Abstract
We sought to examine placentas enriched for trophoblast inclusions (TIs) in order to characterize, quantify, and examine the interrelations between subtypes of TIs to better understand their underlying biology. We examined a cohort of 600 placentas from deliveries between 206 and 430 weeks of gestation. Forty-five percent of the placentas had at least one TI in the two slides examined. Four percent of the placentas had 10 or more TIs and two placentas had more than 70 TIs. Four distinct TI types were observed: inclusionoids (early forming inclusions), inclusions, calcified inclusions, and calcified bodies. We suggest this reflects a developmental trajectory of TI maturation, the timing of which might be useful when comparing TI expression to clinical outcomes.
Keywords: Placenta, trophoblast inclusions, pathology, genetic markers, folding
Graphical Abstract
1. Introduction
In the last 10–15 years, researchers have shown that TIs are found with increased frequency in placentas from children with autism spectrum disorder (ASD) [1] and from children with increased familial risk for ASD [2]. TIs are also more prevalent in cases of placenta accreta, increta, and percreta [3] as well as premature delivery [4]. This study aimed to identify, characterize, quantitate, and study the interrelations between trophoblast inclusion types to better understand their biology. To achieve this, we performed histologic examination on a subset of 600 placentas from the Safe Passage Study (SPS) of the Prenatal Alcohol and SIDS and Stillbirth (PASS) Network [5, 6]. This sample is highly enriched for risk of pregnancy loss and prematurity, outcomes which are associated with TIs [4, 7–20].
2. Materials and Methods
2.1. Subjects and study design
Placentas were obtained from 591 pregnancies that were part of the NIAAA/NICHD-funded SPS, a prospective, multicenter cohort study designed to evaluate the hypothesis that prenatal exposure to alcohol is associated with increased risk of SIDS or stillbirth [5]. This secondary analysis includes only data obtained from the South African site [6]. Paraffin-embedded tissue sections were cut and two hematoxylin and eosin stained slides were produced for histological examination of each placenta. Of the 591 pregnancies included in this analysis, there were 582 singleton pregnancies and 9 twin pregnancies, resulting in a total of 600 placentas. Gestational age (GA) at delivery ranged from 20.9 to 43.0 weeks with a mean of 35.9 weeks (SD = 5.01).
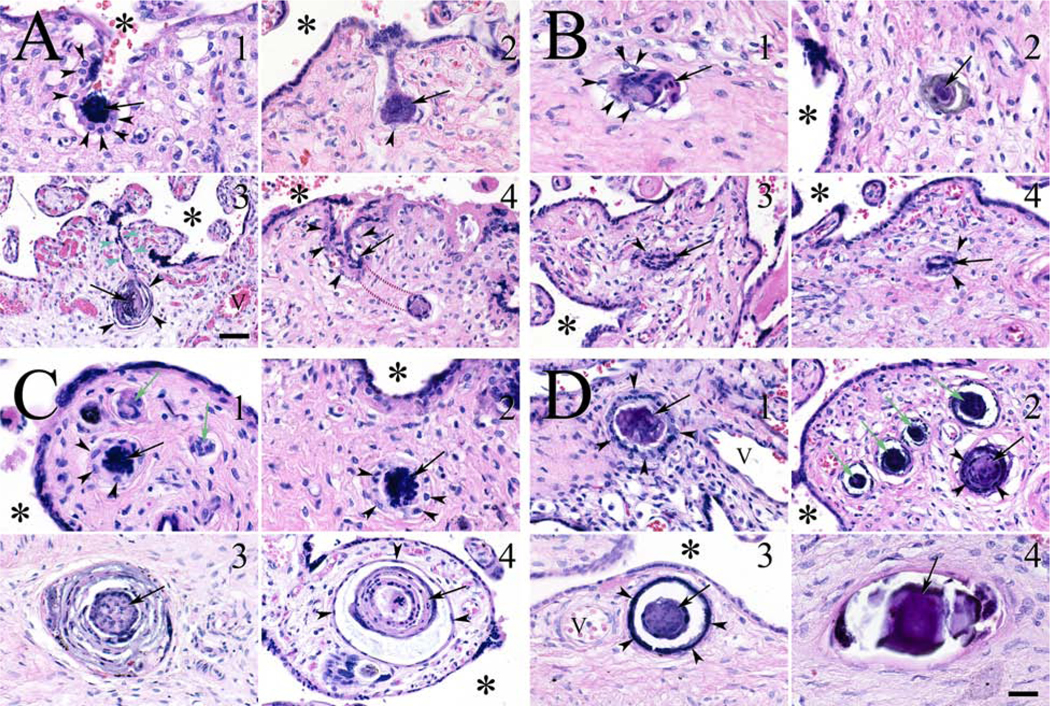
The primary outcome of interest was the frequency of TIs per slide. TIs are defined as cross sections of invaginations of the trophoblast bilayer, resulting in the appearance of trophoblasts within the villous core characterized by central syncytiotrophoblast nuclei surrounded by one or more cytotrophoblasts, always away from the villus edge [1, 2, 4, 21, 22]. In our previous studies, two types of TIs were assessed: inclusions and calcified inclusions [2, 4]. For this study we further subdivided the TIs into four groups: inclusionoids (incipient inclusions), inclusions, calcified inclusions, calcified bodies (Fig. 1).
