Abstract
Colorectal carcinoma (CRC) is one of the most common forms of malignancy in the Western world. Accumulating evidence indicates that colon carcinogenesis is tightly controlled by tumour-associated immune cells and stromal cells, which can either stimulate or suppress CRC cell growth and survival, mainly via the production of cytokines. Interleukin-34 (IL-34), a cytokine known to regulate mainly monocyte/macrophage survival and function, is highly produced within the CRC microenvironment by several cell types, including cancer cells, tumour-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs), and regulates the pro-tumoural functions of such cells. In this article, we summarize the available data supporting the multiple effects of IL-34 in human CRC.
Subject terms: Preclinical research, Inflammatory bowel disease
Facts
IL-34 is a positive regulator of colorectal cancer (CRC) cell growth.
IL-34 acts on CRC cells as well as on other immune cells and non-immune cells (i.e. tumour-associated macrophages and cancer-associated fibroblasts) in the CRC microenvironment.
Association between high tissue expression of IL-34 and unfavourable prognosis and poor survival in CRC patients has been documented.
Open questions
Can IL-34 blockers enhance the properties of other anti-tumoural drugs?
Can circulating IL-34 serve as a prognostic biomarker in colorectal cancer?
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed form of cancer globally, accounting for approximately 10% of all annually diagnosed cancers worldwide. Incidence rates vary geographically with highest frequencies in developed countries [1, 2]. Despite significant advances in prevention and diagnosis, CRC is still one of the most deadly cancers worldwide, and this is because in about one fourth of the patients CRC diagnosis is made when cancer has metastasized and surgery, which remains the primary course of treatment in cases of early diagnosis, is no longer effective [2].
The CRC actually comprises a heterogeneous group of neoplasias, which are associated with different risk factors. More than two thirds of CRC arise sporadically and environmental and demographic factors (e.g. smoking habit, red meat consumption and obesity, age, positive family history of CRC) are supposed to play a key role in the pathogenesis of this form of CRC [3]. A small group of patients (5–7%) have a well-defined hereditary CRC syndrome, such as hereditary nonpolyposis colorectal cancer and familial adenomatous polyposis [4], while in 2–3% of cases CRC arises in patients with long-standing inflammatory bowel diseases (IBD) [5, 6].
CRC arises when colonic epithelial cells acquire a series of genetic or epigenetic mutations that increase cell growth and survival. Support to the abnormal behaviour of cancer cells is given by immune cells and stromal cells, which produce several pro-tumorigenic factors. On the other hand, cancer cells secrete several chemoattractants for immune cells [7]. Moreover, CRC cells synthesize a large array of cytokines, which enhance the pro-tumoural functions of immune cells and stromal cells, thus contributing to generate a microenvironment that favours disease progression [7]. One such a molecule is interleukin-34 (IL-34), a cytokine that was initially known as a factor regulating survival, proliferation and differentiation of monocytes, macrophages, and osteoclasts [8].
We here review the data about the expression and role of IL-34 in CRC.
Interleukin-34 expression and signalling
In 2008, Lin and colleagues showed that macrophage colony-stimulating factor receptor (M-CSF-1-R) could bind, in addition to the well-known ligand macrophage colony-stimulating factor (M-CSF-1), IL-34 [8]. The mature, full-length human IL-34 protein comprises 242 amino acids with a molecular mass of 39 KDa [8]. Non-covalently linked IL-34 homodimer recruits two M-CSF1-R. Despite IL-34 and M-CSF-1 share the same receptor, they bind different anchorage points of M-CSFR-1 and activate distinctive signalling pathways thus mediating unique biological functions [9–13]. The distinct biological functions of IL-34 and M-CSF-1 are dependent on the different hydrophobic/hydrophilic interactions of each ligand with M-CSF1-R. In particular, the M-CSF-1:M-CSF1-R complex depends on hydrophilic interactions, while the IL-34:M-CSF1-R interface contains a large number of hydrophobic regions, which stabilize the cytokine-receptor binding and favour a prolonged and strong transmembrane signalling [8–11].
Binding of IL-34 to M-CSF1-R activates different signalling pathways [(i.e. nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphoinositide 3-kinase (PI3K)/AKT, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK), Janus kinase (JAK), signal transducer and activator of transcription (STAT)3], mostly depending on the cell type analysed [12–16]. In human primary monocytes, IL-34-induced signals can also activate caspase-3/8 and promote autophagy through an AMP-activated protein kinase-UNC-51-like Kinase 1-dependent mechanism [17].
A second receptor of IL-34 is receptor-type protein-tyrosine phosphatase zeta (PTP-ζ). PTP-ζ is a cell surface chondroitin sulfate proteoglycan primarily expressed on neuronal progenitors and glial cells and to a lesser extent on B cells and kidney tubular cells [18]. After interaction with IL-34, PTP-ζ induces a series of intracellular events that inhibit motility, clonogenicity, and proliferation of glioblastoma cells via tyrosine phosphorylation of paxillin and focal adhesion kinase [18]. The third and last functional IL-34 receptor identified is Syndecan-1 (also known as CD138) that, once engaged by IL-34, stimulates myeloid cell migration [19] (Fig. 1).
Fig. 1. Schematic view of interleukin (IL)-34 receptors and IL-34-induced signalling pathways.
