Abstract
The levels of reproduction-associated hormones in females, such as estrogen, progesterone, prolactin, and oxytocin, change dramatically during pregnancy and postpartum. Reproduction-associated hormones can affect adult hippocampal neurogenesis (AHN), thereby regulating mothers' behavior after delivery. In this review, we first briefly introduce the overall functional significance of AHN and the methods commonly used to explore this front. Then, we attempt to reconcile the changes of reproduction-associated hormones during pregnancy. We further update the findings on how reproduction-related hormones influence adult hippocampal neurogenesis. This review is aimed at emphasizing a potential role of AHN in reproduction-related brain plasticity and its neurobiological relevance to motherhood behavior.
1. Introduction
During pregnancy, the levels of reproduction-related hormones, including human chorionic gonadotropin (hCG), estrogen, progesterone, relaxin, prolactin (PRL), and oxytocin (OT), exhibit sharp variations [1–12] (Figure 1). Striking changes in cognition and behavior also occur during pregnancy and postpartum, involving multiple neuropsychological aspects such as learning, memory, mood, and childcare. Studies in animal models such as rats, mice, and sheep have shown that hormonal changes during pregnancy and postpartum may significantly modulate the process of neuroplasticity, including adult neurogenesis, in many brain regions such as the hypothalamus and hippocampal formation [13–17].
Figure 1.
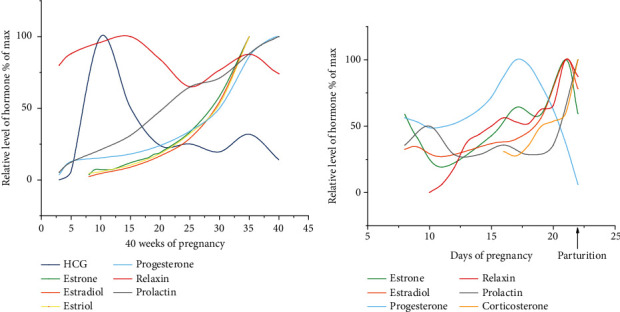
Schematic illustration of the changes in the levels of serum hCG [3], estrone [4], estradiol [4], estriol [4], progesterone [5], relaxin [6], and prolactin (PRL) [5, 7] during gestational weeks 6-40 in humans according to literature as indicated (the numbers in the x-axis indicate the gestational week). Blood samples were obtained through venipuncture of pregnant human females, and the diagnosis of pregnancy was confirmed by urine hCG determination and/or ultrasonography. HCG was determined via the double-antibody beta radioimmunoassay. Estrogens (estrone, estradiol, and estriol) were determined with an antibody against estrone-17-oxime coupled with bovine serum albumin. Progesterone was measured via the Bayer ADVIA Centaur assay. Relaxin was measured via radioimmunoassay with iodine 125-labeled polytyrosyl-relaxin and rabbit anti-porcine relaxin serum R6. PRL was determined via radioimmunoassay with homologous double antibodies. (b) Summary of the changes in the levels of estrone [8], estradiol [8], progesterone [9], relaxin [10], PRL [11], and corticosterone [12] in rat from gestational days 8-22 according to existing reports as indicated (the x-axis indicates the gestational days). The samples were derived from the peripheral blood of primiparous Sprague-Dawley rats (estrone, estradiol, progesterone, relaxin, and PRL) or the arterial blood of Wistar rats (corticosterone). Estrone and estradiol were determined via radioimmunoassay with estradiol-17β antiserum. Progesterone was quantified by protein-binding displacement. Relaxin was measured via the interpubic ligament bioassay. PRL was determined via radioimmunoassay. Corticosterone was measured using the fluorometric method.
Adult neurogenesis refers to the proliferation and differentiation of neural stem cells or neuron precursor cells into new neurons in the adult brain [18, 19]. So far, adult neurogenesis is considered to occur principally in the subventricular zone (SVZ) in the wall of the lateral ventricle and subgranular zone (SGZ) in the dentate gyrus of the hippocampal formation [20, 21], which has been known to play an essential role in olfactory and spatial learning and memory. Adult hippocampal neurogenesis (AHN) appears also important for other cognitive functions, mood regulation, and behavioral processes in normal conditions. Importantly, impairment of AHN is related to the pathogenesis of many psychiatric disorders and neurological diseases affecting different age groups [22–24].
The role of adult neurogenesis in shaping neuroplasticity thereby modulating reproduction or motherhood-related cognition and motherhood behavior is a topic of broad interest involving both the basic and clinical neuroscience fields. However, researches in the front remain fairly limited, with data from a number of animal species available. This review is aimed at updating the current understanding regarding the potential effects of reproductive hormones on adult hippocampal neurogenesis. We first briefly introduce the general functional significance of AHN and the methods commonly used to explore its role in vivo. Next, we attempt to reconcile the changes of reproduction-associated hormones during pregnancy based on animal and human studies. We further update the findings on how various reproduction-related hormones may influence AHN. Overall, this review attempts to emphasize a potential neurobiological role of AHN in reproduction-related brain plasticity that may modulate motherhood behavior.
2. Functional Studies on Adult Hippocampal Neurogenesis
2.1. Functional Significance of Adult Hippocampal Neurogenesis
AHN is considered to underlie numerous hippocampus-dependent fuctions, including learning and memory, spatial navigation, new memory formation, and old memory forgetting functions [25, 26]. The newly generated granule cells may contribute to significant structural and functional plasticity in the trisynaptic hippocampal circuits in response to internal and external stimuli thereby also fulfilling new functional demands [27].
AHN can be alteredin the context of stress, physical training, changes in the levels of multiple hormones, sleep deprivation, and even some diseases, excitement has been fueled regarding whether and how the neonatal neurons integrate into the relevant neural circuits and influence action and whether neurogenesis improves hippocampus-dependent memory and learning. Most studies to date have demonstrated that adult neurogenesis enhances hippocampal-dependent functions [26, 28]. For instance, several approaches have been adopted to assess alterations in spatial learning and memory, including low-dose irradiation, antimitotic treatment technology, and genetic modification to ablate adult newborn neurons. Mice submitted to these procedures exhibited pronounced impairment of certain cognitive functions after the adult newborn neurons were eliminated when the ablation was performed after memory formation [29, 30]. Zhang et al. treated mice in the postpartum estrogen withdrawal (EW) group with anti–N-methyl-D-aspartate receptor stimulants and the resting mice with inhibitors; the former exhibited a significant increase in BrdU-immunolabeled-positive cells coupled with an increased rate of depression and anxiety, whereas the latter did not [31]. The excitability and synaptic plasticity of the CA1 and CA3 regions of the hippocampus were enhanced when the estrogen level reached its maximum (proestrus), at which point the proliferation of hippocampal dentate gyrus cells increased [32]. Berdugo-Vega et al. reported that increasing neurogenesis refines hippocampal activity, thereby rejuvenating navigational learning strategies and contextual memory throughout life [29]. Furthermore, senescence is an important negative biological factor in hippocampal adult neurogenesis. Studies have shown that aging-associated loss of cognition can be reversed by increasing adult hippocampal newborn neurons [33]. In addition to aging, growing evidence has indicated that aging-related neurological diseases such as Alzheimer's and Parkinson's disease may impair adult hippocampal neurogenesis. Radad et al. demonstrated that adult neurogenesis has great therapeutic potential for the treatment of Parkinson's disease and Alzheimer's disease [34]. Additionally, depression and anxiety are also associated with adult hippocampal neurogenesis. Research has indicated that both hippocampal volume and adult hippocampal neural progenitor cells decreased in patients with depression, and antidepressant treatment increased the volume of the adult hippocampal dentate gyrus and the number of hippocampal neural progenitor cells [35].
Conversely, some studies have indicated that hippocampus-dependent memory and cognition did not decline or exhibit any observable changes in the context of increased hippocampal neurogenesis. For example, no effect on learning and memory was observed when adult newborn neurons were ablated by X-ray radiation or/and genetic ablation [26]. In contrast with the view that synaptic plasticity is positively correlated with learning ability, cell proliferation increases in the preestrus period, whereas the spatial learning ability decreases [36]. Estrogen improves the survival rate of newborn neurons; however, it impairs spatial learning and memory [37]. Some studies have shown that extremely high levels of cell proliferation may disrupt the neural activity of the hippocampus and interfere with the normal function of the hippocampal dentate gyrus, which is likely due to the lower activation thresholds and higher resting potential of newborn neurons compared with mature neuron synapses [38, 39]. As such, the signal-to-noise ratio of the hippocampal dentate gyrus is reduced, making it difficult to detect excitatory signals [40]. Additionally, accumulating evidence suggests that dysregulation of adult hippocampal neurogenesis may be associated with cognitive decline in neurological disorders and psychological symptoms in psychiatric disorders. Several studies have demonstrated that epileptic patients have an increased number of new neurons in the brain and weakened hippocampus-dependent task performance [41, 42]. These studies indicated that adult-born neurons generated by seizure activity exhibit aberrant cell migration, morphogenesis, and synaptic integration through several signaling pathways and eventually establish recurrent networks. Therefore, seizure-induced enhanced adult neurogenesis substantially reorganizes the local neural network in the dentate gyrus and may impair cognitive functions.
In recent years, although significant progress has been made in the functional study of adult neurogenesis, the understanding of how these diseases interact with adult hippocampal neurogenesis remains very limited. Therefore, although demonstrating the correlations between adult hippocampal neurogenesis and various neuropsychiatric disorders has garnered increasing interest, achieving this goal represents a considerable challenge. One major limitation of studying adult hippocampal neurogenesis in humans is the inability to access live samples. However, some signs of a connection have been elucidated from existing animal models. In these studies, newborn adult neurons would be partly integrated into the neural network to influence neuronal activity; however, the functional significance of adult newborn neurons is not entirely determined by the number of new neurons. Therefore, more in-depth and comprehensive functional studies of adult neurogenesis are required to establish a correlation between the activity of distinct brain circuits, cell types, and cognition.
2.2. Methods for the Functional Characterization of Adult Hippocampal Neurogenesis
Hippocampal adult neurogenesis has recently been demonstrated to occur in the adult brain, and it has been proposed to participate in a myriad of behavioral responses, both in physiological states and in the context of neuropsychiatric disorders. However, current studies have not conclusively determined whether adult-born neurons provide important contributions to hippocampal function and, ultimately, how they impact behavior. Disrupting these newborn neurons combined with the evaluation of behavioral changes in animals provides a powerful approach to investigate the function of adult neurogenesis. Common methods for the ablation of newborn neurons include X-ray radiation, genetic ablation, and mitotic inhibitor ablation.
