Abstract
Background
Sequential Organ Failure Assessment (SOFA) score predicts probability of in-hospital mortality. Many crisis standards of care suggest the use of SOFA scores to allocate medical resources during the COVID-19 pandemic.
Research question
Are SOFA scores elevated among Non-Hispanic Black and Hispanic patients hospitalized with COVID-19, compared to Non-Hispanic White patients?
Study design and methods
Retrospective cohort study conducted in Yale New Haven Health System, including 5 hospitals with total of 2681 beds. Study population drawn from consecutive patients aged ≥18 admitted with COVID-19 from March 29th to August 1st, 2020. Patients excluded from the analysis if not their first admission with COVID-19, if they did not have SOFA score recorded within 24 hours of admission, if race and ethnicity data were not Non-Hispanic Black, Non-Hispanic White, or Hispanic, or if they had other missing data. The primary outcome was SOFA score, with peak score within 24 hours of admission dichotomized as <6 or ≥6.
Results
Of 2982 patients admitted with COVID-19, 2320 met inclusion criteria and were analyzed, of whom 1058 (45.6%) were Non-Hispanic White, 645 (27.8%) were Hispanic, and 617 (26.6%) were Non-Hispanic Black. Median age was 65.0 and 1226 (52.8%) were female. In univariate logistic screen and in full multivariate model, Non-Hispanic Black patients but not Hispanic patients had greater odds of an elevated SOFA score ≥6 when compared to Non-Hispanic White patients (OR 1.49, 95%CI 1.11–1.99).
Interpretation
Given current unequal patterns in social determinants of health, US crisis standards of care utilizing the SOFA score to allocate medical resources would be more likely to deny these resources to Non-Hispanic Black patients.
Introduction
Prior to the first wave of Coronavirus-2019 (COVID-19), models predicted that a pandemic respiratory virus might require ventilators, intensive care unit (ICU) beds, and other life-sustaining medical resources far in excess of available supplies [1]. On January 30th 2020, the World Health Organization (WHO) declared a Public Health Emergency of International Concern which, in some countries, early on led to formal and informal restrictions on the allocation of critical medical resources on the basis of advanced age [2, 3]. These policies and recommendations were quickly criticized within their home countries and revised to promote equal access to care, but nonetheless informed the development of early COVID-19 crisis standards of care (CSC) in the United States (US) [4].
In response to early shortages and high rates of infection and mortality in Europe and the Northeastern US, a number of healthcare systems and states in the US developed CSC: guidelines that advise hospitals and providers how to operate in a public health disaster, outside of their normal operating standards of care. CSC include triage protocols for the allocation of scarce life-sustaining medical resources [5–9]. The primary goal of published protocols was to establish a consistent system for allocating resources to save as many lives as possible during public health emergencies. A potential alternative goal of saving as many life-years as possible was widely rejected as being likely to unjustly discriminate against marginalized racial and ethnic groups, people with disabilities, people of advanced age, and others with a shorter long-term life expectancy [10].
Most publicly available US triage protocols, prior to and during the pandemic, used the Sequential Organ Failure Assessment (SOFA) score, with or without additional prognostic factors, to assess patients’ likelihood of benefiting (surviving) as a result of receiving medical resources [9, 11]. The rationale was that if a severe shortage of critical medical resources did occur, then the limited resources would be directed to those most likely to survive as a result of receiving them, thereby saving the most lives possible. Without standardized protocols the allocation of scarce resources is likely to be highly variable and inequitable. The SOFA score is a validated prognostic score ranging from 0–24, with points assigned for evidence of organ failure within 6 different organ systems, with higher scores correlating with a higher likelihood of in-hospital mortality [12, 13]. Originally developed and validated among septic patients in the medical ICU, subsequent research during the COVID pandemic has shown that the SOFA score is actually poorly predictive of mortality among patients with acute respiratory distress syndrome (ARDS) in the setting of COVID-19 infection; it is less accurate than either the Acute Physiology and Chronic Health Evaluation (APACHE) II score or chronologic age [14, 15]. Triage protocols utilizing the SOFA score were not widespread in Europe and even in the US these CSC were generally not actually activated during the pandemic. Nonetheless, most published US disaster triage protocols prioritized patients who require medical resources but have lower SOFA scores to receive resources, on the grounds that such patients are more likely to benefit (survive), and there is concern that these protocols will guide medical decision making in the US in the event of future public health emergencies [9].
In addition to threatening to overwhelm existing medical resources, the COVID-19 pandemic has also highlighted and exacerbated existing racial, ethnic, and socioeconomic health disparities. Marginalized populations, including racial and ethnic minorities and individuals of lower socioeconomic status, are more likely to become infected with COVID-19, more likely to be hospitalized, and more likely to die as a result [16–18]. Disparities in social determinants of health, including safe access to adequate nutritious food, exercise options, stable housing, and economic opportunities likely contribute to disparities in COVID-19 outcomes.
Marginalized populations are more likely to work in service-sector jobs that cannot be conducted remotely, are more likely to depend on public transportation, and are more likely to live in small and densely packed housing units and in group-living situations including homeless shelters, prisons, jails, and detention facilities [19–22]. They are less likely to have access to preventive healthcare and more likely to experience bias when they do access the healthcare system, resulting in higher rates of chronic comorbidities including diabetes, hypertension, and chronic pulmonary diseases [23, 24]. These pervasive inequalities in social determinants of health, and the health inequities that they cause, constitute a structure of systemic racism and contribute to higher rates of COVID-19 infection, more severe acute illness due to preexisting conditions, and higher mortality rates [25, 26].
Given that marginalized populations are more likely to become sicker with COVID-19, utilization of CSC triage protocols, which rely on the SOFA score, have the potential to disproportionately deny medical resources to racial and ethnic minorities [27, 28]. Racial, ethnic, and socioeconomic inequities in health outcomes are a consequence of inequalities in social determinants of health, and they can potentially be exacerbated by triage protocols, as documented in patient cohorts with sepsis and ARDS but not previously examined in patients with COVID-19 [29]. The use of SOFA as a criterion to allocate scarce medical resources has the potential to exacerbate inequities caused by social determinants of health. We conducted a retrospective cohort study to assess whether SOFA scores are disproportionately elevated among members of racial and ethnic minorities, and specifically Non-Hispanic Black and Hispanic patients, in comparison to Non-Hispanic White patients with COVID-19. The existence of such a disparity would raise significant concerns about the use of triage protocols relying on SOFA scores and the potential for exacerbating racial and ethnic health inequities during future waves of the COVID-19 pandemic and other public health emergencies.
