Abstract
In the present study restriction fragment length polymorphism analyses with the recently described insertion sequence IS1245 as a probe was performed with clinical Mycobacterium avium complex strains cultured in Denmark during a 2-year period. The overall aim of the study was to disclose potential routes of transmission of these microorganisms. As a first step, the genetic diversity among isolates from AIDS patients and non-human immunodeficiency virus (HIV)-infected patients was described. In addition, a number of isolates from nonhuman sources cultured during the same period were analyzed and compared to the human isolates. A total of 203 isolates from AIDS patients (n = 90), non-HIV-infected patients (n = 91), and nonhuman sources (n = 22) were analyzed. The presence of IS1245 was restricted to Mycobacterium avium subsp. avium isolates. The majority of human isolates had large numbers of IS1245 copies, while nonhuman isolates could be divided into a high-copy-number group and a low-copy-number group. Groups of identical strains were found to be geographically widespread, comprising strains from AIDS patients as well as strains from non-HIV-infected patients. Samples of peat (to be used as potting soil) and veterinary samples were found to contain viable M. avium isolates belonging to genotypes also found in humans.
The incidence of Mycobacterium avium complex (MAC) infections has increased during the last decade mainly due to disseminated infections in severely immunocompromised patients infected with the human immunodeficiency virus (HIV) (13). MAC infections in humans have been known for years mainly as pulmonary infections in patients with chronic lung diseases and as lymph node infections in otherwise healthy children. However, little is still known about the pathogenesis of the infection. Apparently, the HIV-infected, immunocompromised individual is an adequate host for MAC since as many as 40% of HIV-positive patients develop disseminated MAC infection (11, 23), whereas 5% of other severely immunocompromised patients develop disseminated MAC infection (14). However, not all HIV-infected patients contract MAC infection. The route of infection is believed to be the respiratory or gastrointestinal tract (5, 6, 15), but the reservoir of human infection is basically unknown, although several hypotheses have been brought forward over the years (14, 22, 37). Since MAC can be isolated form environmental sources like water and soil (37–39) and is capable of causing infection in animals, i.e., birds and pigs, it is usually postulated that the source of human infection is either the environment or animals. Yet, a more precise determination of the sources of infection is still needed, since detailed knowledge might help in the identification of risk factors so that prophylactic measures could be taken to avoid infection in HIV-infected patients. In recent years a number of new techniques for the subtyping of MAC have been described, i.e., pulsed-field gel electrophoresis (4, 21, 31), ribotyping (24), PCR (27), and restriction fragment length polymorphism (RFLP) analyses based on various insertion sequences (9, 29). In this study RFLP analysis with IS1245 as a probe was used to genotype MAC strains from Danish AIDS patients, non-HIV-infected patients, and animal and environmental sources. The aim of the study was to determine the genetic diversity of the MAC strains infecting Danish AIDS patients, to compare them with isolates cultured from HIV-negative patients, and to explore possible relations between the genotype and the clinical manifestation or demographics. In addition, we compared the clinical isolates to a number of isolates cultured from animals and the environment during the same period in order to study possible sources of infection.
MATERIALS AND METHODS
Bacterial strains.
The Department of Mycobacteriology at Statens Serum Institut functions as a central laboratory for the diagnosis of mycobacterioses including tuberculosis for all of Denmark, Greenland, and the Faroe Islands. All human isolates were cultured in this laboratory. Isolates retrieved from HIV-positive patients with culture-proven MAC infection from January 1994 through March 1996 were included in the study. The patients were identified as HIV positive by the Danish AIDS register. This procedure was approved by the Danish Data Protection Agency. MAC isolates retrieved from HIV-negative patients from 1994 and 1995 were also included. The first culture of a specimen from each patient was selected for RFLP analysis.
We obtained the MAC strains cultured from animals and the environment by the Danish Veterinary Laboratory (DVL) during 1994 and 1995. This laboratory cultures a small number of specimens for the detection of mycobacteria. In most cases the specimens are from animals (pigs and occasionally from pets or wild animals). The environmental isolates from DVL were cultured from specimens sent from producers of peat and bedding material. The MAC strains in these specimens consequently represent a selected fraction of the MAC strains from animals and environment.
Culture.
Blood and bone marrow specimens were inoculated directly onto BACTEC 13A broth medium (Becton-Dickinson, Sparks, Md.). Other specimens (except specimens from sterile sites) were decontaminated with NaOH and centrifuged. The pellet was resuspended in 1 ml of phosphate-buffered saline. Smears were prepared from a portion of the sediment, and the remaining sediment was inoculated onto one slant of Middlebrook 7H10 agar and into one vial of BACTEC 12B broth medium. Stool and urine specimens were inoculated onto solid media only. Specimens were incubated at 35°C for 8 weeks. Species identification was performed by DNA-RNA hybridization with Accu-Probe (GenProbe Inc., San Diego, Calif.).
