Abstract
Sex differences in the organization of large-scale resting-state brain networks have been identified using traditional static measures, which average functional connectivity over extended time periods. In contrast, emerging dynamic measures have the potential to define sex differences in network changes over time, providing additional understanding of neurobiological sex differences. To meet this goal, we used a Coactivation Pattern Analysis (CAP) using resting-state functional magnetic resonance imaging data from 181 males and 181 females from the Human Connectome Project. Significant main effects of sex were observed across two independent imaging sessions. Relative to males, females spent more total time in two transient network states (TNSs) spatially overlapping with the dorsal attention network and occipital/sensory-motor network. Greater time spent in these TNSs was related to females making more frequent transitions into these TNSs compared to males. In contrast, males spent more total time in TNSs spatially overlapping with the salience network, which was related to males staying for longer periods once entering these TNSs compared to females. State-to-state transitions also significantly differed between sexes: females transitioned more frequently from default mode network (DMN) states to the dorsal attention network state, whereas males transitioned more frequently from DMN states to salience network states. Results show that males and females spend differing amounts of time at rest in two distinct attention-related networks and show sex-specific transition patterns from DMN states into these attention-related networks. This work lays the groundwork for future investigations into the cognitive and behavioral implications of these sex-specific network dynamics.
Keywords: sex differences, functional magnetic resonance imaging, resting-state, functional network dynamics, coactivation pattern analysis, Human Connectome Project
Introduction
Biological sex is one factor associated with individual differences in cognition and behavior. Sex differences have been identified in basic neurocognitive processes such as attention and spatial processing (Bayliss et al., 2005), but also in cognitive development (Gur et al., 2012) and in psychiatric disease (Naninck et al., 2011). Although it is difficult to disentangle physiological from social aspects of sex, investigating sex differences in neurobiological functioning may improve our understanding of key neurocognitive domains that contribute to mental health and daily functioning. One way to evaluate fundamental neurobiological sex differences is to investigate the brain’s resting-state. Imaging the brain at rest can reveal brain regions with correlated fluctuations in the functional magnetic resonance imaging (fMRI) signal, forming resting-state networks associated with known brain systems such as those involved in attention, visuospatial, auditory, motor, and affective processing (Yeo et al., 2011). The use of resting-state data may therefore identify sex-specific differences in the intrinsic organization and function of the brain underlying cognition.
Males and females differ in their functional connectivity within and between large-scale brain networks at rest. For example, males and females have demonstrated differences in the resting-state functional connectivity (rsFC) of several networks involved in cognitive processes including self-referential processing (Allen et al., 2011; Biswal et al., 2010; de Lacy et al., 2019), executive function (Hjelmervik et al., 2014), salience processing (Wang et al., 2014), attention (Filippi et al., 2013), and sensory-motor functioning (Allen et al., 2011; de Lacy et al., 2019). This literature suggests that males and females differ in their intrinsic brain organization, which may underlie differences in core cognitive functions. However, this prior work used only traditional static measures of FC, which average correlated brain activity across the entirety of the resting-state scan (typically 6 minutes or more). While informative, static FC analyses only provide insight into functional brain organization as a snapshot in time and do not provide insight into the temporal or dynamic properties of resting brain function. In contrast, evaluating dynamic features of the resting brain will inform how such brain networks perform at rest, thus providing another piece of information regarding sex differences in neurobiology.
Several emerging techniques assess temporal dynamic properties of brain networks at rest. However, only a few studies have begun to evaluate sex-specific temporal differences. For example, one approach for assessing dynamic functional connectivity (dFC) includes measuring changes in correlated fMRI activity over time (e.g., FC using sliding windows). Sliding window methods parcellate the functional time series of regions of interest into shorter, often overlapping windows to probe regional connectivity changes over time. Collectively, studies using this method suggest that males make more frequent transitions into different networks while providing some evidence that women are more likely to remain in specific network states over time (Cai et al., 2020; de Lacy et al., 2019; Yaesoubi et al., 2015). Other methods evaluate temporal patterns of resting data by examining changes in simultaneous activation, or the coactivation patterns (CAP) to probe functional network dynamics. CAP analysis is a data-driven technique that uses both spatial distribution and magnitude of activation to identify transient network states (TNSs) of coactivation (Liu et al., 2018). In a study that identified 30 TNSs, the occurrence rate of a TNS overlapping with the sensorimotor cortex was greater in males relative to females (Liu et al., 2013). However, the CAPs procedure allows for a range of additional temporal dynamic analyses, including total time spent in a network, time persisting in a network per transition into that network, the frequency of transitions into that network, as well as transition patterns between networks. Collectively, evaluating these metrics will provide a more nuanced understanding of sex differences in the temporal dynamics of the brain.
To provide a comprehensive evaluation of resting temporal dynamics, we recently used CAP analysis to characterize the dynamic features of the brain across two resting-state fMRI scans in a large sample of adults from the Human Connectome Project (HCP) (Janes et al., 2020). We computed measures of functional network dynamics, including total time spent in individual TNSs, persistence (i.e., time spent in a TNS before transitioning to another TNS), and the transition frequency into different TNSs. Our findings identified 8 TNSs that overlapped with canonical large-scale brain networks typically defined by static measures of resting-state fMRI data including the default mode network (DMN; 3 TNSs), salience network (SN; 2 TNSs), dorsal attention network (DAN; 1 TNS), occipital/sensory-motor network (1 TNS), and frontoparietal network (FPN; 1 TNS) (Janes et al., 2020). The eight TNSs defined in Janes et al. (2020) contrast with the 30 TNSs defined by Liu et al. (2013) in which many individual TNSs were comprised of subregions of these larger networks. Methodological differences in TNS derivation between these studies is worth noting, as the approach by Liu et al. (2013) is well suited for identifying novel associations between regions or groups of regions, whereas the TNSs identified in Janes at al. (2020) spatially map onto large resting-state networks, allowing for CAP to clarify dynamic properties of well-established neurocognitive networks.
