Abstract
The goal of clinical research is to improve clinical practice. In progressive neurodegenerative conditions without any disease-slowing therapies, this will result in eventual approval of a first disease-modifying treatment. Clinical trials will still be needed to discover treatments that are more effective, safer, or more convenient. This will generate controversies over how to design these trials; specifically, controversies about the use of a placebo control. We consider ethical guidance for these studies with attention to 3 designs: placebo-controlled trials in the absence of the new drug, placebo-controlled trials with the approved drug as background therapy, and trials with the new drug as an active control. To understand the practical implications of these designs, we examine experiences in drug development in multiple sclerosis. We conclude by contemplating the future of clinical trials in Alzheimer disease.
Introduction
The goal of clinical research is to improve clinical care. Among the most unbiased and therefore impactful research study designs to achieve this goal is the randomized controlled trial. Trials compare an intervention to something else: either another treatment or, if no treatment is available, a placebo. In trials to discover treatments that slow the progression of neurodegenerative diseases that cause dementia, placebo is the default design because no intervention has been shown to slow disease progression.
What will happen when the US Food and Drug Administration (FDA) approves a therapy as one that slows disease progression, also known as a disease-modifying therapy? This welcome event will generate an ethically complex question. How should this new treatment affect subsequent clinical trials for that condition? To answer this, we outline the ethical guidance and its effect on trial design options. We examine how this guidance and options played out in the real world using the decades-long example of multiple sclerosis (MS). We close with specific recommendations for trials in Alzheimer disease (AD), as the first disease-modifying therapies may well be within reach.
Ethical Guidance
Three ethical principles guide the discussion. A study must be valid and cannot trade off or otherwise sacrifice validity for value.1 Especially in later phases, it should be designed to change clinical practice (logic of clinical purpose).2 A study cannot expose participants to unnecessary or unreasonable risks, including potential risks resulting from withholding available treatments (nonexploitation).3 The application of these principles should attend to the phases of drug development, treatment risks and benefits, and assessments of the implications of withholding available therapeutics.
Item 33 of the Declaration of Helsinki offers widely accepted international guidance that translates these principles into practical decisions about the choice of a control group. A new treatment must be tested against “the best proven intervention” and trial participants who receive placebo must not be “subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.”4 Together, these principles and item 33 provide a common set of terms and discussion points to address.
Scholars have debated how to use these principles and item 33 to decide whether a placebo-controlled trial is ethical.5-8 That debate has centered on the level of evidence necessary to achieve adequate, valid evidence of effectiveness and what is “standard of care.”7
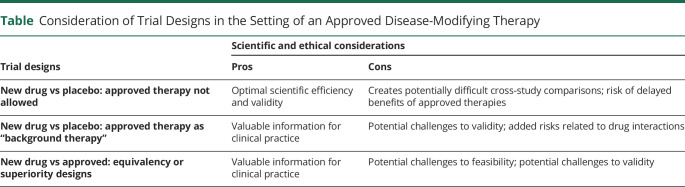
There are 3 possible designs of clinical trials in the setting of a first-approved disease-slowing therapy (Table): placebo-controlled trials in the absence of the new drug, placebo-controlled trials with the new drug as background therapy, and active comparator trials with the newly approved drug as the control arm. Each design engages interrelated scientific and ethical considerations.
Table.
Consideration of Trial Designs in the Setting of an Approved Disease-Modifying Therapy
Design 1: New Drug vs Placebo: No Newly Approved Therapy Allowed
Placebo-controlled trials are considered by many as the gold standard to assess efficacy. The design minimizes the risk of bias, including bias from participants as well as investigators.
It also offers efficiency because, compared to the other 2 designs, statistically significant effects of a new treatment can be achieved with smaller sample sizes followed for shorter durations. Answering scientific questions efficiently is valuable to patients, society, and the sponsors of the research.
