Abstract
Programmed cell death protein 1 (PD-1) inhibitors have demonstrated promising activity among patients with advanced soft tissue sarcomas (STS) in phase II trials. The purpose of this study was to assess the efficacy and safety of toripalimab (a novel PD-1 inhibitor) combined with doxorubicin as first-line treatment in patients with metastatic STS between December 2018 and September 2019. A total of 30 patients with metastatic STS were included and followed up retrospectively. One patient had complete response (CR), 10 patients obtained partial response, and 13 patients achieved stable disease. The objective response rate was 36.7% and the disease control rate was 80%. The median progression-free survival (PFS) was 8 months (95% CI: 6.30–10.64). The most frequent any grade adverse events were nausea (66.7%), fatigue (60%), and vomiting (40%). Neutropenia (20%) was the most common grade 3/4 adverse events, followed by leucopenia (13.3%) and febrile neutropenia (6.7%). No death related to treatment was observed during the drugs administration. Toripalimab combined with doxorubicin is effective in patients with metastatic STS as first-line treatment with manageable adverse events.
Keywords: doxorubicin, efficacy, safety, soft tissue sarcomas, toripalimab
Introduction
Soft tissue sarcomas (STS), comprising 1% of all solid tumors, account for more than 50 histologic subtypes and differ in clinical and pathological features, with an incidence of 5–6/100 000 per year [1]. Doxorubicin monotherapy has been the standard first-line chemotherapy for most advanced STS for four decades, yet the prognosis is generally unsatisfactory with objective response rates (ORRs) of 12–20%, median progression-free survival (PFS) of 4.1–6.8 months, and median overall survival (OS) ranging from 12.8 to 20.4 months [2,3]. Several combined treatments with doxorubicin and other antitumor drugs have been explored for survival benefit in a wide range of patients with advanced STS. However, they rarely lead to a high ORR and prolonged PFS, especially OS [4]. Thus, it is an urgent need to discover a novel therapeutic strategy against the disease.
Recently, increasing evidence showed that the interaction between programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) was the main pathway by which tumors regulate immune control. Toripalimab is a newly developed humanized IgG4 mAb, which exhibits the similar properties as pembrolizumab (a PD-1 inhibitor) and nivolumab (a PD-1 inhibitor) [5]. It targets the PD-1/PD-L1 interaction and shows promising antitumor activity in several malignancies [6,7], including melanoma, lymphoma, and STS. It has been approved by China Food and Drug Administration (CFDA) for unresectable or metastatic treatment-refractory melanoma based on its safety and efficacy since 2018. A phase I study of toripalimab in patients with refractory malignancies showed that 12 patients with alveolar soft part sarcoma treated with toripalimab had an ORR of 25.0% (3 in 12) and disease control rate (DCR) of 91.7% (11 in 12) [6]. Clinical trials of other PD-1 inhibitors have shown encouraging antitumor activity and favorable safety profile in advanced STS patients. In a multicenter randomized phase II trial of 85 patients with advanced sarcomas [8], nivolumab with or without ipilimumab (a CTLA-4 inhibitor) achieved response rates of 16% and 5%, respectively. Another multicenter phase II trial showed pembrolizumab monotherapy obtained an ORR of 18% with tolerable toxicity in 40 patients with advanced STS [9]. Interestingly, responses in patients in that study were generally durable, with a median duration of 33 weeks. However, the efficacy of PD-1 inhibitors alone in most STS patients is still limited because of their low ORR.
The success of PD-1 inhibitors combined with chemotherapy in other malignancies allows us to assert that the combined treatment may be a promising treatment strategy for advanced STS. Preclinical studies showed that doxorubicin not only killed tumor cells, but also regulated tumor microenvironment and increased tumor antigen presentation to enhance the antitumor effect of immunotherapy [10]. A cohort of patients was treated with toripalimab combined with doxorubicin in two hospitals since 2018. Herein, we conducted this observational study to assess the efficacy and safety of toripalimab and doxorubicin as first-line treatment for patients with metastatic STS.
Methods
Study design and ethics
This is a multicenter observational study. This study was in line with the Declaration of Helsinki, and approved by Institutional Review Board and Ethics Committees of Henan Cancer Hospital and The Affiliated People Hospital of Zhengzhou University. Informed consent was obtained from all patients before the treatment procedures.
