Abstract
It is a sad fact that despite being almost completely preventable through human papillomavirus (HPV) vaccination and screening, cervical cancer remains the fourth most common cancer to affect women worldwide. Persistent high-risk HPV infection (hrHPV) is the primary etiological factor for cervical cancer. Upwards of 70% of cases are driven by HPV types 16 and 18, with a dozen other hrHPV associated with the remainder of cases. Current standard-of-care treatments include radiotherapy, chemotherapy, and/or surgical resection. However, they have significant side effects and limited efficacy against advanced disease. There are few treatment options for recurrent or metastatic cases. Immunotherapy offers new hope, as demonstrated by the recent approval of PD1 blocking antibody for recurrent or metastatic disease. This might be augmented by combination with antigen-specific immunotherapy approaches, such as vaccines or adoptive cell transfer, to enhance the host cellular immune response targeting HPV-positive cancer cells. As cervical cancer progresses, it can foster an immunosuppressive microenvironment and counteract host anticancer immunity. Thus, approaches to reverse suppressive immune environments and bolster effector T cell functioning are likely to enhance the success of such cervical cancer immunotherapy. The success of non-specific immunostimulants like imiquimod against genital warts also suggest the possibility of utilizing these immunotherapeutic strategies in cervical cancer prevention to treat precursor lesions (cervical intraepithelial neoplasia) and persistent hrHPV infections against which the licensed prophylactic HPV vaccines have no efficacy. Here we review the progress and challenges in the development of immunotherapeutic approaches for the prevention and treatment of cervical cancer.
Keywords: Human papillomavirus, cervical cancer, CIN2/3, immunotherapy
1. Introduction
Cervical cancer is the fourth most common female cancer worldwide (1,2) and one of the top three cancers to affect women younger than 45 (3). Globally there were approximately 570,000 cases of cervical cancer and 311,000 deaths in 2018, demonstrating the significant global burden of the cancer (3). For countries with a high Human Development Index (HDI), cervical cancer has a 5-year survival rate of 60–70%, whereas in countries with low HDIs it falls to <20% (2,4). In the United States, survival has not significantly improved for cervical cancer patients since the 1970s (5) demonstrating the urgent need to improve on current treatment approaches for cervical cancer.
The precursors of cervical cancer are categorized into grades of increasing severity of dysplasia: Cervical Intraepithelial Neoplasia grades 1, 2, and 3 (CIN1, CIN2, and CIN3, respectively). CIN1, also called low-grade squamous intraepithelial lesions (LSIL), represents a productive HPV infection, while CIN2/3, also called high-grade squamous intraepithelial lesions (HSIL), and are considered to be the immediate precursor lesion. CIN1 lesions frequently spontaneously regress due to immune system clearance after HPV infection. However, CIN2/3 is associated with lower rates of regression. Individuals with CIN2/3 are at a high risk for developing cervical cancer if left untreated by ablation by conization/LEEP (6). Unfortunately, conization and LEEP are associated with risk of recurrence, cervical incompetence and premature delivery of future pregnancies (and associated risks to the child), so an immunotherapeutic treatment modality could be a complementary approach or even a better alternative.
Treatment approaches and outcomes for cervical cancer patients are highly dependent on disease stage at diagnosis (7,8), with five year survival ranging from over 90% if diagnosed in an early, localized stage to less than 20% if diagnosed as distant or metastatic (8). Treatment options for early-stage and locally invasive cervical cancer include radical hysterectomy or radical trachelectomy with pelvic lymphadenectomy and concurrent chemotherapy and radiation therapy. For distant metastatic cervical cancer, the treatment focuses on systemic therapies (for review see (7,9–12)). With the low cure rates for advanced disease and side effects of current therapies, new therapeutic options for cervical cancer patients are desperately needed. Immunotherapy is an anti-cancer strategy that aims to modify and recruit the host immune system to more effectively and specifically target cancer cells; it is a growing field that offers hope for improved cervical cancer treatment and survival. Currently, the use of immunotherapy for the treatment of cervical cancer is being actively investigated, although only one immunotherapeutic drug (pembrolizumab) has so far been approved by the FDA for use against cervical cancer.