M-CSF-1-R macrophage colony-stimulating factor receptor, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, PI3K phosphoinositide 3-kinase, MAPK mitogen-activated protein kinase, ERK1/2 extracellular signal-regulated protein kinases 1 and 2, JNK c-Jun N-terminal kinase, JAK Janus kinase, STAT3 signal transducer and activator of transcription 3, ULK1 UNC-51-like Kinase 1, PTP-ζ protein-tyrosine phosphatase zeta, FAK focal adhesion kinase, TAMs tumour-associated macrophages.
IL-34 is produced by a wide range of cell types, including macrophages, endothelial cells, fibroblasts, adipocytes, neurons, cancer cells, and epithelial cells and is constitutively expressed in adult human tissues, such as heart, brain, testis, ovary, prostate, spleen, liver, thymus, small intestine, and colon [20–26]. IL-34 levels can change under pathological conditions [27]. Increased IL-34 RNA and protein expression was documented in various diseases, including autoimmune and inflammatory disorders, infections, metabolic diseases, neurological disorders, and fibrosis while a reduced expression of the cytokine was documented in Alzheimer’s disease, atopic dermatitis, hepatitis B viral infection, and periodontal diseases [23, 26, 28–38] (Table 1). Changes in IL-34 expression have also documented in various neoplastic diseases, where the cytokine is supposed to either limit or enhance the carcinogenetic processes [20, 39–51] (Table 2). IL-34 expression can be upregulated by several stimuli [23, 26, 34, 52]. Inflammatory cytokines, such as tumour necrosis factor (TNF)-α, IL-6 and IL-1β, activate NF-κB, JNK, and ERK1/2 signalling pathways and enhance IL-34 synthesis in a wide range of cell types including fibroblasts, epithelial cells, intestinal lamina propria mononuclear cells (LPMC), periodontal ligament cells, osteosarcoma cells, and adipocytes [15, 26, 34, 53]. Pathogen-associated molecular patterns, such as peptidoglycan, lipopolysaccharide and nucleic acids mimickers, poly I:C, and CpG, bind to Toll like receptors and induce IL-34 expression in macrophages, intestinal LPMC, and adipocytes. Iα,25(OH)2D3, a hormonally active form of vitamin D, increases IL-34 expression in neuroblastoma cells and normal gastric epithelial cells [26, 34, 52]. Infectious agents (i.e. hepatitis C virus) can increase IL-34 expression in infected cells [23]. On the contrary, transforming growth factor (TGF)-β1 and bone morphogenetic protein-2 can downregulate IL-34 expression in TNF-α-stimulated synovial fibroblasts and mesenchymal stem cells [54].
Table 1.
Changes of IL-34 expression in human diseases.
| Disease | IL-34 expression | References |
|---|---|---|
| Alzheimer’s disease | ↓ mRNA | [29] |
| Ankylosing spondylitis | ↑ protein | [31] |
| Atopic dermatitis | ↓ mRNA | [30] |
| Periodontal diseases | ↓ protein | [35] |
| Hepatitis B viral infection | ↓ mRNA and protein | [32, 33] |
| Hepatitis C viral infection | ↑ protein | [23] |
| Inflammatory bowel diseases | ↑ mRNA and protein | [26] |
| Influenza A viral infection | ↑ protein | [89] |
| Steatosis | ↑ protein | [23] |
| Obesity | ↑ protein | [34] |
| Psoriasis and psoriatic arthritis | ↑ protein | [90] |
| Rheumatoid arthritis | ↑ protein | [36, 37] |
| Sjogren’s syndrome | ↑ mRNA and protein | [38] |
Data are indicated as compared to controls (↑increased compared to controls; ↓decreased compared to controls).
Table 2.
Role of IL-34 in different cancer types.
| Cancer type | Function | References |
|---|---|---|
| Bone giant cell tumour | IL-34 promotes RANKL-induced osteoclastogenesis | [20] |
| Mammary cancer | IL-34 enhances M-CSF1-R–dependent macrophage infiltration and promotes tumour growth | [39] |
| Lung and brain metastases derived from breast cancer express M-CSF-1 and IL-34 | [39] | |
| Expression of IL-34 is associated with a favourable prognosis in luminal and HER2, but not basal, breast cancer patients | [40] | |
| Human ovarian cancer | High IL-34 expression correlates with worse survival in patients with ovarian cancer | [41] |
| Hepatocellular carcinoma | IL-34 promotes HCC cell growth and metastasis | [42] |
| High IL-34 serum levels associate with poor prognosis in patients with non-viral HCC | [43] | |
| Osteosarcoma | IL-34 enhances osteosarcoma growth and metastasization | [44] |
| Cholangiocarcinoma | IL-34 promotes stemness features in cancer stem cells | [45] |
| Lung cancer | IL-34 and M-CSF-1 expression correlates with advanced tumour stages and poor survival in lung cancer patients | [46] |
| IL-34 enhances the tumorigenic and immunosuppressive functions of TAMs and promotes the survival of chemoresistant cancer cells | [47] | |
| Multiple myeloma | IL-34 accelerates osteoclast formation increasing the severity of bone lesions | [48] |
| Sporadic vestibular schwannoma | IL-34 is expressed in sporadic vestibular schwannoma | [49] |
| Adult T-cell leukemia/lymphoma | IL-34 co-expression with M-CSF-1 may be related to the aggressiveness of this cancer type | [50] |
| Refractory melanoma | Enhanced expression of IL-34 in refractory melanoma tissues | [51] |
RANKL receptor activator of nuclear factor kappa-Β ligand, M-CSF1-R macrophage colony-stimulating factor-1 receptor, HCC hepatocellular carcinoma, TAMs tumour-associated macrophages, M-CSF-1 macrophage colony-stimulating factor-1.