Patients
Patients were eligible for the study who needed to meet the following criteria: (a) patients with metastatic confirmed STS who received toripalimab combined with doxorubicin in Henan Cancer Hospital and The Affiliated People Hospital of Zhengzhou University between December 2018 and September 2019; (b) patients between the ages of 18 and 65 years; (c) patients who had adequate blood, renal, liver, and cardiac functions; (d) patients with a performance status of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) performance scale; (e) patients with a life expectancy of at least 3 months; (f) presence of at least one measurable metastatic disease lesion according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; (g) patients who did not have prior systemic treatment including chemotherapy, immune checkpoint inhibitors, or targeted small molecule drugs.
All data, including demographic and clinical characteristics, were obtained and collected from patients’ medical history.
Treatment
During the first six 3-week cycles, patients were treated with toripalimab 240 mg intravenously on day 2 of each cycle, and doxorubicin on a dose of 37.5 mg/m2 per day on days 1 and 2 of each cycle. After the first six cycles of toripalimab combined with doxorubicin, if a complete response (CR), partial response (PR), or stable disease occurred, toripalimab was administrated continually until disease progression or unacceptable adverse events occurred. The study was permitted two doses reduction levels doxorubicin, from 37.5 mg/day to 31.9 mg/day and 27.1 mg/day, while toripalimab was not allowed to be reduced but could be delayed due to adverse events. When serious adverse events (SAEs) were observed, either toripalimab or doxorubicin was suspended until recovery to grade 1 or better adverse events. The treatment termination was recommended if disease progressed or adverse events remained unacceptable after the dose reduction and treatment interruption. Primary prophylaxis with PEGylated G-CSF was routinely given after each combined treatment. Adverse events were collected and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Efficacy and safety
Tumor responses were categorized as CR, PR, stable disease, and progressive disease (PD) according to the RECIST version 1.1. The efficacy of toripalimab combined with doxorubicin was assessed including ORR, DCR, and PFS. ORR was determined as the proportion of patients with CR and PR. DCR indicated the proportion of patients who achieved CR, PR, and stable disease. PFS referred to the time from the beginning of treatment to the first recorded date of progression or death or the date of the last tumor assessment showing no tumor progression for those patients without progression. The efficacy of the combined treatment was assessed using computed tomography (CT) or MRI scans. Tumor response was evaluated at baseline, every 2 months during the combined treatment, and then every 3 months during maintenance toripalimab. To evaluate the rate of tumor growth, the percentage change in size of target lesions was measured by professional radiologists using CT/MRI during the treatment.
Adverse events were monitored and collected from patients’ medical history and laboratory examination results or from telephone follow-up. Adverse events were graded using the CTCAE, v4.0.
Statistical analysis
All statistical data were analyzed by GraphPad Prism 8.11 software and SPSS 22.0 software. Quantitative variables were presented as medians (range) or numbers (percentage). The descriptive variables regarding treatment-related adverse events and patient characteristics were directly calculated from the database. PFS was plotted with Kaplan–Meier method.
Results
Patients and tumor characteristics
A total of 30 patients with metastatic STS who received toripalimab and doxorubicin as first-line treatment were included in this study. The median age was 52 years (range 20–65) and 12 patients were females. STS histological subtypes included undifferentiated pleomorphic sarcoma (n = 9), dedifferentiated liposarcoma (n = 6), angiosarcoma (n = 3), synovial sarcoma (n = 3), leiomyosarcoma (n = 3), myxofibrosarcoma (n = 2), epithelioid sarcoma (n = 2), clear cell sarcoma (n = 1), and malignant peripheral nerve sheath tumor (n = 1). The primary tumors occurred all over the body, and majority (n = 18) of them originated from the extremities. Twenty-five patients had undergone primary lesion resection, and 10 patients had a history of radiotherapy for local lesions. Lung metastasis occurred in 26 patients (86.7%). Table 1 shows patient characteristics at baseline and Table 2 lists patient demographics and characteristics.
Table 1.