Methods
Study design and data source
We conducted a retrospective cohort study of patients with COVID-19 within the Yale-New Haven Health System (YNHH) from March 29th, 2020 to August 1, 2020. YNHH includes 5 hospitals and a large physician practice base, serving racially, ethnically, and socioeconomically diverse communities across Connecticut and Rhode Island. The hospitals range from primary community hospitals to a tertiary academic medical center, with a total of 2,681 beds. During the pandemic, the healthcare system worked as a unified network, with sicker patients transferred from smaller hospitals within the network to Yale New Haven Hospital, all operating under a single protocol. Across the system, patients are 61% White, 17% Black, 3% Asian, 0.3% Native American, 0.3% Hawaiian or other Pacific Islander, and 19% other or unknown race. Patients are 78% Non-Hispanic, 20% Hispanic, and 2% other or unknown ethnicity. Forty-six percent of patients have primary private insurance, 19% have primary Medicare, 28% have primary Medicaid, and 7% are uninsured.
Connecticut and Rhode Island Medicaid cover low-income residents who are US nationals, citizens, permanent residents, or legal aliens [30, 31] Emergency Medicaid, available to all residents regardless of immigration status, during the COVID-19 public health emergency was expanded to include COVID-19 treatment and hospitalization [32, 33]. Personal asset and income limits vary across states and within states and are available [30, 31, 34]. Generally, Medicaid enrollees in Connecticut and Rhode Island do not pay premiums, deductibles, or copayments [34].
Data from the YNHH electronic medical record (EMR, Epic Systems Corporation, Verona, WI) database was used for analyses. The study was approved by the Yale University Human Subjects Committee (study number 2000028081), which judged that the study was exempt from the requirement for consent because data were analyzed anonymously.
Participants
We included EMR data for all patients age ≥18 with COVID-19 admitted to YNHH hospitals during the study period. Patients were considered positive for COVID-19 if they had a positive PCR test or clinical markers including fever, cough and chest radiographs considered to be consistent with COVID-19 infection in the setting of the first wave of the pandemic in the northeastern United States, and designated as COVID-19 positive by an attending physician. Patients <18 years of age were excluded as the SOFA score is not validated in pediatric patients. Patients were excluded from the analysis if they did not have a SOFA score recorded within 24 hours of admission, if it was not their first admission with COVID-19, or if their race and ethnicity data were not Non-Hispanic Black, Non-Hispanic White, or Hispanic (Fig 1). While YNHH serves significant Hispanic and Non-Hispanic Black patient populations, it serves relatively smaller numbers of Asian, Native American, Pacific Islander, and other patient populations, and these small samples statistically prevented inclusion in the analysis. Because prior COVID-19 studies show that Black and Hispanic patients experience higher rates of critical illness and mortality [16–18], we hypothesized that Non-Hispanic Black and Hispanic patients will be more likely to have elevated SOFA scores within 24 hours of admission compared to Non-Hispanic White patients.
Fig 1. Construction of study cohort.
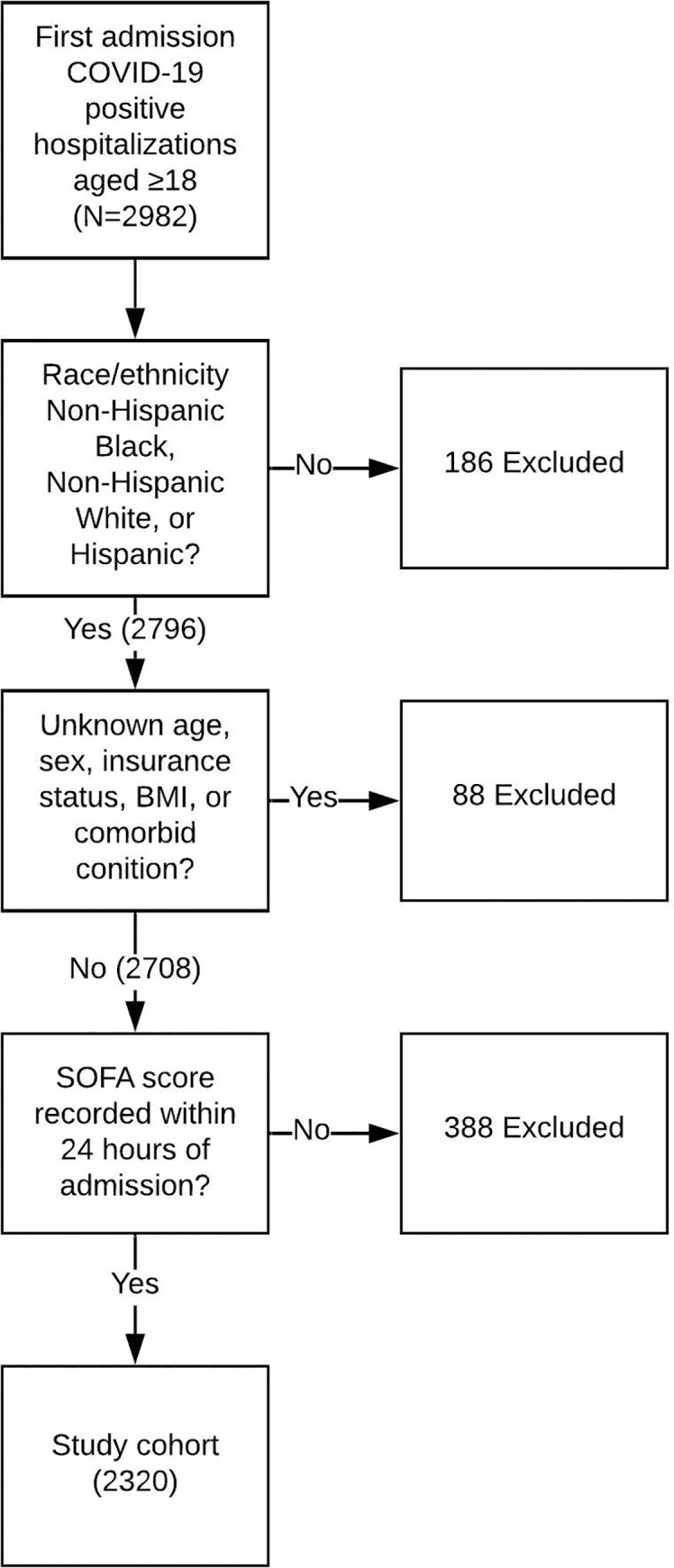
Abbreviations: BMI: Body Mass Index; COVID-19: Coronavirus Disease 2019; SOFA: Sequential Organ Failure Assessment.
Predictor variables
Data extracted from the EMR included sociodemographic and clinical variables. Our main predictor variables were age, sex, race, ethnicity, and insurance status. These variables are recorded by admitting clerks at YNHH hospitals. Other variables included clinical characteristics like body mass index (BMI) and comorbid conditions known to be associated with mortality in the setting of COVID-19 and accurately captured in the electronic medical record (including chronic pulmonary disease, congestive heart failure, diabetes, coronary artery disease, hypertension, advanced renal disease, and advanced liver disease) [18]. Smoking status was not included in the analysis, because in our clinical experience there is a significant desirability bias, leading patients to report themselves to clinicians as non-smokers or former smokers rather than current smokers [35].
