Abstract
The region around the plant root referred to as the rhizosphere, is the zone where various microbial activity occurs. It performs crucial functions such as increasing the uptake of nutrients for plant development and preventing plant against plant pathogens. Keeping in mind the beneficial role performed by rhizospheric microorganisms, rhizobacterial species were isolated from the maize and soybean plant's rhizosphere. The isolated microorganisms were evaluated for their biochemical characteristics, plant growth-promoting potentials, tolerance to different environmental conditions, and their antifungal activity against Fusarium graminearum, a fungal pathogen that infects maize. The rhizobacterial isolates with multiple plant growth-promoting potentials were identified as Bacillus spp (80.77%), Rhodocyclaceae bacterium (3.85%), Enterococcus spp (3.85%). Massilia spp (3.85%. and Pseudomonas (7.69%) species based on their 16S rRNA molecular characterization. The bacterial isolates possessed antifungal activities against Fusarium graminearum, promote maize and soybeans seed under laboratory conditions, and exhibited different levels of tolerance to pH, temperature, salt, and heavy metal. Based on this, the whole genome sequencing of Bacillus sp. OA1, Pseudomonas rhizosphaerea OA2, and Pseudomonas sp. OA3 was performed using Miseq Illumina system to determine the functional genes and secondary metabolites responsible for their plant growth-promoting potential Thus, the result of this research revealed that the selected bacterial isolates possess plant growth-promoting potentials that can make them a potential candidate to be employed as microbial inoculants for protecting plants against phytopathogens, environmental stress and increasing plant growth and productivity.
Keywords: 16S rRNA gene, Bioinformatics, Antifungal activity, Beneficial bacterial, Microbial inoculant, Plant growth
Introduction
The rhizosphere is a region in the soil near the plant root, it is an interface between the soil and plant roots (Fasusi et al. 2021). It can be described as a home for plant roots where it is colonized by an enormous number of microbial species such as bacteria which are influenced by the organic compound that are present in the plant root, which is of great economic importance in promoting plant growth (Odelade and Babalola 2019). During plant germination, an interaction occurs between the plant root and the soil around it. This results in the release of organic matter via the soil, which promotes the activities of microorganisms like bacteria in the plant root region, which could be beneficial to enhance plant growth (Gouda et al. 2018). Plant growth-promoting rhizobacteria (PGPR) are beneficial bacteria inhabiting the rhizosphere of plants (Prasad et al. 2019). The plant rhizosphere consists of numerous PGPR species such as Bacillus Azospirillum, Klebsiella, and Paenibacillus azotofixans., Pseudomonas, Rhizobium, Azotobacter Acinetobacter, and Bacillus, which are recognized for their potential to stimulate plant growth (Goswami et al. 2016). These PGPR species possess plant growth-promoting potentials that stimulate native plant growth, yield, and defense through different mechanisms such as phytohormones production indole-3-acetic acid (IAA) production (Asari et al. 2017), Phosphate solubilization (Majeed et al. 2015), production of hydrogen cyanide to suppress the deleterious effect of plant pathogens, production of metabolites, production of siderophore which promotes the uptake of iron and nutrient from the environment and making it available for plant growth through the root system, production of lytic enzyme and antimicrobial compounds (Kejela et al. 2016) or production of the fungicidal compound, competitive with plant pathogens and enhancing plant tolerance to biotic and abiotic stress like metal, temperature, water, and salinity (Tewari and Arora 2013; Zolla et al. 2013). The mechanisms of promoting plant growth and defense by these rhizobacteria could be direct or indirect.
Globally, with an increase in the human population, challenges of food security, soil infertility, and the pressure of increasing food production are emerging (Raklami et al. 2019). Cereals and legumes such as soybean and maize are regarded as food crops of great importance and value in human diet. Their production is reduced due to soil infertility; hence, the demand for food by the increasing world population is not entirely met (Raklami et al. 2019). Therefore, the application of agrochemicals in the modern agricultural system to supply the nutrients needed for plant growth and protection of plants against pathogen attacks worldwide arises. However, the cost of purchase and the harmful effects of these chemicals on the environment is of great concern in agricultural practices (Yadav et al. 2018). Therefore, there is an urgent need to research beneficial soil bacteria as alternative means of increasing plant growth and health, intensifying soil fertility, and protecting plants from pathogenic infections that will ensure environmental safety and provide a long-time balance to the ecosystem (Chauhan et al. 2016).
Interest in the PGPR associated with food crops has dramatically increased. It is considered an alternative in promoting plant growth in different areas such as biofertilizers, biopesticides, and probiotics under favorable and unfavorable conditions. The application of PGPR in ensuring agricultural sustainability is a practice that is cost-effective, environmentally friendly, productive, and more efficient over the use of agrochemicals (Itelima et al. 2018). However, little is known on the functional genes and secondary metabolites that are responsible for plant growth promotion. Consequently, knowledge of PGPR, specific to food crops such as soybeans and maize, their characterization, and plant growth potential in promoting plant growth toward ensuring sustainable agricultural practices is needed.
In light of the detrimental effect of chemical fertilizer application on human health and the environment, this study aimed to isolate and characterize rhizobacterial with multiple plant growth-promoting activity from soybeans and maize rhizosphere, evaluate their response to environmental stress, and antagonistic activities against Fusarium graminearum a pathogenic fungus that infects cereals. The ability of the rhizobacteria to enhance maize and soybean in vitro was evaluated and the whole genome sequencing of the best isolates was performed to determine the functional genes and secondary metabolites responsible for plant growth promotion in the bacteria genome. Thus, this study can be a basis for application of rhizobacterial strains for biofertilizers production to enhance plant growth and in ensuring agricultural sustainability.
Materials and methods
Study site and collection of soil samples
Soil samples were collected from maize and soybean rhizosphere planted in a cultivated field at North-West University Agricultural-Farm, Molelwane, North-West Province, South Africa. The geographical coordinate of the soil sampling sites is (25° 47′ 25.24056″ S 25° 37′ 8.17464″ E) and (25° 47′ 30.14056″ S 25° 37′ 9.27464″ E). The intact root system of the plants was dug out and the rhizospheric soil that attaches to it was collected in sterile plastic bags using a sterile technique method, labelled, and transported to the Microbial Biotechnology Research Laboratory, North-West, University South Africa in the icebox. The samples were stored at − 20 °C for further analysis.
Isolation of rhizobacteria from the rhizospheric soil
Isolation of rhizospheric bacteria from maize (Zea mays L.) and soybeans (Glycine max) rhizosphere was carried out using the agar plate method (Karnwal and Kumar 2012). Approximately 10 g of the soil sample from the rhizosphere of maize was collected aseptically and transferred to 90 ml of sterile distilled water inside a 250 ml conical flask with proper stirring. Exactly, 1 ml from the stock was used for a serial dilution with seven test tubes filled with 9 ml of sterile distilled water and 0.1 ml of the suspension in the test tubes was transferred from suitable dilution on Luria Bertani agar (Sigma-Aldrich, Saint Louis, MO, USA) plate in triplicate and incubated at 28 ± 2 °C for 24 h until colonies developed. After incubation, distinct colonies were picked with a sterile wire loop and streaked onto a pre-sterilized Luria Bertani agar plate, incubated at 28 ± 2 °C for 24 h for purification of the bacterial isolates.
Morphological and biochemical characterization of rhizobacterial isolates
The isolated rhizobacterial morphological features were examined on Luria Bertani agar plates using pure cultures of the bacterial isolates which were incubated at 28 ± 2 °C for 24 h. The morphology of the colonies like the size, shape, colour, and pigmentation was recorded after incubation. Gram reaction for the bacterial isolates was conducted. The biochemical characterization of the bacterial isolates was conducted this includes catalase test, oxidase test, starch hydrolysis, citrate, nitrate reduction test, Voges Proskauer test, casein test, indole test, using the standard procedure described by (Clarke and Cowan 1952). The utilization of carbohydrates by the bacterial isolates was conducted using different carbon sources such as maltose, lactose, glucose, sucrose, fructose, xylose, and galactose.
In vitro plant growth-promoting traits test
Production of indole-3-acetic acid (auxin activity test)
Indole-3-acetic acid production by the rhizobacterial species was determined using the method described by Gordon and Weber (1951) with little modifications. Precisely 0.05 L Luria Bertani broth enriched with 0.1% (D)l-tryptophan inside test tubes was inoculated with a freshly grown overnight culture of each bacterial isolate (500 µl) incubated using a shaking incubator at 30 °C and 180 rpm for 48 h in the dark. After incubation, the bacterial culture was centrifuged at 1000 rpm using a cold centrifuge at 4 °C for 10 min. After centrifugation, 2–3 drops of orthophosphoric acid and 4000 µl of Salkowski reagent (0.05 L of 35% perchloric acid and 1 ml of 0.5 M FeCl3 · 6H2O) was added to 2000 µl of the supernatant (Ahmad et al. 2008). The mixture was incubated in the dark for 30 min and observed for pink coloration which is an indication of IAA production.
Determination of phosphate solubilization
Phosphate solubilization by the bacterial isolates was evaluated according to the method described by Verma et al. (2001). Pikovskaya agar compounded consisting of the following composition/litres: (KCl 200 mg, MgSO4.7H2O 100 mg, MnSO4.H2O 500 mg, Ca3(PO4)2 5 g, FeSO4.7H2O 1 mg, yeast extract 500 mg, glucose 10 g, agar 15 g, (NH4)2SO4 0.5 g) the pH of the mixture was adjusted to pH 7 using digital pH meter and sterilized at 121 °C for 15 min. Exactly 20 ml of the sterilized medium was poured inside the Petri dishes and left for a while to solidify. Pure culture of the bacterial colony was inoculated on the Pikovskaya agar using the spot inoculation method and Petri plates were incubated at 30 °C for 5–7 days. The observation of clear zone around the bacterial colony was an indication of positive results.
Hydrogen cyanide production
Production of hydrogen cyanide (HCN) by the bacterial isolates was conducted using the method described by Castric (1975). Each bacterial isolate was inoculated on sterile LB agar supplemented with glycine (4.4 g/L) using the streaking method. Whatman filter papers were sterilized and dipped inside an alkaline picric acid solution (2% Na2CO3 in 0.5% picric acid). The filter paper containing the picric acid solution was placed on the upper lid of the Petri plates containing the bacterial inoculum. Before incubation, the Petri plates were sealed with parafilm and incubated at 30 ± 1 °C for 96 h. After incubation, the filter paper was examined for colour change from yellow to red-brown, which was an indication for hydrogen cyanide production.
Ammonia production
The bacterial isolates were examined for ammonia production using the method described by Cappuccino and Sherman (1992). Evaluation for ammonia production was conducted by inoculating an overnight culture of each bacterial isolates in 10 ml of sterile peptone water prepared according to the manufacturer's instruction and were incubated at 30 °C for 48 h on a shaking incubator. After incubation, 0.5 ml Nessler’s reagent with the following composition (H2O 79.65%, KI 3.49%, NaOH 12.49%, HgI2 4.37%) was added to the samples. The development of slight yellow to brownish colour was an indicator of ammonia production.
