Abstract
Background
Zika virus (ZIKV) is associated with severe congenital abnormalities and laboratory diagnosis of antenatal infection is difficult. Here we evaluated ZIKV neutralizing antibody (nAb) kinetics in infants born to mothers with PCR-confirmed ZIKV infection during pregnancy.
Methods
Neonates (n = 98) had serum specimens tested repeatedly for ZIKV nAb over the first 2 years of life using virus neutralization test (VNT). ZIKV neonatal infection was confirmed by RT-PCR in blood or urine and/or presence of ZIKV IgM antibodies, and results were correlated with infant clinical features.
Results
Postnatal laboratory evidence of ZIKV vertical transmission was obtained for 60.2% of children, while 32.7% exhibited clinical abnormalities. Congenital abnormalities were found in 37.3% of children with confirmed ZIKV infection and 31.0% of children without confirmed infection (P = .734). All but 1 child displayed a physiologic decline in ZIKV nAb, reflecting maternal antibody decay, despite an early ZIKV-IgM response in one-third of infants.
Conclusions
Infants with antenatal ZIKV exposure do not develop ZIKV nAb despite an early IgM response. Therefore, ZIKV VNT in children is not useful for diagnosis of congenital infection. In light of these findings, it remains to be determined if children infected in utero are potentially susceptible to reinfection.
Keywords: Zika virus, vertical transmission, neutralizing antibodies, infection status, biomarker
Infants with in utero ZIKV exposure do not develop an autochthonous production of anti-ZIKV IgG neutralizing antibodies, even those with confirmed vertical transmission by detection of ZIKV RNA or an early IgM response.
The Zika epidemic in Brazil revealed the teratogenic potential of Zika virus (ZIKV), placing the virus in the same group as other TORCH pathogens. These include Toxoplasma gondii, rubella virus, cytomegalovirus (CMV), herpes simplex virus, and other pathogens, which include Treponema pallidum, human immunodeficiency virus (HIV), hepatitis B and C viruses, varicella-zoster virus, and parvovirus B19. Diagnosis of congenital infections following maternal infection during pregnancy can often be challenging. Unsurprisingly, the risk of vertical transmission varies greatly across TORCH pathogens over the course of pregnancy [1].
In the case of ZIKV, based on data generated by major cohort studies, the overall risk of microcephaly as the most severe manifestation of congenital Zika has been estimated to range between 3% and 8% [2–8]. The determination of the vertical transmission rate in utero, however, has not been conclusively shown. The overall risk estimate for microcephaly might thus be a product of 2 different risks: (1) the risk of vertical transmission, and (2) the risk of congenital abnormalities following fetal infection. Although some studies attempted to estimate the rate of ZIKV vertical transmission, assessments were limited by short follow-up periods (often not beyond the first week of life) and limited number of diagnostic evaluations [6, 7, 9].
In some congenital infections, persistence of immunoglobulin G (IgG) antibodies after the first year of life is indicative of the true infection status of an infant. Passively transferred maternal IgG antibodies decline physiologically over time and disappear between 9 and 15 months of age [10]. The aim of the present study was to describe ZIKV neutralizing antibody (nAb) kinetics in infants with polymerase chain reaction (PCR)-confirmed maternal ZIKV infection during pregnancy, and to evaluate whether persistence of ZIKV-specific neutralizing antibodies beyond the first year of life is associated with infection status.
METHODS
Study Population
The study population has been previously described [2, 11] and included neonates born to mothers with PCR-confirmed ZIKV infection during pregnancy. Briefly, 182 children were followed, and 131 underwent imaging and neurodevelopmental and sensory organ assessment. A subset of 98 children, for whom the necessary specimens were available, were tested repeatedly for titration of ZIKV nAb during the first 2 years of life. All infants were born in 2016, after the peak of the ZIKV epidemic in Rio de Janeiro, Brazil. Birth outcomes were classified as normal or abnormal within the first 3 months of life. Abnormal outcomes were defined as: prematurity; small for gestational age (SGA); microcephaly; neurosensory alterations including abnormal complete eye exams or hearing deficits detected by brain-steam auditory evoked response (BERA); or any structural brain abnormality detected by imaging exams [2, 12]. The study was approved by the institutional review boards of the Fundação Oswaldo Cruz (FIOCRUZ) in Rio de Janeiro and University of California Los Angeles.
Laboratory Diagnosis
ZIKV RT-PCR Detection
Infant serum and urine specimens were obtained following parental signed informed consent by standard phlebotomy and via bagged urine collection, respectively. Serum was processed immediately for PCR while additional aliquots were stored at −80°C for subsequent testing for Zika IgM, as previously described [2]. Laboratory testing sites included the research laboratory of the Instituto Fernandes Figueira Hospital, FIOCRUZ and the Laboratory of Molecular Biology of Flaviviruses, FIOCRUZ. Briefly, ZIKV RNA was investigated by reverse transcription PCR (RT-PCR) targeting the env gene as described by Lanciotti et al [13]. Viral RNA was extracted from 140 μL of biological fluids using QIAmp MiniElute Virus Spin (QIAgen), following the manufacturer’s recommendations. ZIKV RNA detection was performed using One Step TaqMan RT-PCR (Thermo Fisher Scientific) on a 7500 Real-time PCR System (Applied Biosystems) and conditions as described elsewhere [13]. A limit of detection of approximately 1000 copies/mL of clinical specimen was obtained since a minimum of 3.2 RNA copies is detected in the reaction, as shown by Corman et al [14].