Outcome variable
The main outcome, SOFA score, was continuously and automatically calculated for all admitted patients and recorded every 4 hours within the YNHH EMR. Triage decisions were never made at YNHH on the basis of this calculated SOFA score, because the Triage Protocol was approved but never activated during the pandemic [7]. SOFA scores were determined by an automated algorithm within the EMR system, assigning 0–4 points for each of 6 organ systems (neurologic, pulmonary, cardiovascular, renal, hepatic, hematologic), based on laboratory, respiratory and nursing flowsheet data in the EMR, following previously specified and validated rules [12]. The total SOFA score ranges from 0–24, with higher scores indicating a higher likelihood of in-hospital mortality. A binary SOFA variable (peak score within 24 hours <6, ≥6) was created to examine variation in illness severity by patient sociodemographic characteristics. We focused on this dichotomous outcome because published triage protocols categorize patients with a SOFA score <6 as being in the most prioritized group, most likely to receive scarce medical resources in a disaster situation, whereas patients with SOFA score ≥6 are deprioritized, resulting in lower likelihoods of receiving scarce medical resources [5, 7]. We focused on peak SOFA score within the first 24 hours because in a public health emergency in which life-sustaining medical resources are fully occupied, it is initial SOFA scores that will determine whether a newly admitted critically ill patient receives scarce resources.
Statistical analysis
We used Analysis of Variance (ANOVA) tests to examine mean differences in peak 24-hour SOFA score by sociodemographic and clinical characteristics. Chi-square tests were used to examine differences in the proportion of COVID positive patients with SOFA score ≥6 and <6 by patient characteristics. Finally, we conducted logistic regression analyses to assess racial differences in SOFA score adjusting for sociodemographic and clinical covariates. We considered candidate covariates based on clinical experience and emerging evidence regarding associations with clinical outcomes in COVID-19. The final multivariate model was then refined through the exclusion of collinear covariates. We conducted a univariate screen followed by a multivariate regression adjusting for all sociodemographic and clinical covariates listed in Table 4 (race/ethnicity, age, sex, BMI, insurance status, comorbid conditions including chronic pulmonary disease, congestive heart failure, diabetes, coronary artery disease, hypertension, advanced renal disease, and advanced liver disease). Race-stratified models were also constructed to assess whether factors associated with SOFA score varied according to race and ethnicity. We also conducted a multiple mixed-effects logistic regression, including random intercepts placed on hospital of admission to account for potential inter-hospital variability.
Table 4. Univariate and multivariate regression model results for factors associated with SOFA score within 24 hours ≥ 6.
| Characteristic | Model 1- Combined Univariate (unadjusted) | Model 1- Combined Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Race/Ethnicity | ||||||||
| Hispanic | 0.78 | 0.58 | 1.06 | 0.1077 | 0.87 | 0.62 | 1.24 | 0.4501 |
| Black Non-Hispanic | 1.58 | 1.21 | 2.06 | 0.0008* | 1.49 | 1.11 | 1.99 | 0.0075* |
| White Non-Hispanic | reference | Reference | ||||||
| Age | ||||||||
| 18–34 | reference | Reference | ||||||
| 35–64 | 2.55 | 1.44 | 4.52 | 0.0013* | 1.95 | 1.07 | 3.54 | 0.0288* |
| > = 65 | 2.84 | 1.62 | 4.98 | 0.0003* | 2.57 | 1.32 | 4.98 | 0.0052* |
| Sex | ||||||||
| Men | 1.83 | 1.45 | 2.32 | < .0001* | 1.94 | 1.51 | 2.50 | < .0001* |
| Women | Reference | Reference | ||||||
| Insurance status | ||||||||
| Private | Reference | Reference | ||||||
| Medicare | 1.53 | 1.09 | 2.14 | 0.0135* | 1.07 | 0.70 | 1.63 | 0.757 |
| Medicaid | 1.40 | 0.94 | 2.07 | 0.0955 | 1.35 | 0.89 | 2.04 | 0.1536 |
| Uninsured | 1.01 | 0.58 | 1.76 | 0.9678 | 1.19 | 0.66 | 2.13 | 0.566 |
| BMI | ||||||||
| <25 | Reference | Reference | ||||||
| 25–29.9 | 1.07 | 0.79 | 1.45 | 0.6708 | 1.22 | 0.88 | 1.68 | 0.2317 |
| 30–34.9 | 1.08 | 0.77 | 1.52 | 0.6628 | 1.30 | 0.91 | 1.87 | 0.1561 |
| 35+ | 1.17 | 0.85 | 1.62 | 0.3372 | 1.52 | 1.07 | 2.18 | 0.0207* |
| Comorbid conditions | ||||||||
| Chronic pulmonary disease | 1.27 | 0.99 | 1.62 | 0.0603 | 1.07 | 0.81 | 1.40 | 0.6453 |
| CHF | 1.86 | 1.45 | 2.38 | < .0001* | 1.19 | 0.86 | 1.63 | 0.288 |
| Diabetes | 1.65 | 1.31 | 2.08 | < .0001* | 1.07 | 0.82 | 1.41 | 0.6082 |
| CAD | 1.89 | 1.48 | 2.42 | < .0001* | 1.18 | 0.86 | 1.61 | 0.3151 |
| Hypertension | 1.67 | 1.27 | 2.19 | 0.0003* | 0.95 | 0.67 | 1.34 | 0.7594 |
| Advance renal disease | 3.15 | 2.29 | 4.34 | < .0001* | 2.35 | 1.62 | 3.40 | < .0001* |
| Advance liver disease | 2.96 | 1.58 | 5.54 | 0.0007* | 2.51 | 1.29 | 4.89 | 0.0068* |
Abbreviations
*: p-value < 0.05; BMI: Body Mass Index; CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; CI: Confidence Interval; OR: Odds Ratio; SOFA: Sequential Organ Failure Assessment.
Results
From March 29th to August 1st, there were 3362 admissions of COVID-19-positive patients aged ≥18 to YNHH hospitals. Of these, 2982 were first admissions (Fig 1) and 2796 were Non-Hispanic Black, Non-Hispanic White, or Hispanic. Of these, 88 had missing baseline demographics or clinical data, and 388 had missing SOFA scores, and were excluded. Two thousand three hundred and twenty patients had complete race/ethnicity and baseline characteristics, were either Hispanic, Non-Hispanic Black, or Non-Hispanic White, and were included in the analysis. There were no statistically significant differences in demographic or clinical characteristics between patients with and without SOFA scores.