IL-34 is detectable at low concentrations in serum/plasma, cerebral spinal fluid, synovial fluid, and saliva [35, 55, 56] and there exists a correlation between levels of IL-34 secreted into these extracellular biofluids and disease parameters in rheumatoid arthritis, heart failure, viral infections, sepsis, periodontal disease, non-alcoholic fatty liver disease, obesity, and type 2 diabetes mellitus [35, 55–57]. Ding and colleagues showed that, in Chinese patients with rheumatoid arthritis, low serum level of IL-34 (≤194.12 pg/ml) at baseline was a good predictor of response at 3-month following anti-TNF-α treatment [58].
Expression of IL-34 in colorectal cancer
As mentioned above, colon carcinogenesis is a tightly controlled phenomenon in which many cell types contribute to either stimulate or suppress CRC cell proliferation and death through the production of cytokines. We have recently shown that IL-34 is constitutively produced by human intestinal LPMC and its production is markedly increased in patients with IBD [26]. Moreover, high expression of IL-34 RNA and protein was found in tumour samples of patients with sporadic CRC as compared to non-tumour samples of the same CRC patients and normal controls [59]. In tumour areas, IL-34 was mostly produced by cancer cells and to lesser extent by mucosal mononuclear cells. Our observations are in line with those published by Kobayashi and co-workers, who documented high levels of IL-34 mRNA transcripts in various CRC cell lines including SW48, SW480, SW620, SW948, Caco2, CoLo205, and HT29 as compared to fetal human colon cells [60]. IL-34 mRNA was also overexpressed in primary CRC tissues taken from a cohort of 292 Japanese patients compared to the normal colorectal epithelium [60]. In the same study, the authors evaluated the impact of IL-34 expression in cancer tissues on patients’ survival. There was a strong positive correlation between high expression of IL-34 and unfavourable prognosis and poor survival. Similar findings were seen in a cohort of CRC patients registered at The Cancer Genome Atlas [60, 61]. In contrast, Wang and co-workers showed that lower expression of IL-34 gene was associated with poor survival in a cohort of 55 CRC patients [62].
Taken together these findings indicate that CRC cells produce IL-34 and suggest the possible prognostic value of the cytokine in this neoplasia.
IL-34 as a regulator of colon cancer cell growth
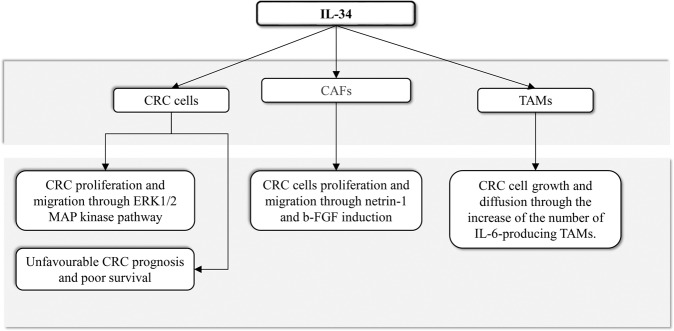
M-CSFR-1 expression was found to be more pronounced in the tumour areas as compared to the non-tumour areas of CRC patients and immunohistochemical studies showed that CRC cells were strongly positive for this receptor [59]. PTP-ζ was also constitutively expressed in the human colon with no apparent difference between tumour and non-tumour samples [59]. Altogether these data support the hypothesis that CRC cells are functionally able to respond to locally produced IL-34. Indeed, stimulation of CRC cells with recombinant IL-34 resulted in enhanced CRC cell proliferation and migration [59]. No change in cell growth was seen in normal intestinal epithelial cells following IL-34 stimulation, clearly indicating that the proliferative effect of IL-34 is restricted to the neoplastic cells. The mitogenic effect of IL-34 on CRC cells was preventable by a pharmacologic inhibitor of ERK1/2 MAP kinase pathway. Consistent with this, IL-34 knockdown in CRC cells with an antisense oligonucleotide (ASO) inhibited ERK1/2 activation, thereby resulting in reduced cell proliferation [59] (Fig. 2). In contrast, IL-34 did not affect the rate of apoptosis/necrosis of CRC cells either left untreated or treated with FAS Ligand or TNF. However, IL-34 knockdown enhanced the susceptibility of CRC cells to oxalipaltin-induced death, in line with the demonstration that IL-34, produced during chemotherapy, increases lung cancer cell survival [47].
Fig. 2. Schematic view of IL-34 as a regulator of CRC cells proliferation.
IL-34 knockdown in CRC cells with an antisense oligonucleotide inhibits ERK1/2 thereby reducing cell proliferation. CRC colorectal cancer, ASO antisense oligonucleotide.
IL-34 activated also p38 MAP kinases in CRC cells but pharmacological inhibition of this pathway did not influence the mitogenic effect of IL-34. It is, however, conceivable that IL-34-induced p38 MAP kinase activation can contribute to regulate other CRC cell functions. In this context, it is noteworthy that IL-34 activates p38 MAP kinase signal pathway in bone-marrow-derived macrophages thus resulting in enhanced expression of CD36 [63], a scavenger receptor for fatty acid uptake that modulates cell-to-extracellular matrix attachment and has pro-metastatic functions in several cancers [64].