Human Papillomavirus (HPV) is a necessary but not sufficient etiological factor of cervical cancer. HPV is a common sexually transmitted infection, but most HPV infections are cleared by the body’s immune system. However, certain genotypes are associated with greater oncogenicity (this group are termed high risk HPV (hrHPV)). When these genital mucosal hrHPV infections persist they drive cell proliferation and genomic instability and ultimately progression to malignancy if unchecked. HrHPV infection thus produces high grade CIN (aka squamous intraepithelial lesions (SIL)), which represent the precursor lesion of cervical cancer (13). The remainder of the >200 known HPV genotypes do not cause cancer and are considered low risk (14). HPV are also classified by their tropism toward mucosal and cutaneous epithelia and these low risk HPVs cause benign genital and skin warts. HPV present in more than 95% of all cervical cancers (1,15), with high risk types being associated with over 85% of cervical cancer cases, and notably HPV16 and HPV18 are the most common high-risk types (16). Furthermore, hrHPVs are also responsible for the malignant transformation of other mucosa and have been associated with penile, vaginal, vulvar, anal, and head and neck (oropharyngeal) cancers, although HPV16 is by far the dominant type (16–21).
HPV is a small (~8000 base pair), non-enveloped, double-stranded, circular DNA virus (22). The HPV genome expresses an early transcriptional program encoding E1, E2, E4, E5, E6, and E7, which regulate the viral life cycle, and late transcriptional program encoding proteins, major and minor capsid proteins L1 and L2, which are structural components for genome encapsidation and virion assembly. While E1 is a replication factor, E2 is a transcription regulator of all HPV viral proteins, capable of regulating viral DNA replication and viral RNA transcription. E4 regulates the cytoskeleton structure of infected epithelial cells to promote virion release. E5, E6, and E7 mediate cellular transformation. E6 and E7 are especially important, as they are oncoproteins that repress tumor suppressors p53 and pRb, respectively. They disrupt the activation of apoptotic pathways and promote cell proliferation, ultimately leading to the progression of HPV-associated malignancies (17,19). During cancer development, viral integration into the host genome often leads to the loss of E2, E4, E5, L1, and L2 expression, whereas constitutive expression of E6 and E7 oncogenes is obligate for cancer cell survival and growth.
Identifying hrHPV as the primary and necessary etiological factor of cervical cancer and other HPV-associated cancerous malignancies has been central to the development of immunization strategies to prevent its associated cancers. There are preventative vaccines that generate neutralizing antibodies against HPV and establish protective immunity using L1 virus like particles (23). Commercially available prophylactic vaccines include the bivalent Cervarix® (GlaxoSmithKline) and multi-valent Gardasil® and Gardasil-9® (Merck) (24–26). Gardasil-9® has expanded coverage to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 (24,27) and is the dominant vaccine in the United States. These vaccines protect naive individuals from contracting HPV infections targeted by the vaccine; (28,29) however, these preventive vaccines have not been successful in treating established HPV infections (30). Unfortunately, prophylactic HPV vaccination rates have been low globally, especially in low-income countries (31). In 2016, only 50% of females aged 13–17, the target demographic for the vaccine, were vaccinated with an HPV vaccine in the United States (5), and vaccination rates can vary widely globally and between racial groups (32). Barriers to starting and completing the full preventative vaccine series include parental attitudes, race/ethnicity, cultural beliefs regarding sexual activity, insurance status, geographic location, socioeconomic status, low knowledge among healthcare providers, and logistical challenges towards acquiring all three doses (32–34). Vaccine rates are especially suboptimal for male adolescents (33). Furthermore, given the relatively recent introduction of HPV vaccination, older cohorts of women and men remain unprotected and HPV infection remains highly prevalent in these generations; however, in 2018 the FDA approved of raising the age cap for Gardasil-9 administration from 26 to 45 for men and women (35). Nonetheless, overall low prophylactic vaccination rates among adolescents and pre-existing infections in older women demonstrates the urgent need for the development of therapeutic HPV vaccines to treat HPV-associated cervical dysplasia and cervical cancer. Due to their role in the majority of cervical cancer cases, high-risk types HPV16 and HPV18 have been the main focus for developing HPV antigen specific immunotherapies (36).