Within the study cohort of 2320, 1058 (45.6%) were Non-Hispanic White, 645 (27.8%) were Hispanic, and 617 (26.6%) were Non-Hispanic Black (Table 1). The median age was 65.0, and 1226 (52.8%) were female. Six-hundred and fifty-nine (28.4%) had Medicaid or no insurance. Nine-hundred and sixty-nine (41.7%) were obese. A total of 1829 (78.8%) had one or more comorbid conditions thought to increase risk of mortality in the setting of COVID-19. Patients with peak SOFA scores ≥6 within the first 24 hours were disproportionately common among Non-Hispanic Black patients, older patients, males, and patients with Congestive Heart Failure (CHF), diabetes, coronary artery disease (CAD), hypertension, advanced renal disease, and advanced liver disease. Baseline characteristics broken down by race/ethnicity are given in Table 2. Non-Hispanic White patients were significantly older than average while Hispanic patients were significantly younger. Non-Hispanic Black patients had higher rates of elevated BMI and most comorbid conditions, including chronic pulmonary disease, diabetes, hypertension, and advanced renal disease.
Table 1. Characteristics of COVID+ patients with SOFA within 24 hours of admission; SOFA <6 and ≥6.
| Characteristic | Total (n = 2,320) | 24-hour Sofa < 6 (n = 1,985) | 24-hour Sofa ≥ 6 (n = 335) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Race/Ethnicity | < .0001 | ||||||
| Hispanic | 645 | 27.8 | 575 | 29.0 | 70 | 20.9 | |
| Black Non-Hispanic | 617 | 26.6 | 495 | 24.9 | 122 | 36.4 | |
| White Non-Hispanic | 1058 | 45.6 | 915 | 46.1 | 143 | 42.7 | |
| Age | 0.0008 | ||||||
| 18–34 | 224 | 9.7 | 210 | 10.6 | 14 | 4.2 | |
| 35–64 | 895 | 38.6 | 765 | 38.5 | 130 | 38.8 | |
| > = 65 | 1201 | 51.8 | 1010 | 50.9 | 191 | 57.0 | |
| Sex | < .0001 | ||||||
| Men | 1094 | 47.2 | 893 | 45.0 | 201 | 60.0 | |
| Women | 1226 | 52.8 | 1092 | 55.0 | 134 | 40.0 | |
| Language preference | 0.2679 | ||||||
| English | 1850 | 79.7 | 1572 | 79.2 | 278 | 83.0 | |
| Spanish | 418 | 18.0 | 368 | 18.5 | 50 | 14.9 | |
| Other | 52 | 2.2 | 45 | 2.3 | 7 | 2.1 | |
| Insurance status | 0.0472 | ||||||
| Private | 434 | 18.7 | 386 | 19.4 | 48 | 14.3 | |
| Medicare | 1227 | 52.9 | 1031 | 51.9 | 196 | 58.5 | |
| Medicaid | 480 | 20.7 | 409 | 20.6 | 71 | 21.2 | |
| Uninsured | 179 | 7.7 | 159 | 8.0 | 20 | 6.0 | |
| BMI | 0.8194 | ||||||
| <25 | 677 | 29.2 | 585 | 29.5 | 92 | 27.5 | |
| 25–29.9 | 674 | 29.1 | 577 | 29.1 | 97 | 29.0 | |
| 30–34.9 | 455 | 19.6 | 389 | 19.6 | 66 | 19.7 | |
| 35+ | 514 | 22.2 | 434 | 21.9 | 80 | 23.9 | |
| Comorbid conditions | |||||||
| Chronic pulmonary disease | 702 | 30.3 | 586 | 29.5 | 116 | 34.6 | 0.0599 |
| CHF | 573 | 24.7 | 454 | 22.9 | 119 | 35.5 | < .0001 |
| Diabetes | 1001 | 43.1 | 821 | 41.4 | 180 | 53.7 | < .0001 |
| CAD | 594 | 25.6 | 470 | 23.7 | 124 | 37.0 | < .0001 |
| Hypertension | 1601 | 69.0 | 1341 | 67.6 | 260 | 77.6 | 0.0002 |
| Advance renal disease | 206 | 8.9 | 141 | 7.1 | 65 | 19.4 | < .0001 |
| Advance liver disease | 46 | 2.0 | 31 | 1.6 | 15 | 4.5 | 0.0004 |
Abbreviations: BMI: Body Mass Index; CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; SOFA: Sequential Organ Failure Assessment
Table 2. Characteristics of COVID+ patients by race/ethnicity.
| Characteristic | Total | Hispanic (n = 645) | Non-Hispanic Black | Non-Hispanic White | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 2,320) | (n = 617) | (n = 1,058) | |||||||
| n | % | n | % | n | % | N | % | ||
| Age | < .0001 | ||||||||
| 18–34 | 224 | 9.7 | 121 | 18.8 | 58 | 9.4 | 45 | 4.3 | |
| 35–64 | 895 | 38.6 | 344 | 53.3 | 284 | 46.0 | 267 | 25.2 | |
| > = 65 | 1201 | 51.8 | 180 | 27.9 | 275 | 44.6 | 746 | 70.5 | |
| Sex | 0.0002 | ||||||||
| Men | 1094 | 47.2 | 348 | 54.0 | 268 | 43.4 | 478 | 45.2 | |
| Women | 1226 | 52.8 | 297 | 46.0 | 349 | 56.6 | 580 | 54.8 | |
| Language preference | < .0001 | ||||||||
| English | 1850 | 79.7 | 226 | 35.0 | 597 | 96.8 | 1027 | 97.1 | |
| Spanish | 418 | 18.0 | 415 | 64.3 | 0 | 0.0 | 3 | 0.3 | |
| Other | 52 | 2.2 | 4 | 0.6 | 20 | 3.2 | 28 | 2.6 | |
| Insurance status | < .0001 | ||||||||
| Private | 434 | 18.7 | 116 | 18.0 | 134 | 21.7 | 184 | 17.4 | |
| Medicare | 1227 | 52.9 | 166 | 25.7 | 296 | 48.0 | 765 | 72.3 | |
| Medicaid | 480 | 20.7 | 224 | 34.7 | 158 | 25.6 | 98 | 9.3 | |
| Uninsured | 179 | 7.7 | 139 | 21.6 | 29 | 4.7 | 11 | 1.0 | |
| BMI | < .0001 | ||||||||
| <25 | 677 | 29.2 | 153 | 23.7 | 151 | 24.5 | 373 | 35.3 | |
| 25–29.9 | 674 | 29.1 | 211 | 32.7 | 148 | 24.0 | 315 | 29.8 | |
| 30–34.9 | 455 | 19.6 | 152 | 23.6 | 129 | 20.9 | 174 | 16.4 | |
| 35+ | 514 | 22.2 | 129 | 20.0 | 189 | 30.6 | 196 | 18.5 | |
| Comorbid conditions | |||||||||
| Chronic pulmonary disease | 702 | 30.3 | 137 | 21.2 | 222 | 36.0 | 343 | 32.4 | < .0001 |
| CHF | 573 | 24.7 | 68 | 10.5 | 177 | 28.7 | 328 | 31.0 | < .0001 |
| Diabetes | 1001 | 43.1 | 230 | 35.7 | 354 | 57.4 | 417 | 39.4 | < .0001 |
| CAD | 594 | 25.6 | 81 | 12.6 | 171 | 27.7 | 342 | 32.3 | < .0001 |
| Hypertension | 1601 | 69.0 | 294 | 45.6 | 500 | 81.0 | 807 | 76.3 | < .0001 |
| Advance renal disease | 206 | 8.9 | 27 | 4.2 | 100 | 16.2 | 79 | 7.5 | < .0001 |
| Advance liver disease | 46 | 2.0 | 15 | 2.3 | 14 | 2.3 | 17 | 1.6 | 0.4917 |
Abbreviations: BMI: Body Mass Index; CAD: Coronary Artery Disease; CHF: Cogestive Heart Failure; SOFA: Sequential Organ Failure Assessment
Mean peak SOFA score within the first 24 hours was (2.4±3.0) overall, ranging from 0 to 18 (Table 3). Mean SOFA score was significantly elevated among Non-Hispanic Black patients (3.0±3.1), but not among Hispanic patients (2.2±3.1) in comparison to Non-Hispanic White patients (2.5±2.8). SOFA score was also significantly elevated among patients aged 35–64 (2.5±3.0) and ≥65 (2.8±3.0) in comparison to those aged 18–34 (1.3±2.3), among Men (3.0±3.2) in comparison to Women (2.2±2.6), and among those with Medicare insurance (2.9±3.0) but not Medicaid (2.3±3.0), or no insurance (2.0±3.1) compared to those with private insurance (2.0±2.8). The SOFA score was also significantly elevated among those with comorbid conditions including CHF (3.4±3.2) compared to those without (2.3±2.8), diabetes (3.0±3.1) compared to those without (2.2±2.8), CAD (3.3±3.2) compared to those without (2.3±2.8), hypertension (2.8±3.0) compared to those without (1.9±2.7), advanced renal disease (4.7±3.1) compared to those without (2.3±2.9), and advanced liver disease (4.8±3.9) compared to those without (2.5±2.9).
