Abstract
The COVID-19 lockdown had a series of intended and unintended consequences, including reduced infections and changes in activities and behaviours. Some of these changes may have been beneficial to perinatal outcomes; however, other factors such as reduced access to face-to-face healthcare may have contributed negatively to antenatal care. The aim of this audit was to evaluate neonatal admissions in the South-West of England during the COVID-19 pandemic in 2020 and the previous two years 2018–2019. Anonymised birth and neonatal admission rates from January to December 2020 was obtained and compared to data from 2018 to 2019. The results demonstrate a decreasing in neonatal unit admissions between 2018 and 2020, 9.48% of live births in 2018 (95% CI 9.17, 9.80) to 8.89% (95% CI 8.65, 9.13) in 2020 (p = 0.002).
Conclusion: There were no significant differences across gestational groups. It is unclear without nationwide data whether our observed trends, decreased neonatal admissions over the past 3 years, are generalisable and related to the COVID-19 pandemic. Future research exploring the impact of lockdowns on behaviour change during pregnancy and support services is warranted to understand the implications of pandemics on pregnancy and preterm birth.
|
What is Known: • The COVID-19 lockdown had a series of intended and unintended consequences; some of which may have been beneficial to perinatal outcomes. • Research suggests that preterm births have not significantly changed overall, but they have decreased in high-income countries. | |
|
What is New: • In our audit, analysing retrospective data of regional birth and neonatal admission from the South-West of England, we observed a decrease in live birth rates between 2018 and 2020. • A reduction in neonatal unit admissions was observed from 2018 to 2020 with no significant differences across gestational groups. The reduction from 2019 to 2020 was smaller than that from 2018 to 2019 implying that the COVID-19 pandemic in 2020 was not necessarily implicated. |
Keyword: Birth, COVID-19, Neonatal intensive care units, Neonatology, Patient admissions
Background
Since the start of COVID-19 in December 2019, the UK initiated national lockdowns in March 2020 with restrictions gradually released from June 2020 and reinstated in November 2020. The lockdown had a series of intended and unintended consequences, including reduced infections, changes in activities, and work practices. During the first lockdown from March to June 2020, pregnant women were considered a vulnerable group and were recommended to ‘shield’, by remaining at home at all times. Some of these changes may have been beneficial to perinatal outcomes; however, other factors such as reduced access to face-to-face healthcare and/or reluctance to attend hospital for fear of exposure to infection may have contributed negatively to antenatal care [1, 2].
Recent studies reported changes in infants born preterm. However, findings have been inconsistent, likely due to heterogeneous populations, outcomes, and timeframes [3–5]. For example, a study using a nationwide Danish register comparing data from 5162 births between March and April 2020 to the previous 5 years, a significantly lower rate of extremely premature infants (0.9 vs 2.19/1000 births) was observed. However, there was no significant difference between 2020 and previous years for other gestational age categories [5]. A study from California of 123,853 births comparing data from April to July 2020 to the same timeframe in 2016–2019 found that preterm birth rates remained unchanged except the 28–32-week subset, which increased from 6.09 to 11.22/1000 births [4]. This change appeared to be driven primarily by Hispanic or Latin population suggesting there may be ethnic differences in preterm birth/access to healthcare. In the UK, a study from a London hospital comparing data from February–June 2020 to October 2019–January 2020 reported no significant differences in preterm births or neonatal units (NNU) admissions [3]. Overall, the first systematic review on this topic concluded that preterm births before 37 weeks’ gestation were not significantly changed overall but were decreased in high-income countries where preterm birth was also decreased [6].
In our NNUs in the South-West of England, staff perceived to be less busy in 2020 and speculated whether there had been a reduction in preterm birth in the region in response to the COVID-19 pandemic. The aim of this audit was therefore to compare neonatal admissions during 2020 to previous years (2018–2019) to determine whether staff perceptions were corroborated by numbers, considering previous publications finding conflicting results.