ZIKV IgM Detection
Infant ZIKV IgM antibody testing was performed in serum aliquots using IgM antibody capture Zika enzyme-linked immunosorbent assay (MAC-ELISA) from the Centers for Disease Control and Prevention (CDC) according to manufacturer’s instructions [15]. Plates were coated with 75 μL of goat anti-human IgM (Kirkegaard and Perry Laboratories) in carbonate/bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Blocking step was done with phosphate-buffered saline (PBS; pH 7.2) containing 5% non-fat dried milk/0.05% Tween 20, for 30 minutes at room temperature and washing (carried out after every step). After blocking, 50 μL of serum samples diluted at 1/400 in PBS pH 7.2 with 0.05% Tween 20, or negative (pooled flavivirus-negative serum) or positive controls (CDC humanized 6B6C-1 pan-flavivirus) were added to plates and incubated at 37°C for 1 hour. Fifty-microliters of viral Zika antigen (CDC Vero E6 derived, inactivated ZIKV antigen) or normal antigen (CDC Vero E6 derived, mock-infected normal antigen) were added to each well and incubated overnight at 4°C. Detection antibody conjugate (horseradish peroxidase-conjugated monoclonal antibody 6B6C-1; CDC) diluted in blocking buffer was added and incubated for 1 hour at 37°C. 3′3′5′5′tetramethylbenzidine base (Becton Dickson) was added to wells. After 10 minutes incubation at room temperature, the reaction was stopped with 1 N sulfuric acid solution and the optical density (OD) read at 450 nm. The ratio (P/N) was calculated as follows: mean OD of the test sample reacted on viral antigen (P) divided by the mean OD of the negative control reacted on viral antigen (N). P/N value < 3.0 was considered negative and positive when >3.0. This ELISA protocol has a sensitivity rate of 90.9%–100% and a specificity of 93.2%–100% [16].
Virus Neutralization Test
Titration of ZIKV nAb in serum samples was performed by virus neutralization test (VNT), as previously described [17]. Briefly, 2-fold serial dilution (1:10 to 1:2560) of heat inactivated sera (56°C/30 minutes) was performed in 96-well U-bottom plates with 199 medium, and then mixed vol/vol with 100 TCID50 (50% tissue culture infectious dose) of ZIKV (Rio U1 strain [18]), resulting in final testing dilutions of 1:20 to 1:5120. After 1 hour of incubation at 37°C with 5% CO2, serum/virus mixtures were transferred in duplicates onto monolayers of Vero cells (ATCC CCL-81) in 96-well plates, and further incubated for 6 days. In each VNT, a positive serum control from the French National Reference Centre for Arboviruses was used (VNT titer, 80). Later, direct observation of cytopathic effect (CPE) was carried out under a light microscopy. Serum dilutions associated with CPE were considered as negative, while the absence of CPE indicated a positive result, representing complete neutralization of the ZIKV inoculum. The VNT titer was considered as the highest serum dilution where virus neutralization was observed in both test duplicates; a threshold was set at 40 and serum specimens with a titer <40 were considered negative. As previously reported [17], the VNT shows 98.1% sensitivity and 98.8% specificity when compared with 90% plaque reduction neutralization test.
Statistical Analysis
Descriptive statistics included the frequency of categorical variables and summary measures of quantitative variables: mean, median, interquartile range (IQR), minimum, and maximum values. Fisher exact test was used to identify associations between outcomes (major abnormalities and laboratory findings) and confirmed ZIKV vertical transmission (by at least 1 positive result in the detection of ZIKV RNA and/or ZIKV IgM response). The Kaplan-Meyer method was used to determine the median time of IgM responses: (1) from birth to the first positive ZIKV IgM result, and (2) until disappearance of IgM antibodies in a subset of children.
ZIKV nAb titers as determined by VNT were compared by age groups at the time of specimen collection using the Dunn test. ZIKV nAb titers from the first specimen in the first 6 months of life were used for comparison between serum IgM results, confirmed ZIKV infection, and gestational age at the time of Zika infection using the Mann-Whitney test. Statistical analysis and boxplots were performed with GraphPad Prism version 5 to explore differences between groups. Line graphs and polynomial regression (lowess) were used to describe the profile of ZIKV nAb titers during follow-up. Statistical analysis was performed with R version 3.6.0.