For instance, by utilizing acute or fractionated X-ray irradiation (2 Gy) in mice at postnatal day 21, Peng et al. observed impairments of proliferation and neurogenesis in the subgranular zone of the dentate gyrus but insignificant cognitive dysfunction in subsequent behavioral tests [43]. In another study, using X-ray radiation to inhibit neurogenesis, the relationship between neurogenesis and morphine-induced addiction-related learning and memory was detected [44, 45]. Specifically, the authors reported that reduced neurogenesis caused morphine-related reward memory either to fade or to increase in persistence. These differences in outcomes may be attributed to the morphine dose, the radiation level, and the timing of the detection. Mitotic inhibitors such as cytosine-β-D-arabinofuranoside (AraC) could inhibit hypothalamic neurogenesis and could therefore be exploited to observe changes in the behavior of model animals in the context of decreased neurogenesis. Compared to the controls, pups infused with AraC displayed reduced sleep duration, increased sleep interruptions, and a sleep-wake rhythm similar to that of aging mice, favoring the emerging concept that decreased hypothalamic neurogenesis could induce sleep/wake disturbances and senescence-generated sleep disorders [46]. Additionally, genetic ablation is gaining popularity as a tool to characterize the mechanisms of disease onset. Houben et al. evaluated the neurogenesis of tau-expressing disease models and tau-knockout mice and linked tau pathology in the granule cells of the dentate gyrus with the suppression of adult hippocampal neurogenesis, supporting the view that reducing tau expression may promote injury suppression [47]. Given that the role of newborn neurons in the development of chronic epilepsy and related cognitive deficits remains somewhat vague, a study used Nestin-δ-HSV-thymidine kinase-EGFP (Nestin-TK) transgenic mice to ablate adult newborn neurons prior to the onset of acute epileptic seizures in mice. The authors found that neuronal ablation decreased the frequency of the seizures, suggesting that abnormal neurogenesis may contribute to epilepsy [41]. The CaM/Tet-DTA transgenic mouse model of hippocampal cell loss has been utilized to examine the impact of neuronal loss on endogenous neurogenesis in aged animals [48]. Moreover, the ablation of neurogenesis regulatory proteins can also reduce neurogenesis. For example, activator protein 2γ (AP2γ), which exists in a subpopulation of hippocampal transiently expanded progenitor cells, acts as a modulator of adult hippocampal glutamatergic neurogenesis in mice. The ablation of activator protein 2γ can significantly reduce hippocampal neurogenesis and disrupt neural continuity between the ventral hippocampus and the medial frontal cortex [49].
Some studies have indicated that various strategies to ablate neurogenesis produce a limited impairment in some hippocampal-dependent learning and memory tasks. Moreover, due to the lack of spatial and cellular specificity of most ablation techniques, it is difficult to ascertain whether the consequent behavioral effects were caused by ablation of neurogenesis or other impairments [50]. To circumvent these problems, our group was the first to utilize iodine-125 seed brachytherapy for neuron ablation in the hippocampus (Figure 2). This procedure not only is simpler and safer than conventional X-ray ablation but also has greater spatial specificity and produces less trauma than other mitotic inhibitors and genetic neuron ablation. We not only confirmed that 125I brachytherapy could ablate newborn neurons in the hippocampus but also evaluated its effect on the ablation of hippocampal adult neurogenesis under different doses, time, and space (unpublished work).
Figure 2.
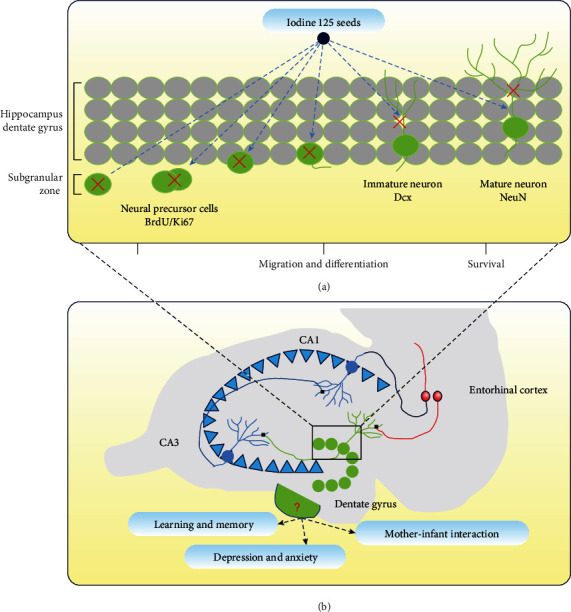
Schematic diagram of 125I brachytherapy for ablation of hippocampal neonatal neurons (a), functional changes in hippocampus (b). Red crosses in (a) indicate ablation of newborn neurons.
3. Effect of Reproduction-Associated Hormones on Adult Hippocampal Neurogenesis
3.1. Estrogen
3.1.1. Distribution of Estrogen and Its Receptors in the Brain
Estrogen is involved in numerous physiological processes including the development and maturity of follicles and female sex organs, the formation and differentiation of breast acini and milk production, the maintenance of female secondary sexual characteristics, the movement and maturation of eggs in the fallopian tube, and the metabolism of the endometrium and smooth muscle. Estrogen is in fact a group of female sex hormones, which encompasses three major endogenous hormones: estrone, estriol, and estradiol [1]. Estrogen is mainly synthesized by the corpus luteum of the ovary and the fetal placental unit. Its levels increase continuously from the onset of pregnancy until just prior to labor, after which they decrease sharply and remain low during lactation [51].
Estrogen receptors are comprised of nuclear estrogen receptors (ERα and ERβ) and membrane estrogen receptors (GPER1, ER-X, and Gq-mER) [52]. ERα (with a molecular weight of 67 kDa) and ERβ (with a molecular weight of 60 kDa) are located in the cytoplasm when inactive and are distributed throughout the brain in the hippocampus, hypothalamus, amygdala, and preoptic area [53]. GPER1, a membrane-bound G protein-coupled estrogen receptor mainly located on the plasma membrane, was originally identified in the endoplasmic reticulum of neurons and was found to be ubiquitous in various cortical areas including the hippocampus, olfactory bulb, hypothalamus, motor and somatosensory centers, piriform cortex, and the nucleus of the epithalamus, particularly in the CA3 area of female rats [54, 55]. Unlike ERα and ERβ, GPER1 mainly mediates estrogen to activate MAPK/ERK and other signaling pathways [52]. ER-X (with a molecular weight of 63 kDa), which was detected during research on the development of the cerebral neocortex, shows high affinity to the isomers of E2, 17α-E2, and 17β-E2 compared with other estrogen receptors [56]. Gq-mER was first discovered in the arcuate nucleus of the hypothalamus and promoted estrogenic activity associated with hypothalamic reproduction and energy homeostasis [52, 57]. So far, ER-X and Gq-mER have only been reported to be distributed in the cerebral cortex and the hypothalamus.
Estrogens (estradiol and estrone) regulate multiple cellular processes including cell proliferation and survival, which underlies their impact on neurogenesis [31, 60, 61]. Their regulatory effects are related to the time and dosage of the estrogen treatment, as well as the age and sex of the subjects (Table 1). Despite many reports on the effect of estrogen on adult hippocampal neurogenesis in female mammals, data on its effect on male mammals are scarce [64, 68]. However, the available data has indicated that estradiol has a slight influence on hippocampal neurogenesis in males. The sections below provide an overview of the correlation between endogenous and exogenous estrogens and adult hippocampal neurogenesis.
Table 1.
Effects of different estrogen and progesterone treatments on the adult hippocampal neurogenesis of mammals.
| Treatment | Model organism | Neuron proliferation | Neuron survival | Reference |
|---|---|---|---|---|
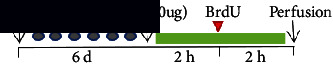
|
SD rats | ↑ | [58] | |
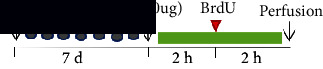
|
↑ | |||
|
SD rats | ↔ | [59] | |
|
↔ | |||

|
SD rats | ↓ | [60] | |
|
SD rats | ↑ | [61, 62] | |
|
SD rats | ↑ | [61, 63] | |
|
↑ | |||
|
SD rats | ↑ | ↓ | [64] |
|
SD rats | ↔ | ↑ | [65] |
|
↔ | ↓ | ||
|
SD rats | ↔ | ↓ | [66] |
|
↔ | ↔ | ||
|
Wistar rats | ↔ | ↔ | [67] |
|
ICR mice | ↓ | ↓ | [31] |
|
↔ | |||
|
↑ | ↑ | ||
|
↑ |
Note: “OVX” indicates bilateral ovarian resection; the green bar indicates estrogen or estradiol benzoic acid; the red bar indicates estrone; the orange bar indicates progesterone; the purple bar indicates benzoic acid and progesterone. The up arrows indicate upregulation, the down arrows indicate downregulation, and the horizontal bars indicate no change. Abbreviations: E2: estradiol; BrdU: bromodeoxyuridine; EB: estradiol benzoate; FST: forced swimming test; P4: progesterone; SD rats: Sprague-Dawley rats.
3.1.2. Endogenous Dynamic Estrogen and Adult Hippocampal Neurogenesis
Natural fluctuations in ovarian hormones persist throughout the female lifecycle and are particularly visible in women's menstrual cycle, reproductive stage, and aging transition.
The estrous cycle of mammals is divided into four stages: proestrus, estrus, late estrus, and diestrus. Hormone level variations exist at each stage. In the proestrus period, serum estrogen and progesterone levels increase and peak, whereas they decrease in the diestrus period [69]. Hormone levels can affect the proliferation of neurons in the dentate gyrus of the hippocampus, which in turn influences adult hippocampal neurogenesis. During the proestrus period, increased cell proliferation was observed in the hippocampal dentate gyrus of rats [58, 70, 71], whereas the cell proliferation rate decreased during the diestrus. Conversely, studies in mice showed only a minor change in cell proliferation in each estrus stage [70], which was likely because the ovarian hormones only fluctuated for a few hours and the fluctuation was not captured. Additionally, some studies have characterized the seasonal and nonseasonal breeding of prairie voles and have demonstrated that estrogens have different effects on neurogenesis at different periods in their lifecycle [72].
Diminished estrogen secretion is strongly correlated with decreased gonadal function in female menopause [73]. In aging female rats, the estrus cycle becomes irregular and prolonged from midlife onwards and eventually reaches the noncirculatory stage of a continuous estrus interval where the proliferation and survival rates of neurons decrease [74, 75], indicating that the decline in neurogenesis in aging rats is related to the natural reduction in estrogen secretion. The influence of endogenous ovarian hormone fluctuations on cell proliferation is highly debated, and studies on the influence of endogenous ovarian hormone fluctuations on newborn neuron survival are scarce.
Taken together, current findings indicate that endogenous estrogen could promote the proliferation of hippocampal dentate gyrus nerve cells. However, in-depth research on the effect of single-estrogen treatment on neurogenesis is needed to eliminate the potential confounding effects of other hormones.
3.1.3. Exogenous Estrogen and Adult Hippocampal Neurogenesis
(1) Effect of Estrogen on the Proliferation of Hippocampal Dentate Gyrus Cells. Adult bilateral ovariectomized (OVX) animal models were used to investigate the effects of estrogen on neurogenesis. Compared with the sham operation group, the serum estradiol level in the short-term OVX rats decreased sharply within 24 hours, with a concomitant decline in cell proliferation and the number of newly born immature neurons [31, 76], which could be reversed by acute exposure to estrogens [58, 59]. In contrast, no observable change in neuronal proliferation was detected after long-term estrogen deprivation (4 weeks) in rats and mice by ovariectomy and subsequent high-dose estrogen treatment [59], possibly owing to the presence of locally synthesized estrogen in the hippocampus leading to the resumption of cell proliferation. There is evidence that topical estradiol application affects hippocampal cell proliferation in 5-day-old rats [77]; however, several studies in which little or no estradiol was detected in the hippocampus of adult rats 2-3 weeks after ovariectomy deny this hypothesis. Goel et al. indicated that the decline of estrogen, which could stimulate the hypothalamic-pituitary-adrenal axis, may lead to a decrease in cortisol. This in turn inhibits cell proliferation, resulting in an increased proliferation of neural cells [78], and therefore, the limited alteration in the cell proliferation rate observed after long-term estrogen deprivation might have been due to the involvement of cortisol [79]. Additionally, the serotonin in the hippocampus may also have important effects [80]. Cell proliferation was unaffected after long-term estrogen deprivation and subsequent short-term high-dose estrogen treatment, which was likely due to the decreased sensitivity to estrogen in the neuron precursor cells.
