Abstract
Background and Objectives
Prevalence of nursing home residents with Alzheimer’s disease and related dementias (ADRD) has increased along with a growing consensus that person-centered ADRD care in nursing homes should maximize quality of life (QoL). However, concerns about whether residents with ADRD can make appropriate QoL judgments persist. This study assesses the stability and sensitivity of a self-reported, multidomain well-being QoL measure for nursing home residents with and without ADRD.
Research Design and Methods
This study linked the 2012–2015 Minnesota Nursing Home Resident QoL and Satisfaction with Care Survey, Minimum Data Set 3.0 (nursing home assessments), and Minnesota Department of Human Services Cost Reports. The QoL survey included cohort–resident pairs who participated for 2 consecutive years (N = 12 949; 8 803 unique residents from 2012–2013, 2013–2014, and 2014–2015 cohorts). Change in QoL between 2 years was conceptualized as stable when within 1.5 SD of the sample average. We used linear probability models to estimate associations of ADRD/Cognitive Function Scale status with the stability of QoL summary and domain scores (eg, social engagement) and the absolute change in QoL summary score, controlling for resident and facility characteristics.
Results
Most (86.82%) residents had stable QoL summary scores. Residents with moderate to severe cognitive impairment, irrespective of ADRD, were less likely to have stable summary scores than cognitively capable residents without ADRD (p < .001), but associations varied by QoL domains. Among those with stable summary QoL scores, changes in health/functional status were associated with absolute changes in summary QoL score (p < .001), suggesting sensitivity of the QoL measure.
Discussion and Implications
QoL scores were similarly stable over time for most residents with and without ADRD diagnoses and were sensitive to changes in health/functional status. This self-reported QoL measure may be appropriate for nursing home residents, regardless of ADRD diagnosis, and can efficaciously be recommended to other states.
Keywords: ADRD, Long-term care, Measurement, Person-centered care, Policy, Surveys
Translational Significance: Residents in skilled nursing facilities complete quality of life (QoL) surveys to assess their well-being and care experience, but these scales may not be adequate for people with Alzheimer’s disease and related dementias (ADRD). In fact, our results show that self-reported QoL measures from the Minnesota Nursing Home Resident QoL Survey were similarly stable and sensitive for nursing home residents with and without ADRD. QoL assessment is appropriate for nursing home residents, regardless of ADRD diagnosis, and should be adopted nationally.
In 2020, an estimated 5.8 million Americans aged 65 and older lived with Alzheimer’s disease and related dementias (ADRD), and this number is projected to increase across the United States (1). Between 75% and 90% of persons with ADRD will need a nursing home (NH)-level care (2,3), and up to 50% of NH residents have ADRD, creating a mandate to understand quality of care in NHs among residents with ADRD (4).
There is growing recognition that person-centered measures are important for dementia care (5), and consensus that person-centered dementia care in NH settings needs to maximize residents’ quality of life (QoL) (6). QoL is a multidimensional measure of overall well-being and is best measured by self-report because objectively coded and proxy-reported measures do not fully capture the phenomenological experience of residents (7). By tailoring care to provide the greatest possible QoL, the quality of that care, by definition, improves in the ways that will best enhance the experience of NH residents. Yet, because QoL is fundamentally subjective, some have questioned its validity of reporting for persons with ADRD whose declines in cognitive, social, and physical functions may reduce their ability to make complex judgments about their QoL (8,9). If QoL is not assessed in NH residents with ADRD, then care cannot be tailored to meet residents’ unique preferences and needs. Health care providers and staff cannot assume that their values are the same as those of NH residents with ADRD and may not otherwise know what persons living with dementia need for their well-being and care. To examine whether persons with ADRD can make subjective QoL judgments, this work will compare the stability and sensitivity of a self-reported, multidomain QoL measure for NH residents with and without ADRD.
Background: Assessing QoL for Persons With ADRD
In part due to concerns about recall and ability to answer self-reported questions, several QoL questionnaires have been developed specifically for persons with ADRD. These include Dementia Quality of Life, EQ-5D, Quality of Life in Alzheimer’s Disease (QoL-AD), and Quality of Life for Dementia (9,10). Yet, generic QoL questionnaires are also sometimes used to study QoL in persons with ADRD (9). Studies examining the consistency of information from persons with ADRD and studies evaluating the validity of ADRD-specific QoL questionnaires have demonstrated that persons with ADRD are able to provide consistent and accurate responses to fact-based questions (9,11–14). These studies examined interrater reliability and test–retest reliability of the QoL instruments, typically over a period of a few weeks. However, a 2014 systematic review for QoL measures used in NHs (the most recent we located) found no studies that validated the use of QoL measures across years (10). The few studies that examined the stability of QoL scores over 12 months in persons with ADRD used small samples (n < 129) (15,16). Studies examining changes in QoL scores among NH residents with ADRD found statistically nonsignificant changes over time (17–22). Gaps in knowledge persist following these studies because their small sample sizes may have been underpowered to find true differences, and those studies did not explicitly assess the impact of health/functional changes on QoL.
Unfortunately, when NH residents have greater functional limitations, those limitations can decrease their ability to participate in the kinds of activities that might otherwise improve QoL while requiring increasingly invasive clinical interventions (7). Thus, a self-reported QoL measure should be sensitive to changes in function. At the same time, a resident’s QoL scores should typically be stable across time when there is no considerable change in a person’s function (23). If self-reported QoL for NH residents with ADRD shows this pattern of sensitivity to changes in capacity for function, that sensitivity would be a strong indicator of the validity of the QoL measure. Two ways to assess functional changes over time are through health status (eg, functional decline, new medical conditions) and hospitalizations. Health status may be the strongest predictor of subjective well-being (24) and is closely tied to hospitalizations for NH residents and older persons (25,26). Hospitalizations could result in further functional decline and complications that require further clinical interventions, reducing QoL (27).