Clinical characteristics of patients (N = 30)
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| Median | 52 |
| Range | (20–65) |
| Sex | |
| Female | 12 (40%) |
| Male | 18 (60%) |
| ECOG status | |
| 0 | 20 (66.7%) |
| 1 | 10 (33.3%) |
| Histologic subtype | |
| UPS | 9 (30%) |
| DDP | 6 (20%) |
| Angiosarcoma | 3 (10%) |
| Synovial sarcoma | 3 (10%) |
| Leiomyosarcoma | 3 (10%) |
| Other | 6 (20%) |
| Primary lesion | |
| Extremities | 20 (66.7%) |
| Trunk | 9 (30%) |
| Head and neck | 1 (3.3%) |
| Prior therapy | |
| Surgery | 28 (93.3%) |
| Radiotherapy | 10 (33.3%) |
| Metastatic site | |
| Lung | 27 (90%) |
| Lymph | 6 (20%) |
DDP, dedifferentiated liposarcoma; ECOG, Eastern Cooperative Oncology Group performance status; Other included myxofibrosarcoma, epithelioid sarcoma, clear cell sarcoma, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma.
Table 2.
Demographics and characteristics of patients (N = 30)
| Patient no | Gender | Age | ECOG PS | Histological type | Stage | Primary site | Metastatic site | Prior therapy | Best response | PFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 50 | 0 | UPS | IV | Upper limb | Lung | Surgery, radiotherapy | PR | 21 |
| 2 | Male | 55 | 0 | UPS | IV | Lower limb | Lung | Surgery, radiotherapy | SD | 8 |
| 3 | Male | 63 | 0 | UPS | IV | Thoracic wall | Lung | Surgery | PR | 15 |
| 4 | Female | 45 | 1 | UPS | IV | Lower limb | Lung | Surgery | SD | 12 |
| 5 | Male | 56 | 1 | UPS | IV | Lower limb | Lung | Surgery, radiotherapy | CR | 21 |
| 6 | Female | 52 | 0 | UPS | IV | Lower limb | Lung | Surgery | SD | 4 |
| 7 | Male | 58 | 0 | UPS | IV | Paravertebral | Lung | Surgery, radiotherapy | PR | 16 |
| 8 | Male | 62 | 0 | UPS | IV | Upper limb | Lung | Surgery, radiotherapy | PD | 2 |
| 9 | Male | 53 | 1 | UPS | IV | Upper limb | Lung | Surgery | PR | 18 |
| 10 | Male | 40 | 0 | Dedifferentiated liposarcoma | IV | Lower limb | Lung | Surgery, radiotherapy | PR | 10 |
| 11 | Female | 54 | 0 | Dedifferentiated liposarcoma | IIIB | Upper limb | Lymph | Surgery, radiotherapy | PD | 2 |
| 12 | Male | 63 | 0 | Dedifferentiated liposarcoma | IV | Lower limb | Lung | Surgery | SD | 3 |
| 13 | Male | 54 | 1 | Dedifferentiated liposarcoma | IV | Lower limb | Lung | Surgery | PD | 2 |
| 14 | Male | 61 | 0 | Dedifferentiated liposarcoma | IV | Lower limb | Lung | Surgery | SD | 4 |
| 15 | Male | 52 | 1 | Dedifferentiated liposarcoma | IV | Pelvic girdle | Lung | Surgery | SD | 10 |
| 16 | Female | 65 | 1 | Angiosarcoma | IV | Thoracic wall | Lung | Surgery | PR | 14 |
| 17 | Male | 64 | 0 | Angiosarcoma | IIIB | Head and neck | Lymph | Surgery | SD | 7 |
| 18 | Male | 60 | 0 | Angiosarcoma | IV | Lower limb | Lung | Surgery | PD | 2 |
| 19 | Female | 48 | 0 | Leiomyosarcoma | IV | Lower limb | Lung | Surgery, radiotherapy | PR | 10 |
| 20 | Male | 26 | 1 | Leiomyosarcoma | IV | Pelvic girdle | Lung | No | SD | 8 |
| 21 | Female | 48 | 0 | Leiomyosarcoma | IV | Lower limb | Lung | Surgery | SD | 5 |
| 22 | Female | 48 | 1 | Synovial sarcoma | IV | Pelvic girdle | Lung | Surgery | PR | 14 |
| 23 | Female | 40 | 0 | Synovial sarcoma | IV | Pelvic girdle | Lung | Surgery, radiotherapy | PR | 9 |
| 24 | Female | 26 | 1 | Synovial sarcoma | IIIB | Lower limb | Lymph | No | PR | 10 |
| 25 | Male | 20 | 1 | Epithelioid sarcoma | IIIB | Lower limb | Lymph | No | PD | 2 |
| 26 | Male | 32 | 0 | Epithelioid sarcoma | IV | Lower limb | Lung, Lymph | Surgery | PD | 2 |
| 27 | Female | 36 | 0 | Myxofibrosarcoma | IV | Paravertebral | Lung | Surgery, radiotherapy | SD | 6 |
| 28 | Female | 42 | 0 | Myxofibrosarcoma | IV | Paravertebral | Lung | No | SD | 8 |
| 29 | Female | 35 | 0 | Clear cell sarcoma | IV | Lower limb | Lung | No | SD | 7 |
| 30 | Male | 55 | 0 | Malignant peripheral nerve sheath tumor | IV | Upper limb | Lung, lymph | Surgery | SD | 4 |
CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; UPS, undifferentiated pleomorphic sarcoma.