Figure 1.
Trophoblast invaginations and inclusion types. (A) Panel showing four examples of invaginations of the trophoblast bilayer. (A1) A concentrated mass of syncytiotrophoblast nuclei (arrow) at the base of an invagination formed by an excess of cytotrophoblasts (arrowheads). Intervillous space (*). (A2) Dumbbell shaped invagination with syncytiotrophoblast core (arrow) and only a rare surrounding cytotrophoblast (arrowhead). (A3) Multilayered TI core (arrow), surrounded by thinned out cytotrophoblasts (arrowheads). Note the tenuous invagination channel (green arrowheads) demonstrating the connection between the surface trophoblast layers and a forming inclusion. (A4) Portion of an invagination with a central syncytiotrophoblast core (arrow), surrounded by cytotrophoblasts (arrowheads) that leaves the plane of the section (red dashed lines), to eventually make contact with an inclusion. (B1–4) Panel showing four examples of inclusionoids. Irregular inclusions with syncytiotrophoblast cores (arrows), surrounded in some cases by cytotrophoblasts (arrowheads). (C1–4) Panel showing fully developed inclusions, with syncytiotrophoblast cores (arrows), surrounded by cytotrophoblasts of varying thicknesses (arrowheads). Inclusionoids also seen in (C1; green arrows). (D) Panel showing calcified inclusions and an inclusion body. (D1) What appeared to be an inclusion, had, on closer inspection, a calcified core (arrow) with surrounding cytotrophoblasts (arrowheads). (D2) Calcified inclusion with calcified core with persistent syncytiotrophoblast nuclei (black arrow), surrounded by a dense ring of cytotrophoblasts (arrowheads). The remaining calcified inclusions (green arrows) had such dense calcified cores that syncytiotrophoblast nuclei could not be identified. (D3) Single calcified inclusion with only faint syncytiotrophoblast nuclei seen in the core (arrow), surrounded by a very dense layer of calcified cytotrophoblasts (arrowheads). (D4) Calcified body with a completely calcified core with no evidence of nuclei present (arrow). Magnification bar = 50 μ for all images except image (A3), for which it = 100 μ.
2.2. Statistical Analysis
We examined the frequencies of all trophoblast inclusion types in the 600 placentas and the associations between the TI types and other pathologic findings in these placentas. Correlations were determined using the Spearman rank-order method to avoid undue influence from outliers. To get approximate confidence intervals for the correlation, as well as approximate p-values, we used statistical bootstrapping [23].