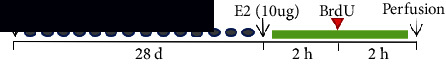
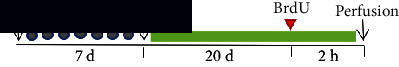
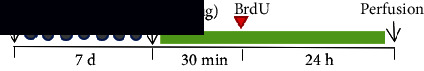
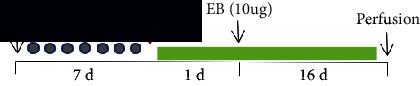
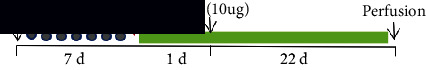
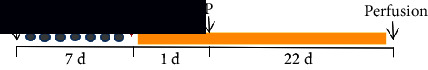
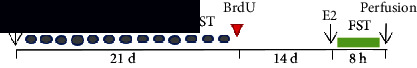
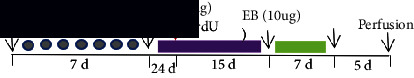
Further, the duration of estrogen exposure also plays a regulatory role in hormone activity. Seven days after bilateral ovariectomy, estradiol and estrone (10 μg) could increase the cell proliferation rate after exposure for 30 minutes and 2 h, whereas the cell proliferation rate slightly increased after exposure to estradiol benzoic acid (EB) for 4 h [58, 59, 61, 63]. Ormerod et al. found that cell proliferation increased in female prairie voles after acute EB administration for 4 hours but was inhibited 48 hours later [60]. Seven days after bilateral ovariectomy, rats were exposed to EB for 20 days. Subsequently, they were injected with BrdU for 2 hours and showed no change in proliferation. The same result was reported under long-term exposure to various estrogens [31, 65, 66]. As previously noted, the inhibition of cell proliferation is mediated by the stimulation by estrogen with adrenal steroids. Zhang et al. established a hormone-simulated pregnancy (HSP) mouse model. Seven days after bilateral ovariectomy, the HSP postpartum estrogen nonwithdrawal group and the postpartum EW group were exposed to both EB (0.5 μg/day) and progesterone (0.8 mg/day) for 16 days [31]. The EW group was treated with EB for 7 consecutive days, and samples were taken after 5 days of survival (day 28). In contrast, the HSP group was treated with EB for 12 consecutive days (day 28). The expression of S28dBrdU+ was downregulated in the EW group compared to the control, whereas the expression of S10dBrdU+ remained unchanged. The expression of both S28dBrdU+ and S10dBrdU+ in the HSP group was upregulated, indicating that postpartum EW could impair hippocampal neurogenesis in mice. We observed that the rapid withdrawal of estrogen may lead to the massive death of newborn neural progenitor cells. In other cases, estradiol could stimulate cortisol secretion, thereby restraining cell proliferation. In summary, short-term exogenous exposure to estrogen stimulates the proliferation of neurons, whereas long-term exposure has negligible or even inhibitory effects.
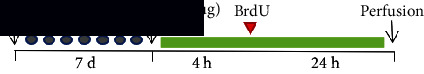
In addition to exposure duration, estrogen species is another factor that influences neuronal proliferation. In female rats, proliferative activity increased after exposure to 17α-estradiol, 17β-estradiol, and estrone for 30 minutes but remained constant after exposure to EB, which was likely due to the slower metabolism of EB in comparison to other estrogens [61, 81].
Additionally, the effect of estrogen on the proliferation of neurons is dose-dependent. An injection of 0.3 μg or 10 μg of estradiol is equivalent to the circulating serum levels of estradiol in the diestrus and proestrus stages, respectively [82]. This treatment could decrease cell proliferation rates after ovariectomy, thus reverting them to their preovariectomy rate [61]. In contrast, a medium dose of 1 μg or a high dose of 50 μg was generally ineffective in restoring the cell proliferation rate [59, 61].
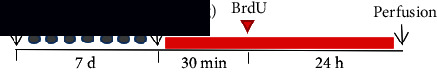
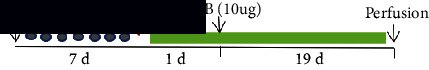
(2)Effect of Estrogen on the Survival of Hippocampal Dentate Gyrus Cells. Neural cell proliferation refers to the production of newborn neurons, whereas neuronal survival emphasizes the long-term process by which neurons are generated, differentiate, and mature. However, most studies have thus far focused primarily on neuron proliferation, whereas neuron survival has remained largely understudied. Neuronal survival was reduced in OVX rats that were bilaterally ovariectomized (day 0), labeled with BrdU (day 6), exposed to EB (day 7) for 16 consecutive days, and then perfused [64]. The same conclusion was reached after 22 days of continuous exposure to EB [66]. In another study, 7 days after bilateral oophorectomy, rats were exposed to estradiol or estrone for 19 consecutive days and BrdU was injected after 24 hours of hormone exposure [65]. The results showed a diminished proliferation rate in rats exposed to estrone, which was likely due to the difficulty of surviving in an estrone-rich environment [83]. Additionally, Zhang et al. reported that the survival rate of neurons rose in OVX mice exposed chronically to EB and progesterone but decreased in an estradiol retreatment group [31]. It is worth noting that the hormone affected both neuron proliferation and survival after estrogen exposure, whereas it only affected the survival of neurons produced prior to hormone exposure. In summary, the survival of newborn neurons is inhibited before estradiol treatment but enhanced thereafter due to an increased proliferation rate [64–66], showing that an estradiol-rich environment is ideal for the survival of neurons produced under the stimulation of this hormone. This was further validated by the decline in neuronal survival upon rapid postpartum estradiol retreatment in HSP. Additionally, prior behavioral tests or spatial training of subjects may interfere with the proliferation and survival of nerve cells, which should be taken into consideration when designing future experiments [65, 67].
3.2. Progesterone
3.2.1. Distribution of Progesterone and Its Receptors in the Brain
Progesterone is a reproduction-associated steroid hormone that is mainly produced by the corpus luteum in the first 8 weeks of pregnancy and secreted by the placenta at 8-12 weeks. Importantly, this hormone can coordinate multiple estrogen-mediated physiological and behavioral processes. During pregnancy, the progesterone level rises continuously, peaks within 4 weeks before delivery, and rapidly decreases after delivery or in the third trimester [69, 84]. Progesterone regulates the smooth muscle of the uterus, reduces its sensitivity to OT, and ensures the normal growth and development of fertilized eggs [1]. PRA and PRB are representative progesterone nuclear receptors, which are broadly distributed in several brain regions including the hippocampus, the frontal cortex, the hypothalamus, the cerebellum, the medial inner side of the amygdala, and the bed nucleus of the stria terminalis [85]. Another class of progesterone receptors called membrane progesterone receptors (mPRs) include mPRα, mPRβ, and mPRγ [86, 87], among which mPRα is the most pharmacologically active and enriched in the olfactory bulb, cortex, hypothalamus, hippocampus, and cerebellum. In contrast, mPRβ is mainly distributed in the hippocampus, hypothalamus, midbrain, and forebrain of mammalian brains [88]. Additionally, the b5-like heme/steroid binding protein family includes progesterone membrane receptor module 1 (PGMRC1) [89], which is distributed in the forebrain and regulates neuroendocrine function, as well as the hippocampus, cortex, and cerebellum [90].
3.2.2. Progesterone and Adult Hippocampal Neurogenesis
Progesterone regulates spinal cord production, synaptogenesis, neuron survival, and dendritic growth and plays a neuroprotective role in animal models of neurodegenerative diseases and acute brain damage [85, 91]. However, few studies have thus far characterized the effects of progesterone on the proliferation and survival of nerve cells. Previous studies have revealed that in vitro treatment with progesterone may increase the proliferation of hippocampal nerve cells in female rats in a dose-dependent manner [92]. The same is true for tetrahydroprogesterone, a progesterone metabolite [93]. Additionally, exposure to progesterone (4 mg/kg) for 1 hour could increase cell proliferation in the granulosa cell layer of the hippocampal dentate gyrus in OVX rats [90, 93]. However, with prolonged exposure to progesterone (chronic treatment), cell proliferation and survival remained constant in OVX rats, and female OVX rats showed almost no change in neurogenesis after long-term administration (for 21 days) of progesterone (1 or 4 mg/kg) or medroxyprogesterone acetate (MPA) [66]. Brinton et al. found that exposing 18-day-old rat embryos to progesterone alone for 24 hours could induce a 20% increase in rat hippocampal neurons [87], suggesting that treatment with progesterone alone may promote the proliferation of hippocampal neural progenitor cells similar to tetrahydroprogesterone. Zhang et al. exposed mice to EB and progesterone for 17 consecutive days, treated them with EB alone for 5 days to establish an HSP model [31] (Table 1), and found that the number of BrdU+ cells did not decrease in the HSP mice, indicating that progesterone withdrawal did not lead to the death of immature newborn neurons. Instead, EB treatment might diminish the number of mature newborn neurons in the brain of mice in the HSP postpartum EW group, which remains to be confirmed. Additionally, treating male rats with progesterone for 7 consecutive days could increase the cell proliferation in the dentate gyrus of the hippocampus but had no detectable effect on the survival rate [94]. Male mice were exposed to progesterone for 3, 7, 28, and 56 days, but the survival rate of hippocampal dentate gyrus cells only increased after 3 days of treatment with progesterone, indicating that progesterone increased the survival rate of relatively young newborn neurons [95].
During the menstrual period, estrus cycle, and reproductive period, progesterone and estrogen exhibited similar dynamic changes. Given that progesterone mediates the effect of estrogen on neurogenesis, scholars prefer to use estrogen and progesterone in combination to observe changes in neurogenesis. Tanapat et al. inhibited the estradiol-induced proliferation of hippocampus dentate gyrus cells in adult female ovariectomized rats through 2 injections of estradiol (10 μg each) with an interval of 2 hours, followed by progesterone treatment after 48 hours [59]. Progesterone and BrdU were injected two days after the female rats were injected with 17β-estradiol or EB (10 μg), which inhibited estradiol-induced cell proliferation [59, 90]. When estradiol and progesterone were injected simultaneously into the OVX rats, the increase in cell proliferation induced by estradiol was not blocked but was lower than the level of cell proliferation induced by the injection of estradiol alone [93]. Although chronic MPA treatment had no observable effect on the neurogenesis of the female hippocampal dentate gyrus, the injection of MPA combined with EB (10 μg) for 21 days reduced the cell survival rate of adult female rats [66]. The proliferation of hippocampal cells in the OVX rats increased after exposure to a high estradiol dose but was inhibited upon exposure to a progesterone antagonist. Simultaneous acute exposure to estradiol and progesterone would not lead to synergistic effects [93]. Importantly, progesterone alone could enhance the proliferation of nerve cells, while its combination with other sex hormones would have an inhibitory effect [59, 92].
In the context of cerebral ischemia and acute brain injury, not only could progesterone inhibit brain injury-induced cell proliferation in male rats, but it also enhanced the survival of newborn neurons inhibited by ischemia [96]. Additionally, progesterone treatment after traumatic brain injury could restore cell proliferation and cell death to normal levels in the dentate gyrus of the hippocampus [94].