Methods
Anonymised data were obtained from the South-West Neonatal Network (SWNN) registry, which covers an area of 9000 square miles with a population of 4.7 million and a birth rate of ~ 45,000/year. The SWNN records all births that occur across the region, irrespective of the setting such as hospital or home births. The registry includes admissions of 12 NNUs: three Neonatal Intensive Care Units admitting infants of all gestations including referrals from the other units, six local NNUs admitting infants from 27-week gestation, and three Special Care Units admitting babies from 32-week gestation.
We analysed data of live births and neonatal admissions during 2020 and compare these with data from 2018 to 2019. The number of admissions of all extremely and very preterm (< 31+6 weeks), late preterm (31+6–36+6 weeks), and term (> 36+6 weeks) births occurring between the 1 January and 31 December 2020 was determined and compared to the 12 months in 2018 and 2019. All non-viable at birth with gestational age ≥ 22 weeks and late termination for foetal abnormalities were excluded. The number of neonatal admissions as a percentage of live births was calculated as a whole and per gestational subgroup. The relationship between the number and category of neonatal admissions and year was assessed using chi-squared tests.
Results
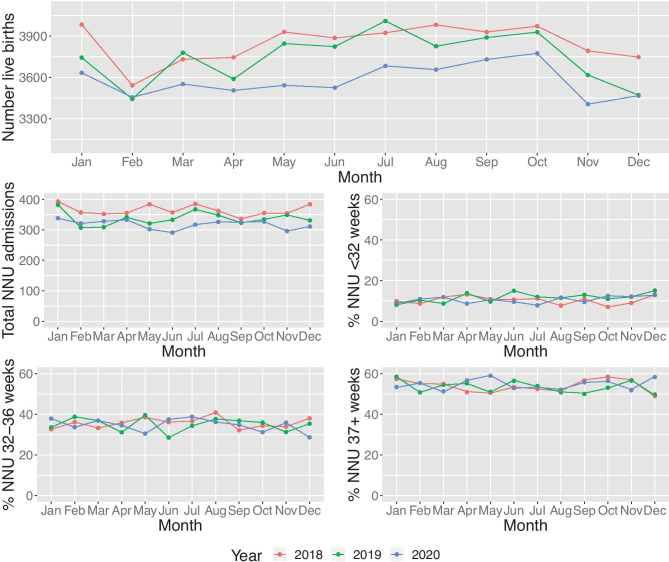
Overall, we observed a decrease in live birth rates over the 3-year period. There were no significant differences across gestational groups (p = 0.27) when comparing subcategories of early preterm, late preterm, and term (Fig. 1).
Fig. 1.
Admission trends to South-West Neonatal Units between 2018 and 2020, overall and by gestational subgroup. NNU neonatal unit
The results demonstrate a decrease of NNU admissions between 2018 and 2020 (p = 0.002) (Table 1). The drop in total number of NNU admissions is observed from 2018 (n = 4374 in 2018 to 3815 in 2020). This equates to a percentage of live birth decrease from 9.48% (95% CI 9.17, 9.80) to 8.89% (95% CI 8.65, 9.13) respectively. However, the decrease between 2018 and 2019 (n = 329) is larger than the difference between 2019 and 2020 (n = 230).
Table 1.
Number of live births and admissions in SWNN region by gestation from 2018 to 2020
| Year | Total number of live births | NNU admissions (n) (% of live births [CI]) | Infants < 32 weeks admitted to NNU (n) (% of live births [CI]) | Infants 32–36 weeks admitted to NNU (n) (% of live births [CI]) | Infants 37 + weeks admitted to NNU (n) (% of live births [CI]) |
|---|---|---|---|---|---|
| 2018 | 46,160 |
4374 9.48 [9.17,9.80] |
454 0.99 [0.85,1.12] |
1563 3.39 [3.17,3.60] |
2357 5.11 [4.92,5.30] |
| 2019 | 44,961 |
4045 9.01 [8.61,9.41] |
473 1.06 [0.91,1.20] |
1411 3.14 [2.97,3.32] |
2161 4.81 [4.48,5.15] |
| 2020 | 42,926 |
3815 8.89 [8.65,9.13] |
404 0.94 [0.85,1.04] |
1324 3.08 [2.89,3.28] |
2087 4.86 [4.67,5.06] |
CI confidence interval, NNU neonatal units
Discussion
The aim of this audit was to compare neonatal admissions in the South-West of England during 2020 to previous years. We found a decrease in NNU admissions from 2018 to 2020, confirming neonatal staff perceptions [7]. However, the decrease between 2018 and 2019 was larger than the difference between 2019 and 2020, implying that the COVID-19 pandemic was not necessarily implicated. Additionally, we found no significant difference in admissions across gestational groups, similar to a single-site UK-based study [3] and data from Philadelphia and Israel [8, 9]. However, these findings are in disagreement with data from other developed countries such as Italy, Ireland, and the Netherlands [6, 10–12].