Serotyping.
Serotyping of the nonhuman strains was performed as part of a previous study (19) by a tube agglutination test with 28 antiserum specimens as described by Jørgensen (17). In cases of cross-reactions, an antibody absorption test was performed (30). No further examination of nonagglutinable strains or strains showing spontaneous agglutination was performed.
RFLP analysis.
MAC strains were grown for 3 to 4 weeks in 5 ml of Tween-albumin medium. The cultures were centrifuged, heat killed, and resuspended in 400 μl of TE (Tris-EDTA) buffer. DNA was extracted as described previously (35). The only minor modification was the use of phenol-chloroform-isoamyl alcohol (ratios, 25/24/1) instead of chloroform-isoamyl alcohol (24/1) for extraction. Approximately 4 μg of DNA was digested with PvuII and was separated by electrophoresis in a 0.7% agarose gel. After electrophoresis, the DNA was blotted onto a nylon membrane (Hybond N+; Amersham). The membrane was hybridized with a chemiluminescence-labelled (ECL Direct System; Amersham) 427-bp probe generated by PCR as described previously (9).
Analysis of RFLP patterns.
The RFLP patterns were compared with GelCompar software (Applied Maths, Kortrijk, Belgium) and visually. An internal marker of the molecular mass (a PvuII-digested supercoiled DNA ladder [Boehringer] mixed with HaeIII-digested φX174 [Boehringer]) was used to standardize the patterns as described previously (35).
Patient information.
Personal data (nationality, age, sex, race, HIV risk group, and zip code) and clinical information (date of AIDS diagnosis, dates of MAC infection and other infections, symptoms of MAC disease, and paraclinical data) were obtained from the hospital records of the HIV-infected patients. Permission to collect data was obtained by the Scientific Ethical Committees for Copenhagen and Frederiksberg, Denmark. For all patients microbiological data (type of specimens, results of microscopy, number of colonies, number of positive specimens, and drug susceptibility patterns) were obtained from the computerized patient database in the Department of Mycobacteriology.
Statistical analyses.
For comparison of HIV status, HIV risk group, sex, nationality, and clinical presentation the chi-square test was used, and Fisher’s exact test was used when appropriate. For comparison of the CD4 levels, the Student t test was used.
RESULTS
Bacterial isolates.
Bacterial isolates were obtained from 90 AIDS patients with MAC infection admitted to Danish hospitals. These patients represented 92% of all AIDS patients (n = 98) with culture-proven MAC infection in Denmark from January 1994 to ultimo March 1996. The number of person-months of AIDS patients in this period was calculated to be 7,805 (32), making the rate of isolation of MAC from AIDS patients in Denmark during this period approximately 15% per person-year. Of the 90 isolates, 85 were identified as M. avium subsp. avium and 5 were positive with the MAC-specific probe but negative with the probes specific for Mycobacterium intracellulare and M. avium (8, 33). Isolates exhibiting these characteristics are, in the following, designated MAC positive.
MAC isolates were cultured from 130 HIV-negative patients from January 1994 to January 1996. Of these 130 isolates, 13 were identified as M. intracellulare and were excluded from the study since our results confirmed the observations made by Guerrero et al. (9) that IS1245 is not present in M. intracellulare (4 M. intracellulare strains were analyzed). Of the remaining 117 isolates, 91 (78%) were available for RFLP analysis. A total of 73 (80%) of the isolates were identified as M. avium subsp. avium and 18 (20%) were identified as MAC positive.
During 1994 and 1995, DVL had cultured 25 MAC isolates. DNAs for RFLP analyses were obtained from 22 isolates. Of these 22 isolates, 21 were identified as M. avium subsp. avium and 1 was identified as M. intracellulare. Table 1 presents the sources and the serovars of the isolates from DVL.
TABLE 1.
Sources of isolates, serovars, and distributions of low-copy-number and high-copy-number strains for nonhuman MAC isolates
| Source | No. of isolates | Serovar (no. of isolates) | No. of isolates with the following characteristics:
|
||
|---|---|---|---|---|---|
| Low IS1245 copy no. | High IS1245 copy no. | No copies of IS1245 | |||
| Pig | 14 | 2 (7), 3 (1), 3/9 (1), 4 (2), 6 (1), 9 (1), NAa (1) | 9 | 5 | |
| Deer | 2 | 3/9 (1), 8/21 (1) | 0 | 2 | |
| Bird | 1 | 8/21 (1) | 0 | 1 | |
| Peat | 4 | 4 (3), 6 (1) | 0 | 4 | |
| Beddingb | 1 | 3 (1) | 1 | ||
NA, nonagglutinable.