Treatment
The median number of doxorubicin cycles administered was 6 (range 1–6). Twenty patients received six cycles of doxorubicin. Similarly, the median number of toripalimab cycles administered was 10 (range 1–30). Twenty-one patients received six cycles of toripalimab. At the cut-off time (15 September 2020), six patients remained on the combined treatment with toripalimab and doxorubicin, while the other 24 (80%) had discontinued. The main reason for treatment discontinuation was the disease progression. Among 10 patients who discontinued during combined treatment with toripalimab and doxorubicin, nine discontinued for tumor progression and one stopped for adverse events. About 60% (6 in 10) treatment discontinuations occurred between cycle 1 and cycle 2. Additionally, doxorubicin reduction occurred in four patients, the initial dose of doxorubicin was reduced to 31.9 mg/day in three patients, and the dose reduced to 27.1 mg/day in one patient. Most reasons for doxorubicin dose reductions were grade 3/4 neutropenia and leucopenia.
Efficacy
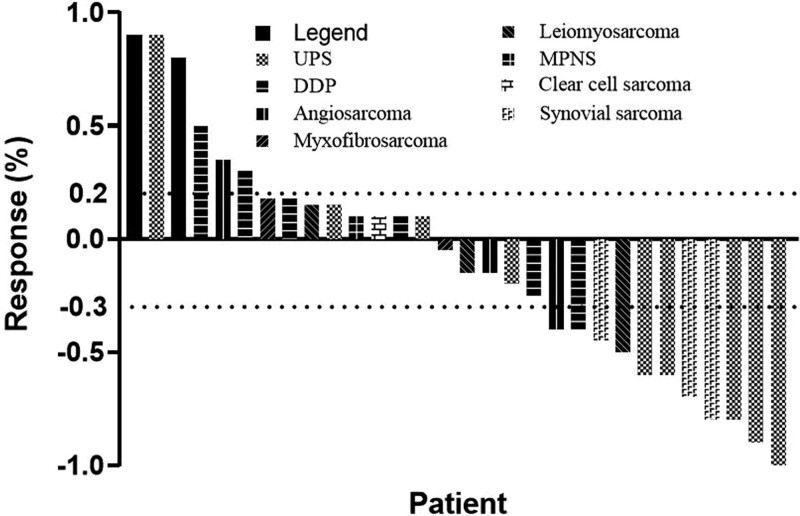
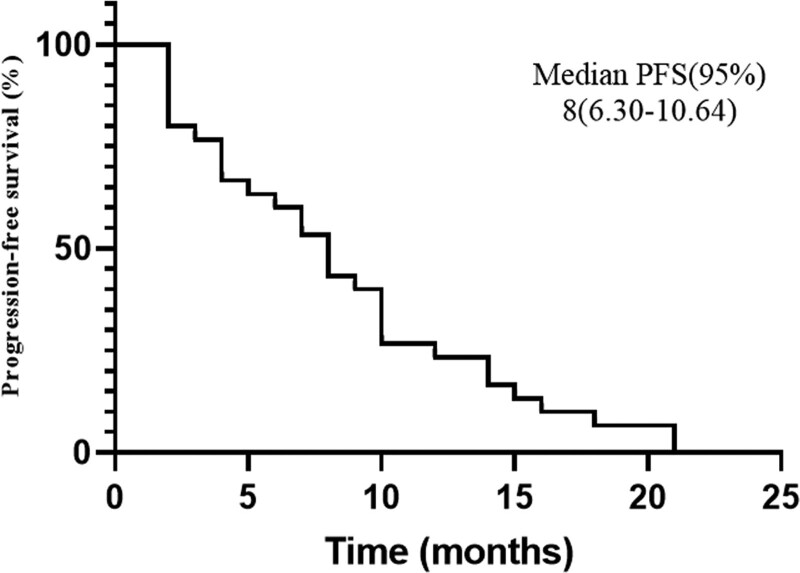
The median follow-up was 13.5 months (range 8–21), one patient had CR, and 10 patients obtained PR. The ORR was 36.7% (11 in 30). Their median duration of response was 12 months (range 6–19), and six (54.5%) of them showed response duration longer than 12 months. Thirteen patients achieved stable disease. The DCR was 80% (24 in 30). The best percentage change in tumor burden is shown in Fig. 1. The median PFS was 8 months (95% CI: 6.30–10.64). The Kaplan–Meier curve of PFS is shown in Fig. 2. The progression-free rates (PFRs) at 3 and 6 months were 80% and 63.3%, respectively. The details of the tumor responses are listed in Table 3.