Minnesota is one of the 2 states (the other is Ohio) where the Minnesota Department of Health conducts validated in-person surveys of NH residents’ QoL. Since 2005, the state of Minnesota has administered the Minnesota NH Resident QoL and Satisfaction with Care Survey to a random sample of residents in all Medicaid-certified NHs, which are reported via the Minnesota NH Report Card (28). QoL scores from the Minnesota QoL survey have been used as an indicator for NH care performance evaluation. The annual Minnesota Resident QoL and Satisfaction with Care Survey* (see Author Note) is not tailored to the ADRD context, but includes domains often seen in dementia-specific QoL instruments, including positive and negative mood and engagement in activities (9,10). Previous research has demonstrated the high validity of this survey (7). However, the stability and sensitivity of the QoL scores from this survey have not yet been examined for residents with ADRD.
New Contribution
The survey’s large sample size and inclusion of all Medicare–Medicaid certified NHs in the state allow well-powered examination of the stability and sensitivity of QoL scores among NH residents with and without ADRD diagnoses. It also allows for the identification of factors related to the stability of QoL scores, a gap in previous work. This study aims to address the following research questions:
(1) Are QoL scores in the Minnesota NH Resident QoL measure similarly stable among persons with and without ADRD?
(2) Are QoL score changes in the Minnesota NH Resident QoL measure sensitive to major health/functional changes? If so, how sensitive are they and what is the role of other resident and facility characteristics?
Research Design and Methods
Data
This study linked 3 sources of data: (a) the annual Minnesota Resident QoL and Satisfaction with Care Survey from 2012 to 2015, (b) the Minimum Data Set 3.0 (MDS) NH resident assessments conducted immediately before the resident’s Minnesota QoL survey date, and (c) facility characteristics from Minnesota Department of Human Services Cost Reports. The Minnesota QoL survey is administered as part of a wider 37-item interview that takes approximately 20 minutes to administer, is conducted in-person annually with approximately 13 000 residents of Medicare/Medicaid certified NHs in Minnesota. A random sample of eligible residents is selected to participate in the survey. Residents who are severely cognitively impaired (Cognitive Performance Scale [CPS] score of 6 and/or Brief Interview for Mental Status [BIMS] score of 0 or 1) or ill are ineligible; a resident’s guardian can also refuse participation on the resident’s behalf. Residents with ADRD are not excluded from participation. Trained interviewers use a standardized questionnaire that measures QoL over multiple domains.
Study Sample
Our analytic sample included residents who participated in the QoL survey in 2 consecutive years to allow us to capture changes in QoL over time. This resulted in 3 cohorts: (a) participants in both 2012 and 2013 surveys, (b) participants in both 2013 and 2014 surveys, and (c) participants in both 2014 and 2015 surveys. In sensitivity analyses, the 3 cohorts had similar cognitive health, functional health, and demographic characteristics. Given their comparability, we pooled the 3 cohorts to obtain a larger sample and better represent small nursing facilities. In this pooled sample, residents who participated in more than 2 surveys were counted more than once, resulting in an increased representation of long-stay residents. Analyses were at the cohort–resident level. We excluded residents whose ADRD and race information were missing (N < 11). The final sample contained 12 949 cohort–resident pairs (8 803 unique residents and 3 120 residents participated in more than 2 surveys). Overall, more than 90% of respondents in each year had less than 10 question items missing. Residents with ADRD diagnoses and/or cognitive impairment were more likely to have more QoL survey items missing. Factors associated with having missing survey items and QoL were included in our imputation models, including ADRD diagnoses and cognitive status. We used multiple imputation by chained equations to address missingness on survey items (0.77% had more than half of items missing) and resident and facility characteristics (all <2% missing).
Measurements
Dependent variable
Stability of QoL scores.
—QoL summary scores were created from 31 items that assessed 6 QoL domains: environment, attention from staff, food enjoyment, social engagement, negative mood, and positive mood. Summary scores ranged from 0 to 100 with higher scores indicating higher QoL (additional information about the Minnesota NH resident QoL measure is provided in Supplementary Appendix). We considered changes within 1.5 SD as a typical fluctuation range representing stable QoL scores. This decision was based on a similar approach used to identify differences in quality of care Star Ratings in the Minnesota NH Report Card, which also used the 1.5 SD limit as a threshold to define high- and low-performing facilities. We also conducted sensitivity analyses using 2 SD as our cutoff in line with other recommendations to measure the difference in scores (29), which did not significantly alter results.
Sensitivity of QoL scores.
—A justifiable QoL instrument should change in response to changes in health and function status. To examine the sensitivity of the QoL measure, we used absolute unit changes in QoL summary score (QoL score changes from Year 1 to Year 2, either an increase or decrease, with a range of 0–100) as an outcome variable to understand how QoL changes in response to changes in health and functional conditions.
Independent variables
We created all resident characteristic variables using MDS and all facility characteristic variables using quarterly MDS reports and the Department of Human Services Nursing Facility Cost Report. For each cohort–resident pair, we created resident characteristic variables based on assessments matched with the first-year survey. To capture health status change between the 2 surveys, we also included variables indicating the number of all-cause hospitalizations and significant changes in status (defined by the MDS as a decline or improvement in resident’s status that requires clinical interventions, affects health status, or requires review and/or revision of care plan) (30).
Resident characteristics: ADRD and cognitive function status.
