Abstract
Dance is a complex sensorimotor activity with positive effects on physical fitness, cognition, and brain plasticity in the aging population. We explored whether individual levels of cognitive reserve (CR) proxied by education moderate dance intervention (DI)-induced plasticity assessed by resting-state functional connectivity (rs-FC) changes of the sensorimotor network (SMN), and between the dorsal attention network (DAN) and anterior default mode network (aDMN). Our cohort consisted of 99 subjects, randomly assigned to either a DI group who underwent a 6-month intervention (n = 49, Mage = 69.02 ± 5.40) or a control group (n = 50, Mage = 69.37 ± 6.10). Moderation analyses revealed that CR moderated DI-induced increase of the SMN rs-FC with significant changes observed in participants with ≥ 15 years of education (b = 0.05, t(62) = 3.17, p = 0.002). Only DI alone was a significant predictor of the DAN–aDMN crosstalk change (b = 0.06, t(64) = 2.16, p = 0.035). The rs-FC increase in the SMN was correlated with an improved physical fitness measure, and changes in the DAN–aDMN connectivity were linked to better performance on figural fluency. Consistent with the passive CR hypothesis, we observed that CR correlated only with baseline behavioral scores, not their change.
Subject terms: Cognitive ageing, Neural ageing, Regeneration and repair in the nervous system, Neurological disorders
Introduction
Dance is a complex sensorimotor activity that involves learning new motor skills, utilizes attentional action observation and imitation, and integrates sensory, motor, and cognitive demands1 that bestow rehabilitating effects even on an aging brain. Considerable experimental research on dance intervention (DI) in the elderly has shown compelling benefits in postural and gait parameters2, physical fitness3, and cognition in the memory4,5, attention2,6, and psychosocial domains7. A recent meta-analysis supported the rich benefits of DI on global cognition and memory, but not on the inhibition and task-switching aspects of executive functioning8. Overall, DI-induced behavioral benefits are key in preserving mobility and independence in older age9 and the importance of studying them stems particularly from the low efficacy of current pharmacological treatment for dementia patients10.
Our previous research of an optimized, structured 6-month-long dance intervention (DI) on non-demented seniors demonstrated its positive effects in comparison with “life activities as usual” (LAU) on the performance of the 8-Foot Up-and-Go (8UG) and the 30-Second Chair Stand (30CS) tests11 which target dynamic balance, agility, lower body strength, and physical endurance9; and of the Five Point Test (FPT)12,13, which assesses figural fluency, i.e. the ability of executive functions to provide information about divergent reasoning, divided attention, planning, and mental flexibility14. Interestingly, despite the fact that figural fluency is known to decline in the healthy elderly and in patients with Alzheimer’s disease (AD)15, the observed improvements were independent of hippocampal volumes12. This finding indicates an individual capacity to recruit additional neural resources in order to meet the demands of the intervention. To test this hypothesis, the current work aims at studying neural changes associated with the described behavioral improvements in terms of neural compensation. This accords well with Lövdén and colleagues, who postulated that any acquisition of new skills (dancing, in our case) requires changes in neuronal connections provided by the brain’s capacity for plasticity termed as a cognitive reserve (CR)16. CR is usually proxied by lifetime exposure to cognitively enriching activities17,18 with “years of education” being the most common proxy of CR19. The CR bridges the disjunction between brain pathology load and preserved cognition, and promotes the optimization and recruitment of brain networks20. This is achieved by various mechanisms, among which the increased engagement and connectivity of large-scale brain networks plays an important role18,21. Several authors attempted to examine CR-related changes at the level of resting-state brain networks with inconclusive results22. Others showed that the level of CR has an impact on the association between the magnitude of resting-state connectivity and cognitive outcomes. For example, lower anti-correlation between an anterior (frontal) part of the default mode network (aDMN) and the dorsal attention network (DAN) was linked with decreased memory performance in those amnestic mild cognitive impairment (aMCI) patients who had low CR (proxied by education and IQ), but not in those with high CR levels23. In other words, higher levels of CR alleviated the impact of disrupted inter-network crosstalk on cognition. A rich body of research also supported CR effects in healthy elderly subjects, particularly on preserving inhibition, flexibility, working memory, and visuo-perceptive functions24, but also on alleviating the negative impact of white matter lesions on motor functions25.
In the current study, we aimed to explore the extent to which the individual level of CR moderates the intervention-induced ability to recruit behaviorally-relevant neural resources as assessed by resting-state fMRI. We were specifically interested in the resting-state functional connectivity (rs-FC) changes of the sensorimotor network (SMN) and in the inter-network connectivity changes between the DAN and the aDMN, with the ventromedial prefrontal cortex (vmPFC) as a representative region. To provide a comprehensive picture, changes of other large-scale network pairings26 are analyzed in an exploratory manner. The rationale behind the first network of interest was the fact that the SMN connectivity increases in response to motor training27,28. The SMN is a set of highly interconnected somatosensory, primary motor, and premotor regions that coordinate action and operate in a hierarchical fashion to translate visual and rule-based information into appropriate motor responses29. Regarding the DAN–aDMN, activity within the aDMN node in the anterior cingulate cortex encompassed in the vmPFC has been associated with the modulation of reaction times30, as well conflict resolution and responses within the constraints of a task, such as during fluency tasks31. The DAN consists particularly of the bilateral superior parietal lobules/ intraparietal sulci and the frontal eye fields, and since it contributes to the formation of task rules and goals by top-down orienting, it is engaged especially during externally directed tasks32. Conversely, the DMN is a set of highly interconnected brain regions active when the mind is not engaged in specific behavioral tasks and suppressed (deactivated) during goal-directed behavior with focused attention33. The anterior part of the DMN is particularly connected to the parietal regions of the DAN, and it has been associated with attention and memory functions34. It has been well documented that the magnitude of the rs-FC between the task-positive DAN and the task-negative DMN plays a central regulating role within functional networks underlying cognition35. Patterns of the DAN–DMN connectivity reflect cognitive control efficiency and working memory33 as well as episodic memory performance23,36. Finally, we used the graph metrics of global efficiency and modularity to assess whether CR or the DI modulate general age-related network changes37.