Production of siderophore
The production of siderophore by the bacterial isolates was determined using chrome-azurol S (CAS) as an indicator dye following the method of Schwyn and Neilands (1987). Briefly, the sterile CAS agar plate was prepared with the following composition (60.5 mg chrome-azurol S dye in 50 ml H2O) and mixed with 10 ml iron (III) solution prepared as follows (1 Mm FeCl3.6H2O in 10 mM HCl). The solution mixture was gently added to hexadecyltrimethylammonium bromide (HDTMA) (72.9 mg in 40 ml H2O) and sterilized at 121 °C for 15 min. Approximately100 ml of CAS dye solution was poured into 900 ml of LB agar. Before sterilization of the LB agar, the pH was adjusted to 6.8 using a digital pH meter, sterilized at 121 °C for 15 min, the sterile CAS agar medium was poured into the Petri plate and allowed to solidify. After solidification, the CAS agar plate was spot inoculated with a 24 h old bacteria colony and incubated at 30 °C for 48–72 h. After incubation, the plate was observed for colour change in the medium from blue to an orange or yellow zone of clearance around the bacterial colonies, which was an indication for the production of siderophore.
Exopolysaccharide production
The production of exopolysaccharides by the bacterial isolates was evaluated following the method described by Paulo et al. (2012) with few modifications. LB agar was prepared according to the manufacturer's instruction and was amended with 10% sucrose. The pH of the mixture was adjusted to 7 using a digital pH meter before sterilization. The medium was sterilized at 121 °C for 15 min using an autoclave. Sterile Whatman filter paper was cut into 6 mm in diameter and was placed on solidified nutrient agar amended with 10% sucrose in Petri dishes. After that, 2 µl of 24 h old bacterial culture was inoculated on the sterile filter paper placed on agar inside the Petri plate, and the plates were incubated at 30 °C for 2 days. After incubation, plates were observed for the formation of mucoid colonies around the filter paper, which was an indication of the production of exopolysaccharide.
Evaluation of antifungal activity of the bacteria isolates
The bacterial isolates were evaluated for antagonistic activities against pathogenic fungi belonging to the genera of Fusarium using the dual culture techniques described by Skidmore and Dickinson (1976). Fusarium graminearum was collected from the Microbial Biotechnology Laboratory, North-West University, South Africa. The fungus was grown on potato dextrose agar (PDA) plates and incubated at 30 °C for 7 days to ensure that the mycelium of the fungus fully grew on the plate. The fungus was picked using a sterile cock borer and syringe and placed at the centre of a Petri plate containing solidified sterile PDA. Simultaneously, an overnight grown culture of the bacterial isolate was inoculated by streaking method at 2.5 cm from the center of the Petri plate. The control plate was maintained by placing the pathogenic fungus on the PDA agar plate without bacteria. The plates were incubated at 30 °C in an incubator for 7 days and were observed for inhibition zone. The percentage of the fungal mycelial inhibition by the bacterial was calculated using the formula described by Noumavo et al. (2015):
where V1 = The diameter of the fungus growth (control) and V2 = the diameter of the fungus growth in the dual culture plate.
Genomic sequencing of the bacterial isolates using 16S rRNA analysis
The genomic DNA from each bacterial isolate was extracted using the Zymo soil microbe’s extraction kit (Zymo Research, USA) following the manufacturers instruction.
PCR gene amplification was conducted using a reaction volume of 25 µl containing (0.5 µl forward primer, 0.5 µl reverse primer, 12.5 µl PCR master mix, 2 µl DNA template, 9.5 µl nuclease-free water). Amplification was conducted using 16S rRNA universal primer for bacteria (341F) (5′-CCTACGGGAGGCAGCAG-3′), and (907 R) (5′-CCCCGTCAATTCCTTTGAGTTT-3′) (Fukuda et al. 2016). PCR was performed using the Bio-Rad C 1000 touch thermal cycler and it was programmed at 94 °C for 2 min for initial denaturation, then 94 °C for 30 s, annealing temperature at 59 °C for 1 min, extension at 72 °C in 2 min for 35 cycles, and a final extension at 72 °C in 8 min.
A 1.5% agarose gel containing ethidium bromide was prepared and used to check the quality of the PCR products. The gel was observed using a gel documentation system (Gel Doc 2000, Bio-Rad, USA). Thereafter, PCR products were analyzed for sequencing with the specific universal 16S rRNA primers (341 F) (5′-CCTACGGGAGGCAGCAG-3′), and (907 R) (5′-CCCCGTCAATTCCTTTGAGTTT-3′) at Inqaba Biotechnology Laboratory, South Africa. The sequence from each region of each bacterial isolates was cleaned using Chromas Lite Version 2.1 and edited with Bio Edit Sequence Alignment Editor (Aremu and Babalola 2015; Hall 1999). The consensus sequence was generated and Blast on the NCBI database (www.ncbi.nlm.nih.gov/Blast.cgi) using the Basic Alignment Search Tool (Blastn) for identification of the closest representative strains of the bacterial isolates. The result of the sequence obtained was compared with other sequences in the NCBI GenBank data. The consensus sequence of the bacterial isolates was deposited in the NCBI GenBank database.
Effect of the rhizobacterial strains on in vitro seed germination
The effect of the rhizobacteria strains on in vitro seed germination of maize and soybean seed was evaluated using the method described by Gholami et al. (2009) with little modification. Petri dishes were prepared by placing sterile filter paper inside them. Thereafter, the Petri dishes were inoculated with 10 ml of each rhizobacterial suspension (107 CFU/ml) in replicates and were inoculated with 10 sterile maize (Capstone seed, Nelson Choice variety) and soybean (Pannar seed., PAN 1532 variety) seeds as described by Madhaiyan et al. (2007) while the control plate was inoculated with sterile distilled water. The seeds were incubated at 28 °C for 7 days in a growth chamber. After incubation, the percentage seed germination, of the maize and soybean seedlings were measured using the formula below:
where GS = Number of seeds that germinated after 7 days and.
Z = The total number of seeds in each Petri dish.
Bacterial tolerance to different pH
Tolerance of bacterial isolates to different pH was evaluated by preparing LB broth according to the manufacturer’s instruction, the pH of the LB broth was adjusted to acidic, neutral, and alkaline (4,7,10) using 1MHCl or 1 M NaOH and sterilized at 121 °C for 15 min. Exactly 20 µl from an overnight culture of each bacterial isolate was inoculated into sterile 10 ml LB broth and homogenized. Inoculation of the bacterial isolate was done in triplicate, and the inoculated LB broth was incubated at 30 °C on a rotary shaker at 150 rpm for 48 h. The optical density of the bacterial growth was taken at 600 nm using a UV Spectrophotometer (Thermo Spectronic; Merck).
Tolerance of bacterial isolates to different temperatures
The bacterial isolates' tolerance to different temperatures was evaluated by preparing LB broth and sterilized at 121 °C for 15 min. Approximately 5 µl of each bacterial strain was inoculated into 25 ml LB broth and gently vortexed. Inoculation of each bacterial isolate was replicated in triplicate and the inoculated LB broth was incubated at 30, 35, 40 °C. At 24 h of incubation optical density of the bacterial growth was taken at 600 nm using a spectrophotometer (Thermo Spectronic; Meck, South Africa) (Ndeddy Aka and Babalola 2016).
Bacterial growth response to different salt concentrations
The effect of bacterial strain tolerance to different salt concentrations (NaCl) was evaluated by preparing LB broth according to the manufacturer's instruction and supplemented with different salt concentrations (1%, 3%, and 5%). The tubes containing 10 ml of the LB broth supplemented with different salt concentrations were inoculated with 100 µl overnight culture of bacterial and were incubated at 30 °C on a shaking incubator at 150 rpm for 72 h. The absorbance of the bacterial growth was measured at 600 nm using a UV Spectrophotometer (Thermo Spectronic; Merck).
Bacterial tolerance to different heavy metals
The tolerance of each bacterial isolate to different heavy metals was evaluated in test tubes by preparing the LB broth and supplemented with 100 mg/l of different heavy metals (PbCl2, K2Cr2O7, and CdSO4. 8H2O). Exactly 10 ml of LB broth containing different heavy metals was dispensed inside test tubes, sterilized at 121 °C for 15 min, inoculated with 1 ml overnight culture of the bacterial strain, and incubated at 30 °C on a rotary shaker at 150 rpm for 72 h. The bacterial growth's optical density was taken after incubation at 600 nm using a UV Spectrophotometer (Thermo Spectronic; Merck) (Ndeddy Aka and Babalola 2016).
Whole-genome sequencing of rhizobacterial strains
Extraction of deoxyribonucleic acid (DNA), sequencing and reads assembly
Based on the result of the in vitro plant growth-promoting test and seed germination potentials of the rhizobacterial strains. Three rhizobacterial strains (Bacillus sp. strain OA1, Pseudomonas rhizosphaerae strain OA2 and Pseudomonas sp. strain OA3) were selected for whole-genome sequencing to determine the functional genes and secondary metabolites that are responsible for plant growth promotion in these rhizobacterial genomes. Fresh culture of the rhizobacterial strains was prepared by streaking the strains separately on sterile LB agar plates incubated at 28 °C for 24 h. Thereafter, the DNA from the fresh bacterial isolates was extracted using Zymo microbe’s extraction kit following the manufacturer's instruction. The extracted DNA was checked for purity by gel electrophoresis and the concentration of the DNA was also checked using the NanoDrop spectrophotometer. Approximately, 4 µl of the extracted DNA from each bacterial isolate was sent for sequencing in an ice pack at Agricultural Research Council (ARC) Biotechnology Platform Pretoria, South Africa for MiSeq system (illumina) sequencing.
Prior, to the DNA sequencing the library was prepared using Illumina Nextera DNA Flex library preparation kit following the manufacturer’s instruction and sequencing was conducted using the MiSeq illumina system. The sequenced read generated from each DNA was in 2 × 250 bp and was visualized using KBase (Arkin et al. 2018). The quality of the reads was checked using Fast QC version 1.0.4 (Andrews 2010), Trimmomatic version 1.2.14 (Bolger et al. 2014) was used to trim low-quality reads and adapter, and the high-quality reads were assembled using SPAdes version 1.2.4 (Nurk et al. 2013). Default parameters were used for all the software unless otherwise stated.
Annotation
The genome of Bacillus sp. strain OA1, Pseudomonas rhizosphaerae strain OA2, and Pseudomonas sp. strain OA3 were annotated using Rapid Annotation using Subsystem Technology (RAST) (Overbeek et al. 2014). The RAST permits the annotation of functional genes (manual curation of gene annotation) that are present in the bacterial genome. The result of the annotation was used to determine the contigs location where the genes responsible for plant growth-promoting potentials are located in each rhizobacteria genome. Thereafter, antiSMASH was used to determine the secondary metabolites that are responsible for the plant growth promotion in the bacterial genome. All the data generated have been deposited in the National Center for Biotechnology Information (NCBI) database. The raw reads are available under the Bioproject accession numbers (PRJNA683742- Bacillus sp. OA1, PRJNA683763- Pseudomonas rhizosphaerae OA2 and PRJNA683768- Pseudomonas sp. OA3) and Biosample numbers (SAMN17036205- Bacillus sp. OA1, SAMN17036358- Pseudomonas rhizosphaerea OA2 and SAMN17036469- Pseudomonas sp. OA3). The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number (SRX9654894- Bacillus sp. OA1, SRX9654938- Pseudomonas rhizosphaerae OA2 and SRX9654998- Pseudomonas sp. OA3).
Statistical analysis
All the data obtained from experiments were conducted in triplicates. Data were imputed using Microsoft excel and analysis of variance (ANOVA) was used to analyze the difference among the other parameters and Duncan multiple tests at 5% (P < 0.05) probability level was used to check the significance level using SPSS statistical package (v 20.0).