—MDS assessments report active diagnoses in the last 7 days. We used assessments matched with first-year QoL surveys and the most recent comprehensive assessments before the QoL survey date to assess if a resident in a cohort was diagnosed with ADRD. We created a binary variable to indicate whether a cohort–resident pair had ADRD diagnoses. We measured cognitive function using the Cognitive Function Scale (CFS) developed by Thomas et al. (31), which combined the BIMS summary score and the CPS score. Due to the small number of residents with severe cognitive impairment (1.58% with CPS score of 5 or 6, a different definition of severe impairment from the survey exclusion criteria), we combined severe impairment with moderate impairment, creating 3 CFS categories: capable, mildly impaired, and moderately to severely impaired. CFS was strongly positively correlated with ADRD diagnoses (correlation = 0.43, p < .001). Because ADRD residents with different cognitive function may respond to surveys differently, we also created a 6-category variable to indicate the interaction between ADRD diagnoses and CFS statuses (ie, ADRD/Cognitively Capable, No ADRD/Cognitively Capable, ADRD/Mildly Impaired, No ADRD/Mildly Impaired, ADRD/Severely Impaired, and No ADRD/Severely Impaired).
Other resident characteristics.
—We included 12 other resident characteristics that are associated with QoL scores based on previous research (7). Age and length of NH stay were continuous variables. We used 4 categories for race/ethnicity: White, Black, American Indian, and Other (Asians, Hispanics, and those who chose multiple races were combined due to low representation). Sex and marital status were binary variables. Activities of daily living (ADL) scores ranged from 0 to 28 (higher scores indicated greater impairment). We included 3 binary variables to indicate (a) whether a resident had an anxiety or depression diagnosis, (b) any other severe mental illness diagnoses (bipolar, psychotic, schizophrenia, or posttraumatic stress disorder), and (c) any behavioral symptoms. Chronic condition count ranged from 0 to 5 indicating the number of conditions a resident had (congestive heart failure, diabetes, stroke, asthma, and cancer). Health status change was measured with (a) the number of all-cause hospitalizations between the 2 surveys and (b) the number of MDS significant changes in status between 2 surveys. Each of these 2 variables had 3 categories, indicating whether a cohort-resident pair had 0, 1, or more than 1 hospitalization or significant change in status.
Facility characteristics
We included 9 characteristics of the cohort resident’s facility that are associated with QoL based on previous studies on this topic. We calculated the proportion of racial/ethnic minority residents in the facility as the average proportion of racial/ethnic minority residents over the 4 quarterly MDS census reports in the year of a cohort resident’s first survey. Other facility characteristics were measured in the Department of Human Services Nursing Facility Cost Report, which included the facility’s proportion of Medicaid patient-days, the facility’s proportion of Medicare patient-days, whether the facility was in a metro area (vs other), the facility’s ownership (for profit, nonprofit, and government-owned), number of beds in the facility, the facility’s staff retention rate, resident acuity in the facility, and the facility’s proportion of private rooms.
Statistical Analyses
We first explored characteristics of our sample at a cohort–resident level for cohort–residents with and without ADRD diagnoses for the first survey year. We then assessed changes in QoL summary and domain scores between 2 years for residents with and without ADRD diagnoses. We plotted changes in QoL summary scores against the 2-year average of the scores. We also graphed the correlation between ADRD and CFS. We used linear probability models to estimate the associations of ADRD and CFS status with the stability of QoL summary and domain scores, while controlling for resident and facility factors. To examine whether QoL was sensitive to health status change, we used linear regression to estimate the effects of changes in resident and facility characteristics on the absolute change in QoL summary scores. For these analyses, we divided our sample into 2 groups: those with stable summary QoL scores versus those with unstable QoL scores and examined the associations of resident and facility characteristics with absolute QoL summary score changes separately for these 2 groups.
Results
Table 1 compares the characteristics of residents with ADRD diagnoses versus residents without ADRD diagnoses in our sample at a cohort–resident level for the first survey year. Compared to residents without ADRD diagnoses, residents with ADRD diagnoses had higher QoL summary scores, higher environment, food enjoyment, and negative mood domain scores, and lower engagement and positive mood domain scores. Given our large sample size, resident characteristics and most residing facility characteristics did not differ substantially between residents with and without ADRD diagnoses though statistical tests were significant. Compared to residents without ADRD diagnoses, residents with ADRD diagnoses were more likely to have greater cognitive impairment, anxiety or depression diagnosis, severe mental illness, and behavioral symptoms. Moreover, residents with ADRD diagnoses were more likely to have significant changes in status, but less likely to have hospitalizations between the 2 surveys than those without ADRD diagnoses. Residents with ADRD diagnoses were more likely to be in facilities in metro areas than residents without ADRD diagnoses.
Table 1.