This isolate was identified as M. intracellulare.
HIV-infected patients.
The mean age of the patients included in the study was 38 years (age range, 21 to 63 years). The demographic data for the patients are presented in Table 2. The mean time to MAC infection from the time of diagnosis of HIV infection was 12 months (range, 0 to 90 months). For 34 (38%) of the patients, disseminated MAC infection was the AIDS-defining diagnosis. The clinical presentations of the patients with MAC infections were comparable to those described by others (5, 10, 12). Anemia, fever, and weight loss were the most frequently observed symptoms (95, 80, and 73%, respectively). Less frequently observed symptoms were elevated liver enzyme levels, cough, diarrhea, lymphadenopathy, and abdominal pain. The average CD4 count at the time of MAC diagnosis was 21 × 106/liter (range, 0 to 258 × 109/liter). MAC was cultured from blood, bone marrow, and/or liver biopsy specimens from 84% of the patients. MAC was isolated only from pulmonary specimens from 10% of the patients and was isolated only from stool specimens from 6% of the patients.
TABLE 2.
Demographic data for HIV-positive and HIV-negative patients
| Characteristic | No. (%) of patients
|
||
|---|---|---|---|
| HIV-positive patients | HIV-negative patients
|
||
| Lunga | Lymph node | ||
| Sex | |||
| Male | 84 (93) | 31 (63) | 16 (38) |
| Female | 6 (7) | 18 (37) | 26 (62) |
| Nationality | |||
| Danes | 79 (88) | 47 (96) | 40 (95) |
| Immigrantsb | 11 (12) | 2 (4) | 2 (5) |
| HIV risk group | |||
| Homo- or bisexual | 63 (70) | NAc | NA |
| Heterosexual | 15 (16) | NA | NA |
| Intravenous drug abuser | 6 (7) | NA | NA |
| Blood transfusion | 1 (1) | NA | NA |
| Unknown | 5 (6) | NA | NA |
All isolates were pulmonary isolates except for one isolate from a bone marrow specimen and one isolate from a urine sample.
The immigrants were from Scandinavia (n = 3), Europe (n = 7), Africa (n = 2), the United States (n = 1), Australia (n = 1), and Greenland (n = 1).
NA, not applicable.
Non-HIV-infected patients.
Demographic data for the 91 HIV-negative patients are presented in Table 2. Of the 42 isolates from children with lymph node infections, 39 were identified as M. avium subsp. avium and 3 were identified as MAC positive. Of the 47 pulmonary isolates, 33 were identified as M. avium subsp. avium and 14 were identified as MAC positive. One bone marrow isolate from a patient with leukemia was identified as MAC positive and one isolate from urine was identified as M. avium subsp. avium. Of the isolates from the 47 patients with pulmonary infections, 16 (34%) were smear positive, 8 (17%) were smear negative but had more than two specimens that were positive by culture, and 23 (49%) were smear negative and had only one or two specimens that were positive by culture.
IS1245 patterns.
Of the 23 MAC isolates, 20 carried no copies of IS1245. The remaining three isolates carried 10, 6, and 5 copies, respectively. All these isolates were from non-HIV-infected patients. A closer investigation revealed that the species identification was uncertain for these isolates and was based primarily on colony morphology and biochemical tests due to repeatedly unclear results with
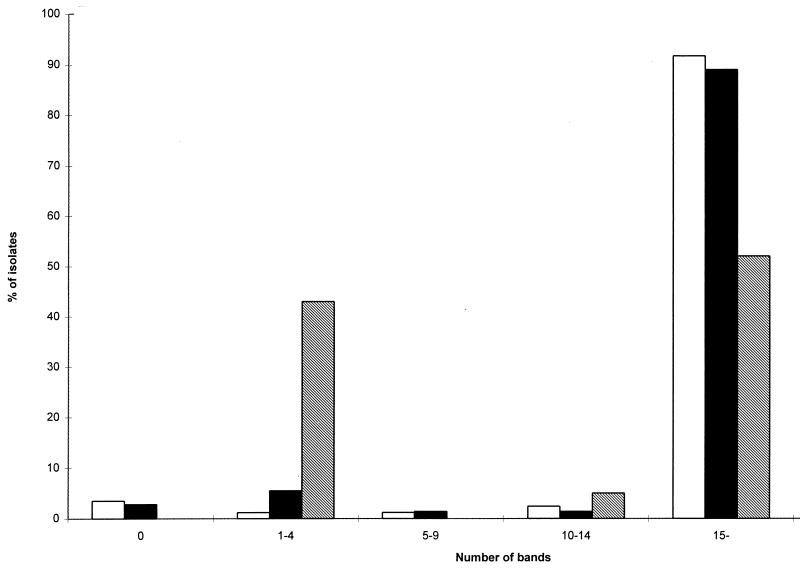
Of the 179 M. avium subsp. avium isolates, 5 (2.8%) carried no copies of IS1245 and were consequently not typeable by RFLP analysis. Of the remaining 174 isolates, the majority of the human isolates carried multiple copies, while the nonhuman strains could be divided into a high-copy-number group (11 to 23 bands) and a low-copy-number group (one isolate with 1 band and 8 isolates with three bands). The nonhuman strains had been serotyped previously (19). The serotypes in relation to copy number and source of isolate are shown in Table 1. Figure 1 indicates the number of bands for isolates from AIDS patients, isolates from non-HIV-infected patients, and nonhuman isolates. Comparison of the 174 typeable M. avium subsp. avium isolates revealed 99 different patterns. Seventy-six patterns were unique, and 23 patterns among 98 isolates were observed more than once.