Result
Isolation, biochemical and molecular characterization of rhizobacteria
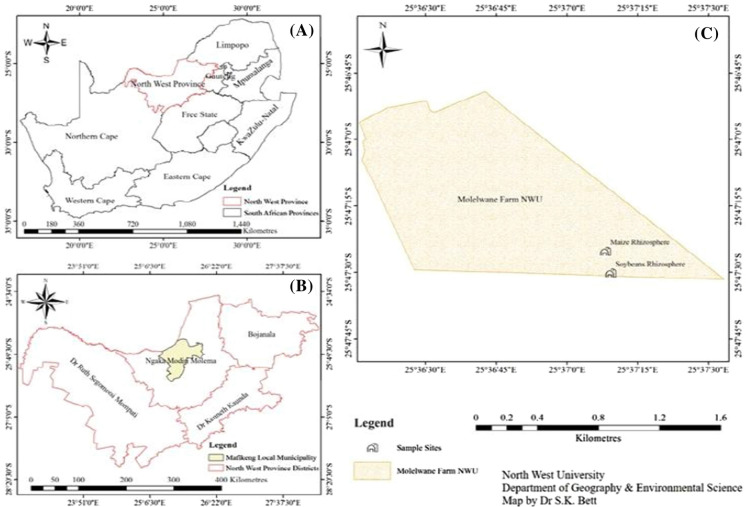
As indicated in Fig. 1, the map showed the geographical location where the rhizospheric soil sample was collected. In this study, twenty-six plant growth-promoting rhizobacteria isolated from maize and soybeans plant rhizosphere were evaluated for their morphological, biochemical characteristics, and plant growth-promoting traits. The bacterial isolates possessed multiple plant growth-promoting characteristics as revealed by their in vitro plant growth-promoting characteristics test conducted. The cultural and biochemical characteristics of the twenty-six bacterial species isolated revealed that the strains were Bacillus spp. (21)., Rhodocyclaceae bacterium (1), Enterococcus spp. (1), Massilia spp. (Oxalobacteriaceae) (1), and Pseudomonas spp. (2). The number in bracket represent the number of the bacterial species. Twenty-two strains were found to be gram-positive MNWU1, SNWU1, MNWU2, MNWU3, SNWU3, SNWU4 MNWU4, MNWU5, SNWU5, MNWU6, MNWU7, SNWU7, SNWU8, SNWU9, MNWU10, SNWU10 SNWU14, MNWU3, MNWU14, MNWU16, MNWU15, SNWU15, except MNWU4, SNWU13, MNWU16, SNWU17 which are gram-negative. All the twenty-six strains were able to show different morphological and biochemical characteristics (Table 1).
Fig. 1.
Geographical location of maize and soybeans rhizosphere soil used for isolation of rhizobacterial. a The map of South Africa showing North West Province (red sketch) b map of North West Province showing the map of Ngaka Modiri Molema district where Mafikeng Local Municipal is located (light yellow region) c map of the North-West University Molelwane where maize and soybeans rhizospheric soil sample was collected for rhizobacteria isolation. (Color figure online)
Table 1.
Biochemical characteristics of selected plant growth-promoting rhizobacteria isolates
| Isolate code | Cellular shape | Colonial elevation | Colonial edge | Colonial opacity | Colonial surface | Colonial pigmentation | Cellular arrangement |
|---|---|---|---|---|---|---|---|
| MNWU14 | Rod | Raised | Lobate | Translucent | Dull | Whitish Cream | Clusters |
| SNWU3 | Rod | Raised | lobate | Opaque | Dull | Whitish Cream | Clusters |
| SNWU14 | Rod | Raised | Lobate | Translucent | Dull | Whitish Cream | Chain |
| SNWU15 | Rod | Raised | Entire | Opaque | Dull | Whitish Cream | Clusters |
| SNWU5 | Rod | Raised | Lobate | Opaque | Dull | Whitish Cream | Clusters |
| SNWU13 | Rod | Raised | Lobate | Translucent | Smooth | Whitish Cream | Clusters |
| MNWU5 | Rod | Flat | Entire | Opaque | Dull | Whitish Cream | Chain |
| MNWU16 | Rod | Raised | Lobate | Opaque | Pigmented | Whitish Cream | Clusters |
| SNWU1 | Rod | Raised | Entire | Opaque | Smooth | Whitish Cream | Clusters |
| MNWU4 | Rod | Raised | Lobate | Opaque | Smooth | Whitish Cream | Clusters |
| MNWU15 | Rod | Raised | Entire | Opaque | Smooth | Whitish Cream | Clusters |
| SNWU9 | Rod | Raised | Entire | Translucent | Dull | Whitish Cream | Clusters |
| MNWU2 | Rod | Raised | Entire | Translucent | Dull | Whitish Cream | Clusters |
| MNWU3 | Cocci | Flat | Entire | Opaque | Dull | Whitish | Cluster |
| MNWU6 | Rod | Raised | Entire | Opaque | Dull |
Creamy White |
Clusters |
| SNWU7 | Rod | Raised | Lobate | Translucent | Smooth |
Creamy White |
Clusters |
| MNWU1 | Rod | Raised | Lobate | Opaque | Dull | Cream | Custer |
| SNWU8 | Rod | Raised | Lobate | Opaque | Dull | Whitish | Clusters |
| MNWU10 | Rod | Flat | Lobate | Opaque | Dull | Whitish | Clusters |
| SNWU16 | Rod | Raised | Lobate | Opaque | Smooth |
Creamy White |
Clusters |
| MNWU4 | Rod | Raised | Lobate | Opaque | Dull | White | Clusters |
| MNWU7 | Rod | Raised | Entire | Opaque | Smooth |
Creamy White |
Clusters |
| MNWU13 | Rod | Raised | Entire | Opaque | Smooth |
Creamy White |
Clusters |
| SNWU4 | Rod | Raised | Entire | Opaque | Smooth |
Creamy White |
Clusters |
| SNWU10 | Cocci | Raised | Entire | Opaque | Smooth |
Creamy White |
Clusters |
| SNWU17 | Rod | Raised | Lobate | Opaque | Pigmented | Grey | Clusters |
| Gram’s staining | Motility test | Spore staining | Catalase | Starch Hydrolysis | Citrate | VP | Casein | Indole |
|---|---|---|---|---|---|---|---|---|
| + | + | + | + | + | + | + | + | − |
| + | + | + | + | + | + | + | + | − |
| + | + | + | + | − | + | + | + | − |
| + | + | + | + | + | + | − | − | − |
| + | + | + | + | + | + | − | + | − |
| − | − | − | + | + | − | + | − | − |
| + | + | + | + | + | − | − | + | − |
| − | − | − | + | − | + | − | − | − |
| + | − | − | + | + | + | − | + | − |
| − | + | − | + | + | + | − | + | − |
| + | + | + | + | − | − | − | − | − |
| + | + | + | + | + | + | − | + | − |
| + | + | + | + | + | − | − | + | − |
| + | − | − | + | − | + | − | + | − |
| + | + | + | + | + | − | − | + | − |
| + | − | + | + | + | − | − | − | − |
| + | + | + | + | + | − | − | + | − |
| + | + | + | + | + | + | − | + | − |
| + | + | + | + | + | + | − | + | − |
| + | + | + | + | − | − | − | − | − |
| + | + | + | + | − | + | − | + | − |
| + | − | − | + | + | + | − | + | − |
| + | + | + | + | − | + | − | + | − |
| + | + | + | + | + | + | − | + | − |
| + | + | + | + | + | + | − | + | − |
| − | − | − | + | − | + | − | + | − |
| Isolate code | Nitrate reduction | Oxidase | Carbohydrates Utilization | Fructose | Xylose | Galactose | |||
|---|---|---|---|---|---|---|---|---|---|
| Maltose | Lactose | Glucose | Sucrose | ||||||
| MNWU14 | + | + | + | + | + | + | + | − | − |
| SNWU3 | + | − | + | − | + | − | − | − | − |
| SNWU14 | + | + | + | − | + | + | − | − | − |
| SNWU15 | + | + | + | − | + | − | + | − | − |
| SNWU5 | + | + | + | + | + | + | + | − | − |
| SNWU13 | + | − | + | − | + | + | − | − | − |
| MNWU5 | + | + | + | − | + | − | − | − | − |
| MNWU16 | + | + | + | − | − | + | − | − | − |
| SNWU1 | + | − | + | + | + | + | + | − | − |
| MNWU4 | + | + | + | − | + | − | + | − | + |
| MNWU15 | + | − | + | − | + | − | − | − | − |
| SNWU9 | + | + | + | + | + | + | + | − | − |
| MNWU2 | + | + | + | − | + | + | + | − | − |
| MNWU3 | + | + | + | + | + | + | + | − | − |
| MNWU6 | + | + | + | − | + | + | + | − | − |
| SNWU7 | + | − | + | + | + | + | + | − | − |
| MNWU1 | + | + | + | − | + | + | + | + | − |
| SNWU8 | + | − | + | − | + | + | − | − | − |
| MNWU10 | + | − | + | + | + | + | + | − | − |
| SNWU16 | + | + | + | − | + | + | − | − | − |
| MNWU4 | + | + | + | + | + | + | + | − | + |
| MNWU7 | + | − | + | + | + | − | + | − | − |
| MNWU13 | − | + | + | − | + | − | + | − | − |
| SNWU4 | + | + | + | − | + | + | + | − | − |
| SNWU10 | + | + | + | − | + | + | + | − | − |
| SNWU17 | + | + | + | − | + | + | + | − | − |
− = Negative, + = Positive
In vitro plant growth-promoting activities of the bacterial isolates
Rhizobacteria promote plant growth by different mechanisms, which include phosphate solubilization, ammonia production, production of hydrogen cyanide production of exopolysaccharide, production of siderophore that make iron unavailable to plant pathogens and enhancing the secretion of plant hormones like indole-3- acetic acid. Among the bacterial species isolated from maize and soybean rhizosphere, 46% showed a significant zone of clearance on Pikovskaya agar used in the identification of phosphate solubilization. The result of the IAA production revealed that all the rhizobacterial strains were able to produce IAA in a medium amended with L-tryptophan. Moreover, all the bacterial strains showed a positive reaction for ammonia and siderophore test using peptone water. In this study, the bacterial isolates were all positive to the exopolysaccharide test responsible for their tolerance to different environmental stress (Table 2).
Table 2.