Fig. 1.
Waterfall plots for the maximum percentage change from baseline in size of target lesions during the combined treatment. The dashed lines represent the criteria for progressive disease (20% increase in target lesion size) and partial response (30% decrease in target lesion size). DDP, dedifferentiated liposarcoma; MPNST, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma.
Fig. 2.
Kaplan–Meier curve of progression-free survival (PFS) of 30 patients with advanced soft tissue sarcomas (STS) with a median PFS of 8 months. CI, confidence interval.
Table 3.
Tumor responses (N = 30)
| Responses | Toripalimab and doxorubicin | |
|---|---|---|
| No | % | |
| CR | 1 | 3.3 |
| PR | 10 | 33.3 |
| SD | 13 | 43.3 |
| PD | 6 | 20 |
| ORR | 11 | 36.7 |
| DCR | 24 | 80 |
| Median PFS | 8 months | |
| PFR 3 months | 80 | |
| PFR 6 months | 63.3 | |
CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; PFR, progression-free rate; PFS, progression-free survival; SD, stable disease.
Safety
Overall, the adverse events associated with the combined treatment with toripalimab and doxorubicin were mild but manageable. All patients suffered at least one adverse event related to the treatment. The most common any grade adverse events were nausea (66.7%), fatigue (60%), and vomiting (40%). The most frequent grade 3/4 adverse events were neutropenia in six (20%) patients, leucopenia in four (13.3%) patients, and febrile neutropenia in two (6.7%) patients. This result was consistent with the previous reports. Grade 3/4 adverse events resulted in dose reduction in four patients (13.3%) and termination of treatment in one patient (3.3%). Among 15 patients who had immune-related adverse events, only one patient had immune-related adverse events of grade 3. No death related to treatment was reported during the study period. All adverse events are described in Table 4.
Table 4.
Adverse events (N = 30)
| G1 | G2 | G3 | G4 | Grade ≥ 1 | Percentage | Grade ≥ 3 | Percentage | |
|---|---|---|---|---|---|---|---|---|
| Nausea | 16 | 4 | 0 | 0 | 20 | 66.7 | 0 | 0 |
| Fatigue | 10 | 8 | 0 | 0 | 18 | 60 | 0 | 0 |
| Leucopenia | 2 | 2 | 3 | 1 | 7 | 23.3 | 4 | 13.3 |
| Neutropenia | 2 | 2 | 4 | 2 | 10 | 33.3 | 6 | 20 |
| Vomiting | 9 | 3 | 0 | 0 | 12 | 40 | 0 | 0 |
| Anemia | 2 | 1 | 1 | 0 | 4 | 13.3 | 1 | 3.3 |
| Thrombocytopenia | 2 | 1 | 1 | 0 | 4 | 13.3 | 1 | 3.3 |
| Transaminase increased | 3 | 2 | 0 | 0 | 5 | 16.7 | 0 | 0 |
| Diarrhea | 5 | 3 | 0 | 0 | 8 | 26.7 | 0 | 0 |
| Cough | 5 | 1 | 1 | 0 | 7 | 23.3 | 1 | 3.3 |
| Limb edema | 2 | 1 | 0 | 0 | 3 | 10 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 2 | 0 | 2 | 6.7 | 2 | 6.7 |
| Hypokalemia | 2 | 1 | 0 | 0 | 3 | 10 | 0 | 0 |
| Hypothyroidism | 2 | 1 | 0 | 0 | 3 | 10 | 0 | 0 |
| Hyperthyroidism | 1 | 1 | 0 | 0 | 2 | 6.7 | 0 | 0 |
Discussion
This study evaluated the efficacy and safety of toripalimab combined with doxorubicin in patients with metastatic STS as the first-line treatment. The ORR of 36.7% (11 in 30), DCR of 80% (24 in 30), and median PFS of 8 months (95% CI: 6.30–10.64) were observed, and there was no death related to adverse events, which suggests that the combined treatment is feasible and associated with well-tolerable adverse events.