The values of rigor and efficiency must be balanced against risks to participants. Would placebo-controlled studies present unreasonable, that is, exploitative, risks to trial participants in the setting of a new approved treatment? The answer depends on the quality of evidence for the new treatment but would likely take years of clinical practice to answer fully. Why? The very trial that discovered the new therapy—an efficient design optimal for testing efficacy—lacks generalizability into the wide scope of clinical practice. Standard of care requires more comprehensive assessments of effectiveness in general practice, such as with patients who have the disease but also have other diseases, or a severity of their neurodegenerative disease that made patients like them not eligible for the breakthrough trial. For these patients, other studies are needed. They may be placebo-controlled trials or observational studies and clinical experiences that assess clinician as well as patient behaviors, such as adherence to treatment, risks, and clinical course.
Design 2: New Drug vs Placebo: Approved Treatment as “Background Therapy”
Trials of new interventions in patients already on the approved treatment would have clinical value. Such trials could examine potential synergies in disease-slowing and safety. In fact, combination approaches may ultimately be needed to meaningfully curb the individual and public health impact of neurodegenerative diseases.9
Testing new agents in patients on an approved background therapy would have challenges and shortcomings. Drug–drug interactions could lead to unforeseen risks, challenging data interpretation, or even the need to stop trials early. Stratified randomization by treatment status would aid but not necessarily resolve potential challenges in data interpretation. Observed drug effects could be erroneous if the drug under study works only in the presence of the approved treatment or if benefit were masked by negative interactions with background therapy. It could also be challenging to power trials without knowledge of whether a new treatment will offer benefit above and beyond that presented by the approved drug.
Optimal designs might incorporate the approved treatment, rather than adjust for it later. A 2 × 2 factorial design would randomly assign participants to the approved drug or placebo and an investigational treatment or placebo in a balanced manner. This design permits rigorous examination of the risks and benefits of each treatment as well as the combination of treatments, while also needing to adhere to the ethical requirements of design 1 (above), given that a proportion of participants would be randomized to a placebo/placebo arm.
Design 3: New Drug vs Approved Drug: Equivalency and Superiority Designs
If the approved drug becomes an accepted standard of care for most patients with the disease, new therapies will need to be tested against it using 1 of 2 designs: equivalency and superiority studies.
Equivalency designs are best called noninferiority trials because it is impossible to prove that 2 drugs are equal. Equivalency designs aim to demonstrate whether a new treatment is within a given confidence interval of being as efficacious as the comparison standard. Equivalency designs that do not incorporate a randomized placebo arm introduce a vexing possibility: the 2 treatments are not different from each other but neither is effective. Numerous examples exist of trials that discovered a new drug was equivalent to an approved standard but neither drug was better than placebo.7
Superiority trials are designed to show that the new drug outperforms the comparator. Superiority trials against a newly approved treatment would provide compelling clinical and scientific evidence for efficacy. Even if the positive results for the approved drug were deemed spurious, outperforming it might reasonably be concluded as outperforming a drug that is effectively a placebo.
Superiority trials are typically unattractive to the companies developing drugs and seeking FDA approval to market them. A competitor's drug becomes the standard to compare a new treatment and efficacy could be more difficult to demonstrate than in a placebo-controlled trial.
The ethics of equivalency and superiority trials include questions about the just allocation of resources. The designs might necessitate sample sizes so large as to make the trials time-consuming and expensive to complete.8 Fewer participants are available for other studies. In the case of trials conducted by private companies, the claim the expense of such trials is “too much” is challenging because upfront costs are balanced by the promise of profit. A public discussion of these private interests is inherently limited in the information available and the authority to decide what design to pursue.
MS Example
The development of treatments for relapsing-remitting MS provides a unique opportunity to examine how, as the armamentarium of approved therapies changed from none to at least one, trial design choices also changed, including the use of each of the 3 designs outlined above.
The 1993 approval of interferon-β as the first disease-modifying therapy for relapsing-remitting MS was a milestone event. Lay media characterized interferon-β as a cure. It of course was not. It slowed disease. It also had risks and hassles, including subcutaneous injection and side effects of flu-like symptoms and leukopenia. The need for better therapies remained. FDA approval did, however, immediately call into question the appropriateness of the use of placebo controls.10 Clinical practice informed the answer. In the years to follow, clinical uptake of interferon-β was sufficiently variable that it was not a widely accepted standard of care. For several years, trials continued to use a placebo-only control arm.