FIG. 1.
Numbers of bands for M. avium isolates cultured from AIDS patients (□), non-HIV-infected patients (■), and nonhuman sources (▧).
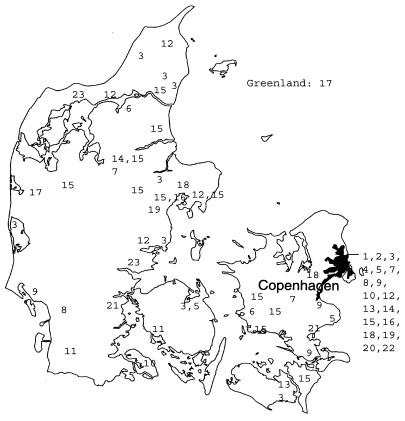
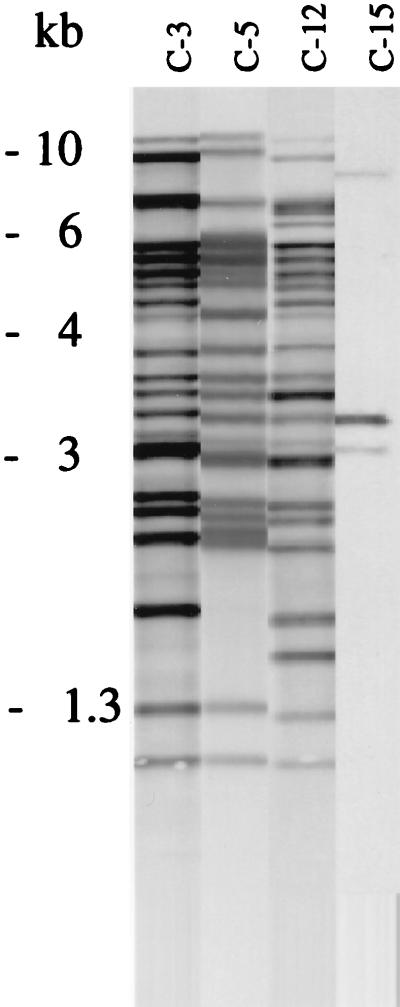
The isolates in several of the 23 clusters (defined as two or more strains exhibiting indistinguishable patterns) comprised human as well as nonhuman isolates. Table 3 presents the number of isolates in the clusters and the distributions for isolates from AIDS patients, non-HIV-infected patients, and nonhuman sources. The clusters were geographically widespread, as shown in Fig. 2. The three most prevalent patterns observed among isolates from humans exhibited a high degree of similarity. When compared by GelCompar, approximately two-thirds of the typeable strains exhibited at least 70% homology. Figure 3 provides examples of the three most prevalent patterns among human isolates and of the most prevalent pattern observed among nonhuman isolates. This pattern (cluster 15) was observed for 8 of 14 porcine isolates, 7 of which belonged to serovar 2. The pattern was not observed among isolates from AIDS patients but was found for isolates from two non-HIV-infected patients with smear-positive pulmonary disease, in one patient with lymphadenitis, and in a urine sample. Approximately half of the human isolates belonged to a cluster (isolates from AIDS patients, 54%; isolates from patients with pulmonary infection, 53%, patients with lymphadenitis, 49%), and there were no significant differences in the odds ratios that the isolates from AIDS patients, patients with lymphadenitis, and patients with pulmonary infections were part of a cluster. The odds ratio that isolates were part of a cluster was not related to nationality (i.e., native Danes compared to immigrants). Among the isolates from the AIDS patients, the CD4 levels and clinical presentations of the patients were compared for patients infected with the clustered and the nonclustered groups of isolates. No significant differences were observed. Isolates from AIDS patients were part of 18 of the 23 clusters. Sixteen of these clusters comprised isolates from patients treated in different hospitals. In order to assess whether bacterial load would correlate to certain genotypes, isolates from patients with pulmonary infections were divided into a group consisting of those from patients with only one or two specimens that were positive by culture and a group consisting of isolates from patients who were smear positive or who had more than two specimens that were positive by culture. The cluster frequency was not influenced by bacterial load. Two of the most frequent clusters (clusters 3 and 12) were represented in both groups.
