Abstract
Chronic rheumatological manifestations similar to those of rheumatoid arthritis (RA) are described after chikungunya virus infection. We aimed to compare the relevance of joint counts and symptoms to clinical outcomes in RA and chronic chikungunya disease. Forty patients with chronic chikungunya arthralgia and 40 patients with RA were enrolled in a cross-sectional study. The association of tenderness and swelling, clinically assessed in 28 joints, and patient evaluations of pain and musculoskeletal stiffness with modified Health Assessment Questionnaire (HAQ) and quality of life (QoL) assessments were investigated. Tender and swollen joint counts, pain and stiffness scores were all associated with the HAQ disability index in RA (all r > 0.55, p ≤ 0.0002), but only stiffness was significantly associated with disability in chikungunya (r = 0.38, p = 0.02). Joint counts, pain and stiffness were also associated with most QoL domains in RA patients. In contrast, in chikungunya disease, tender joint counts were associated only with one QoL domain and swollen joints for none, while pain and stiffness were associated with several domains. Our results confirm the relevance of joint counts in RA, but suggest that in chronic chikungunya disease, joint counts have more limited value. Stiffness and pain score may be more important to quantify chikungunya arthritis impact.
Subject terms: Viral infection, Rheumatoid arthritis, Outcomes research
Introduction
Infection with chikungunya virus (CHIKV) is typically announced by a fever accompanied by a rapid onset of painful and swollen joints1. The fever and joint involvement often resolve after 7–10 days, but in an estimated 40% (95% CI 31–49) of patients painful and stiff joints persist for months or years2,3. The chronic joint pain and inflammation occurring after infection with chikungunya virus is less well characterized than rheumatoid arthritis (RA), but shares many of the same features, such as pain and swelling in multiple joints accompanied by stiffness with the smaller joints of the upper and lower limbs most commonly affected4–6. Instruments used for evaluating clinical status in RA may therefore also be useful in CHIKV arthritis.
The rheumatologist has many different instruments at his disposal to evaluate the severity of disease in a patient with RA, instruments which have been extensively tested and proven to be of value. Tender and swollen joint counts are well established in RA as standard measures of joint involvement7, and recent work has addressed the comprehensive assessment of joint stiffness in RA, a previously understudied area8,9. These same measures and instruments have also been employed in CHIKV arthritis studies, but their usefulness in chikungunya disease is less clear10.
As chikungunya outbreaks have become more widespread, threatening not only tropical, but also semi-tropical and some temperate regions of the world, there is an increasing need to identify effective therapeutics for chronic chikungunya disease. It is important, therefore, to know whether the instruments used in RA are equally appropriate for the assessment of post-chikungunya arthritis.
Following an outbreak of CHIKV infections from the CHIKV ECSA (East, Central and South African) genotype in Roraima State, Brazil, our group has reported a cross-sectional study comparing the characteristics of patients with post-chikungunya arthritis with three different local control groups: patients without chronic arthritis following chikungunya infection, chikungunya negative patients with RA and healthy subjects11. We reported arthritis severity 2 years after CHIKV infection similar to that in the patients with RA. Chronic chikungunya disease, like RA, results in measurable disability and significantly impaired quality of life12,13. Counts of involved joints have been shown to correlate well with these patient-reported outcome measures in RA14–17, but based on our own previous work in chikungunya disease10, we hypothesized that the relationship of these disease measures to patient-reported outcomes may differ in the two diseases. In the present analysis, we further explored the relationship of disease and symptom severity measures with patient-reported outcomes in RA and CHIKV arthritis, with the objective of determining whether joint counts and symptom measures are associated with disability and health-related quality of life (HRQoL) in a similar manner in RA and in patients with post-chikungunya arthritis.
Patients and methods
Study design
This was a cross-sectional analysis investigating the relationship of arthritis severity measures to patient outcomes in two groups of patients with arthritis of different origins.