Building on Janes et al. (2020), a critical next step is to investigate sex differences in functional network dynamics, as doing so will contribute to our understanding of individual variance in neurocognitive function in healthy adults. The current study incorporated a subsample of participants from the HCP and the eight TNSs identified previously (Janes et al., 2020) to examine potential sex differences in four measures of functional network dynamics: (1) total time spent in each TNS, (2) persistence in each TNS, (3) transition frequency into each TNS, and (4) frequency of specific state-to-state transitions (e.g., frequency of State 3 to 4 transitions).
METHOD
Participants
We investigated a subsample of participants included in Janes et al. (2020). Individuals from the HCP 1200 subject release were excluded from the Janes et al. sample if they reported a history of major psychiatric disorder, neurological disorder, or medical disorder known to influence brain function, a family history of schizophrenia, met DSM-IV criteria for alcohol dependence, reported a lifetime history of repeated substance use, or had a positive drug or alcohol test on the scanning day, leaving a sample of 462 participants (n=181 males, n=281 females). To achieve equal groups for the current analyses, we included the n=181 males from the Janes et al. sample, and a randomly selected subsample of n=181 females who did not significantly differ from males on age or education. The final data set included resting-state fMRI scans from 362 individuals. Participants were on average 27.46 years of age (SD=3.13, range: 22–35) and reported completing an average of 15.27 years of education (SD=1.58, range: 11–17). Participants largely self-identified as White (n=271), with the remaining self-identifying as Black/African-American (n= 49), Asian, Native Hawaiian, or Other/Pacific Islander (n=32), or multi-racial (n=6). Four participants did not disclose their race.
fMRI Data Acquisition
Resting-state fMRI data was acquired with a 32-channel head coil on a Siemens 3T Skyra, with a gradient-echo strength of 100 mT/m. Gradient-echo echo-planar imaging (EPI) images were collected using the following parameters: TR = 720 ms, TE = 33.1 ms, flip angle = 52°, FOV = 208 × 180 mm (PO x PE), Matrix = 104 × 90 (RO x PE), echo spacing = 0.58 ms, BW = 2290 Hz/Px. Slice thickness was set to 2.0 mm, 72 slices, 2.0 mm isotropic voxels, with a multi-band acceleration factor of 8 (Glasser et al., 2016; Uğurbil et al., 2013; Van Essen et al., 2013).
Over two scan sessions across consecutive days, four 14.4-minute runs of resting-state fMRI were collected. Within each scan session there were two runs, one run was acquired with a left-to-right phase encoding direction and the other was acquired with a right-to-left phase encoding direction. This resulted in two scan sessions equaling 28.8 minutes. During resting-state acquisition, participants were instructed to lie on their back with their eyes open and fixate on a bright cross-hair, which was projected against a dark background.
Preprocessing
“Fix-extended” resting-state fMRI data was used from the HCP 1200 subject release with the following preprocessing steps: gradient unwarping, motion correction, fieldmap-based EPI distortion correction, brain-boundary-based registration to EPI and structural T1-weighted images, non-linear FNIRT registration to MNI space, and grand-mean intensity normalization using FSL and FreeSurfer (Glasser et al., 2013). The FSL FIX program was used to identify and remove noise (Griffanti et al., 2014; Salimi-Khorshidi et al., 2014).
Time courses were extracted from resting-state fMRI data using 129 regions of interest (ROIs) (Choi et al., 2012; Yeo et al., 2011). An amygdala ROI was obtained from the automated anatomical labeling atlas (http://www.gin.cnrs.fr/en/tools/aal). At the beginning of each time series, twenty volumes were removed to allow for signal stabilization. Finally, time series were concatenated within and across participants.
Co-Activation Pattern Analysis
CAP analyses are described in extended detail in Janes et al. (2020). Briefly, after an initial data reduction step using principal component analysis, a k-means clustering analysis was performed on the first resting-state fMRI scan from all participants to identify brain states corresponding to resting-state brain activity. Silhouette scores were calculated to evaluate the optimal clustering solution, which was deemed to be k=8. CAP analyses were conducted using the open-source “capcalc” package (https://github.com/bbfrederick/capcalc/). After identifying recurring TNSs, four measures of functional network dynamics were conducted for each TNS during the first resting-state fMRI run: (1) total time spent in each TNS, (2) persistence, (3) frequency of transitions into each TNS, and (4) frequency of individual TNS-to-TNS transitions (Kaiser et al., 2019). Initial CAP classification was conducted on the sample described in Janes et al. (2020).
