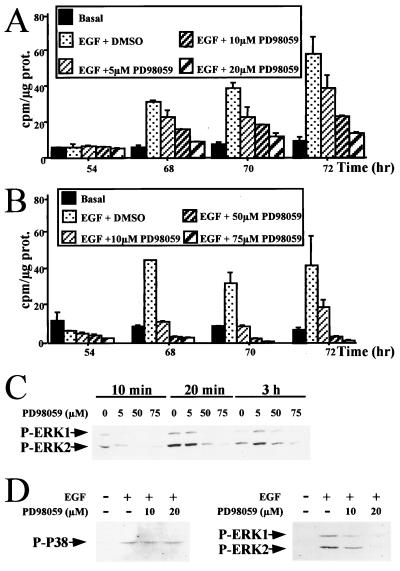
To examine the role of the MEK/ERK cascade activation in hepatocyte progression through the restriction point after mitogen stimulation, we analyzed the effects of the MEK inhibitor PD 98059 on DNA synthesis in primary hepatocyte cultures. When both PD 98059 and EGF were added to 48-h-old hepatocyte cultures, hepatocyte DNA replication was abolished (Fig. 5A). A PD 98059 dose-dependent response was obtained: 10 and 25 μM inhibited DNA synthesis, by 50%, whereas 50 μM decreased [methyl-3H]thymidine incorporation to 80% and 75 μM abolished DNA replication. However, renewal of the medium with 50 μM inhibitor added every 12 h resulted in complete inhibition of DNA synthesis (see Fig. 7A). Moreover, using optimal electrophoretic conditions, we showed that PD 98059 specifically inhibited MEK1 phosphorylation whereas MEK2 isoforms were not affected. Indeed, Western blotting using anti-phospho-MEK1/2 revealed three bands in EGF-stimulated cells, whereas one band disappeared upon addition of PD 98059. This band was attributed specifically to the phosphorylated MEK1 form, as observed by comparing the expression patterns of MEK1 and MEK2 proteins obtained from the same gel (Fig. 5B). As expected, ERK1/2 was found to be strongly inhibited by PD 98059 in EGF-stimulated hepatocytes (Fig. 5C).
FIG. 5.
Inhibition of DNA replication and of MEK/ERK phosphorylation by the specific MEK inhibitor PD 98059. (A) Hepatocyte primary cultures were maintained for 48 h in basal conditions and then stimulated with EGF plus pyruvate in the presence of increasing concentrations (10, 25, 50, and 75 μM) of PD 98059. PD 98059 was added 1 h before EGF stimulation. DMSO, control of inhibitor solvent added at the highest concentration used in the 75 μM PD 98059 solution. (B) Western blotting of MEK1 and MEK2 10 and 20 min after addition of EGF plus pyruvate alone (EGF) or with PD 98059 (EGF+inhi). The membrane was probed with anti-phospho-MEK, stripped, and reprobed with anti-MEK1 or anti-MEK2. Bands I and III correspond to MEK1 and MEK2, respectively, whereas band II represents both MEK1 and MEK2. Note the disappearance of a phosphorylated MEK1 form (band I) in the presence of EGF plus inhibitor (arrows on the right). (C) Western blotting of ERK1 and ERK2 in hepatocytes cultured as described for panel B. The membrane was probed with anti-phospho-ERK1/2 antibody. PD 98059 was used at the concentration of 75 μM.
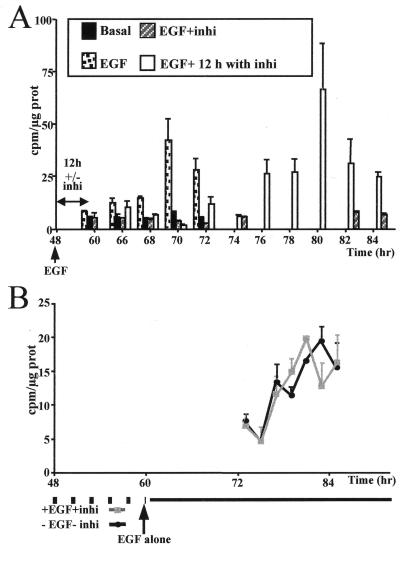
FIG. 7.