Infected basal epithelial cells that harbor the virus only express the early genes. When HPV integrates into the host genome during progression, typically, the oncogenic E6 and E7 genes are integrated and expressed whereas the other HPV genes are lost or not expressed (37,38). As a result, neutralizing antibodies (and L1-specific T cells) generated by prophylactic vaccines are not effective against these HPV-infected cells. In an attempt to clear HPV infections or pre-existing HPV-associated lesions, therapeutic HPV vaccines are being developed. Therapeutic vaccines generate T cell-mediated immunity by specifically targeting HPV early antigens that are constitutively expressed across both infected and cancerous cells (39,40). Specifically, the E6 and E7 proteins represent two logical therapeutic HPV vaccine targets because they are constantly expressed and required to maintain malignant transformation of HPV-associated cancers (38,41). Thus, most immunotherapeutic HPV approaches focus on generating and enhancing T cells specific for the HPV E6 and E7 antigens, but other viral antigens can potentially be targeted when treating precursor lesions or infection (e.g. E1 and E2).
Another major immunotherapeutic approach is adoptive cell therapy (ACT). This strategy is generally personalized to the individual patient, and involves removing naturally occurring tumor-reactive and targeted lymphocytes (tumor-infiltrating lymphocytes, TIL), expanding those with or engineering desired phenotypes in vitro, then re-administering the lymphocytes in significantly higher quantity (42,43). Although the efficacy of ACT has generally been limited against solid tumors (44), there are ongoing studies that apply this strategy to cervical cancers. For cervical cancer TILs are generally selected for HPV E6 and E7 reactivity, although recent evidence suggest that the cancers also contain additional neoepitopes that can be targeted (45).
As cervical cancer cells continue to evade immune surveillance, many immunosuppressive factors are upregulated in the TME. Immune escape is associated with both downregulation of the immune system locally and evasion of detection, including an increase in regulatory T cells (Tregs), loss of major histocompatibility complex (MHC) antigen presentation, chronic inflammation, and upregulated immune checkpoint molecules (for review see (46)). Therefore, beyond targeting tumor antigen, a major immunotherapeutic strategy is to reverse immunosuppression or effector T cell suppression. These approaches are not HPV antigen specific and if successful, they can be effective in most cervical cancers regardless of HPV type or association. For example, immune checkpoint blockade targets cell surface checkpoint molecules such as PD-1/PD-L1 and CTLA-4 with antibodies (for review see (47)). The upregulation of immune checkpoint molecules in the TME is a factor in cancer immune escape as increased PD-1/PD-L1 activity downregulates T cell function. Therefore, by blocking immune checkpoint function, T cells will continue to proliferate within the TME and clear cancer cells. In 2018, the FDA approved pembrolizumab (Keytruda®, Merck) for PD-L1 positive metastatic or recurrent cervical cancer. While pembrolizumab has a response rate for these patients of only ~15% (48) this represents an important first step.
In all, immunotherapy is a promising approach for cervical cancer, especially as it presents unique, non-self-viral antigen targets. Due to the near ubiquity of HPV in cervical cancer, antigen specific treatments targeting hrHPV oncoproteins can prime the host immune system to target HPV-expressing cancer cells. Secondly, as cervical cancer progresses, alters the TME, and suppresses the immune system, strategies to support effector immune cells and reverse immune suppression remain attractive. Current standard-of-care for cervical cancer patients is woefully inadequate, as there is a lack of treatment options, low survival rate for metastatic/recurrent patients, and significant side effects. When compared to other gynecological cancers such as ovarian or endometrial, cervical cancer patients tend to be younger and have fewer treatment options. Developing novel, highly effective immunotherapeutic strategies, either independently or in combination with standard-of-care or other immunotherapies, is therefore of great clinical interest to improve outcomes for these patients.