The use of progesterone alone has few negative effects on neurogenesis, whereas it may serve as a suppressor when combined with other hormones. Regardless, the effects of progesterone (alone or combined with other hormones) on neurogenesis seem to correlate with the concentration of progesterone, the sex of the subject, and the hormone treatment time; however, this remains partly ambiguous and requires further investigation.
3.3. Prolactin (PRL)
3.3.1. Distribution of PRL and Its Receptors in the Brain
PRL belongs to a hormone family that includes growth hormone and placental PRL and is synthesized and secreted by anterior pituitary cells as well as extrapituitary regions such as the breast, placenta, and uterus [97]. This pleiotropic pituitary hormone is known to have more than 300 physiological effects and has a regulatory role in reproduction, immune regulation, angiogenesis, energy metabolism, osmotic balance, and development [98, 99]. For instance, PRL could promote the proliferation of breast cells, stimulate lactation and the synthesis of steroid hormones, accelerate bone metabolism, and regulate the composition of amniotic fluid and osmotic pressure in the uterus. It also plays a fundamental role in promoting the secretion of progesterone and the maturing of follicles [100]. The PRL level increases early in pregnancy, rises rapidly just before and immediately after delivery, and remains high under the stimulation of breastfeeding [101].
PRL receptors exist in many nuclei and subregions of the hippocampus, SVZ, ventricular choroid plexus, and hypothalamus (including the anterior medial nucleus, ventrolateral preoptic nucleus, supraoptic nucleus, paraventricular nucleus, periventricular nucleus, dorsal medial hypothalamic nucleus, ventromedial hypothalamus, and arcuate nucleus) [102, 103]. Moreover, the expression level of PRL receptor genes changes during different periods. For instance, it is significantly upregulated during pregnancy and lactation [104].
3.3.2. PRL and Adult Hippocampal Neurogenesis
PRL plays numerous roles in the central nervous system. To cite a few examples, this hormone induces parenting behaviors in females, stimulates OT neurons, promotes appetite and food intake, facilitates the secretion of ACTH in response to stress, inhibits fertility, and protects neurogenesis under stress [105–108].
PRL is generally thought to serve as a neurogenesis activator in SVZ neurogenesis [100, 109]. However, little correlation was initially observed between PRL and hippocampal neurogenesis. On the 7th day of pregnancy and the 7th day after delivery (under stress), PRL could enhance the proliferation of SVZ cells but had little effect on the proliferation of hippocampal dentate gyrus cells [110], which was possibly due to the method of PRL administration or dose. Later, Lajud et al. found that injecting PRL into rats for 14 consecutive days at the immediate postnatal stage could reduce the neurogenesis of the dentate gyrus and olfactory bulb and aggravate the depressive state of adult male and female rats, thus demonstrating that the effect of PRL on neurogenesis depends on the age of the animal and that PRL is involved in mood regulation [111]. Conversely, other authors have proposed that PRL plays a protective role in adult hippocampal neurogenesis. Torner et al. demonstrated that PRL not only protected hippocampal neurogenesis from stress or glucocorticoids by acting as a regulator of brain cell proliferation but also could perform its functions without perturbing the cell proliferation of the hippocampal dentate gyrus under stress-free conditions [102, 112]. PRL counteracts the adverse effects of glucocorticoids on hippocampal neurogenesis through PRL receptor-mediated mechanisms, and this might be the mechanism by which PRL protects adult neurogenesis during chronic stress [102]. Mak et al. demonstrated that PRL injection into the lateral ventricle of male mice could increase the proliferation of neuron precursor cells in the dentate gyrus and the PRL signal may be associated with parent identification and enhanced neurogenesis in the olfactory bulb and hippocampus [113]. Together with the findings of Wagner et al., the aforementioned results suggested that PRL affects hippocampal neurogenesis through indirect mechanisms [114]. Walker et al. extended prior research and found that exogenous PRL treatment could increase the number of hippocampal dentate gyrus neuron precursor cells both in vivo and in vitro [115]. Compared with the control, PRL gene-deficient mice exhibited a reduced number of hippocampal neuron precursor cells, which impaired hippocampal-dependent learning and memory, confirming that PRL could promote the division of neuron precursor cells in the hippocampal dentate gyrus.
PRL binds to its receptors in the hippocampus to regulate neurogenesis [102, 115]. Most studies have demonstrated that PRL either promotes or has no effect on hippocampal neurogenesis, and only few studies have indicated that PRL functions as an inhibitor. These contradictions may be due to differences in the PRL dose, the age of the subject, and the route of administration, among other factors. Overall, these findings provide preliminary evidence that PRL promotes hippocampal neurogenesis to some extent and influences mood disorders such as depression and anxiety.
3.4. Human Chorionic Gonadotropin (hCG)
hCG, a glycoprotein secreted by the trophoblast cells of the placenta, is comprised of α and β dimers, of which the α subunit is similar to the follicle-stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH) secreted by the pituitary. Therefore, these hormones can cross-react with each other, whereas the structure of the β subunits is apparently divergent [1, 116]. In the nonpregnant state, hCG is produced by the pituitary gland and plays a crucial role in the establishment, promotion, and maintenance of human pregnancy [117]. For instance, hCG could promote the production of progesterone, cytotrophoblast cell differentiation, placental and uterine endothelial angiogenesis, the formation and differentiation of fetal organs, and the growth and development of the umbilical cord, in addition to maintaining the quiescence of the myometrium and the growth of the uterus in line with that of the fetus [116]. Additionally, hCG orchestrates the immune response in vivo and serves as a biomarker for a variety of tumors [116]. During pregnancy, there is a sharp increase in hCG levels in the first trimester, which peaks at approximately 10 weeks of gestation, slowly decreases until the 20th week, and then remains stable until the end of pregnancy [1]. Its specific receptors are distributed in the hippocampus, dentate gyrus, hypothalamus, cortex, brainstem, posterior fossa, cerebellum, choroid plexus, ependymal cells, glial cells, neural retina, pituitary gland, and spinal cord of adult mammals [118]. Nevertheless, few studies have assessed the effects of hCG on the neurogenesis of the adult hippocampus.
3.5. Relaxin
Relaxin, a small peptide hormone belonging to the insulin/relaxin family, is mainly produced in the corpus luteum in the ovaries of pregnant mammals [119]. It was named after its relaxing and lengthening effect on the pubic symphysis during guinea pig delivery. As a vasodilator in mammalian delivery, relaxin facilitates ligament relaxation and birth canal expansion before delivery, adjusts the hemodynamic changes in both the nonpregnant state and the pregnant state, regulates the secretion of OT and antidiuretic hormone, promotes the development of mammary glands, and regulates the lactation reflex and plasma osmotic pressure. Additionally, this hormone is involved in the establishment of pregnancy and adjusts the mother's cardiovascular system to support pregnancy and the maternal-fetal interface in preparation for postpartum breastfeeding [120]. It also has antifibrosis, organ protection, and antiallergic effects [121, 122].
The relaxin peptide family consists of relaxin receptor 1, receptor 2, receptor 3, and other relaxin family peptide receptors [123, 124] whose cell signals are mediated through their corresponding homologous G protein-coupled receptors [125]. The circulating concentration of relaxin begins to rise in the first trimester, peaks at approximately the 12th week of pregnancy, then decreases until roughly the 17th week of pregnancy, remains constant during the rest of the pregnancy, and decreases sharply after delivery [126].
Relaxin plays an important role in memory consolidation and motor activities and is involved in somatosensory and autonomic endocrine pathways [127, 128]. Notably, relaxin 3, the most highly expressed relaxin peptide family member preferentially distributed in the midline cap layer near the fourth ventricle [129, 130], was confirmed to have pivotal biological functions in both reproduction and central nervous system [131]. Its receptor, GPCR135, is expressed in the hippocampus, septum, intergenic lobules, fourth ventricle, and amygdala [128, 132]. Relaxin 3 has been proposed to function as a mediator of emotions and behavior due to its role in activating arousal and behavior, as well as regulating appetite, the stress response, anxiety, memory, sleep, and circadian rhythms [130, 133]. Silvertown et al. demonstrated that relaxin 3 derived from macaques has a neuroprotective effect on human neuronal cell cultures [128]. Few reports have described the correlation between relaxin and neurogenesis. In light of the above-described relaxin 3-mediated activities in the central nervous system, future studies should determine whether and how relaxin 3 mediates neurogenesis in the adult hippocampus dentate gyrus.
3.6. Glucocorticoids
3.6.1. Distribution of Glucocorticoids and Their Receptors in the Brain
Glucocorticoids are a type of steroid hormone secreted by the fascicular zone in the adrenal cortex, which have strong effects on the metabolism of carbohydrates; regulate the biosynthesis and metabolism of sugar, fats, and proteins; suppress the immune response; and dampen inflammatory responses, in addition to exhibiting antitoxic and antishock behavior. The serum level of glucocorticoids in rats decreases slightly in the first trimester, rises sharply from the second trimester, peaks in the third trimester, and finally decreases to nonpregnancy levels after delivery [51]. Glucocorticoid receptors are located in the hippocampus, striatum, amygdala, forebrain, paraventricular nucleus of the hypothalamus, preoptic area, ventromedial nucleus of the thalamus, the periventricular zone of the optic tectum, and the pituitary gland [134, 135].
3.6.2. Glucocorticoids and Adult Hippocampal Neurogenesis
High levels of corticosterone could inhibit the proliferation of hippocampal neuron precursor cells and the survival of neurons [136]. Brummelte and Galea found that high levels of glucocorticoids inhibited hippocampal neurogenesis in both male and female rats [79]. Torner et al. found that the neurogenesis in the hippocampus dentate gyrus of mice was significantly diminished in cases of high glucocorticoid levels induced by chronic stress, the extent to which was closely correlated with the glucocorticoid level [102]. Studies in rats, tree shrews, and marmosets have confirmed these findings [137–139].
Apart from the aforementioned studies on the effect of glucocorticoid levels on neurogenesis in the nonpregnant state, scholars have also investigated the effect of glucocorticoid levels on neurogenesis in the reproductive state due to the influence of reproduction on the hypothalamus-pituitary-adrenal axis and hippocampal plasticity [136, 140]. In light of the high sensitivity of the maternal brain to high concentrations of glucocorticoids during pregnancy and postpartum, elevated levels of glucocorticoids induced by stress would inhibit neurogenesis in the hippocampal dentate gyrus of rats. Leuner and Sabihi observed a decrease in this hormone and a subsequent reduction in the blockage of cell proliferation by virtue of adrenalectomy or evacuation of pups during childbirth, further confirming that high glucocorticoid levels have an inhibitory effect on hippocampal neurogenesis [141].
3.7. Oxytocin (OT)
3.7.1. Distribution of OT and Its Receptors in the Brain
OT is a brain pituitary neurohormone secreted by giant cells in the supraoptic nucleus and paraventricular nucleus of the hypothalamus, which is transported to the posterior pituitary through the nerve fibers of the hypothalamic-pituitary axis [142]. This hormone can be projected along axons of hypothalamic neurons to diverse areas, such as the amygdala, hippocampus, striatum, suprachiasmatic nucleus, terminal striatal nucleus, and brainstem, and acts as a neuromodulator or neurotransmitter, thereby affecting neurotransmission in these areas [143]. In addition to its release in the axonal terminal, OT could be released into the extracellular space via dendrites, and therefore, its effects are not only local but also spread through the brain to reach distant targets. Some peripheral tissues such as the uterus, corpus luteum, amniotic membrane, and placenta can also synthesize OT [144]. This hormone is commonly used clinically to induce labor, maintain postpartum homeostasis, and shorten the third stage of labor. OT also stimulates lactation, promotes milk ejection, induces gravid uterine contraction, and reduces the level of stress hormones such as corticosterone to lower blood pressure [107, 144–146]. Regarded as an initiator of intimate relationships, OT has been confirmed to regulate maternal behavior to enhance self-confidence and establish cooperation [142, 147]. OT neurons have extensive projections throughout the brain regions including the medial preoptic area, the bed nucleus of the stria terminalis, the ventral tegmental area, the ventral striatum, and the amygdala, all of which are critical areas of maternal behavior [143, 148]. The functional level of OT is negatively correlated with postpartum depression and anxiety [149].