Taken together, there is a sufficient evidence that CR buffers the impact of normal or pathological aging on cognition. Individuals with high CR are more capable of drawing brain plasticity changes on the scale of rs-FC22, and hence meet demands of an intervention. Moreover, dancing requires participants to learn complex motor sequences, and thus may interact with previous levels of education. The abovementioned studies have motivated our aim to study the moderating effects of CR in the context of intervention-induced benefits. More specifically, we directly probe the effect of DI on rs-FC changes at different levels of education as a proxy of CR.
Results
Baseline measures and their changes: comparisons of the groups
No significant baseline differences were found between the DI (n = 49) and the LAU (n = 50) groups in sex (X2(1) = 2.77; p = 0.10) and MCI (n = 21; X2(1) = 1.24; p = 0.27) incidence, demographic data, screening of general cognition (MoCA), behavioral tests of interest, or in the connectivity of networks of interest. Note that 68 participants (DI: 36, LAU: 32) had complete fMRI data. All relevant baseline measurements and their changes are depicted in Table 1. As reported in our previous works11,12, changes in the FPT, 8-Foot Up-and-Go, and 30-Second Chair Stand test scores significantly differed between the DI and the LAU groups. Similarly, the change in the rs-FC of DAN–aDMN significantly differed between both groups. For baseline and follow-up comparisons of cognitive tests that were not included in the present text, see Kropacova et al.12; no significant baseline differences were found. Baseline and changes were compared separately for the MCI vs HC and DI vs LAU groups using Mann Whitney U test (see “Supplementary Materials”, Tables S3a and S3b). No variables of interest were driven by the MCI group.
Table 1.
Baseline and follow-up demographic comparison of two experimental groups based on Mann–Whitney U test for independent samples.
| Variables | Baseline variables | Change of variables (time2-time1) | ||||||
|---|---|---|---|---|---|---|---|---|
| DI | LAU | t(df) | p | DI | LAU | t(df) | p | |
| M ± SD | M ± SD | M ± SD | M ± SD | |||||
| Age | 69.10 ± 5.43 | 68.37 ± 6.10 | − 0.63 (97) | 0.53 | – | – | – | – |
| Education | 14.53 ± 2.58 | 14.80 ± 3.10 | 0.47 (97) | 0.64 | – | – | – | – |
| MoCA | 26.92 ± 2.85 | 26.06 ± 2.71 | − 1.53 (97) | 0.13 | − 0.20 ± 2.42 | 0.66 ± 2.37 | 1.80 (97) | 0.075 |
| FPT | 28.39 ± 8.36 | 31.12 ± 9.48 | 1.52 (97) | 0.13 | 0.54 ± 0.92 | − 0.02 ± 1.09 | − 2.81 (97) | 0.006 |
| 8UG | 5.08 ± 1.21 | 5.23 ± 1.28 | 0.60 (96) | 0.55 | − 0.31 ± 0.76 | 0.31 ± 1.22 | 2.98 (93) | 0.004 |
| 30CS | 15.85 ± 3.51 | 16.43 ± 4.53 | 0.70 (95) | 0.49 | 1.58 ± 2.62 | 0.31 ± 2.36 | − 2.46 (91) | 0.02 |
| SMN | 0.54 ± 0.10 | 0.58 ± 0.18 | 1.17 (48.25) | 0.25 | 0.04 ± 0.16 | − 0.01 ± 0.15 | − 1.27 (66) | 0.21 |
| DAN–aDMN | 0.06 ± 0.08 | 0.08 ± 0.12 | 1.03 (53.51) | 0.31 | 0.02 ± 0.10 | − 0.03 ± 0.11 | − 2.23 (66) | 0.03 |
DI dance-intervention group, LAU life-as-usual group, Age and Education in years, MoCA Montreal Cognitive Assessment score, FPT Five-Point Test score, 8UG 8-Foot Up-and-Go score in seconds, 30SC 30-Second Chair Stand test score, SMN internetwork connectivity of the sensorimotor network, DAN–aDMN connectivity between the dorsal attention network and the anterior default mode network, M mean, SD standard deviation.
Correlation analyses
Table 2 display one-tailed Pearson correlations of networks and tests of interest and education; bold values are significant correlations after adjusting for age and sex. Baseline fitness scores were mutually correlated, as well as the FPT with the 8UG task and with the 30SC test. The baseline rs-FC of the SMN and of the DAN–aDMN were not correlated with any of the baseline behavioral tests of interest. Finally, the improvement in the 8UG correlated with increased connectivity within the SMN and improvement in the FPT correlated with the rs-FC change between the DAN–aDMN, but the latter correlation reached significance only after adjusting for age (r = 0.21, p = 0.050).
Table 2.