In vitro MEK inhibitor signal localization. (A) Time course of [methyl-3H]thymidine incorporation into DNA of 48-h-old hepatocyte primary cultures maintained in basal medium (basal), stimulated by EGF (EGF), or treated with EGF plus 50 μM PD 98059 throughout the culture period (EGF+inhi) or only for 12 h and then exposed to EGF alone, i.e., without inhibitor (EGF+ 12 h with inhi). Media with or without MEK inhibitor were renewed every 12 h. (B) Time course of [methyl-3H]thymidine incorporation into DNA in hepatocyte primary cultures untreated (−EGF−inhi) or pretreated with EGF and PD 98059 (+EGF+inhi) between 48 and 60 h before stimulation by EGF alone.
Since insulin and pyruvate used as comitogens in the basal culture medium for hepatocytes could contribute to the MEK/ERK signal activation, the same experiments were performed in the absence of these factors. Under these conditions, hepatocytes were able to replicate DNA after EGF stimulation, and PD 98059 also inhibited this replication in a dose-dependent manner (Fig. 6A and B). Increased cell sensitivity to the inhibitor was noticeable, as PD 98059 used at a concentration as low as 20 μM was able to block most of DNA synthesis following EGF stimulation. In addition, this inhibition was found to correlate with a disappearance of ERK1/2 phosphorylation also in a dose-dependent manner (Fig. 6C).
FIG. 6.
Specificity of the PD 98059 inhibitory effect toward EGF growth signal (A and B) and MEK/ERK cascade signalling (C and D). Dose-dependent inhibition by PD 98059 of DNA replication in EGF-stimulated hepatocytes in the absence of the comitogen insulin and pyruvate is shown. Hepatocyte primary cultures were maintained for 48 h in a basal medium lacking insulin and then stimulated with EGF alone in the presence of increasing concentrations (5, 10, and 20 μM [A] and 10, 50, and 75 μM [B]) of PD 98059. (C) Western blotting of activated ERK proteins. The membrane was probed with an anti-phospho-ERK1/2 antibody. Hepatocyte culture conditions were as described in panels A and B. Cells were harvested 10 min, 20 min, and 3 h after EGF stimulation. PD 98059 was used at concentrations of 5, 50, and 75 μM. (D) P38 and ERK1/2 phosphorylations were analyzed by using anti-phospho-P38 and anti-phospho ERK1/2 antibodies in 48-h-old hepatocytes stimulated for 20 min with EGF in the absence (−) or presence (+) of 10 and 20 μM PD 98059. Culture conditions were as described for panels A and B.
To demonstrate that PD 98059 specifically inhibited the MEK/ERK cascade, we analyzed P38 protein belonging to another signalling pathway. P38 activation was induced by EGF treatment. However, addition of MEK inhibitor did not influence the phosphorylation activity of this pathway even at the concentration (20 μM) at which DNA replication was strongly abolished while ERK1/2 phosphorylation was inhibited at this dose (Fig. 6D).
MEK inhibition suppressed cyclin D1 induction at the restriction point.
To provide evidence that cells remained blocked at the restriction point in the presence of the MEK inhibitor, reversion experiments were carried out. As shown in Fig. 7A, upon removal of the inhibitor after 12 h of exposure, the cells were able to progress to S phase and to undergo DNA synthesis with a corresponding 12-h delay. This result indicated that cells treated with the MEK inhibitor were unable to respond to EGF and behaved as if they remained blocked at the restriction point. This inference was assessed by comparing the kinetics of DNA synthesis in 48-h-old hepatocytes cultured in the absence of mitogen and maintained for an additional 12-h period at the restriction point without EGF and MEK inhibitor to those in cultures treated for the same time with EGF plus MEK inhibitor before stimulation by EGF alone (Fig. 7B). The kinetics of DNA synthesis were strictly identical in both conditions.