IL-34 and tumour-associated macrophages (TAMs)
Tumour-associated macrophages (TAMs) are one of the most abundant immune cell populations in the tumour microenvironment [65]. TAMs can be differentiated into two main subsets with distinctive phenotypes and functions, referred to as M1 (or classic) and M2 (or alternative). M1 macrophages are pro-inflammatory, while M2 macrophages are anti-inflammatory, and this distinction corresponds, respectively, to the anti-tumour and pro-tumour functions of such cell types in the tumour microenvironment [66]. Indeed, M2 stimulate tumour cell proliferation, migration, invasion, and metastasis and high numbers of these cells often correlate with a bad prognosis and therapeutic resistance [67]. TAMs originate mainly from the blood compartment and chemotactic factors produced by tumour cells or by normal cells present in the cancer microenvironment enhance recruitment of monocytic precursors at the tumour site. Then, differentiation and activation of TAMs is favoured by further factors released by cancer cells, stromal cells and/or immune cells [67]. IL-34 promotes differentiation of monocytes into IL-10-expressing, immunoregulatory macrophages, which exhibit similarities to TAMs seen in ovarian cancer [68, 69]. Moreover, macrophages stimulated with IL-34 promote differentiation of CCR4 + CCR6 + CD161 + Th17 cells, a phenomenon occurring in many cancers [67]. Overall, these findings raise the possibility that IL-34 can contribute to the differentiation and activation of TAMs in CRC. This hypothesis is also supported by the demonstration that, in CRC, IL-34 expression correlates with the content of CD163, a marker of TAMs [60]. By flow-cytometry analysis of tumour-infiltrating cells (TICs) and LPMC isolated from normal adjacent mucosa of CRC patients, we have recently shown that CD68/HLA-DRII-expressing TICs and LPMC expressed M-CSF-1-R [70]. Both these cell types produced IL-34 even though IL-34 expression was more pronounced in TICs as compared to normal LPMC. IL-34 was produced by CD68/HLA-DRII-positive cells either expressing or not M-CSF-1-R, suggesting that IL-34 can regulate TAMs functions by acting in a paracrine and/or autocrine manner. Indeed, stimulation of both TICs and LPMCs with IL-34 enhanced the expression of CD163 and CD206, two markers of type-2-polarized macrophages [71]. Moreover, stimulation of both TICs and LPMCs with IL-34 enhanced IL-6 synthesis [70], a cytokine that activates proliferative and survival signals in CRC cells. Consistently, knockdown of IL-34 in TICs with a specific ASO decreased IL-6 production and the number of IL-6-producing TAMs [70]. These findings are in agreement with the demonstration that expression of IL-34 is associated with increased infiltration and function of type-2-polarized TAMs in other cancer types [72].
IL-34 and cancer-associated fibroblasts
The stromal compartment of CRC contains numerous activated fibroblasts, termed cancer-associated fibroblasts (CAFs), which promote CRC growth and progression, resistance to chemotherapy, and relapse of cancer through the synthesis of various molecules targeting the neoplastic cells [73, 74]. By real-time PCR, immunohistochemistry, and flow-cytometry we showed that IL-34 RNA transcripts and protein were significantly increased in CAFs compared to the fibroblasts isolated from the normal, adjacent colonic mucosa of the same patients with sporadic CRC [75]. IL-34 was also abundantly expressed in CAFs isolated from ulcerative colitis-associated CRC as compared to normal fibroblasts [75]. Moreover, CAFs and normal fibroblasts expressed both M-CSFR-1 and PTP-ζ [75]. Our data indicate also that, in the human gut, IL-34 promotes differentiation of CAFs with tumorigenic properties. Indeed, stimulation of normal colonic fibroblasts with IL-34 enhanced the expression of typical markers of CAFs, such α-SMA, Vimentin, and fibroblast activation protein (FAP) and induced cell proliferation, while inhibition of IL-34 expression in CAFs with a specific ASO decreased the expression of CAFs markers and proliferation [75]. CRC cells cultured in the presence of IL-34 AS-treated CAFs supernatants exhibited a significant reduction in proliferation as compared to CRC cells cultured in the presence of supernatants of CAFs treated with control AS. Moreover, a scratch test revealed that the supernatants of IL-34 AS-treated CAFs had reduced ability to stimulate CRC cell migration as compared to the supernatants of untreated CAFs [75]. These findings suggest that IL-34 stimulates CAFs to synthesize factors promoting CRC cell proliferation and migration (Fig. 3). Indeed, IL-34 regulates positively expression of netrin-1 and basic fibroblast growth factor (b-FGF) [75]. Netrin-1 is a multifunctional secreted glycoprotein involved in the control of several biological processes, such as angiogenesis, neuronal navigation, cell survival, and migration, and accumulating evidence suggests a role for this protein in many pathologies, including cardiovascular diseases, diabetes, and cancer [76–78]. Netrin-1 is highly expressed by CAFs in CRC tissue and regulates CRC cell stemness [79, 80]. Similarly, b-FGF stimulates the acquisition of metastatic capacity by CAFs and regulates positively the growth of CRC cells [81].
Fig. 3. Schematic view of interleukin-34 effects on specific cell types in CRC microenvironment.
CRC colorectal cancer, TAMs tumour-associated macrophages, CAFs cancer-associated fibroblasts, b-FGF basic fibroblast growth factor.