The subsequent approvals of glatiramer acetate (also subcutaneously injected) in 1996 and additional interferons (including subcutaneous and intramuscular injections with varying schedules of administration) in the early 2000s necessitated reconsideration of the ethical use of placebo-controlled trials. These trials became more difficult to conduct, particularly in achieving full recruitment. They required more participating sites and steadily increased in the incorporation of global enrollment, including in underresourced nations where approved therapies were less available.11 These events should be interpreted as signals the available treatments were becoming standard of care.
Guidelines published in 2000 notably warned against shortening placebo-controlled trials or using too small sample sizes that could risk the scientific integrity of a study. These guidelines placed considerable emphasis on the role of informed consent.12 Patient preferences, particularly declining available therapies for reasons such as tolerability, were an important consideration in the ethics of these trials. The role of patient preference increased with the development and eventual approval (starting in 2010) of oral therapies. MS treatment guidelines pointed to patients who might not fit the regulatory indications of approval (e.g., those with primary or secondary progressive disease and other clinical subgroups) as appropriate for placebo-controlled trials. Clinical subcategorization of patients into populations who responded, and did not respond, to varying therapies began. This phenotyping of patients and acknowledged changes in trial populations over time made comparing agents tested in separate trials and the use of noninferiority studies fraught. It also illustrated the steady accretion of standard of care into the general patient population.
As more agents became approved, a widely accepted standard of care took shape. The acceptable duration of placebo controls came under increased scrutiny. In 2008, an international group of clinicians, ethicists, statisticians, regulators, and industry representatives continued to advocate for placebo-controlled MS studies but noted that 1–2 year delays in initiating available therapies could have lasting harm.13
In 2014, 21 years after the approval of interferon-β, the first agent was approved based solely on trials incorporating active interferon therapy control.14 Active-control trials have since become the standard in relapsing-remitting MS, with contemporary approvals based primarily on equivalency and superiority studies.15
Guiding Other Neurologic Conditions
How can the experiences of MS drug development instruct other areas of neurologic clinical research? Trials must be designed to achieve specific scientific objectives. These objectives may be driven by the phase of development as well as unique characteristics of the new drug under study. Nevertheless, several general points should be emphasized.
First, science is incremental. It is unlikely, though not impossible, that the approval of a first disease-modifying therapy for a neurodegenerative disease will immediately make the conduct of placebo-controlled trials for that disease unethical. Over time, scientists, clinicians, patients, and their caregivers will interpret and reinterpret data. Their behaviors—such as scientists' willingness to run a placebo-controlled trial, patients and caregivers' decisions to enroll in one, and clinicians' willingness to prescribe new therapies to patients who do not match the kinds of patients who were in studies—will contribute to an evolving standard of care that decides whether placebo-controlled trials should be continued.
Starting a clinical trial requires a state of clinical equipoise—professional disagreement about what arm of a study offers the greatest clinical value to a patient.16 In the case of placebo-controlled trials, this means consensus does not yet exist that a newly available therapy is effective (can slow disease) and that withholding the therapy results in unacceptable harm to patients. Even if professional consensus exists around the benefit of a new therapy, it may be that placebo-controlled trials can continue if they enroll patients who decline or fail to respond. In all cases, careful attention to ethical principles and item 33 of the Declaration of Helsinki discussed above and ensuring thorough informed consent will be essential. A particularly troubling case is patients who enroll in a trial because treatment cost or other nonclinical matters make them unable to be treated with the new drug. This is the concern raised by trials that enroll in underdeveloped and underresourced nations, as was the case in MS drug development. Such patients are being exploited in the manner of an employer who uses unpaid or underpaid workers.
The willingness of clinician investigators to conduct trials and of eligible patients to enroll are key data to inform conversations about the ethics of varying trial designs. Other informative data include long-term assessments of clinical outcomes from those who delayed therapy for the sake of participation and were enrolled in a placebo arm.13,14 Accordingly, ethical conduct and scientific success will require frequent reassessment of the state of the science and of clinical practice.17
AD Considerations
It has been more than 15 years since the FDA approved a new drug for the treatment of AD. None of the approved treatments slows disease progression.18 Biogen has recently submitted to the FDA an application for approval of the anti–β-amyloid (Aβ) monoclonal antibody aducanumab for the treatment of AD. Another anti-Aβ monoclonal antibody, donanemab, under development by Eli Lilly,19 recently demonstrated clinical benefit in a relatively small phase 2 trial. Thus, disease-modifying therapies for AD may, at long last, become part of clinical practice. If aducanumab or donanemab (or another drug) achieves FDA approval, what are the implications for AD trial designs? The example of MS informs answers to this question.