Pearson correlations of behavioral and rs-FC outcomes, and CR proxy.
| CR | MoCA | FPT | 30SC | 8UG | SMN | aDMN–DAN | DMN–DAN | |
|---|---|---|---|---|---|---|---|---|
| CR | 1 | |||||||
| MoCA | 0.22b | 1 | ||||||
| FPT | 0.18b | 0.07 | 1 | |||||
| 30SC | 0.37a | 0.12 | 0.21b | 1 | ||||
| 8UG | − 0.22b | − 0.17 | − 0.24b | − 0.68a | 1 | |||
| SMN | 0.01 | 0.02 | − 0.02 | 0.11 | 0.08 | 1 | ||
| aDMN–DAN | − 0.03 | − 0.04 | 0.10 | 0.22b | − 0.13 | 0.08 | 1 | |
| DMN–DAN | − 0.08 | 0.10 | 0.17 | 0.21b | − 0.14 | 0.29a | 0.76a | 1 |
| CR | Δ MoCA | Δ FPT | Δ 30SC | Δ 8UG | Δ SMN | Δ aDMN–DAN | Δ DMN–DAN | |
|---|---|---|---|---|---|---|---|---|
| CR | 1 | |||||||
| Δ MoCA | 0.22b | 1 | ||||||
| Δ FPT | 0.00 | − 0.23b | 1 | |||||
| Δ 30SC | 0.12 | − 0.09 | 0.01 | 1 | ||||
| Δ 8UG | − 0.11 | − 0.07 | − 0.01 | − 0.40a | 1 | |||
| Δ SMN | 0.02 | 0.09 | − 0.02 | − 0.07 | − 0.21b | 1 | ||
| Δ aDMN–DAN | 0.03 | − 0.13 | 0.19 | − 0.01 | − 0.18 | 0.01 | 1 | |
| Δ DMN–DAN | 0.08 | 0.03 | 0.18 | 0.05 | − 0.07 | 0.22b | 0.70a | 1 |
Bold values suggest significant partial correlations after adjusting for age and sex.
∆-change time2 − time1, CR cognitive reserve, MoCA Montreal Cognitive Assessment test, FPT Five-Point Test, 8UG 8-Foot Up-and-Go score in seconds, 30SC 30-Second Chair Stand test score, SMN internetwork connectivity of the sensorimotor network, DAN–(a)DMN connectivity between the dorsal attention network and the (anterior) default mode network.
aCorrelation is significant at the 0.01 level (1-tailed).
bCorrelation is significant at the 0.05 level (1-tailed).
Education was positively related to all baseline behavioral scores: FPT, the 8-Foot Up-and-Go, the 30-Second Chair Stand test, and the MoCA, but only to the change of the MoCA score. Consistent with the moderation model (see below), education correlated only with the rs-FC change within the SMN in the DI group (r = 0.41, p = 0.007) but not with its baseline; it did not correlate with either the baseline rs-FC between DAN–aDMN nor with the between-network rs-FC change.
Exploratory Pearson correlations between all cognitive tests administered in the study and education are reported in the “Supplementary Materials” (Tables S4a, S4b).
Moderation analyses
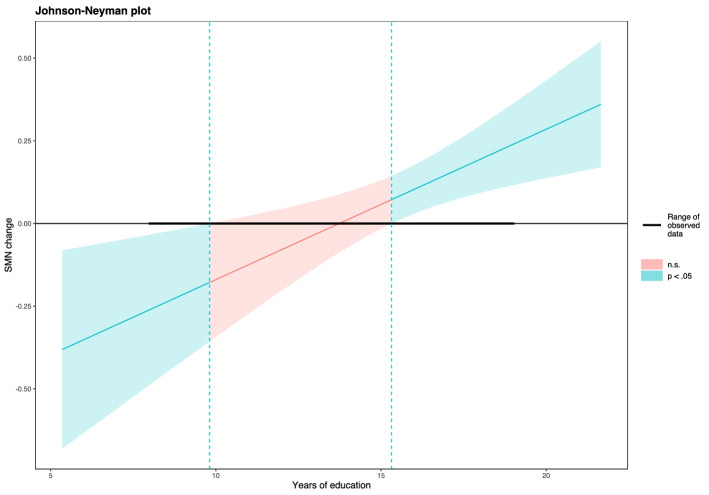
The moderation analysis of rs-FC change within the SMN revealed that education moderates the effect of DI on the network change, i.e. the effect of dance depended on years of education. No main effect was significant in this model and only the interaction effect was observed. Upon closer inspection of the Johnson–Neyman zone of significance (Fig. 1), we observed a significant positive effect in higher values of CR (cut-off 15.34 years of education; operationalized as W = 0.423; 44.12% of cases), which diminished in moderate values of education. Conversely, as education decreased (cut-off 9.80 years of education; W = − 4.885; 4.41% of cases) the relationship between DI and rs-FC change was significantly negative.
Figure 1.
Johnson–Neyman plot depicting conditional effects of dance intervention on change within the sensorimotor network (SMN change) at values of the moderator cognitive reserve (years of education). The plot shows the range of observed data (bold line) and the zone of significance.
As for the second hypothesized DAN–aDMN between-network rs-FC, the moderation analysis revealed that changes were dependent on the main effect of program; the effects of the covariates, education or the interaction were not significant. The regression coefficients and t values that emerged from the moderation analyses of rs-FC changes are presented in Table 3.
Table 3.