IL-34 in other types of cancer
As already stated above, an elevated expression of IL-34 was seen in other cancer types. For example, a higher expression of IL-34 was found in lung cancer compared to normal lung tissues and this was correlated with a poor patients’ prognosis [46]. Moreover, in lung adenocarcinoma, IL-34 protein levels increased upon treatment with doxorubicin and cisplatin, suggesting a possible regulation of IL-34 in response to therapy [47]. Elevated levels of IL-34 were also documented in cell lines of human osteosarcoma and were associated with disease progression [20]. Many other studies documented the contribution of IL-34 in other malignancies, such as haematological tumours, brain, breast, neck, biliary, and ovarian cancers (Table 2).
Conclusions
Studies in human CRC tissue and experimental models of cancer support the view that locally produced cytokines regulate critical steps of the colon carcinogenesis [82, 83]. The findings discussed in this article suggest that IL-34 regulates positively the function of CRC cells as well as other immune cells and non-immune cells in the CRC microenvironment, with the downstream effect of enhancing the growth and invasion of CRC cells [59, 70, 75], (Fig. 3). The possibility to use IL-34 inhibitors to block its pro-tumorigenic effects could thus open up a challenging opportunity for a new treatment option in CRC [59, 70, 75]. In this context, however, further work is needed to confirm the in vitro data generated using cells isolated from human CRC samples in pre-clinical models of CRC and ascertain whether IL-34 blockers can enhance the properties of other anti-tumoural drugs, including chemotherapeutics and biologics. It remains, also, unclear whether the marked expression of IL-34 in CRC tissue is paralleled by high circulating levels of the cytokine and whether IL-34 may serve as a prognostic biomarker in this neoplasia. Indeed, elevated levels of IL-34 have been associated with poor prognosis in primary lung cancer and such levels significantly correlate with the development of chemoresistance and progression in non-viral hepatocellular carcinoma and in basal breast cancer [40, 43, 46].
We also need to know much more about the factors/mechanisms underlying the high expression of IL-34 in CRC and to ascertain if there exists a cell-specific regulation of IL-34. Since M-CSF-1-R is expressed by additional cell types other than cancer cells, macrophages, and fibroblasts, it is likely that IL-34 can regulate the function of other immune and non-immune cells in the CRC microenvironment [84]. The fact that PTP-ζ is expressed in CRC tissue suggests that this receptor can mediate additional functions of the cytokine. Another possibility is that following IL-34 stimulation, signals driven by both PTP-ζ and M-CSF1-R are necessary to influence CRC cell behaviour. Support to this hypothesis comes from the observation that no change in CRC cell proliferation and survival was seen in cultures stimulated with M-CSF-1, the other ligand of M-CSF1R.
Overall, the data here described underline the role of IL-34 in positively regulate the function of CRC cells as well as other cell types (i.e. TAMs and CAFs), which ultimately sustain CRC cell behaviour. It is relevant to point out that the pro-tumorigenic role of IL-34 could also rely on additional functions of the cytokine. For instance, IL-34 induces immune cells to produce TNF-α [26], a cytokine exerting proliferative effects on CRC cells [85]. IL-34 has also been involved in the suppressive function of regulatory T cells, the activity of which associates with the progression of CRC cancer cells [86]. Finally, IL-34 stimulates macrophages to switch non-Th17 committed memory CD4(+) T cells into Th17 cells [87], which are known to enhance CRC cell growth and migration [88].
Supplementary information
Author contributions
EF, SS, and ET searched literature for relevant articles; EF, IM, and GM wrote the manuscript.
Funding information
The study was supported by the Associazione Italiana per la Ricerca sul Cancro (IG2016-19223).
Competing interests
GM has served as an advisory board member for ABBVIE. The remaining authors declare no competing interests.
Footnotes
Edited by Dr Ivano Amelio
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eleonora Franzè, Irene Marafini.
Supplementary information
The online version contains supplementary material available at 10.1038/s41420-021-00636-4.