Selected rhizobacterial isolates with multiple plant growth-promoting traits
| Isolates code | Phosphate solubilization | HCN production | Siderophore production | Ammonia production | IAA production | Exopolysaccharide production |
|---|---|---|---|---|---|---|
| MNWU14 | − | − | + | + | + | + |
| SNWU3 | + | + | + | + | + + | + |
| SNWU14 | + | − | + | + | + | + |
| SNWU15 | + | + | + + | + | + | + |
| SNWU5 | + | − | + | + | + + | + |
| SNWU13 | − | − | + | + | + + | + |
| MNWU5 | − | + | + | + | + + | + |
| MNWU16 | + | − | + | + | + + | + |
| SNWU1 | + | − | + | + | + + | + |
| MNWU4 | − | + | + | + | + | + |
| MNWU15 | + | − | + + | + | + | + |
| SNWU9 | + | − | + | + | + | + |
| MNWU2 | − | − | + | + | + + | + |
| MNWU3 | + | + | + | + | + | + |
| MNWU6 | − | + | + | + | + + | + |
| SNWU7 | − | − | + | + | + + | + |
| MNWU1 | − | − | + | + | + | + |
| SNWU8 | − | − | + | + | + + | + |
| MNWU10 | − | − | + | + | + | + |
| SNWU16 | − | − | + + | + | + | + |
| MNWU4 | + | − | + | + | + + | + |
| MNWU7 | − | − | + | + | + | + |
| MNWU13 | + | + | + | + | + + | + |
| SNWU4 | − | + | + + | + | + | + |
| SNWU10 | − | − | + | + | + + | + |
| SNWU17 | + | + | + | + | + + | + |
+ = presence of trait, + + = highly presence of trait, − = absence of trait
Antifungal activity
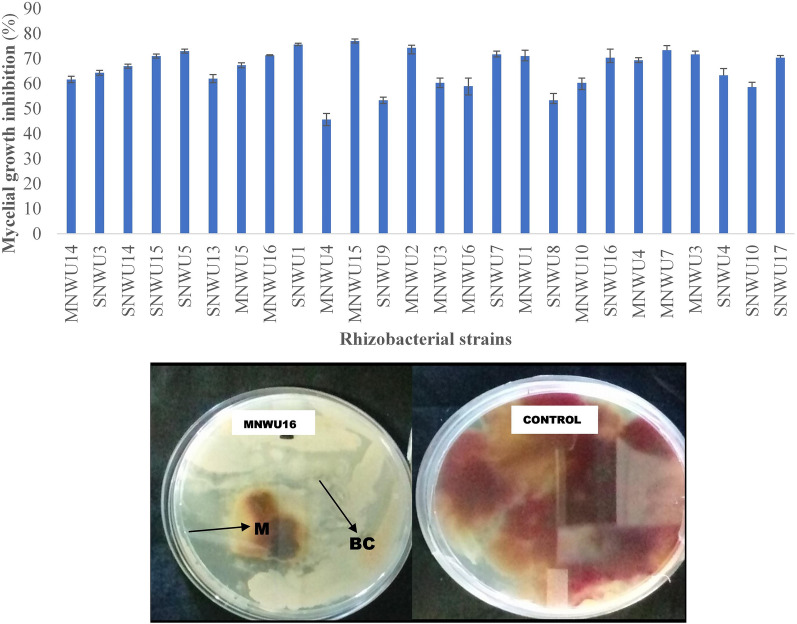
The antifungal activity of the bacterial isolates against Fusarium graminearium, a fungus pathogen that infects maize using a dual assay culture method, was positive for all the bacterial. Based on the in vitro antifungal activity,46.1% of rhizobacterial strains recorded a percentage of fungal mycelium inhibition of 70% and above while 50.9% of the isolates resulted in the percentage of fungal mycelium inhibition of less than 70% (Fig. 2).
Fig. 2.
Antifungal activity of the rhizobacterial isolates against Fusarium graminearium. M = Fungal mycelia, BC = bacterial colony, and the control plate
Molecular characterization of the bacterial isolates using 16S rRNA primer
The identification of the rhizobacterial strains with multiple plant growth-promoting traits characterize with molecularly using 16S rRNA universal primer for bacterial characterization, as shown in (Table 3). The consensus sequence obtained from each isolate were BLAST using Blastn and submitted to NCBI GenBank. Based on 16S rRNA gene characterization, the bacterial species isolated from the rhizosphere of maize and soybean plants were divided into Firmicutes, Beta, and Gammaproteobacteria classes. The bacterial isolates belong to different genera such as Bacillus, Enterococcus, Massilia, Pseudomonas, and Rhodocyclaceae. All the sequences generated by the bacteria isolates were deposited in the National Center for Biotechnology Information (NCBI) GenBank database with different accession numbers, in which the Bacillus genera have the highest diversity with different species which include Bacillus simplex MT982681 (MNWU14), Bacillus megaterium MT982682 (SNWU3), Bacillus paramycoides MT982683 (SNWU14), Bacillus thuringiensis MT982684 (SNWU15), Bacillus sp MT989395 (SNWU5), Bacillus paramycoides MT982685 (MNWU5), Bacillus megaterium MT982687 (SNWU1), Bacillus aryabhattai MT982688 (MNWU15), Bacillus cereus MT982689 (SNWU9), Bacillus thuringiensis MT982690 (MNWU2), Bacillus thuringiensis MT982691 (SNWU6), Bacillus subtilis MT982692 (SNWU7), Bacillus thuringiensis MT982693 (MNWU1), Bacillus cereus MT982694 (SNWU8), Bacillus cereus MT982695 (MNWU10), Bacillus pumilus MT982696 (SNWU16), Bacillus albus MT801081 (MNWU4), Bacillus paramycoides MT801080 (MNWU7), Bacillus siamensis MT801076 (MNWU3), Bacillus atrophaeus MT801077 (SNWU4),, Bacillus wiedmannii MT801078 (SNWU10). Follow by, Pseudomonas genera with the diversity of two species, which are Pseudomonas sp. MT982686 (MNWU16), and Pseudomonas rhizosphaerae MT801079 (SNWU17), while Rhodocyclaceae bacterium MT989397 (MNWU4), Enterococcus spp. MT989398 (MNWU3), and Massilia spp. MT989396 (SNWU13) were represented by one isolate.
Table 3.
16S rRNA sequence identification of the species closely associated with bacterial isolate using the Blastn program
| S/N | Isolates Code | Source of Isolate | Accession No | Percentage Similarity (%) | E-value | Query cover | Strains | Genus (Closest representative species) |
|---|---|---|---|---|---|---|---|---|
| 1 | MNWU14 | Maize | MT982681 | 98 | 0.0 | 98 | AO4 | Bacillus simplex |
| 2 | SNWU3 | Soybean | MT982682 | 100 | 0.0 | 99 | AO5 | Bacillus megaterium |
| 3 | SNWU14 | Soybean | MT982683 | 99.02 | 0.0 | 100 | AO6 | Bacillus paramycoides |
| 4 | SNWU15 | Soybean | MT982684 | 98 | 0.0 | 100 | AO7 | Bacillus thuringiensis |
| 5 | SNWU5 | Soybean | MT989395 | 100 | 0.0 | 99 | AO1 | Bacillus spp |
| 6 | SNWU13 | Soybean | MT989396 | 99 | 1e−173 | 87 | AO8 | Massilia spp |
| 7 | MNWU5 | Maize | MT982685 | 95.60 | 0.0 | 97 | AO9 | Bacilluss paramycoides |
| 8 | MNWU16 | Maize | MT982686 | 100 | 0.0 | 100 | AO3 | Pseudomonas spp |
| 9 | SNWU1 | Soybean | MT982687 | 99.60 | 0.0 | 100 | AO10 | Bacillus megaterium |
| 10 | MNWU4 | Maize | MT989397 | 99 | 1e−148 | 96 | AO11 | Rhodocyclaceae bacterium |
| 11 | MNWU15 | Maize | MT982688 | 99.80 | 0.0 | 100 | AO12 | Bacillus aryabhattai |
| 12 | SNWU9 | Soybeans | MT982689 | 100 | 0.0 | 100 | AO13 | Bacillus cereus |
| 13 | MNWU2 | Maize | MT982690 | 100 | 0.0 | 99 | AO14 | Bacillus thuringiensis |
| 14 | MNWU3 | Maize | MT989398 | 100 | 0.0 | 97 | AO15 | Enterococcus sp |
| 15 | MNWU6 | Maize | MT982691 | 99.80 | 0.0 | 100 | AO16 | Bacillus thuringiensis |
| 16 | SNWU7 | Soybean | MT982692 | 97.68 | 0.0 | 100 | AO17 | Bacillus subtilis |
| 17 | MNWU1 | Maize | MT982693 | 99.56 | 0.0 | 100 | AO18 | Bacillus thuringiensis |
| 18 | SNWU8 | Soybean | MT982694 | 99.26 | 0.0 | 100 | AO19 | Bacillus cereus |
| 19 | MNWU10 | Maize | MT982695 | 99.81 | 0.0 | 100 | AO20 | Bacillus cereus |
| 20 | SNWU16 | Soybean | MT982696 | 97.60 | 0.0 | 100 | AO21 | Bacillus pumilus |
| 21 | MNWU4 | Maize | MT801081 | 100 | 0.0 | 100 | AO22 | Bacillus albus |
| 22 | MNWU7 | Maize | MT801080 | 97 | 0.0 | 98 | AO23 | Bacillus paramycoides |
| 23 | MNWU13 | Maize | MT801076 | 100 | 0.0 | 100 | AO24 | Bacillus siamensis |
| 24 | SNWU4 | Soybean | MT801077 | 100 | 0.0 | 100 | AO25 | Bacillus atrophaeus |
| 25 | SNWU10 | Soybean | MT801078 | 100 | 0.0 | 100 | AO26 | Bacillus wiedmannii |
| 26 | SNWU17 | Maize | MT801079 | 99 | 0.0 | 97 | AO2 | Pseudomonas rhizosphaerae |
Tolerance to heavy metal, salt, temperature and pH
The bacterial isolate's tolerance to heavy metal was shown in Table 4. Based on the result obtained from this study, the bacterial isolate exhibited different tolerance to the heavy metals used. In this study, the bacterial isolate with an optical density above 0.45 was considered tolerant to heavy metal. The highest percentage in the bacterial tolerance to heavy metal was reported in chromium (61.53%), followed by lead (30.76%), while the lowest percentage tolerance was recorded in cadmium (3.8%), respectively. Cadmium was reported in this study to be toxic to the bacterial isolates. Therefore, the tolerance of the bacterial isolates characterized in this finding reduced as follows:
Table 4.
Tolerance of bacterial isolates to heavy metals
| Isolates code | Pb | Cr2+ | Cd+ |
|---|---|---|---|
| MNWU14 | 0.58 ± 0.06cde | 0.66 ± 0.06cd | 0.11 ± 0.01jk |
| SNWU3 | 0.38 ± 0.05fgh | 0.30 ± 0.05kl | 0.26 ± 0.03def |
| SNWU14 | 0.28 ± 0.06hij | 0.56 ± 0.05def | 0.15 ± 0.02hijk |
| SNWU15 | 0.21 ± 0.06jkl | 0.45 ± 0.05ghi | 0.1 ± 0.01ijk |
| SNWU5 | 0.15 ± 0.04klm | 0.52 ± 0.03fgh | 0.20 ± 0.04fghij |
| SNWU13 | 0.04 ± 0.01m | 0.10 ± 0.01n | 0.1 ± 0.05fghij |
| MNWU5 | 0.15 ± 0.02klm | 0.54 ± 0.02efg | 0.14 ± 0.02hijk |
| MNWU16 | 0.32 ± 0.05hij | 0.43 ± 0.05hij | 0.26 ± 0.03defg |
| SNWU1 | 0.68 ± 0.06bc | 0.58 ± 0.06def | 0.23 ± 0.01defgh |
| MNWU4 | 0.73 ± 0.07b | 0.87 ± 0.07a | 0.23 ± 0.01defgh |
| MNWU15 | 0.46 ± 0.03efg | 0.578 ± 0.03def | 0.26 ± 0.02defg |
| SNWU9 | 0.26 ± 0.04hijkl | 0.36 ± 0.03ijk | 0.17 ± 0.03ghijk |
| MNWU2 | 0.35 ± 0.05ghi | 0.48 ± 0.05fgh | 0.07 ± 0.01k |
| MNWU3 | 0.63 ± 0.01bcd | 0.72 ± 0.01bc | 0.45 ± 0.02b |
| MNWU6 | 0.26 ± 0.03hijkl | 0.42 ± 0.03hij | 0.12 ± 0.01ijk |
| SNWU7 | 0.28 ± 0.03hijk | 0.22 ± 0.02lm | 0.36 ± 0.02c |
| MNWU1 | 0.21 ± 0.03jkl | 0.57 ± 0.03defg | 0.19 ± 0.05fghij |
| SNWU8 | 0.22 ± 0.01ijkl | 0.52 ± 0.01fgh | 0.16 ± 0.02hijk |
| MNWU10 | 0.13 ± 0.01lm | 0.33 ± 0.01jkl | 0.24 ± 0.01defgh |
| SNWU16 | 0.50 ± 0.03def | 0.57 ± 0.03def | 0.30 ± 0.05cde |
| MNWU4 | 0.30 ± 0.03hij | 0.32 ± 0.03jkl | 0.11 ± 0.01jk |
| MNWU7 | 0.95 ± 0.02a | 0.81 ± 0.02ab | 0.32 ± 0.01cd |
| MNWU13 | 0.14 ± 0.02klm | 0.47 ± 0.02fgh | 0.17 ± 0.01ghijk |
| SNWU4 | 0.98 ± 0.04a | 0.71 ± 0.04bc | 0.22 ± 0.01efghi |
| SNWU10 | 0.19 ± 0.01jkl | 0.16 ± 0.01mn | 0.21 ± 0.01efghi |
| SNWU17 | 0.49 ± 0.05ef | 0.62 ± 0.05cde | 0.67 ± 0.01a |
Values are expressed as mean ± SE (n = 3), there is no significant difference (P > 0.05) with values having the same letter within a column
The bacterial isolate tolerance to different salt concentrations was shown in Table 5. In this study, the tolerance revealed that virtually all the bacterial strains showed high tolerance in a culture medium amended with a salt concentration of 1% and 3%. The percentage of bacterial isolates that recorded increased in their optical density in culture medium amended with 5% salt concentration was 19.23%, while the percentage of the bacterial isolate with decreased in their optical density in medium amended with 5% salt concentration 80.76%.