RESULTS
ZIKV Vertical Transmission and Association With Infant Clinical and Laboratorial Findings
Infants born to women with PCR-confirmed ZIKV infection during pregnancy were tested throughout the first year of life, and vertical transmission of Zika was confirmed after birth in 59 of 98 infants (60.2%) by RT-PCR detection of ZIKV RNA in urine or blood, or by the demonstration of ZIKV-specific IgM antibodies; 88.1% of infants (52 of 59 infants) tested positive before 6 months of age (Table 1). The presence of ZIKV RNA in blood and/or urine as a direct evidence of Zika infection was shown in 45 of 98 children (45.9%), while 14 (14.3%) only had detectable ZIKV IgM (Table 1). The median age of infants at the time of the first sample collection was 71 days (IQR, 30–105 days), and the median age for a positive IgM result was 41 days (IQR, 20–78 days). ELISA was performed consecutively in 9 children with a positive test result; in these children results turned negative after a median 64 days (IQR, 23–96 days).
Table 1.
Laboratory Diagnosis of Zika Infection in Antenatally Exposed Infants
| Laboratory Diagnosis of Zika Infection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Positivea | ||||||||||
| Age at Sample Collection | ZIKV IgM Only | ZIKV RNA, Blood Only | ZIKV RNA, Urine Only | ZIKV IgM + ZIKV RNA in Blood | ZIKV IgM + ZIKV RNA in Urine | ZIKV RNA in Blood and Urine | ZIKV IgM + ZIKV RNA in Blood and Urine | Total | No. Negativeb | No. Not Done |
| 0–3 mo | 10 | 3 | 9 | 7 | 6 | 3 | 1 | 39 | 17 | … |
| 3–6 mo | 2 | 2 | 6 | 2 | 1 | … | … | 13 | 9 | … |
| 6–9 mo | 2 | … | 2 | 1 | … | … | … | 5 | 3 | … |
| 9–12 mo | … | 1 | 1 | … | … | … | … | 2 | … | … |
| Total | 14 | 6 | 18 | 10 | 7 | 3 | 1 | 59 | 29 | 10 |
Infants (n = 98) were tested for Zika infection within the first year of life to determine vertical transmission. Zika infection was confirmed in infants presenting at least 1 positive result for the detection of ZIKV IgM, and ZIKV RNA in blood or urine. Consecutive samples were sequentially tested until a positive result for ZIKV IgM and/or ZIKV RNA was obtained.
Abbreviations: IgM, immunoglobulin M; ZIKV, Zika virus.
aAge at the first sample with a positive result.
bAge when the first sample was collected.
Thirty-two children (32.7%) were found to have congenital abnormalities or other adverse health outcomes within the first 3 months of life. Prematurity was present in 15 cases (15.3%) (Table 2). Microcephaly was observed in 7 cases (7.1%). The frequency of other abnormalities, such as altered eye fundus or abnormal BERA, evidence of SGA, or structural abnormalities on imaging are reported in Table 2, as well as their distribution according to Zika vertical transmission. The frequency of abnormal findings after birth was not significantly higher in children with confirmed vertical transmission of Zika infection (37.3%) as compared to those without laboratory confirmation of infection (31.0%) (P = .734; Table 2).
Table 2.
Frequency of Severe Abnormalities in Children Stratified by Laboratory Diagnostic Testing
| Laboratory-Confirmed Zika Infectiona | |||||||
|---|---|---|---|---|---|---|---|
| Frequency | Positive | Negative | |||||
| Outcome | No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | P Valueb |
| Microcephaly | |||||||
| Yes | 7 (7.14) | 2.92–14.17 | 6 (10.17) | 3.82–20.83 | 1 (3.45) | .09–17.77 | .499 |
| No | 91 (92.86) | 85.84–97.08 | 53 (89.83) | 79.17–96.18 | 28 (96.55) | 82.23–99.91 | |
| SGA | |||||||
| Yes | 7 (7.14) | 2.92–14.17 | 4 (6.78) | 1.88–16.46 | 3 (10.34) | 2.19–27.35 | .871 |
| No | 91 (92.86) | 85.84–97.08 | 55 (93.22) | 83.54–98.12 | 26 (89.66) | 72.64–97.81 | |
| Abnormal eye fundus | |||||||
| Yes | 4 (4.08) | 1.12–10.12 | 4 (6.78) | 1.88–16.46 | 0 (0.00) | .00–11.97 | … c |
| No | 72 (73.47) | 63.59–81.88 | 41 (69.49) | 56.13–80.82 | 26 (89.66) | 72.64–97.81 | |
| Unknown | 22 (22.45) | 14.63–31.99 | 14 (23.73) | 13.62–36.60 | 3 (10.34) | 2.19–27.35 | |
| Abnormal BERA | |||||||
| Yes | 7 (7.14) | 2.92–14.17 | 6 (10.17) | 3.82–20.83 | 1 (3.45) | .09–17.77 | .540 |
| No | 55 (56.12) | 45.73–66.14 | 33 (55.93) | 42.40–68.85 | 18 (62.07) | 42.26–79.32 | |
| Unknown | 36 (36.73) | 27.22–47.08 | 20 (33.90) | 22.08–47.39 | 10 (34.48) | 17.94–54.33 | |
| Structural brain abnormality | |||||||
| Yes | 12 (12.24) | 6.49–20.41 | 10 (16.95) | 8.44–28.97 | 1 (3.45 | .09–17.77 | .178 |
| No | 71 (73.47) | 62.50–81.00 | 42 (71.19) | 57.92–82.24 | 25 (86.21) | 68.34–96.11 | |
| Unknown | 15 (14.29) | 8.83–23.99 | 7 (11.86) | 4.90–22.93 | 3 (10.34) | 2.19–27.35 | |
| Premature birth | |||||||
| Yes | 15 (15.31) | 8.83-23-99 | 11 (18.64) | 9.69–30.92 | 4 (13.79) | 3.89–31.67 | .789 |
| No | 83 (84.69) | 76.01–91.17 | 48 (81.36) | 69.08–90.31 | 25 (86.21) | 68.34–96.11 | |
| Any of the above abnormalities | |||||||
| Yes | 32 (32.65) | 23.52–42.87 | 22 (37.29) | 25.04–50.86 | 9 (31.03) | 15.28–50.83 | .734 |
| No | 66 (67.35) | 57.13–76.49 | 37 (62.71) | 49.14–74.96 | 20 (68.97) | 49.17–84.72 | |
| Total | 98 (100) | 59 | 29 | ||||
Abbreviations: BERA, brain-steam auditory evoked response; CI, confidence interval; IgM, immunoglobulin M; SGA, small for gestational age; ZIKV, Zika virus.