Sample Characteristics Upon the First Year Survey by ADRD (N = 12 949)
| Variables | Non-ADRD (N = 7 392) | ADRD (N = 5 557) | P * |
|---|---|---|---|
| Quality of life scores | |||
| Summary score | 81.20 | 81.96 | .003 |
| Environment | 85.33 | 87.88 | <.001 |
| Attention from staff | 93.75 | 93.98 | .409 |
| Food enjoyment | 80.78 | 86.21 | <.001 |
| Engagement | 81.02 | 78.71 | <.001 |
| Negative mood † | 67.08 | 68.01 | .024 |
| Positive mood | 79.22 | 76.97 | <.001 |
| Resident characteristics | |||
| Race | |||
| White | 95.64% | 96.72% | .001 |
| Black | 1.92% | 1.76% | .508 |
| American Indians | 0.93% | 0.85% | .598 |
| Other ‡ | 1.50% | 0.67% | <.001 |
| Age | 81.05 | 84.40 | <.001 |
| Female | 69.95% | 70.72% | .344 |
| Married | 18.82% | 20.74% | .007 |
| Length of stay (days) | 757.66 | 748.24 | .577 |
| ADL score (0–28) | 12.66 | 13.82 | <.001 |
| Cognitive Function Scale | |||
| Capable | 66.82% | 27.31% | <.001 |
| Mildly impaired | 22.53% | 31.12% | <.001 |
| Moderately to severely impaired | 10.65% | 41.57% | <.001 |
| Anxiety or depression Dx | 56.45% | 62.82% | <.001 |
| Severe mental illness Dx § | 14.37% | 17.11% | <.001 |
| Behavioral symptoms ‖ | 11.60% | 18.58% | <.001 |
| Chronic conditions (0–5) ¶ | 0.99 | 0.86 | <.001 |
| Hospitalizations between 2 surveys | |||
| 0 | 74.96% | 82.65% | <.001 |
| 1 | 17.07% | 13.57% | <.001 |
| >1 # | 7.97% | 3.78% | <.001 |
| MDS significant status change between 2 surveys | |||
| 0 | 82.35% | 80.62% | .013 |
| 1 | 13.28% | 14.94% | .008 |
| >1 # | 4.37% | 4.44% | .837 |
| Facility characteristics | |||
| Proportion of non-White residents | 4.76% | 3.75% | <.001 |
| Proportion of Medicaid patient-days | 57.95% | 56.98% | <.001 |
| Proportion of Medicare patient-days | 7.71% | 8.14% | <.001 |
| Metro area | 33.00% | 41.03% | <.001 |
| Ownership | |||
| For profit | 28.27% | 25.90% | .003 |
| Nonprofit | 60.90% | 63.92% | <.001 |
| Government | 10.82% | 10.19% | .241 |
| Number of beds | 78.34 | 83.14 | <.001 |
| Staff retention | 0.70 | 0.70 | .678 |
| Acuity | 1.00 | 1.02 | <.001 |
| Proportion of private rooms | 33.19% | 33.99% | .132 |
Note: ADRD = Alzheimer’s disease and related dementias; ADL = activities of daily living; MDS = Minimum Data Set.
*p Values of t tests comparing the means for non-ADRD versus ADRD.
†Reverse-coded. A higher value indicates better quality.
‡Other includes Asians, Hispanics, and those who chose multiple races.
§Bipolar, psychotic, schizophrenia, and posttraumatic stress disorder.
‖Physical or verbal behavioral symptoms directed toward others, or other behavioral symptoms not directed toward others.
¶Congestive heart failure, diabetes, stroke, asthma, and cancer.
#Less than 11 pairs with this information missing were included in this category.
Table 2 presents 2-year average QoL summary and domain scores, as well as change scores from Year 1 to Year 2 for cohort residents with and without ADRD diagnoses. On average, QoL summary scores declined by 1.01 for residents without ADRD diagnoses and by 0.69 for those with ADRD diagnoses, but the difference between the 2 groups was not statistically significant (p = .166). Average changes in domain scores were statistically significant for 2 domains: (a) Residents with ADRD diagnoses were more likely to have an increase in food enjoyment, while residents without ADRD diagnoses were more likely to have a decline in food enjoyment. (b) Residents with ADRD diagnoses had larger positive changes in social engagement than those without ADRD diagnoses. On average, the 2-year summary score means were higher for residents with ADRD diagnoses than those without ADRD diagnoses. Compared to residents without ADRD diagnoses, residents with ADRD diagnoses had higher 2-year means for environment satisfaction, attention from staff, food enjoyment, and negative mood domains and lower means for social engagement and positive mood.
Table 2.
Average of and Difference in QoL Scores for Consecutive 2 Years (N = 12 949)
| Variables | Non-ADRD (N = 7 392) | ADRD (N = 5 557) | ||
|---|---|---|---|---|
| Two-Year Average | Change in Second Year | Two-Year Average | Change in Second Year | |
| Summary score | 80.71 | −1.01 | 81.66 | −0.69 |
| Environment | 84.72 | −1.13 | 87.13 | −1.44 |
| Attention from staff | 93.12 | −1.29 | 93.62 | −0.84 |
| Food enjoyment | 80.17 | −1.45 | 86.40 | 0.08 |
| Engagement | 81.09 | 0.09 | 79.18 | 0.90 |
| Negative mood * | 66.83 | −0.59 | 67.93 | −0.45 |
| Positive mood | 78.49 | −1.51 | 75.99 | −2.20 |
Note: ADRD = Alzheimer’s disease and related dementias; QoL = quality of life.
*Reverse-coded. A higher value indicates better quality.