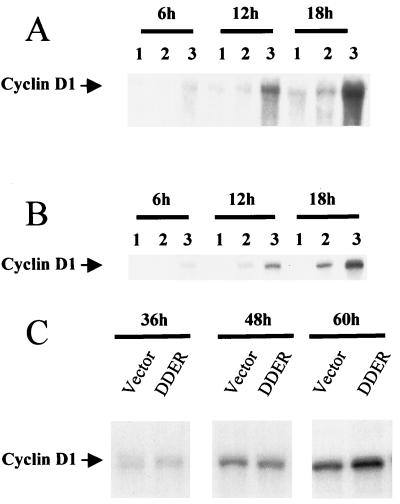
In different model systems, cyclin D1 transcription was recently reported to directly depend on MEK/ERK activation after mitogenic stimulation (2, 36, 37). Therefore, we compared the cyclin D1 mRNA accumulation in EGF-stimulated hepatocytes in the presence or absence of MEK inhibitor. As expected, in 48-h-old cultures arrested at the restriction point, cyclin D1 mRNAs rapidly accumulated after mitogen addition. In contrast, in cells exposed to a medium with EGF plus MEK inhibitor renewed every 6 h, cyclin D1 mRNAs were maintained at levels as low as in mitogen-unstimulated control cultures (Fig. 8A). This effect correlated with the complete inhibition of DNA synthesis (data not shown). The same results were obtained with a medium deprived of insulin and pyruvate in the presence of 20 μM MEK inhibitor (Fig. 8B), indicating that agonist induction pathways did not interfere in cyclin D1 mRNA regulation of cultured hepatocytes. As an additional argument, evidence of a direct relationship between MEK/ERK activation and cyclin D1 induction was provided by performing transfection of hepatocytes by a constitutively activated MEK1 known to exhibit elevated kinase activity (22). Twenty-four-hour hepatocyte cultures were transfected by the MEK1 (DDER) or by the empty vectors and analyzed 36, 48, and 60 h later for cyclin D1 mRNA expression by Northern blotting. From the kinetics of three independent experiments, a twofold increase of cyclin D1 expression was observed around 60 h in activated MEK1 (DDER) transfected hepatocytes compared to that of control cells (empty vector) (Fig. 8C).
FIG. 8.
Cyclin D1 mRNA expression in 48-h-old hepatocyte primary cultures stimulated by EGF in the presence or absence of MEK inhibitor (PD 98059). Experiments were performed in presence (A) or absence (B) of insulin and pyruvate. The MEK inhibitor at a concentration of 75 (A) or 20 (B) μM was added at 48 h of culture and renewed every 6 h. Cyclin D1 mRNA expression was analyzed by Northern blotting in unstimulated control hepatocytes (lanes 1) and EGF-stimulated hepatocytes in the presence (lanes 2) or absence (lanes 3) of MEK inhibitor at the indicated times after stimulation. (C) Transfection of 24-h-old hepatocycte primary cultures maintained in basal medium without EGF by the constitutively activated MEK1 cDNA (DDER) or the empty vector (vector) for 6 h. Cyclin D1 mRNA accumulation was defined by Northern blotting at the indicated times after transfection. Three independent experiments were performed.
Localization of a growth signalling-dependent point in regenerating liver.
To determine whether the MEK/ERK cascade activation was associated with a growth signalling in mid-late G1 in regenerating hepatocytes in vivo as observed in cells stimulated in vitro, we designed the two following experiments.
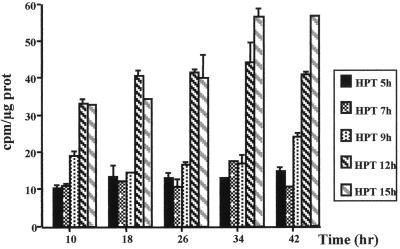
First, hepatocytes were isolated from regenerating livers during the G1 phase at 5, 7, 9, 12, and 15 h after PHT and placed in culture up to 2 or 3 days in the absence of growth factor (Fig. 9). Hepatocytes obtained from regenerating livers 5, 7, and 9 h after PHT synthesized only very low levels of DNA, while cells isolated 12 and 15 h after PHT synthesized high levels of DNA without addition of growth factor. [3H]thymidine incorporation into DNA started 2 to 10 h after establishment of the culture and remained high until 42 h. These results demonstrated that hepatocytes 12 h after PHT had crossed a growth factor restriction point in vivo and were programmed to synthesize DNA without additional growth factor stimulation in vitro. This growth factor regulatory checkpoint located in mid-late G1 phase correlated well with the peak of MEK/ERK activation which also closely preceded cyclin D1 mRNA induction.
FIG. 9.
Time course of [methyl-3H]thymidine incorporation into DNA of hepatocytes isolated from livers 5, 7, 9, 12, and 15 h after PHT and cultured in the absence of growth factor. Two independent experiments were performed for each time after PHT.
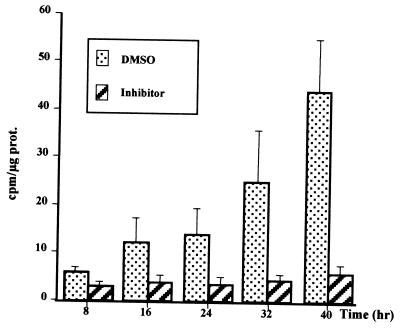
Second, intravenous administration of PD 98059 was performed in vivo 10 h after PHT, a time closely preceding the growth factor regulatory checkpoint. Hepatocytes were isolated from these animals 15 h after PHT and placed in culture without EGF. In contrast to cells from untreated animals (Fig. 9), 15 h after PHT, thymidine incorporation was completely abolished by MEK inhibitor (Fig. 10). DMSO used for PD 98059 solution at the concentration of 0.37% was injected to animals as controls. It resulted only in slightly delaying the DNA replication compared to untreated animals. These results suggested that the MEK/ERK activation pathway is essential for hepatocyte proliferation in vivo.