aZika infection was confirmed in infants presenting at least 1 positive result for the detection of ZIKV IgM, and ZIKV RNA in blood or urine. Data were not available for 10 infants.
bStatistical analysis of the frequency of confirmed Zika infection in infants according to the presence or absence of congenital abnormalities was performed by Fisher exact test, and P value < .05 was considered significant.
cP value not determined due to cells with zero values.
ZIKV nAb Titer Kinetics in Infants
Infants were followed for 12.5 months on average (ranging from 0 to 30 months). Serum samples were collected (n = 228) and tested for the presence of ZIKV nAb titers. All infants had at least 1 serum sample collected throughout the study; 33 infants had 1 sample and 65 had 2 or more consecutive samples (Table 3). Most children had the first sample collected before 6 months of age (n = 72), and only 6 had the first sample collected after 18 months of life (Table 3). In addition, 55 unique children had samples tested after 1 year of life, in which 36 were evaluated at older than 18 months (Table 3).
Table 3.
Distribution of Serum Samples by Infant Age at the Time of Blood Collection
| Age at Sample Collection, mo | |||||||
|---|---|---|---|---|---|---|---|
| Samples | 0–3 | 3–6 | 6–9 | 9–12 | 12–18 | >18 | No. of Infants (%) |
| Serum samples, No. of infants | |||||||
| 1st | 42 | 30 | 8 | 5 | 7 | 6 | 98 (100) |
| 2nd | 3 | 18 | 10 | 15 | 10 | 9 | 65 (66.3) |
| 3rd | 0 | 2 | 5 | 10 | 8 | 11 | 36 (36.7) |
| 4th | 0 | 0 | 0 | 3 | 4 | 10 | 17 (17.3) |
| 5th | 0 | 0 | 0 | 0 | 2 | 6 | 8 (8.1) |
| 6th | 0 | 0 | 0 | 0 | 1 | 1 | 2 (2.0) |
| 7th | 0 | 0 | 0 | 0 | 0 | 2 | 2 (2.0) |
| No. of samples | 45 | 50 | 23 | 33 | 32 | 45 | 228 |
| No. of infantsa | 42 | 48 | 22 | 33 | 31 | 36 | … |
| No. of unique infants testedb | 72 | 47 | 55 | … | |||
The number of serum samples obtained per infant varied from 1 to 7. All infants (n = 98) had at least 1 sample collected during the study, while 65 children (66.3%) had 2 samples, and 36 (36.7%) had 3 or more consecutive serum samples, resulting in a total of 228 samples.
Abbreviation: VNT, virus neutralization test.
aNumber of infants tested by Zika VNT according to age at the time of sample collection.
bNumber of unique infants tested by Zika VNT according to age at the time of sample collection: before 6 months of age, from 6 to 12 months, and above 12 months of age.