Linear model of predictors of change in SMN connectivity (R2 = 0.166; F(5,62) = 3.065; p = 0.016) and DAN–aDMN connectivity (R2 = 0.088; F(5,62) = 1.023; p = 0.412).
| Model terms | B [95% CI] | SE B (HC4) | t | p |
|---|---|---|---|---|
| SMN change | ||||
| Constant | − 0.071 [− 0.643, 0.500] | 0.286 | − 0.250 | 0.803 |
| Program | 0.053 [− 0.021, 0.127] | 0.037 | 1.430 | 0.158 |
| Cognitive reserve (centered) | − 0.016 [− 0.035, 0.003] | 0.010 | − 1.709 | 0.093 |
| Moderator CR*program | 0.045 [0.017, 0.074] | 0.014 | 3.169 | 0.002 |
| Age | 0.001 [− 0.007, 0.009] | 0.004 | 0.285 | 0.777 |
| Sex | − 0.016 [− 0.100, 0.069] | 0.042 | − 0.375 | 0.709 |
| DAN–aDMN change | ||||
| Constant | − 0.064 [− 0.353, 0.225] | 0.145 | − 0.441 | 0.661 |
| Program | 0.062 [0.005, 0.119] | 0.029 | 2.160 | 0.035 |
| Cognitive reserve (centered) | − 0.001 [− 0.018, 0.015] | 0.008 | − 0.182 | 0.856 |
| Moderator CR*program | 0.008 [− 0.014, 0.029] | 0.011 | 0.717 | 0.476 |
| Age | 0.001 [− 0.003, 0.005] | 0.002 | 0.315 | 0.754 |
| Sex | − 0.021 [− 0.102, 0.059] | 0.040 | − 0.523 | 0.603 |
Results are unstandardized beta coefficients with 95% confidence intervals from moderation models estimating the association of program, CR (years of education), and their interaction with rs-FC change. Note: only continuous variables that contributed to the outcomes were centered. Cribari-Neto model was used for standard error of variance and F-statistics. Variable program coded as LAU: 0, DI: 1; SMN inter-network rs-FC of the sensorimotor network; DAN–aDMN between-network rs-FC of dorsal attention network and anterior part of the default mode network.
The exploratory analyses of other networks are presented in detail in the “Supplementary Materials” (Table S5). DI (without the contribution of education or the covariates) was a significant positive predictor of the (whole) DAN–DMN between-network connectivity increase t(df) = 2.17, p = 0.034. The models predicting change between the DMN and the FPCN, VN, and SMN were not significant.
Additionally, we performed moderation analyses on the global efficiency and modularity. These exploratory analyses were carried out in a similar fashion, and a detailed report can be found in the “Supplementary Materials” (Table S6). In brief, global efficiency increased in response to the DI (t(62) = 2.20, p = 0.032) invariantly on education or the covariates. We did not observe significant changes in the global modularity.
Discussion
Dance is a joyful complex activity combining physical exercise with cognitive, social, musical, and artistic stimulation38. The benefits of dance intervention stem from the recruitment of higher-order cognitive functions that require enhanced engagement and coordination of large-scale brain networks39. This study followed up on our previous findings showing that DI elicits distinct motor11 and cognitive improvements12; it focused on the moderating effects of CR proxied by education on the DI-induced rs-FC changes involving the large-scale brain networks, namely the SMN and the DAN–aDMN.
We observed that the rs-FC increase of DAN–aDMN was dependent on the DI, while the changes in the SMN intra-network connectivity depended on the interaction between the DI and education. In other words, the follow-up changes within the SMN network were not significant across the whole DI group but only in those with higher education. This result is discussed in more detail in the context of CR moderating effects in the text below.
All the baseline behavioral (fitness and cognitive) scores as well as their follow-up improvements were mutually correlated, sharing approximately 10% of their variance. This variance can represent a shared component of psychomotor speed, which declines throughout the lifespan40.
Regarding our neuroimaging results, previous literature showed that dance practice may modify brain plasticity as evaluated by structural MRI13,41, but little is known about the rs-FC changes induced by the DI. By comparing professional and naïve dancers, Burzynska et al.38 demonstrated differences between both groups in the engagement of the general motor learning network, including major nodes of the SMN, basal ganglia structures, and frontoparietal regions. The current study employed rs-fMRI and for the first time explored the CR moderation of brain plasticity changes resulting from the DI. Our major finding supported the significant CR moderation (proxied by education) of the DI effect on the rs-FC changes within the SMN. Specifically, the observed increase of the SMN rs-FC was dependent on ≥ 15 years of education (which equals the minimum of secondary education with graduation in the Czech schooling system). This effect dissolved in moderate levels and significantly reversed in low levels of education (≤ 10 years of education). Note that only 4.41% of the cases had such a low education level, and thus this latter result cannot be further interpreted. The observed DI-induced increase of rs-FC of the SMN is clinically relevant as it was associated with improved performance in dynamic balance and mobility, known to decline with aging9. The SMN is particularly engaged in motor learning and execution of specific motor actions42; although the SMN has not been assessed in the context of DI, studies on aerobic exercise interventions have consistently reported a reactive increase of rs-FC of the SMN in healthy28 and diseased subjects43, as well as significant differences in its structural connectivity among professional ballet dancers in comparison to a control group44. The relation between education level and motor network involvement may seem peculiar; nevertheless, associations between motor aspects and education levels have been demonstrated. For instance, in Parkinson’s disease patients (i.e. the typical patient group with a movement disorder) the CR (proxied by education) was inversely correlated with motor symptom severity despite greater reductions in dopamine levels45,46. In healthy elderly subjects, higher CR buffered the impact of white matter lesions on walking speed at baseline but did not influence the follow-up assessment25.