TABLE 3.
Numbers and sources for the 23 groups of identical M. avium strains
| Cluster (no. of bands) | No. of isolates
|
||||
|---|---|---|---|---|---|
| HIV-positive patients | Isolates from non-HIV-infected patients
|
Non-human isolates | Total | ||
| Lunga | Lymph node | ||||
| 1 (17) | 2 | 2 | |||
| 2 (20) | 2 | 1 | 3 | ||
| 3 (21) | 8 | 3 | 4 | 15 | |
| 4 (22) | 2 | 1 | 3 | ||
| 5 (20) | 8 | 8 | |||
| 6 (22) | 2 | 1 | 3 | ||
| 7 (18) | 4 | 1 | 1b | 6 | |
| 8 (19) | 3 | 1 | 4 | ||
| 9 (19) | 3 | 2 | 5 | ||
| 10 (18) | 2 | 1 | 1 | 4 | |
| 11 (19) | 1 | 1 | 2 | ||
| 12 (23) | 3 | 3 | 1 | 2c | 9 |
| 13 (16) | 1 | 1 | 2 | ||
| 14 (20) | 2 | 2 | |||
| 15 (3) | 3 | 1 | 8b | 12 | |
| 16 (22) | 1 | 1 | 2 | ||
| 17 (9) | 1 | 1d | 2 | ||
| 18 (18) | 1 | 1 | 1c | 3 | |
| 19 (17) | 1 | 1 | 1 | 3 | |
| 20 (20) | 1 | 1 | 2 | ||
| 21 (22) | 1 | 1 | 2 | ||
| 22 (21) | 1 | 1 | 2 | ||
| 23 (21) | 1 | 1b | 2 | ||
All isolates were pulmonary isolates except for one isolate from one urine sample.
Porcine isolate.
Isolates were cultured from peat.
This patient was a child from Greenland (native inhabitant).
FIG. 2.
Geographical distribution of 23 M. avium clusters. The numbers indicate cluster numbers.
FIG. 3.
Examples of the four most frequent IS1245 patterns found among 179 human and nonhuman M. avium isolates.
DISCUSSION
During recent years a number of methods have been used to study the molecular epidemiology of MAC (4, 9, 21, 24, 27, 29), especially in order to gain an understanding of the sources of infections for humans. Seroagglutination with polyclonal sera was previously used to subgroup MAC species into 28 different serovars. MAC isolates cultured from AIDS and non-AIDS patients during a 7-year period in Denmark were serotyped in a previous study (1). No major differences in the serovars infecting AIDS patients versus those infecting non-AIDS patients were found in that study (1) or in a U.S. study (34). As pointed out by Denner et al. (7) the classical seroagglutination assay should be combined with assays that use monoclonal antibodies and other methods such as thin-layer and gas chromatographies for better typing results. We chose to use RFLP analysis with the recently described insertion sequence IS1245 to characterize Danish MAC strains. Ninety-two and 78% of the M. avium and MAC-positive strains, respectively, cultured from AIDS patients and non-AIDS patients during a 2-year period were analyzed. Furthermore, we obtained a number of MAC isolates cultured from the environment and animals during the same period.
As concluded in previous studies (3, 26, 28), IS1245 was found to be a useful tool for the differentiation of M. avium strains, and a considerable degree of polymorphism among strains was observed. However, the large number of IS1245 copies in M. avium strains and the fact that many fragments of apparently identical or similar lengths complicate the interpretation of the results. A proposal for an optimized and standardized procedure has been established (36). This procedure will allow future comparison of results obtained in different laboratories, as has been done for Mycobacterium tuberculosis (35), with great benefit.
An important issue for the value of IS1245 as an epidemiological tool is the stability of this insertion element. Recent studies have demonstrated that the stability in vivo is good (25, 28). Differences between single colonies of the same isolate, in most cases of 1 to 2 bands, have been observed by Pestel-Caron and Arbeit (25), and this observation has been confirmed in our laboratory (2). In some but not all isolates we observed similar differences following several subcultures in liquid media, while no differences could be observed following subcultures on solid media in the study of Pestel-Caron and Arbeit (25). Thus, the stability of IS1245 appears to be similar to the stability of IS6110 (40).