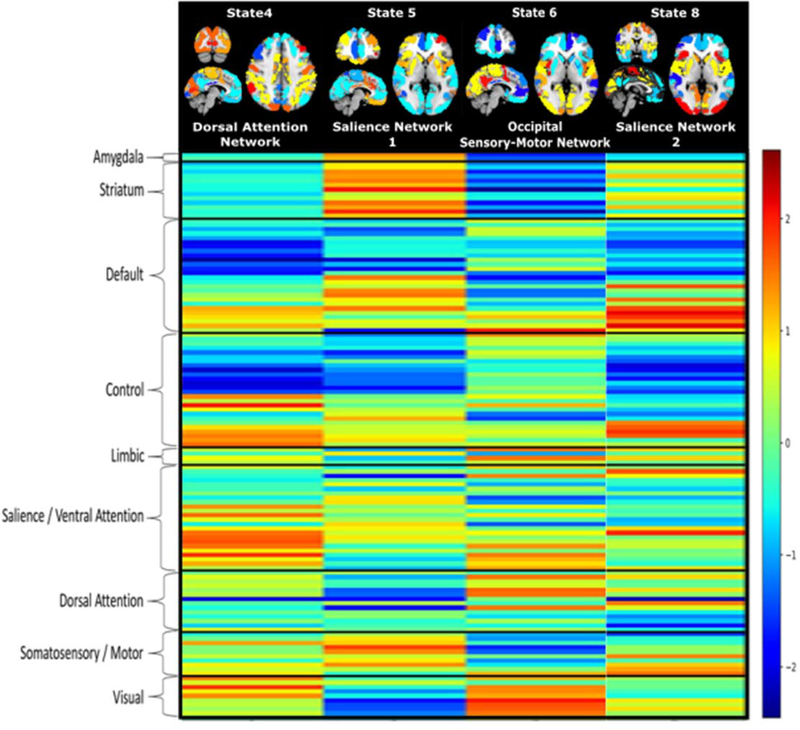
Detailed descriptions of each of the patterns of relative brain activation and deactivation for the eight TNSs are reported in extended detail in Janes et al. (2020). Briefly, the spatial pattern of each TNS overlapped with standard large-scale brain networks (Laird et al., 2011; Smith et al., 2009): three TNSs showed high spatial overlap with the DMN (States 1, 3, and 7), two TNSs showed high spatial overlap with the SN (States 5 and 8), whereas the remaining TNSs showed spatial overlap with the FPN (State 2), DAN (State 4), and the occipital cortex and sensory-motor brain regions (State 6).
Statistical Analyses
A staged analysis was implemented. First, we examined sex differences relating to the total time spent in each TNS. Subsequent analyses focused only on the TNSs showing significant sex differences in total time spent in each TNS. Analyses employed repeated-measures ANOVAs evaluating persistence, transition frequency, and frequency of TNS-to-TNS. In each repeated-measures ANOVA, one measure of functional network dynamics was the dependent measure, with session (scanning session #1 or #2) as a within-subject factor and sex as a between-subjects factor. Significant effects survived a Bonferroni multiple comparison correction within each family of statistical tests performed. Additionally, for all significant ANOVAs, two-sample Kolmogorov-Smirnov tests were used to examine whether these results also extended to significant differences in the functional network dynamics distributions between males and females.
RESULTS
Repeated-Measures ANOVA Analyses
Total Time Spent in Each TNS:
We conducted eight repeated-measures ANOVAs (i.e., one for each TNSs). Significant effects survived a Bonferroni multiple comparison correction (i.e., .05/8, p ≤ .00625).
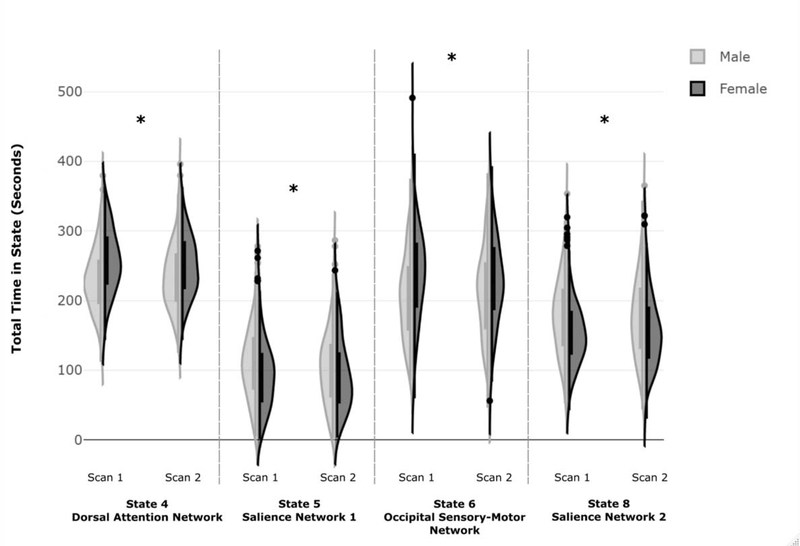
Significant main effects of sex were observed for total time spent in four TNSs: a state overlapping with DAN (State 4) (F(1,360)=25.67, p < .001, partial η2= .067), an occipital/sensory-motor state (State 6) (F(1,360)= 16.48, p < .001, partial η2= .044), and the states overlapping with the SN (State 5: (F(1,360)= 8.33, p = .004, partial η2= .023) and State 8: (F(1,360)= 18.64, p < .001, partial η2= .049). There were no significant main effects of session or sex by session interactions. In post-hoc t-tests, females spent more time in State 4 (t(722)= −6.26, p < .001) and State 6 (t(722)= −4.85, p < .001), whereas males spent more time in State 5 (t(722)= 3.51, p < .001) and State 8 (t(722)= 5.28, p < .001) (Figure 2). Supplemental analyses controlling for variance in global mean signal were similar, however the sex differences in State 5 were no longer significant after multiple comparison correction (see Supplement). Kolmogorov-Smirnov tests revealed significant sex-differences in the distribution of total time in TNS for both scan sessions for State 4 (Scan 1: Z=2.63, p<.001, Scan 2: Z=1.63, p=.010) and State 8 (Scan 1: Z=2.26, p<.001, Scan 2: Z=1.79, p=.003) and for the first scan session only for State 5 (Scan 1: Z=1.52, p=.019, Scan 2: Z=1.31, p=.063) and State 6 (Scan 1: Z=2.21, p<.001, Scan 2: Z=1.16, p=.138).
Figure 2: Sex-Related Differences in Total Time Spent in TNSs of Interest.