Table 3. Mean SOFA within 24 hours of admission.
| Characteristic | Total | SOFA within 24 hours | p-value | ||
|---|---|---|---|---|---|
| n | % | Mean | SD | ||
| Race/Ethnicity | < .0001 | ||||
| Hispanic | 645 | 27.8 | 2.2 | 3.1 | |
| Black Non-Hispanic | 617 | 26.6 | 3.0 | 3.1 | |
| White Non-Hispanic | 1058 | 45.6 | 2.5 | 2.8 | |
| Age | < .0001 | ||||
| 18–34 | 224 | 9.7 | 1.3 | 2.3 | |
| 35–64 | 895 | 38.6 | 2.5 | 3.0 | |
| > = 65 | 1201 | 51.8 | 2.8 | 3.0 | |
| Sex | < .0001 | ||||
| Men | 1094 | 47.2 | 3.0 | 3.2 | |
| Women | 1226 | 52.8 | 2.2 | 2.6 | |
| Language preference | 0.2923 | ||||
| English | 1850 | 79.7 | 2.6 | 2.9 | |
| Spanish | 418 | 18.0 | 2.3 | 3.3 | |
| Other | 52 | 2.2 | 2.7 | 2.7 | |
| Insurance status | < .0001 | ||||
| Private | 434 | 18.7 | 2.0 | 2.8 | |
| Medicare | 1227 | 52.9 | 2.9 | 3.0 | |
| Medicaid | 480 | 20.7 | 2.3 | 3.0 | |
| Uninsured | 179 | 7.7 | 2.0 | 3.1 | |
| BMI | 0.805 | ||||
| <25 | 677 | 29.2 | 2.6 | 2.8 | |
| 25–29.9 | 674 | 29.1 | 2.6 | 2.9 | |
| 30–34.9 | 455 | 19.6 | 2.4 | 3.1 | |
| 35+ | 514 | 22.2 | 2.6 | 3.1 | |
| Comorbid conditions | |||||
| Chronic pulmonary disease | 0.086 | ||||
| No | 1618 | 69.7 | 2.5 | 3.0 | |
| Yes | 702 | 30.3 | 2.7 | 3.0 | |
| CHF | < .0001 | ||||
| No | 1747 | 75.3 | 2.3 | 2.8 | |
| Yes | 573 | 24.7 | 3.4 | 3.2 | |
| Diabetes | < .0001 | ||||
| No | 1319 | 56.9 | 2.2 | 2.8 | |
| Yes | 1001 | 43.1 | 3.0 | 3.1 | |
| CAD | < .0001 | ||||
| No | 1726 | 74.4 | 2.3 | 2.8 | |
| Yes | 594 | 25.6 | 3.3 | 3.2 | |
| Hypertension | < .0001 | ||||
| No | 719 | 31.0 | 1.9 | 2.7 | |
| Yes | 1601 | 69.0 | 2.8 | 3.0 | |
| Advance renal disease | < .0001 | ||||
| No | 2114 | 91.1 | 2.3 | 2.9 | |
| Yes | 206 | 8.9 | 4.7 | 3.1 | |
| Advance liver disease | < .0001 | ||||
| No | 2274 | 98.0 | 2.5 | 2.9 | |
| Yes | 46 | 2.0 | 4.8 | 3.9 | |
Abbreviations: BMI: Body Mass Index; CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; SOFA: Sequential Organ Failure Assessment; SD: Standard Deviation.
In a univariate logistic screen and in a full multivariate model (Table 4), Non-Hispanic Black patients had greater odds of an elevated SOFA score ≥6 when compared to Non-Hispanic White patients (OR 1.49, 95%CI 1.11–1.99). In contrast, Hispanic patients did not have increased odds of an elevated SOFA score. Advanced age was also associated with increased odds of elevated SOFA score (OR 1.95, 95%CI 1.07–3.54 for age 35–64, OR 2.57, 95%CI 1.32–4.98 for age ≥65), as was male sex (OR 1.94, 95%CI 1.51–2.50), body-mass index ≥35 (OR 1.52, 95%CI 1.07–2.18), advanced renal disease (OR 2.35, 95%CI 1.62–3.40), and advanced liver disease (OR 2.51, 95%CI 1.29–4.89). Medicare was associated with increased odds of elevated SOFA score, but dropped out in the multivariate model, when other variables such as age were included. Race stratified models were also constructed but did not identify new covariates associated with elevated SOFA scores in both univariate screen and multivariate logistic analysis. We conducted a multiple mixed-effects logistic regression, including random intercepts placed on hospital of admission to account for potential inter-hospital variability, with unchanged results. We also reran the analysis looking at peak 48 hour SOFA score with unchanged results.