Setting and participants
The study was conducted in Boa Vista, capital city of Roraima State in northern Brazil. This area was the scene of a large outbreak of chikungunya virus infections associated with the ECSA genotype of the virus, whose emergence in Roraima is estimated to date from mid-201618. Between June and August 2019, two to three years after the outbreak, 40 patients with chronic chikungunya arthritis and 40 CHIKV-negative patients with RA were enrolled into the study. Chikungunya virus (CHIKV) cases were identified by the sudden onset of fever and severe arthralgia or acute onset arthritis in a person residing in or travelling to an endemic or epidemic area within 14 days prior to symptom onset. CHIKV infection was confirmed at the Roraima Central Laboratory/Laboratório Central do Estado de Roraima (LACEN) using RT-qPCR. The diagnoses of arthritis in chikungunya patients and rheumatoid arthritis were made by rheumatologists at Coronel Mota Hospital. Rheumatoid arthritis was diagnosed using the American College of Rheumatology (ACR) criteria19.
The study protocol was approved by the Committee for Ethics and Research at the Federal University of Roraima (Protocol number 3.406.0703). All research procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 1983. All patients gave informed consent prior to their inclusion in the study.
Clinical evaluation and outcome measures
Patients were assessed by a rheumatologist at a face-to-face interview. Twenty-eight joints were assessed for tenderness and swelling. The specified 28-joint counts of tender and swollen joints used have been validated with respect to more comprehensive joint counts in RA and are now a widely used standard in this indication20–22. Symptoms were evaluated by the patient with a pain intensity visual analogue scale (0–100) and a 21-item musculoskeletal stiffness questionnaire (overall score expressed as a percentage) which incorporated several aspects of stiffness severity as well as the physical and psychological impacts of stiffness9,23. Functional disability was assessed with the modified Stanford Health Assessment Questionnaire (HAQ). This questionnaire is widely used in rheumatologic diseases and asks the degree of difficulty experienced by subjects in a number of daily activities, with responses consolidated to generate an overall score between zero for no disability and three for maximum disability24. The Euroqol EQ5D-5L questionnaire, a generic health-related quality of life instrument used in many areas of health outcomes research, was employed to measure the patients’ quality of life25. The EQ5D-5L assesses quality of life in five domains: mobility, self-care, usual activities, pain-discomfort and anxiety-depression.
Statistical analysis
Symptoms, scores and joint counts in the two groups were compared using the Mann–Whitney non-parametric test. Associations of joint pain, joint stiffness, tender joint count and swollen joint count with the HAQ disability score and the five domains of the EQ5D-5L quality of life instrument were analysed using Spearman’s rank correlation test for each disease individually. Regression analysis was performed to determine whether there was a statistically significant difference between the associations in RA and in CHIKV arthritis.
Ethics approval
The study protocol was approved by the Committee for Ethics and Research at the Federal University of Roraima (Protocol number 3.406.0703). All research procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 1983. All persons gave informed consent prior to their inclusion in the study. No animals were used in this study.
Consent to participate
All research procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 1983. All persons gave informed consent prior to their inclusion in the study.
Consent for publication
Not applicable.
Results
Demographic and clinical characteristics of participants
In both the RA and CHIKV arthritis groups the patients were predominantly female (93% and 90%, respectively), with an education level at least of secondary school (in 65% and 58%, respectively) and median ages of 51.5 years (interquartile range 40.5–62) and 49.5 years (interquartile range 42–55), respectively. Patients were of 85% and 88% Pardo (mixed) ethnicity, the majority ethnic group in Roraima State, in the RA group and CHIKV arthritis group, respectively. The chikungunya patients were assessed a median of 27 months (interquartile range 22.5–29) after infection. None of this group had reported arthritis prior to chikungunya infection.
The RA and CHIKV arthritis patients had similar symptom scores for pain intensity and stiffness severity, and similar levels of disability as measured by the HAQ (Table 1). However, compared to the RA patients, the CHIKV arthritis patients had significantly fewer swollen joints and a slightly smaller number of tender joints on average (Table 1). The use of medications for arthritis at the time of clinical evaluation also differed significantly with more patients in the RA group taking steroids (60%) or methotrexate (40%) compared to the CHIKV arthritis group (15% and 5% respectively).
Table 1.