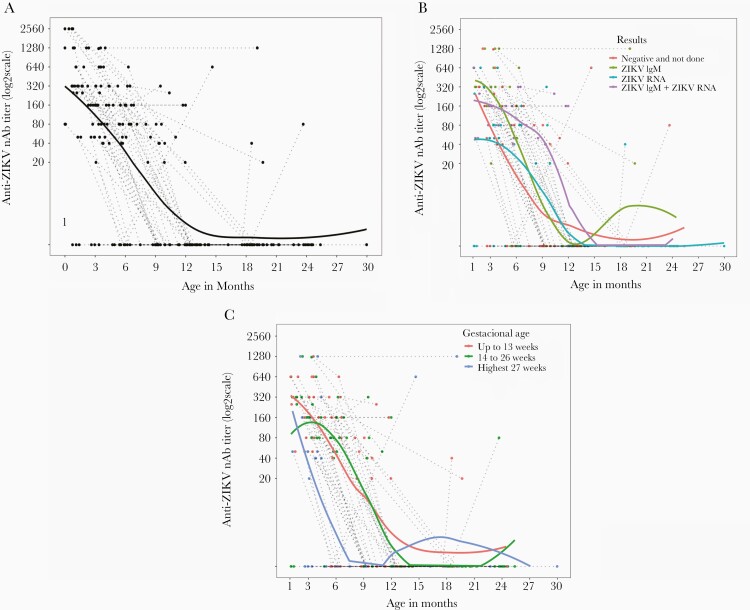
Thirty-one infants had indeterminate ZIKV nAb titers under the threshold of detection (<1:40) at all time points. These children with undetectable ZIKV nAb had a mean age of 10.4 months when the first sample was collected (ranging from 0.8 to 25 months of age). The remaining 67 infants showed a physiological decline in ZIKV nAb over the first 6 to 12 months of life (Figure 1A). Antibody levels progressively decreased over time until undetectable levels (<1:20) were attained at a median of 12 months. After 12 months of age, only 5 children had detectable nAb levels in serum, and after 18 months of age only 3 of 36 tested children had detectable ZIKV nAb levels. Two of these children had intermittent levels of detectable nAb in the first year of life (Figure 1A). With the exception of 1 child, all other children lost detectable ZIKV nAb levels by 20 months of age (Figure 1A), demonstrating that an autochthonous antibody response was not mounted in the vast majority of children, despite the presence of an early IgM response in some infants. Although the absence of ZIKV nAb titers was higher in children who had detectable ZIKV RNA in blood and/or urine and no ZIKV IgM (χ 2 test, P = .027; Table 4), this was due to a higher number of samples being obtained from children older than 6 months. After 6 months of age, a higher frequency of undetectable ZIKV nAb titers was observed in the population, due to the gradual loss of maternal antibodies (Table 4).
Figure 1.
Serum ZIKV nAb titers over time in infants born to women with laboratory-confirmed infection during pregnancy. Titers were determined in consecutive serum samples by the virus neutralization test, and log2 values are shown. Fitted lines represents the polynomial regression (lowess) of (A) all measures (n = 98); (B) cases stratified by the confirmation of Zika infection only by detection of ZIKV IgM (green, n = 14), ZIKV RNA in blood and/or urine (blue, n = 27), or both ZIKV IgM and ZIKV RNA (purple, n = 18), or without confirmation of infection (red, n = 39); and (C) cases stratified by the gestational age at infection, occurring in the first trimester (up to 13 weeks, red, n = 33), in the second trimester (from 14 to 26 weeks, green, n = 45), or during the third trimester (after 27 weeks, blue, n = 20). Abbreviations: nAb, neutralizing antibodies; ZIKV, Zika virus.
Table 4.
Detection of Zika Virus Neutralizing Antibodies According to Diagnosis of Zika Infection
| Laboratory Diagnosis of Zika Infectionc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Positived | ||||||||||
| ZIKV IgM Only | ZIKV IgM + ZIKV RNA | ZIKV RNA Only | No. Negative | No. Not Done | ||||||
| Age, moa | VNTb + | VNT − | VNT + | VNT − | VNT + | VNT − | VNT + | VNT − | VNT + | VNT − |
| 0–3 | 7 | … | 12 | 1 | 8 | 2 | 9 | 1 | … | 1 |
| 3–6 | 4 | 2 | 5 | … | 7 | 2 | 9 | 1 | … | 1 |
| 6–12 | … | … | … | … | 1 | 3 | 3 | 2 | 1 | 3 |
| > 12 | … | 1 | … | … | … | 4 | … | 4 | … | 4 |
| Total | 11 | 3 | 17 | 1 | 16 | 11 | 21 | 8 | 1 | 9 |
Abbreviations: IgM, immunoglobulin M; VNT, virus neutralization test; ZIKV, Zika virus.
aAge of infants at sample collection to carry out ZIKV VNT.
bVNT was considered positive in samples with ZIKV neutralizing antibodies titer >1:40.
cLaboratory diagnosis of Zika infection was performed by detection of ZIKV IgM and ZIKV RNA in blood and/or urine. Investigation was positive in 59 children, negative in 29, and not done in 10 infants.
dDistribution analysis of ZIKV VNT results was performed between infants with positive results for only ZIKV IgM, ZIKV IgM and ZIKV RNA, and only ZIKV RNA. χ 2 test, P = .027.
Children with confirmed Zika infection by detection of both ZIKV RNA in blood/urine and a ZIKV IgM response displayed a slower rate of nAb decay over time in comparison to infants who had infection confirmed by detection of only ZIKV RNA or ZIKV IgM, or without confirmation of infection (Figure 1B). Conversely, a faster antibody decay was observed in children born to women with laboratory-confirmed Zika infection in the third gestational trimester compared to the first and second trimesters (Figure 1C).