Table 5.
Response of bacterial isolates to different salt concentration
| Isolates code | 1% | 3% | 5% |
|---|---|---|---|
| MNWU14 | 1.80 ± 0.06cd | 1.84 ± 0.05ab | 1.45 ± 0.05gh |
| SNWU3 | 1.72 ± 0.05d | 1.74 ± 0.07ab | 1.67 ± 0.06efg |
| SNWU14 | 2.06 ± 0.03abcd | 1.81 ± 0.06 ab | 2.01 ± 0.29abcd |
| SNWU15 | 2.05 ± 0.03abcd | 1.77 ± 0.05ab | 1.92 ± 0.01abcde |
| SNWU5 | 1.99 ± 0.06abcd | 1.99 ± 0.51ab | 1.89 ± 0.04abcdef |
| SNWU13 | 1.04 ± 0.02e | 1.50 ± 0.28ab | 1.36 ± 0.03hi |
| MNWU5 | 1.99 ± 0.11abcd | 1.99 ± 0.51 ab | 1.78 ± 0.03cdef |
| MNWU16 | 2.13 ± 0.02abc | 2.05 ± 0.02ab | 1.96 ± 0.05abcd |
| SNWU1 | 1.32 ± 0.16e | 1.82 ± 0.01ab | 2.16 ± 0.09a |
| MNWU4 | 2.24 ± 0.02ab | 2.00 ± 0.29ab | 1.75 ± 0.02def |
| MNWU15 | 1.94 ± 0.02bcd | 2.01 ± 0.29ab | 1.79 ± 0.04bcdef |
| SNWU9 | 1.78 ± 0.04cd | 1.50 ± 0.22ab | 1.39 ± 0.04hi |
| MNWU2 | 1.93 ± 0.05abcd | 1.97 ± 0.30ab | 1.61 ± 0.01fgh |
| MNWU3 | 2.00 ± 0.29abcd | 1.94 ± 0.02ab | 1.85 ± 0.02bcdef |
| MNWU6 | 2.11 ± 0.06abcd | 2.08 ± 0.04 ab | 2.03 ± 0.01abc |
| SNWU7 | 1.76 ± 0.41cd | 1.88 ± 0.04ab | 1.18 ± 0.03i |
| MNWU1 | 1.87 ± 0.04bcd | 1.97 ± 0.05ab | 1.79 ± 0.05bcdef |
| SNWU8 | 2.36 ± 0.17a | 1.94 ± 0.06ab | 1.16 ± 0.03i |
| MNWU10 | 2.03 ± 0.02abcd | 2.11 ± 0.05ab | 2.07 ± 0.03ab |
| SNWU16 | 2.06 ± 0.03abcd | 1.61 ± 0.06ab | 1.60 ± 0.11fgh |
| MNWU4 | 1.96 ± 0.03abcd | 1.92 ± 0.05ab | 1.94 ± 0.02abcde |
| MNWU7 | 1.99 ± 0.05abcd | 2.01 ± 0.29ab | 1.84 ± 0.01bcdef |
| MNWU13 | 1.76 ± 0.03cd | 1.83 ± 0.05ab | 1.91 ± 0.11abcde |
| SNWU4 | 2.07 ± 0.02abcd | 2.15 ± 0.02ab | 1.86 ± 0.05bcdef |
| SNWU10 | 1.05 ± 0.03e | 1.42 ± 0.01b | 1.39 ± 0.17hi |
| SNWU17 | 2.02 ± 0.01abcd | 2.17 ± 0.03a | 2.04 ± 0.02abc |
Values are expressed as mean ± SE (n = 3), there is no significant difference (P > 0.05) with values having the same letter within a column
In this study, the bacterial species isolated were evaluated for their tolerance to a different temperature ranging from 30 to 40 °C as shown in Table 6. The growth of the bacterial isolate to diverse temperature range evaluated in this study was significant (P < 0.05). At temperature of 30 °C and 35 °C most of the bacterial exhibited high optical density, which could be regarded as the optimum temperature for their growth. At the temperature of 40 °C, the optical density of the bacterial isolate was reduced, respectively.
Table 6.
Response of bacterial isolates to different temperature
| Isolate code | 30 °C | 35 °C | 40 °C |
|---|---|---|---|
| MNWU14 | 0.30 ± 0.05jk | 0.58 ± 0.04ghijk | 0.12 ± 0.01hi |
| SNWU3 | 0.42 ± 0.01hi | 0.31 ± 0.01m | 0.49 ± 0.05a |
| SNWU14 | 0.51 ± 0.01gh | 0.58 ± 0.04ghijk | 0.33 ± 0.01de |
| SNWU15 | 0.24 ± 0.02k | 0.85 ± 0.02bc | 0.33 ± 0.01de |
| SNWU5 | 0.59 ± 0.04k | 0.48 ± 0.04jkl | 0.29 ± 0.04def |
| SNWU13 | 0.27 ± 0.01k | 0.44 ± 0.03l | 0.09 ± 0.01i |
| MNWU5 | 0.77 ± 0.03d | 0.82 ± 0.05cd | 0.24 ± 0.05efg |
| MNWU16 | 0.40 ± 0.06ij | 0.86 ± 0.03bc | 0.19 ± 0.04fghi |
| SNWU1 | 0.39 ± 0.03ij | 0.57 ± 0.03hijk | 0.25 ± 0.02efg |
| MNWU4 | 0.95 ± 0.02bc | 0.53 ± 0.01ijkl | 0.37 ± 0.03cdd |
| MNWU15 | 1.03 ± 0.01b | 0.60 ± 0.05ghijk | 0.34 ± 0.02de |
| SNWU9 | 0.57 ± 0.03fg | 0.87 ± 0.01bc | 0.38 ± 0.04bcd |
| MNWU2 | 0.26 ± 0.03k | 0.42 ± 0.01lm | 0.19 ± 0.05fghi |
| MNWU3 | 1.19 ± 0.05a | 0.67 ± 0.03fgh | 0.47 ± 0.03ab |
| MNWU6 | 0.66 ± 0.05ef | 0.47 ± 0.03kl | 0.33 ± 0.01de |
| SNWU7 | 0.21 ± 0.01k | 0.16 ± 0.02n | 0.10 ± 0.03hi |
| MNWU1 | 0.26 ± 0.03k | 0.59 ± 0.04ghijk | 0.16 ± 0.02ghi |
| SNWU8 | 0.46 ± 0.03hi | 0.58 ± 0.04ghijk | 0.21 ± 0.01fgh |
| MNWU10 | 0.29 ± 0.04jk | 0.96 ± 0.03a | 0.15 ± 0.02ghi |
| SNWU16 | 0.46 ± 0.03hi | 0.71 ± 0.05def | 0.17 ± 0.03ghi |
| MNWU4 | 0.72 ± 0.01de | 0.61 ± 0.06ghij | 0.15 ± 0.02ghi |
| MNWU7 | 0.96 ± 0.01bc | 0.65 ± 0.02fghi | 0.34 ± 0.02de |
| MNWU13 | 0.93 ± 0.02bc | 0.59 ± 0.05ghijk | 0.45 ± 0.01abc |
| SNWU4 | 0.92 ± 0.01c | 0.76 ± 0.03cde | 0.37 ± 0.03bcd |
| SNWU10 | 0.25 ± 0.02k | 0.19 ± 0.04n | 0.21 ± 0.01fgh |
| SNWU17 | 1.20 ± 0.01a | 0.60 ± 0.05gh | 0.35 ± 0.02jk |
Values are expressed as mean ± SE (n = 3), there is no significant difference (P > 0.05) with values having the same letter within a column
The tolerance of the bacterial isolate to different pH range (4, 7, and 10) was evaluated in this study, as shown in Table 7. At pH 4, the optical density of the bacterial isolate above 0.4 was considered tolerance to acidic medium. In this case, 46.15% of the bacterial isolate was tolerant to pH 4, 84.61% tolerance to pH 7, and 7.6% tolerance to pH 10. The result could also enhance the classification of the bacterial isolate into acid and alkaline tolerance microorganisms.
Table 7.