In contrast, the moderation model of the DAN–aDMN rs-FC changes revealed that even when accounting for age and sex, the DI alone, without CR contribution, is a significant predictor of its change. This was not specific just to the anterior node of the DMN, since we observed the same effects for the whole DAN–DMN. In contrast, we found that when accounting for age and sex, rs-FC change between the DAN–aDMN was significantly related to improvement in the figural fluency task; this was not true for the whole DAN–DMN. The DMN–DAN connectivity plays an important role in cognitive control and working memory30,33, which is significantly altered with aging47. Anthony and Lin48 speculate that individual hub seeds of the DMN, including the anterior cingulate region, underlie the core hub of neural reserve in the context of CR, while the DAN regions are rather related to neural compensations (i.e. engaged in brain maintenance to compensate for brain pathology). Therefore, our results are in line with the notion that by increasing the DAN–DMN crosstalk, dancing may facilitate neuroplasticity and the preservation of CR49. We also observed DI-induced increase of network efficiency, which has been linked to higher CR capacity in pathological aging37,50. A prospective 21-year study demonstrated that regular participation in dancing was the only physical activity among the 11 studied (e.g. bicycling, playing tennis or swimming) that was associated with a lower risk of dementia in an elderly cohort, presumably by increasing plasticity and CR51.
Interestingly, while higher levels of education proxy were related to better baseline behavioral scores, they were not correlated with their follow-up changes. Even though ours is the first study to observe such discrepancies resulting from an intervention, many longitudinal observational studies in healthy elderly subjects, and particularly those conducted on samples with a degenerative brain disease, found that CR (proxied by education, occupation, or premorbid IQ) was related to baseline behavioral outcomes, but not to their changes52,53. For instance, higher education among PD patients predicted lower incidence of high Hoehn–Yahr stage, better cognitive and motor baseline scores as estimated by MMSE and gait speed with UPDRS-III respectively, but not their annual progression of 6 years54. This phenomenon has been dubbed a passive reserve hypothesis and highlights the CR contribution to better cognitive and motor performance scores resulting from the persistence of differences that appear at younger ages, rather than from ongoing changes (e.g. lifestyle or pathology) that influence differential rates of cognitive decline54.
There are limitations to our study. We used a static proxy of CR which may not be reflective of the dynamic nature of the CR and its pathology-induced depletion55. Estimating dynamic CR using a latent or residual CR index56,57 in future research might deepen our understanding of its reactive nature. Besides, educational attainment is contaminated with socioeconomic factors, such as income, access to health care, gender, and healthy lifestyle habits. Second, by using a non-active “life activities as usual” control group, we were unable to control for other significant factors, e.g. the social aspect of this collective intervention. Finally, adaptive testing is a more sensitive approach to training or evaluation in uncovering post-intervention effects.
In conclusion, the protective effects of cognitive reserve in nondemented older adults have been suggested by several lines of research. We showed that an intensive 6-month DI can induce clinically-relevant changes in brain plasticity, physical fitness, and cognition, and importantly, that some of the brain plasticity changes depend on education, a proxy of CR, suggesting that higher capacity for plasticity applies to better intervention outcomes. Our study also demonstrated that the DAN–(a)DMN rs-FC, a potential neural representation of CR, can be modulated by DI. Future studies should employ multimodal comprehensive programs to benefit people across different CR levels40. Despite our clinically-relevant results, it is unknown whether short-term engagement in any set of activities is sufficient to elicit changes that last several months or even years after the intervention completion. Therefore, long-term behavioral outcomes of such interventions should be examined and long-term moderation effects of CR should be tested.
Materials and methods
Sample
A total of 99 community-dwelling, non-demented elderly (MCI and healthy) subjects completed the main study and were described in detail previously10,11. All subjects were over 60 years of age without any medical, neurological, or psychiatric disorders that may have an impact on cognition (such as major depression, drug and/or alcohol abuse), or would interfere with DI or with MRI scanning. The absence of dementia was assessed by a screening of cognitive decline (MoCA), the Functional Activities Questionnaire, and a detailed cognitive battery (see Table S1 in the “Supplementary Materials”). Subjects were randomized to a dance intervention group (DI) (N = 49) or a control (LAU) group (N = 50). For detailed information about the enrolment and randomization process, see Kropacova et al.12. Informed consent was obtained from each subject. The study was approved by the ethics committee of Masaryk University and the experiment was performed in accordance with relevant guidelines and regulations. Each subject underwent a neurological examination, detailed neuropsychological evaluation, MRI, and physical fitness examination prior to the program and six months after the program completed.
Dance intervention
The DI program was designed and supervised by specialists from the Faculty of Sports Studies, Masaryk University, Brno, Czech Republic. The whole study lasted for three years with the yearly rotation of a group of 20 subjects. Each intervention took six months and included three training units (each of 60 min) per week. The DI program was supervised and conducted at a medium physical load intensity which was monitored each session using the Borg Rating of Perceived Exertion (RPE) scale, a user-friendly numerical scale that evaluates an individual’s subjective effort, physical exertion, and fatigue during exercise on a 15-point scale58. The DI sessions included folk, country, African, Greek, and tango dancing. The choreographies were divided into smaller blocks that were gradually taught in individual lessons and modified and developed over time into the final choreography. Only subjects who completed at least 60% of the DI program were included in the final cohort12. The real average completion of the DI program was 78.1%.
Physical fitness examination
The effect of the DI was evaluated using two tests from the functional fitness assessment9. The 8-Foot Up-and-Go Test evaluates agility and dynamic balance. It measures the time (in seconds) required to get up from a seated position, walk an eight-foot distance, return to the chair, and sit down. Lower values indicate better performance. The 30-Second Chair Stand test evaluates lower body strength and physical endurance by measuring the number of repetitions of full stands from a chair in 30 s. Higher values indicate better performance. Scores on both tests improved in the DI group as compared to the LAU group11.