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology. 2020;158:322–40. doi: 10.1053/j.gastro.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62; quiz 263. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071–84. doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 9.Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. J Bone Miner Metab. 2013;31:486–95. doi: 10.1007/s00774-013-0476-3. [DOI] [PubMed] [Google Scholar]
- 10.Felix J, Elegheert J, Gutsche I, Shkumatov AV, Wen Y, Bracke N, et al. Human IL-34 and CSF-1 establish structurally similar extracellular assemblies with their common hematopoietic receptor. Structure. 2013;21:528–39. doi: 10.1016/j.str.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Lin WY, Chen Y, Stawicki S, Mukhyala K, Wu Y, et al. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure. 2012;20:676–87. doi: 10.1016/j.str.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Leo C, Chen X, Wong BR, Williams LT, Lin H, et al. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys Acta. 2012;1824:938–45. doi: 10.1016/j.bbapap.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, et al. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–27. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- 14.Eda H, Shimada H, Beidler DR, Monahan JB. Proinflammatory cytokines, IL-1beta and TNF-alpha, induce expression of interleukin-34 mRNA via JNK- and p44/42 MAPK-NF-kappaB pathway but not p38 pathway in osteoblasts. Rheumatol Int. 2011;31:1525–30. doi: 10.1007/s00296-010-1688-7. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Yang D, Qiu L, Okamura H, Guo J, Haneji T. Tumor necrosis factor-alpha induces interleukin-34 expression through nuclear factorkappaB activation in MC3T3-E1 osteoblastic cells. Mol Med Rep. 2014;10:1371–6. doi: 10.3892/mmr.2014.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Sun X, Zhang J, Yang Y, Chen D, Cao J. IL-34 regulates IL-6 and IL-8 production in human lung fibroblasts via MAPK, PI3K-Akt, JAK and NF-kappaB signaling pathways. Int Immunopharmacol. 2018;61:119–25. doi: 10.1016/j.intimp.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Boulakirba S, Pfeifer A, Mhaidly R, Obba S, Goulard M, Schmitt T, et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci Rep. 2018;8:256. doi: 10.1038/s41598-017-18433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandi S, Cioce M, Yeung YG, Nieves E, Tesfa L, Lin H, et al. Receptor-type protein-tyrosine phosphatase zeta is a functional receptor for interleukin-34. J Biol Chem. 2013;288:21972–86. doi: 10.1074/jbc.M112.442731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segaliny AI, Brion R, Mortier E, Maillasson M, Cherel M, Jacques Y, et al. Syndecan-1 regulates the biological activities of interleukin-34. Biochim Biophys Acta. 2015;1853:1010–21. doi: 10.1016/j.bbamcr.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Baud’huin M, Renault R, Charrier C, Riet A, Moreau A, Brion R, et al. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J Pathol. 2010;221:77–86. doi: 10.1002/path.2684. [DOI] [PubMed] [Google Scholar]
- 21.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–60. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–60. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, et al. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879–90. doi: 10.1002/hep.27328. [DOI] [PubMed] [Google Scholar]
- 24.Franze E, Dinallo V, Laudisi F, Di Grazia A, Di Fusco D, Colantoni A, et al. Interleukin-34 stimulates gut fibroblasts to produce collagen synthesis. J Crohns Colitis. 2020;14:1436–45. [DOI] [PubMed]
- 25.Franze E, Marafini I, De Simone V, Monteleone I, Caprioli F, Colantoni A, et al. Interleukin-34 induces Cc-chemokine ligand 20 in gut epithelial cells. J Crohn Colitis. 2016;10:87–94. doi: 10.1093/ecco-jcc/jjv181. [DOI] [PubMed] [Google Scholar]
- 26.Franze E, Monteleone I, Cupi ML, Mancia P, Caprioli F, Marafini I, et al. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci. 2015;129:271–80. doi: 10.1042/CS20150132. [DOI] [PubMed] [Google Scholar]
- 27.Baghdadi M, Endo H, Tanaka Y, Wada H, Seino KI. Interleukin 34, from pathogenesis to clinical applications. Cytokine. 2017;99:139–47. doi: 10.1016/j.cyto.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, et al. Interleukin-34, a comprehensive review. J Leukoc Biol. 2018;104:931–51. doi: 10.1002/JLB.MR1117-457R. [DOI] [PubMed] [Google Scholar]
- 29.Walker DG, Tang TM, Lue LF. Studies on colony stimulating factor receptor-1 and ligands colony stimulating factor-1 and interleukin-34 in Alzheimer’s disease brains and human microglia. Front Aging Neurosci. 2017;9:244. doi: 10.3389/fnagi.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esaki H, Ewald DA, Ungar B, Rozenblit M, Zheng X, Xu H, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol. 2015;135:153–63. doi: 10.1016/j.jaci.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang SH, Choi BY, Choi J, Yoo JJ, Ha YJ, Cho HJ, et al. Baseline serum interleukin-34 levels independently predict radiographic progression in patients with rheumatoid arthritis. Rheumatol Int. 2015;35:71–79. doi: 10.1007/s00296-014-3056-5. [DOI] [PubMed] [Google Scholar]
- 32.Cheng ST, Tang H, Ren JH, Chen X, Huang AL, Chen J. Interleukin-34 inhibits hepatitis B virus replication in vitro and in vivo. PLoS ONE. 2017;12:e0179605. doi: 10.1371/journal.pone.0179605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YQ, Cao WJ, Gao YF, Ye J, Zou GZ. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2018;24:1312–20. doi: 10.3748/wjg.v24.i12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang EJ, Lee SK, Song YS, Jang YJ, Park HS, Hong JP, et al. IL-34 is associated with obesity, chronic inflammation, and insulin resistance. J Clin Endocrinol Metab. 2014;99:E1263–1271. doi: 10.1210/jc.2013-4409. [DOI] [PubMed] [Google Scholar]