FIG. 10.
In vivo inhibition by MEK inhibitor of DNA replication after PHT. Ten hours after PHT, 5 ml of a solution containing either PD 98059 (75 μM in 0.37% DMSO) or 0.37% DMSO alone was administered via jugular vein injection. Five hours later (15 h after PHT), hepatocytes were isolated by collagenase perfusion and cultured in basal medium in the absence of growth factor. Two independent experiments were performed for each injection. Each experimental value corresponded to eight different thymidine incorporations, and experiments were done twice for each injection.
DISCUSSION
In this study, we show that the MEK/ERK cascade is activated at two time points of the G1 phase progression in mature rat hepatocytes. The first occurs in early G1 between 30 min and 4 h after partial hepatectomy; the second occurs between 10 and 11 h in mid-late G1 phase and is associated with induction of cyclin D1 (3, 33, 40). Evidence is provided that induction of the MEK/ERK cascade is strictly restricted to hepatocytes undergoing a prereplicative competency and contributes to the control of hepatocyte progression up to the G1/S boundary through the induction of cyclin D1 at the mitogen-dependent restriction point.
MEK/ERK is transiently activated in early G1 phase in parenchymal cells from regenerating liver but not in cells from sham-operated control animals.
It is generally assumed that the G0/G1 transition occurs immediately after PHT. Then, the priming step in early G1 is an initiating event that is presumably induced by tumor necrosis factor alpha and other cytokines (14, 44, 57). Hepatocytes then enter in a state of replicative competence before they can fully respond to growth factors (17, 39). Hepatocyte entry into and progression through G1 are characterized by the early induction of oncogenes such as c-fos, c-jun, and then others, including c-myc and D-jun, as previously described (21, 34, 45, 54). Early changes of several transcription factors such as NF-κB, C/EBPs, and STAT3 and cell cycle proteins such as cyclin D1 have also been reported (3, 40, 57). We show here that cyclin D1 accumulates early both after PHT and in sham-operated animals. The significance of this cyclin induction is not clear. Since the MEK/ERK cascade is not activated in sham-operated animals, this early expression should be not dependent on MAPK MEK/ERK but associated with another transduction signal such as JAK/STAT or P38, which can be early induced by different cytokines (14, 36, 46, 58). Recent results showing changes in P38 phosphorylation status immediately after surgery in both sham and PHT liver argue for the role of this pathway in early G1 phase (unpublished results). Cyclin D1, as well as several other early oncogenes, is observed in liver from sham-operated animals; therefore, it can be postulated that while their expression might be necessary for cell entry in G1, they are not sufficient by themselves for further progression in G1 phase.
The MEK/ERK cascade is rapidly and transiently activated between 30 min and 4 h in early G1 phase of hepatocytes, as shown by Western blotting and specific substrate phosphorylation analyses. Because MEK/ERK activation is observed only in regenerating hepatocytes, it appears to represent one of the first markers of regeneration after PHT and could therefore reflect an early control of hepatocyte cycling. Prolonged activation up to 2 h of the MEK/ERK cascade has also been reported for other cell types such as breast cancer and Rat-1 cells (11) and appeared necessary for further progression in G1 phase (30). Chen et al. also reported recently that p42/p44 reached a peak at 5 h after PHT (8). However, this first peak of MEK/ERK activation restricted to cells from regenerating liver is not sufficient to induce their progression through the restriction point up to the G1/S boundary. This was assessed by our experiments showing that hepatocytes isolated 5, 7, and 9 h after PHT were not able to progress up to S phase in the absence of growth factor.
A second MEK/ERK activation located at the mitogen-dependent restriction point in mid-late G1 is critical for progression to the G1/S boundary.