Intra-rater and inter-rater test-retest reliability was established by re-reading 10% of both slides from a randomly-selected subset of the placentas (60 out of 600 placentas, yielding 120 out of the original 1,200 slides) and quantitating the four TI types described above in each slide. The percent agreement between reads for both the expert (HJK) and novice (KMH) reader were calculated. Using the stringent criteria of an exact numeric intra-rater agreement, HJK had an exact agreement ranging between 81.5 and 94.1% for the four TI types, while KMH’s exact agreement ranged between 69.2 and 91.7%. We also evaluated the percent agreement between reads using a Poisson derived standard deviation. The two slides were considered to be in agreement if both were no further from their average than the square root of their average. Using this approach we found that HJK had an adjusted percent agreement ranging between 99.2 and 100%, while KMH’s agreement increased to between 95.8 and 100%. Analyses were carried out using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Of the 600 placentas examined, 270 (45%) had at least 1 TI of any type in the two slides examined, while 330 (55%) had no TIs in the two slides. 438 placentas (73%) had fewer than 2 TIs across the two slides (our previous a priori definition of a negative placenta [1]). Twenty-four placentas (4%) had 10 or more TIs of any type and two placentas had more than 70 TIs in the two slides examined.
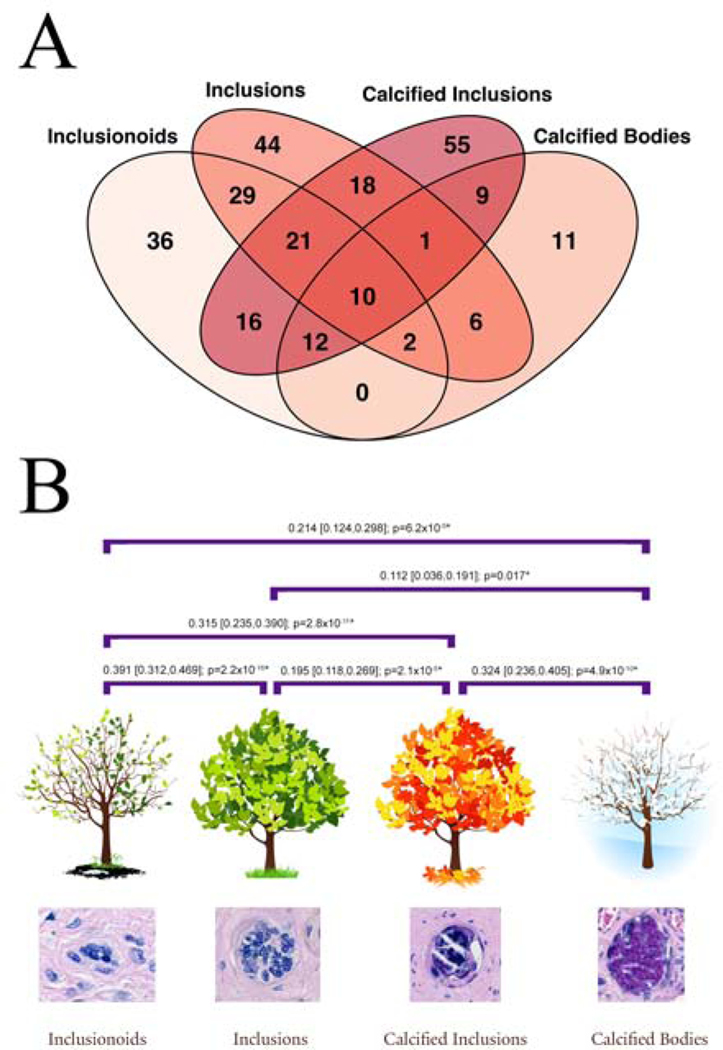
Having parsed the TIs at the time of data collection, we decided to examine the relationships among these TI types. A Venn diagram (see Fig. 2A) revealed that although many of the 270 placentas with TIs only had one type of TI, there was significant overlap with placentas simultaneously having two or more TI types. This suggested that there might be significant relationships between these four types. Therefore, we next calculated the correlation coefficients of each TI type compared to the other three types, resulting in six correlation coefficients (see Fig. 2B). There were statistically significant correlations between all six pair-wise comparisons, the most significant three being between the inclusionoids and inclusions (Spearman’s rank correlation coefficient [ρ] = 0.391, 95% confidence interval [CI] 0.320, 0.467; p=0), followed by calcified inclusions and calcified bodies (ρ = 0.324, 95% CI 0.237, 0.406; p=4.9×10−10), and finally, inclusionoids and calcified inclusions (ρ = 0.315, 95% CI 0.239, 0.390; p=1.1×10−11). These correlations, and their ordering, suggests the possibility of a temporal relationship between these inclusion types, namely that inclusionoids form first, followed by inclusions, then calcified inclusions, and finally, calcified bodies (see Fig. 2B).