Stability of QoL Scores
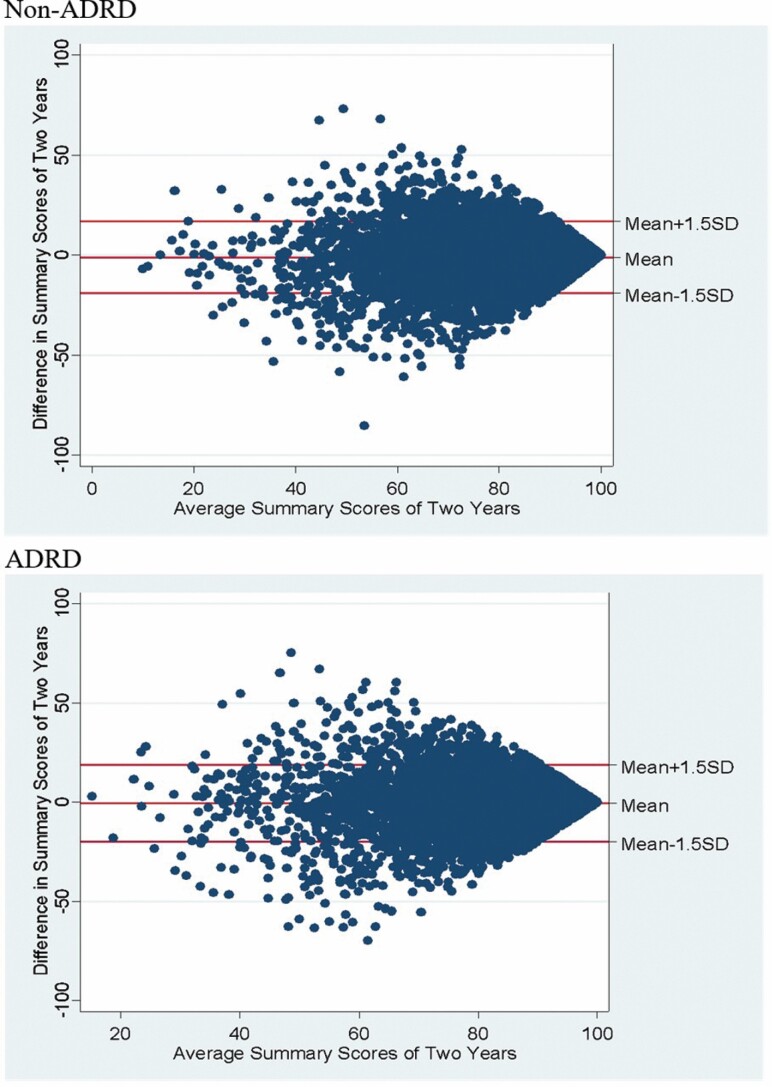
To understand the distribution of QoL summary score changes, we created scatter plots of the changes against 2-year summary score means for each cohort–resident pair with versus without ADRD diagnoses (Figure 1).
Figure 1.
Difference in summary score between 2 years against mean for the scores for 2 years. Note: ADRD = Alzheimer’s disease and related dementias.
Residents with and without ADRD diagnoses shared similar patterns in their distributions of QoL summary score changes. Stable QoL scores should be expected to have average changes of approximately zero. The sample mean of the changes in QoL summary score was −0.80 (p < .001), and the standard deviation was 12.37, suggesting that the changes mostly clustered around zero. Among 12 949 cohort–residents, 86.82% had changes in QoL summary scores that fell within the range of ±1.5 SD (between −19.36 and 17.76, defined as stable QoL scores). However, a higher proportion of residents with ADRD diagnoses experienced changes outside of this range (p < .001). Specifically, among 7 392 residents without ADRD diagnoses, 87.58% had stable summary QoL scores from 1 year to another. Among 5 557 residents with ADRD diagnoses, 85.80% had stable summary QoL scores.
Figure 2 displays how ADRD diagnoses and CFS interact in their association with the stability of QoL summary scores. Residents without ADRD diagnoses who were cognitively capable had the highest proportion (89.06%) of stable QoL summary scores, while residents without ADRD whose cognition was moderately to severely impaired had the lowest proportion (81.82%) of stable QoL summary scores. Residents with and without ADRD diagnoses who had the same level of cognitive function had a similar percent of residents with stable summary scores.
Figure 2.
Stability by ADRD and Cognitive Function Scale status. Note: ADRD = Alzheimer’s disease and related dementias.
Because ADRD diagnoses and CFS interacted in their association with the stability of QoL scores, we used the 6-category indicator of ADRD and CFS statuses in our regression analyses to understand determinants of the stability of QoL scores. Figure 3 shows coefficient plots of the associations of ADRD and CFS status with the stability of QoL scores by domain. Residents without ADRD diagnoses and with mild cognitive impairment had 2.06 percentage points lower probability of having stable summary scores, compared to residents without ADRD and cognitively capable (p = .033). Regardless of ADRD diagnoses, residents with moderate or severe cognitive impairment were less likely to have stable summary scores than cognitively capable residents without ADRD diagnoses (p < .001), though there was variation by QoL domain. Residents with ADRD diagnoses and any level of cognitive impairment were more likely to have stable QoL scores for satisfaction with the environment (mildly impaired: p = .023, moderately to severely impaired: p = .001) and food enjoyment (p < .001 for both levels of cognition) than cognitively capable residents without ADRD diagnoses. Regardless of ADRD diagnoses, residents with moderate or severe cognitive impairment were less likely to have stable QoL scores in satisfaction with attention from staff (p = .001), social engagement, negative mood, and positive mood (ps < .001) than cognitively capable residents without ADRD diagnoses. Regardless of ADRD diagnoses, residents with mild cognitive impairment were less likely to have stable scores in social engagement, negative mood, and positive mood (ps < .001) than cognitively capable residents without ADRD diagnoses.
Figure 3.
Estimated effects of ADRD and Cognitive Function Scale status on quality of life scores by domains. Note: ADRD = Alzheimer’s disease and related dementias. Reference group: Cognitively capable residents without ADRD diagnoses.
Sensitivity of QoL Scores
Table 3 includes results from linear regression models that estimate the effects of changes in resident and facility characteristics on the absolute change in QoL summary scores.
Table 3.