Disease characteristics of patients with rheumatoid and chikungunya arthritis.
| Severity or symptom measure | Rheumatoid arthritis (n = 40) | CHIKV arthritis (n = 40) | p value |
|---|---|---|---|
| Tender joint count | 14 (7–24) | 12 (4–14) | 0.03 |
| Swollen joint count | 12 (3–24) | 3 (0–12) | 0.002 |
| Joint pain (VAS) | 75 (50–90) | 73 (50–82) | 0.97 |
| Stiffness severity (%) | 55 (32–64) | 54 (30–61) | 0.95 |
| HAQ | 0.56 (0.25–1.25) | 0.50 (0.25–0.75) | 0.22 |
Results expressed as median (interquartile range).
Association of joint counts and symptoms with clinical outcomes
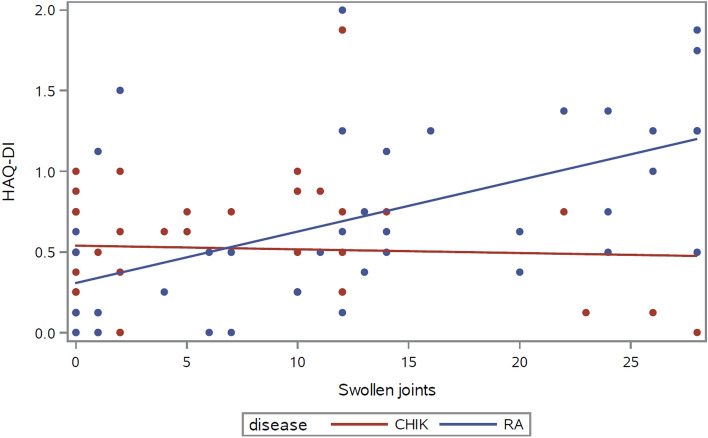
Tender and swollen joint counts, pain and stiffness were all correlated with the HAQ disability index in RA, but only stiffness was significantly associated with disability in chikungunya patients (Table 2). Regression analysis revealed a statistically significant interaction term between the disease diagnosis and swollen joint count, confirming that the effect of swollen joint count on disability significantly changes between RA and CHIKV arthritis (p = 0.003 for the interaction of diagnosis with swollen joint count) (Fig. 1). For tender joint counts, pain intensity and stiffness, the interaction term with disease showed a similar trend without reaching statistical significance (0.05 < p < 0.10).
Table 2.
Association of disease severity with HAQ disability index in rheumatoid and chikungunya arthritis.
| Severity or symptom measure | Rheumatoid arthritis (n = 40) | Chikungunya arthritis (n = 40) |
|---|---|---|
| r (p) | r (p) | |
| Tender joint count | 0.56 (0.0002) | 0.24 (0.14) |
| Swollen joint count | 0.60 (< 0.0001) | 0.002 (0.99) |
| Joint pain (VAS) | 0.55 (0.0002) | 0.29 (0.07) |
| Stiffness severity | 0.57 (0.0001) | 0.38 (0.02) |
Results expressed as Spearman’s rank correlation coefficients.
Figure 1.
Relationship of swollen joint count to the HAQ-disability index in rheumatoid arthritis and in chikungunya arthritis; p = 0.003 for the interaction disease*swollen joint count.