Figure 2. Frequency distributions of males and females for the total time spent in each TNS of interest. Females exhibited increased total time spent in States 4 and 6, whereas males exhibited increased total time spent in States 5 and 8. There were no significant sex differences in total time spent in the other four TNSs investigated. Independent samples Kolmogorov-Smirnov tests were used to examine differences in the frequency distributions between males and females (State 4: Scan 1: K-S Z=2.63, p<.001, Scan 2: K-S Z=1.63, p=.010; State 5: Scan 1: K-S Z=1.52, p=.019, Scan 2: K-S Z=1.31, p=.063; State 6: Scan 1: K-S Z=2.21, p<.001, Scan 2: K-S Z=1.16, p=.138; State 8: Scan 1: K-S Z=2.26, p<.001, Scan 2: K-S Z=1.79, p=.003).
Persistence:
We performed four repeated-measures ANOVAs to investigate sex differences in persistence in the four TNSs of interest defined by our initial analyses (States 4, 5, 6, and 8). Significant effects survived a Bonferroni multiple comparison correction (i.e., .05/4, p ≤ .0125).
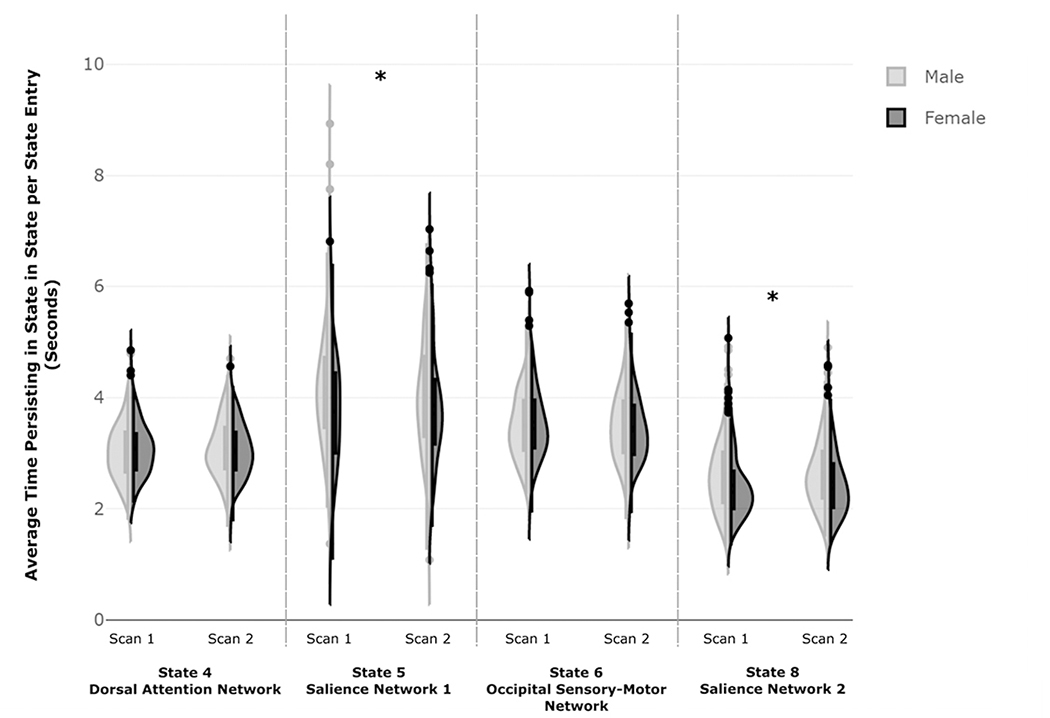
Significant main effects of sex were observed for persistence in both SN states (State 5: (F(1,360)= 10.73, p = .001, partial η2= .029) and State 8: (F(1,360)= 10.67, p = .001, partial η2= .029). There were no significant main effects of session or sex by session interactions. In post-hoc t-tests, males persisted for longer periods of time in State 5 (t(722)= 3.88, p < .001) and State 8 (t(722)= 3.91, p < .001)) compared to females (Figure 3). Kolmogorov-Smirnov tests revealed significant sex-differences in the distribution of TNS persistence for both scan sessions for State 8 (Scan 1: Z=2.05, p<.001, Scan 2: Z=1.58, p=.014) and in the first scan session only for State 5 (Scan 1: Z=1.73, p=.005, Scan 2: Z=1.21, p=.108).
Figure 3: Sex-Related Differences in Persistence in TNSs of Interest.
Figure 3. Frequency distributions of males and females for the persistence in each TNS of interest. Males exhibited greater persistence in both TNSs overlapping with the salience network (State 5 and 8) compared to females. Independent samples Kolmogorov-Smirnov tests were used to examine differences in the frequency distributions between males and females (State 5: Scan 1: K-S Z=1.73, p=.005, Scan 2: K-S Z=1.21, p=.108; State 8: Scan 1: K-S Z=2.05, p<.001, Scan 2: K-S Z=1.58, p=.014).
Frequency of Transitions into Specific TNSs:
We performed four repeated-measures ANOVAs to investigate sex differences relating to the overall frequency of transitions into the four TNSs of interest. Significant effects survived a Bonferroni multiple comparison correction (i.e., .05/4, p ≤ .0125).