Like MS, AD is a progressive neurologic disease that has been an active area of drug development. An FDA-approved drug for AD, as was the case for interferon-β, will undoubtedly generate substantial attention. The media coverage of aducanumab, including the controversies around its unusual path to FDA submission,20 support this. The data for aducanumab do not describe a treatment that will be a broadly accepted standard of care. Aducanumab has only one positive registration trial and an FDA advisory panel was uniformly negative. The studies enrolled patients with a narrow range of disease severity and showed mixed results. For example, subgroup efficacy analyses suggested differential effects among unique subpopulations enrolled in the studies.21 In the event that aducanumab is approved, until more data become available, placebo-controlled trials will remain ethical.
As was the case in MS, it is likely that years will pass before sufficient evidence shows a new therapy is the standard of care for persons living with AD. The kinds of evidence that will do this will include the results of studies that show the benefits and risks of a new treatment in general, standard clinical practice, whether the benefits of therapy are maintained over time, and the risks of withholding therapy.22 Even with such evidence, absent a risk-free, convenient, disease-arresting therapy, better treatments will still be needed that offer greater efficacy or effectiveness, improved safety, or easier modes of administration (both aducanumab and donanemab require intravenous infusions). As improved therapies are discovered, there may be particular types of patients for whom placebo-controlled trials become unacceptable.
Placebo-controlled studies will also serve as a kind of test of AD participants' views about the approved drug's efficacy, safety, and convenience. If a new AD therapy is approved, an immediate and important learning will come from the patients in the more than 135 ongoing AD trials.23 Essentially all of these trials are placebo-controlled. After their participants learn about the new approval, what do they do? The count of those who drop out to begin aducanumab (or another drug) therapy, as has happened with previous AD approvals,24 will be an important measure of the patient and caregiver perception a new treatment is becoming a standard of care. Researchers must carefully monitor and share these drop-out rates because patient behaviors, particularly the choice between available treatment vs enrolling in a trial, are an important determinant of what is a clinically appropriate, hence ethical, trial design. Similar assessments will be needed within clinical practice, which will bring ethical complications related to the triumvirate of individuals involved in treatment decisions: the patient, the physician, and the family members.25 Indeed, ensuring that cognitively impaired patients' best interests are ensured in therapeutic and research decisions will be critical as the field adopts new treatments.
Regardless of the FDA's decision on aducanumab or the results of phase 3 studies of donanemab, recent events are a wake-up call to clinicians, researchers, patients, and their care partners to address an important question: how to discover better treatments in the setting of effective disease-slowing therapies for AD and other neurodegenerative diseases. As new data and new drugs become available, reconsideration of the ethics of placebo-controlled trials should be frequent. The scientific and clinical value of combination trials and superiority designs warrant consideration because they will assess the benefits and risks of a new drug as well as the value of approved treatments. The field should expect several years of likely contentious but hopefully productive debate.
Acknowledgment
J.D.G. and J.K. are supported by NIA AG057437. J.D.G. is supported by NIA AG066519 and NCATS UL1 TR001414. J.K. is supported by NIA AG010124 and NIA U54-AG-063546. The authors thank Dr. Gaby Thai for comments during the development of this manuscript.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- FDA
US Food and Drug Administration
- MS
multiple sclerosis
Study Funding
The authors report no targeted funding.
Disclosure
Joshua D. Grill reports research grants from Lilly, Biogen, and Genentech, and consulting for SiteRx. Jason Karlawish reports research grants from Lilly Inc. (A4 Study) and Novartis (Generation program). Go to Neurology.org/N for full disclosures.
