Abstract
Peripheral nerve injuries (PNIs) are some of the most common types of traumatic lesions affecting the nervous system. Although the peripheral nervous system has a higher regenerative ability than the central nervous system, delayed treatment is associated with disturbances in both distal sensory and functional abilities. Over the past decades, adult stem cell-based therapies for peripheral nerve injuries have drawn attention from researchers. This is because various stem cells can promote regeneration after peripheral nerve injuries by differentiating into neural-line cells, secreting various neurotrophic factors, and regulating the activity of in situ Schwann cells (SCs). This article reviewed research from the past 10 years on the role of stem cells in the repair of PNIs. We concluded that adult stem cell-based therapies promote the regeneration of PNI in various ways.
1. Introduction
Peripheral nerve injuries (PNIs) are one of the most common types of traumatic lesions affecting the nervous system. They have an incidence of between 13 and 23 per 100,000 persons per year in developed countries [1], although it has a relatively higher impact in developing countries [2]. PNI usually involves partial or total loss of motor, sensory, and autonomic functions as well as neuropathic pain owing to the loss of structure and function of peripheral nerves from trauma, accidents, and other causes.
After PNI, a series of cellular and molecular events called Wallerian degeneration initially occurs. This is a process that clears debris from degeneration by degrading Schwann cells (SCs) and inducing the infiltration of microphages [3, 4]. Meanwhile, protein metabolism is altered, resulting in the activation of SCs [5]. These cells start forming structures known as “bands of Büngner” in order to provide guidance for axon regeneration. They also produce neurotrophic factors and extracellular matrix (ECM) molecules that promote axonal regeneration [6]. Therefore, SCs play a crucial role in peripheral nerve repair. Despite peripheral nerve axons possessing an intrinsic capability to generate and reconnect with their targets, in crucial nerve gaps, it is difficult to achieve complete functional and structural recovery [7]. This is because of the slow rate of axonal regeneration (1 mm/d) [8] as well as Wallerian degeneration occurring in the distal axon. Different therapeutic approaches have been investigated for handling lesions that cause large nerve gap, ranging from autologous nerve grafting, the gold standard treatment, up to various designed nerve guidance conduits (NGCs) combined with SC treatment [9]. The former is limited by poor functional outcomes, caused by scarce tissue graft availability and donor site morbidity. Furthermore, the latter is limited by difficulties in the harvesting and expansion of SCs. In this regard, researchers have begun to search for stem cells from different cell lineages, which are able to transform into SCs. We know that SCs are differentiated from the ectoderm, thus, this article reviewed the role and mechanisms of multifunctional adult stem cells from the ectoderm, as well as from the mesoderm and the neural crest of the fourth germ layer in peripheral nerve regeneration (Figures 1 and 2).
Figure 1.
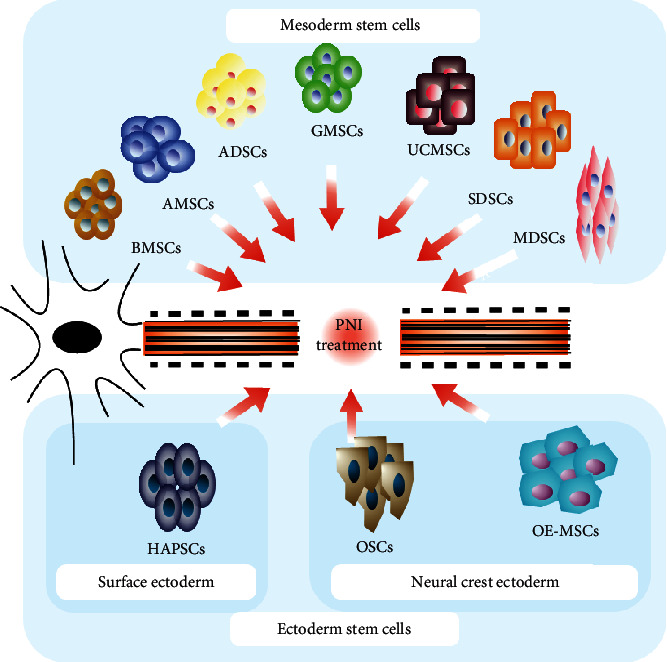
Adult stem cell types.
Figure 2.
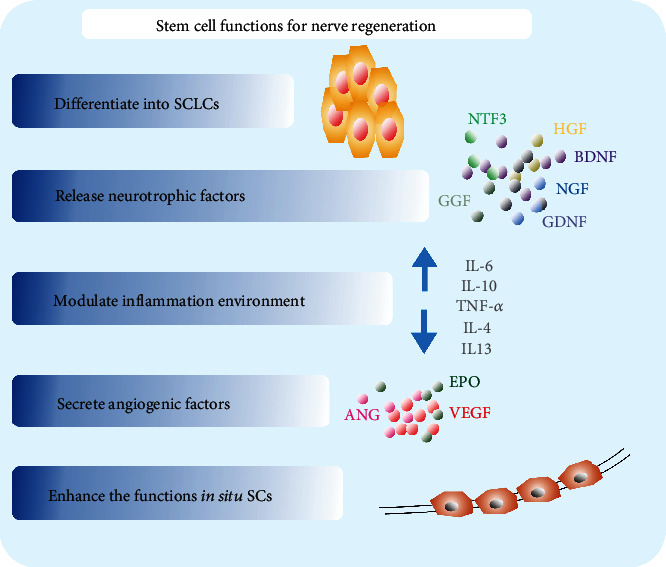
Functions of adult stem cells.
2. Adult Stem Cell-Based Therapies
Adult stem cells (ASCs), or somatic stem cells, are a collection of undifferentiated cells that are present in the postnatal body. Owing to their considerable self-renewal and multipotent ability, ASCs play an important role in PNIs. Recently, the beneficial effects of ASCs in the treatment of PNIs have been well studied, and comprehensive reports demonstrate their multilineage differentiation potential, secretion of neurotrophic factors, and immunomodulatory properties. Herein, we discuss the characteristics and functions of mesoderm-derived, surface ectoderm-derived, and fourth germ layer-derived stem cell types.
2.1. Mesodermal Cells: Mesenchymal Stem Cells
After a report in 1999 indicated that mesenchymal stem cells (MSCs) could be induced to transdifferentiate exclusively into adipocytic, chondrocytic, or osteocytic lineages [10], MSCs have been considered a hot topic in regenerative medicine. MSCs belong to the mesodermal lineage, but they are able to cross boundaries between mesodermal and ectodermal lineages, which include neural lineages [11]. Recently, MSCs are regarded as an important source of SCs because they are easily harvested from either the patient or donor-derived mesenchymal tissues, such as the bone marrow, adipose tissue, and umbilical cord. Furthermore, SCs are the main supportive cells for peripheral nerve regeneration; however, the SC buildup causes new damage to other peripheral nerve segments and also has several technical limitations regarding their cell-based therapy application. Therefore, MSCs are satisfactory candidates for use in PNI.
2.1.1. Bone Marrow Mesenchymal Stem Cells
Bone marrow mesenchymal stem cells (BMSCs) have abundant sources and the potential to self-renew; however, they can also differentiate into several different lineages, including neuronal cell types such as SCs [12, 13]. Several studies have indicated that undifferentiated bone marrow mesenchymal stem cell (u-BMSC) transplantation promotes nerve regeneration in different animal models [14, 15]; however, some reports demonstrated that u-BMSCs failed to promote any significant changes in regeneration outcome [16, 17]. SC-like differentiated bone marrow stem cells (d-BMSCs) have been shown to be more effective at nerve functional and histological recovery [18, 19]. This suggests that d-BMSCs are functionally close to authentic SCs. The characteristics of the mechanisms underlying the BMSC regeneration enhancement are thought to be directly related to the release of axonal regeneration proteins such as growth-associated protein 43 (GAP-43) [20] and neurotrophic factors such as nerve growth factors (NGF) and brain-derived neurotrophic factors (BDNF) [21]. These exert immunomodulatory effects by changing the inflammatory environment [22, 23], while protecting the injured nerve area from fibrous tissue infiltration [24]. Other research groups also reported vital improvements in the regeneration of injured peripheral nerves following the transplantation of neurotrophic factor-transfected BMSCs, when compared to normal BMSCs. Therefore, gene-based cell therapies have attracted the attention of many scientists. Furthermore, Chen et al. [25] reported that the Hippo, Wnt, transforming growth factor-beta, and hedgehog signaling pathways are potentially associated with BMSC neural differentiation. Additionally, overexpression of microRNA-124 promotes the neuronal differentiation of BMSCs.