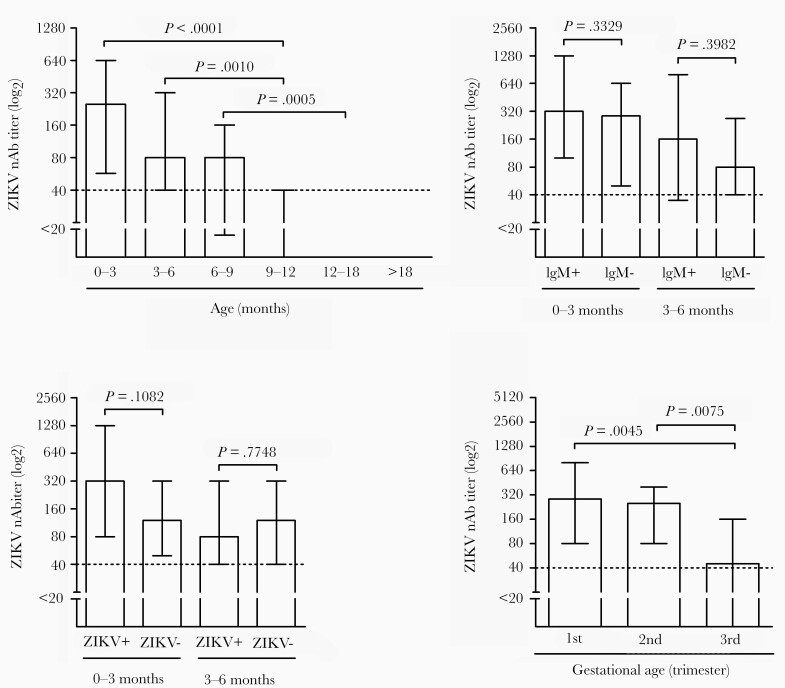
Because infant nAb titers are likely associated with maternal nAb levels, higher ZIKV nAb titers were noted in early infant specimens, particularly within the first 3 months of life. nAb levels were significantly higher in early samples in comparison to specimens obtained between 9 and 12 months of age (Kruskal-Wallis, P < .0001; Figure 2A). However, no significant difference was observed in nAb titers of children with laboratory-confirmed ZIKV infection (detection of ZIKV RNA and/or ZIKV IgM), or ZIKV IgM response only, when comparing the first nAb results obtained in samples before 3 months or between 3 and 6 months of age (both at the time of the first sample collection; (Figure 2B and 2C). On the other hand, ZIKV nAb titers were significantly lower in the first 6 months of life in infants born to women infected by Zika during the third gestational trimester compared with the first and second trimesters (Figure 2D), indicating that the faster antibody decay observed in this group was associated with a lower initial ZIKV nAb titer.
Figure 2.
Maternal anti-ZIKV nAb titers in the serum of infants born to women with confirmed infection in pregnancy. A, Anti-ZIKV nAb titers in infants according to age at the time of sample collection, analyzed by Dunn test. Differences in nAb titers in the first 3 months and between 3 to 6 months of life evaluated according to (B) the presence or absence of ZIKV IgM, and (C) the confirmation of Zika infection (ZIKV+). D, ZIKV nAb titers in the first 6 months of life evaluated according to the gestational age at the time of Zika infection. Statistical analysis was performed with Mann-Whitney test. Log2 titers are shown. Bars represent median values with interquartile range. Abbreviations: nAb, neutralizing antibodies; ZIKV, Zika virus.
DISCUSSION
Vertical transmission was documented in 60.2% of our cohort of antenatally ZIKV exposed children, while congenital abnormalities were present in 37.3% of the children with laboratory-confirmed infection by PCR or IgM detection. We did not observe continuous shedding of ZIKV in the majority of children followed in our cohort, and not all clinically affected infants had detectable levels of ZIKV-specific IgM [19].
A considerable proportion of infants with abnormal birth outcomes or obvious clinical pathology, that is congenital Zika syndrome, did not exhibit confirmatory laboratory markers (eg, ZIKV detection by PCR at birth or ZIKV IgM in the serum or cerebrospinal fluid). However, even in the absence of laboratory evidence for determining the infant infection status, the presence of congenital abnormalities in infants born to mothers with documented ZIKV infection in pregnancy is suggestive of ZIKV vertical transmission. On the other hand, a number of ZIKV-infected infants with laboratory documentation of infection did not show any congenital abnormalities at birth (37 of 59 children, 62.7%), which has been reported before [19]. In addition, a proportion of children with antenatal ZIKV exposure may experience health problems later in childhood [20–23].
It has been shown that immune complexes between ZIKV and cross-reactive antibodies to dengue virus enhance in vitro infection of Hofbauer cells, and it is speculated this could implicate higher rates of mother to child ZIKV transmission in utero in dengue-endemic areas [24]. A history of prior dengue was reported for 88% of pregnant women in our cohort [2]. It is possible this process contributed to the elevated rates of ZIKV vertical transmission described in our study. Nevertheless, our study design precludes us from exploring potential associations on this matter.