References
- 1.Freedman B. Scientific value and validity as ethical requirements for research: a proposed explication. IRB. 1987;9(6):7-10. [PubMed] [Google Scholar]
- 2.Freedman B. Placebo-controlled trials and the logic of clinical purpose. IRB. 1990;12(6):1-6. [PubMed] [Google Scholar]
- 3.Miller FG, Brody H. What makes placebo-controlled trials unethical? Am J Bioeth. 2002;2(2):3-9. [DOI] [PubMed] [Google Scholar]
- 4.World Medical Association. WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Accessed February 23, 2021. wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/2018 [Google Scholar]
- 5.Rothman KJ, Michels KB. The continuing unethical use of placebo controls. N Engl J Med. 1994;331(6):394-398. [DOI] [PubMed] [Google Scholar]
- 6.Ellenberg SS, Temple R. Placebo-controlled trials and active-control trials in the evaluation of new treatments: part 2: practical issues and specific cases. Ann Intern Med. 2000;133(6):464-470. [DOI] [PubMed] [Google Scholar]
- 7.Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments: part 1: ethical and scientific issues. Ann Intern Med. 2000;133(6):455-463. [DOI] [PubMed] [Google Scholar]
- 8.Emanuel EJ, Miller FG. The ethics of placebo-controlled trials: a middle ground. N Engl J Med. 2001;345(12):915-919. [DOI] [PubMed] [Google Scholar]
- 9.Salloway SP, Sevingy J, Budur K, et al. Advancing combination therapy for Alzheimer's disease. Alzheimers Dement. 2020;6(1):e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodkin DE, Kanoti GA. Ethical considerations raised by the approval of interferon beta-1b for the treatment of multiple sclerosis. Neurology. 1994;44(1):166-170. [DOI] [PubMed] [Google Scholar]
- 11.Glickman SW, McHutchison JG, Peterson ED, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360(8):816-823. [DOI] [PubMed] [Google Scholar]
- 12.Lublin FD, Reingold SC. Placebo-controlled clinical trials in multiple sclerosis: ethical considerations: National Multiple Sclerosis Society (USA) Task Force on placebo-controlled clinical trials in MS. Ann Neurol. 2001;49(5):677-681. [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Barkhof F, et al. Ethics of placebo-controlled clinical trials in multiple sclerosis: a reassessment. Neurology. 2008;70(13 pt 2):1134-1140. [DOI] [PubMed] [Google Scholar]
- 14.Solomon AJ, Bernat JL. A review of the ethics of the use of placebo in clinical trials for relapsing-remitting multiple sclerosis therapeutics. Mult Scler Relat Disord. 2016;7:109-112. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Salter A, Wallstrom E, Cutter G, Stuve O. Evolution of clinical trials in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419826547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141-145. [DOI] [PubMed] [Google Scholar]
- 17.Kawas CH, Clark CM, Farlow MR, et al. Clinical trials in Alzheimer disease: debate on the use of placebo controls. Alzheimer Dis Assoc Disord. 1999;13(3):124-129. [DOI] [PubMed] [Google Scholar]
- 18.Cummings J. Lessons learned from Alzheimer disease: clinical trials with negative outcomes. Clin Transl Sci. 2018;11(2):147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eli Lilly. Lilly's donanemab slows clinical decline of Alzheimer's disease in positive phase 2 Tria [online]. Accessed February 11, 2021. investor.lilly.com/news-releases/news-release-details/lillys-donanemab-slows-clinical-decline-alzheimers-disease
- 20.Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2020;17(4):693-701. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement [online]. Accessed February 23, 2021. fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting [Google Scholar]
- 22.Liu-Seifert H, Siemers E, Holdridge KC, et al. Delayed-start analysis: mild Alzheimer's disease patients in solanezumab trials, 3.5 years. Alzheimers Dement. 2015;1(2):111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2020. Alzheimers Dement. 2020;6(1):e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolkowitz OM, Kramer JH, Reus VI, et al. DHEA treatment of Alzheimer's disease: a randomized, double-blind, placebo-controlled study. Neurology. 2003;60(7):1071-1076. [DOI] [PubMed] [Google Scholar]
- 25.Hirschman KB, Joyce CM, James BD, Xie SX, Casarett DJ, Karlawish JH. Would caregivers of Alzheimer disease patients involve their relative in a decision to use an AD-slowing medication? Am J Geriatr Psychiatry. 2005;13(11):1014-1021. [DOI] [PubMed] [Google Scholar]