2.1.2. Adipose-Derived Mesenchymal Stem Cells
Within the past decade, adipose-derived mesenchymal stem cells (ADSCs) have attracted the attention of researchers and clinicians in PNI repair. This is because they have the ability to differentiate into Schwan cell-like cells (SCLCs), downregulating inflammation [26]. Furthermore, they induce the direct effect of paracrine growth factors including NGF, BDNF, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin-like growth factor (IGF) [27], which indirectly affect endogenous SC activity [28–30]. It has been previously shown that ADSC-derived exosomes promote peripheral nerve regeneration by providing NGFs, optimizing the function of SCs in situ and reducing SC apoptosis in vitro and in vivo [25]. Regarding these functions, many experimental studies have been performed to demonstrate the beneficial effects of ADSCs (Table 1). Nevertheless, accumulating evidence indicates that differentiated ADSCs (dADSCs) show better axonal regeneration and functional recovery results than for undifferentiated-ADSCs (uADSCs) [31]. Several studies have reported that dADSCs express higher levels of neurotrophic factors, including NFG, BNDNF, GDNF, and NT4 [32–35], angiogenic factor (VEGF1), and ECM related protein (COL3A1) [36, 37] when compared with uADSCs, thus, accelerating axonal regeneration. Experimental studies in rat nerve injury models have investigated the effects of dADSCs (Table 2). However, because the dADSCs rapidly dedifferentiate in the absence of a stimulating medium [38], the differentiation process and method of cell transplantation to an injured environment must be studied prior to the use of dADSCs in PNI therapies. Thus, researchers applied ECM scaffolds containing fibronectin or laminin to create a similar microenvironment in order to maintain SC-like features for cell survival [39]. Interestingly, there was no significant difference between ADSCs and BMSCs when used in rat sciatic nerve injury. Both showed satisfactory results in terms of histological and functional recovery [40]. Furthermore, another study indicated that ADSCs successfully reduced neuropathic pain in the PNI model when compared with the BMSC group [41]. Recently, overexpressed neurotrophic factor ADSCs, via lentivirus transfection, became a new method to enhance PNI repair [42]. Without the donor site morbidity limitations associated with the isolation of SCs or BMSCs, ADSCs may provide a more effective cell population, thus, translating into the clinic to enhance PNI repair methods.
Table 1.
Effect of ADSCs in PNI animal models.
| Stem cell characteristics | Animal/nerve | Experimental model | Delivery system | Contribution to PNI regeneration | Reference |
|---|---|---|---|---|---|
| ADSCs | Mice sciatic nerve | 5 mm nerve gap | Gelatin hydrogel tube | Promoted formation of myelin, restoration of denervation muscle atrophy | [95] |
| hADSCs | Rabbit peroneal | 40 mm nerve gap | Vein conduit refilled with fibrin | Promoted myelination and axonal regeneration | [96] |
| ADSCs | Rat sciatic nerve | Nerve crush model | Perineural transplantation by cell injection | Gained better motor functional recovery and early proregeneration | [97] |
| ADSCs | Mouse sciatic nerve | Nerve crush model | Intravenous administration of ADSCs | Accelerated the functional recovery and reduced inflammatory infiltrates | [26] |
| ADSCs | Dog facial nerve | 7 mm nerve gap | Core-Tex tube filled alginate hydrogel | Promoted nerve diameter, nerve number, and electrophysiological recovery | [39] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | Collagen conduit filled with fibrin-agarose hydrogels | Enhanced sensory and motor functional recovery | [98] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | Silicon rubber conduit | Augmented the functional recovery and axonal regeneration | [99] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | GGT (genipin-gelatin-tricalcium phosphate) nerve conduit | Promoted SFI and CMAPs (compound muscle action potentials) | [100] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | Acellular nerve tube | Increased walking behavior, nerve conduct velocity, myelinated fiber density | [101] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | Fibrin conduit | Enhanced axonal regeneration and angiogenesis | [36] |
| ADSCs | Rat sciatic nerve | 10 mm nerve gap | 3D-engineering of cellularized conduits | Augmented functional and histological assessment | [102] |
| ADSCs | Rabbit sciatic nerve | 20 mm nerve gap | Acellular allogenic nerve | Improved recovery of nerve function, morphology, and tensile mechanical properties. | [103] |
| ADSCs | Rat sciatic nerve | Nerve transection | Fibrin glue | Enhanced the process of nerve regeneration and angiogenesis | [104] |
| ADSCs | Rat sciatic nerve | Nerve transection | Fibrin-hydrogel nerve conduits (FNC) | Promoted early nerve regeneration | [105] |
Table 2.
Effect of dADSCs in PNI animal models.
| Stem cell characteristics | Cell markers | Animal/nerve | Experimental model | Delivery system | Contribution to PNI regeneration | Reference |
|---|---|---|---|---|---|---|
| Neuronally differentiated ADSCs | βIII-tubulin | Rat sciatic nerve | 10 mm nerve gap | Aligned PBHV nanofiber nerve scaffold | Improved motor functional and histological recovery | [106] |
| ADSCs-SC-like cells | GFAP, S100 | Rat sciatic nerve | 10 mm nerve gap | Acellular nerve allograft | Enhanced walking-track and electrophysiological result | [42] |
| Differentiated ADSCs | GFAP, S100 | Rat sciatic nerve | 15 mm nerve gap | Acellular nerve | Augmented histological and electrophysiological recovery | [107] |
| ADSCs-SC-like cells | S100, NGFR p75 | Rat sciatic nerve | 10 mm nerve gap | Biodegradable chitin conduit | Promoted motor functional and histological recovery | [108] |
| Differentiated ADSCs | S100, GFAP, p75 | Rat sciatic nerve injury | 15 mm nerve gap | NeuraWrap™ filled with EngNT-dADSC sheets | Supported neuronal regeneration in regard to myelination thickness and number of axons | [109] |
| Differentiated ADSCs | GFAP, S100 | Rat sciatic nerve injury | 15 mm nerve gap | PGA-c tube | Improved myelin formation and functional recovery | [110] |
| Differentiated ADSCs | GFAP, S100 | Rat sciatic nerve injury | 10 mm nerve gap | GGT nerve conduit | Promoted SFI, electrophysiological recovery, and gained equally result with autologous in histological analysis | [111] |
| Differentiated ADSCs | GFAP, βIII-tubulin | Rat facial nerve | 8 mm | Decellularized allogeneic artery conduits | Promoted nerve regeneration and functional restoration. | [112] |
| Differentiated ADSCs | GFAP, S100, p75 | Rat sciatic nerve injury | 10 mm nerve gap | PHB tube filled with fibrin glue | Increased axon myelination and functional recovery | [113] |
2.1.3. Gingiva-Derived Mesenchymal Stem Cells
As an alternative and readily accessible source of stem cells for the repair of nerve tissues, gingiva-derived mesenchymal stem cells (GMSCs) have attracted the attention of researchers in recent years. GMSCs can be directly induced to become multipotent and expandable neural progenitor-like cell (NPCs) through nongenetic approaches [43, 44]. NPCs displayed better therapeutic effects on peripheral nerve regeneration than their parental GMSC counterparts. A study demonstrated that GMSCs and induced-NPCs promote peripheral nerve repair by promoting remyelination regulators, c-JUN, and Krox-20/EGR2 [44]. Furthermore, the same group suggested that the Yes-associated protein (YAP) signaling plays an important role in orchestrating the induction of NPCs from GMSCs [45]. Other researchers have used exosomes from the supernatant of cultured GMSCs for the treatment of PNI. Their results indicated that GMSC-derived vesicles or exosomes preferentially promote SC dedifferentiation and have the potential to activate the repair phenotype of SCs by regulating the expression of key transcription factors [46, 47]. These findings suggest that the practical advantages of GMSCs make them applicable for peripheral nerve injuries.
2.1.4. Skin-Derived Stem Cells
Skin-derived stem cells (SDSCs) are an accessible source of multipotent stem cells extracted from the dermis. These cells were reported to generate both endothelial and neural derivatives in vitro and in vivo [48, 49]. Furthermore, research has shown that SDSCs can differentiate into SDSC-SCs by 95% in vitro as well as survive, migrate, and maintain the expression of SC markers, over long-term periods, when transplanted into acellular isografts in vivo [50]. Several studies have reported that SDSC-SC treatment provides PNIs with immediate axon regeneration, myelination, and functional recovery. They do this via secreting neurotrophic factors and promoting proliferation of denervated host SCs or recruiting them to the sites of injury [51]. Moreover, studies have suggested the beneficial effects of SDSC-SCs in delayed PNI and in enhancing muscle reinnervation [52, 53]. Regarding immunomodulatory function, Stratton et al. [54] transplanted SDSC-SCs into nerve-injury site. The results indicated that SDSC-SCs enhanced debris clearance and inflammation following injury by secreting cytokines such as IL-6. Moreover, another group proved that advanced debris clearance implies a less inhibitory microenvironment, which in turn contributes to axon regeneration [53]. Additionally, treatment of the SDSC-SCs sensory neurons [55] or motoneurons [52] could be significantly improved in PNI animal models. In a clinical environment, patient-derived SDSCs alongside a transplanted collagen artificial nerve graft promoted motor and sensory functions of the median nerve during the case follow-up period [56]. In addition, Brandenburger and Kruse's group [57] described a protocol in which a coculture system of peripheral nerve cells with sweat gland-derived stem cells promoted neurite outgrowth. Given their ease of accessibility, ability to differentiate, and their capacity to enhance axon regeneration, SDSCs are a strong candidate for therapeutic PNI.