A second peak of MEK/ERK activation was observed in regenerating liver. This peak occurred around 10 h after PHT and lasted for at least 1 h. Similarly, a peak of MEK phosphorylation was detected in primary hepatocyte cultures stimulated by EGF. This observation is in agreement with other reports showing an acute MAPK activation by EGF, HGF, and nerve growth factor in primary hepatocyte cultures (1, 19, 29, 55, 56, 59, 61) and indicated that MEK activation in mid-G1 could be directly related to growth factor signalling. On the other hand, a chronic activation was recently found to inhibit this process (59). The p42/p44 MAPK cascade has been shown to be essential for the propagation of growth factors and differentiating signals. The fact that addition of PD 98059, a MEK inhibitor, to EGF-stimulated hepatocytes inhibited DNA synthesis in hepatocytes strongly suggests a crucial role of this kinase in regulatory processes that control progression to the G1/S boundary in these cells. Various data argue for the high specificity of PD 98059: it efficiently inhibits DNA synthesis in the absence of any comitogens such as insulin and pyruvate; it selectively blocks MEK1 activation, while MEK2 remains intact. This observation agrees with previous reports showing that PD 98059 binds to MEK1 and to a lesser extent MEK2 form, thus preventing their activation by upstream activators, while it fails to inhibit MEK3, SEK (MKK4), or MKK6 and related family members (4, 48). Furthermore, it does not influence phosphorylation of other proteins like P38, which belongs to another, parallel signalling pathway. In addition, MEK inhibitor was found to inhibit hepatocyte proliferation in vivo during regeneration. Altogether, these results lead to the conclusion that MEK1 activation is a major factor for late G1 progression and G1/S transition of proliferating hepatocytes.
The precise location in mid-late G1 phase of the second peak of MEK activity in regenerating liver appears to strictly correlate with the mitogen-dependent restriction point, a view supported by several observations: (i) phosphorylation occurred within 5 min after EGF stimulation in 48-h-old hepatocyte cultures blocked at this restriction point; (ii) in reversion experiments, the time interval after removal of the inhibitor and DNA synthesis was the same as in uninhibited cells, which are known to be blocked at the restriction point in the absence of mitogen; and (iii) MEK activation closely preceded cyclin D1 induction located in mid-late G1 of hepatocytes both in vivo and in vitro.
Accumulation of cyclin D1 mRNAs at the restriction point was found to depend on MEK activation by growth factor stimulation. In the presence of MEK inhibitor, no cyclin D1 mRNA accumulation was observed and the cells did not progress to S phase. Furthermore, transfection of a constitutively activated MEK1 mutant resulted in a twofold increase in cyclin D1 mRNA expression. Induction of cyclin D proteins, mainly cyclin D1, at the restriction point has been reported for various types of cells, including hepatocytes (3, 33, 39, 41). In association with Cdks, it could be one of the major events that drive the cells toward S phase. ERK1/2 activation has been proposed to be required for cyclin D1 up-regulation following cell growth induction after platelet-derived growth factor stimulation of Chinese hamster fibroblasts (36, 37). In these cells and in human breast cancer cells, inhibition of MEK by PD 98059 resulted in a loss of cyclin D1 expression (18, 60). Albanese et al. also reported that overexpression of either p42 MAPK or c-Ets-2 was able to stimulate the cyclin D1 promoter (2). Interestingly, a recent report shows that constitutively activated MEK results in up-regulation of cyclin D1 expression and assembly of dependent kinase, thus establishing a direct link between MEK1 and the cell cycle machinery in 3T3 fibroblasts (9, 22). Moreover, 22 of 25 cases of human hepatocellular carcinoma exhibited positive correlation between MAPK/ERK activation and cyclin D1 expression (27).
Finally, our observation that a growth factor dependency might exist up to mid-late G1 phase, but not after this stage, in hepatocytes from regenerating liver in vivo provides an additional argument in favor of the crucial role in vivo of the MEK/ERK cascade activation and cyclin D1 induction in the cell decision to progress to the G1/S boundary. Indeed, contrary to hepatocytes isolated 5, 7, or 9 h after PHT, cells from 12- and 15-h regenerating livers were able to progress in late G1 and replicated DNA without additional growth factor stimulation. A growth factor dependency could be defined in vivo in mid-late G1 between 9 and 12 h after PHT. Moreover, DNA synthesis could be blocked in regenerating hepatocytes isolated 15 h after PHT by intravenous administration of PD 98059 performed 10 h after PHT, a time preceding the growth factor restriction point. These results indicated a MEK/ERK growth factor dependency in mid-late G1 of proliferating hepatocytes in vivo like that described in vitro. The apparent discrepancy with the recent data of Spector et al. (55), who concluded that the MEK cascade does not play a role in hepatocyte growth, is probably due to the late stage (24 h after PHT) corresponding to S phase chosen for analysis.