Units of Absolute Summary Score Change Associated With Each Factor by Stability Group
| Variables | Residents With Stable Quality of Life Scores | Residents Without Stable Quality of Life Scores |
|---|---|---|
| ADRD and CFS status | ||
| No ADRD, cognitively capable | Ref | Ref |
| ADRD, cognitively capable | −0.0105 | 0.855 |
| No ADRD, mildly impaired | 0.224 | 0.234 |
| ADRD, mildly impaired | −0.324* | 1.43 |
| No ADRD, moderately or severely impaired | −0.0765 | 1.028 |
| ADRD, moderately or severely impaired | −0.194 | 1.812* |
| Race | ||
| White | Ref | Ref |
| Black | 0.204 | −0.667 |
| American Indians | 0.31 | −4.386* |
| Other | 0.258 | 0.886 |
| Age | 0.00698 | −0.0384 |
| Female | −0.111 | −0.55 |
| Married | 0.0223 | 0.467 |
| Length of stay (100 days) | −0.0195*** | 0.039 |
| ADL score | 0.0609*** | 0.0465 |
| Anxiety or depression Dx | 0.157 | −0.0271 |
| Severe mental illness Dx | 0.419** | 0.266 |
| Behavioral symptoms | 0.24 | 0.524 |
| Chronic conditions | 0.0517 | 0.408 |
| Hospitalizations between 2 surveys | ||
| 0 | Ref | Ref |
| 1 | 0.123 | 1.084 |
| >1 | 0.213 | 2.337* |
| MDS significant status change between 2 surveys | ||
| 0 | Ref | Ref |
| 1 | 0.559*** | 0.19 |
| >1 | 0.285 | 0.232 |
| Facility level | ||
| Proportion of non-White residents | 0.00558 | 0.053 |
| Proportion of Medicaid patient-days | 0.21 | −2.719 |
| Proportion of Medicare patient-days | 1.988 | −14.36* |
| Metro area | 0.147 | 0.181 |
| Ownership | ||
| For profit | 0 | 0 |
| Nonprofit | −0.384** | −1.642** |
| Government | −0.293 | −3.416*** |
| Number of beds | 0.394*** | 0.522 |
| Staff retention | −0.078 | −3.505 |
| Acuity | −0.0723 | −1.073 |
| Proportion of private rooms | 0.0699 | 0.245 |
| Constant | 4.500*** | 33.21*** |
| N | 11242 | 1707 |
Note: ADRD = Alzheimer’s disease and related dementias; CFS = Cognitive Function Scale; ADL = activities of daily living; MDS = Minimum Data Set.
*p < .05, **p < .01, ***p < .001.
Residents with stable scores
Among residents with stable QoL summary scores, health and functional status changes resulted in up to 1 unit of absolute changes in QoL summary scores. Residents without ADRD diagnoses, but with mild cognitive impairment, had 0.32 smaller absolute score change, compared to cognitively capable residents without ADRD diagnoses (p = .023). Having one MDS significant change in status between the 2 surveys was associated with 0.56 larger absolute changes in the summary score (p < .001) than no status changes. Moreover, greater ADL impairment and severe mental illness were associated with larger absolute changes, while the longer length of stay was associated with smaller absolute score changes.
Residents with unstable scores
Among residents with unstable QoL summary scores, resident characteristic changes resulted in up to 4.39 absolute unit changes in QoL summary scores. Residents with ADRD diagnoses and moderate or severe cognitive impairment had a 1.81 larger absolute score change, compared to residents without ADRD diagnoses and cognitive impairment (p = .014). American Indian residents without stable QoL had 4.39 more absolute unit changes, compared to White residents (p = .014), indicating that their QoL was less stable than White residents’ QoL.
Discussion and Implications
Our study aimed to determine whether the stability and sensitivity of the Minnesota NH Resident QoL measure differed by ADRD. In the study sample, scores from a validated, multidomain measure of QoL of NH residents for those with and without ADRD were similarly stable over time and their QoL score changes were sensitive to health and functional status changes.
Previously, interest in improving person-centered care and QoL among NH residents with ADRD has led to the design of dementia-specific QoL measures, such as QoL-AD (8–10). These important contributions demonstrated the ability of persons with dementia and mild or moderate cognitive impairment to consistently answer questions and state preferences (8). While dementia-specific measures are valuable, the ability to use a generic measure reduces costs and increases the uptake of the measure. For instance, Minnesota’s QoL survey is already implemented statewide. A nondementia-specific instrument also improves our ability to include residents with ADRD in other NH measures, such as state report cards. Other states and organizations seeking to implement quality improvement tools for system-wide change and better person-centered care may consider using a well-developed survey such as the Minnesota Resident QoL and Satisfaction with Care Survey.
While the ability of persons with dementia to answer questions consistently has been documented (8,11), our study filled a gap in the understanding of the stability of QoL scores across time and the sensitivity of QoL scores to health and functional changes in a large NH population. Previous studies of the stability of QoL scores among persons with dementia or cognitive impairment relied on smaller samples but found largely similar results. Hickey and Bourgeois (16) found stable scores in residents with and without dementia diagnoses over a period of 4 months. While Carpenter et al. (15) found decreased stability after 1 year with increased cognitive impairment, the clinical dementia rating (no, very mild, or mild dementia) is akin to our measure of cognitive impairment. Our data provided a unique opportunity to (a) include a large population of NH residents statewide and those with moderate cognitive impairment, (b) better understand the relationship between ADRD diagnosis and cognitive impairment, and (c) understand the sensitivity of QoL to health status change among NH residents with ADRD diagnoses.
We found that QoL score changes in the Minnesota NH Resident QoL measure across a year largely fell within 1.5 SD of the mean. Among residents with stable QoL summary scores (86.82% of all residents), significant changes in health status, severe mental illness, and needs for assistance with ADLs were significantly associated with absolute changes in summary QoL scores, indicating the sensitivity of the measure to health and functional changes. In contrast, among residents whose QoL scores were not stable (13.18% of all residents), absolute changes in QoL summary scores were not significantly associated with these indicators of health and functional status.