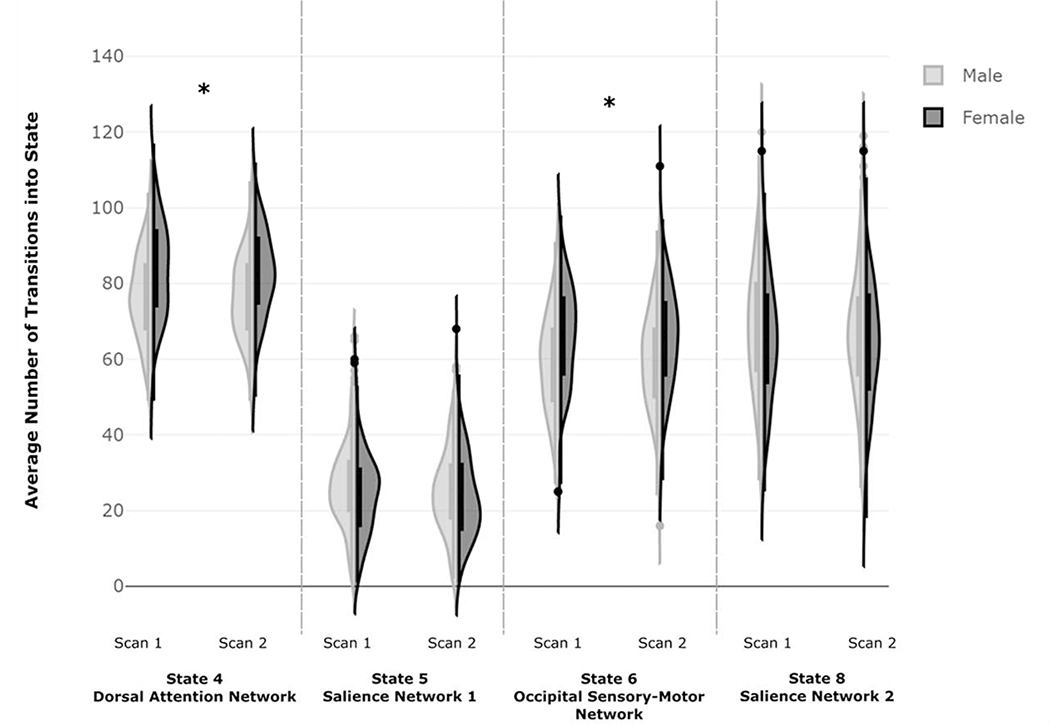
Significant main effects of sex were observed for frequency of transitions into the State 4 (F(1,360)= 40.60, p < .001, partial η2= .101) and State 6 (F(1,360)= 30.31, p < .001, partial η2= .078). There were no significant main effects of session or sex by session interactions. In post-hoc t-tests, females made more frequent transitions into State 4 (t(722)= −7.74, p < .001) and State 6 (t(722)= −6.58, p < .001) compared to males (Figure 4). Kolmogorov-Smirnov tests revealed significant sex differences in the distribution of frequency of transitions into TNS for both scan sessions for State 4 (Scan 1: Z=2.37, p<.001, Scan 2: Z=2.31, p<.001) and State 6 (Scan 1: Z=2.26, p<.001, Scan 2: Z=2.00, p<.001).
Figure 4: Sex-Related Differences in Frequency of Transitions into TNSs of Interest.
Figure 4. Frequency distributions of males and females for the frequency of transitions into each TNS of interest. Compared to males, females transitioned more frequently into the TNSs overlapping with DAN and occipital/sensory-motor states (State 4 and State 6). Independent samples Kolmogorov-Smirnov tests were used to examine differences in the frequency distributions between males and females (State 4: Scan 1: K-S Z=2.37, p<.001, Scan 2: K-S Z=2.31, p<.001; State 6: Scan 1: K-S Z=2.26, p<.001, Scan 2: K-S Z=2.00, p<.001).
Frequency of Specific TNS-to-TNS Transitions:
We next investigated sex differences in the state-to-state transition patterns. We examined the frequency of transitions into each of the four TNSs of interest from the remaining TNSs (e.g., transitions into State 4 from the other seven TNSs). Significant effects survived Bonferroni multiple comparison correction (i.e., .05/28, p ≤ .002; Supplemental Figure 1).
Main effects of sex were observed regarding frequency of transitions into State 4 from two TNSs spatially overlapping with the DMN; State 3 (F(1,360)= 15.99, p < .001, partial η2= .043) and State 7 (F(1,360)= 20.37, p < .001, partial η2= .054). There were no significant main effects of session or sex by session interactions. Post-hoc t-tests revealed that females made more frequent transitions into State 4 from State 3 (t(722)= −4.74, p < .001) and State 7 (t(722)= −5.13, p < .001) compared to males. Kolmogorov-Smirnov tests revealed significant sex differences in the frequency distributions for transitions into State 4 for both scan sessions from State 3 (Scan 1: Z=2.16, p<.001, Scan 2: Z=1.79, p=.003) and from State 7 (Scan 1: Z=2.05, p<.001, Scan 2: Z=1.47, p=.026).
Main effects of sex were observed regarding frequency of transitions into State 6 from a DMN state (State 1: (F(1,360)= 23.79, p < .001, partial η2= .062), the FPN state (State 2; F(1,360)= 12.49, p < .001, partial η2= .034), the DAN state (State 4; F(1,360)= 33.09, p < .001, partial η2= .084), and a SN state (State 8; F(1,360)= 14.90, p < .001, partial η2= .040). There were no significant main effects of session or sex by session interactions. Post-hoc t-tests revealed that females transitioned more frequently into State 6 from State 1 (t(722)= −5.54, p < .001), State 2 (t(722)= −3.79, p < .001), State 4 (t(722)= −6.36, p < .001), and State 8 (t(722)= −4.48, p < .001) compared to males. Kolmogorov-Smirnov tests revealed significant sex differences in the frequency distributions for transitions into State 6 for both scan sessions from State 1 (Scan 1: Z=2.94, p<.001, Scan 2: Z=1.63, p=.010), from State 2 (Scan 1: Z=1.37, p<.048, Scan 2: Z=1.95, p=.001) and from State 6 (Scan 1: Z=2.05, p<.001, Scan 2: Z=2.05, p<.001).