Taken together, these results lead to the conclusion that the MEK/ERK cascade activation in proliferating liver in vivo is biphasic and could control two main points of G1 progression in the hepatocyte cycle. Other pathways must be important for hepatocyte proliferation (5, 29, 55, 56), but our work shows that the MEK/ERK cascade is a key signalling pathway essential for late G1 progression. The first activation, occurring very early in G1, could be associated with the prereplicative competence status, which enables the primed hepatocyte to progress in G1. The second activation in mid-late G1 resulting from growth factor stimulation is linked to cyclin D1 induction, which allows for progression to the G1/S boundary. These observations have helped us to determine a growth factor dependency in mid-late G1 and to define a crucial role of MEK signalling pathway in the process of liver regeneration and raise the question of the control of its activation in hepatocarcinogenesis.
ACKNOWLEDGMENTS
We thank R. L. Erikson and H. Greulich for providing us the constitutively activated MEK1 cDNA vector. We also thank J.-Y. Robert for expert technical assistance, F. McKenzie for fruitful suggestions, and A. Guillouzo for critical reading of the manuscript.
This research was supported by the Institut National de la Santé et de la Recherche Médicale, by EEC grant BIO4-CT 960052, and by the Association pour la Recherche contre le Cancer. H.T. is a recipient of a fellowship from the Association pour la Recherche contre le Cancer.
REFERENCES
- 1.Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. Possible involvement of pertussis toxin-sensitive G protein in hepatocyte growth factor-induced signal transduction in cultured rat hepatocytes: pertussis toxin treatment inhibits activation of phospholipid signalling, calcium oscillation, and mitogen-activated protein kinase. Hepatology. 1997;26:295–300. doi: 10.1002/hep.510260207. [DOI] [PubMed] [Google Scholar]
- 2.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21 ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht J H, Hu M Y, Cerra F B. Distinct patterns of cyclin D1 regulation in models of liver regeneration and human liver. Biochem Biophys Res Commun. 1995;209:648–655. doi: 10.1006/bbrc.1995.1548. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 98059 is a specific inhibitor of activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 5.Auer K L, Contessa J, Brenz-Verca S, Pirola L, Rusconi S, Cooper G, Abo A, Wymann M P, Davis R J, Birrer M, Dent P. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of hepatocytes. Mol Biol Cell. 1998;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block G D, Locker J, Bowen W C, Petersen B E, Katyal S, Storm S C, Riley T, Howard T A, Michalopoulos G K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan J M, Gruppuso P A. In vitro and in vivo regulation of hepatic mitogen-activated protein kinases in foetal rats. Am J Physiol. 1994;267:G1078–G1086. doi: 10.1152/ajpgi.1994.267.6.G1078. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Ishac E J N, Dent P, Kunos G, Gao B. Effects of ethanol on mitogen-activated protein kinase and stress-activated protein kinase cascades in normal and regenerating liver. Biochem J. 1998;334:669–676. doi: 10.1042/bj3340669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin-dependent kinase and titration of p27kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark-Lewis I, Sanghera J S, Pelech S L. Definition of a consensus sequence for peptide substrate recognition by p44 mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991;266:15180–15184. [PubMed] [Google Scholar]
- 11.Cook S J, Beltman J, Cadwallader K A, McMahon M, McCornick F. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca2+-dependent signal pathways in rat-1-cells. J Biol Chem. 1997;272:13309–13319. doi: 10.1074/jbc.272.20.13309. [DOI] [PubMed] [Google Scholar]
- 12.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase is necessary and sufficient for PC 12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furth E E, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukine-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 14.Diehl A M, Rai R M. Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215–227. doi: 10.1096/fasebj.10.2.8641555. [DOI] [PubMed] [Google Scholar]
- 15.Etienne P L, Baffet G, Desvergne B, Boisnard-Rissel M, Glaise D, Guguen-Guillouzo C. Transient expression of c-fos and constant expression of c-myc in freshly isolated and cultured normal adult rat hepatocytes. Oncogene Res. 1988;3:255–262. [PubMed] [Google Scholar]