During pregnancy, serum OT concentration may vary widely among individuals depending on emotions and the mother-infant relationship. The serum OT level, which gradually rises throughout pregnancy, rises substantially in the third trimester and after delivery due to stimulation from mother-infant interactions [1, 150]. From the third trimester to postpartum, the basal activity of OT receptors and OT-energetic cells increases with the strengthening of the mother-infant relationship [151]. The OT signal is mediated by the OT receptor (OTR), which belongs to the G protein-coupled receptor family and exists in the prefrontal cortex, the preoptic area, the lateral septum, the hippocampus, the hypothalamus, and the medial amygdala of mammals [152–154].
3.7.2. OT and Adult Hippocampal Neurogenesis
Little is known regarding the correlation between OT and adult neurogenesis. Existing studies have shown that exogenous OT could stimulate hippocampal cell proliferation and neurogenesis in rats. Further, OT could prevent the inhibitory effect of stress hormones on hippocampal neurogenesis and enhance the proliferation of newborn neurons in rats under cold water swimming stress [155]. Opendak et al. proposed that in vitro OT treatment could stimulate the neurogenesis of the dentate gyrus in adult male rats [156]. Taken together, these observations indicate that OT promotes the proliferation of nerve cells; however, its influence on hippocampal dentate gyrus neurogenesis in pregnant mammals remains ambiguous. Given the neurological effects of OT in mediating maternal nurturing behavior, cognition, and mood disorders, the effect of OT on the neurogenesis of the hippocampal dentate gyrus in the reproductive state is worth studying in more detail.
4. Potential Mechanisms Underlying the Role of Hormones on Adult Hippocampal Neurogenesis Taking Estrogens as an Example
4.1. Receptors
Researches have proposed that the effects of estrogen on hippocampal neurons are partly mediated by estrogen receptors [62, 157] (which encompass ERα, ERβ, and GPER in the hippocampus as discussed in previous sections), with the regulation of cell proliferation being the most affected. A series of experiments was performed using estradiol receptor agonists and inhibitors, which suggested that estradiol receptor agonists promoted the proliferation of nerve cells [62]. In contrast, estrogen inhibitors reversed the upregulation of cell proliferation induced by estradiol [158], resulting in the coexpression of estrogen receptors in neural progenitor cells in the granular cell layer of the dentate gyrus [62], thus confirming the receptor-mediated effect by which estrogen affected hippocampal neurogenesis. Genomic and nongenomic mechanisms have been proposed to explain the regulatory effects of estrogen on neurogenesis.
4.1.1. Genomic Mechanism (Figure 3)
Figure 3.
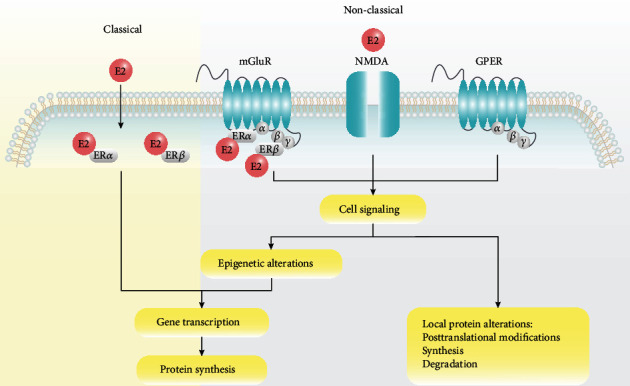
Genomic and nongenomic mechanisms underlying the regulatory effects of estrogen on neurogenesis. Classical receptors, ERα and ERβ, are located in the cytoplasm or nucleus and induce genomic effects when combined with estradiol. Membrane-associated receptors, GPER, mGluR, and NMDA, are localized in the plasma membrane of neurons (e.g., tether proteins on cell membranes) and trigger rapid nongenomic effects primarily through kinase activation. Abbreviations: E2: estradiol; ER: estrogen receptor; mGluR: metabotropic glutamate receptor; NMDA: N-methyl-D-aspartate; GPER: G protein-coupled estrogen receptor.
Estrogen binds to its receptor, ERα or ERβ, which is distributed in the cytoplasm or nucleus, resulting in the formation of a homodimer or heterodimer. It is then transported to the cell nucleus and binds to estrogen response elements (EREs) located in the target promoter region on the DNA to regulate gene expression and protein synthesis as a transcription factor. Given that some estrogen-regulated genes do not contain ERE sequences [159], gene expression is regulated through mechanisms involving other transcription factors and corresponding response elements, such as protein-protein interactions [159, 160].
4.1.2. Nongenomic Mechanism (Figure 3)
In addition to the genomic mechanism in which estrogen binds to nuclear receptors, nongenomic effects in the hippocampus are mediated by estrogen receptors in the plasma membrane (extranuclear receptors) [161]. This process is rapid, occurring in a few seconds or minutes, and transient (1-4 hours) [56]. For instance, enzymes through which estrogens regulate cell signaling associated with hippocampal memory were rapidly phosphorylated by multiple membrane receptors on the dorsal side of the hippocampus, including metabotropic glutamate receptor (mGluR) 1a and its interaction with ERα and ERβ, N-methyl-D-aspartate (NMDA) receptor [162], and G protein-coupled estrogen receptor (GPER) [163]. The activation of cell signaling pathways may trigger epigenetic changes such as histone acetylation, thereby regulating gene transcription and protein synthesis. Alterations in cell signal transduction may also affect the local protein levels of internal structures such as dendritic spines.
4.2. Neuroimmune Mechanisms
Beyond receptor mechanisms, other factors may influence the onset of adult hippocampal neurogenesis [19, 65]. For instance, microglia, the innate immune cells in the brain, provide support for adult neurogenesis under physiological conditions in the hippocampus [164, 165]. The majority of newborn cells in the hippocampus cannot survive to maturity [166]. Instead, most cells undergo apoptosis and microglial phagocytosis within the first 1-4 days of birth. Additionally, microglia are the predominant mediator of neuroinflammation, which has been found to correlate with the regulation of adult hippocampal neurogenesis based on accumulating evidence [167]. The nervous system becomes populated with activated microglia in cases of pathological inflammation, which is accompanied by a decreased newborn neuron survival rate, whereas the proliferation of nerve cells remains unaffected. However, microglia inhibitors could restore the survival rate of neurons [165]. The anti-inflammatory effects of estrogen on the brain have been broadly documented in central nervous system diseases, including stroke and neurodegenerative diseases [168–171]. In vitro studies have confirmed that estrogens significantly affected the microglia function of rat primary microglia cell cultures or the N9 microglia cell line. For example, estradiol could suppress lipopolysaccharide-induced microglial activation [172]. Given the role of inflammation and microglia in neurogenesis, as well as the anti-inflammatory effects of estrogen, it is conceivable that the effect of estrogen on neurogenesis is partly achieved by regulating microglial inflammatory processes. Further, estrogen-deficient microenvironments may lead to immune dysregulation and perturbed neurogenesis. However, to the best of our knowledge, a direct link between estrogens and neurogenesis has not been established.
4.3. Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) is an influential neurotrophin during neurogenesis and binds to a membrane receptor, TrkB, mediating the survival, growth, and maintenance of neurons in key brain circuits that contribute to emotion and cognitive functions. Therefore, alterations in the BDNF level generally typically affect neurogenesis rates [173, 174]. Many studies have indicated that BDNF has a critical role in neurogenesis [175, 176]. For example, BDNF plays an important role in regulating the basal level of neurogenesis in the dentate gyrus of adult mice, reversing dietary restriction-induced decreases in neurogenesis by enhancing the survival of newborn neurons. Sairanen et al. proposed that BDNF signaling was required for the long-term survival of mouse hippocampal neonatal neurons. Moreover, rising BDNF levels in the dentate gyrus coincided with an increase in hippocampal dentate gyrus neurogenesis [177]. Decreases in BDNF levels associated with depression are thought to be one of the main pathological mechanisms underlying impaired hippocampal neurogenesis [178]. Additionally, given that the effects of estrogen paralleled those of BDNF [179], further studies demonstrated that estrogens directly regulated BDNF, including ERE recognition on the BDNF gene. For instance, BDNF mRNA levels were significantly reduced after bilateral ovariectomy in rodents, whereas the opposite outcome was observed after estradiol treatment [180, 181]. The activation of estrogen receptors selectively reinforced BDNF signaling and BDNF expression, thereby promoting hippocampal dentate gyrus neurogenesis [182, 183]. The expression of BDNF was enhanced in the hippocampus of HSP mice and OVX/EB mice but decreased in the EW and OVX groups [31]. Therefore, we hypothesize that the effect of estrogen on adult hippocampal neurogenesis is largely mediated by the regulation of BDNF.
5. Conclusions and Future Perspectives
The effect of reproduction-associated hormones on the renewal of adult hippocampal neurons varies with hormone dose, the time of administration, experimental treatment, and subject gender and age, among other factors. Short-term estrogen exposure stimulated the proliferation of nerve cells, whereas long-term treatment may have the opposite effect. Progesterone alone has no inhibitory effect on neurogenesis but reduces the effects of other hormones on neurogenesis when combined with other hormones. Generally, short-term estrogen exposure, progesterone treatment alone, and PRL and OT activity have a positive effect on adult hippocampal neurogenesis, whereas glucocorticoids may have inhibitory effects. Moreover, little research has been conducted on the effects of relaxin or hCG.
Most current studies have focused on the effects of multiple reproductive hormones in isolation. Further, except for the combination of estrogen and progesterone, research on the combined effects of multiple hormones on neurogenesis is scarce. Hormones change constantly in vivo and show complex interactions during pregnancy and postpartum. Given that the impact of these hormones on neurogenesis and the functional significance of the newborn neurons, it is hoped that more research will be conducted in these areas.
Although there have been tremendous advances in the understanding of adult hippocampal neurogenesis in the past few years, few studies have assessed whether the neonatal neurons in the hippocampus are activated and what influence they have on spatial learning and memory, particularly in reproductive state mammals, which are likely to exhibit a low proportion of activated newborn neurons. Therefore, future studies should focus on establishing a causal relationship between reproduction-related hormones and brain plasticity, in addition to determining whether newborn neurons have been integrated into the neural circuits and are involved in memory and spatial learning.
Acknowledgments
This work was supported by funding from the National Natural Science Foundation, China (Grant number: #81974206 to BX) and the Scientific Research Project of Hunan Provincial Health Commission (Grant number: 20200475 to JG).
Contributor Information
Lily Wan, Email: wanll1203@csu.edu.cn.
Bo Xiao, Email: xiaobo_xy@126.com.
Data Availability
All data and material files are available upon request.