2.1.5. Muscle-Derived Mesenchymal Stem Cells
Under certain conditions, muscle-derived mesenchymal stem cells (MDSCs) can not only differentiate into mesoderm cells, including myocytes, adipocytes [58], and cartilage [59], but they can also differentiate into ectoderm cells such as neurocytes [60, 61] in vitro. Moreover, in vivo, some studies indicated that MDSCs could ameliorate critical-sized sciatic nerve injury in a murine model by differentiating into myelin-producing SCs [62, 63]. Meanwhile, Kazuno et al. [64] transplanted MDSCs into bioabsorbable polyglyconate (PGA) in a recurrent laryngeal nerve (RLN) transected mouse model and described good recovery of the RLN. Furthermore, Tamaki et al.'s group also suggested that MDSCs preferentially differentiate into perineurial/endoneurial cells, as well as SCs, in vivo [60]. They further sorted MDSCs into CD34(-)/CD45(-)/CD29(+) (Sk-DN/29(+)) and CD34(+)/CD45(-) (Sk-34) cells. This was followed by their expansion and then cotransplantation; it was reported that the latter type of MDSCs can differentiate into vascular endothelial cells and pericytes in vivo [65], which significantly improved functional recovery by promoting axon growth and vascular formation [66]. Furthermore, other studies also transplanted MDSCs with overexpressed the neurotrophic factor and achieved high-quality PNI healing [67]. Thus far, the main problem is that more reliable MDSC expansion protocols are needed for future clinical applications.
2.1.6. Amniotic-Derived Mesenchymal Stem Cells
Amniotic-derived mesenchymal stem cells (AMSCs) are a kind of multipotent stem cell with the capabilities of MSCs [68]. During the early years, intravenous administration of AMSCs provided beneficial effect on PNI because they express stromal cell-derived factor-1α (SDF-α) and its receptor chemokine receptor type-4 (CXCR-4) to enhance nerve regeneration by recruiting progenitor cells [69]. And another study also showed the therapy could alleviate the neuropathic pain and suppress the inflammation response by expressing IL-1β, TNF-α [70]. Furthermore, AMSCs produce a number of neurotrophic factors such as GAP-43, NGF, BDNF, and GDNF, which promote the nerve injury recovery in different animal models [71]. Additionally, Li et al.'s group indicated that AMSC transplantation display neurovascular tropism, which could aid in the recovery of sciatic nerve injury [72]. Besides, when compared to traditional BMSCs, AMSCs showed higher proliferative capacity and more efficiency nerve growth factor secretion both in vivo and vitro [73]. Therefore, AMSCs are a promising alternation for therapy of PNI.
2.1.7. Umbilical Cord-Derived Mesenchymal Stem Cells
Umbilical cord-derived mesenchymal stem cells (UCMSCs) are able to give rise to multiple cell types of neural lineage both in vivo and vitro [74]. Recent studies have showed that UCMSCs can be effectively used for peripheral nerve regeneration due to its paracrine [75], immunomodulatory, antioxidative [76], as well as inflammation modulatory characteristics [77]. Those properties could supply a favorable microenvironment for nerve regeneration. Furthermore, when combined with the biomaterial nerve conduit, UCMSCs exhibit beneficial effect in PNI. Cui et al. utilized the collagen conduit loaded with UCMSCs to the sciatic nerve defect in dogs and suggested that the therapy improved functional and histological recovery [78]. Another study indicated that associating a hybrid chitosan membrane with UCMSCs enhanced the motor and sensory functional recovery in rat model by stimulating the UCMSC differentiation in to SCLCs [79]. With regarding clinical application, one study reported radial nerve injury patients treated with UCMSC-loaded amniotic membrane displayed obvious improvement in muscular strength after 12 weeks when compared with control patients [80]. However, even though the UCMSCs are easy to harvest and purify, the main disadvantage is that it is hard to collect enough UCMSCs to transplant an adult.
2.2. Ectodermal Cells
The fact that embryonic origin is shared with the peripheral nervous system allows us to argue that ectodermal cells are much closer to nerve cells than mesodermal MSCs. In this section, two types of ectodermal cells, the surface ectoderm and neural crest ectoderm, are discussed. The latter is regarded as the fourth germ layer because of its multipotency, long-range migration throughout the embryo, and its capacity to generate a prodigious number of differentiated cell types. In the following subsections, we review the contribution of ectodermal cells originating from various adult tissue types for PNI regeneration.
2.2.1. Hair Follicle-Associated-Pluripotent Stem Cells
Hair follicle-associated pluripotent stem cells (HAPSCs) are a typical source of surface ectodermal cells. In 2004, researchers showed that neural crest cells (NCCs) grew out when the hair follicle bulge was explanted, resulting in differentiation into a variety of cell types, including neurons, smooth muscle cells, rare SCs, and melanocytes [81]. Furthermore, Amoh et al. (2005, 2010, 2012) proved that HAPSCs could differentiate into glial fibrillary acidic protein-positive SCs in vivo and form a myelin sheath surrounding axons while the severed sciatic nerve regrew in mice [82, 83]. Furthermore, this same group implanted mouse green fluorescent protein- (GFP-) expressing HAPSC spheres, encapsulated in polyvinylidene fluoride- (PVDF-) membrane cylinders, into the severed sciatic nerve of immunocompetent and immunocompromised (nude) mice. Eight weeks after treatment, the transplanted group showed greater improvement both in hematoxylin and eosin (H&E) staining and quantitative walking analysis than with the transplantation of empty cylinders. These findings suggest that HAPSCs provide a potentially accessible, autologous source of stem cells for PNI regeneration therapy.
2.2.2. Olfactory Stem Cells
Olfactory stem cells (OSCs) are a type of neural crest cell in mammals, including humans. During the past decade, OSCs have attracted considerable interest due to their self-renewal ability, as well as their ability to express different glial markers [84] and myelin constituents in adult mammals [85], thereby providing paracrine factors and a favorable microenvironment for neurogenesis [86]. Their therapeutic potential has been successfully tested in various PNI animal models (Table 3). One study confirmed that stem cells from the olfactory mucosa produce various growth factors and cytokines such as Galectin-1, growth arrest-specific 1 (GAS1), insulin-like growth factor-binding proteins 2 and 3 (IGF-BP2 and IGF-BP3), soluble tumor necrosis factor receptor I (sTNF-RI), and tumor necrosis factor-related weak inducer of apoptosis receptor (TWEAKR), all of which accelerate functional recovery after facial nerve injury in mice [87]. Additionally, when combined with biodegradation nerve conduits, olfactory ectomesenchymal stem cells (OE-MSCs) are also regarded as a good option. Salehi et al. (2019) utilized an alginate/chitosan hydrogel saturated with OSCs to treat sciatic nerve defects. They found that the therapy enhanced regeneration when compared to both the control and hydrogel without cells groups [88]. Furthermore, OSCs can be easily obtained from olfactory mucosa biopsies with limited risk, making it possible to envisage autologous cell transplantation strategies in future clinical work.
Table 3.