Guerrero et al. (9), who first described IS1245 (9), investigated the host range of the insertion element and concluded that it was present only in M. avium and not in M. intracellulare or 16 other species investigated. Of four M. intracellulare strains analyzed during this study, none were found to contain IS1245 copies, nor did 20 of 23 MAC-positive strains investigated. The remaining three strains exhibited unique patterns, with 5, 6, and 10 bands, respectively. These three isolates might have been classified incorrectly. The use of IS1245 for strain differentiation is consequently restricted to the M. avium subsp. avium species. However, even among these strains, 2.8% were found not to carry any copies. This does imply a limitation of the technique. The majority of the human M. avium subsp. avium isolates carried a large number of IS1245 copies, while the isolates from nonhuman sources divided into a high-copy-number group and a low-copy-number group. The latter consisted of nine strains, eight of which exhibited the same three-band pattern, most likely the pattern described previously (9, 28) and designated the “bird” pattern (28). Seven of the eight isolates belonged to serovar 2, supporting the observations made by Ritacco et al. (28) that the bird type constitutes a highly conserved group of M. avium strains. This pattern was observed among four human isolates from non-HIV-infected patients. In at least three cases, the data suggest that the isolation of this strain might have been of clinical relevance, since one patient had lymphadenitis and two patients had smear-positive cultures of pulmonary specimens. However, it has so far not been observed among isolates from AIDS patients. In general, the level of similarity between isolates from HIV-positive and HIV-negative patients and the presence of clusters comprising isolates from patients from both groups suggest that the two groups of patients are likely to share the same sources of infection. This is in agreement with previous studies which have demonstrated a lack of correlation between serotype and HIV infection status (1, 16).
A recent Danish study describing serotypes, IS901 genotypes, and expression of a 40-kDa protein in MAC strains cultured during 1993 concluded that strains isolated from animals differed distinctly from strains isolated from humans (19). This is not fully consistent with our results, since five of the patterns observed among the 23 isolates from nonhuman sources were found to be identical to those observed among human isolates. Two of these patterns were exhibited by isolates from peat and three were exhibited by porcine isolates. This suggests either that peat is a common source of infection for humans and animals or that animals and peat are potential sources of infection for humans. The peat samples were sent to DVL from producers of peat who wanted to control the growth of MAC before and after heat treatment of their products. The isolates from these samples were all cultured before heat treatment, and no growth was observed after heat treatment. However, these products are treated with heat only when they are to be used for bedding material in piggerys to avoid MAC infections in pigs. When used for sale as potting soil the products are not heat treated (18). The peat products are sold as potting soil throughout Denmark. The clustered isolates were found to be widely distributed geographically. This could reflect the equal distributions of genotypes in the environment in Denmark, but it might also be explained by a single source of infection, e.g., food or potting soil for potted plants.
Two studies from other countries have demonstrated that potable water is the source of infection for humans and animals. In one study potable water from two hospitals was demonstrated to be the source of MAC infection for four AIDS patients (37), and another study suggested that potable water in a biolevel 3 containment facility was the source of infection for at least six rhesus macaques (20). In Denmark, AIDS patients are mainly treated at only four hospital departments, two of which are located in Copenhagen. Water from three of these hospitals was cultured, but no growth of MAC was identified (data not shown). Furthermore, we collected water samples from a large Danish lake whose surface water is used as drinking water during the summer months (data not shown). None of these samples exhibited growth of MAC. Furthermore, AIDS patients whose isolates belonged to the same clusters were generally treated in different hospitals. In conclusion, we have not been able to demonstrate water as a possible source of MAC infection.
Human-to-human transmission of MAC strains is generally thought to be unlikely, and the geographical distributions of similar strains found in this study support this assumption. Isolates from two HIV-positive, MAC-infected intravenous drug-abusing patients who lived together exhibited distinctly different patterns (data not shown).
A question of interest is whether MAC disease is due to recent infection or reactivation of a latent infection. The answer to this questions might have implications for the type of prophylaxis chosen for AIDS patients, e.g., vaccination or chemoprophylaxis. In our study we found that isolates from several small children, who must have been infected recently, exhibited the same patterns as isolates from AIDS patients and elderly patients with lung infections. Furthermore, approximately half of the foreign-born AIDS patients, who originated from very different geographical areas, were infected with strains exhibiting the same patterns as those found among isolates from Danish patients. These observations support the assumption that MAC disease is due to recent infection.