For most patients with metastatic diseases, the latest NCCN guidelines recommend that doxorubicin alone remains the standard first-line chemotherapy. Due to the limited effective drugs and treatment options, the treatment of the group of heterogeneous advanced STS is challenging. Recently, some clinical trials have focused on the potential improved outcomes of addition other antitumor drugs to doxorubicin (Table 5) [11]. In a phase III trial of patients with advanced, metastatic STS [4], the combination of doxorubicin and ifosfamide achieved higher response rates and median PFS than doxorubicin alone, but did not improve median OS. The results are similar to those of two other clinical trials in similar population. Doxorubicin in combination with palifosfamide or evofosfamide only increased overall response rates [12,13], but showed no significant improvement in PFS and OS compared with doxorubicin alone. Although these important palliative achievements are achieved at the cost of greater drug toxicity, it is justified for patients receiving combined treatment to shrink the tumor rapidly in order to relieve symptoms, delay tumor progression as long as possible, and require neoadjuvant chemotherapy. A randomized phase Ib/II trial showed doxorubicin combined with olaratumab improved PFS and OS over doxorubicin alone in patients with advanced STS [14]. The magnitude of improvement in median PFS and median OS were 2.5 and 11.8 months, respectively. Unfortunately, in a following phase III trial [15], the combined treatment failed to achieve survival benefits compared with doxorubicin and placebo. Therefore, it is still necessary to look for new treatments to improve survival in patients with advanced disease.
Table 5.
Previous randomized controlled trials comparing doxorubicin with doxorubicin and other drugs in the treatment of soft tissue sarcoma
| Drug | Disease | Phase | The first authors last name |
Year of publication |
Trial sponsor | Number of patients |
Clinical outcome |
|---|---|---|---|---|---|---|---|
| Dox vs. CYVADIC vs. Ifo and Dox | Advanced STS | III | Santoro | 1995 | EORTC Soft Tissue and Bone Sarcoma Group | 263 vs. 142 vs. 258 | RR 21.3% vs. 26.8% vs. 25.2%; m-DOR 46 weeks vs. 48 weeks vs. 44 weeks; m-OS 52 weeks vs. 51 weeks vs. 55 weeks |
| Dox vs. Dox and Ifo | Locally advanced, unresectable, or metastatic high-grade STS | III | Judson | 2014 | EORTC | 228 vs. 227 | ORR 14% vs. 26%; m-PFS 4.6m vs. 7.4m; m-OS 12.8m vs. 14.3m |
| Dox vs. Dox and Tra | Advanced STS | II | Martin-Broto | 2015 | The Spanish Group for Research on Sarcoma | 55 vs. 60 | ORR:17% vs. 17%; m-PFS 5.5m vs. 5.7m; m-OS 13.7m vs. 13.3m |
| Dox vs. Dox and Pal | Metastatic STS | III | Ryan | 2016 | Ziopharm | 221 vs. 226 | ORR 19.9% vs. 28.3%; m-PFS 5.2m vs. 6.0m; m-OS 16.9m vs. 15.9m |
| Dox vs. Dox and Evo | Advanced, unresectable, or metastatic STS | III | Tap | 2017 | Threshold Pharmaceuticals | 323 vs. 317 | ORR 18% vs. 28%; m-PFS 6.0m vs. 6.3m; m-OS 19m vs. 18.4m |
| Dox vs. Dox and Ola | Unresectable or metastatic STS | Ib/II | Tap | 2017 | Eli Lilly and Company | 67 vs. 66 | ORR 11.9% vs. 18.2%; m-PFS 6.6m vs. 4.1m; m-OS 26.5m vs. 14.7m (P < 0.001) |
| Dox vs. Dox and Ola | Unresectable locally advanced or metastatic STS | III | Tap | 2020 | Eli Lilly and Company | 251 vs. 258 | ORR 14% vs. 18.3%; m-PFS 6.8m vs. 5.4m; m-OS 19.7m vs. 20.4m (P < 0.001) |
CYVADIC, cyclophosphamide, vincristine, doxorubicin, and dacarbazine; Dox, doxorubicin; EORTC, European Organisation for Research and Treatment of Cancer; Evo, evofosfamide; Ifo, ifosfamide; m-DOR, median duration of response; m-OS, median overall survival; m-PFS, median progression-free survival; Ola, olaratumab; ORR, objective response rate; Pal, palifosfamide; RR, response rates; STS, soft tissue sarcoma; Tra, trabectedin.
