Coronavirus disease 2019 (COVID-19) is a multisystem condition associated with cytokine-mediated hyperinflammation and coagulopathy due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The nose is usually the first portal of viral entry to the respiratory tract, followed by rapid nasociliary clearance of secretions to the oropharynx and aspiration into the lungs. Early involvement of the nose explains why patients with COVID-19 often experience an alteration in the sense of smell as a presenting symptom. Indeed, the nasopharynx is the most common site for SARS-CoV-2 swab testing. In more severe cases, often in susceptible older individuals with relevant comorbidities, SARS-CoV-2 infection may result in pneumonitis with hypoxia followed by adult respiratory distress syndrome, requirement for mechanical ventilation, and ultimately death.
The cascade of pro-inflammatory cytokine activation includes interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor alpha, with tissue-specific cellular inflammation. This, in turn, points to a putative role for local or systemic corticosteroid therapy to dampen down the resulting hyperinflammatory response. The PRINCIPLE (Platform Randomised Trial of Treatments in the Community for Epidemic and Pandemic Illnesses) trial using high-dose budesonide 1.6 mg via dry powder inhaler in ambulatory patients infected with SARS-CoV-2 and at high risk for severe disease showed a shortening of disease duration by 3 days but no significant influence in disease progression in terms of obviating hospital admission, mechanical ventilation, or death.1 One possible explanation for this earlier recovery with inhaled budesonide might be a putative systemic glucocorticoid effect, in addition to its local activity in the oropharynx and proximal airways, bearing in mind most of the emitted corticosteroid particles would have been too large to reach the alveoli. Other observational data from a U.K. database found that patients with asthma taking various inhaled corticosteroids (ICS) hospitalized with COVID-19 had a significant 14% mean reduction in mortality.2 Furthermore, the use of a medium-dose systemic corticosteroid such as dexamethasone 6 mg in the RECOVERY (Randomized Evaluation of COVID-19 Therapy Trial) trial in patients with severe hospitalized COVID-19 was associated with reduced mortality when the drug was administered more than 7 days after symptom onset.3 This amounted to having to treat 25 patients requiring supplemental oxygen or 8 mechanically ventilated patients in order to prevent 1 death.
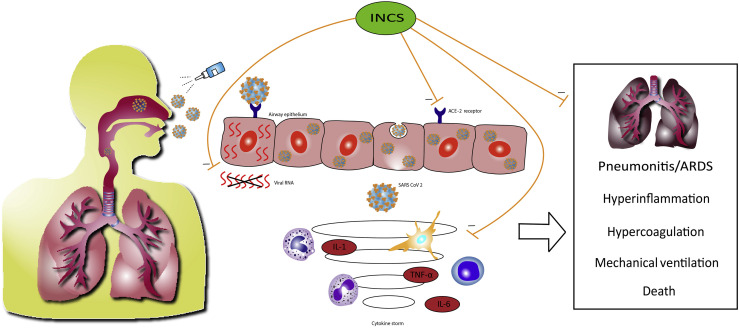
One could argue from a mechanistic point of view that it would be preferable to intercept SARS-CoV-2 in the nose before it travels to the lung with attendant systemic involvement (Figure 1 ).2 Angiotensin-converting enzyme-2 (ACE-2) is the epithelial entry receptor responsible for binding to the SARS-CoV-2 spike protein. Expression of ACE-2 is greatest in the nose with reducing levels down the respiratory tract, which is accompanied by a similar gradient for SARS-CoV-2 infection load along the airway epithelium.4 There is age-related expression of ACE-2 in the nose with older people having higher levels, which along with elderly comorbidities, perhaps explains why such patients often experience more COVID-19 disease.5 Inhaled corticosteroids as a class effect have been found to reduce levels of ACE-2 in induced sputum in a dose-dependent fashion,6 and ICS also suppress pro-inflammatory cytokines including IL-6. Another more specific mechanism of mometasone and ciclesonide invokes inhibition of in vitro replication of SARS-CoV-2.7 Hence, there appear to be cogent reasons to postulate that using intranasal corticosteroids (INCS) as prophylaxis early on in COVID-19 might be effective in preventing disease progression, through inhibiting viral entry and suppressing the inflammatory cascade related to severe COVID-19 disease.
Figure 1.
Schematic depiction of SARS-CoV-2 binding to the ACE-2 receptor in airway epithelium with the nose being the first portal of entry. This may be followed in more severe cases by subsequent involvement of the lung with development of pneumonitis and adult respiratory distress syndrome (ARDS), a need for invasive ventilation, and ultimately death. In tandem, there is a cytokine-mediated systemic hyperinflammatory and hypercoagulopathic state. It is postulated that intranasal corticosteroids might attenuate disease progression by preventing epithelial cell entry via ACE-2, suppressing local cytokine release, and inhibiting viral replication (mometasone furoate and ciclesonide). TNF-α, Tumor necrosis factor-alpha.