- 16.Fabrikant J I. The kinetic of cellular proliferation in regenerating liver. J Cell Biol. 1968;36:551–565. doi: 10.1083/jcb.36.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fausto N, Laird A D, Webber E M. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–1536. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- 18.Fiddes R J, Janes P W, Sivertsen S P, Sutherland R L, Musgrove E A, Daly R J. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene. 1998;16:2803–2813. doi: 10.1038/sj.onc.1201815. [DOI] [PubMed] [Google Scholar]
- 19.Gines P, Li X, Brown S E S, Nakamura T, Guzelian P S, Heasley L E, Schirer R W, Nemenoff R A. Inhibitory actions of cyclic adenosine monophosphate and pertussis toxin define two distinct epidermal growth factor-regulated pathways leading to activation of mitogen-activated protein kinase in rat hepatocytes. Hepatology. 1996;23:1167–1173. doi: 10.1002/hep.510230535. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Lechon M J, Guillen I, Ponsoda X, Fabra R, Trullenque R, Nakamura T, Castell J V. Cell cycle progression proteins (cyclins), oncogene expression, and signal transduction during the proliferative response of human hepatocytes to hepatocyte growth factor. Hepatology. 1996;23:1012–1019. doi: 10.1002/hep.510230511. [DOI] [PubMed] [Google Scholar]
- 21.Goyette M, Petropoulos C J, Shank P R, Fausto N. Expression of a cellular oncogene during cellular regeneration. Science. 1983;219:510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- 22.Greulich H, Erikson R L. An analysis of MEK1 signaling in cell proliferation and transformation. J Biol Chem. 1998;273:13280–13288. doi: 10.1074/jbc.273.21.13280. [DOI] [PubMed] [Google Scholar]
- 23.Grisham J W. A morphological study of deoxyribonucleic acid synthesis and cell proliferation in regenerating liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- 24.Guguen C, Guillouzo A, Boisnard M, Le Cam A, Bourel M. Etude ultrastructurale de monocouches d’hépatocytes de rat cultivés en présence d’hémisuccinate d’hydrocortisone. Biol Gastroenterol. 1975;8:223–231. [PubMed] [Google Scholar]
- 25.Higgins G M, Anderson R M. Experimental pathology of liver; restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 26.Ikeda T, Sawada N, Fujinga K, Minase T, Mori M. c-Ha-ras gene is expressed at the G1 phase in primary cultures of hepatocytes. Exp Cell Res. 1989;185:292–296. doi: 10.1016/0014-4827(89)90058-x. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, Okamoto E, Hasashi N, Hori M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 28.Janknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis W D, Auer K L, Spector M, Kunos G, Grant S, Hylemon P, Mikkelsen R, Dent P. Positive and negative regulation of JNK1 by protein kinase C and p42 MAP kinase in adult rat hepatocytes. FEBS Lett. 1997;412:9–14. doi: 10.1016/s0014-5793(97)00705-9. [DOI] [PubMed] [Google Scholar]
- 30.Kahan C, Seuwen K, Meloche S, Pouysségur J. Coordinate, biphasic activation of p44 mitogen-activated protein kinase and S6 kinase by growth factors in hamster fibroblasts. J Biol Chem. 1992;267:13369–13375. [PubMed] [Google Scholar]
- 31.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 32.Kitano S, Fawcett T W, Yo Y, Roth G S. Molecular mechanisms of impaired stimulation of DNA synthesis in cultured hepatocytes of aged rats. Am J Physiol. 1998;275:C146–C154. doi: 10.1152/ajpcell.1998.275.1.C146. [DOI] [PubMed] [Google Scholar]
- 33.Koch K S, Lu X P, Leffert H L. Primary rat hepatocytes express cyclin D1 messenger RNA during their growth cycle and during mitogenic transitions induced by transforming growth factor-alpha. Biochem Biophys Res Commun. 1994;204:91–97. doi: 10.1006/bbrc.1994.2430. [DOI] [PubMed] [Google Scholar]
- 34.Kruijer W, Skelly H, Botteri F, Van der Putten H, Barber J R, Verma I M, Leffert H L. Proto-oncogene expression in regenerating liver is stimulated in culture of primary adult rat hepatocytes. J Biol Chem. 1986;261:7929–7933. [PubMed] [Google Scholar]
- 35.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie J N, L’Allemain G, Brunnet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOG MAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 37.Lavoie J N, Rivard N, L’Allemain G, Pouysségur J. A temporal and biochemical link between growth factor-activated MAP kinases, cyclin D1 induction and cell cycle entry. Prog Cell Cycle Res. 1996;2:49–58. doi: 10.1007/978-1-4615-5873-6_5. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Guyton K Z, Gorospe M, Xu Q, Kokkonen G C, Mock Y D, Roth G S, Holbrook N J. Age-related decline in mitogen-activated protein kinase in epidermal growth factor-stimulated rat hepatocytes. J Biol Chem. 1996;271:3604–3607. [PubMed] [Google Scholar]