The presence of rather large groups of identical strains could be explained by a higher level of virulence of these strains. However, we were not able to detect any differences in clinical presentation or immunological status among AIDS patients infected with clustered strains and AIDS patients infected with unique strains. Similar observations were made for non-HIV-infected patients with pulmonary infections. Isolates from patients believed to have clinically significant M. avium infection, since they were smear positive or had at least three specimens that were positive by culture, did not exhibit the most frequent patterns more often than isolates from patients with only one or two specimens that were positive by culture. Thus, our data do not support the assumption of a correlation between IS1245 genotype and a virulent phenotype.
ACKNOWLEDGMENT
We thank Else Smith, Department of Epidemiology, Statens Serum Institut, for help with the data from the AIDS register.
REFERENCES
- 1.Askgaard D S, Giese S B, Thybo S, Lerche A, Bennedsen J. Serovars of Mycobacterium avium complex isolated from patients in Denmark. J Clin Microbiol. 1994;32:2880–2882. doi: 10.1128/jcm.32.11.2880-2882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer J, Andersen A B. Stability of insertion sequence IS1245, a marker for differentiation of M. avium strains. J Clin Microbiol. 1999;37:442–444. doi: 10.1128/jcm.37.2.442-444.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bono M, Jemmi T, Bernasconi C, Burki D, Telenti A, Bodmer T. Genotypic characterization of Mycobacterium avium strains recovered from animals and their comparison to human strains. Appl Environ Microbiol. 1995;61:371–373. doi: 10.1128/aem.61.1.371-373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burki D R, Bernasconi C, Bodmer T, Telenti A. Evaluation of the relatedness of strains of Mycobacterium avium using pulsed-field gel electrophoresis. Eur J Clin Microbiol Infect Dis. 1995;14:212–217. doi: 10.1007/BF02310358. [DOI] [PubMed] [Google Scholar]
- 5.Chin D P, Hopewell P C, Yajko D M, Vittinghoff E, Horsburgh C R, Jr, Hadley W K, Stone E N, Nassos P S, Ostroff S M, Jacobson M A, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289–295. doi: 10.1093/infdis/169.2.289. [DOI] [PubMed] [Google Scholar]
- 6.Damsker B, Bottone E J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985;151:179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- 7.Denner J C, Tsang A Y, Chatterjee D, Brennan P J. Comprehensive approach to identification of serovars of Mycobacterium avium complex. J Clin Microbiol. 1992;30:473–478. doi: 10.1128/jcm.30.2.473-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GenProbe Inc. AccuProbe Mycobacterium avium complex culture identification test. Package insert. San Diego, Calif: GenProbe Inc.; 1992. [Google Scholar]
- 9.Guerrero C, Bernasconi C, Burki D, Bodmer T, Telenti A. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J Clin Microbiol. 1995;33:304–307. doi: 10.1128/jcm.33.2.304-307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havlik J A, Jr, Horsburgh C R, Jr, Metchock B, Williams P P, Fann S A, Thompson S E., 3d Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis. 1992;165:577–580. doi: 10.1093/infdis/165.3.577. [DOI] [PubMed] [Google Scholar]
- 11.Havlik J A, Jr, Metchock B, Thompson III S E, Barrett K, Rimland D, Horsburgh C R., Jr A prospective evaluation of Mycobacterium avium complex colonization of the respiratory and gastrointestinal tracts of persons with human immunodeficiency virus infection. J Infect Dis. 1993;168:1045–1048. doi: 10.1093/infdis/168.4.1045. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins C C, Gold J W, Whimbey E, Kiehn T E, Brannon P, Cammarata R, Brown A E, Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- 13.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 14.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson M A, Hopewell P C, Yajko D M, Hadley W K, Lazarus E, Mohanty P K, Modin G W, Feigal D W, Cusick P S, Sande M A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991;164:994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- 16.Julander I, Hoffner S, Petrini B, Ostlund L. Multiple serovars of Mycobacterium avium complex in patients with AIDS. APMIS. 1996;104:318–320. [PubMed] [Google Scholar]
- 17.Jørgensen J B. Serological investigations of strains of Mycobacterium avium and Mycobacterium intracellulare isolated from animal and non-animal sources. Nord Veterinaermed. 1978;30:155–162. [PubMed] [Google Scholar]
- 18.Karh, B. (Pindstrup Mosebrug). 1998. Personal communication.