Discussion
In our cohort of COVID-19 positive patients admitted to YNHH hospitals, Non-Hispanic Black race/ethnicity, male sex, advanced age, stage II or greater obesity, advanced renal disease, and advanced liver disease were all independently associated with significantly higher odds of elevated peak SOFA score ≥6 during the first 24-hours of admission. Hispanic ethnicity was not associated with increased risk of elevated SOFA score. Neither Medicaid, Medicare nor lack of insurance were independently associated with increased odds of elevated SOFA score. Non-Hispanic Black patients were more likely to suffer from chronic comorbidities associated with elevated peak SOFA scores such as obesity and advanced renal disease (Table 2), and their risk of an elevated SOFA score persisted even when such comorbidities and other risk factors were controlled for in the multivariate analysis (Table 4). Neither insurance status nor exposure to chronic comorbidities fully explained the increased risk faced by Non-Hispanic Black patients with COVID-19.
These findings are consistent with prior studies showing that Black race, older age, obesity, and chronic medical comorbidities are associated with increased rates of mortality in COVID-19 [18]. These findings are also consistent with prior findings that SOFA overestimates mortality among Black patients and underestimates mortality among White patients with sepsis and ARDS prior to the COVID-19 pandemic [29]. The existing literature suggests that both differences in individual characteristics (income, comorbid conditions) and hospital characteristics contribute to racial disparities in COVID-19 outcomes in the US [36].
The racial disparities in SOFA scores we found among patients with COVID might be due to the systemic overestimation of mortality among Black persons or underestimation of mortality among White persons. This might occur for example because Black patients at baseline have higher creatinine levels than White patients, leading to an elevation of the renal component of the SOFA score unrelated to any illness [37]. Alternatively, Black persons with COVID-19 might have higher SOFA scores in the hospital because COVID-19 affects them more severely, for example because they are subjected to higher levels of discrimination and stress or because they have less access to long-term preventive care, quality education, economic stability, and other social determinants of health [23, 24, 38]. Finally, Black patients might have higher SOFA scores at the time of admission because they present or are admitted to hospitals only when they are sicker [39, 40]. This could occur because of current or prior discrimination within the healthcare system that might discourage patients from seeking medical attention with mild or moderate symptoms [41].
Of these potential contributing factors, prior or current anti-Black discrimination, leading to distrust of the healthcare system and delays in hospital admission, could explain our current findings. Of note, Non-Hispanic Black patients but neither Hispanic patients, patients with Medicaid, nor uninsured patients demonstrated a greater risk of an elevated SOFA score within 24-hours of admission. This would suggest an etiology, such as anti-Black stigma and resulting delays in hospital admission, that affects Black patients in the US to a greater degree than other marginalized populations such as patients with Medicaid or without insurance, who would also be expected to have reduced income, access to preventative care, and other social determinants of health. This potential mechanism accords with prior reports of Black patients in the US presenting to medical attention at more advanced stages of illness [39, 42]. This proposed mechanism is tentative because our analysis does not directly examine income or wealth but uses the imperfect proxy of Medicaid status. Further study, involving more detailed socioeconomic data will be necessary to support or refute this proposed mechanism.
Because published US triage protocols utilized the SOFA score to allocate scarce medical resources, and prioritized patients with lower SOFA scores over other patients, such protocols–if implemented–would be more likely to triage Non-Hispanic Black people to not receive scarce resources such as ventilators and ICU beds during future waves of the COVID-19 pandemic. As part of a system that predictably leads to racial disparities in health outcomes, triage protocols have the potential to become a component of systemic racism.
Given these findings and the possibility that crisis standards of care may be implemented during the future pandemics, it is important to prospectively consider and implement measures to reduce systemic racism, as well as socioeconomic and disability barriers to equal access to healthcare. The ideal would be to minimize or prevent entirely the need for triage, particularly among marginalized populations. This might be achieved in the short term through vaccination support, public health education, distribution of personal protective equipment, stockpiling of critical medical resources, targeted COVID-19 testing, contact tracing, social distancing, and even lockdowns coupled with financial support. The manifest injustice of the systemic racism and health inequities that COVID-19 has highlighted should also motivate long-term efforts to achieve more equitable health outcomes in the United States. These might include universal health insurance [43], a more redistributive system of taxation [44], housing support [45], elimination of food deserts and neighborhood segregation [46], anti-racism trainings for clinicians [47], robust collection of race and ethnicity health data [48], expanded access to higher and medical education, and recruitment of marginalized populations into the medical workforce [49]. Such interventions, while requiring large investments on a societal level, could reduce health disparities on a much broader and more lasting scale than interventions tailored specifically to the COVID-19 or other pandemics.
It is also possible to make crisis standards of care and triage protocols themselves more equitable. Such efforts would not be expected to reduce disparities beyond the setting of a pandemic, and then only for those patients who were subjected to rationing. The development, revision, and oversight of these protocols might be made more open and transparent to patients, community members, and to the general public. Healthcare systems and states might recruit triage advisory and oversight committees that specifically include robust representation from ethnic and racial minorities, as well as individuals with disabilities and other marginalized populations [7]. Committees might specifically recruit advocacy organizations, faith leaders, institutional diversity officers, and other community leaders to ensure adequate representation of community concerns. The triage teams that implement protocols in hospitals might also be mandated to include representation of diverse perspectives.
In addition, the SOFA score might be supplemented to achieve more equitable outcomes. Prioritarian triage protocols might still use mortality probability scoring, such as the SOFA score, but might give marginalized populations a bonus or prioritization in these assessments. For example, patients might have their priority score improved slightly on the basis of their home address, using the Area Deprivation Index [50]. The impact of such modifications to SOFA scores on identified racial disparities would be an important area for future study. Potential comparative advantages and disadvantages of alternative triage systems are reviewed elsewhere [51].
Our study is limited in that it was conducted within a single healthcare system in the Northeastern United States. Our healthcare system experienced a surge of COVID patients relatively early in the pandemic, with a peak on April 22, 2020 followed by relatively lower numbers, and medical care for COVID-19 has evolved over the course of the pandemic. The disparities that this study documents may not be generalizable to other regions with different racial and ethnic demographics within the United States or globally. Our study is also limited by the relatively small number of patients with very elevated SOFA scores, which prevented analysis of more detailed SOFA score categories. Future research should include larger sample sizes which would allow for these types of analyses.
Perhaps most importantly, our study is limited by the data available within the clinical EMR. For example, racial and ethnic data is generally documented by unit clerks based on their observation of patients rather than on patient’s self-identification. Patients classified as Hispanic often lack race data, which prevents us from identifying Hispanic Black vs. Hispanic White patients in our analyses. Prior studies have shown that “socially assigned” race does associate closely with health outcomes [52]. Another limitation is that we did not investigate potential disparities in SOFA scores in other marginalized populations. In particular, our EMR does not include socioeconomic data beyond insurance status, which is a poor surrogate for income, wealth, education, or occupation. Our insurance data are further limited because home care and disability data are not available. Evaluating the degree to which racial and ethnic disparities in the US, such as those identified in this study, are caused by or independent of socioeconomic inequality is a critical question that will require future study with detailed socioeconomic data [53]. Our study was also limited in the paucity of data available on some important clinical comorbidities. Future research in this area should include more detailed and complete data on clinical comorbidities that may affect mortality, such as dementia and cancer diagnoses. In addition, future research is needed to examine the effects of disability, psychiatric comorbidities, substance use disorders, unstable housing, or incarceration on SOFA scores.