Neuropsychological examination
Global cognition (MoCA), activities of daily living, and five cognitive domains were evaluated by complex neuropsychological testing (see Table S1 in the “Supplementary Materials”). The five domains included memory, attention, executive, visuospatial, and language domain. In the current study, we focus on the Five-Point Test (FPT) performance which significantly improved in the DI group as compared to the LAU group12.
MRI examination
All subjects were scanned using the 3T Siemens Prisma MRI scanner (Siemens Corp., Erlangen, Germany) employing various sequences including the T1 anatomical and diffusion tensor imaging sequences11,13. For the purpose of this study, we used resting-state fMRI data, employing gradient-echo echo-planar imaging sequence (200 scans, 34 transversal slices, slice thickness = 3.5 mm, TR = 1990 ms, TE = 35 ms, FA = 70°, FOV = 192 mm, matrix size 64 × 64).
fMRI data processing
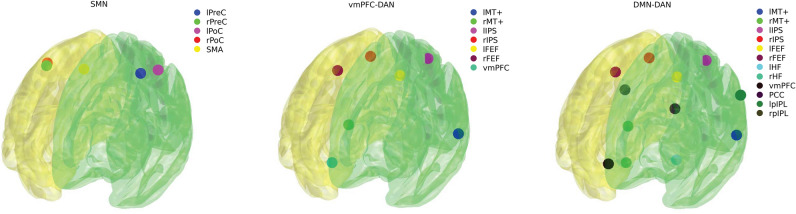
Resting-state fMRI data were preprocessed using the SPM 12 toolbox and Matlab 2014b. Preprocessing started with realignment and unwarping. Next, cardiac and respiratory signals were regressed out using RETROICOR59. Then, normalization into standard anatomical space (MNI) and spatial smoothing with 5 mm FWHM was performed. The level of motion was thoroughly checked in terms of frame-wise displacement (FD)60. No FD was higher than 3 mm and scans that displayed FD > 0.75 mm were scrubbed60. No more than 2.5% of subject scans were removed. Moreover, the six movement regressors (obtained during realignment and unwarping), FD, and extracted signals from white matter and cerebrospinal fluid were regressed out of the data in the subsequent analysis. Representative regions of interest (ROIs; spheres with 6 mm radius) of large-scale functional brain networks of interest (SMN, DAN) and a vmPFC ROI representing the aDMN were chosen (see Fig. 2), together with the whole DMN, VN and FPCN for exploratory purposes based on a literature review26. MNI coordinates for each ROI and network are listed in the “Supplementary Materials” (Table S2). Mean ROI signals were extracted and a correlation matrix was calculated for each subject. Pearson’s correlation coefficients were converted to z values using Fisher’s r-to-z transformation. The connectivity within the SMN and between the DAN–aDMN were calculated as the average of z values within the network ROI pairs61.
Figure 2.
Representative ROIs of the SMN sensorimotor network, vmPFC–DAN ventromedial prefrontal cortex–dorsal attention network, and DMN–DAN default mode network–dorsal attention network.
Graph theory
The whole brain (except the cerebellum) was parcelled into 90 regions of interest (ROIs) according to the AAL atlas62. These ROIs were intersected with previously calculated masks to ensure high signal quality in every subject. ROIs that contained more than 50% of signal dropouts in more than 10% of subjects were removed (4 in total). The time-series of each ROI was averaged and cross-correlated using Pearson’s correlation coefficient to form a 86 × 86 correlation matrix for each subject. Pearson’s correlation coefficients were converted into z-values using Fisher’s r-to-z transformation. Weighted networks were analysed so that the useful information about the strength of a particular connection was preserved. Negative correlations were replaced with zeros. Finally, global efficiency and modularity were computed using the Brain Connectivity toolbox63.
Analyses of the CR effects on DI-induced changes in rs-FC
Demographics between the two study groups were compared using t-tests for continuous variables and chi-square tests for categorical variables. The CR was represented by years of education19; the program variable had two dimensions—dance intervention (DI) or control life as usual (LAU); changes in the outcome variables of interest were computed as timepoint2 (a follow-up visit after 6 months)—timepoint1 (baseline).
As discussed briefly, we also conducted one-tailed Pearson correlations between baseline and follow-up change of behavioral and rs-FC outcomes, and with education independently of the intervention. This was to test the association of behavioral outcomes and the rs-FC changes of interest, as well as their relationship with years of education.
In the moderation analyses, the effect of CR (moderator variable) was tested on the relationship between the program (independent variable) and change in functional outcomes of interest. The moderations were performed on 68 subjects who had fMRI data of sufficient quality (36 DI and 32 LAU) to assess whether and to what degree the relationship between DI and rs-FC changes within the SMN and between the DAN–aDMN depend on CR levels. Moderation was selected because it enables inferring the effect of CR, program, and their interaction (CR*program), as well as modeling the DI effect on different levels of the CR moderator. This approach simplifies the interpretation of results when such effects are present based on the zone of significance without the necessity of subsequent sets of single main effect tests. The significant moderations are plotted using the CRAN R package v 1.1.0. All data were normally distributed.
All statistical analyses were performed using IBM SPSS Statistics 27. For moderation analyses, we used the PROCESS macro for SPSS v 3.4. Finally, the Šidák-Dunn correction was set for each dataset independently in the following manner: 1 − (1 − α)½ = 0.025 for the two moderation models; the correlation analyses were exploratory and uncorrected.