Response of bacteria isolates to different pH
| Isolate code | 4 | 7 | 10 |
|---|---|---|---|
| MNWU14 | 0.13 ± 0.05gh | 0.63 ± 0.06cd | 0.08 ± 0.01j |
| SNWU3 | 0.16 ± 0.03gh | 0.63 ± 0.01cd | 0.15 ± 0.02fghij |
| SNWU14 | 0.39 ± 0.04de | 0.52 ± 0.02def | 0.13 ± 0.01ghij |
| SNWU15 | 0.16 ± 0.03gh | 0.80 ± 0.06b | 0.14 ± 0.02fghij |
| SNWU5 | 0.44 ± 0.02d | 0.58 ± 0.01cde | 0.14 ± 0.01 fghij |
| SNWU13 | 0.22 ± 0.01fg | 0.35 ± 0.03fgh | 0.13 ± 0.01 fghij |
| MNWU5 | 0.16 ± 0.03gh | 0.34 ± 0.01hi | 0.10 ± 0.01ij |
| MNWU16 | 0.25 ± 0.02fg | 0.42 ± 0.05fgh | 0.23 ± 0.01defg |
| SNWU1 | 0.63 ± 0.01b | 0.71 ± 0.05bc | 0.10 ± 0.01ij |
| MNWU4 | 0.65 ± 0.02b | 0.70 ± 0.06bc | 0.15 ± 0.02fghij |
| MNWU15 | 0.09 ± 0.01h | 0.48 ± 0.01efg | 0.17 ± 0.03efghij |
| SNWU9 | 0.42 ± 0.06de | 0.51 ± 0.05def | 0.30 ± 0.05cd |
| MNWU2 | 0.68 ± 0.05b | 0.68 ± 0.04bc | 0.19 ± 0.04efghi |
| MNWU3 | 0.45 ± 0.02d | 0.68 ± 0.04bc | 0.34 ± 0.02c |
| MNWU6 | 0.58 ± 0.04bc | 0.61 ± 0.05cde | 0.16 ± 0.02fghij |
| SNWU7 | 0.49 ± 0.04cd | 0.65 ± 0.05cd | 0.16 ± 0.02fghij |
| MNWU1 | 0.16 ± 0.03gh | 0.72 ± 0.01bc | 0.23 ± 0.01def |
| SNWU8 | 0.46 ± 0.03d | 0.71 ± 0.06bc | 0.17 ± 0.03 efghij |
| MNWU10 | 0.16 ± 0.03gh | 0.61 ± 0.05cde | 0.16 ± 0.03 efghij |
| SNWU16 | 0.28 ± 0.02fg | 0.48 ± 0.05efg | 0.21 ± 0.04efgh |
| MNWU4 | 0.13 ± 0.01gh | 0.35 ± 0.02ghi | 0.12 ± 0.01hij |
| MNWU7 | 0.30 ± 0.05ef | 1.06 ± 0.03a | 0.26 ± 0.04cde |
| MNWU13 | 0.44 ± 0.02d | 0.68 ± 0.04bc | 0.13 ± 0.01fghij |
| SNWU4 | 0.87 ± 0.01a | 1.16 ± 0.03a | 0.73 ± 0.01a |
| SNWU10 | 0.37 ± 0.03de | 0.25 ± 0.02i | 0.09 ± 0.01ij |
| SNWU17 | 0.60 ± 0.06b | 1.05 ± 0.02a | 0.51 ± 0.05b |
Values are expressed as mean ± SE (n = 3), there is no significant difference (P > 0.05) with values having the same letter within a column
Effect of the rhizobacterial strains on seed germination
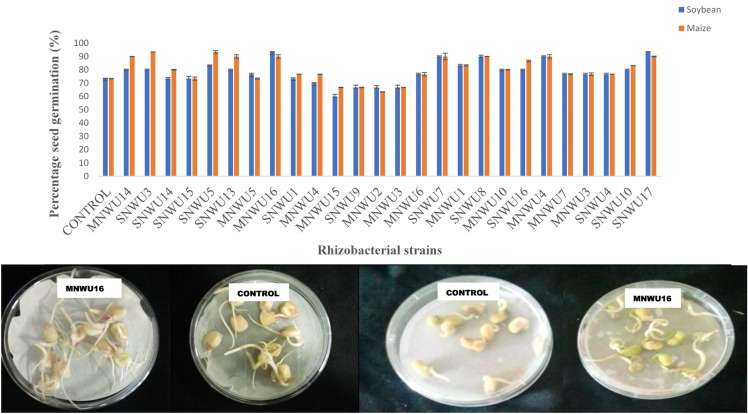
The rhizobacterial strains characterized in this study were able to promote maize and soybean seed under laboratory conditions where this experiment was conducted as shown in Fig. 3. The result of the seed germination test revealed the highest seed germination percentage (above 80%) in the Bacillus sp. OA1, Pseudomonas rhizosphaerae OA2 and Pseudomonas sp. OA3. However, some of the bacterial isolates as well as the control showed a lower percentage of germination (below 80%).
Fig. 3.
Effect of rhizobacterial strains on in vitro maize and soybeans seed germination
Whole-genome analysis of plant growth-promoting functional genes and secondary metabolite in the rhizobacterial
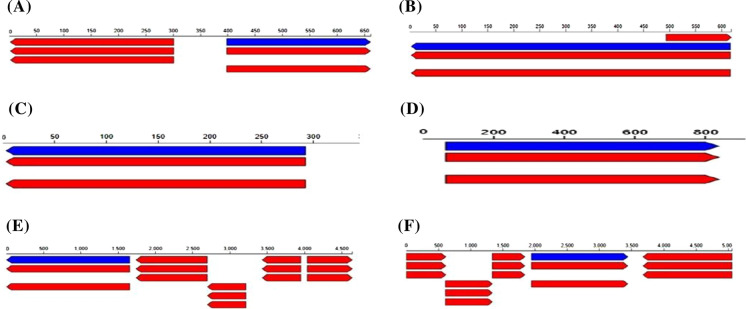
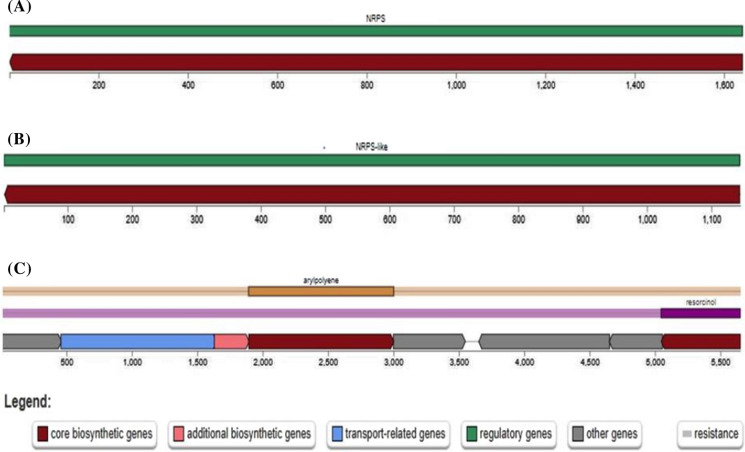
The functional genes and secondary metabolite that are responsible for plant growth-promoting potential in the rhizobacterial strain's genome as revealed by whole-genome sequencing were reported in Table 8. The region where the plant growth-promoting functional gene and the metabolites are located was reported in Figs. 4 and 5. The blue bar in Fig. 4 represents the contigs location where the plant growth-promoting are located. Among the plant growth-promoting, functional genes selected are genes responsible for phosphate solubilization, siderophore production, and exopolysaccharide production. For the secondary metabolite that is present in the rhizobacterial genome, NRPS, arypolyene, and resorcinol which are crucial in enhancing plant growth promotion were selected.
Table 8.
Some selected plant growth-promoting functional genes revealed by whole-genome sequencing of the rhizobacterial strains
| Rhizobacterial stains | Feature/ ID Type | Function | Ontology | Start | Strand | Protein length | Location |
|---|---|---|---|---|---|---|---|
| Bacillus sp. OA1 | CDS.10026/Gene | Phosphoglycolate phosphatase (EC 3.1.3.18) | SSO:000,005,983- Phosphoglycolate phosphatase (EC 3.1.3.18) | 398 | + | 264 | NODE_2883_length_662_cov_3.704673 |
| CDS.10284/Gene | Siderophore biosynthesis non-ribosomal peptide synthetase modules @ Bacillibactin synthetase component F (EC 2.7.7.-) | SSO:000,001,137- Bacillibactin synthetase component F (EC 2.7.7.–) SSO:000,007,486- Siderophore biosynthesis non-ribosomal peptide synthetase modules | 617 | + | 615 | NODE_3079_length_619_cov_2.752033 | |
| Pseudomonas rhizospherea OA2 | CDS.10018/Gene | Anthranilate phosphoribosyltransferase (EC 2.4.2.18) | SSO:000,000,995- Anthranilate phosphoribosyltransferase (EC 2.4.2.18) | 293 | − | 291 | NODE_5433_length_509_cov_1.801047 |
| CDS.1005/Gene | Iron siderophore sensor protein | SSO:000,004,003- Iron siderophore sensor protein | 65 | + | 777 | NODE_239_length_2787_cov_2.212782 | |
| Pseudomonas sp. OA3 | CDS.1624/Gene | Ferrichrome-iron receptor FiuA @ Ferric siderophore receptor, TonB dependent | SSO:000,002,737- Ferric siderophore receptor, TonB dependent | 1658 | − | 1656 | NODE_229_length_4642_cov_1.975415 |
| CDS.1404/Gene | Exopolyphosphatase (EC 3.6.1.11) | SSO:000,002,629- Exopolyphosphatase (EC 3.6.1.11) | 1944 | + | 1503 | NODE_187_length_5062_cov_3.014995 |
Fig. 4.
Contigs location of the plant growth promote functional genes in the rhizobacterial genome a Phosphoglycolate phosphatase (EC 3.1.3.18) b Siderophore biosynthesis non-ribosomal peptide synthetase modules @ Bacillibactin synthetase component F (EC 2.7.7.–) c Anthranilate phosphoribosyltransferase (EC 2.4.2.18) d Iron siderophore sensor protein e Ferrichrome-iron receptor FiuA @ Ferric siderophore receptor, TonB dependent f Exopolyphosphatase (EC 3.6.1.11). The blue bars represent the region where the genes are located. (Color figure online)
Fig. 5.
Selected secondary metabolites in the rhizobacterial genome a NRPS-Bacillus sp. OA1 b NRPS-like Pseudomonas rhizospherea OA2 c Arypolyene, resorcinol- Pseudomonas sp. OA3
Discussion
The application of agrochemicals in agrarian practices has become deleterious to humans and the environment. Therefore, there is a need to intensify research on new advances in the application of biological and ecologically friendly approaches which involve the use of rhizospheric beneficial bacterial to promote plant growth and improve plant tolerance to environmental stress and phytopathogen (Fasusi and Babalola 2021). The molecular characterization of the bacterial isolates evaluated in this study is crucial for proper identification and description of the bacterial isolates, and it gives vital information about the novelty of the organisms (Adegboye and Babalola 2012). The similarity of the bacterial isolates evaluated in the study met the standard and the specification of the National Center for Biotechnology Information (NCBI), which could enhance the consensus sequence generated from each isolate to be deposited in the NCBI GenBank for allocation of an accession number.
The plant growth-promoting rhizobacterial colonizes plant roots by promoting plant growth and competitively limit the population of harmful soil microorganisms using various mechanisms such as the production of hydrogen cyanide, phosphate solubilization, ammonia production, antibiotics production, siderophore production, and stimulating the production of phytohormones such as IAA (Olanrewaju et al., 2017; Meena et al., 2017).
In this study, it was shown that the rhizosphere of soybeans and maize harbors beneficial soil bacteria with multiple plant growth-promoting features. In previous studies, the presence of rhizospheric bacteria that are of importance in promoting plant growth has been reported (Josephine and Thomas 2019). In this study, the bacterial were screened for in vitro plant growth-promoting characteristics, antifungal potential, and response to environmental stress. The presence of beneficial bacterial in the plant rhizosphere is well established to be crucial for plant growth promotion and defense. For bacterial isolate to be an efficient plant growth-promoting rhizobacteria strain, the bacterial strain must be able to colonize the plant rhizosphere at high population density, which will enable it to exact its beneficial effect on plant development, tolerance to stress, and defense. The isolated and characterized rhizobacterial evaluated in this study were likely to contribute to the promotion of plant growth and enhance plant tolerance to different environmental stress.
On the other hand, rhizobacterial with plant growth-promoting potentials such as bacteria that solubilize phosphate play a crucial in promoting plant growth by converting insoluble phosphorus to soluble phosphatase for plant absorption (Alori et al. 2017). Therefore, soil fertility and plant growth promotion can be enhanced by the application of these phosphate solubilizing microorganisms, and the widespread commercialization of these microorganisms can reduce the cost of purchase of chemical fertilizer, the ill effect associated with its usage, and its application for increasing plant growth. The phosphate solubilization potentials of the rhizobacterial isolates in this study were in agreement with the previous research conducted by Rajkumar et al. (2006) that attributed plant root proliferation and absorption of nutrient such as phosphorus by the plant to the potential of phosphate solubilizing Bacillus sp. BA32 and Pseudomonas sp. PSA4.