In conclusion, Non-Hispanic Black patients admitted to hospitals with COVID-19 had increased odds of an elevated SOFA score ≥6 within the first 24-hours of admission. Therefore, published triage protocols utilizing the SOFA score to allocate scarce medical resources would be more likely to deny Non-Hispanic Black patients scarce medical resources such as ventilators and ICU beds if implemented during the COVID-19 pandemic. Governments and healthcare systems should prospectively consider and implement measures to reduce systemic racism, protect marginalized populations, and promote racial and ethnic equity during pandemics.
Acknowledgments
The authors wish to acknowledge the support of the Center for Medical Informatics and the Equity Research and Innovation Center at Yale School of Medicine. In particular we are indebted to Indira Flores, and Olamide Olawoyin for assisting in literature review and project planning.
Data Availability
We have discussed the sharing of our data with the Yale University Privacy Office which made the determination that we are legally and ethically restricted from sharing data because the extent of data poses a risk of re-identification of patients and their HIPAA protected data through deductive disclosure. Susan Bouregy, PhD (susan.buregy@yale.edu) is Yale’s chief privacy officer, and will serve as the contact for the Yale University Privacy Office, to which data requests may be sent.
Funding Statement
The authors received no specific funding for this work. BT receives research support from the US Department of Veterans Affairs (https://www.newengland.va.gov/research/v1cda/) and the C.G. Swebilius Foundation (https://fconline.foundationcenter.org/fdo-grantmaker-profile/?key=SWEB001). KN and MS are supported by the National Clinician Scholars Program and the CTSA Grant Number TL1 TR001864 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). (https://ncats.nih.gov/) The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. 2015. [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–6. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 3.Vergano M, Bertolini G, Giannini A, Gristina GR, Livigni S, Mistraletti G, et al. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Società Italiana Anestesia Analgesia Rianimazione e Terapia Intensiva. DECISIONI PER LE CURE INTENSIVE IN CASO DI SPROPORZIONE TRA NECESSITÀ ASSISTENZIALI E RISORSE DISPONIBILI IN CORSO DI PANDEMIA DA COVID-19 January 13th, 2021 [Available from: https://snlg.iss.it/wp-content/uploads/2021/01/2021_01_13__LINEE-GUIDA_DECISIONI-CURE-INTENSIVE_Def.pdf. [Google Scholar]
- 5.White DB, Lo B. A Framework for Rationing Ventilators and Critical Care Beds During the COVID-19 Pandemic. JAMA. 2020;323(18):1773–4. doi: 10.1001/jama.2020.5046 [DOI] [PubMed] [Google Scholar]
- 6.Biddison ELD, Faden R, Gwon HS, Mareiniss DP, Regenberg AC, Schoch-Spana M, et al. Too many patients… a framework to guide statewide allocation of scarce mechanical ventilation during disasters. Chest. 2019;155(4):848–54. doi: 10.1016/j.chest.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 7.Tolchin B, Latham SR, Bruce L, Ferrante LE, Kraschel K, Jubanyik K, et al. Developing a Triage Protocol for the COVID-19 Pandemic: Allocating Scarce Medical Resources in a Public Health Emergency. The Journal of clinical ethics. 2020;31(4):303–17. [PubMed] [Google Scholar]
- 8.Manchanda ECC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. Journal of Racial and Ethnic Health Disparities. 2020:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manchanda ECC, Molina MF, Rodriguez RM. Racial Equity in Crisis Standards of Care—Reassuring Data or Reason for Concern? JAMA network open. 2021;4(3):e214527–e. doi: 10.1001/jamanetworkopen.2021.4527 [DOI] [PubMed] [Google Scholar]
- 10.Supady A, Curtis JR, Abrams D, Lorusso R, Bein T, Boldt J, et al. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. The Lancet Respiratory Medicine. 2021. doi: 10.1016/S2213-2600(20)30580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antommaria AHM, Gibb TS, McGuire AL, Wolpe PR, Wynia MK, Applewhite MK, et al. Ventilator triage policies during the COVID-19 pandemic at US hospitals associated with members of the association of bioethics program directors. Ann Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 13.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328 [DOI] [PubMed] [Google Scholar]
- 14.Zou X, Li S, Fang M, Hu M, Bian Y, Ling J, et al. Acute Physiology and Chronic Health Evaluation II Score as a Predictor of Hospital Mortality in Patients of Coronavirus Disease 2019. Crit Care Med. 2020. doi: 10.1097/CCM.0000000000004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raschke RA, Agarwal S, Rangan P, Heise CW, Curry SC. Discriminant accuracy of the SOFA score for determining the probable mortality of patients with COVID-19 pneumonia requiring mechanical ventilation. JAMA. 2021;325(14):1469–70. doi: 10.1001/jama.2021.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowger TL, Davis BA, Etkins OS, Makofane K, Lawrence JA, Bassett MT, et al. Comparison of Weighted and Unweighted Population Data to Assess Inequities in Coronavirus Disease 2019 Deaths by Race/Ethnicity Reported by the US Centers for Disease Control and Prevention. JAMA Network Open. 2020;3(7):e2016933–e. doi: 10.1001/jamanetworkopen.2020.16933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO Housing and Health Guidelines. 2018. [Google Scholar]
- 20.McCormack G, Avery C, Spitzer AK-L, Chandra A. Economic Vulnerability of Households With Essential Workers. JAMA. 2020. doi: 10.1001/jama.2020.11366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emeruwa UN, Ona S, Shaman JL, Turitz A, Wright JD, Gyamfi-Bannerman C, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324(4):390–2. doi: 10.1001/jama.2020.11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhart E, Chen D. Incarceration And Its Disseminations: COVID-19 Pandemic Lessons From Chicago’s Cook County Jail: Study examines how arrest and pre-trial detention practices may be contributing to the spread of COVID-19. Health Aff (Millwood). 2020: doi: 10.1377/hlthaff.2020.00652 [DOI] [PubMed] [Google Scholar]
- 23.Cookson R, Propper C, Asaria M, Raine R. Socio‐economic inequalities in health care in England. Fiscal Studies. 2016;37(3–4):371–403. [Google Scholar]
- 24.National Center for Health Statistics. Health, United States, 2018. 2019. [PubMed] [Google Scholar]