Effect of OSCs in PNI animal models.
| OSC origin | Surface marker expression in vitro | Models of PNI | Delivery system | Contribution to PNI regeneration | Reference |
|---|---|---|---|---|---|
| hOE-MSCs | CD13, CD44, CD90, CD166, CD146, CD73, CD29, CD105 | 2 mm facial nerve gap in rats | Nerve stumps | Promoted the movement score and electrophysiological results | [114] |
| OE-MSCs | Nestin | 20 mm inferior laryngeal nerve gap in rats | Cells are injected into nerve graft | Enhanced laryngeal mobility and function score | [115] |
| OE-MSCs | CD73, CD90, CD105, nestin, vimentin | Sciatic nerve crush in rats | Alginate/chitosan hydrogel | Improved motor and sensory nerve regeneration | [88] |
| OSCs | β-tubulin, nestin, and GFAP | 10 mm sciatic nerve gap in rats | Biphasic conduit | Contributed functional, electrophysiological, and histological recovery | [116] |
| OSCs | β-tubulin, GFAP, nestin, OMP, Musashi-1, sox-2, Nanog | Facial nerve crush model in mice | Biodegradable hydrogel | Accelerated the recovery from facial palsy and enhanced nerve regeneration | [87] |
2.2.3. Dental Ectomesenchymal Stem Cells
Dental ectomesenchymal stem cells (DE-MSCs), which originate from most craniofacial structures, such as the dental pulp, periodontal ligament, and exfoliated deciduous teeth, have the same origin as neural crest cells [89]. Over the past decade, dental pulp stem cells (DPSCs) have been confirmed to be capable of differentiating into functional oligodendrocytes in vitro [90], which highlights their potential application in PNI repair. Previous findings have shown that DPSCs promote axonal regeneration by releasing growth factors, including BDNF, GDNF, NGF, NTF3, ANGPT1, and VEGFA [91]. Furthermore, Sanen et al. [92] applied differentiated DPSCs in a rat sciatic nerve injury model and reported the proangiogenic effects of differentiated human DPSCs (d-hDPSCs) in PNI treatment. Recently, several investigations have also proven that DPSCs combined with different artificial nerves favor the peripheral nervous system [93]. They highlighted that both customized 3D nanofibrous scaffolds and chitosan-scaffolds support proliferation and neural differentiation of DPSCs, thus, they could be utilized for PNI repair. A few studies have also described the paracrine activity and differentiation potential of stem cells from exfoliated deciduous teeth (SHED) and periodontal ligaments (PDLSCs) [94]. Data from different studies (Table 4) suggest that these DE-MSCs not only show pluripotent differentiation potential but also have promising regenerative potential.
Table 4.
Effect of different ED-MSCs types in PNI animal models.
| ED-MSC type | Surface marker expression in vitro | Models of PNI | Delivery system | Contribution to PNI regeneration | Reference |
|---|---|---|---|---|---|
| hDPSCs | P75NTR, GFAP, S100b, nestin, SOX-10, STRO-1, c-Kit, CD34 | 6 mm nerve gap in a rat sciatic nerve model | Collagen scaffold | Differentiated into Schwan cells in vitro, promoted myelin formation and functional recovery in vivo | [91] |
| DPSCs, PDLSC | CD73, CD90, CD105, CD146 | 10 mm nerve gap in a rat sciatic nerve model | Fibrin glue conduit | Beneficial effects on neurite outgrowth in vitro. Enhanced axonal regeneration in vivo | [117] |
| d-DPSCs | - | 15 mm nerve gap in a rat sciatic nerve model | EngNT | Showed angiogenic properties in vitro and positive effect in nerve regeneration in vivo | [92] |
| DPSCs | - | 7 mm nerve gap in a rat facial nerve model | PLGA tube | Promoted histological recovery in vivo | [118] |
| SHED | CD73, CD90, CD105, nestin, doublecortin, β-III-tubulin, NeuN, GFAP, S-100, A2B5, CNPase | 12 mm nerve gap in a rat sciatic nerve model | Silicon conduit | Stimulated angiogenesis and neurite growth in vitro, enhanced functional and histological recovery in vivo | [94] |
| SHED | CD29, CD73, CD90, CD105, CD166, S100 | 5 mm nerve gap in a rat facial nerve model | PGAt nerve tube | Enhanced axonal regeneration and functional recovery | [119] |
| PDLSCs | POU4F2 | Rat optic nerve crush model | Cells were injected into the vitreous chamber | Promoted neurite growth in vitro, improved optic nerve regeneration in vivo. | [120] |
2.3. Endodermal Cell
Primary cells from endodermal organs such as the liver, lung, pancreas, and digestive tract are often difficult to grow in vitro, while the procurement of primary tissue is often ethically questionable, especially from healthy donors. Thus, nerve regenerative medicine applications are currently limited by the lack of high-quality endodermal adult stem cells.
3. Conclusions and Future Perspectives
Due to their multipotential, paracrine, and ethically friendly properties, ASC therapies are gradually garnering more attention as an efficient solution to healing PNIs. In this review, we sorted ASCs based on the three germ layers. Ideally, in this application, the ASCs should originate from mesodermal tissue because they not only retain the characteristics of mesodermal cells but can also differentiate into neural lineage cells, which are beneficial for peripheral regeneration. In addition to MSCs, ectoderm-derived stem cells also enable nerve regeneration in preclinical treatments. In particular, neural crest stem cells (NCDSs), which have the same germ layer as neurocytes, can express nerve-specific markers. Furthermore, with their multipotent ability, NCSCs expedite the development of ectodermal stem cell-based therapies to treat PNI. Over the past decade, combining ASCs with tissue-engineered nerve conduits has accelerated the therapeutic effects of peripheral nerve repair.
Despite great promise in ASCs, some issues still exist that affect the efficiency of ASC-based therapy (Table 5). For instance, when ASCs are transplanted into PNI animal models, the percentage of ASCs differentiating directly into supportive cells and the ratio of surviving cells should be noted. Furthermore, some data support that differentiated cells rapidly dedifferentiated when there is a lack of stimulation [38]. Thus, the stimulator, mobilization, homing, and delivery system should be taken into consideration to improve the quantity and quality of stem cells in therapeutic environments.
Table 5.
Summary of pros and cons for stem cells.
| Pros | Cons | Reference | ||
|---|---|---|---|---|
| Mesodermal cells | BMSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Immunomodulatory effects |
(i) Require invasive surgical procedures (ii) Donor site morbidity |
[15, 18–21, 23, 121–126] |
| ADSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Downregulating inflammation (v) Abundant source |
(i) Require invasive surgical procedures (ii) Rapidly dedifferentiated |
[25, 36, 38, 95, 103, 110, 127–132] | |
| GMSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Promote situ SC dedifferentiation (iv) Phenotypically stable paracrine ability (v) Less invasive procedure |
(i) Require invasive surgical procedures | [43–47] | |
| SDSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Promote situ SC dedifferentiation (iv) Immunomodulatory effects |
(i) Require invasive surgical procedures (ii) Low efficiency of isolation |
[48, 49, 52–54, 56, 133–137] | |
| MDSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Promote vascular formation (iv) Produce neurotrophic factors |
(i) Require invasive surgical procedures (ii) Donor site morbidity (iii) Low efficiency of expansion |
[60, 62, 63, 65, 67, 138] | |
| AMSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Promote vascular formation (v) Downregulating inflammation |
(i) Difficult to isolation (ii) Unstable phenotype |
[73, 139–144] | |
| UCMSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Downregulating inflammation (v) Easy to harvest and purify |
(i) Risk of tumorgenesis (ii) Not abundance |
[2, 75, 76, 78, 145–153] | |
| Ectodermal cells | HAPSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Readily accessible (iv) Abundance |
(i) Unclear mechanism and condition for differentiation | [5, 81–83, 154, 155] |
| OSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Readily accessible (v) Abundance |
(i) Limited migration ability under hypoxic environment at the site of injury | [86, 87, 88, 156] | |
| DE-MSCs | (i) Self-renew ability (ii) Differentiate into neural lineage (iii) Produce neurotrophic factors (iv) Ease isolation procedure |
(i) Low yield of stem cells | [89, 92–94, 157] | |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li R., Liu Z., Pan Y., Chen L., Zhang Z., Lu L. Peripheral nerve injuries treatment: a systematic review. Cell Biochemistry and Biophysics. 2014;68(3):449–454. doi: 10.1007/s12013-013-9742-1. [DOI] [PubMed] [Google Scholar]
- 2.Jiang L., Jones S., Jia X. Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. International Journal of Molecular Sciences. 2017;18(1):p. 94. doi: 10.3390/ijms18010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman M. P., Conforti L., Buckmaster E. A., et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proceedings of the National Academy of Sciences. 1998;95(17):9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waller A. Experiments on the section of the glosso-pharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Edinburgh Medical and Surgical Journal. 1851;76(189):369–376. [PMC free article] [PubMed] [Google Scholar]
- 5.Amoh Y., Katsuoka K., Hoffman R. M. Peripheral-nerve and spinal-cord regeneration in mice using hair-follicle-associated pluripotent (HAP) stem cells. Methods in Molecular Biology. 2016;1453:21–32. doi: 10.1007/978-1-4939-3786-8_4. [DOI] [PubMed] [Google Scholar]