Recent comparative and prospective trials confirmed that PD-1 inhibitors combined with chemotherapy showed promising antitumor activity in several malignant tumors [16,17], including squamous cell carcinoma and non-small cell lung cancer. Notably, pembrolizumab combined with chemotherapy showed high response rates and survival benefits compared with chemotherapy alone even in patients with low PD-L1 tumor proportion score (<1%) [17]. Preclinical studies showed that doxorubicin could not only directly kill tumor cells and suppressive immunogenic cell, which resulted in release of tumor antigens and danger associated molecular patterns in the tumor microenvironment, but also induced type I interferons and T cell homing through induction of the chemokine CXCL10, and exposed calreticulin on dying cells. These mechanisms exerted positive immunomodulatory effects, enhancing the antitumor activity [10,18,19]. A recent study showed immunotherapy in combination with doxorubicin leaded to superior therapeutic responses and durable tumor control in mice tumor models [20]. Additionally, no significant increase in SAEs was observed in the treatment of PD-1 inhibitors combined with chemotherapy in most previous trials. Although the expression of PD-L1 is low in STS, toripalimab combined with doxorubicin may have synergistic antitumor effect in advanced STS. So, we began to treat patients with the combined treatment with toripalimab and doxorubicin for survival benefit after consideration of patient-specific factors.
To our best knowledge, this is the first study of toripalimab combined and doxorubicin in patients with advanced STS. OS was not achieved in this study. Hence, ORR, DCR, and median PFS were chosen as primary end points. The ORR was 36.7% (11 in 30) and DCR was 80% (24 in 30) with median PFS of 8 months (95% CI: 6.30–10.64). Although this study had the limitation of the nature of a retrospective study, the result suggested that the activity of the combined treatment may be slightly better than either PD-1 inhibitor or doxorubicin alone. So far, the highest response rate of immunotherapy for advanced STS in prospective studies has been reported by SARC028, which reported that pembrolizumab had the ORR of 18% and median PFS of 18 weeks [9]. In large randomized trials, doxorubicin alone had the ORR of 14–23.3% and median PFS of 4.6–6.0 months [11,12,15]. The result of this study is similar to that reported in a recent study. In a nonrandomized phase I/II trial of 37 patients with advanced anthracycline-naive sarcoma who were treated with doxorubicin and pembrolizumab [21], leiomyosarcoma (n = 11) was the most common histologic subtype. Although the ORR was of 13%, which failed to reach the primary end point, PFS and OS were favorable, with median PFS and OS being 8.1 months and 27.6 months, respectively. The PFS rates at 12 and 24 weeks were 81% and 73%, respectively. Some patients with undifferentiated pleomorphic sarcoma and undifferentiated liposarcoma had durable responses. Another phase II clinical trial showed that pembrolizumab combined with pegylated liposomal doxorubicin (PLD) achieved a higher ORR and median PFS than either PLD alone or anti-PD-1/PD-L1 agents alone in platinum resistant ovarian cancer [22]. The great efficacy may be mainly attributed to the additive or synergistic effects of PD-1 inhibitors and doxorubicin. However, there are also studies of limited efficacy of PD-1 inhibitor and chemotherapy. In a phase II trial [23], the combination of pembrolizumab and cyclophosphamide showed that only three of 50 patients with advanced sarcomas had clinical benefit. The poor efficacy may be related to the inhibition of pembrolizumab activity by cyclophosphamide and low PD-1 expression of STS. There are one ongoing trial of PD-1 inhibitor and doxorubicin in advanced STS. It is an open-label phase II study that recruited 30 patients with advanced sarcomas to assess the safety, tolerability, and efficacy of doxorubicin in combination with pembrolizumab in metastatic or unresectable soft tissue sarcoma (NCT03056001) [24]. The primary end point was the serious or life-threatening treatment emergent adverse event rate. An early report of the study showed that the combined treatment had manageable toxicity and promising activity, with an ORR of 33% and median PFS of 6.9 months.