2. Strategies to Generate and Enhance Cervical Cancer Specific T Cells
A plethora of vaccine strategies are being tested for cervical cancer immunotherapy, many of which target HPV early proteins, predominantly E6 and/or E7. Vaccine types include live vector, protein/peptide, nucleic acid, and cell based vaccines. These antigen-specific forms of immunotherapy aim to stimulate antigen presentation via MHC class I and II, which leads to the generation of CD8+ cytotoxic and CD4+ helper T cell responses. A crucial consideration with therapeutic vaccines is their reliance on an intact immune system, which is potentially problematic in patients with immunosuppression (organ transplant recipients, or HIV+ patients for example) who are at elevated risk for HPV-associated cancers.
2.1. Vaccine Strategies
2.1.1. Live Vector Based Vaccines
Live vector-based vaccines use either bacterial or viral vectors, depending on the selected platform. They can induce strong cellular and humoral immune responses, potentially with a single dose (for review see (30)). However, live-vector based vaccines can pose as a safety risk, especially in immunocompromised patients, and immunity to the vector can dominate and/or prevent boosting with the same vector.
Several HPV therapeutic vaccine candidates have bacterial vector bases, including Listeria monocytogenes, Lactococcus lactis, Lactobacillus plantarum, and Lactobacillus casei (49–52). Listeria is an especially promising vector due to its ability to infect macrophages and secrete listeriolysin O (LLO), a pore-forming toxin to escape phagosomal lysis, permitting it to replicate in the cytoplasm of the host cell (53). Since the bacteria can be present in the cytoplasm and endosomal compartments, antigen peptides can be presented via MHC class I and MHC class II in order to recruit both cytotoxic and helper T cells (54). Listeria based vaccines carrying E7 antigen have been shown to elicit significant immune response against E6/E7-expressing tumors (49,55). Listeria vaccines for the treatment of cervical cancer are clinically encouraging. ADXS11–001 vaccine, a Listeria-based bacterial vector vaccine encoding HPV16 E7, has shown promise for patients with recurrent or persistent cervical cancer (56). It is currently in a Phase III clinical trial for cervical cancer patients (NCT02853604, Table 1).
Table 1.
Summary of clinical trials using strategies to generate and enhance T-cell priming: vaccines
| Immunotherapy Treatment(s) | Additional Therapy (chemo, radiation, chemical therapies) | Patient Population (n) | Phase of Clinical Trial | NCT Number |
|---|---|---|---|---|
| ADXS11–001 vaccine (attenuated Lm-LLO-HPV16E7 viral vaccine) | High risk locally advanced cervical cancer (450 participants) | Phase 3 | NCT02853604 | |
| HB-201, HB202 (Viral vectors coding against HPV16 E6/E7) | HPV-associated squamous cell carcinoma (140 participants) | Phase 1/2 | NCT04180215 | |
| Vvax001 (Semliki Forest Virus based HPV vaccine) | CIN 2/3, Cervical cancer (12 participants) | Phase 1 | NCT03141463 | |
| ISA101/ISA101b (therapeutic synthetic long peptide vaccine targeting HPV16 E6 and E7) Bevacizumab (anti-VEGF antibody) | Carboplatin and Paclitaxel | HPV16+ advanced, metastatic, or recurrent cancer patients (93 participants) | Phase 1/2 | NCT02128126 |