- 6.Pabari A., Yang S. Y., Mosahebi A., Seifalian A. M. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. Journal of Controlled Release. 2011;156(1):2–10. doi: 10.1016/j.jconrel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Sachanandani N. F., Pothula A., Tung T. H. Nerve gaps. Plastic and Reconstructive Surgery. 2014;133(2):313–319. doi: 10.1097/01.prs.0000436856.55398.0f. [DOI] [PubMed] [Google Scholar]
- 8.Trojaborg W. Rate of recovery in motor and sensory fibres of the radial nerve: clinical and electrophysiological aspects. Journal of Neurology, Neurosurgery & Psychiatry. 1970;33(5):625–638. doi: 10.1136/jnnp.33.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho C. R., Oliveira J. M., Reis R. L. Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Frontiers in Bioengineering and Biotechnology. 2019;7:p. 337. doi: 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittenger M. F., Mackay A. M., Beck S. C., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Dezawa M., Kanno H., Hoshino M., et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. The Journal of Clinical Investigation. 2004;113(12):1701–1710. doi: 10.1172/JCI200420935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M., Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells International. 2013;2013:8. doi: 10.1155/2013/132642.132642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitada M. Mesenchymal cell populations: development of the induction systems for Schwann cells and neuronal cells and finding the unique stem cell population. Anatomical Science International. 2012;87(1):24–44. doi: 10.1007/s12565-011-0128-4. [DOI] [PubMed] [Google Scholar]
- 14.Duan X. H., Cheng L. N., Zhang F., et al. In vivo MRI monitoring nerve regeneration of acute peripheral nerve traction injury following mesenchymal stem cell transplantation. European Journal of Radiology. 2012;81(9):2154–2160. doi: 10.1016/j.ejrad.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Abbas O. L., Özatik O., Gönen Z. B., et al. Bone marrow mesenchymal stem cell transplantation enhances nerve regeneration in a rat model of hindlimb replantation. Plastic and Reconstructive Surgery. 2019;143(4):758e–768e. doi: 10.1097/PRS.0000000000005412. [DOI] [PubMed] [Google Scholar]
- 16.Seyed Foroutan K., Khodarahmi A., Alavi H., Pedram S., Baghaban Eslaminejad M. R., Bordbar S. Bone marrow mesenchymal stem cell and vein conduit on sciatic nerve repair in rats. Trauma Monthly. 2015;20(1, article e23325) doi: 10.5812/traumamon.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eren F., Öksüz S., Küçükodaci Z., et al. Targeted mesenchymal stem cell and vascular endothelial growth factor strategies for repair of nerve defects with nerve tissue implanted autogenous vein graft conduits. Microsurgery. 2016;36(7):578–585. doi: 10.1002/micr.22401. [DOI] [PubMed] [Google Scholar]
- 18.Ladak A. O., Olson J., Tredget E. E., Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Experimental Neurology. 2011;228(2):242–252. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Hou B., Ye Z., Ji W., et al. Comparison of the effects of BMSC-derived Schwann cells and autologous Schwann cells on remyelination using a rat sciatic nerve defect model. International Journal of Biological Sciences. 2018;14(13):1910–1922. doi: 10.7150/ijbs.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y., Ge S., Zhang J., et al. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. International Journal of Clinical and Experimental Pathology. 2017;10(9):10032–10039. [PMC free article] [PubMed] [Google Scholar]
- 21.Mahay D., Terenghi G., Shawcross S. G. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Experimental Cell Research. 2008;314(14):2692–2701. doi: 10.1016/j.yexcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Wakao S., Hayashi T., Kitada M., et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Experimental Neurology. 2010;223(2):537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Cooney D. S., Wimmers E. G., Ibrahim Z., et al. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Scientific Reports. 2016;6(1, article 31306) doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Luo E., Li Y., Hu J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Research. 2011;1383:71–80. doi: 10.1016/j.brainres.2011.01.098. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Ren S., Duscher D., et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. Journal of Cellular Physiology. 2019;234(12):23097–23110. doi: 10.1002/jcp.28873. [DOI] [PubMed] [Google Scholar]
- 26.Marconi S., Castiglione G., Turano E., et al. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Engineering Part A. 2012;18(11-12):1264–1272. doi: 10.1089/ten.tea.2011.0491. [DOI] [PubMed] [Google Scholar]
- 27.Sowa Y., Imura T., Numajiri T., Nishino K., Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells and Development. 2012;21(11):1852–1862. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- 28.Erba P., Mantovani C., Kalbermatten D. F., Pierer G., Terenghi G., Kingham P. J. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63(12):e811–e817. doi: 10.1016/j.bjps.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Carlson K. B., Singh P., Feaster M. M., et al. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cell-derived laminin. Glia. 2011;59(2):267–277. doi: 10.1002/glia.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh S. C., Chang C. J., Cheng W. T., Tseng T. C., Hsu S. H. Effect of an epineurial-like biohybrid nerve conduit on nerve regeneration. Cell Transplantation. 2016;25(3):559–574. doi: 10.3727/096368915X688920. [DOI] [PubMed] [Google Scholar]
- 31.Orbay H., Uysal A. C., Hyakusoku H., Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2012;65(5):657–664. doi: 10.1016/j.bjps.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Tse K. H., Sun M., Mantovani C., Terenghi G., Downes S., Kingham P. J. In vitro evaluation of polyester-based scaffolds seeded with adipose derived stem cells for peripheral nerve regeneration. Journal of Biomedical Materials Research Part A. 2010;95(3):701–708. doi: 10.1002/jbm.a.32889. [DOI] [PubMed] [Google Scholar]
- 33.Reid A. J., Sun M., Wiberg M., Downes S., Terenghi G., Kingham P. J. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–522. doi: 10.1016/j.neuroscience.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 34.Tomita K., Madura T., Sakai Y., Yano K., Terenghi G., Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 35.Lopatina T., Kalinina N., Karagyaur M., et al. Correction: adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2019;14(7, article e0219946) doi: 10.1371/journal.pone.0219946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kingham P. J., Kolar M. K., Novikova L. N., Novikov L. N., Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells and Development. 2014;23(7):741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 37.Mathot F., Rbia N., Thaler R., Bishop A. T., van Wijnen A. J., Shin A. Y. Gene expression profiles of differentiated and undifferentiated adipose derived mesenchymal stem cells dynamically seeded onto a processed nerve allograft. Gene. 2020;724, article 144151 doi: 10.1016/j.gene.2019.144151. [DOI] [PubMed] [Google Scholar]
- 38.Faroni A., Smith R. J. P., Lu L., Reid A. J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. European Journal of Neuroscience. 2016;43(3):417–430. doi: 10.1111/ejn.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.di Summa P. G., Kalbermatten D. F., Raffoul W., Terenghi G., Kingham P. J. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue Engineering Part A. 2013;19(3-4):368–379. doi: 10.1089/ten.tea.2012.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.di Summa P. G., Kingham P. J., Campisi C. C., Raffoul W., Kalbermatten D. F. Collagen (NeuraGen®) nerve conduits and stem cells for peripheral nerve gap repair. Neuroscience Letters. 2014;572:26–31. doi: 10.1016/j.neulet.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Haselbach D., Raffoul W., Larcher L., Tremp M., Kalbermatten D. F., di Summa P. G. Regeneration patterns influence hindlimb automutilation after sciatic nerve repair using stem cells in rats. Neuroscience Letters. 2016;634:153–159. doi: 10.1016/j.neulet.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Fu X. M., Wang Y., Fu W. L., et al. The combination of adipose-derived Schwann-like cells and acellular nerve allografts promotes sciatic nerve regeneration and repair through the JAK2/STAT3 signaling pathway in rats. Neuroscience. 2019;422:134–145. doi: 10.1016/j.neuroscience.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Nguyen P. D., Shi S., Burrell J. C., Cullen D. K., le A. D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Scientific Reports. 2018;8(1):p. 6634. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q., Nguyen P., Xu Q., et al. Neural progenitor-like cells induced from human gingiva-derived mesenchymal stem cells regulate myelination of Schwann cells in rat sciatic nerve regeneration. Stem Cells Translational Medicine. 2017;6(2):458–470. doi: 10.5966/sctm.2016-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q., Nguyen P. D., Shi S., et al. Neural crest stem-like cells non-genetically induced from human gingiva-derived mesenchymal stem cells promote facial nerve regeneration in rats. Molecular Neurobiology. 2018;55(8):6965–6983. doi: 10.1007/s12035-018-0913-3. [DOI] [PubMed] [Google Scholar]