- 35.Martinez GL, Majster M, Bjurshammar N, Johannsen A, Figueredo CM, Bostrom EA. Salivary colony stimulating factor-1 and interleukin-34 in periodontal disease. J Periodontol. 2017;88:e140–e149. doi: 10.1902/jop.2017.170081. [DOI] [PubMed] [Google Scholar]
- 36.Hwang SJ, Choi B, Kang SS, Chang JH, Kim YG, Chung YH, et al. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012;14:R14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon SJ, Hong YS, Ju JH, Kwok SK, Park SH, Min JK. Increased levels of interleukin 34 in serum and synovial fluid are associated with rheumatoid factor and anticyclic citrullinated peptide antibody titers in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1842–9. doi: 10.3899/jrheum.130356. [DOI] [PubMed] [Google Scholar]
- 38.Ciccia F, Alessandro R, Rodolico V, Guggino G, Raimondo S, Guarnotta C, et al. IL-34 is overexpressed in the inflamed salivary glands of patients with Sjogren’s syndrome and is associated with the local expansion of pro-inflammatory CD14(bright)CD16+ monocytes. Rheumatology. 2013;52:1009–17. doi: 10.1093/rheumatology/kes435. [DOI] [PubMed] [Google Scholar]
- 39.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zins K, Heller G, Mayerhofer M, Schreiber M, Abraham D. Differential prognostic impact of interleukin-34 mRNA expression and infiltrating immune cell composition in intrinsic breast cancer subtypes. Oncotarget. 2018;9:23126–48. doi: 10.18632/oncotarget.25226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endo H, Hama N, Baghdadi M, Ishikawa K, Otsuka R, Wada H, et al. Interleukin-34 expression in ovarian cancer: a possible correlation with disease progression. Int Immunol. 2020;32:175–86. doi: 10.1093/intimm/dxz074. [DOI] [PubMed] [Google Scholar]
- 42.Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, et al. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. 2016;63:1560–75. doi: 10.1002/hep.28445. [DOI] [PubMed] [Google Scholar]
- 43.Noda Y, Kawaguchi T, Korenaga M, Yoshio S, Komukai S, Nakano M, et al. High serum interleukin-34 level is a predictor of poor prognosis in patients with non-viral hepatocellular carcinoma. Hepatol Res. 2019;49:1046–53. doi: 10.1111/hepr.13350. [DOI] [PubMed] [Google Scholar]
- 44.Segaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. 2015;137:73–85. doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- 45.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–15. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baghdadi M, Endo H, Takano A, Ishikawa K, Kameda Y, Wada H, et al. High co-expression of IL-34 and M-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep. 2018;8:418. doi: 10.1038/s41598-017-18796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baghdadi M, Wada H, Nakanishi S, Abe H, Han N, Putra WE, et al. Chemotherapy-induced IL34 enhances immunosuppression by tumor-associated macrophages and mediates survival of chemoresistant lung cancer cells. Cancer Res. 2016;76:6030–42. doi: 10.1158/0008-5472.CAN-16-1170. [DOI] [PubMed] [Google Scholar]
- 48.Baghdadi M, Ishikawa K, Nakanishi S, Murata T, Umeyama Y, Kobayashi T, et al. A role for IL-34 in osteolytic disease of multiple myeloma. Blood Adv. 2019;3:541–51. doi: 10.1182/bloodadvances.2018020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Vries WM, Briaire-de Bruijn IH, van Benthem PPG, van der Mey AGL, Hogendoorn PCW. M-CSF and IL-34 expression as indicators for growth in sporadic vestibular schwannoma. Virchows Arch. 2019;474:375–81. [DOI] [PMC free article] [PubMed]
- 50.Komohara Y, Noyori O, Saito Y, Takeya H, Baghdadi M, Kitagawa F, et al. Potential anti-lymphoma effect of M-CSFR inhibitor in adult T-cell leukemia/lymphoma. J Clin Exp Hematop. 2018;58:152–60. doi: 10.3960/jslrt.18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han N, Baghdadi M, Ishikawa K, Endo H, Kobayashi T, Wada H, et al. Enhanced IL-34 expression in Nivolumab-resistant metastatic melanoma. Inflamm Regen. 2018;38:3. doi: 10.1186/s41232-018-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Li M, Dong Y, Zhang X, Liu X, Chen Z, et al. 1alpha,25-Dihydroxyvitamin D3 up-regulates IL-34 expression in SH-SY5Y neural cells. Innate Immun. 2017;23:584–91. doi: 10.1177/1753425917725391. [DOI] [PubMed] [Google Scholar]
- 53.Bostrom EA, Lundberg P. The newly discovered cytokine IL-34 is expressed in gingival fibroblasts, shows enhanced expression by pro-inflammatory cytokines, and stimulates osteoclast differentiation. PLoS ONE. 2013;8:e81665. doi: 10.1371/journal.pone.0081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chemel M, Brion R, Segaliny AI, Lamora A, Charrier C, Brulin B, et al. Bone morphogenetic protein 2 and transforming growth factor beta1 inhibit the expression of the proinflammatory cytokine IL-34 in rheumatoid arthritis synovial fibroblasts. Am J Pathol. 2017;187:156–62. doi: 10.1016/j.ajpath.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–13. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian Y, Shen H, Xia L, Lu J. Elevated serum and synovial fluid levels of interleukin-34 in rheumatoid arthritis: possible association with disease progression via interleukin-17 production. J Interferon Cytokine Res. 2013;33:398–401. doi: 10.1089/jir.2012.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge Y, Huang M, Yao YM. Immunomodulation of interleukin-34 and its potential significance as a disease biomarker and therapeutic target. Int J Biol Sci. 2019;15:1835–45. doi: 10.7150/ijbs.35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding R, Li P, Song D, Zhang X, Bi L. Predictors of response to TNF-alpha antagonist therapy in Chinese rheumatoid arthritis. Clin Rheumatol. 2015;34:1203–10. doi: 10.1007/s10067-015-2973-3. [DOI] [PubMed] [Google Scholar]