Supplementary Information
Acknowledgements
This project has received funding from the Internal grant agency MU under Masaryk University CZ.02.2.69/0.0/0.0/19_073/0016943 (The role of cognitive reserve in brain maintenance in healthy aging and neurodegenerative diseases) and from the Czech Ministry of Health NU21-04-00652 (Implication of cognitive reserve in non-pharmacological intervention outcomes). We also acknowledge the core facility MAFIL of CEITEC supported by the MEYS CR (LM2018129 Czech-BioImaging). We thank Anne Johnson for English editing.
Author contributions
Study conception and design: K.M., I.R., P.V., A.S., R.G; data collection: K.M., A.S.M., S.K., Z.B., P.K., P.V., A.S., R.G; analysis and interpretation of results: K.M., P.K., J.T.; draft manuscript preparation: K.M.; All authors reviewed the results and approved the final version of the manuscript.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-97323-2.
References
- 1.Kattenstroth JC, Kolankowska I, Kalisch T, Dinse HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front. Aging Neurosci. 2010;2:1–9. doi: 10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kattenstroth JC, Kalisch T, Holt S, Tegenthoff M, Dinse HR. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front. Aging Neurosci. 2013;5:1–16. doi: 10.3389/fnagi.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang PWN, Braun KL. The effectiveness of dance interventions to improve older adults’ health: A systematic literature review phoebe. Altern. Ther. Health Med. 2015;21:64–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Müller P, et al. Evolution of neuroplasticity in response to physical activity in old age: The case for dancing. Front. Aging Neurosci. 2017;9:1–8. doi: 10.3389/fnagi.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehfeld K, et al. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE. 2018;13:1–15. doi: 10.1371/journal.pone.0196636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coubard OA, Duretz S, Lefebvre V, Lapalus P, Ferrufino L. Practice of contemporary dance improves cognitive flexibility in aging. Front. Aging Neurosci. 2011;3:1–12. doi: 10.3389/fnagi.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers, D. K. et al. Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Front. Aging Neurosci.9, 1–12. 10.3389/fnagi.2017.00110 (2017). [DOI] [PMC free article] [PubMed]
- 8.Meng X, et al. Effects of dance intervention on global cognition, executive function and memory of older adults: A meta-analysis and systematic review. Aging Clin. Exp. Res. 2020;32:7–19. doi: 10.1007/s40520-019-01159-w. [DOI] [PubMed] [Google Scholar]
- 9.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 10.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 11.Sejnoha Minsterova, A. et al. Multishell diffusion MRI reflects improved physical fitness induced by dance intervention. Neural Plast.2020, 1–9. 10.1155/2020/8836925 (2020). [DOI] [PMC free article] [PubMed]
- 12.Kropacova S, et al. Cognitive effects of dance-movement intervention in a mixed group of seniors are not dependent on hippocampal atrophy. J. Neural Transm. 2019;126:1455–1463. doi: 10.1007/s00702-019-02068-y. [DOI] [PubMed] [Google Scholar]
- 13.Rektorova I, et al. Brain structure changes in nondemented seniors after six-month dance-exercise intervention. Acta Neurol. Scand. 2020;141:90–97. doi: 10.1111/ane.13181. [DOI] [PubMed] [Google Scholar]
- 14.Johanidesova S, Bolcekova E, Stepankova H, Preiss M. The five point test—A test of nonverbal fluency: normative data for adults. Ces. Slov. Neurol. Neurochir. 2014;110:704–713. [Google Scholar]
- 15.Fama R, et al. Fluency performance patterns in Alzheimer’s disease and Parkinson’s disease. Clin. Neuropsychol. 1998;12:487–499. doi: 10.1076/clin.12.4.487.7235. [DOI] [Google Scholar]
- 16.Lövdén M, Bäckman L, Lindenberger U, Schaefer S, Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychol. Bull. 2010;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2018 doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabeza, R. et al. Cognitive neuroscience of healthy aging: Maintenance, reserve, and compensation. Nat. Rev. Neurosci. 19, 701–710 (2018). [DOI] [PMC free article] [PubMed]
- 19.Tucker AM, Stern Y. Cognitive reserve and aging. Curr. Alzheimer Res. 2010;8:354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern Y. Cognitive reserve: Implications for assessment and intervention. Folia Phoniatr. Logop. 2014;65:49–54. doi: 10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002;26:819–825. doi: 10.1016/S0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 22.Barulli D, Stern Y. Emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:1–17. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzmeier N, et al. Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol. Aging. 2017;50:152–162. doi: 10.1016/j.neurobiolaging.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Roldán-Tapia MD, Cánovas R, León I, García-Garcia J. Cognitive vulnerability in aging may be modulated by education and reserve in healthy people. Front. Aging Neurosci. 2017;9:1–8. doi: 10.3389/fnagi.2017.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elbaz A, Tavernier B. The decreases in motor function in the elderly. Am. Acad. Neurol. 2013;81:417–426. doi: 10.1212/WNL.0b013e31829d8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor KM, et al. Effects of a 12-week aerobic spin intervention on resting state networks in previously sedentary older adults. Front. Psychol. 2018;9:1–13. doi: 10.3389/fpsyg.2018.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss MW, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010;2:1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb GE, Brown IE, Cheng EJ. A hierarchical foundation for models of sensorimotor control. Exp. Brain Res. 1999;126:1–18. doi: 10.1007/s002210050712. [DOI] [PubMed] [Google Scholar]