Third, a significant main effect of sex was observed regarding frequency of transitions into State 5 from a DMN state (State 3; F(1,360)= 11.65, p = .001, partial η2= .031). There were no significant main effects of session or sex by session interactions. In post-hoc t-tests, males made more frequent transitions into State 5 from State 3 (t(722)= 3.97, p < .001) compared to females. Kolmogorov-Smirnov tests revealed significant sex differences in the frequency distributions for transitions into State 5 for both scan sessions from State 3 (Scan 1: Z=1.73, p=.005, Scan 2: Z=1.47, p=.026).
Finally, main effects of sex were observed regarding frequency of transitions into State 8 from one of the DMN states (State 1; F(1,360)= 34.26, p < .001, partial η2= .087) and the occipital/sensory-motor state (State 6; F(1,360)= 14.90, p < .001, partial η2= .040). There were no significant main effects of session or sex by session interactions. Post-hoc t-tests revealed that males transitioned into State 8 from State 1 (t(722)= 7.05, p < .001) and State 6 (t(722)= 4.39, p < .001) more frequently compared to females. Kolmogorov-Smirnov tests revealed significant sex differences in the frequency distributions for transitions into State 8 for both scan sessions from State 1 (Scan 1: Z=2.52, p<.001, Scan 2: Z=2.10, p<.001) the first scan session only from State 6 (Scan 1: Z=1.73, p=.005, Scan 2: Z=1.26, p=.083).
DISCUSSION
The current study is among the first to investigate sex differences in functional network dynamics at rest. We found that males and females exhibited different dynamic properties in several large-scale functional networks. Specifically, in contrast with males, females showed increased total time spent in TNSs spatially overlapping with the DAN and occipital/sensory-motor brain regions. The difference can be explained by females making more frequent transitions into the DAN state and the occipital/sensory-motor state than males. Relative to females, males spent more total time in two TNSs overlapping with the SN. This difference can be explained by males persisting for longer periods upon entering both SN TNSs. Together, these results indicate that males and females display distinct dynamic patterns in established neurocognitive networks at rest, suggesting sex-related differences in intrinsic brain function.
In addition to sex differences in the total time and the frequency of transitions into DAN and SN states, we also identified sex differences in the transition patterns into these distinct attentional states. We found that State 3 (overlapping with the canonical DMN) preferentially transitions into the DAN and SN states in a sex-specific manner. Females were more likely to transition from DMN State 3 into the DAN TNS (State 4) whereas males were more likely to transition from DMN State 3 into a SN TNS (State 5). Of the three DMN-like states identified by CAP, State 3 most closely fits the prototypical DMN as this state did not extend beyond standard DMN regions including the dorsal and ventral medial prefrontal cortex, posterior cingulate, and precuneus (Janes et al., 2020; Smith et al., 2009). The current work therefore indicates a sex-specific transition pattern from the standard DMN into distinct attentional networks. Further, our findings are in line with previous research indicating that females have greater static rFC within the DAN (Filippi et al., 2013) and males have greater static rFC between regions of the SN (Wang et al., 2014), suggesting that males and females have distinct functional connectivity and display different dynamic properties in these networks.
In addition to differences in transitions from the prototypical DMN into attentional states, we also found sex-specific transition patterns for other states that spatially overlapped with complex-DMN states (Janes et al., 2020; Kaiser et al., 2019). Our prior work (Janes et al., 2020) considered States 1 and 7 to represent “complex-DMN” states, as they involved the coactivation of additional regions beyond the standard DMN. Specifically, State 1 co-activated with frontoinsular regions involved in goal-directed behavior, including the anterior insula, lateral prefrontal cortex, and frontal pole, while State 7 co-activated with visual and sensory-motor regions. We speculated that these DMN states represent different modes of interoception relating to goal-related processing (i.e., State 1) and sensorimotor integration (i.e., State 7). The concept that different DMN states correspond with specific neurocognitive functions is supported by our prior findings where the frontoinsular-DMN, but not the “classic” DMN, was associated with major depressive disorder and rumination (Kaiser et al., 2019). The current findings show that these complex DMN states have sex-specific transition patterns; females were more likely to transition from sensory-motor DMN State 7 to the DAN state compared to males, whereas males were more likely to transition from frontoinsular-DMN State 1 to a SN state.
Beyond sex differences, examining the transition patterns of complex-DMN states may improve our understanding of cognition, behavior, and psychopathology. In the current study, we found that the frontoinsular-DMN State 1 transitions to the SN state more so in males, which also plays a role in goal-related motivation and orientation. However, the sensorimotor DMN (State 7) transitions to the DAN state more so in females, which is thought to regulate sensory information to guide cognitive resources and attention. These findings suggest that the DMN may coordinate with other networks to facilitate specific cognitive functions. Thus, the current findings not only inform sex differences, but also provide insight into how different DMN states engage other networks in a neurobiologically meaningful way. Relatedly, Kaiser et al. found that specific patterns of DMN state engagement were related to maladaptive cognition in depression. Specifically, greater transition frequency between a frontoinsular-DMN state and a prototypical DMN state was associated with rumination and depression (Kaiser et al., 2019). Thus, examining the dynamic features of complex-DMN states may elucidate novel relationships between resting-state networks and inform the understanding of cognition and psychopathology.