While residents with severe cognitive impairment were excluded from the Minnesota survey, at each remaining level of cognitive status (capable, mildly impaired, moderately to severely impaired), the presence of ADRD diagnoses did not substantially decrease the probability of a resident having a stable summary score. Residents with increasing levels of cognitive impairment but without ADRD diagnoses may signal a true cognitive impairment without dementia, such as delirium and personality disorders (32). Alternatively, this could also indicate missed ADRD diagnoses or the onset of progressive cognitive decline not yet diagnosed as dementia, which could lead to inadequate or even inappropriate care planning and interaction with staff/facility. Thus, we might expect cognitively impaired residents without ADRD diagnoses to have wide variations in perceived QoL, as nondementia causes of cognitive impairment tend to be more transient, and those who are undiagnosed may experience greater fluctuations in QoL domains that involve interaction with staff or administration. Indeed, cognitive impairment, regardless of ADRD diagnoses, was associated with a smaller probability of having stable QoL in the domains of attention from staff, engagement, negative mood, and positive mood. Cognitively impaired residents with ADRD diagnoses were more likely to have stable QoL scores in the environment and food enjoyment domains than cognitively impaired residents with no ADRD diagnoses. Consequently, residents with ADRD diagnoses had QoL scores for most domains that were as stable as residents without ADRD diagnoses; cognitive impairment might be the main factor affecting the stability of QoL scores.
Also, residents living with ADRD had higher QoL summary scores and higher QoL domains for environment, food, and negative mood. Previous analyses of these data found similar trends of higher satisfaction with food among people living with ADRD, indicating that people with ADRD may experience food and meal times differently (ie, they may look forward to meals more as an event in the day) compared to those without ADRD. Looking at mood, people with ADRD were more likely to report “never” having negative emotions. They also were less likely to report “often” having positive emotions. These together suggest a trend to a “flatter” emotional experience for these residents as measured by this mood scale (which is also consistent with the difficulty in diagnosing memory loss vs depression).
Our unique data linkage provided the opportunity to understand the roles of ADRD diagnoses and cognitive impairment in stability and sensitivity of a generic QoL measure. The multidomain measure of QoL allows for further understanding the stability of QoL measure in different domains and its associations with ADRD and cognitive impairment. Despite these strengths, we acknowledge some limitations. First, our definition of stability considered changes within 1.5 SD of sample mean as reasonable fluctuation in QoL within a year and changes larger than that as potential measurement errors. Our sensitivity analyses using 2 SD and analyses on the sensitivity of QoL scores supported the validity of this operationalization. However, there is currently no evidence for how much change in QoL over a year is reasonable. Second, the survey excluded most residents with severe cognitive impairment. We do not know whether residents with ADRD and severe cognitive impairment can self-report QoL using this measure; our findings, however, show an inverse relationship between score stability and level of cognitive impairment. Third, our study focused only on NH residents in Minnesota; as this QoL survey expands to other states, this analysis could be repeated. Fourth, because we focused on residents who had at least 2 surveys in 2 consecutive years, we oversampled long-stay residents. However, in our population, long-stay and short-stay residents had similar characteristics. Future research should investigate thresholds for reasonable yearly fluctuation in QoL scores, especially for those who had health status changes. Finally, while the results suggest that the Minnesota NH Resident QoL measure may show stability and sensitivity regardless of ADRD status, it is not clear whether other QoL measures will operate the same and we encourage future researchers to investigate whether these properties are present for other measures of QoL.
Our study demonstrated that self-report QoL scores can reliably detect meaningful changes in QoL in NH residents with ADRD diagnoses using the Minnesota NH QoL measure. Thus, QoL surveys in NHs should not exclude participants based exclusively on ADRD diagnoses if those measures can appropriately be used with residents with ADRD. These results have policy implications for potentially revising CMS 5-star compare measures to include QoL as well as other states looking to not only measure but improve QoL for NH residents living with ADRD. The utility of nondementia-specific QoL surveys has the potential to increase QoL measurement among NH residents with ADRD, with the goal of improving person-centered care and to decrease the likelihood that QoL experiences of residents with ADRD will be excluded from consumer tools such as NH report cards.
Supplementary Material
Author Note
*There are 2 separate tools as part of this larger survey including both resident reports (ie, Quality of Life) and family reports (ie, satisfaction). This study concerns only the resident-report (ie, Quality of Life) tool because (a) the overarching research question is concerned with whether residents with ADRD would be able to self-report QoL, not whether families of residents would be able to report satisfaction, and (b) because the resident and family samples are conducted with different methods and drawn separately (and therefore cannot be matched).
Funding
This study was funded by the National Institute of Minority Health Disparities (5R01MD010729, PI: Tetyana P. Shippee, PhD). Research reported in this publication was supported by the Robert L. Kane Endowed Chair in Long-Term Care and Aging.
Conflict of Interest
None declared.