Figure 2.
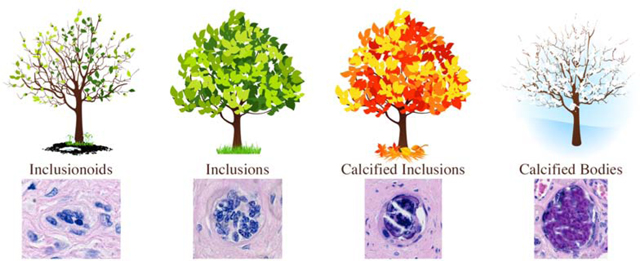
Interrelations between inclusion types. (A) Venn diagram showing the number of placentas with each inclusion type and their overlaps with the other inclusion types. The total adds up to 270, the number of placentas with at least one TI in two slides. (B) Spearman correlations between inclusion types (with 95% confidence limits and p values), all of which are significant (*). The inclusion types have been placed in their temporal formation order with inclusionoids first (analogous to the buds forming on a tree), inclusions next (as in the leaves on a tree), calcified inclusions (now aging like the colored leaves of autumn), and finally calcified bodies (as in a tree in winter).
4. Discussion
The cohort examined in this study was highly enriched in TIs, allowing us to assess the interrelations between the four TI subtypes we identified (see Fig. 2). The pregnancies included in this secondary analysis were derived from the PASS Study, which was itself enriched to study maternal prenatal alcohol use, SIDS, and stillbirth [5]. With this enrichment, we were able to detect what appears to be the developmental relationships between the four TI types.
Inclusionoids are imperfect inclusions due to several factors. They are frequently smaller than what a classic inclusion would look like (compare Fig. 1, panel B to panel C), more often irregular in shape, and may not have had an obvious cytotrophoblast outer layer, one of the features of a fully developed inclusion. Although our current approach is not sufficient to prove this hypothesis, we speculate that inclusionoids are the earliest form of TIs, possibly representing cross sections of very early invaginations. As such, we have placed them as occurring prior to the development of a full-fledged TI (Fig. 2B). Inclusionoids are highly correlated with both inclusions and calcified inclusions (see Fig. 2), suggesting that these TI types are closely related. Although it is speculative to suggest that inclusionoids precede the other TI types, the temporal relationship between calcified inclusions and calcified bodies is much more certain. The calcification process itself is irreversible. Therefore, once the syncytiotrophoblast core of an inclusion starts to become calcified, it cannot revert to either an inclusion, nor an inclusionoid, but will remain as a calcified inclusion or progress to a calcified body.
The slides in the present cohort were examined previously by other pathologists as part of the PASS study, however, TIs were not initially identified in these slides under the examination protocol based on the Amsterdam Placental Workshop Group Consensus Statement [24]. TIs are not part of routine pathologic examination, and therefore, they are often not noted by pathologists. To establish the feasibility and reliability of including TIs as a standard pathologic feature of placental examination, we performed an analysis of intra-rater and inter-rater test-retest reliability. Our results suggest that TIs can be easily identified—if they are looked for.
Trophoblast inclusions are formed by abnormal folding of the trophoblast bilayer and represent dysmorphic development in the placenta, which is most likely associated with a genetic abnormality. As such, TIs may serve as a proxy/indicator for matched genetic abnormalities in the associated conceptus and newborn. Our study validates the reliability of identifying TIs in placentas and suggests that it may be useful to prospectively include TIs in the examination of all placentas to identify those pregnancies that may be associated with an occult genetic abnormality. More generally, TIs may be an important tool for both clinical and research applications. It is also clear from our study that when TIs are present—and specifically looked for—they will be seen.