- 46.Mao Q., Nguyen P. D., Shanti R. M., et al. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate Schwann cell repair phenotype and promote nerve regeneration. Tissue Engineering Part A. 2019;25(11-12):887–900. doi: 10.1089/ten.tea.2018.0176. [DOI] [PubMed] [Google Scholar]
- 47.Rao F., Zhang D., Fang T., et al. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells International. 2019;2019:12. doi: 10.1155/2019/2546367.2546367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toma J. G., Akhavan M., Fernandes K. J. L., et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biology. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 49.Belicchi M., Pisati F., Lopa R., et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. Journal of Neuroscience Research. 2004;77(4):475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- 50.Walsh S., Biernaskie J., Kemp S. W. P., Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164(3):1097–1107. doi: 10.1016/j.neuroscience.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 51.Kitada M., Murakami T., Wakao S., Li G., Dezawa M. Direct conversion of adult human skin fibroblasts into functional Schwann cells that achieve robust recovery of the severed peripheral nerve in rats. Glia. 2019;67(5):950–966. doi: 10.1002/glia.23582. [DOI] [PubMed] [Google Scholar]
- 52.Walsh S. K., Gordon T., Addas B. M. J., Kemp S. W. P., Midha R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Experimental Neurology. 2010;223(1):221–228. doi: 10.1016/j.expneurol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 53.Khuong H. T., Kumar R., Senjaya F., et al. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Experimental Neurology. 2014;254:168–179. doi: 10.1016/j.expneurol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Stratton J. A., Shah P. T., Kumar R., et al. The immunomodulatory properties of adult skin-derived precursor Schwann cells: implications for peripheral nerve injury therapy. European Journal of Neuroscience. 2016;43(3):365–375. doi: 10.1111/ejn.13006. [DOI] [PubMed] [Google Scholar]
- 55.Shakhbazau A., Mohanty C., Kumar R., Midha R. Sensory recovery after cell therapy in peripheral nerve repair: effects of naïve and skin precursor-derived Schwann cells. Journal of Neurosurgery. 2014;121(2):423–431. doi: 10.3171/2014.5.JNS132132. [DOI] [PubMed] [Google Scholar]
- 56.Grimoldi N., Colleoni F., Tiberio F., et al. Stem cell salvage of injured peripheral nerve. Cell Transplantation. 2015;24(2):213–222. doi: 10.3727/096368913X675700. [DOI] [PubMed] [Google Scholar]
- 57.Brandenburger M., Kruse C. Fabrication of a co-culture system with human sweat gland-derived cells and peripheral nerve cells. Skin Tissue Engineering. 2019;1993:139–148. doi: 10.1007/978-1-4939-9473-1_11. [DOI] [PubMed] [Google Scholar]
- 58.Aguiari P., Leo S., Zavan B., et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proceedings of the National Academy of Sciences. 2008;105(4):1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H., Johnson N. R., Usas A., et al. Sustained release of bone morphogenetic protein 2 via coacervate improves the osteogenic potential of muscle-derived stem cells. Stem Cells Translational Medicine. 2013;2(9):667–677. doi: 10.5966/sctm.2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamaki T., Hirata M., Soeda S., et al. Preferential and comprehensive reconstitution of severely damaged sciatic nerve using murine skeletal muscle-derived multipotent stem cells. PLoS One. 2014;9(3, article e91257) doi: 10.1371/journal.pone.0091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vojnits K., Pan H. Y., Mu X., Li Y. Characterization of an injury induced population of muscle-derived stem cell-like cells. Scientific Reports. 2015;5(1, article 17355) doi: 10.1038/srep17355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavasani M., Thompson S. D., Pollett J. B., et al. Human muscle-derived stem/progenitor cells promote functional murine peripheral nerve regeneration. The Journal of Clinical Investigation. 2014;124(4):1745–1756. doi: 10.1172/JCI44071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Z. X., Lu H. B., Jin X. L., Feng W. F., Yang X. N., Qi Z. L. Skeletal muscle-derived cells repair peripheral nerve defects in mice. Neural Regeneration Research. 2020;15(1):152–161. doi: 10.4103/1673-5374.264462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kazuno A., Maki D., Yamato I., et al. Regeneration of transected recurrent laryngeal nerve using hybrid-transplantation of skeletal muscle-derived stem cells and bioabsorbable scaffold. Journal of Clinical Medicine. 2018;7(9):p. 276. doi: 10.3390/jcm7090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamaki T., Uchiyama Y., Hirata M., et al. Therapeutic isolation and expansion of human skeletal muscle-derived stem cells for the use of muscle-nerve-blood vessel reconstitution. Frontiers in Physiology. 2015;6:p. 165. doi: 10.3389/fphys.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamaki T., Hirata M., Nakajima N., et al. A long-gap peripheral nerve injury therapy using human skeletal muscle-derived stem cells (Sk-SCs): an achievement of significant Morphological, Numerical and Functional Recovery. PLoS One. 2016;11(11, article e0166639) doi: 10.1371/journal.pone.0166639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halum S. L., McRae B., Bijangi-Vishehsaraei K., Hiatt K. Neurotrophic factor-secreting autologous muscle stem cell therapy for the treatment of laryngeal denervation injury. Laryngoscope. 2012;122(11):2482–2496. doi: 10.1002/lary.23519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Coppi P., Bartsch G., Jr., Siddiqui M. M., et al. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology. 2007;25(1):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 69.Yang D. Y., Sheu M. L., Su H. L., et al. Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1α in a sciatic nerve injury model. Journal of Neurosurgery. 2012;116(6):1357–1367. doi: 10.3171/2012.2.JNS111360. [DOI] [PubMed] [Google Scholar]
- 70.Chiang C. Y., Liu S. A., Sheu M. L., et al. Feasibility of human amniotic fluid derived stem cells in alleviation of neuropathic pain in chronic constrictive injury nerve model. PLoS One. 2016;11(7, article e0159482) doi: 10.1371/journal.pone.0159482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C. J., Cheng F. C., Su H. L., et al. Improved neurological outcome by intramuscular injection of human amniotic fluid derived stem cells in a muscle denervation model. PLoS One. 2015;10(5, article e0124624) doi: 10.1371/journal.pone.0124624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y., Guo L., Ahn H. S., Kim M. H., Kim S. W. Amniotic mesenchymal stem cells display neurovascular tropism and aid in the recovery of injured peripheral nerves. Journal of Cellular and Molecular Medicine. 2014;18(6):1028–1034. doi: 10.1111/jcmm.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan Z. J., Hu Y. Q., Zhang H. T., et al. Comparison of the neural differentiation potential of human mesenchymal stem cells from amniotic fluid and adult bone marrow. Cellular and Molecular Neurobiology. 2013;33(4):465–475. doi: 10.1007/s10571-013-9922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gärtner A., Pereira T., Alves M. G., et al. Use of poly(DL-lactide-ε-caprolactone) membranes and mesenchymal stem cells from the Wharton's jelly of the umbilical cord for promoting nerve regeneration in axonotmesis: in vitro and in vivo analysis. Differentiation. 2012;84(5):355–365. doi: 10.1016/j.diff.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Guo Z. Y., Sun X., Xu X. L., Zhao Q., Peng J., Wang Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regeneration Research. 2015;10(4):651–658. doi: 10.4103/1673-5374.155442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., Li D., Ju X., Shi Q., Wang D., Wei F. Umbilical cord-derived mesenchymal stem cells retain immunomodulatory and anti-oxidative activities after neural induction. Neural Regeneration Research. 2012;7(34):2663–2672. doi: 10.3969/j.issn.1673-5374.2012.34.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma Y., Dong L., Zhou D., et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. Journal of Cellular and Molecular Medicine. 2019;23(4):2822–2835. doi: 10.1111/jcmm.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui Y., Yao Y., Zhao Y., et al. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(5):1285–1296. doi: 10.1002/term.2660. [DOI] [PubMed] [Google Scholar]