Furthermore, rhizobacteria possessing multiple plant growth-promoting potential can be employed in enhancing plant growth promotion and protecting plant health for an optimum increase in agricultural productivity (Zaidi et al. 2015). The numerous plant growth-promoting ability of Bacillus spp. and Pseudomonas spp. characterized in the study are consistent with the finding of other researchers (Ahmad et al. 2016; Ndeddy Aka and Babalola 2016). Likewise, the characterization of Rhodocyclaceae bacterium with plant growth-promoting potentials from soybeans rhizosphere in this study has been reported by Liu et al. (2019) as rhizosphere soil microbiomes associated with soybeans plant which could contribute to plant growth enhancement. In this study, the production of plant growth-promoting trait by Enterococcus spp. agreed with the study of (Anzuay et al. 2017; Mussa et al. 2018) that reported the isolation of Enterococcus spp growth-promoting potential and antifungal activity against pathogenic fungi from the rhizosphere of grass pea. Massilia spp. (Oxalobacteriaceae) characterization from plant rhizosphere in this study for plant growth-promoting activity corroborates with the finding of Soares et al. (2020) that reported the characterization of Massilia spp. among the bacterial isolate with plant growth-promoting trait and biocontrol activity isolated from a leguminous plant (Lotus parviflorus).
These bacterial isolates characterized in this study was reported to produce IAA which is among the mechanisms employed by PGPR in exerting their positive effect on the plant which corroborates with the previous study (Rana et al. 2011). The elongation of the plant root system for uptake of water and nutrient needed for plant growth is enhanced by the production of IAA by plant growth-promoting rhizobacteria (Egamberdieva et al. 2017). All the bacteria isolates were able to produce ammonia (NH3), which corroborates with the finding of Geetha et al. (2014). In the findings, they reported the production of ammonia by rhizobacteria isolated from the green gram rhizosphere. Ammonia production by rhizobacterial was also reported by Kandjimi et al. (2015) to promote plant growth by making nitrogen available for plant growth. Likewise, the production of ammonia by rhizobacteria isolates was reported by Bhattacharyya and Jha (2012) to promote plant growth by preventing the growth of fungal pathogens. In this study, bacterial isolates were all positive to the exopolysaccharide test responsible for their tolerance to different environmental stress. This result corroborates with the finding of Deka et al. (2019) that reported the production of exopolysaccharide by Bacillus spp. to be responsible for their acid tolerance and increase the soil aggregate when they are applied to the soil.
All the bacterial isolate characterized in this study were siderophore-producing microbes, which agreed with the previous research study of Shen et al. (2013) they reported that siderophore producing microbes increase plant growth by promoting the acquisition of iron, inhibit the growth of other microorganisms through anabiosis, and inhibit the growth of fungi phytopathogens by limiting the amount of iron need for their development. The isolation and characterization of Bacillus spp. and Pseudomonas spp from various maize rhizosphere in this study have been reported by Figueroa-López et al. (2016) for their plant growth potential, biocontrol activity.
In contrast to the plant growth-promoting characteristic, the biocontrol activity of the bacterial isolates against Fusarium graminarium pathogens can be attributed to the production of the lytic enzyme by the bacterial isolates, which disrupts the component of the fungal cell wall, inhibit spore germination and mycelia growth and disrupt protein synthesis (Mardanova et al. 2016).
More so, the antifungal activity of Bacillus, Enterococcus, and Pseudomonas species highlighted in this study have previously been reported to be harmful to fungal pathogens (Brown et al. 2019; Khan et al. 2018; Kurniawan et al. 2018). The plant-growth-promoting and biocontrol activity of Massilia spp reported in this study agreed with the study of Araujo et al. (2019). All the bacteria isolate was able to produce catalase which was reported in the previous study to help maintain the ROS level and protect plants against stress (chemical and environmental) (Kumar and Sharma 2012). In the study, all the bacterial isolates showed a different level of tolerance to pH, temperature, salinity, and heavy metal stress. The tolerance of the bacterial isolates characterized in this study to various environmental stress, which could be detrimental to plant health is important for evaluating the effectiveness of the bacterial isolate and the result obtained corroborates with previous findings (Abedinzadeh et al. 2019; Ndeddy Aka and Babalola 2016). Plant growth promotion, photosynthesis, enzyme activity, and protein synthesis are negatively affected by abiotic stress (Enebe and Babalola 2018). Therefore, the co-inoculation of these stress tolerance bacteria strains as an inoculant for plant growth will help the plant to tolerate stress conditions.
Regarding the in vitro potential of the rhizobacterial to enhance maize and soybean seed growth under laboratory condition. Some of the Bacillus and Pseudomonas species were able to enhance the seed germination effectively than other species. This finding corroborates with the result of Almaghrabi et al. (2014) that reported the effect of Bacillus species and Pseudomonas putida in enhancing maize seed under laboratory condition. The identification of the functional genes and secondary metabolites that are responsible for plant growth promotion in the genome of the rhizobacterial strains using whole-genome sequencing is an important method to validate in vitro plant growth potential of the rhizobacteria strains, but this a less studied in previous research finding. More so the whole genome sequencing method should be conducted to ascertain if plant growth-promoting genes are present in the bacterial genome. In this study, the rhizobacterial strain possessed plant growth-promoting functional genes in their genome. This agreed with the findings of Crovadore et al. (2020) that reported the whole genome analysis to reveal the plant growth-promoting functional genes in Pseudomonas and Bacillus species. The production of secondary metabolites by plant growth-promoting rhizobacteria has to be reported to enhance plant growth (Lucke et al. 2020). In this study, the genomic analysis of the rhizobacterial strains revealed production of different secondary metabolites that are responsible for plant growth promotion among which Non-Ribosomal Peptide Synthetase (NRPS), arypolyene, and resorcinol are selected. This study corroborates with the finding of Rieusset et al. (2020) that reported the production of secondary metabolites arypolyene and resorcinol by Pseudomonas strains to enhance plant growth. Also, the production of Non-Ribosomal Peptide Synthetase (NRPS) secondary metabolites by Pseudomonas and Bacillus species had previously been reported (Gu et al. 2020; Martínez-Núñez and y López 2016).
Conclusion
In the present study, isolation, characterization, stress tolerance, and antifungal activity of PGPR from soybean and maize plant rhizosphere have been studied. Based on the result obtained from this study, the bacteria strains isolated from the rhizosphere of maize and soybeans exhibited more than one plant growth-promoting potentials such as hydrogen cyanide production, IAA production, production of ammonia, antifungal activity, phosphate solubilization, and tolerance to abiotic stress. Furthermore, these microorganisms have shown in vitro antagonistic activity against phytopathogen Fusarium graminearum, causing fusariosis in maize, reducing maize production in South Africa, and were able to enhance maize and soybean seed germination under laboratory condition. More so, the whole genome analysis of the rhizobacterial strain performed in this study reveals the presence of functional genes and secondary metabolites responsible for plant growth promotion in the bacteria genome. In evaluating the result obtained from this research study, it can be deduced that there are several plants beneficial bacterial associated with maize and soybeans rhizosphere, and it can be concluded that the plant growth-promoting rhizobacteria characterized in this study possessed biofertilization and biocontrol trait that can make them to be used as bioinoculant for plant growth to ensuring agricultural sustainability and reduce the application of chemical fertilizer which are deleterious to human health and environment.
Acknowledgements
OAF appreciates the National Research Foundation, South Africa, The World Academy of Science (NRF-TWAS) for (UID116095) Ph.D. stipend. OOB acknowledges the National Research Foundation (UID123634 and UID132595) that supported research in her laboratory.
Authors’ contribution
OAF designed, performed the experiment, did the bioinformatic analysis and wrote the first draft; OOB secured funding, edited and provided academic input in writing the manuscript, and AEA thoroughly critiqued the article. All authors approved the article for publication.
Funding
The study was funded by the National Research Foundation of South Africa (Grant Ref: UID 123634).
Availability of data and materials
All the bacteria strains data characterized using I6S rRNA in this study with their accession number are available in the NCBI database.
For the whole genome sequence data:
Bacillus sp. OA1: The raw reads are available under the Bioproject accession number (PRJNA683742)and Biosample numbers (SAMN17036205). The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number (SRX9654894). The genome sequence of Bacillus sp. OA1 has been deposited at DDBJ/ENA/GenBank under the accession JAEKPB000000000.1
Pseudomonas rhizosphaerae OA2: The raw reads are available under the Bioproject accession number PRJNA683763 and Biosample numbers SAMN17036358. The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number SRX9654938.
Pseudomonas sp. OA3: The raw reads are available under the Bioproject accession number PRJNA683768- Pseudomonas sp. OA3)and Biosample number SAMN17036469- Pseudomonas sp. OA3). The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number SRX9654998- Pseudomonas sp. OA3. The genome sequence of Pseudomonas sp. OA3 has been deposited at DDBJ/ENA/GenBank under the accession JAEMOM000000000.1.