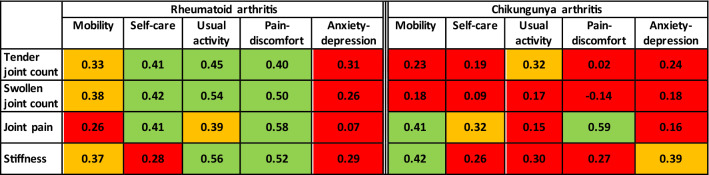
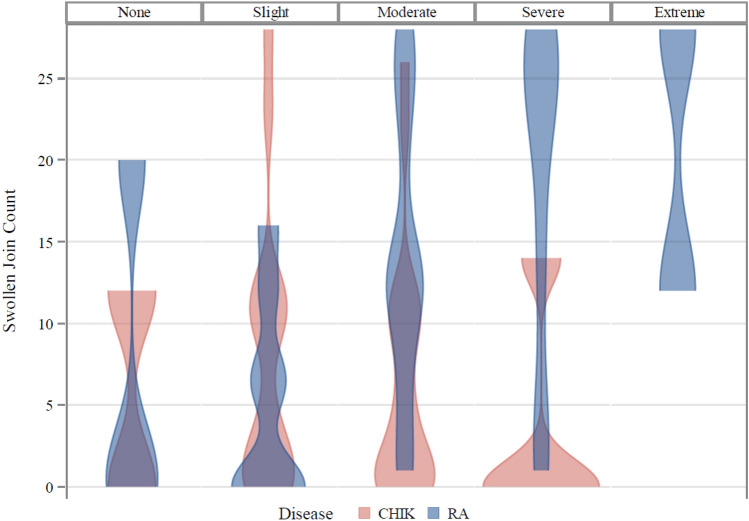
Tender and swollen joint counts, pain and stiffness were all associated with the majority of EQ5D quality of life domains (except anxiety/depression) in RA patients (Fig. 2). In contrast, in CHIKV arthritis, tender joint counts were significantly correlated (p < 0.05) only with one quality of life domain, while pain intensity score correlated with impaired mobility, self-care and pain/discomfort domains, and stiffness was associated with the mobility, usual activity and anxiety/depression domains (Fig. 2). Swollen joint counts were not significantly correlated with any of the quality of life domains in chikungunya disease. Regression analysis further confirmed that the effect of swollen joint count on the pain/discomfort domain was significantly different in RA and CHIKV arthritis (p = 0.006 for the statistical interaction of underlying disease with number of swollen joints), the degree of pain and discomfort increasing with the number of swollen joints only in RA patients (Fig. 3).
Figure 2.
Association of disease severity with EQ5D quality of life domains in rheumatoid and chikungunya arthritis. Spearman’s Rank Correlation coefficient: red indicates not significant, orange indicates p < 0.05 and green indicates p < 0.01.
Figure 3.
Swollen joint counts shown by EQ5D level of pain/discomfort for chikungunya arthritis (CHIK) and rheumatoid arthritis (RA); p = 0.006 for the interaction disease*swollen joint count in multiple regression analysis.
Discussion
Chronic post-chikungunya rheumatism, like rheumatoid arthritis, has a large impact on HRQoL13,26–28 and results in a moderate disability even more than two years after infection12. The present study compared patients with CHIKV arthritis 2–3 years post-infection with a group of RA patients who had similar levels of self-reported joint pain and stiffness and similar levels of disability. In the patients with RA, we were able to confirm the well-established strong association of tender and swollen joint counts and patient-reported symptoms with disability using the HAQ disability index14–16 and with HRQoL using the EQ-5D17. In contrast, in the patients with CHIKV arthritis, only patient-reported symptoms were clearly associated with the disability and HRQoL instruments. Despite the patients with CHIKV arthritis having only slightly fewer tender joints, the association of tender joint counts with disability and HRQoL was markedly weaker than that in RA.
The weak relationship of swollen joint counts with both disability and quality of life in post-chikungunya rheumatism was observed in a previous study, in which patients had very low numbers of swollen joints13. In the present study, swollen joints were more frequent, but the weak relationship of swollen joint count to patient-reported outcomes in chronic chikungunya disease was confirmed. Pain and stiffness occurring in the absence of notable swelling may be indicative of subclinical synovitis, a finding which has been reported following ultrasound examination of joints in patients with chronic musculoskeletal symptoms after chikungunya infection29. Incorporation of musculoskeletal ultrasound in the assessment of chronic chikungunya may help to explain the impact of the disease on function and quality of life.
Self-report scales in rheumatology commonly intercorrelate well30, so the association of patients’ subjective assessment of symptoms (e.g. pain and stiffness) with HRQoL scores might be expected to be stronger than that seen with more objective disease measures (e.g. joint scores or serum biomarkers of inflammation). Patients’ perceptions may influence in a similar fashion reporting of symptoms and outcomes, and this may be accentuated in the case of affliction by a poorly understood disease such as chikungunya. The potential role of subjectivity in reporting symptoms, influenced by the preceding intense acute fever has been discussed in this disease31. In this scenario, many symptoms and disabilities may be attributed to chikungunya. Indeed, compared to the joint counts, the self-assessment of symptom severity by the patients with CHIKV arthritis in the present study was more closely related to disability and some quality of life domains. This was particularly true for joint stiffness, which seems to be a frequent and relevant symptom in chikungunya patients deserving careful assessment. However, even allowing for this explanation of joint counts correlating less strongly than symptom scores with patient-reported outcomes, the association of joint counts with outcomes in patients with CHIKV arthritis was notably weak compared to our findings in the RA group.