References
- 1.Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. 2020. Accessed December 26, 2020. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
- 2.Sury L, Burns K, Brodaty H. Moving in: adjustment of people living with dementia going into a nursing home and their families. Int Psychogeriatr. 2013;25(6):867–876. doi: 10.1017/S1041610213000057 [DOI] [PubMed] [Google Scholar]
- 3.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ. Lethality of Alzheimer’s disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2010;24(1):90–95. doi: 10.1097/WAD.0b013e31819fe7d1 [DOI] [PubMed] [Google Scholar]
- 4.Gaugler J, James B, Johnson T, Marin A, Weuve J. 2019. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. [Google Scholar]
- 5.Gitlin LN, Maslow K, Khillan R.. National Research Summit on Care, Services and Supports for Persons With Dementia and Their Caregivers: Final Summit Report. Office of the Assistant Secretary for Planning and Evaluation; 2018. [Google Scholar]
- 6.Gaugler JE, Yu F, Davila HW, Shippee T. Alzheimer’s disease and nursing homes. Health Aff (Millwood). 2014;33(4):650–657. doi: 10.1377/hlthaff.2013.1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shippee TP, Henning-Smith C, Kane RL, Lewis T. Resident- and facility-level predictors of quality of life in long-term care. Gerontologist. 2015;55(4):643–655. doi: 10.1093/geront/gnt148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–519. doi: 10.1097/00006842-200205000-00016 [DOI] [PubMed] [Google Scholar]
- 9.Ettema TP, Dröes RM, de Lange J, Mellenbergh GJ, Ribbe MW. A review of quality of life instruments used in dementia. Qual Life Res. 2005;14(3):675–686. doi: 10.1007/s11136-004-1258-0 [DOI] [PubMed] [Google Scholar]
- 10.Aspden T, Bradshaw SA, Playford ED, Riazi A. Quality-of-life measures for use within care homes: a systematic review of their measurement properties. Age Ageing. 2014;43(5):596–603. doi: 10.1093/ageing/afu089 [DOI] [PubMed] [Google Scholar]
- 11.Clark PA, Tucke SS, Whitlatch CJ. Consistency of information from persons with dementia: an analysis of differences by question type. Dementia. 2008;7(3):341–358. doi: 10.1177/1471301208093288 [DOI] [Google Scholar]
- 12.Feinberg LF, Whitlatch CJ. Are persons with cognitive impairment able to state consistent choices? Gerontologist. 2001;41(3):374–382. doi: 10.1093/geront/41.3.374 [DOI] [PubMed] [Google Scholar]
- 13.Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord. 2003;17(4):201–208. doi: 10.1097/00002093-200310000-00002 [DOI] [PubMed] [Google Scholar]
- 14.Trigg R, Jones RW, Skevington SM. Can people with mild to moderate dementia provide reliable answers about their quality of life? Age Ageing. 2007;36(6):663–669. doi: 10.1093/ageing/afm077 [DOI] [PubMed] [Google Scholar]
- 15.Carpenter BD, Kissel EC, Lee MM. Preferences and life evaluations of older adults with and without dementia: reliability, stability, and proxy knowledge. Psychol Aging. 2007;22(3):650–655. doi: 10.1037/0882-7974.22.3.650 [DOI] [PubMed] [Google Scholar]
- 16.Hickey EM, Bourgeois MS. Health-related quality of life (HR-QOL) in nursing home residents with dementia: stability and relationships among measures. Aphasiology. 2000;14(5–6):669–679. doi: 10.1080/026870300401388 [DOI] [Google Scholar]
- 17.Castro-Monteiro E, Forjaz MJ, Ayala A, et al. Change and predictors of quality of life in institutionalized older adults with dementia. Qual Life Res. 2014;23(9):2595–2601. doi: 10.1007/s11136-014-0706-8 [DOI] [PubMed] [Google Scholar]
- 18.Hoe J, Hancock G, Livingston G, Woods B, Challis D, Orrell M. Changes in the quality of life of people with dementia living in care homes. Alzheimer Dis Assoc Disord. 2009;23(3):285–290. doi: 10.1097/WAD.0b013e318194fc1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mjørud M, Røsvik J, Rokstad AM, Kirkevold M, Engedal K. Variables associated with change in quality of life among persons with dementia in nursing homes: a 10 months follow-up study. PLoS One. 2014;9(12):e115248. doi: 10.1371/journal.pone.0115248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyketsos CG, Gonzales-Salvador T, Chin JJ, Baker A, Black B, Rabins P. A follow-up study of change in quality of life among persons with dementia residing in a long-term care facility. Int J Geriatr Psychiatry. 2003;18(4):275–281. doi: 10.1002/gps.796 [DOI] [PubMed] [Google Scholar]
- 21.Oudman E, Veurink B. Quality of life in nursing home residents with advanced dementia: a 2-year follow-up. Psychogeriatrics. 2014;14(4):235–240. doi: 10.1111/psyg.12062 [DOI] [PubMed] [Google Scholar]
- 22.van der Zon A, Wetzels RB, Bor H, Zuidema SU, Koopmans RTCM, Gerritsen DL. Two-year course of quality of life in nursing home residents with dementia. Am J Geriatr Psychiatry. 2018;26(7):754–764. doi: 10.1016/j.jagp.2018.01.202 [DOI] [PubMed] [Google Scholar]
- 23.Atkinson T. The stability and validity of quality of life measures. Soc Indic Res. 1982;10(2):113–132. doi: 10.1007/BF00302506 [DOI] [Google Scholar]
- 24.George LK. Perceived quality of life. In: Binstock R, George L, Cutler S, Hendricks J, Schulz J, eds. Handbook of Aging and the Social Sciences. Elsevier; 2006:320–336. [Google Scholar]
- 25.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45(3):265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x [DOI] [PubMed] [Google Scholar]
- 26.Wu AW, Yasui Y, Alzola C, et al. Predicting functional status outcomes in hospitalized patients aged 80 years and older. J Am Geriatr Soc. 2000;48(S1):S6–S15. doi: 10.1111/j.1532-5415.2000.tb03142.x [DOI] [PubMed] [Google Scholar]
- 27.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. doi: 10.7326/0003-4819-118-3-199302010-00011 [DOI] [PubMed] [Google Scholar]
- 28.Minnesota Departments of Health and Human Services. Minnesota Nursing Home Report Card Technical User Guide. Published online August 28, 2020. Accessed January 24, 2021. https://nhreportcard.dhs.mn.gov/technicaluserguide.pdf
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual Version 1.13. Published online October 2015. Accessed February 12, 2016. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursinghomeQualityInits/MDS30RAIManual.html
- 31.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. Published online 2015. Accessed February 12, 2016. http://europepmc.org/abstract/med/25763665 [DOI] [PMC free article] [PubMed]
- 32.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.