- 79.Gärtner A., Pereira T., Simões M. J., et al. Use of hybrid chitosan membranes and human mesenchymal stem cells from the Wharton jelly of umbilical cord for promoting nerve regeneration in an axonotmesis rat model. Neural Regeneration Research. 2012;7(29):2247–2258. doi: 10.3969/j.issn.1673-5374.2012.29.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z., Qin H., Feng Z., et al. Human umbilical cord mesenchymal stem cell-loaded amniotic membrane for the repair of radial nerve injury. Neural Regeneration Research. 2013;8(36):3441–3448. doi: 10.3969/j.issn.1673-5374.2013.36.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sieber-Blum M., Grim M., Hu Y. F., Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Developmental Dynamics: An Official Publication of the American Association of the Anatomists. 2004;231(2):258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 82.Amoh Y., Li L., Campillo R., et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proceedings of the National Academy of Sciences. 2005;102(49):17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amoh Y., Aki R., Hamada Y., et al. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. The Journal of Dermatology. 2012;39(1):33–38. doi: 10.1111/j.1346-8138.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 84.Franceschini I. A., Barnett S. C. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Developmental Biology. 1996;173(1):327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- 85.Gordon Boyd J., Doucette R., Kawaja M. D. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. The FASEB Journal. 2005;19(7):694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 86.Ge L., Jiang M., Duan D., et al. Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells International. 2016;2016:16. doi: 10.1155/2016/1243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esaki S., Katsumi S., Hamajima Y., Nakamura Y., Murakami S. Transplantation of olfactory stem cells with biodegradable hydrogel accelerates facial nerve regeneration after crush injury. Stem Cells Translational Medicine. 2019;8(2):169–178. doi: 10.1002/sctm.15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salehi M., Bagher Z., Kamrava S. K., et al. Alginate/chitosan hydrogel containing olfactory ectomesenchymal stem cells for sciatic nerve tissue engineering. Journal of Cellular Physiology. 2019;234(9):15357–15368. doi: 10.1002/jcp.28183. [DOI] [PubMed] [Google Scholar]
- 89.Janebodin K., Horst O. V., Ieronimakis N., et al. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6(11, article e27526) doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Askari N., Yaghoobi M. M., Shamsara M., Esmaeili-Mahani S. Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience. 2015;305:197–208. doi: 10.1016/j.neuroscience.2015.07.088. [DOI] [PubMed] [Google Scholar]
- 91.Carnevale G., Pisciotta A., Riccio M., et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(2):e774–e785. doi: 10.1002/term.2378. [DOI] [PubMed] [Google Scholar]
- 92.Sanen K., Martens W., Georgiou M., Ameloot M., Lambrichts I., Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? Journal of Tissue Engineering and Regenerative Medicine. 2017;11(12):3362–3372. doi: 10.1002/term.2249. [DOI] [PubMed] [Google Scholar]
- 93.Zheng K., Feng G., Zhang J., et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. International Journal of Neuroscience. 2021;131(7):625–633. doi: 10.1080/00207454.2020.1744592. [DOI] [PubMed] [Google Scholar]
- 94.Sugimura-Wakayama Y., Katagiri W., Osugi M., et al. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells and Development. 2015;24(22):2687–2699. doi: 10.1089/scd.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sowa Y., Kishida T., Imura T., et al. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plastic and Reconstructive Surgery. 2016;137(2):318e–330e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 96.Lasso J. M., Pérez Cano R., Castro Y., Arenas L., García J., Fernández-Santos M. E. Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2015;68(12):e189–e197. doi: 10.1016/j.bjps.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez Sánchez D. N., de Lima Resende L. A., Boff Araujo Pinto G., et al. Canine adipose-derived mesenchymal stromal cells enhance neuroregeneration in a rat model of sciatic nerve crush injury. Cell Transplantation. 2019;28(1):47–54. doi: 10.1177/0963689718809045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carriel V., Garrido-Gómez J., Hernández-Cortés P., et al. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. Journal of Neural Engineering. 2013;10(2, article 026022) doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 99.Mohammadi R., Azizi S., Amini K. Effects of undifferentiated cultured omental adipose-derived stem cells on peripheral nerve regeneration. Journal of Surgical Research. 2013;180(2):e91–e97. doi: 10.1016/j.jss.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 100.Shen C. C., Yang Y. C., Liu B. S. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. Journal of Biomedical Materials Research Part A. 2012;100A(1):48–63. doi: 10.1002/jbm.a.33227. [DOI] [PubMed] [Google Scholar]
- 101.Liu G. B., Cheng Y. X., Feng Y. K., et al. Adipose-derived stem cells promote peripheral nerve repair. Archives of Medical Science. AMS. 2011;7(4):592–596. doi: 10.5114/aoms.2011.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Y., Wu Y., Gou Z., et al. 3D-engineering of cellularized conduits for peripheral nerve regeneration. Scientific Reports. 2016;6(1, article 32184) doi: 10.1038/srep32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piao C., Li Z., Ding J., Kong D. Mechanical properties of the sciatic nerve following combined transplantation of analytically extracted acellular allogeneic nerve and adipose-derived mesenchymal stem cells. Acta Cirúrgica Brasileira. 2020;35(4, article e202000405) doi: 10.1590/s0102-865020200040000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reichenberger M. A., Mueller W., Hartmann J., et al. ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery. 2016;36(6):491–500. doi: 10.1002/micr.30018. [DOI] [PubMed] [Google Scholar]
- 105.Prautsch K. M., Degrugillier L., Schaefer D. J., Guzman R., Kalbermatten D. F., Madduri S. Ex-vivo stimulation of adipose stem cells by growth factors and fibrin-hydrogel assisted delivery strategies for treating nerve gap-injuries. Bioengineering (Basel) 2020;7(2):p. 42. doi: 10.3390/bioengineering7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu F., Zhang X., Liu H., et al. Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochemical and Biophysical Research Communications. 2017;489(2):171–178. doi: 10.1016/j.bbrc.2017.05.119. [DOI] [PubMed] [Google Scholar]
- 107.Jiang L., Zheng Y., Chen O., Chu T., Ding J., Yu Q. Nerve defect repair by differentiated adipose-derived stem cells and chondroitinase ABC-treated acellular nerves. International Journal of Neuroscience. 2016;126(6):1–9. doi: 10.3109/00207454.2015.1048547. [DOI] [PubMed] [Google Scholar]
- 108.Sun X., Zhu Y., Yin H. Y., et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: potential advantage of cellular transient memory function. Stem Cell Research & Therapy. 2018;9(1):p. 133. doi: 10.1186/s13287-018-0884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Georgiou M., Golding J. P., Loughlin A. J., Kingham P. J., Phillips J. B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials. 2015;37:242–251. doi: 10.1016/j.biomaterials.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto D., Tada K., Suganuma S., et al. Differentiated adipose-derived stem cells promote peripheral nerve regeneration. Muscle & Nerve. 2020;62(1):119–127. doi: 10.1002/mus.26879. [DOI] [PubMed] [Google Scholar]
- 111.Liu B. S., Yang Y. C., Shen C. C. Regenerative effect of adipose tissue-derived stem cells transplantation using nerve conduit therapy on sciatic nerve injury in rats. Journal of Tissue Engineering and Regenerative Medicine. 2014;8(5):337–350. doi: 10.1002/term.1523. [DOI] [PubMed] [Google Scholar]
- 112.Sun F., Zhou K., Mi W. J., Qiu J. H. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials. 2011;32(32):8118–8128. doi: 10.1016/j.biomaterials.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 113.Schaakxs D., Kalbermatten D. F., Pralong E., Raffoul W., Wiberg M., Kingham P. J. Poly-3-hydroxybutyrate strips seeded with regenerative cells are effective promoters of peripheral nerve repair. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(3):812–821. doi: 10.1002/term.1980. [DOI] [PubMed] [Google Scholar]
- 114.Batioglu-Karaaltin A., Karaaltin M. V., Oztel O. N., et al. Human olfactory stem cells for injured facial nerve reconstruction in a rat model. Head & Neck. 2016;38(Supplement 1):E2011–E2020. doi: 10.1002/hed.24371. [DOI] [PubMed] [Google Scholar]
- 115.Saïd Z., Pauline C., Claire B., Celia D., Jean-Paul M., Nicolas B. M. Olfactory ecto-mesenchymal stem cells in laryngeal nerve regeneration in rats. Journal of Voice. 2021;35(3):349–359. doi: 10.1016/j.jvoice.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 116.Roche P., Alekseeva T., Widaa A., et al. Olfactory derived stem cells delivered in a biphasic conduit promote peripheral nerve repair in vivo. Stem Cells Translational Medicine. 2017;6(10):1894–1904. doi: 10.1002/sctm.16-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kolar M. K., Itte V. N., Kingham P. J., Novikov L. N., Wiberg M., Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Scientific Reports. 2017;7(1, article 12605) doi: 10.1038/s41598-017-12969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasaki R., Aoki S., Yamato M., et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2011;5(10):823–830. doi: 10.1002/term.387. [DOI] [PubMed] [Google Scholar]
- 119.Pereira L. V., Bento R. F., Cruz D. B., et al. Stem cells from human exfoliated deciduous teeth (SHED) differentiate in vivo and promote facial nerve regeneration. Cell Transplantation. 2019;28(1):55–64. doi: 10.1177/0963689718809090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cen L. P., Ng T. K., Liang J. J., et al. Human periodontal ligament-derived stem cells promote retinal ganglion cell survival and axon regeneration after optic nerve injury. Stem Cells. 2018;36(6):844–855. doi: 10.1002/stem.2812. [DOI] [PubMed] [Google Scholar]