Overall, our results suggest that males and females exhibit different functional network dynamic properties at rest pertaining to large-scale sensory orienting systems. Broadly, the DAN and SN are separable attention networks involved in deploying attentional resources. The DAN is involved in coordinating with frontoparietal systems to orient attention towards external stimuli of interest to successful task performance. The SN is involved in bottom-up, largely involuntary attention orienting towards salient external or internal stimuli (Menon and Uddin, 2010). Static resting-state FC within these networks has been previously linked to task performance. Specifically, greater FC within the DAN is related to better performance on sustained attention (Hampson et al., 2006), while higher static FC within the SN has been associated with greater cognitive flexibility (Chen et al., 2016; Tomiyama et al., 2019). How differences in temporal dynamic properties of these networks relate to cognitive performance is unclear. However, one possibility is that spending more time in a specific state at rest supports the ability to engage that network during a task. Such a conjecture, coupled with the current findings, may support findings suggesting that females have superior DAN-mediated attentional capacity relative to males (Conners et al., 2003; Dumais et al., 2018; Yuan et al., 2008).
Our findings contrast with a recent study by de Lacy and colleagues (2019). This previous study used a sliding-windows approach and identified that male participants as making more switches between various brain states whereas females were shown to exhibit greater persistence in a brain state corresponding to the DMN (de Lacy et al., 2019). Here, we found males and females displayed unique transition patterns, depending on the specific TNS examined. Specifically, we found that females transitioned more frequently into the DAN and occipital sensory/motor states whereas males exhibited greater persistence in two TNSs overlapping with the SN. Study-specific differences may be due to methodological variation as de Lacy et al. (2019) investigated functional network dynamics using spatial independent component analysis using a sliding windows approach, whereas we incorporated CAPs which evaluated large-scale brain networks. Sliding windows approaches examine average correlated activity of brain regions within a specified time window, whereas CAP analysis identifies TNSs by using both signal magnitude and spatial distribution to identify co-activated brain regions at each TR. To our knowledge, there has not been a direct comparison of these methodologically different approaches to identifying and examining dynamic features of functional networks. In addition, an early study using the CAP approach reported that males had a greater occurrence rate of a TNS overlapping with the sensorimotor cortex than females (Liu et al., 2013). In our study, we found that females spent more time in, and transitioned more frequently into, a TNS spatially overlapping with occipital/sensory-motor regions. Such variance is likely explained by the fact that Liu and colleagues (2013) defined a larger number of TNSs whereas our analysis defined a fewer TNSs that overlapped with large-scale networks, which were not parcellated into sub-networks. Thus, the spatial pattern of our occipital/sensory-motor TNS, which included large regions of the occipital cortex, differed substantially from the sensorimotor CAP identified in Liu et al. and may have contributed to the different findings.
Limitations
The current study has several limitations to consider when interpreting the results. First, while we investigated sex differences in specific transitions between various TNSs (e.g., DMN-to-DAN), we did not investigate more complex transition patterns regarding TNSs. Second, while our results help provide a normative baseline of sex differences in functional network dynamics, we did not investigate whether these differences related to neurocognitive performance in the current investigation. Recently, de Lacy et al. (2019), linked functional network dynamics to superior performance in response inhibition and mental rotation in males compared to females (de Lacy et al., 2019). Our prior findings also showed that resting-state temporal properties relate to psychopathology, cognition, and brain reactivity (Kaiser et al., 2019; Wang et al., 2020), supporting the need to determine whether there is also an influence of sex on these relationships.
Finally, given the high correspondence between biological sex and the social construct of gender in the general population, we are unable to disentangle whether our findings are driven by physiological or social components of sex and gender. Indeed, differences between the sexes are often subtle, often with higher variance within a sex than between (Hyde, 2007). We also found larger variance in dynamic measures within a sex than between the sexes (e.g., for total time in State 4, the mean difference between males and females was 22.0 seconds, whereas the within-sex standard deviation was 48.3 seconds for males and 46.5 seconds for females). Large within-sex variability may have contributed to the small to medium effect sizes for most dynamic properties in our study. To explore the point raised by Hyde (2007), we used violin plots to display the within- and between-sex variance of the dynamic features investigated in the current study. Despite the large within-sex variability, we did find significant between-sex differences in the distributions of functional network dynamics (i.e., two-sample K-S tests). Nevertheless, exploring subtle sex differences in neural function may still inform our understanding of domains in which larger sex differences emerge such as psychopathology (Naninck et al., 2011).
Conclusions
The current findings showed that males and females exhibited differing functional network dynamic properties at rest. Specifically, females exhibited increased total time spent in states which overlap with the DAN and occipital/sensory-motor network, whereas males exhibited increased total time spent in states overlapping with the SN. Furthermore, males and females made unique transitions from various TNSs to distinct attentional states. Results obtained provide novel insights into sex differences regarding the temporal dynamics of large-scale brain networks at rest and may help inform future research investigating sex differences in neurocognitive functioning and psychopathology.
Supplementary Material
Figure 1: Transient Network States (TNSs) Displaying Sex-Related Differences in Total Time Spent in State.
Figure 1. (A) Transient Network States (TNSs) with significant sex-related differences in total time spent in each state. Warm colors represent activation while cool colors represent deactivation (relative to within-state global average). State numbers are identified at the top of the figure, while the resting state network each state spatially overlaps with is listed below the brain image. (B) Raw unnormalized representation of each state grouped by prototypical resting state network divisions. The color bar represents the relative activation (warm colors, higher activation relative to raw average) and deactivation (cool colors, lower activation relative to raw average) for each region, within each transient network state. Figure adapted, with permission, from Janes et al., 2020, under a creative commons attribution license https://creativecommons.org/licenses/by/4.0/.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA) K02 DA042987 (Janes), the National Institute on Neurological Disorders and Stroke R01 NS097512 (Frederick), and the NARSAD Young Investigator Award 24879 (Kaiser). Dr. Maurer and Dr. Murray were supported by T32 DA015036 (PI: Scott Lukas, Ph.D.). Data were provided by the Human Connectome Project 1200 subject release, WU-Minn Consortium (National Institutes of Health U54 MH091667 (PIs: David Van Essen, Ph.D. & Kamil Ugurbil, Ph.D.) https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release.