The hematological adverse events in this study were less than those reported in the previous studies. The most common grade 3–4 hematological adverse events were neutropenia, leucopenia, and febrile neutropenia. The prophylactic use of PEGylated G-CSF might be helpful to reduce hematological adverse events in this study. However, the nonhematological adverse events were similar or slightly higher than those treated with doxorubicin. This may be due to the addition of pembrolizumab to doxorubicin treatment. A total of four patients reduced doxorubicin dose due to grades 3 and 4 adverse events, and one of them stopped treatment.
Although the limited number of patients and retrospective nature made it impossible to draw a definite conclusion, this study showed that toripalimab combined with doxorubicin as first-line treatment for patients with advanced STS might be effective and feasible. Large-scale comparative trials of the two drugs are needed to clarify the benefits of the addition of toripalimab to doxorubicin.
Conclusion
Taken together, the results suggest that toripalimab combined with doxorubicin is effective in the treatment of metastatic STS as the first-line treatment, with well-tolerated adverse events. It needs to conduct a randomized controlled trial to establish a more specific and accurate description.
Acknowledgements
We are grateful to all the patients and their families for their participation in this study.
H.G., S.G., C.L., and Z.L.: conception and design. Z.L., J.W., and P.Z.: collection and assembly of data. Z.L. and C.L.: data analysis and interpretation. Z.L. and W.Y.: article preparation. Z.L. and W.Y.: revision of the article; All authors approved the article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000; 1:75–85. [DOI] [PubMed] [Google Scholar]
- 2.Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017; 18:1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Graaf WTA, Jones RL. Neoadjuvant chemotherapy in localised soft-tissue sarcomas: where do we go from here? Lancet Oncol. 2017; 18:706–707. [DOI] [PubMed] [Google Scholar]
- 4.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. ; European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014; 15:415–423. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Guo L, Zhang J, Zhou Y, Zhou J, Yao J, et al. Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy. MAbs. 2019; 11:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Dong L, Yang S, Han X, Han Y, Jiang S, et al. Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer. 2020; 130:182–192. [DOI] [PubMed] [Google Scholar]
- 7.Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res. 2020; 26:4250–4259. [DOI] [PubMed] [Google Scholar]
- 8.Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013; 24:257–263. [DOI] [PubMed] [Google Scholar]
- 9.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017; 18:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013; 62:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano K, Takahashi S. Precision medicine in soft tissue sarcoma treatment. Cancers (Basel). 2020; 12:E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan CW, Merimsky O, Agulnik M, Blay JY, Schuetze SM, Van Tine BA, et al. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016; 34:3898–3905. [DOI] [PubMed] [Google Scholar]
- 13.Tap WD, Papai Z, Van Tine BA, Attia S, Ganjoo KN, Jones RL, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017; 18:1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016; 388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, et al. ; ANNOUNCE Investigators. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA. 2020; 323:1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, et al. ; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019; 394:1915–1928. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. ; KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018; 379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva CG, Rueda F, Löwik CW, Ossendorp F, Cruz LJ. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials. 2016; 83:308–320. [DOI] [PubMed] [Google Scholar]
- 19.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007; 7:139–147. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva CG, Camps MGM, Li TMWY, Zerrillo L, Löwik CW, Ossendorp F, Cruz LJ. Effective chemoimmunotherapy by co-delivery of doxorubicin and immune adjuvants in biodegradable nanoparticles. Theranostics. 2019; 9:6485–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack SM, Redman MW, Baker KK, Wagner MJ, Schroeder BA, Loggers ET, et al. Assessment of doxorubicin and pembrolizumab in patients with advanced anthracycline-naive sarcoma: a phase 1/2 nonrandomized clinical trial. JAMA Oncol. 2020; 6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EK, Xiong N, Cheng S-C, Barry WT, Penson RT, Konstantinopoulos PA, et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: a phase 2 clinical trial. Gynecol Oncol. 2020; 159:72–78. [DOI] [PubMed] [Google Scholar]
- 23.Lien IC, Pollack SM. Limited activity of metronomic cyclophosphamide and pembrolizumab for soft tissue sarcomas. Transl Gastroenterol Hepatol. 2018; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livingston M. A pilot study evaluating the safety, tolerability, and efficacy of doxorubicin and pembrolizumab in patients with metastatic or unresectable soft tissue sarcoma. https://meetinglibrary.asco.org/record/186764/abstract. [Accessed 5 May 2021]