- 39.Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Guguen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. J Biol Chem. 1996;271:11484–11492. doi: 10.1074/jbc.271.19.11484. [DOI] [PubMed] [Google Scholar]
- 40.Loyer P, Glaise D, Cariou S, Baffet G, Meijer L, Guguen-Guillouzo C. Expression and activation of Cdks (1 and 2) and cyclins in the cell cycle progression during liver regeneration. J Biol Chem. 1994;269:2491–2500. [PubMed] [Google Scholar]
- 41.Lu X P, Koch K S, Lew D J, Dulic V, Pines J, Reed S I, Hunter T, Leffert H L. Induction of cyclin mRNA and cyclin-associated histone H1 kinase during liver regeneration. J Biol Chem. 1992;267:2841–2844. [PubMed] [Google Scholar]
- 42.Marshall M S. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 43.McKillop I H, Schmidt C M, Cahill P A, Sitzmann J V. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;26:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 44.Michalopoulos G K, DeFrances M C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 45.Morello D, Lavenu A, Babinet C. Differential regulation and expression of c-jun, c-fos and c-myc protooncogenes during mouse liver regeneration and after inhibition of protein synthesis. Oncogene. 1990;5:1511–1519. [PubMed] [Google Scholar]
- 46.Musgrove E A, Hamilton J A, Lee C S L, Sweeney K J E, Watts C K W, Sutherland R L. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagès G, Lenormand P, L’Allemain G, Chambard J-C, Meloche S, Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang L, Sawada T, Decker S T, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 49.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 50.Sasse J, Hemmann U, Schwartz C, Schniertshauer U, Heesel B, Landgraf C, Schneider-Mergener J, Heinrich P C, Horn F. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol. 1997;17:4677–4686. doi: 10.1128/mcb.17.8.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaper F, Siewert E, Gomez-Lechon M J, Gatsios P, Sachs M, Birchmeier W, Heinrich P C, Castell J. Hepatocyte growth factor/scatter factor (HGF/SF) signals via the STAT3/APRF transcription factor in human hepatoma cells and hepatocytes. FEBS Lett. 1997;405:99–103. doi: 10.1016/s0014-5793(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt C M, McKillop I H, Cahill P A, Sitzmann J V. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997;236:54–58. doi: 10.1006/bbrc.1997.6840. [DOI] [PubMed] [Google Scholar]
- 53.Seger R, Seger D, Reszka A A, Munar E S, Eldar-Finkelman H, Dobrowolska G, Jensen A M, Campbell J S, Fisher E F, Krebs E G. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- 54.Sobczak J, Mechti N, Tournier M F, Blanchard J M, Duguet M. c-myc and c-fos genes in regulation during mouse liver regeneration. Oncogene. 1989;4:1503–1508. [PubMed] [Google Scholar]
- 55.Spector M S, Auer K L, Jarvis W D, Ishac E J N, Gao B, Kunos G, Dent P. Differential regulation of mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stolz D B, Michalopoulos G K. Comparative effects of hepatocyte growth factor and epidermal growth factor on motility, morphology, mitogenesis, and signal transduction of primary rat hepatocytes. J Cell Biol. 1994;55:445–464. doi: 10.1002/jcb.240550405. [DOI] [PubMed] [Google Scholar]
- 57.Taub R. Transcriptional control of liver regeneration. FASEB J. 1996;10:413–427. [PubMed] [Google Scholar]
- 58.Taub R. Blocking NF-kappaB in the liver: the good and bad news. Hepatology. 1998;27:1445–1446. doi: 10.1002/hep.510270538. [DOI] [PubMed] [Google Scholar]
- 59.Tombes R M, Auer K L, Mikkelsen R, Valerie K, Wymann M P, Marshall C J, McMahon M, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber J D, Raben D M, Phillips P J, Baldassare J J. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westwick J K, Fleckenstein J, Yin M, Yang S Q, Bradham C A, Brenner D A, Diehl A M. Differential regulation of hepatocyte DNA synthesis by cAMP in vitro and in vivo. Am J Physiol. 1996;271:G780–G790. doi: 10.1152/ajpgi.1996.271.5.G780. [DOI] [PubMed] [Google Scholar]
- 62.Yamada Y, Kirillowa I, Peschon J J, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan M, Templeton D J. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994;269:19067–19073. [PubMed] [Google Scholar]