Highlights.
TIs are formed by abnormal invagination of the trophoblast bilayer
TIs represent dysmorphic placental development
TIs are easily identified, if they are looked for
TIs progress through four morphologic stages
Acknowledgements
We wish to thank Handre Carstens of the Division of Anatomical Pathology of Stellenbosch University for preparing the slides.
Funding
Grants U01HD055154, U01HD045935, U01HD055155, U01HD045991, and U01AA016501 issued by the National Institute on Alcohol Abuse and Alcoholism, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders; The Sackler Institute for Developmental Psychobiology; and the Reproductive and Placental Research Unit, Yale University.
Ethical approval
Patient recruitment into the Safe Passage Study (SPS) and PASS Network was approved by Stellenbosch University Health Research Ethics Committee (project #9317) and the New York State Psychiatric Institute Institutional Review Board (protocol #5338). Microscopic examination of the deidentified slides sent for evaluation to the Yale Reproductive and Placental Research Unit was approved by the Stellenbosch University Ethics Board and the Yale University Human Investigation Committee (protocol #1003006495).
Abbreviations
- TIs
Trophoblast inclusions
- ASD
Autism spectrum disorder
- SIDS
Sudden infant death syndrome
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- NICHD
National Institute of Child Health and Human Development
- SPS
Safe Passage Study
- PASS
Prenatal Alcohol and SIDS and Stillbirth
- GA
Gestational age
- CalcTIs
Calcified TIs
Footnotes
Declaration of competing interest
The authors declare no competing interests.
Declarations of interest: none
Conflict of Interest Statement
PLAC-S-20-00597R1
Kliman et al
Trophoblast inclusions in the human placenta: identification, characterization, quantification, and interrelations of subtypes
All the authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ, Placental trophoblast inclusions in autism spectrum disorder, Biol Psychiatry 61(4) (2007) 487–91. [DOI] [PubMed] [Google Scholar]
- [2].Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ, Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism, Biol Psychiatry 74(3) (2013) 204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adler E, Madankumar R, Rosner M, Reznik SE, Increased placental trophoblast inclusions in placenta accreta, Placenta 35(12) (2014) 1075–8. [DOI] [PubMed] [Google Scholar]
- [4].Firestein MR, Abellar R, Myers MM, Welch MG, Increased trophoblast inclusions in placentas from prematurely born infants: A potential marker of risk for preterm neurodevelopmental outcomes, Placenta 60 (2017) 61–63. [DOI] [PubMed] [Google Scholar]
- [5].Shuffrey LC, Myers MM, Isler JR, Lucchini M, Sania A, Pini N, Nugent JD, Condon C, Ochoa T, Brink L, du Plessis C, Odendaal HJ, Nelson ME, Friedrich C, Angal J, Elliott AJ, Groenewald C, Burd L, Fifer WP, Network P, Association Between Prenatal Exposure to Alcohol and Tobacco and Neonatal Brain Activity: Results From the Safe Passage Study, JAMA Netw Open 3(5) (2020) e204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GD, Hereld D, Hoffman HJ, Myers MM, Odendaal HJ, Signore C, Sullivan LM, Willinger M, Wright C, Kinney HC, Network PR, The safe passage study: design, methods, recruitment, and follow-up approach, Paediatr Perinat Epidemiol 28(5) (2014) 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mall FP, Meyer AW, Studies on abortuses: A survey of pathologic ova in the Carnegie Embryological collection, Carnegie Institution of Washington; 1921. [Google Scholar]