There are certain limitations of the present study, of which one was the restriction of the clinical examination to 28 joints. Although a commonly used standard in recent RA studies, this 28-joint count excludes the more distal joints of the lower limb, joints which are frequently involved in chikungunya disease32. This opens the possibility that involvement of certain joints not assessed in this study may have had an important impact on disability and some quality of life domains. It would be of interest to re-investigate the association of joint counts with patient outcomes using counts expanded to include the more distal lower limb joints. It may also be important to consider that joint counts evaluated here offer only a snapshot at a single time-point and that post-CHIKV musculoskeletal disorders can result in a variety of phenotypes ranging from rapidly resolving inflammatory disease to chronic inflammatory rheumatic disorders and include those with a relapsing–remitting pattern of symptoms, which may be inconsistently captured by a physical examination.
Matching groups of patients with RA and chikungunya arthritis for such a study is not straightforward. Our groups were well-matched demographically, which is important in comparing joint stiffness33, and well matched for symptom severity and for disability. However, there were large differences in swollen joint counts and use of medications. We also had incomplete information on the disease history of RA and no possibility to compare objective serum markers. The course of the two diseases is quite different: RA is progressive, while joint symptoms in chikungunya patients are extremely disabling in the acute phase and generally resolve slowly over time2,34, so matching patients for duration of disease would not be meaningful. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are well-characterised in RA, but ESR is not elevated in chikungunya patients and CRP levels are primarily elevated during the acute phase of infection35, thus limiting any opportunity for matching the patient groups on these objective laboratory parameters. There is also no strong evidence-base yet for the treatment of chronic CHIKV arthritis, so certain differences between the groups in disease history and use of disease-modifying drugs for arthritis were to be expected. Nonetheless, the similarities in the symptom and disability scores suggest that our groups were adequately matched in terms of disease impact for the purpose of this study.
In terms of the generalizability of our findings, two further aspects should be considered. Inter-genotype heterogeneity in the prevalence of chronic post-CHIKV symptoms has been suggested36. Our post-CHIKV cohort had most probably been exposed to an ECSA lineage circulating in this part of Brazil18. Although our earlier findings in a Colombian cohort exposed to the Asian genotype were broadly in line with the present study10, it cannot be excluded that the pattern and importance of joint inflammation may differ from patients infected with the Indian Ocean lineage. Secondly, considering the subjective nature of self-reported outcome measures, evaluation bias can play a role and may differ between populations depending on the cultural context. As both the present study and our previous work was performed in South America, confirmation in Asian or African populations would reinforce the conclusions which can be drawn.
Defining the most relevant measures of disease severity in chronic chikungunya rheumatism needs further study. No single measure of disease severity tested here seems to predict very well the impact of chikungunya disease on the patient. Although musculoskeletal manifestations feature prominently in chronic chikungunya disease, other systemic manifestations such as asthenia, fatigue, anxiety and depression are reported4,31,37,38. These are also likely to be associated with HRQoL in chronic chikungunya rheumatism, possibly explaining why measures of joint involvement alone were so poorly predictive of HRQoL in our study. While instruments designed for use in rheumatoid arthritis provide a good starting point in the assessment of CHIKV arthritis, the construction of a disease severity index incorporating additional variables adapted to post-chikungunya rheumatism may be of value in the future study of this condition and in identifying the most effective treatments. Future research efforts in this direction should therefore take into account the impact of chronic fatigue, depression and other neurosensory sequelae of chikungunya infection in order to capture the full burden of the disease.
Author contributions
H.W., R.L.N.H., M.R.M., F.N., K.S., G.S.F., G.S., A.C. contributed to the study conception and design of the study; R.L.N.H., M.R.M., F.N. and A.C. contributed to study conduct and data collection; H.W., G.C., K.S., A.C. contributed to data analysis and interpretation; H.W. and A.C. drafted the manuscript; all authors approved the final manuscript.