Declarations
Conflict interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors approved the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abedinzadeh M, Etesami H, Alikhani HA. Characterization of rhizosphere and endophytic bacteria from roots of maize (Zea mays L.) plant irrigated with wastewater with biotechnological potential in agriculture. Biotechnol Rep. 2019;21:e00305. doi: 10.1016/j.btre.2019.e00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adegboye MF, Babalola OO. Taxonomy and ecology of antibiotic producing actinomycetes. Afr J Agricul Res. 2012;7:2255–2261. [Google Scholar]
- Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181 [DOI] [PubMed]
- Ahmad S, Daur I, Al-Solaimani SG, Mahmood S, Bakhashwain AA, Madkour MH, Yasir M. Effect of rhizobacteria inoculation and humic acid application on canola (Brassica napus L.) crop. Pak J Bot. 2016;48:2109–2120. [Google Scholar]
- Almaghrabi OA, Abdelmoneim T, Albishri HM, Moussa TA. Enhancement of maize growth using some plant growth promoting rhizobacteria (PGPR) under laboratory conditions. Life Sci J. 2014;11:764–772. [Google Scholar]
- Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. FastQC: a quality control tool for high throughput sequence data. Babraham bioinformatics. Cambridge: Babraham Institute; 2010. [Google Scholar]
- Anzuay MS, Ciancio MGR, Ludueña LM, Angelini JG, Barros G, Pastor N, Taurian T. Growth promotion of peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants by single and mixed cultures of efficient phosphate solubilizing bacteria that are tolerant to abiotic stress and pesticides. Microbiol Res. 2017;199:98–109. doi: 10.1016/j.micres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Araujo R, Dunlap C, Barnett S, Franco CMM. Decoding wheat endosphere-rhizosphere microbiomes in Rhizoctonia solani infested soils challenged by Streptomyces biocontrol agents. Front Plnt Sci. 2019;10:1038. doi: 10.3389/fpls.2019.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aremu BR, Babalola OO. Construction of specific primers for rapid detection of South African exportable vegetable macergens. Int J Environ Res Public Health. 2015;12:12356–12370. doi: 10.3390/ijerph121012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin AP, et al. KBase: the United States department of energy systems biology knowledgebase. Nat Biotechnol. 2018;36:566–569. doi: 10.1038/nbt.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari S, Tarkowská D, Rolčík J, Novák O, Palmero DV, Bejai S, Meijer J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta. 2017;245:15–30. doi: 10.1007/s00425-016-2580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. W J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AO, Graham CE, Cruz MR, Singh KV, Murray BE, Lorenz MC, Garsin DA. Antifungal activity of the Enterococcus faecalis peptide EntV requires protease cleavage and disulfide bond formation. Mbio. 2019;10:e01334–e11319. doi: 10.1128/mBio.01334-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccino J, Sherman N (1992) Biochemical activities of microorganisms. In: Microbiology, a laboratory manual. The Benjamin/Cummings Publishing Co., California, pp188–247
- Castric PA. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol. 1975;21:613–618. doi: 10.1139/m75-088. [DOI] [PubMed] [Google Scholar]
- Chauhan AK, Maheshwari DK, Kim K, Bajpai VK. Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can J Microbiol. 2016;62:880–892. doi: 10.1139/cjm-2016-0249. [DOI] [PubMed] [Google Scholar]
- Clarke PH, Cowan S. Biochemical methods for bacteriology. Microbiology. 1952;6:187–197. doi: 10.1099/00221287-6-1-2-187. [DOI] [PubMed] [Google Scholar]
- Crovadore J, Cochard B, Chablais R, Haenzi M, Raffini F, Lefort F. Draft genome sequences of Pseudomonas koreensis strain UASWS1668, Bacillus megaterium strain UASWS1667, and Paenibacillus sp. strain UASWS1643, considered potential plant growth-promoting rhizobacteria. Microbiol Res Announce. 2020;9(33):e00768-00720. doi: 10.1128/MRA.00768-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka P, Goswami G, Das P, Gautom T, Chowdhury N, Boro RC, Barooah M. Bacterial exopolysaccharide promotes acid tolerance in Bacillus amyloliquefaciens and improves soil aggregation. Mol Biol Rep. 2019;46:1079–1091. doi: 10.1007/s11033-018-4566-0. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D, Wirth SJ, Alqarawi AA, Abd-Allah EF, Hashem A. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. 2017;8:2104. doi: 10.3389/fmicb.2017.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enebe MC, Babalola OO. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl Microbiol Biotechnol. 2018;102:7821–7835. doi: 10.1007/s00253-018-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasusi OA, Babalola OO. The multifaceted plant-beneficial rhizobacteria toward agricultural sustainability. Plnt Protect Sci. 2021;57:95–111. doi: 10.17221/130/2020-PPS. [DOI] [Google Scholar]
- Fasusi OA, Cruz C, Babalola OO. Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture. 2021;11:163. doi: 10.3390/agriculture11020163. [DOI] [Google Scholar]
- Figueroa-López AM, et al. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. Springerplus. 2016;5:330. doi: 10.1186/s40064-016-1780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Ogawa M, Taniguchi H, Saito M. Molecular approaches to studying microbial communities: targeting the 16S ribosomal RNA gene. J UOEH. 2016;38:223–232. doi: 10.7888/juoeh.38.223. [DOI] [PubMed] [Google Scholar]
- Geetha K, Rajithasri A, Bhadraiah B. Isolation of plant growth promoting rhizo bacteria from rhizosphere soils of green gram, biochemical characterization and screening for antifungal activity against pathogenic fungi. Int J Pharm Sci Investig. 2014;3:47–54. [Google Scholar]
- Gholami A, Shahsavani S, Nezarat S. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. World Acad Sci Eng Technol. 2009;49:19–24. doi: 10.3923/pjbs.2009.26.32. [DOI] [PubMed] [Google Scholar]
- Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D, Thakker JN, Dhandhukia PC. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agricul. 2016;2:1127500. [Google Scholar]
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin H-S, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Gu Y, Ma YN, Wang J, Xia Z, Wei HL. Genomic insights into a plant growth-promoting Pseudomonas koreensis strain with cyclic lipopeptide-mediated antifungal activity. Microbiol Open. 2020;9:e1092. doi: 10.1002/mbo3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series, vol 41. [London]: Information Retrieval Ltd., c1979–c2000., pp 95–98
- Itelima J, Bang W, Onyimba I, Oj E. A review: biofertilizer; a key player in enhancing soil fertility and crop productivity. Microbiol Biotechnol Rep. 2018;2:22–28. [Google Scholar]
- Josephine R, Thomas J. 16S microbial phylogeny of multifunctional plant-growth-promoting rhizobacteria from the rhizosphere of maize (Zea mays L.) for agricultural soil fortification. Biotechnologia. 2019;100:143–154. doi: 10.5114/bta.2019.85324. [DOI] [Google Scholar]
- Kandjimi OS, Uzabakiriho J, Chimwamurombe PM. Isolation and characterization of culturable bacteria from bulk soil samples and the rhizosphere of arid-adapted Tylosema esculentum (Burchell). A. Schreiber (Marama bean) in Namibia. Afri J Biotechnol. 2015;14:944–952. [Google Scholar]
- Karnwal A, Kumar V. Influence of plant growth promoting rhizobacteria (PGPR) on the growth of chickpea (Cicer arietinum l.) Ann Food Sci Technol. 2012;13:43–48. [Google Scholar]
- Kejela T, Thakkar VR, Thakor P. Bacillus species (BT42) isolated from Coffea arabica L. rhizosphere antagonizes Colletotrichum gloeosporioides and Fusarium oxysporum and also exhibits multiple plant growth promoting activity. BMC Microbiol. 2016;16:277. doi: 10.1186/s12866-016-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, et al. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol. 2018;9:2363. doi: 10.3389/fmicb.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sharma R. Production of alkaline pectinase by bacteria (Cocci sps.) isolated from decomposing fruit materials. J Phytol. 2012;4:1–5. [Google Scholar]
- Kurniawan O, Wilson K, Mohamed R, Avis TJ. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol Control. 2018;126:136–141. doi: 10.1016/j.biocontrol.2018.08.001. [DOI] [Google Scholar]
- Liu F, Hewezi T, Lebeis SL, Pantalone V, Grewal PS, Staton ME. Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol. 2019;19:1–19. doi: 10.1186/s12866-018-1372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke M, Correa MG, Levy A. The role of secretion systems, effectors, and secondary metabolites of beneficial rhizobacteria in interactions with plants and microbes. Front Plant Sci. 2020 doi: 10.3389/fpls.2020.589416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhaiyan M, Poonguzhali S, Sa T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.) Chemos. 2007;69:220–228. doi: 10.1016/j.chemosphere.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol. 2015;6:198. doi: 10.3389/fmicb.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardanova AM, et al. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agricul Sci. 2016;8:1–20. [Google Scholar]
- Martínez-Núñez MA, y López VEL. Nonribosomal peptides synthetases and their applications in industry. Sustain Chem Proc. 2016;4:1–8. doi: 10.1186/s40508-016-0045-x. [DOI] [Google Scholar]
- Meena M, Swapnil P, Zehra A, Aamir M, Dubey MK, Goutam J, Upadhyay R (2017) Beneficial microbes for disease suppression and plant growth promotion. In: Singh D, Singh H, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp. 395–432
- Mussa A, Million T, Assefa F. Rhizospheric bacterial isolates of grass pea (Lathyrus sativus L.) endowed with multiple plant growth promoting traits. J Appl Microbiol. 2018;125:1786–1801. doi: 10.1111/jam.13942. [DOI] [PubMed] [Google Scholar]
- Ndeddy Aka RJ, Babalola OO. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytorem. 2016;18:200–209. doi: 10.1080/15226514.2015.1073671. [DOI] [PubMed] [Google Scholar]
- Noumavo PA, et al. Metabolic and biofungicidal properties of maize rhizobacteria for growth promotion and plant disease resistance. Afr J Biotechnol. 2015;14:811–819. doi: 10.5897/AJB2014.14132. [DOI] [Google Scholar]
- Nurk S, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol. 2013;20:714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelade KA, Babalola OO. Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int J Environ Res Public Health. 2019;16:3873. doi: 10.3390/ijerph16203873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. W J Microbiol Biotechnol 33:197 [DOI] [PMC free article] [PubMed]
- Overbeek R, et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST) Nucl Acid Res. 2014;42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo EM, et al. An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Food Sci Technol. 2012;32:710–714. doi: 10.1590/S0101-20612012005000094. [DOI] [Google Scholar]
- Prasad M, Srinivasan R, Chaudhary M, Choudhary M, Jat LK (2019) Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: perspectives and challenges. In: PGPR amelioration in sustainable agriculture. Elsevier, pp 129–157
- Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere. 2006;62:741–748. doi: 10.1016/j.chemosphere.2005.04.117. [DOI] [PubMed] [Google Scholar]
- Raklami A, Bechtaoui N, Tahiri A-i, Anli M, Meddich A, Oufdou K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol. 2019;10:1106. doi: 10.3389/fmicb.2019.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Saharan B, Kabi SR, Prasanna R, Nain L. Providencia, a PGPR with biocontrol potential elicits defense enzymes in wheat. Ann Plant Prot Sci. 2011;19:138–141. [Google Scholar]
- Rieusset L, et al. Secondary metabolites from plant-associated Pseudomonas are overproduced in biofilm. Microb Biotechnol. 2020;13:1562–1580. doi: 10.1111/1751-7915.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B, Neilands J. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shen X, Hu H, Peng H, Wang W, Zhang X. Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genom. 2013;14:271. doi: 10.1186/1471-2164-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore A, Dickinson C. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans Br Mycol Soc. 1976;66:57–64. doi: 10.1016/S0007-1536(76)80092-7. [DOI] [Google Scholar]
- Soares R, Trejo J, Lorite MJ, Figueira E, Sanjuán J, Videira e Castro I, Diversity, phylogeny and plant growth promotion traits of nodule associated bacteria isolated from Lotus parviflorus. Microorg. 2020;8:499. doi: 10.3390/microorganisms8040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari S, Arora NK. Plant growth promoting rhizobacteria for ameliorating abiotic stresses triggered due to climatic variability. Clim Chan Environ Sustain. 2013;1:95–103. doi: 10.5958/j.2320-642X.1.2.009. [DOI] [Google Scholar]
- Verma SC, Ladha JK, Tripathi AK. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;91:127–141. doi: 10.1016/S0168-1656(01)00333-9. [DOI] [PubMed] [Google Scholar]
- Yadav RS, et al. Seed bio-priming of baby corn emerged as a viable strategy for reducing mineral fertilizer use and increasing productivity. Sci Horticul. 2018;241:93–99. doi: 10.1016/j.scienta.2018.06.096. [DOI] [Google Scholar]
- Zaidi A, Ahmad E, Khan MS, Saif S, Rizvi A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: current perspective. Sci Horticul. 2015;193:231–239. doi: 10.1016/j.scienta.2015.07.020. [DOI] [Google Scholar]
- Zolla G, Badri DV, Bakker MG, Manter DK, Vivanco JM. Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Appl Soil Ecol. 2013;68:1–9. doi: 10.1016/j.apsoil.2013.03.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the bacteria strains data characterized using I6S rRNA in this study with their accession number are available in the NCBI database.
For the whole genome sequence data:
Bacillus sp. OA1: The raw reads are available under the Bioproject accession number (PRJNA683742)and Biosample numbers (SAMN17036205). The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number (SRX9654894). The genome sequence of Bacillus sp. OA1 has been deposited at DDBJ/ENA/GenBank under the accession JAEKPB000000000.1
Pseudomonas rhizosphaerae OA2: The raw reads are available under the Bioproject accession number PRJNA683763 and Biosample numbers SAMN17036358. The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number SRX9654938.
Pseudomonas sp. OA3: The raw reads are available under the Bioproject accession number PRJNA683768- Pseudomonas sp. OA3)and Biosample number SAMN17036469- Pseudomonas sp. OA3). The sequence data obtained in this work have been deposited in the NCBI Sequence Read Archive under accession number SRX9654998- Pseudomonas sp. OA3. The genome sequence of Pseudomonas sp. OA3 has been deposited at DDBJ/ENA/GenBank under the accession JAEMOM000000000.1.