- [8].Philippe E, Boue JG, [The placenta in lethal chromosome aberrations], Ann Anat Pathol (Paris) 14(3) (1969) 249–66. [PubMed] [Google Scholar]
- [9].Philippe E, Boue JG, [Placenta and chromosome aberrations in spontaneous abortion], Presse Med 78(14) (1970) 641–6. [PubMed] [Google Scholar]
- [10].Honore LH, Dill FJ, Poland BJ, The association of hydatidiform mole and trisomy 2, Obstet Gynecol 43(2) (1974) 232–7. [PubMed] [Google Scholar]
- [11].Jacobs PA, Hunt PA, Matsuura JS, Wilson CC, Szulman AE, Complete and partial hydatidiform mole in Hawaii: cytogenetics, morphology and epidemiology, Br J Obstet Gynaecol 89(4) (1982) 258–66. [DOI] [PubMed] [Google Scholar]
- [12].Surti U, Szulman AE, Wagner K, Leppert M, O’Brien SJ, Tetraploid partial hydatidiform moles: two cases with a triple paternal contribution and a 92,XXXY karyotype, Hum Genet 72(1) (1986) 15–21. [DOI] [PubMed] [Google Scholar]
- [13].Novak R, Agamanolis D, Dasu S, Igel H, Platt M, Robinson H, Shehata B, Histologic analysis of placental tissue in first trimester abortions, Pediatr Pathol 8(5) (1988) 477–82. [DOI] [PubMed] [Google Scholar]
- [14].Conran RM, Hitchcock CL, Popek EJ, Norris HJ, Griffin JL, Geissel A, McCarthy WF, Diagnostic considerations in molar gestations, Hum Pathol 24(1) (1993) 41–8. [DOI] [PubMed] [Google Scholar]
- [15].Koenig C, Demopoulos RI, Vamvakas EC, Mittal KR, Feiner HD, Espiritu EC, Flow cytometric DNA ploidy and quantitative histopathology in partial moles, Int J Gynecol Pathol 12(3) (1993) 235–40. [DOI] [PubMed] [Google Scholar]
- [16].Fukunaga M, Ushigome S, Endo Y, Incidence of hydatidiform mole in a Tokyo hospital: a 5-year (1989 to 1993) prospective, morphological, and flow cytometric study, Hum Pathol 26(7) (1995) 758–64. [DOI] [PubMed] [Google Scholar]
- [17].Lorenzato M, Visseaux-Coletto B, Lallemand A, Masure M, Gaillard D, Determination of reliable histological features associated with early triploidy using DNA image cytometry, Pathol Res Pract 191(12) (1995) 1179–85. [DOI] [PubMed] [Google Scholar]
- [18].Silvestre E, Cusi V, Borras M, Antich J, Cytogenetic and morphologic findings in chorionic villi from spontaneous abortions, Birth Defects Orig Artic Ser 30(1) (1996) 353–7. [PubMed] [Google Scholar]
- [19].Sumithran E, Cheah PL, Susil BJ, Looi LM, Problems in the histological assessment of hydatidiform moles: a study on consensus diagnosis and ploidy status by fluorescent in situ hybridisation, Pathology 28(4) (1996) 311–5. [DOI] [PubMed] [Google Scholar]
- [20].Chew SH, Perlman EJ, Williams R, Kurman RJ, Ronnett BM, Morphology and DNA content analysis in the evaluation of first trimester placentas for partial hydatidiform mole (PHM), Hum Pathol 31(8) (2000) 914–24. [DOI] [PubMed] [Google Scholar]
- [21].Kliman HJ, Segel L, The placenta may predict the baby, J Theor Biol 225 (2003) 143–145. [DOI] [PubMed] [Google Scholar]
- [22].Rejniak KA, Kliman HJ, Fauci LJ, A computational model of the mechanics of growth of the villous trophoblast bilayer, Bull Math Biol 66 (2004) 199–232. [DOI] [PubMed] [Google Scholar]
- [23].Streiner DL, Statistics Commentary Series: Commentary No. 26: Dealing With Outliers, J Clin Psychopharmacol 38(3) (2018) 170–171. [DOI] [PubMed] [Google Scholar]
- [24].Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ, Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement, Arch Pathol Lab Med 140(7) (2016) 698–713. [DOI] [PubMed] [Google Scholar]