- 19.Klausen J, Giese S B, Fuursted K, Ahrens P. Distribution of serotypes, IS901 and a 40 kDa protein in Mycobacterium avium complex strains isolated from man and animals in Denmark. APMIS. 1997;105:277–282. doi: 10.1111/j.1699-0463.1997.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield K G, Lackner A A. Simian immunodeficiency virus-inoculated macaques acquire Mycobacterium avium from potable water during AIDS. J Infect Dis. 1997;175:184–187. doi: 10.1093/infdis/175.1.184. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek G H, Hartman S, Zhang Y, Brown B A, Hector J S, Murphy D, Wallace R J., Jr Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993;31:390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meissner G, Anz W. Sources of Mycobacterium avium complex infection resulting in human diseases. Am Rev Respir Dis. 1977;116:1057–1064. doi: 10.1164/arrd.1977.116.6.1057. [DOI] [PubMed] [Google Scholar]
- 23.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Wynne B A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 24.Peillon R, Drouet E B, Bruneau S, Panteix G, Denoyel G A, De Montclos H P. Discrimination of Mycobacterium avium-Mycobacterium intracellulare strains by genomic DNA fingerprinting with a 16S rRNA gene probe. FEMS Microbiol Lett. 1994;124:75–79. doi: 10.1111/j.1574-6968.1994.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 25.Pestel-Caron M, Arbeit R D. Characterization of IS1245 for strain typing of Mycobacterium avium. J Clin Microbiol. 1998;36:1859–1863. doi: 10.1128/jcm.36.7.1859-1863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picardeau M, Varnerot A, Lecompte T, Brel F, May T, Vincent V. Use of different molecular typing techniques for bacteriological follow-up in a clinical trial with AIDS patients with Mycobacterium avium bacteremia. J Clin Microbiol. 1997;35:2503–2510. doi: 10.1128/jcm.35.10.2503-2510.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picardeau M, Vincent V. Typing of Mycobacterium avium isolates by PCR. J Clin Microbiol. 1996;34:389–392. doi: 10.1128/jcm.34.2.389-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritacco V, Kremer K, van der Laan T, Pijnenburg J E M, de Haas P E, van Soolingen D. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. Int J Tuberc Lung Dis. 1997;2:242–251. [PubMed] [Google Scholar]
- 29.Roiz M P, Palenque E, Guerrero C, Garcia M J. Use of restriction fragment length polymorphism as a genetic marker for typing Mycobacterium avium strains. J Clin Microbiol. 1995;33:1389–1391. doi: 10.1128/jcm.33.5.1389-1391.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer W B. Type-specificity of atypical mycobacteria in agglutination and antibody-absorption tests. Am Rev Respir Dis. 1967;96:1165–1168. doi: 10.1164/arrd.1967.96.6.1165. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky A M, Arbeit R D, Barber T W, Rich J, von Reyn C F, Pieciak W, Barlow M A, Maslow J N. Polyclonal infections due to Mycobacterium avium complex in patients with AIDS detected by pulsed-field gel electrophoresis of sequential clinical isolates. J Clin Microbiol. 1994;32:1773–1778. doi: 10.1128/jcm.32.7.1773-1778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, E. 1998. Personal communication.
- 33.Soini H, Eerola E, Viljanen M K. Genetic diversity among Mycobacterium avium complex AccuProbe-positive isolates. J Clin Microbiol. 1996;34:55–57. doi: 10.1128/jcm.34.1.55-57.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang A Y, Denner J C, Brennan P J, McClatchy K. Clinical and epidemiological importance of typing of Mycobacterium avium complex isolates. J Clin Microbiol. 1992;30:479–484. doi: 10.1128/jcm.30.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Soolingen D, Bauer J, Ritacco V, Cardoso Lexo S, Pavlik I, Vincent V, Rastogi N, Bodmer T, Garzelli C, Garcia M J. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. J Clin Microbiol. 1998;36:3051–3054. doi: 10.1128/jcm.36.10.3051-3054.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Reyn C F, Maslow J N, Barber T W, Falkinham J O, Arbeit R D. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 38.von Reyn C F, Waddell R D, Eaton T, Arbeit R D, Maslow J N, Barber T W, Brindle R J, Gilks C F, Lumio J, Lahdevirta J, et al. Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J Clin Microbiol. 1993;31:3227–3230. doi: 10.1128/jcm.31.12.3227-3230.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yajko D M, Chin D P, Gonzalez P C, Nassos P S, Hopewell P C, Reingold A L, Horsburgh C R, Jr, Yakrus M A, Ostroff S M, Hadley W K. Mycobacterium avium complex in water, food, and soil samples collected from the environment of HIV-infected individuals. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:176–182. [PubMed] [Google Scholar]
- 40.Yeh R W, Ponce de Leon A, Agasino C B, Hahn J A, Daley C L, Hopewell P C, Small P M. Stability of Mycobacterium tuberculosis DNA genotype. J Infect Dis. 1998;177:1107–1111. doi: 10.1086/517406. [DOI] [PubMed] [Google Scholar]