- 25.Egede LE, Walker RJ. Structural Racism, Social Risk Factors, and Covid-19—A Dangerous Convergence for Black Americans. N Engl J Med. 2020. doi: 10.1056/NEJMp2023616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia MA, Homan PA, García C, Brown TH. The Color of COVID-19: Structural Racism and the Pandemic’s Disproportionate Impact on Older Racial and Ethnic Minorities. The Journals of Gerontology: Series B. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleveland Manchanda E, Couillard C, Sivashanker K. Inequity in Crisis Standards of Care. N Engl J Med. 2020. doi: 10.1056/NEJMp2011359 [DOI] [PubMed] [Google Scholar]
- 28.White DB, Lo B. Mitigating inequities and saving lives with ICU triage during the COVID-19 pandemic. Am J Respir Crit Care Med. 2021;203(3):287–95. doi: 10.1164/rccm.202010-3809CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashana DC, Anesi GL, Liu VX, Escobar GJ, Chesley C, Eneanya ND, et al. Equitably Allocating Resources During Crises: Racial Differences in Mortality Prediction Models. Am J Respir Crit Care Med. 2021. doi: 10.1164/rccm.202012-4383OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benefits.gov. Connecticut Medicaid 2021. [Available from: https://www.benefits.gov/benefit/1622. [Google Scholar]
- 31.Benefits.gov. Rhode Island Medical Assistance (Medicaid) 2021. [Available from: https://www.benefits.gov/benefit/1639. [Google Scholar]
- 32.The Office of Governor Ned Lamont. Governor Lamont Provides Update on Connecticut’s Coronavirus Response Efforts 2020. [Available from: https://portal.ct.gov/Office-of-the-Governor/News/Press-Releases/2020/04-2020/Governor-Lamont-Coronavirus-Update-April-30. [Google Scholar]
- 33.Rhode Island Department of Health. COVID-19 Information: Resources for Undocumented Immigrants 2020. [Available from: https://covid.ri.gov/public/undocumented-immigrants. [Google Scholar]
- 34.Brooks T, Roygardner L, Artiga S, Pham O, Dolan R. Medicaid and CHIP eligibility, enrollment, and cost sharing policies as of January 2020: Findings from a 50-state survey 2020. [Available from: https://www.kff.org/medicaid/report/medicaid-and-chip-eligibility-enrollment-and-cost-sharing-policies-as-of-january-2020-findings-from-a-50-state-survey/. [Google Scholar]
- 35.Persoskie A, Nelson WL. Just blowing smoke? Social desirability and reporting of intentions to quit smoking. nicotine & tobacco research. 2013;15(12):2088–93. doi: 10.1093/ntr/ntt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asch DA, Islam MN, Sheils NE, Chen Y, Doshi JA, Buresh J, et al. Patient and Hospital Factors Associated With Differences in Mortality Rates Among Black and White US Medicare Beneficiaries Hospitalized With COVID-19 Infection. JAMA Network Open. 2021;4(6):e2112842–e. doi: 10.1001/jamanetworkopen.2021.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peralta CA, Risch N, Lin F, Shlipak MG, Reiner A, Ziv E, et al. The association of African ancestry and elevated creatinine in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Nephrol. 2010;31(3):202–8. doi: 10.1159/000268955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuevas AG, Ong AD, Carvalho K, Ho T, Chan SWC, Allen J, et al. Discrimination and systemic inflammation: A critical review and synthesis. Brain Behav Immun. 2020. doi: 10.1016/j.bbi.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De la Garza Ramos R, Benton JA, Gelfand Y, Echt M, Rodriguez JVF, Yanamadala V, et al. Racial disparities in clinical presentation, type of intervention, and in-hospital outcomes of patients with metastatic spine disease: An analysis of 145,809 admissions in the United States. Cancer Epidemiol. 2020;68:101792. doi: 10.1016/j.canep.2020.101792 [DOI] [PubMed] [Google Scholar]
- 40.Hanchate AD, Paasche-Orlow MK, Baker WE, Lin M-Y, Banerjee S, Feldman J. Association of Race/Ethnicity With Emergency Department Destination of Emergency Medical Services Transport. JAMA Network Open. 2019;2(9):e1910816–e. doi: 10.1001/jamanetworkopen.2019.10816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–13. doi: 10.1016/j.jamcollsurg.2010.02.051 [DOI] [PubMed] [Google Scholar]
- 43.Galvani AP, Parpia AS, Pandey A, Zimmer C, Kahn JG, Fitzpatrick MC. The imperative for universal healthcare to curtail the COVID-19 outbreak in the USA. EClinicalMedicine. 2020;23:100380. doi: 10.1016/j.eclinm.2020.100380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuomala M. Optimal redistributive taxation: Oxford University Press; 2016. [Google Scholar]
- 45.Gibson M, Petticrew M, Bambra C, Sowden AJ, Wright KE, Whitehead M. Housing and health inequalities: a synthesis of systematic reviews of interventions aimed at different pathways linking housing and health. Health & place. 2011;17(1):175–84. doi: 10.1016/j.healthplace.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Block JP, Subramanian S. Moving beyond “food deserts”: reorienting United States policies to reduce disparities in diet quality. PLoS Med. 2015;12(12):e1001914. doi: 10.1371/journal.pmed.1001914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezrukova K, Spell CS, Perry JL, Jehn KA. A meta-analytical integration of over 40 years of research on diversity training evaluation. Psychol Bull. 2016;142(11):1227. doi: 10.1037/bul0000067 [DOI] [PubMed] [Google Scholar]
- 48.Lee WC, Veeranki SP, Serag H, Eschbach K, Smith KD. Improving the Collection of Race, Ethnicity, and Language Data to Reduce Healthcare Disparities: A Case Study from an Academic Medical Center. Perspect Health Inf Manag. 2016;13(Fall):1g. [PMC free article] [PubMed] [Google Scholar]
- 49.The Sullivan Commission. Missing Persons: Minorities in the Health Professions 2016. [Available from: https://campaignforaction.org/wp-content/uploads/2016/04/SullivanReport-Diversity-in-Healthcare-Workforce1.pdf. [Google Scholar]
- 50.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. eGEMs. 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tolchin B, Hull SC, Kraschel K. Triage and justice in an unjust pandemic: ethical allocation of scarce medical resources in the setting of racial and socioeconomic disparities. J Med Ethics. 2020. [DOI] [PubMed] [Google Scholar]
- 52.Jones CP, Truman BI, Elam-Evans LD, Jones CA, Jones CY, Jiles R, et al. Using “socially assigned race” to probe White advantages in health status. Race, Ethnicity, and Health: A Public Health Reader. 2012;26:57. [PubMed] [Google Scholar]
- 53.Nuru-Jeter AM, Michaels EK, Thomas MD, Reeves AN, Thorpe RJ Jr, LaVeist TA. Relative roles of race versus socioeconomic position in studies of health inequalities: a matter of interpretation. Annu Rev Public Health. 2018;39:169–88. doi: 10.1146/annurev-publhealth-040617-014230 [DOI] [PMC free article] [PubMed] [Google Scholar]