- 59.Franze E, Dinallo V, Rizzo A, Di Giovangiulio M, Bevivino G, Stolfi C, et al. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget. 2018;9:3432–45. doi: 10.18632/oncotarget.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi T, Baghdadi M, Han N, Murata T, Hama N, Otsuka R, et al. Prognostic value of IL-34 in colorectal cancer patients. Immunol Med. 2019;42:1–7. [DOI] [PubMed]
- 61.Shi X, Kaller M, Rokavec M, Kirchner T, Horst D, Hermeking H. Characterization of a p53/miR-34a/CSF1R/STAT3 feedback loop in colorectal cancer. Cell Mol Gastroenterol Hepatol. 2020;10:391–418. doi: 10.1016/j.jcmgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Xu W, Tan M, Xiao Y, Yang H, Xia TS. Integrative genomic analyses of a novel cytokine, interleukin-34 and its potential role in cancer prediction. Int J Mol Med. 2015;35:92–102. doi: 10.3892/ijmm.2014.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Q, Fan J, Bai J, Peng L, Zhang T, Deng L, et al. IL-34 promotes foam cell formation by enhancing CD36 expression through p38 MAPK pathway. Sci Rep. 2018;8:17347. doi: 10.1038/s41598-018-35485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enciu AM, Radu E, Popescu ID, Hinescu ME, Ceafalan LC. Targeting CD36 as biomarker for metastasis prognostic: how far from translation into clinical practice? Biomed Res Int. 2018;2018:7801202. doi: 10.1155/2018/7801202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 66.Lankadasari MB, Mukhopadhyay P, Mohammed S, Harikumar KB. TAMing pancreatic cancer: combat with a double edged sword. Mol Cancer. 2019;18:48. doi: 10.1186/s12943-019-0966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNgamma. PLoS ONE. 2013;8:e56045. doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Q, Wu X, Wu Y, Wang X. Interaction between Treg cells and tumor-associated macrophages in the tumor microenvironment of epithelial ovarian cancer. Oncol Rep. 2016;36:3472–8. doi: 10.3892/or.2016.5136. [DOI] [PubMed] [Google Scholar]
- 70.Franze E, Laudisi F, Di Grazia A, Maronek M, Bellato V, Sica G, et al. Macrophages produce and functionally respond to interleukin-34 in colon cancer. Cell Death Discov. 2020;6:117. doi: 10.1038/s41420-020-00350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franze E, Stolfi C, Troncone E, Scarozza P, Monteleone G. Role of interleukin-34 in cancer. Cancers. 2020;12:252. [DOI] [PMC free article] [PubMed]
- 73.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–9. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 74.Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47:312–9. doi: 10.1038/ng.3224. [DOI] [PubMed] [Google Scholar]
- 75.Franze E, Di Grazia A, Sica GS, Biancone L, Laudisi F, Monteleone G. Interleukin-34 enhances the tumor promoting function of colorectal cancer-associated fibroblasts. Cancers. 2020;12:3537. [DOI] [PMC free article] [PubMed]
- 76.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–87. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 77.Layne K, Ferro A, Passacquale G. Netrin-1 as a novel therapeutic target in cardiovascular disease: to activate or inhibit? Cardiovasc Res. 2015;107:410–9. doi: 10.1093/cvr/cvv201. [DOI] [PubMed] [Google Scholar]
- 78.Yimer EM, Zewdie KA, Hishe HZ. Netrin as a novel biomarker and its therapeutic implications in diabetes mellitus and diabetes-associated complications. J Diabetes Res. 2018;2018:8250521. doi: 10.1155/2018/8250521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sung PJ, Rama N, Imbach J, Fiore S, Ducarouge B, Neves D, et al. Cancer-associated fibroblasts produce Netrin-1 to control cancer cell plasticity. Cancer Res. 2019;79:3651–61. doi: 10.1158/0008-5472.CAN-18-2952. [DOI] [PubMed] [Google Scholar]
- 80.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–4. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 81.New BA, Yeoman LC. Identification of basic fibroblast growth factor sensitivity and receptor and ligand expression in human colon tumor cell lines. J Cell Physiol. 1992;150:320–6. doi: 10.1002/jcp.1041500215. [DOI] [PubMed] [Google Scholar]
- 82.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bao S, Hu R, Hambly BD. IL-34, IL-36 and IL-38 in colorectal cancer-key immunoregulators of carcinogenesis. Biophys Rev. 2020;12:925–30. doi: 10.1007/s12551-020-00726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al Obeed OA, Alkhayal KA, Al Sheikh A, Zubaidi AM, Vaali-Mohammed MA, Boushey R, et al. Increased expression of tumor necrosis factor-alpha is associated with advanced colorectal cancer stages. World J. Gastroenterol. 2014;20:18390–6. doi: 10.3748/wjg.v20.i48.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74:4258–69. doi: 10.1158/0008-5472.CAN-13-3065. [DOI] [PubMed] [Google Scholar]
- 87.Foucher ED, Blanchard S, Preisser L, Descamps P, Ifrah N, Delneste Y, et al. IL-34- and M-CSF-induced macrophages switch memory T cells into Th17 cells via membrane IL-1alpha. Eur J Immunol. 2015;45:1092–102. doi: 10.1002/eji.201444606. [DOI] [PubMed] [Google Scholar]
- 88.Razi S, Baradaran Noveiry B, Keshavarz-Fathi M, Rezaei N. IL-17 and colorectal cancer: from carcinogenesis to treatment. Cytokine. 2019;116:7–12. doi: 10.1016/j.cyto.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 89.Yu G, Bing Y, Zhu S, Li W, Xia L, Li Y, et al. Activation of the interleukin-34 inflammatory pathway in response to influenza A virus infection. Am J Med Sci. 2015;349:145–50. doi: 10.1097/MAJ.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 90.Li J, Liu L, Rui W, Li X, Xuan D, Zheng S, et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology. 2017;233:37–46. doi: 10.1159/000471798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.