Funding
Supported by NIH (National Center for Advancing Translational Sciences Grants UL1-TR-001876 and KL2-TR-001877).
Data availability
The datasets generated during and/or analysed during the current study are stored in the REDCap database at The George Washington University. Datasets may be available on reasonable request.
Code availability
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Charrel R, de Lamballerie X, Raoult D. Chikungunya outbreaks—The globalization of vector-borne diseases. N. Engl. J. Med. 2007;356:769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 2.Yaseen HM, Simon F, Deparis X, Marimoutou C. Estimation of lasting impact of a Chikungunya outbreak in Reunion island. Epidemiol. 2012;S2:003. doi: 10.4172/2161-1165S2-003. [DOI] [Google Scholar]
- 3.Rodríguez-Morales AJ, Cardona-Ospina JA, Urbano-Garzón SF, Hurtado-Zapata JS. Prevalence of post-chikungunya infection chronic inflammatory arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2016;68:1849–1858. doi: 10.1002/acr.22900. [DOI] [PubMed] [Google Scholar]
- 4.Marimoutou C, Ferraro J, Javelle E, Deparis X, Simon F. Chikungunya infection: Self-reported rheumatic morbidity and impaired quality of life persist 6 years later. Clin. Microbiol. Infect. 2015;21:688–693. doi: 10.1016/j.cmi.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Miner JJ, et al. Chikungunya viral arthritis in the United States: A mimic of seronegative rheumatoid arthritis. Arthritis Rheum. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgherini G, et al. Persistent arthralgia associated with chikungunya virus: A study of 88 adult patients on Reunion Island. Clin. Infect. Dis. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 7.Sokka T, Pincus T. Quantitative joint assessment in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005;23(Suppl 39):S58–62. [PubMed] [Google Scholar]
- 8.Orbai A-M, et al. More than just a few minutes of stiffness in the morning: Report from the OMERACT rheumatoid arthritis flare group stiffness breakout sessions. J. Rheumatol. 2015;42:2182–2184. doi: 10.3899/jrheum.141172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halls S, et al. Development and testing of candidate items for inclusion in a new rheumatoid arthritis stiffness patient-reported outcome measure. Rheumatology. 2018;57:263–272. doi: 10.1093/rheumatology/kex085. [DOI] [PubMed] [Google Scholar]
- 10.Watson H, et al. Stiffness, pain, and joint counts in chronic chikungunya disease: Relevance to disability and quality of life. Clin. Rheumatol. 2020;39:1679–1686. doi: 10.1007/s10067-019-04919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira Hayd RL, et al. Persistent chikungunya arthritis in Roraima, Brazil. Clin. Rheumatol. 2020;39:2781–2787. doi: 10.1007/s10067-020-05011-9. [DOI] [PubMed] [Google Scholar]
- 12.Bouquillard E, et al. Rheumatic manifestations associated with Chikungunya virus infection: A study of 307 patients with 32-month follow-up (RHUMATOCHIK study) Joint Bone Spine. 2018;85:207–210. doi: 10.1016/j.jbspin.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Tritsch SR, et al. Chronic joint pain 3 years after chikungunya virus infection largely characterized by relapsing-remitting symptoms. J. Rheumatol. 2020;39:1679–1686. doi: 10.3899/jrheum.190162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Heijde D, van‘t Hof M, van Riel PLCM, van der Putte LBA. Validity of single variables and indices to measure disease activity in rheumatoid arthritis. J. Rheumatol. 1993;20:538–541. [PubMed] [Google Scholar]
- 15.Uhlig T, Haavardsholm EA, Kvien TK. Comparison of the Health Assessment Questionnaire (HAQ) and the modified HAQ (MHAQ) in patients with rheumatoid arthritis. Rheumatology. 2006;45:454–458. doi: 10.1093/rheumatology/kei181. [DOI] [PubMed] [Google Scholar]
- 16.Verstappen SMM, et al. Functional Health Assessment Questionnaire (HAQ) and psychological HAQ are associated with and predicted by different factors in rheumatoid arthritis. J. Rheumatol. 2007;34:1837–1840. [PubMed] [Google Scholar]
- 17.Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: Validity, responsiveness and reliability of EuroQol (EQ-5D) Br. J. Rheumatol. 1997;36:551–559. doi: 10.1093/rheumatology/36.5.551. [DOI] [PubMed] [Google Scholar]