Ethical Approval
All procedures were followed in accordance with the ethical standards of the responsible committee on animal and human experimentation (Ethics Review Committee of Xiangya Hospital, numbers 201603296 and 201603297) and with the Helsinki Declaration of 1964 and later versions.
Conflicts of Interest
The authors declare that they have no conflict of interests.
Authors' Contributions
Lily Wan and Rou-Jie Huang drafted the manuscript; Aihua Pan and Zhao-Hui Luo drew the figures; Lily Wan designed the outline and revised the manuscript; Jim Manavis, Xiao-Xin Yan, and Lily Wan proofread and edited the manuscript; Jiao-e Gong and Bo Xiao provided financial support. All authors read and approved the publication of this manuscript. Lily Wan and Rou-Jie Huang contributed equally to this work.
References
- 1.Napso T., Yong H. E. J., Lopez-Tello J., Sferruzzi-Perri A. N. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Frontiers in Physiology. 2018;9:p. 1091. doi: 10.3389/fphys.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grattan D. R., Ladyman S. R. Chapter 2- neurophysiological and cognitive changes in pregnancy. In: Steegers E. A. P., Cipolla M. J., Miller E. C., editors. Handbook of Clinical Neurology. Elsevier; 2020. [DOI] [PubMed] [Google Scholar]
- 3.Braunstein G. D., Rasor J., Adler D., Danzer H., Wade M. E. Serum human chorionic gonadotropin levels throughout normal pregnancy. American Journal of Obstetrics and Gynecology. 1976;126(6):678–681. doi: 10.1016/0002-9378(76)90518-4. [DOI] [PubMed] [Google Scholar]
- 4.Loriaux D. L., Ruder H. J., Knab D. R., Lipsett M. B. Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy. The Journal of Clinical Endocrinology and Metabolism. 1972;35(6):887–891. doi: 10.1210/jcem-35-6-887. [DOI] [PubMed] [Google Scholar]
- 5.Neville M. C., McFadden T. B., Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia. 2002;7(1):49–66. doi: 10.1023/A:1015770423167. [DOI] [PubMed] [Google Scholar]
- 6.Seki K., Uesato T., Tabei T., Kato K. The secretory patterns of relaxin and human chorionic gonadotropin in human pregnancy. Endocrinologia Japonica. 1985;32(5):741–744. doi: 10.1507/endocrj1954.32.741. [DOI] [PubMed] [Google Scholar]
- 7.Biswas S., Rodeck C. H. Plasma prolactin levels during pregnancy. British Journal of Obstetrics and Gynaecology. 1976;83(9):683–687. doi: 10.1111/j.1471-0528.1976.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh A. A. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biology of Reproduction. 1971;5(3):297–307. doi: 10.1093/biolreprod/5.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Pepe G. J., Rothchild I. A comparative study of serum progesterone levels in pregnancy and in various types of pseudopregnancy in the rat. Endocrinology. 1974;95(1):275–279. doi: 10.1210/endo-95-1-275. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- 11.Amenomori Y., Chen C. L., Meites J. Serum prolactin levels in rats during different reproductive states. Endocrinology. 1970;86(3):506–510. doi: 10.1210/endo-86-3-506. [DOI] [PubMed] [Google Scholar]
- 12.Dupouy J. P., Coffigny H., Magre S. Maternal and foetal corticosterone levels during late pregnancy in rats. The Journal of Endocrinology. 1975;65(3):347–352. doi: 10.1677/joe.0.0650347. [DOI] [PubMed] [Google Scholar]
- 13.Rolls A., Schori H., London A., Schwartz M. Decrease in hippocampal neurogenesis during pregnancy: a link to immunity. Molecular Psychiatry. 2008;13(5):468–469. doi: 10.1038/sj.mp.4002126. [DOI] [PubMed] [Google Scholar]
- 14.Pawluski J. L., van den Hove D. L. A., Rayen I., Prickaerts J., Steinbusch H. W. M. Stress and the pregnant female: impact on hippocampal cell proliferation, but not affective-like behaviors. Hormones and Behavior. 2011;59(4):572–580. doi: 10.1016/j.yhbeh.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Brus M., Meurisse M., Franceschini I., Keller M., Lévy F. Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Hormones and Behavior. 2010;58(5):737–746. doi: 10.1016/j.yhbeh.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Bridges R. S. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Hormones and Behavior. 2016;77:193–203. doi: 10.1016/j.yhbeh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina J., Workman J. L. Maternal experience and adult neurogenesis in mammals: implications for maternal care, cognition, and mental health. Journal of Neuroscience Research. 2020;98(7):1293–1308. doi: 10.1002/jnr.24311. [DOI] [PubMed] [Google Scholar]
- 18.Ihunwo A. O., Tembo L. H., Dzamalala C. The dynamics of adult neurogenesis in human hippocampus. Neural Regeneration Research. 2016;11(12):1869–1883. doi: 10.4103/1673-5374.195278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colangelo A. M., Cirillo G., Alberghina L., Papa M., Westerhoff H. V. Neural plasticity and adult neurogenesis: the deep biology perspective. Neural Regeneration Research. 2019;14(2):201–205. doi: 10.4103/1673-5374.244775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman J., Das G. D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of Comparative Neurology. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 21.Wan L., Tu T., Zhang Q. L., Jiang J., Yan X. X. Pregnancy promotes maternal hippocampal neurogenesis in guinea pigs. Neural Plasticity. 2019;2019:17. doi: 10.1155/2019/5765284.5765284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimone J. B., Li Y., Lee S. W., Clemenson G. D., Deng W., Gage F. H. Regulation and function of adult neurogenesis: from genes to cognition. Physiological Reviews. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahay A., Scobie K. N., Hill A. S., et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility -- linking memory and mood. Nature Reviews Neuroscience. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberwirth C., Pan Y., Liu Y., Zhang Z., Wang Z. Hippocampal adult neurogenesis: its regulation and potential role in spatial learning and memory. Brain Research. 2016;1644:127–140. doi: 10.1016/j.brainres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toda T., Parylak S. L., Linker S. B., Gage F. H. The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry. 2019;24(1):67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toda T., Gage F. H. Review: adult neurogenesis contributes to hippocampal plasticity. Cell and Tissue Research. 2018;373(3):693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 28.Baptista P., Andrade J. P. Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Frontiers in Neuroanatomy. 2018;12:p. 44. doi: 10.3389/fnana.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdugo-Vega G., Arias-Gil G., López-Fernández A., et al. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nature Communications. 2020;11(1):p. 135. doi: 10.1038/s41467-019-14026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arruda-Carvalho M., Sakaguchi M., Akers K. G., Josselyn S. A., Frankland P. W. Posttraining ablation of adult-generated neurons degrades previously acquired memories. The Journal of Neuroscience. 2011;31(42):15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Hong J., Zhang S., et al. Postpartum estrogen withdrawal impairs hippocampal neurogenesis and causes depression- and anxiety-like behaviors in mice. Psychoneuroendocrinology. 2016;66:138–149. doi: 10.1016/j.psyneuen.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Scharfman H. E., Mercurio T. C., Goodman J. H., Wilson M. A., MacLusky N. J. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. The Journal of Neuroscience. 2003;23(37):11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAvoy K. M., Scobie K. N., Berger S., et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron. 2016;91(6):1356–1373. doi: 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radad K., Moldzio R., al-Shraim M., Kranner B., Krewenka C., Rausch W. D. Recent advances on the role of neurogenesis in the adult brain: therapeutic potential in Parkinson's and Alzheimer's diseases. CNS & Neurological Disorders Drug Targets. 2017;16(7):740–748. doi: 10.2174/1871527316666170623094728. [DOI] [PubMed] [Google Scholar]
- 35.Micheli L., Ceccarelli M., D’Andrea G., Tirone F. Depression and adult neurogenesis: positive effects of the antidepressant fluoxetine and of physical exercise. Brain Research Bulletin. 2018;143:181–193. doi: 10.1016/j.brainresbull.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Tada H., Koide M., Ara W., et al. Estrous cycle-dependent phasic changes in the stoichiometry of hippocampal synaptic AMPA receptors in rats. PLoS One. 2015;10(6, article e0131359) doi: 10.1371/journal.pone.0131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barha C. K., Galea L. A. The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiology of Aging. 2013;34(3):986–1004. doi: 10.1016/j.neurobiolaging.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Butz M., Lehmann K., Dammasch I. E., Teuchert-Noodt G. A theoretical network model to analyse neurogenesis and synaptogenesis in the dentate gyrus. Neural Networks. 2006;19(10):1490–1505. doi: 10.1016/j.neunet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt-Hieber C., Jonas P., Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 40.Parsons C. G., Stöffler A., Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system - too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Cho K. O., Lybrand Z. R., Ito N., et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nature Communications. 2015;6(1):p. 6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jessberger S., Römer B., Babu H., Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Experimental Neurology. 2005;196(2):342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Peng S., Yang B., Duan M. Y., et al. The disparity of impairment of neurogenesis and cognition after acute or fractionated radiation exposure in adolescent BALB/c mice. Dose Response. 2019;17(1, article 1559325818822574) doi: 10.1177/1559325818822574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera P. D., Simmons S. J., Reynolds R. P., Just A. L., Birnbaum S. G., Eisch A. J. Image-guided cranial irradiation-induced ablation of dentate gyrus neurogenesis impairs extinction of recent morphine reward memories. Hippocampus. 2019;29(8):726–735. doi: 10.1002/hipo.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulin S. E., Mendoza M. L., Richardson D. R., et al. Dentate gyrus neurogenesis ablation via cranial irradiation enhances morphine self-administration and locomotor sensitization. Addiction Biology. 2018;23(2):665–675. doi: 10.1111/adb.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostin A., Alam M. A., McGinty D., Szymusiak R., Alam M. N. Chronic suppression of hypothalamic cell proliferation and neurogenesis induces aging-like changes in sleep-wake organization in young mice. Neuroscience. 2019;404:541–556. doi: 10.1016/j.neuroscience.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 47.Houben S., Leroy K., Ando K., et al. Genetic ablation of tau in postnatal neurons rescues decreased adult hippocampal neurogenesis in a tauopathy model. Neurobiology of Disease. 2019;127:131–141. doi: 10.1016/j.nbd.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Yeung S. T., Myczek K., Kang A. P., Chabrier M. A., Baglietto-Vargas D., LaFerla F. M. Impact of hippocampal neuronal ablation on neurogenesis and cognition in the aged brain. Neuroscience. 2014;259:214–222. doi: 10.1016/j.neuroscience.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mateus-Pinheiro A., Alves N. D., Patrício P., et al. AP2γ controls adult hippocampal neurogenesis and modulates cognitive, but not anxiety or depressive-like behavior. Molecular Psychiatry. 2017;22(12):1725–1734. doi: 10.1038/mp.2016.169. [DOI] [PubMed] [Google Scholar]
- 50.Saxe M. D., Battaglia F., Wang J. W., et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawluski J. L., Brummelte S., Barha C. K., Crozier T. M., Galea L. A. M. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Frontiers in Neuroendocrinology. 2009;30(3):343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J., Bosch M. A., Tobias S. C., et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. The Journal of Neuroscience. 2003;23(29):9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almey A., Milner T. A., Brake W. G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Hormones and Behavior. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters E. M., Thompson L. I., Patel P., et al. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35(6):2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexander A., Irving A. J., Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113:652–660. doi: 10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Micevych P., Dominguez R. Membrane estradiol signaling in the brain. Frontiers in Neuroendocrinology. 2009;30(3):315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Micevych P. E., Kelly M. J. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96(2):103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanapat P., Hastings N. B., Reeves A. J., Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. The Journal of Neuroscience. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanapat P., Hastings N. B., Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. The Journal of Comparative Neurology. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 60.Ormerod B. K., Lee T. T., Galea L. A. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. Journal of Neurobiology. 2003;55(2):247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 61.Barha C. K., Lieblich S. E., Galea L. A. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. Journal of Neuroendocrinology. 2009;21(3):155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 62.Mazzucco C. A., Lieblich S. E., Bingham B. I., Williamson M. A., Viau V., Galea L. A. M. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141(4):1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 63.Duarte-Guterman P., Lieblich S. E., Chow C., Galea L. A. M. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS One. 2015;10(6, article e0129880) doi: 10.1371/journal.pone.0129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker J. M., Galea L. A. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152(4):888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 65.McClure R. E., Barha C. K., Galea L. A. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Hormones and Behavior. 2013;63(1):144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Chan M., Chow C., Hamson D. K., Lieblich S. E., Galea L. A. M. Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. Journal of Neuroendocrinology. 2014;26(6):386–399. doi: 10.1111/jne.12159. [DOI] [PubMed] [Google Scholar]