Studies which have found changes in preterm birth rates have speculated that a number of mechanisms may be responsible for the reduction observed [10, 12]. Although there have undoubtedly been negative consequences on access to perinatal health care, some of the known risk-factors for preterm birth might have positively benefitted from COVID-19 lockdown measures. Specifically, social distancing and self-isolation, awareness of hand hygiene, working from home, and closure of school/childcare facilities contributed to a reduction in contact with pathogens and, accordingly, risk of maternal infection and vertical transmission to the offspring [12]. Also, a decrease in hospitalisations of infants with bronchiolitis was observed during the winter period of the COVID-19 pandemic [13]. However, the recent RSV outbreak in the summer of 2021 has led to a surge in admissions of infants with bronchiolitis.
Between 2010 and 2019, the rate of preterm live births in the UK has been between 7 and 8%. Reducing preterm birth is a national health priority in the UK, with an aim to reduce rates from 8 to 6% by 2025 [14]. The current strategy to improve preterm birth rates focus on three key areas of care provision: prediction, prevention, and preparation of women at high-risk preterm birth. As most morbidity and mortality is associated with births < 34-week gestation, even extending gestation for a few further weeks and reducing a relatively small number of preterm births, could have demonstrable impact on health outcomes, as well as cost and resource savings. The drop in NNU admissions in our audit is already evident from 2018 to 2019 and may be reflective of organisational and policy changes to improve preterm rates, in advance of any potential impact of national lockdown restrictions due to the COVID-19 pandemic.
We recognise that the limitation of our observations is reliant on a crude analysis of retrospective data and does not include possible explanatory factors, including induction of labour and/or other obstetric complications or any socioeconomic factors. Although a time-series analysis or alternative quasi-experimental approach could potentially provide a more robust analysis [15], the utility of such analysis here is limited by the lack of data around specific date cut-points, the unknown timing of potential impact of the various COVID-related lockdowns/restrictions, and relatively few post-lockdown time-points. In addition to lack of weekly data, stillbirths and individual level data were also not available further limiting such an approach. Additionally, we did not include COVID-19 rates in our analysis. The UK incidence admission rate with confirmed SARS-CoV-2-infection in pregnancy has been recorded at 4.9/1000 [16]. However, overall COVID-19 rates in the South-West of England have been relatively low compared to the rest of the UK, concurring the hypothesis that infection is not implicated in our observed trends.
We chose to include a full 12 months of data during the COVID-19 pandemic (January–December 2020), unlike other studies, as there is now emerging evidence that COVID-19 is likely to have been circulating prior to the official lockdown in March 2020. It is also unclear how specific behaviours changed across the whole year as lockdown restrictions in the UK were locally released and reimposed and whether those in a particular stage of pregnancy (or pregnancy planning) were most susceptible to any potential effects of the pandemic. Data from years prior to 2018 were not included to enable us to demonstrate a long-term trend; however, other studies examined a similar timeframe [8, 10, 11]. It is unclear without nationwide data whether the trends observed in our audit are generalisable. We aim to monitor the data of 2021 and beyond to understand and investigate possible effects of the extended lockdown period during the COVID-19 pandemic. Further data on maternal infection rates, the influence of emerging variants, and the effect of vaccination during pregnancy are also critical.
Our audit provided directions to revisit clinical practices such as organisational and workforce, including to ensure optimal neonatal staff to patient ratios and safe cot capacity. Additionally, investigating how best clinical services can adapt to provide family-centred care during the pandemic is important for infant and parental health outcomes [17]. Future research exploring the impact of lockdowns on behaviour change and support services during pregnancy is required to understand the implications of pandemics on pregnancy and preterm birth.