- 30.Novakova L, Gajdos M, Rektorova I. Theta-burst transcranial magnetic stimulation induced cognitive task-related decrease in activity of default mode network: An exploratory study. Brain Stimul. 2020;13:597–599. doi: 10.1016/j.brs.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Crosson B, et al. Activity in the paracingulate and cingulate sulci during word generation: An fMRI study of functional anatomy. Cereb. Cortex. 1999;9:307–316. doi: 10.1093/cercor/9.4.307. [DOI] [PubMed] [Google Scholar]
- 32.Spreng RN, Shoemaker L, Turner GR. Executive Functions and Neurocognitive Aging. Executive Functions in Health and Disease. Elsevier Inc.; 2017. [Google Scholar]
- 33.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole MW, Repovš G, Anticevic A. The frontoparietal control system: A central role in mental health. Neurosci. 2014;20:652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kragel JE, Polyn SM. Functional interactions between large-scale networks during memory search. Cereb. Cortex. 2015;25:667–679. doi: 10.1093/cercor/bht258. [DOI] [PubMed] [Google Scholar]
- 37.Iordan AD, et al. Aging and network properties: Stability over time and links with learning during working memory training. Front. Aging Neurosci. 2018;9:1–18. doi: 10.3389/fnagi.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burzynska, A. Z., Finc, K., Taylor, B. K., Knecht, A. M. & Kramer, A. F. The dancing brain: Structural and functional signatures of expert dance training. Front. Hum. Neurosci.11, 1–20. 10.3389/fnhum.2017.00566 (2017). [DOI] [PMC free article] [PubMed]
- 39.Kullberg-Turtiainen M, Vuorela K, Huttula L, Turtiainen P, Koskinen S. Individualized goal directed dance rehabilitation in chronic state of severe traumatic brain injury: A case study. Heliyon. 2019;5:e01184. doi: 10.1016/j.heliyon.2019.e01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cespón J, Miniussi C, Pellicciari MC. Interventional programmes to improve cognition during healthy and pathological ageing: Cortical modulations and evidence for brain plasticity. Ageing Res. Rev. 2018;43:81–98. doi: 10.1016/j.arr.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira-Machado L, Arida RM, de Jesus Mari J. Dance for neuroplasticity: A descriptive systematic review. Neurosci. Biobehav. Rev. 2019;96:232–240. doi: 10.1016/j.neubiorev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J. Neurosci. 2011;31:16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fling BW, Martini DN, Zeeboer E, Hildebrand A, Cameron M. Neuroplasticity of the sensorimotor neural network associated with walking aid training in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2019;6:1–4. doi: 10.1016/j.msard.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hänggi J, Koeneke S, Bezzola L, Jäncke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum. Brain Mapp. 2010;31:1196–1206. doi: 10.1002/hbm.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sunwoo MK, Hong JY, Lee JJ, Lee PH, Sohn YH. Does education modify motor compensation in Parkinson’s disease? J. Neurol. Sci. 2016;362:118–120. doi: 10.1016/j.jns.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 46.Kotagal V, et al. Educational attainment and motor burden in Parkinson’s disease. Mov. Disord. 2015;30:1143–1147. doi: 10.1002/mds.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders NLJ, Summers MJ. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010;32:350–357. doi: 10.1080/13803390903042379. [DOI] [PubMed] [Google Scholar]
- 48.Anthony M, Lin F. A systematic review for functional neuroimaging studies of cognitive reserve across the cognitive aging spectrum. Arch. Clin. Neuropsychol. 2017;33:937–948. doi: 10.1093/arclin/acx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zilidou VI, et al. Functional re-organization of cortical networks of senior citizens after a 24-week traditional dance program. Front. Aging Neurosci. 2018;10:1–14. doi: 10.3389/fnagi.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alijore O, Lamar M, Anand K. Association of brain network efficiency with aging, depression, and cognition. Am. J. Geriatr. Psychiatry. 2014;22:102–110. doi: 10.1016/j.jagp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the Elderly Joe. N. Engl. J. Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 52.Lo, R. Y., & Jagust, W. J. Effect of cognitive reserve markers on alzheimer pathological progression. Alzheimer Dis. Assoc. Disord. 27, 1–14. 10.1097/WAD.0b013e3182900b2b (2013). [DOI] [PMC free article] [PubMed]
- 53.Gazzina S, et al. Education modulates brain maintenance in presymptomatic frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2019;90:1124–1130. doi: 10.1136/jnnp-2019-320439. [DOI] [PubMed] [Google Scholar]
- 54.Lee PC, et al. Examining the reserve hypothesis in Parkinson’s disease: A longitudinal study. Mov. Disord. 2019;34:1663–1671. doi: 10.1002/mds.27854. [DOI] [PubMed] [Google Scholar]
- 55.Reed BR, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133:2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKenzie C, et al. Cognitive reserve predicts future executive function decline in older adults with Alzheimer’s disease pathology but not age-associated pathology. Neurobiol. Aging. 2020;88:119–127. doi: 10.1016/j.neurobiolaging.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 57.van Loenhoud AC, Habeck C, van der Flier WM, Ossenkoppele R, Stern Y. Identifying a task-invariant cognitive reserve network using task potency. Neuroimage. 2020;210:116593. doi: 10.1016/j.neuroimage.2020.116593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams N. The Borg rating of perceived exertion (RPE) scale. Occup. Med. (Chic. Ill.) 2017;67:404–405. doi: 10.1093/occmed/kqx063. [DOI] [Google Scholar]
- 59.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 60.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klobušiakova P, Mareček R, Fousek J, Výtvarova E, Rektorova I. Connectivity between brain networks dynamically reflects cognitive status of Parkinson’s disease: A longitudinal study. J. Alzheimer’s Dis. 2019;67:971–984. doi: 10.3233/JAD-180834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 63.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.