- 67.Vega-Rivera N. M., Fernández-Guasti A., Ramírez-Rodríguez G., Estrada-Camarena E. Effect of sub-optimal doses of fluoxetine plus estradiol on antidepressant- like behavior and hippocampal neurogenesis in ovariectomized rats. Psychoneuroendocrinology. 2015;57:113–124. doi: 10.1016/j.psyneuen.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 68.Spritzer M. D., Galea L. A. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Developmental Neurobiology. 2007;67(10):1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- 69.Costa M. A. The endocrine function of human placenta: an overview. Reproductive Biomedicine Online. 2016;32(1):14–43. doi: 10.1016/j.rbmo.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Lagace D. C., Fischer S. J., Eisch A. J. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17(3):175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 71.Tzeng W. Y., Chen L. H., Cherng C. G., Tsai Y. N., Yu L. Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology. 2014;42:24–37. doi: 10.1016/j.psyneuen.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Galea L. A., McEwen B. S. Sex and seasonal changes in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89(3):955–964. doi: 10.1016/S0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs E. G., Weiss B. K., Makris N., et al. Impact of sex and menopausal status on episodic memory circuitry in early midlife. The Journal of Neuroscience. 2016;36(39):10163–10173. doi: 10.1523/JNEUROSCI.0951-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Driscoll I., Howard S. R., Stone J. C., et al. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139(4):1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 75.Nacher J., Alonso-Llosa G., Rosell D. R., McEwen B. S. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiology of Aging. 2003;24(2):273–284. doi: 10.1016/S0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- 76.Woolley C. S., McEwen B. S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. The Journal of Comparative Neurology. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 77.Fester L., Ribeiro-Gouveia V., Prange-Kiel J., et al. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. Journal of Neurochemistry. 2006;97(4):1136–1144. doi: 10.1111/j.1471-4159.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- 78.Goel N., Workman J. L., Lee T. T., Innala L., Viau V. Sex differences in the HPA axis. Comprehensive Physiology. 2014;4(3):1121–1155. doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- 79.Brummelte S., Galea L. A. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168(3):680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J., Inazu M., Tsuji K., Yamada E., Takeda H., Matsumiya T. Neurochemical characteristics and behavioral responses to psychological stress in ovariectomized rats. Pharmacological Research. 1999;39(6):455–461. doi: 10.1006/phrs.1999.0468. [DOI] [PubMed] [Google Scholar]
- 81.Mahmoud R., Wainwright S. R., Galea L. A. Sex hormones and adult hippocampal neurogenesis: regulation, implications, and potential mechanisms. Frontiers in Neuroendocrinology. 2016;41:129–152. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Viau V., Meaney M. J. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 83.Duarte-Guterman P., Yagi S., Chow C., Galea L. A. M. Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Hormones and Behavior. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 84.Csapo A. I., Knobil E., van der Molen H. J., Wiest W. G. Peripheral plasma progesterone levels during human pregnancy and labor. American Journal of Obstetrics and Gynecology. 1971;110(5):630–632. doi: 10.1016/0002-9378(71)90242-0. [DOI] [PubMed] [Google Scholar]
- 85.González-Orozco J. C., Camacho-Arroyo I. Progesterone actions during central nervous system development. Frontiers in Neuroscience. 2019;13(503) doi: 10.3389/fnins.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossetti M. F., Cambiasso M. J., Holschbach M. A., Cabrera R. Oestrogens and progestagens: synthesis and action in the brain. Journal of Neuroendocrinology. 2016;28(7) doi: 10.1111/jne.12402. [DOI] [PubMed] [Google Scholar]
- 87.Brinton R. D., Thompson R. F., Foy M. R., et al. Progesterone receptors: form and function in brain. Frontiers in Neuroendocrinology. 2008;29(2):313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meffre D., Labombarda F., Delespierre B., et al. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. doi: 10.1016/j.neuroscience.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 89.Tsai H. W., Ho C. L., Cheng S. W., et al. Progesterone receptor membrane component 1 as a potential prognostic biomarker for hepatocellular carcinoma. World Journal of Gastroenterology. 2018;24(10):1152–1166. doi: 10.3748/wjg.v24.i10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bali N., Arimoto J. M., Iwata N., et al. Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology. 2012;153(2):759–769. doi: 10.1210/en.2011-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yilmaz C., Karali K., Fodelianaki G., et al. Neurosteroids as regulators of neuroinflammation. Frontiers in Neuroendocrinology. 2019;55, article 100788 doi: 10.1016/j.yfrne.2019.100788. [DOI] [PubMed] [Google Scholar]
- 92.Wang J. M., Johnston P. B., Ball B. G., Brinton R. D. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. The Journal of Neuroscience. 2005;25(19):4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L., Zhao L., She H., et al. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology. 2010;151(12):5782–5794. doi: 10.1210/en.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barha C. K., Ishrat T., Epp J. R., Galea L. A. M., Stein D. G. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Experimental Neurology. 2011;231(1):72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z., Yang R., Zhou R., Li L., Sokabe M., Chen L. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus. 2010;20(3):402–412. doi: 10.1002/hipo.20642. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z., Yang R., Cai W., Bai Y., Sokabe M., Chen L. Treatment with progesterone after focal cerebral ischemia suppresses proliferation of progenitor cells but enhances survival of newborn neurons in adult male mice. Neuropharmacology. 2010;58(6):930–939. doi: 10.1016/j.neuropharm.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Ben-Jonathan N., Mershon J. L., Allen D. L., Steinmetz R. W. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocrine Reviews. 1996;17(6):639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 98.Costanza M., Pedotti R. Prolactin: friend or foe in central nervous system autoimmune inflammation? International Journal of Molecular Sciences. 2016;17(12):p. 2026. doi: 10.3390/ijms17122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costanza M., Musio S., Abou-Hamdan M., Binart N., Pedotti R. Prolactin is not required for the development of severe chronic experimental autoimmune encephalomyelitis. Journal of Immunology. 2013;191(5):2082–2088. doi: 10.4049/jimmunol.1301128. [DOI] [PubMed] [Google Scholar]
- 100.Larsen C. M., Grattan D. R. Prolactin, neurogenesis, and maternal behaviors. Brain, Behavior, and Immunity. 2012;26(2):201–209. doi: 10.1016/j.bbi.2011.07.233. [DOI] [PubMed] [Google Scholar]
- 101.Torner L., Neumann I. D. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5(4):249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 102.Torner L., Karg S., Blume A., et al. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. The Journal of Neuroscience. 2009;29(6):1826–1833. doi: 10.1523/JNEUROSCI.3178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bole-Feysot C., Goffin V., Edery M., Binart N., Kelly P. A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Reviews. 1998;19(3):225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 104.Sugiyama T., Minoura H., Toyoda N., et al. Pup contact induces the expression of long form prolactin receptor mRNA in the brain of female rats: effects of ovariectomy and hypophysectomy on receptor gene expression. The Journal of Endocrinology. 1996;149(2):335–340. doi: 10.1677/joe.0.1490335. [DOI] [PubMed] [Google Scholar]
- 105.Sairenji T. J., Ikezawa J., Kaneko R., et al. Maternal prolactin during late pregnancy is important in generating nurturing behavior in the offspring. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(49):13042–13047. doi: 10.1073/pnas.1621196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torner L. Actions of prolactin in the brain: from physiological adaptations to stress and neurogenesis to psychopathology. Frontiers in Endocrinology. 2016;7:p. 25. doi: 10.3389/fendo.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Augustine R. A., Ladyman S. R., Bouwer G. T., et al. Prolactin regulation of oxytocin neurone activity in pregnancy and lactation. The Journal of Physiology. 2017;595(11):3591–3605. doi: 10.1113/JP273712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sackmann-Sala L., Guidotti J. E., Goffin V. Minireview: prolactin regulation of adult stem cells. Molecular Endocrinology. 2015;29(5):667–681. doi: 10.1210/me.2015-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mak G. K., Enwere E. K., Gregg C., et al. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nature Neuroscience. 2007;10(8):1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 110.Shingo T., Gregg C., Enwere E., et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299(5603):117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 111.Lajud N., Gonzalez-Zapien R., Roque A., et al. Prolactin administration during early postnatal life decreases hippocampal and olfactory bulb neurogenesis and results in depressive-like behavior in adulthood. Hormones and Behavior. 2013;64(5):781–789. doi: 10.1016/j.yhbeh.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 112.Albin-Brooks C., Nealer C., Sabihi S., Haim A., Leuner B. The influence of offspring, parity, and oxytocin on cognitive flexibility during the postpartum period. Hormones and Behavior. 2017;89:130–136. doi: 10.1016/j.yhbeh.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mak G. K., Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nature Neuroscience. 2010;13(6):753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 114.Wagner K., Couillard-Despres S., Lehner B., et al. Prolactin induces MAPK signaling in neural progenitors without alleviating glucocorticoid-induced inhibition of in vitro neurogenesis. Cellular Physiology and Biochemistry. 2009;24(5-6):397–406. doi: 10.1159/000257432. [DOI] [PubMed] [Google Scholar]