- 121.Bingham J. R., Kniery K. R., Jorstad N. L., Horkayne-Szakaly I., Hoffer Z. S., Salgar S. K. "Stem cell therapy to promote limb function recovery in peripheral nerve damage in a rat model" - Experimental research. Annals of Medicine and Surgery. 2019;41:20–28. doi: 10.1016/j.amsu.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zou D., Chen Y., Han Y., Lv C., Tu G. Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells. Neural Regeneration Research. 2014;9(12):1241–1248. doi: 10.4103/1673-5374.135333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin F., Meng C., Lu R., et al. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regeneration Research. 2014;9(18):1665–1671. doi: 10.4103/1673-5374.141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lerner M. Z., Matsushita T., Lankford K. L., Radtke C., Kocsis J. D., Young N. O. Intravenous mesenchymal stem cell therapy after recurrent laryngeal nerve injury: a preliminary study. Laryngoscope. 2014;124(11):2555–2560. doi: 10.1002/lary.24798. [DOI] [PubMed] [Google Scholar]
- 125.Feng Y., Wang J., Ling S., et al. Differentiation of mesenchymal stem cells into neuronal cells on fetal bovine acellular dermal matrix as a tissue engineered nerve scaffold. Neural Regeneration Research. 2014;9(22):1968–1978. doi: 10.4103/1673-5374.145378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang C. L., Lv G. Repair of sciatic nerve defects using tissue engineered nerves. Neural Regeneration Research. 2013;8(21):1985–1994. doi: 10.3969/j.issn.1673-5374.2013.21.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou L. N., Wang J. C., Zilundu P. L. M., et al. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Research & Therapy. 2020;11(1):p. 153. doi: 10.1186/s13287-020-01661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bucan V., Vaslaitis D., Peck C. T., Strauß S., Vogt P. M., Radtke C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Molecular Neurobiology. 2019;56(3):1812–1824. doi: 10.1007/s12035-018-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li M., Lei H., Xu Y., et al. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6(6):927–935. doi: 10.1111/andr.12519. [DOI] [PubMed] [Google Scholar]
- 130.Fernandes M., Valente S. G., Sabongi R. G., et al. Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration. Neural Regeneration Research. 2018;13(1):100–104. doi: 10.4103/1673-5374.224378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nishibayashi A., Tomita K., Kiya K., Yano K., Hosokawa K. Differentiated adipose-derived stem cells promote the recovery of nociceptor function in rats. Neuroreport. 2016;27(15):1134–1139. doi: 10.1097/WNR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 132.Tse K. H., Novikov L. N., Wiberg M., Kingham P. J. Intrinsic mechanisms underlying the neurotrophic activity of adipose derived stem cells. Experimental Cell Research. 2015;331(1):142–151. doi: 10.1016/j.yexcr.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 133.Li N., Li X., Chen K., Dong H., Kagami H. Characterization of spontaneous spheroids from oral mucosa-derived cells and their direct comparison with spheroids from skin-derived cells. Stem Cell Research & Therapy. 2019;10(1):p. 184. doi: 10.1186/s13287-019-1283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hagner A., Biernaskie J. Isolation and differentiation of hair follicle-derived dermal precursors. Skin Stem Cells. 2013;989:247–263. doi: 10.1007/978-1-62703-330-5_19. [DOI] [PubMed] [Google Scholar]
- 135.Park B. W., Kang D. H., Kang E. J., et al. Peripheral nerve regeneration using autologous porcine skin-derived mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine. 2012;6(2):113–124. doi: 10.1002/term.404. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z., Pradhan S., Liu C., le L. Q. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells. 2012;30(10):2261–2270. doi: 10.1002/stem.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marchesi C., Pluderi M., Colleoni F., et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007;55(4):425–438. doi: 10.1002/glia.20470. [DOI] [PubMed] [Google Scholar]
- 138.Vojnits K., Pan H., Dai X., et al. Functional neuronal differentiation of injury-induced muscle-derived stem cell-like cells with therapeutic implications. Scientific Reports. 2017;7(1):p. 1177. doi: 10.1038/s41598-017-01311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gwam C., Emara A., Mohamed N., Chughtai N., Plate J., Ma X. Amniotic stem cell-conditioned media for the treatment of nerve and muscle pathology: a systematic review. Surgical Technology International. 2021;38 doi: 10.52198/21.sti.38.hr1387. [DOI] [PubMed] [Google Scholar]
- 140.Chen W., Xiao S., Wei Z., Deng C., Nie K., Wang D. Schwann cell-like cells derived from human amniotic mesenchymal stem cells promote peripheral nerve regeneration through a microRNA-214/c-Jun pathway. Stem Cells International. 2019;2019:13. doi: 10.1155/2019/2490761.2490761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chai H. H., Chen M. B., Chen G. Z., et al. Inhibitory effect of TGF-β gene modified human amniotic mesenchymal stem cells on rejection after xenotransplantation of peripheral nerves. European Review for Medical and Pharmacological Sciences. 2019;23(8):3198–3205. doi: 10.26355/eurrev_201904_17678. [DOI] [PubMed] [Google Scholar]
- 142.Su C. F., Chang L. H., Kao C. Y., et al. Application of amniotic fluid stem cells in repairing sciatic nerve injury in minipigs. Brain Research. 2018;1678:397–406. doi: 10.1016/j.brainres.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 143.Sokol J., Lippert T., Borlongan C. V., Stuppia L. Translating amniotic fluid-derived stem cells for transplantation in stroke. Chinese Neurosurgical Journal. 2016;2(1):p. 35. doi: 10.1186/s41016-016-0055-2. [DOI] [Google Scholar]
- 144.Li D., Wang C., Shan W., Zeng R., Fang Y., Wang P. Human amnion tissue injected with human umbilical cord mesenchymal stem cells repairs damaged sciatic nerves in rats. Neural Regeneration Research. 2012;7(23):1771–1778. doi: 10.3969/j.issn.1673-5374.2012.23.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ülger M., Sezer G., Özyazgan İ., et al. The effect of erythropoietin and umbilical cord-derived mesenchymal stem cells on nerve regeneration in rats with sciatic nerve injury. Journal of Chemical Neuroanatomy. 2021;114, article 101958 doi: 10.1016/j.jchemneu.2021.101958. [DOI] [PubMed] [Google Scholar]
- 146.Li J., Gao F., Ma S., et al. Control the fate of human umbilical cord mesenchymal stem cells with dual-enzymatically cross-linked gelatin hydrogels for potential applications in nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2020;14(9):1261–1271. doi: 10.1002/term.3098. [DOI] [PubMed] [Google Scholar]
- 147.Xiao B., Rao F., Guo Z. Y., et al. Extracellular matrix from human umbilical cord-derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration. Neural Regeneration Research. 2016;11(7):1172–1179. doi: 10.4103/1673-5374.187061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drela K., Lech W., Figiel-Dabrowska A., et al. Enhanced neuro-therapeutic potential of Wharton's Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy. 2016;18(4):497–509. doi: 10.1016/j.jcyt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 149.Park J. W., Kang Y. D., Kim J. S., Lee J. H., Kim H. W. 3D microenvironment of collagen hydrogel enhances the release of neurotrophic factors from human umbilical cord blood cells and stimulates the neurite outgrowth of human neural precursor cells. Biochemical and Biophysical Research Communications. 2014;447(3):400–406. doi: 10.1016/j.bbrc.2014.03.145. [DOI] [PubMed] [Google Scholar]
- 150.Sun T., Ma Q. H. Repairing neural injuries using human umbilical cord blood. Molecular Neurobiology. 2013;47(3):938–945. doi: 10.1007/s12035-012-8388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen H., Zhang Y., Yang Z., Zhang H. Human umbilical cord Wharton's jelly-derived oligodendrocyte precursor-like cells for axon and myelin sheath regeneration. Neural Regeneration Research. 2013;8(10):890–899. doi: 10.3969/j.issn.1673-5374.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin Y. J., Lee Y. W., Chang C. W., Huang C. C. 3D spheroids of umbilical cord blood MSC-derived Schwann cells promote peripheral nerve regeneration. Frontiers in Cell and Development Biology. 2020;8, article 604946 doi: 10.3389/fcell.2020.604946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan D., Chang X., Xu M., et al. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. Journal of Chemical Neuroanatomy. 2019;96:134–139. doi: 10.1016/j.jchemneu.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Yamazaki A., Obara K., Tohgi N., et al. Implanted hair-follicle-associated pluripotent (HAP) stem cells encapsulated in polyvinylidene fluoride membrane cylinders promote effective recovery of peripheral nerve injury. Cell Cycle. 2017;16(20):1927–1932. doi: 10.1080/15384101.2017.1363941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Amoh Y., Hamada Y., Aki R., Kawahara K., Hoffman R. M., Katsuoka K. Direct transplantation of uncultured hair-follicle pluripotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. Journal of Cellular Biochemistry. 2010;110(1):272–277. doi: 10.1002/jcb.22534. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y., Wang W. T., Gong C. R., Li C., Shi M. Combination of olfactory ensheathing cells and human umbilical cord mesenchymal stem cell-derived exosomes promotes sciatic nerve regeneration. Neural Regeneration Research. 2020;15(10):1903–1911. doi: 10.4103/1673-5374.280330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pisciotta A., Bertoni L., Vallarola A., Bertani G., Mecugni D., Carnevale G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regeneration Research. 2020;15(3):373–381. doi: 10.4103/1673-5374.266043. [DOI] [PMC free article] [PubMed] [Google Scholar]