Acknowledgements
We thank the neonatal units in the South-West Neonatal Network for their support.
Authors’ contributions
RMC, JML, KM, CS, and JS initiated and designed the audit. JH and LS contributed to the data. KM and JML drafted the first manuscript. RMC, JH, LS, CS, and JS provided revisions. All authors read and approved the submitted version.
Availability of data and material
Data are available upon reasonable request from the corresponding author.
Declarations
Ethics approval
Ethical approval was not required as anonymised crude (numbers only) data was provided by the South-West Neonatal Network with the approval of the 12 individual neonatal units of the network. All procedures performed in the audit were in accordance with the ethical standards of the Declaration of Helsinki.
Consent to participate
Parents and neonatal units consented for data to be included in the South-West Neonatal Network registry.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kate Maslin, Email: kate.maslin@plymouth.ac.uk.
Roisin McKeon-Carter, Email: roisin.mckeon-carter@nhs.net.
Joanne Hosking, Email: joanne.hosking@plymouth.ac.uk.
Lauren Stockley, Email: lauren.stockley@plymouth.ac.uk.
Clara Southby, Email: clara.southby@nhs.net.
Jill Shawe, Email: jill.shawe@plymouth.ac.uk.
Jos M. Latour, Email: jos.latour@plymouth.ac.uk
References
- 1.Burki T. The indirect impact of COVID-19 on women. Lancet Infect Dis. 2020;20(8):904–905. doi: 10.1016/S1473-3099(20)30568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Curtis M, Villani L, Polo A (2020) Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed fetalneonatal-2020–320682. Online ahead of print. 10.1136/archdischild-2020-320682 [DOI] [PMC free article] [PubMed]
- 3.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L (2020) Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 324(7):705–706. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed]
- 4.Main EK, Chang SC, Carpenter AM, Wise PH, Stevenson DK, Shaw GM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224(2):239–241. doi: 10.1016/j.ajog.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedermann G, Hedley PL, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(6):e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw C, Gallagher K, Petty J, Mancini A, Boyle B. Neonatal nursing during the COVID-19 global pandemic: a thematic analysis of personal reflections. J Neonatal Nurs. 2021;27(3):165–171. doi: 10.1016/j.jnn.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals During the SARS-CoV-2 pandemic, March-June 2020. JAMA. 2021;325(1):87–89. doi: 10.1001/jama.2020.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mor M, Kugler N, Jauniaux E, Betser M, Wiener Y, Cuckle H, et al. Impact of the COVID-19 pandemic on excess perinatal mortality and morbidity in Israel. Am J Perinatol. 2021;38(4):398–403. doi: 10.1055/s-0040-1721515. [DOI] [PubMed] [Google Scholar]
- 10.Berghella V, Boelig R, Roman A, Burd J, Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2(4):100258. doi: 10.1016/j.ajogmf.2020.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonnell S, McNamee E, Lindow SW, O'Connell MP. The impact of the Covid-19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5(11):e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stera G, Pierantoni L, Masetti R, Leardini D, Biagi C, Buonsenso D, et al. Impact of SARS-CoV-2 Pandemic on bronchiolitis hospitalizations: the experience of an Italian tertiary center. Children (Basel) 2021;8(7):556. doi: 10.3390/children8070556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Health (2017) Safer maternity care: next steps towards the national maternity ambition. London. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662969/Safer_maternity_care_-_progress_and_next_steps.pdf. Accessed 5 Jun 2021
- 15.de Vocht F, Vittal Katikireddi S, McQuire C, Tilling K, Hickman M, Craig P. Conceptualising natural and quasi experiments in public health. BMC Med Res Methodol. 2021;21(1):32. doi: 10.1186/s12874-021-01224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green J, Petty J, Whiting L, Fowler C. Exploring modifiable risk-factors for premature birth in the context of COVID-19 mitigation measures: a discussion paper. J Neonatal Nurs. 2021;27(3):172–179. doi: 10.1016/j.jnn.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request from the corresponding author.