In this issue of the Journal, Strauss and colleagues8 performed a retrospective health informatics study using the Cleveland Clinic COVID-19 research registry with propensity score matching, including time-dependent analysis, to evaluate whether use of INCS prior to SARS-CoV-2 infection might ameliorate outcomes, including hospital admission, need for intensive care, or death in hospital, in 72,147 adult patients with confirmed infection who met the inclusion criteria. Among the 12,608 individuals (17.5%) from this cohort who were admitted, 2,935 (4.1%) required intensive care with 1,880 (2.6%) deaths. Within this cohort, there were 10,187 (14.1%) patients prescribed INCS who, compared with non-INCS users, were found to be 22% (95% CI 15-28) less likely to be hospitalized; 23% (95% CI 8-35) less likely to need intensive care; and 24% (95% CI 6-39) less likely to die. Notably similar results were obtained from a sensitivity analysis in which patients taking ICS and those with allergic rhinitis were excluded. Furthermore, when adjusting for baseline eosinophils, the protective effect of INCS remained significant. These findings are relevant because blood eosinophilia appears to be protective against worse COVID-19 outcomes, whereas the presence of increasing immunoglobulin E levels are associated with downregulation of nasal ACE-2 expression.9
These observations suggest the possibility that taking conventional low doses of INCS might be a cogent strategy to reduce the risk of disease progression in COVID-19. Clearly, further replication is indicated from other retrospective observational cohorts including different ethnic populations. Similarly, the interaction between the possible salutary effects of type 2 inflammation in patients with COVID-19 and the use of INCS to reduce that type 2 inflammation should be considered. Prospective evaluation is required in the setting of a large community-based randomized controlled trial in susceptible older individuals with comorbidities and also in immunosuppressed patients, who are more likely to develop severe COVID-19 with emerging variants, despite available vaccinations. Intranasal and inhaled ciclesonide are being considered in the United Kingdom as prophylactic therapy in ambulatory immunocompromised patients with chronic kidney disease (PROTECT-V [Prophylaxis for Vulnerable Patients at Risk of COVID-19 Therapy Trial], Clintrials.gov NCT04870333) to assess whether they might attenuate disease progression of COVID-19. However, such trials should also have an arm comprising INCS alone to tease out their effects aside from ICS. Other practical factors to consider are that INCS are relatively easy to administer, inexpensive, available as over-the-counter medicines, and usually indicated for once-daily dosing. The recent U.K. approval of the casirivimab and imdevimab antibody cocktail for use in vulnerable ambulatory individuals with early COVID-19 might make trials evaluating INCS prophylaxis untenable, whereas cost effectiveness might be another factor in poorer nations.
Several questions remain unanswered from the data of Strauss et al.8 It is important to know whether this is a protective class effect of corticosteroids or particular to certain INCS. For example, more lipophilic drugs such as budesonide and ciclesonide have prolonged mucosal retention owing to formation of intracellular lipid conjugates. The findings of Strauss et al8 were associated with the use of conventional doses of INCS, which are devoid of meaningful systemic bioactivity,10 in turn inferring that any protection against COVID-19 was likely due to topical effects alone.
The optimal timing of INS use is also still not known, but extrapolating from published trials, there may be benefit with preexposure use, early in known infection, and potentially as postexposure prophylaxis for those individuals in recent contact with COVID-19. Studies might also look at the degree of viral load after taking INCS, especially with those agents that suppress replication of SARS-CoV-2.7 In the meantime, the axiom primum non nocere (first do no harm) can now be affirmed to reassure patients with allergic rhinitis or nasal polyps taking maintenance INCS. Furthermore, prescribers should encourage their patients to keep taking their INCS because it might confer some protection against COVID-19 used either alone or in conjunction with ICS.
Footnotes
Funding: No funding has been received for this study.
Conflicts of interest: B. J. Lipworth reports nonfinancial support (equipment) from GSK; grants, personal fees (consulting, talks, and advisory board), other support (attending ATS and ERS), and from AstraZeneca, grants, personal fees (consulting, talks, advisory board), other support (attending ERS) from Teva, personal fees (consulting) from Sanofi, personal fees (consulting, talks, and advisory board) from Circassia in relation to the submitted work; personal fees (consulting) from Lupin, personal fees (consulting) from Glenmark, personal fees (consulting) from Vectura; personal fees (consulting) from Dr. Reddy, personal fees (consulting) from Sandoz; grants, personal fees (consulting, talks, advisory board), other support (attending BTS) from Boehringer Ingelheim; grants and personal fees (advisory board and talks) from Mylan outside of the submitted work; and B. J. Lipworth's son is presently an employee of AstraZeneca. T. F. Carr reports consulting fees from AstraZeneca, Genentech, GSK, Novartis, Regeneron; and royalties from UpToDate outside the submitted work. R. Chan declares no relevant conflicts of interest.
References
- 1.Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom C.I., Drake T.M., Docherty A.B., Lipworth B.J., Johnston S.L., Nguyen-Van-Tam J.S., et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., 3rd, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., et al. COVID-19-related genes in sputum cells in asthma. relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:e01648-20. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss R., Jawhari N., Attaway A.H., Hu B., Jehi L., Milinovich A., et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019 (COVID-19) J Allergy Clin Immunol Pract. 2021;9:3934–3940. doi: 10.1016/j.jaip.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipworth B., Chan R., RuiWen Kuo C. Type 2 asthma inflammation and COVID-19: a double edged sword. J Allergy Clin Immunol Pract. 2021;9:1163–1165. doi: 10.1016/j.jaip.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson A.M., Sims E.J., McFarlane L.C., Lipworth B.J. Effects of intranasal corticosteroids on adrenal, bone, and blood markers of systemic activity in allergic rhinitis. J Allergy Clin Immunol. 1998;102:598–604. doi: 10.1016/s0091-6749(98)70275-1. [DOI] [PubMed] [Google Scholar]