Funding
This work was supported by the National Alliance for Research on Schizophrenia and Depression [NARSAD Young Investigator Award 24879]; National Institute of Neurological Disorders and Stroke [R01 NS097512]; National Institute on Drug Abuse [K02 DA042987,T32 DA015036]. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. (2011) A baseline for the multivariate comparison of resting-state networks, J Frontiers in systems neuroscience, 5, p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss AP, Di Pellegrino G & Tipper SP (2005) Sex differences in eye gaze and symbolic cueing of attention, J The Quarterly Journal of Experimental Psychology, 58(4), pp. 631–650. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. (2010) Toward discovery science of human brain function, Proceedings of the National Academy of Sciences, 107(10), pp. 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Zhang G, Zhang A, Hu W, Stephen JM, Wilson TW, et al. (2020) A GICA-TVGL framework to study sex differences in resting state fMRI dynamic connectivity, J Journal of Neuroscience Methods, 332, p. 108531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Cai W, Ryali S, Supekar K & Menon V (2016) Distinct global brain dynamics and spatiotemporal organization of the salience network, PLoS biology, 14(6), p. e1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT & Buckner RL (2012) The organization of the human striatum estimated by intrinsic functional connectivity, Journal of neurophysiology, 108(8), pp. 2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, Angold A & Klaric J (2003) Continuous performance test performance in a normative epidemiological sample, Journal of abnormal child psychology, 31(5), pp. 555–562. [DOI] [PubMed] [Google Scholar]
- de Lacy N, McCauley E, Kutz JN & Calhoun VD (2019) Sex-related differences in intrinsic brain dynamism and their neurocognitive correlates, Neuroimage, 202, p. 116116. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Chernyak S, Nickerson LD & Janes AC (2018) Sex differences in default mode and dorsal attention network engagement, PloS one, 13(6), p. e0199049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Valsasina P, Misci P, Falini A, Comi G & Rocca MA (2013) The organization of intrinsic brain activity differs between genders: A resting-state fMRI study in a large cohort of young healthy subjects, Human brain mapping, 34(6), pp. 1330–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, et al. (2016) The human connectome project’s neuroimaging approach, Nature neuroscience, 19(9), pp. 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. (2013) The minimal preprocessing pipelines for the Human Connectome Project, Neuroimage, 80, pp. 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, et al. (2014) ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging, Neuroimage, 95, pp. 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. (2012) Age group and sex differences in performance on a computerized neurocognitive battery in children age 8− 21, Neuropsychology, 26(2), p. 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC & Constable RT (2006) Brain connectivity related to working memory performance, Journal of Neuroscience, 26(51), pp. 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik H, Hausmann M, Osnes B, Westerhausen R & Specht K (2014) Resting states are resting traits–an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks, PloS one, 9(7), p. e103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS (2007) New directions in the study of gender similarities and differences, Current Directions in Psychological Science, 16(5), pp. 259–263. [Google Scholar]
- Janes AC, Peechatka AL, Frederick BB & Kaiser RH (2020) Dynamic functioning of transient resting-state coactivation networks in the Human Connectome Project, Human brain mapping, 41(2), pp. 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Kang MS, Lew Y, Van Der Feen J, Aguirre B, Clegg R, et al. (2019) Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: a preliminary resting-state co-activation pattern analysis, Neuropsychopharmacology, 44(9), pp. 1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. (2011) Behavioral interpretations of intrinsic connectivity networks, Journal of Cognitive Neuroscience, 23(12), pp. 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chang C & Duyn JH (2013) Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns, Frontiers in systems neuroscience, 7, p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang N, Chang C & Duyn JH (2018) Co-activation patterns in resting-state fMRI signals, Neuroimage, 180, pp. 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V & Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function, Brain Structure Function, 214(5–6), pp. 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck E, Lucassen P & Bakker J (2011) Sex differences in adolescent depression: do sex hormones determine vulnerability?, Journal of neuroendocrinology, 23(5), pp. 383–392. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L & Smith SM (2014) Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers, Neuroimage, 90, pp. 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. (2009) Correspondence of the brain’s functional architecture during activation and rest, Proceedings of the National Academy of Sciences, 106(31), pp. 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama H, Nakao T, Murayama K, Nemoto K, Ikari K, Yamada S, et al. (2019) Dysfunction between dorsal caudate and salience network associated with impaired cognitive flexibility in obsessive-compulsive disorder: A resting-state fMRI study, NeuroImage: Clinical, 24, p. 102004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, et al. (2013) Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project, Neuroimage, 80, pp. 80–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, et al. (2013) The WU-Minn human connectome project: an overview, Neuroimage, 80, pp. 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Erpelding N & Davis KD (2014) Sex differences in connectivity of the subgenual anterior cingulate cortex, PAIN, 155(4), pp. 755–763. [DOI] [PubMed] [Google Scholar]
- Wang KS, Kaiser RH, Peechatka AL, Frederick BB, Janes ACJBPCN & Neuroimaging (2020) Temporal dynamics of large-scale networks predict neural cue reactivity and cue-induced craving. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaesoubi M, Allen EA, Miller RL & Calhoun VD (2015) Dynamic coherence analysis of resting fMRI data to jointly capture state-based phase, frequency, and time-domain information, Neuroimage, 120, pp. 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity, Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, He Y, Qinglin Z, Chen A & Li H (2008) Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task, Psychophysiology, 45(6), pp. 986–993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.