- 115.Walker T. L., Vukovic J., Koudijs M. M., et al. Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One. 2012;7(9, article e44371) doi: 10.1371/journal.pone.0044371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nwabuobi C., Arlier S., Schatz F., Guzeloglu-Kayisli O., Lockwood C., Kayisli U. hCG: biological functions and clinical applications. International Journal of Molecular Sciences. 2017;18(10):p. 2037. doi: 10.3390/ijms18102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paulesu L., Rao C. V., Ietta F., Pietropolli A., Ticconi C. hCG and its disruption by environmental contaminants during human pregnancy. International Journal of Molecular Sciences. 2018;19(3):p. 914. doi: 10.3390/ijms19030914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao C. V. Physiological and pathological relevance of human uterine LH/hCG receptors. Journal of the Society for Gynecologic Investigation. 2006;13(2):77–78. doi: 10.1016/j.jsgi.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 119.Bathgate R. A. D., Halls M. L., van der Westhuizen E. T., Callander G. E., Kocan M., Summers R. J. Relaxin family peptides and their receptors. Physiological Reviews. 2013;93(1):405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 120.Martin B., Romero G., Salama G. Cardioprotective actions of relaxin. Molecular and Cellular Endocrinology. 2019;487:45–53. doi: 10.1016/j.mce.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 121.Kanai A. J., Konieczko E. M., Bennett R. G., Samuel C. S., Royce S. G. Relaxin and fibrosis: emerging targets, challenges, and future directions. Molecular and Cellular Endocrinology. 2019;487:66–74. doi: 10.1016/j.mce.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaftanovskaya E. M., Soula M., Myhr C., et al. Human relaxin receptor is fully functional in humanized mice and is activated by small molecule agonist ML290. Journal of the Endocrine Society. 2017;1(6):712–725. doi: 10.1210/js.2017-00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ng H. H., Esteban-Lopez M., Agoulnik A. I. Targeting the relaxin/insulin-like family peptide receptor 1 and 2 with small molecule compounds. Molecular and Cellular Endocrinology. 2019;487:40–44. doi: 10.1016/j.mce.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thanasupawat T., Glogowska A., Nivedita-Krishnan S., Wilson B., Klonisch T., Hombach-Klonisch S. Emerging roles for the relaxin/RXFP1 system in cancer therapy. Molecular and Cellular Endocrinology. 2019;487:85–93. doi: 10.1016/j.mce.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Halls M. L., Bathgate R. A. D., Sutton S. W., Dschietzig T. B., Summers R. J. International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacological Reviews. 2015;67(2):389–440. doi: 10.1124/pr.114.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Renegar R. H., Owens C. R., 3rd Measurement of plasma and tissue relaxin concentrations in the pregnant hamster and fetus using a homologous radioimmunoassay. Biology of Reproduction. 2002;67(2):500–505. doi: 10.1095/biolreprod67.2.500. [DOI] [PubMed] [Google Scholar]
- 127.Burazin T. C. D., Johnson K. J., Ma S., Bathgate R. A. D., Tregear G. W., Gundlach A. L. Localization of LGR7 (relaxin receptor) mRNA and protein in rat forebrain: correlation with relaxin binding site distribution. Annals of the New York Academy of Sciences. 2005;1041(1):205–210. doi: 10.1196/annals.1282.031. [DOI] [PubMed] [Google Scholar]
- 128.Silvertown J. D., Neschadim A., Liu H. N., et al. Relaxin-3 and receptors in the human and rhesus brain and reproductive tissues. Regulatory Peptides. 2010;159(1-3):44–53. doi: 10.1016/j.regpep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Ma S., Gundlach A. L. Relaxin-family peptide and receptor systems in brain: insights from recent anatomical and functional studies. Advances in Experimental Medicine and Biology. 2007;612:119–137. doi: 10.1007/978-0-387-74672-2_9. [DOI] [PubMed] [Google Scholar]
- 130.Kumar J. R., Rajkumar R., Jayakody T., et al. Relaxin' the brain: a case for targeting the nucleus incertus network and relaxin-3/RXFP3 system in neuropsychiatric disorders. British Journal of Pharmacology. 2017;174(10):1061–1076. doi: 10.1111/bph.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bathgate R. A., Samuel C. S., Burazin T. C. D., et al. Human Relaxin Gene 3 (H3) and the Equivalent Mouse Relaxin (M3) Gene: The Journal of Biological Chemistry. 2002;277(2):1148–1157. doi: 10.1074/jbc.M107882200. [DOI] [PubMed] [Google Scholar]
- 132.Liu C., Bonaventure P., Sutton S. W., et al. Recent progress in relaxin-3-related research. Annals of the New York Academy of Sciences. 2005;1041(1):47–60. doi: 10.1196/annals.1282.009. [DOI] [PubMed] [Google Scholar]
- 133.Ma S., Smith C. M., Blasiak A., Gundlach A. L. Distribution, physiology and pharmacology of relaxin-3/RXFP3 systems in brain. British Journal of Pharmacology. 2017;174(10):1034–1048. doi: 10.1111/bph.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Senft R. A., Meddle S. L., Baugh A. T. Distribution and abundance of glucocorticoid and mineralocorticoid receptors throughout the brain of the great tit (Parus major) PLoS One. 2016;11(2, article e0148516) doi: 10.1371/journal.pone.0148516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gross M., Romi H., Miller A., Pinhasov A. Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Scientific Reports. 2018;8(1):p. 9595. doi: 10.1038/s41598-018-27988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pawluski J. L., Császár E., Savage E., Martinez-Claros M., Steinbusch H. W. M., van den Hove D. Effects of stress early in gestation on hippocampal neurogenesis and glucocorticoid receptor density in pregnant rats. Neuroscience. 2015;290:379–388. doi: 10.1016/j.neuroscience.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 137.Barha C. K., Brummelte S., Lieblich S. E., Galea L. A. M. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21(11):1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- 138.Gould E., McEwen B. S., Tanapat P., Galea L. A. M., Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. The Journal of Neuroscience. 1997;17(7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gould E., Tanapat P., McEwen B. S., Flugge G., Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brummelte S., Galea L. A. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Hormones and Behavior. 2010;58(5):769–779. doi: 10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 141.Leuner B., Sabihi S. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Frontiers in Neuroendocrinology. 2016;41:99–113. doi: 10.1016/j.yfrne.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim S., Strathearn L. Oxytocin and maternal brain plasticity. New Directions for Child and Adolescent Development. 2016;2016(153):59–72. doi: 10.1002/cad.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 144.Arrowsmith S., Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. Journal of Neuroendocrinology. 2014;26(6):356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 145.Bethlehem R. A., van Honk J., Auyeung B., Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38(7):962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 146.Petersson M., Alster P., Lundeberg T., Uvnäs-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiology & Behavior. 1996;60(5):1311–1315. doi: 10.1016/S0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- 147.Bosch O. J., Neumann I. D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and Behavior. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Gottschalk M. G., Domschke K. Oxytocin and anxiety disorders. Current Topics in Behavioral Neurosciences. 2018;35:467–498. doi: 10.1007/7854_2017_25. [DOI] [PubMed] [Google Scholar]
- 149.Payne J. L., Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Frontiers in Neuroendocrinology. 2019;52:165–180. doi: 10.1016/j.yfrne.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim S. C., Lee J. E., Kang S. S., Yang H. S., Kim S. S., An B. S. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. Journal of Molecular Endocrinology. 2017;59(3):235–243. doi: 10.1530/JME-16-0223. [DOI] [PubMed] [Google Scholar]
- 151.Rilling J. K., Young L. J. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sladek C. D., Song Z. Diverse roles of G-protein coupled receptors in the regulation of neurohypophyseal hormone secretion. Journal of Neuroendocrinology. 2012;24(4):554–565. doi: 10.1111/j.1365-2826.2011.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaidyanathan R., Hammock E. A. Oxytocin receptor dynamics in the brain across development and species. Developmental Neurobiology. 2017;77(2):143–157. doi: 10.1002/dneu.22403. [DOI] [PubMed] [Google Scholar]
- 154.Cilz N. I., Cymerblit-Sabba A., Young W. S. Oxytocin and vasopressin in the rodent hippocampus. Genes, Brain, and Behavior. 2018;18(1, article e12535) doi: 10.1111/gbb.12535. [DOI] [PubMed] [Google Scholar]
- 155.Leuner B., Caponiti J. M., Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Opendak M., Offit L., Monari P., et al. Lasting adaptations in social behavior produced by social disruption and inhibition of adult neurogenesis. The Journal of Neuroscience. 2016;36(26):7027–7038. doi: 10.1523/JNEUROSCI.4435-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vasudevan N., Pfaff D. W. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology. 2008;29(2):238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 158.Nagy A. I., Ormerod B. K., Mazzucco C., Galea L. A. M. Estradiol-induced enhancement in cell proliferation is mediated through estrogen receptors in the dentate gyrus of adult female rats. Drug Development Research. 2005;66(2):142–149. doi: 10.1002/ddr.20053. [DOI] [Google Scholar]
- 159.O’Lone R., Frith M. C., Karlsson E. K., Hansen U. Genomic targets of nuclear estrogen receptors. Molecular Endocrinology. 2004;18(8):1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 160.Björnström L., Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Molecular Endocrinology. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 161.McEwen B. S., Akama K. T., Spencer-Segal J. L., Milner T. A., Waters E. M. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behavioral Neuroscience. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potier M., Georges F., Brayda-Bruno L., et al. Temporal Memory and Its Enhancement by Estradiol Requires Surface Dynamics of Hippocampal CA1 N-Methyl-D-Aspartate Receptors. Biological Psychiatry. 2016;79(9):735–745. doi: 10.1016/j.biopsych.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 163.Correa J., Ronchetti S., Labombarda F., de Nicola A. F., Pietranera L. Activation of the G protein-coupled estrogen receptor (GPER) increases neurogenesis and ameliorates neuroinflammation in the hippocampus of male spontaneously hypertensive rats. Cellular and Molecular Neurobiology. 2020;40(5):711–723. doi: 10.1007/s10571-019-00766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sierra A., Encinas J. M., Deudero J. J. P., et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ueno M., Fujita Y., Tanaka T., et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 166.Cameron H. A., Mckay R. D. G. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of Comparative Neurology. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 167.Sierra A., Beccari S., Diaz-Aparicio I., Encinas J. M., Comeau S., Tremblay M. È. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plasticity. 2014;2014:15. doi: 10.1155/2014/610343.610343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koellhoffer E. C., McCullough L. D. The effects of estrogen in ischemic stroke. Translational Stroke Research. 2013;4(4):390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ritzel R. M., Capozzi L. A., McCullough L. D. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Hormones and Behavior. 2013;63(2):238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chakrabarti M., Haque A., Banik N. L., Nagarkatti P., Nagarkatti M., Ray S. K. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Research Bulletin. 2014;109:22–31. doi: 10.1016/j.brainresbull.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pratap U. P., Patil A., Sharma H. R., et al. Estrogen-induced neuroprotective and anti-inflammatory effects are dependent on the brain areas of middle-aged female rats. Brain Research Bulletin. 2016;124:238–253. doi: 10.1016/j.brainresbull.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 172.Vegeto E., Bonincontro C., Pollio G., et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. The Journal of Neuroscience. 2001;21(6):1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plasticity. 2017;2017:17. doi: 10.1155/2017/7260130.7260130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leal G., Bramham C. R., Duarte C. B. BDNF and hippocampal synaptic plasticity. Vitamins and Hormones. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 175.Xia S. H., Hu S. W., Ge D. G., et al. Chronic pain impairs memory formation via disruption of neurogenesis mediated by mesohippocampal brain-derived neurotrophic factor signaling. Biological Psychiatry. 2020;88(8):597–610. doi: 10.1016/j.biopsych.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 176.Zhang J., Rong P., Zhang L., et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Science Advances. 2021;7(12, article eabb9888) doi: 10.1126/sciadv.abb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. The Journal of Neuroscience. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Colucci-D’Amato L., Speranza L., Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. International Journal of Molecular Sciences. 2020;21(20):p. 7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pan M., Zhang C. Stimulatory effect of gonadal hormones on fetal rat hippocampal neural proliferation requires neurotrophin receptor activation in vitro. Neuroscience Letters. 2013;546:1–5. doi: 10.1016/j.neulet.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 180.Cavus I., Duman R. S. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biological Psychiatry. 2003;54(1):59–69. doi: 10.1016/S0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 181.Singh M., Meyer E. M., Simpkins J. W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136(5):2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 182.Lee J., Duan W., Mattson M. P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 183.Spencer J. L., Waters E. M., Romeo R. D., Wood G. E., Milner T. A., McEwen B. S. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in Neuroendocrinology. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material files are available upon request.