- 18.Naveca FG, et al. Genomic, epidemiological and digital surveillance of Chikungunya virus in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2019;13:e0007065. doi: 10.1371/journal.pntd.0007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aletaha D, et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584(2010). [DOI] [PubMed] [Google Scholar]
- 20.Fuchs HA, et al. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum. 1989;32:531–537. doi: 10.1002/anr.1780320504. [DOI] [PubMed] [Google Scholar]
- 21.van Gestel AM, Haagsma CJ, van Riel PLCM. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs HA, Pincus T. Reduced joint counts in controlled clinical trials in rheumatoid arthritis. Arthritis Rheum. 1994;37:470–475. doi: 10.1002/art.1780370406. [DOI] [PubMed] [Google Scholar]
- 23.Sinnathurai P, et al. Investigating dimensions of stiffness in rheumatoid and psoriatic arthritis: The Australian Rheumatology Association Database registry and OMERACT collaboration. J. Rheumatol. 2019;46:1462–1469. doi: 10.3899/jrheum.181251. [DOI] [PubMed] [Google Scholar]
- 24.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 25.Herdman M, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramachandran V, et al. Impact of Chikungunya on health related quality of life Chennai, South India. PLoS ONE. 2012;7:e51519. doi: 10.1371/journal.pone.0051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couzigou B, et al. Occurrence of chronic stage chikungunya in the general population of Martinique during the first 2014 epidemic: A prospective epidemiological study. Am. J. Trop. Med. Hygiene. 2018;99:182–190. doi: 10.4269/ajtmh.17-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertolotti A, et al. Prevalence of chronic chikungunya and associated risks factors in the French West Indies (La Martinique): A prospective cohort study. PLoS Negl. Trop. Dis. 2020;14:e0007327. doi: 10.1371/journal.pntd.0007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canella C. Imaging findings in chikungunya fever (editorial) Radiol. Bras. 2017;50:V. doi: 10.1590/0100-3984.2017.50.2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gossec L, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: A EULAR initiative. Ann. Rheum. Dis. 2011;70:935–942. doi: 10.1136/ard.2010.142901. [DOI] [PubMed] [Google Scholar]
- 31.Gerardin P, et al. Perceived morbidity and community burden after a chikungunya outbreak: The TELECHIK survey, a population-based cohort study. BMC Med. 2011;9:5. doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP. Long-term sequelae of chikungunya virus disease: A systematic review. Travel Med. Infect. Dis. 2017;15:8–22. doi: 10.1016/j.tmaid.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Watson H, Lynggård Hansen A, Calusi G, Bartels LE. Musculoskeletal stiffness is common in healthy adults and increases with age. Musculoskelet. Care. 2021;19:3–8. doi: 10.1002/msc.1501. [DOI] [PubMed] [Google Scholar]
- 34.De Moraes L, et al. A clinical scoring system to predict long-term arthralgia in Chikungunya disease: A cohort study. PLoS Negl. Trop. Dis. 2020;14:e0008467. doi: 10.1371/journal.pntd.0008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow A, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011;203:149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paixão ES, et al. Chikungunya chronic disease: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hygiene. 2018;112:301–316. doi: 10.1093/trstmh/try063. [DOI] [PubMed] [Google Scholar]
- 37.Elsinga J, et al. Long-term Chikungunya Sequelae in Curaçao: Burden, determinants and a novel classification tool. J. Infect. Dis. 2017;216:573–581. doi: 10.1093/infdis/jix312. [DOI] [PubMed] [Google Scholar]
- 38.Moro, M.L., et al. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: A prognostic cohort study. J. Infect.65, 165–172. 10.1016/j.jinf.2012.04.005 (2012). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are stored in the REDCap database at The George Washington University. Datasets may be available on reasonable request.
Not applicable.