ZIKV-specific nAb decreased over time, which is consistent with the physiological decline of passively transferred maternal antibodies. Even those children with initially detectable ZIKV IgM did not exhibit persisting levels of ZIKV nAb titers over time. It remains to be investigated whether children without persisting IgG nAb response could potentially be susceptible to ZIKV reinfection in spite of their antenatal ZIKV infection.
Evaluation of specific ZIKV memory T- and B-cell responses may be helpful in elucidating whether immunologic responses to ZIKV infection are suppressed in congenitally infected children. In other congenital infections (for example CMV or rubella), virus-specific T- and B-cell responses and development of effective nAb responses are suppressed in infancy, coinciding with intermittent or continuous virus shedding [25]. In fact, patients affected by congenital rubella syndrome may not develop IgG responses to the virus, which was documented during the US rubella epidemic in the 1960s [26]. Five-year follow-up of congenitally infected children demonstrated a 16-fold decline in rubella mean antibody titers over time from the time of birth, and many children had undetectable levels at age 5 years [27]. Interestingly, even after rubella immunization, a number of congenitally infected children did not develop rubella-specific IgG responses. The failure to develop nAb in perinatally HIV-infected children also leads to the lack of control of virologic suppression. Children with perinatally acquired HIV, however, generally develop detectable HIV-specific antibodies after 15 months of age.
Congenital ZIKV infection does not appear to follow the immunologic pattern observed in HIV infections or in toxoplasmosis, when the presence of a positive serology beyond 12–15 months of age indicates perinatally acquired infection [10, 28]. In this sense, ZIKV appears to induce immune responses similar to those observed in congenital CMV and rubella infections, with the difference that children with either of these infections tend to shed the infecting virus intermittently for years, which does not appear to be the case with ZIKV.
The vast majority of infants born to mothers with confirmed ZIKV infection lost ZIKV-specific nAb after 12 months of age, demonstrating an expected decline of passively transferred maternal antibodies and failure to develop autochthonous anti-ZIKV IgG nAb responses. Of interest, children with Zika infection confirmed by a ZIKV-specific IgM response and ZIKV genomic material in serum/urine showed a slower decay of maternal antibodies over time, suggesting those women may have transferred higher nAb titers to their infants before birth.
Thus, virus neutralization assays do not seem to reliably identify vertically infected infants. The alternative of measuring nonneutralizing antibodies by standard ELISA IgG assays is not helpful as these tests suffer from lack of specificity and cross-react with antibodies to closely related flaviviruses such as dengue, which cocirculate in most regions where ZIKV may resurface in the future. Although acute or early convalescent dengue (≤3 months from symptoms onset) secondary sera may neutralize ZIKV, late convalescent sera exhibit poor ZIKV neutralization potential, as reviewed by Priyamvada et al [29]. In our study, none of the pregnant women had symptoms of acute dengue during their pregnancy although 88% reported a history of dengue infection [2]. Therefore, a conservative threshold (titer ≥ 40) was used to minimize the possible interference of cross-reaction, providing sensitivity and specificity rates over 98% for the ZIKV VNT assay used [17].
Limitations to our study include the fact that not all infants were tested sequentially at exactly the same time points nor had the same number of samples collected, which would have greatly facilitated the comparison of nAb titers over time. However, we want to point out that we were able to collect 2 or more samples in two-thirds of children during their follow-up. This study was performed during an epidemic before any structured protocols were available. For this study, we relied entirely on the availability of parents to bring their children to the clinic when possible. The other study limitation is that we did not measure nonneutralizing antibody activity in our population. At the time of the conduct of this study, the available IgG assays for ZIKV detection did not exhibit the requested sensitivity and specificity.
In summary, we conclude that there is no evidence of a sustained anti-ZIKV IgG nAb immune responses in children with PCR-confirmed antenatal exposure to ZIKV, even in the subgroup with confirmed vertical transmission (approximately 60%). This is in contrast to other perinatal pathogens such as toxoplasmosis or HIV, where persistence of IgG antibodies is used as a diagnostic marker of congenital infection [1]. Ensuring regular clinical follow-up of children with in utero exposure to ZIKV is currently the only way to identify early- and late-stage repercussions over time. The detection of a reliable biomarker of ZIKV congenital infection is still elusive and requires further investigation.
Notes
Acknowledgments. We thank all the health professionals that gave supportive care to the mothers and their newborns during the Zika epidemic in Rio de Janeiro, Brazil.
Financial Support. This work was supported by the Department of Science and Technology, Brazilian Ministry of Health (grant number 25000.072811/2016–19); Coordination for the Improvement of Higher Level or Education Personnel (grant number 88887.116627/2016-01); Brazilian National Council for Scientific and Technological Development (grant number 307282/2017-1); Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (grant number E-26/202.862/2018, Cientista do Nosso Estado); Thrasher Research Fund (grant number 20164370); European Union Horizon 2020 Research and Innovation Programme under ZIKAlliance (grant number 734548); and National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (grant numbers AI28697, AI1259534, and AI140718); Fondation Christophe et Rodolphe Mérieux; and National Eye Institute, NIH (grant number AI129847).
Potential conflicts of interest. All authors: No reported conflicts of interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Voordouw B, Rockx B, Jaenisch T, et al. Performance of Zika assays in the context of Toxoplasma gondii, parvovirus B19, rubella virus, and cytomegalovirus (TORCH) diagnostic assays. Clin Microbiol Rev 2019; 33:e00130-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honein MA, Dawson AL, Petersen EE, et al. ; US Zika Pregnancy Registry Collaboration . Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317:59–68. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure—U.S. Zika pregnancy registry, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro-Mendoza CK, Rice ME, Galang RR, et al. ; Zika Pregnancy and Infant Registries Working Group . Pregnancy outcomes after maternal Zika virus infection during pregnancy—U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018; 378:985–94. [DOI] [PubMed] [Google Scholar]
- 7.Pomar L, Vouga M, Lambert V, et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ 2018; 363:k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice ME, Galang RR, Roth NM, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection—U.S. territories and freely associated states, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira ML, Nery Júnior NRR, Estofolete CF, et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin Microbiol Infect 2018; 24:646–52. [DOI] [PubMed] [Google Scholar]
- 10.Remington J, McLeod R, Wilson C, Desmonts G. Toxoplasmosis . In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, eds. Remington and Klein’s infectious disease of the fetus and newborn infant. 8th ed. Philadelphia, USA: Elsevier Saunders, 2015. [Google Scholar]
- 11.Lopes Moreira ME, Nielsen-Saines K, Brasil P, et al. Neurodevelopment in infants exposed to Zika virus in utero. N Engl J Med 2018; 379:2377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pool KL, Adachi K, Karnezis S, et al. Association between neonatal neuroimaging and clinical outcomes in Zika-exposed infants from Rio de Janeiro, Brazil. JAMA Netw Open 2019; 2:e198124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman VM, Rasche A, Baronti C, et al. Assay optimization for molecular detection of Zika virus. Bull World Health Organ 2016; 94:880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger D, Hilgart H, Misner L, et al. Serologic testing for Zika virus: comparison of three Zika virus IgM-screening enzyme-linked immunosorbent assays and initial laboratory experiences. J Clin Microbiol 2017; 55:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado Portilho M, de Moraes L, Kikuti M, et al. Accuracy of the Zika IgM antibody capture enzyme-linked immunosorbent assay from the centers for disease control and prevention (CDC Zika MAC-ELISA) for diagnosis of Zika virus infection. Diagnostics (Basel) 2020; 10:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurtop E, Villarroel PMS, Pastorino B, et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J 2018; 15:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonaldo MC, Ribeiro IP, Lima NS, et al. Isolation of infective Zika virus from urine and saliva of patients in Brazil. PLoS Negl Trop Dis 2016; 10:e0004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasil P, Vasconcelos Z, Kerin T, et al. Zika virus vertical transmission in children with confirmed antenatal exposure. Nat Commun 2020; 11:3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulkey SB, Bulas DI, Vezina G, et al. Sequential neuroimaging of the fetus and newborn with in utero Zika virus exposure. JAMA Pediatr 2019; 173:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdes V, Zorrilla CD, Gabard-Durnam L, et al. Cognitive development of infants exposed to the Zika virus in Puerto Rico. JAMA Netw Open 2019; 2:e1914061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen-Saines K, Brasil P, Kerin T, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019; 25:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulkey SB, Arroyave-Wessel M, Peyton C, et al. Neurodevelopmental abnormalities in children with in utero Zika virus exposure without congenital Zika syndrome. JAMA Pediatr 2020; 174:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman MG, Quicke KM, O’Neal JT, et al. Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe 2018; 24:731–42.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison G. Cytomegalovirus . In: Cherry J, Demmler-Harrison G, Kaplan S, Steinbach W, Hotez P, eds. Feigin and Cherry’s textbook of pediatric infectious diseases. 8th ed. Philadelphia, USA: Elsevier Saunders, 2018. [Google Scholar]
- 26.Cherry J, Baker A. Rubella virus . In: Cherry J, Demmler-Harrison G, Kaplan S, Steinbach W, Hotez P, eds. Feigin and Cherry’s textbook of pediatric infectious diseases. 8th ed. Philadelphia, USA: Elsevier Saunders, 2018. [Google Scholar]
- 27.Cooper LZ, Florman AL, Ziring PR, Krugman S. Loss of rubella hemagglutination inhibition antibody in congenital rubella. Failure of seronegative children with congenital rubella to respond to HPV-77 rubella vaccine. Am J Dis Child 1971; 122:397–403. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen-Saines K, Paul M, Shearer W. Human immunodeficiency virus and acquired immunodeficiency syndrome. In: Cherry J, Demmler-Harrison G, Kaplan S, Steinbach W, Hotez P, eds. Feigin and Cherry’s textbook of pediatric infectious diseases. 8th ed. Philadelphia, USA: Elsevier Saunders, 2018. [Google Scholar]
- 29.Priyamvada L, Hudson W, Ahmed R, Wrammert J. Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology. Emerg Microbes Infect 2017; 6:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]