| PVX-2 DNA prime boost: twice intramuscular administration of pNGVL4a-Sig/E7(detox)/HSP70 DNA (DNA vaccine containing HPV16 E7 antigen), followed by TA-CIN boost (single fusion protein vaccine priming T cells against HPV16 E6, E7, and L2 antigens) | ASC-US, ASC-H, or LSIL patients (122 participants) | Phase 2 | NCT03911076 | |
| PVX-6 DNA Prime Boost: twice intramuscular administration of pNGVL4aCRTE6E7L2 DNA vaccine (DNA vaccine that contains HPV16 E6, E7, L2 antigen), followed by TA-CIN boost (single fusion protein vaccine priming T cells against HPV16 E6, E7, and L2 antigens) | HPV16+ ASC-US/LSIL patients (30 participants) | Phase 1 | NCT03913117 | |
| Intramuscular TA-CIN (single fusion protein vaccine priming T cells against HPV16 E6, E7, and L2 antigens) | HPV16+ cervical cancer (14 participants) | Phase 1 | NCT02405221 | |
| TVGV-1 (Protein pseudomonas exotoxin HPV16E7 vaccine adjuvanted with or without GPI-0100) | HPV+ HSIL (10 participants) | Phase 2 | NCT02576561 | |
| Subcutaneous UCPVax (Universal Cancer Peptide Vaccine, induces DC4 T cells) Atezolizumab (anti-PD-L1 antibody) |
Advanced or metastatic HPV+ head and neck, cervical, or anal cancer patients (47 participants) | Phase 2 | NCT03946358 | |
| Electroporation of GX-188E (DNA vaccine that produces antigen for HPV16/18 E6 and E7) | HPV16/18+ CIN patients (72 participants) | Phase 2 | NCT02139267 | |
| Electroporation of GX-188E (DNA vaccine that produces antigen for HPV16/18 E6 and E7) | HPV16/18+ CIN3 patients (9 participants) | Phase 1 | NCT01634503 | |
| Electroporation of VGX-3100 (DNA vaccine that encodes HPV 16/18 E7 antigen) | HPV16/18+ CIN or HSIL patients (198 participants) | Phase 3 | NCT03721978 | |
| Electroporation of VGX-3100 (DNA vaccine that encodes HPV 16/18 E7 antigen) | HPV16/18+ HSIL patients (201 participants) | Phase 3 | NCT03185013 | |
| Electroporation of pNGVL4a/CRT E6 E7 L2 (DNA vaccine that contains HPV16 E6, E7, L2 antigen) | HPV16+ CIN2/3 patients (48 participants) | Phase 1 | NCT04131413 | |
| BNT113 vaccine (mRNA vaccine encoding HPV16 E6, E7) | HPV16+ head and neck, anogenital, penile, cervical cancer (44 participants) | Phase 1/2 | NCT03418480 | |
| Intramuscular administration of TA-HPV (live vaccinia vaccine encoding HPV16/18 E6 and E7); and pNGVL4a-Sig/E7 (Detox)HSP70 DNA (DNA vaccine encoding HPV16 E7 antigen) Imiquimod (immune modifying cream |
HPV16+ CIN 3 patients (75 participants) | Phase 1 | NCT00788164 |
Live viral vector vaccines express target antigens by infecting cells and hijacking translation machinery. Multiple viral vector vaccines have been developed to target E6 and E7 antigens, including adenoviruses, adeno-associated viruses, alphaviruses, and vaccinia viruses (57–60). Vaccinia is currently among the most promising, due to its high infectivity, safety record, and low risk of integration into host DNA genome (61). Vaccinia-based vaccines include encoding the vector with E7 fused to calreticulin, E7 fused to LLO, and SigE7-LAMP-1, in which E7 is linked to sorting signals and a lysosomal-associated membrane protein (60,62). Human Antigen-Human Papillomavirus (TA-HPV) is a vaccinia-based live vector vaccine that encodes mutated E6 and E7 oncoproteins for HPV16 and HPV18. Early clinical trials with TA-HPV demonstrated its ability to induce HPV-specific cytotoxic T-cell responses in patients with cervical cancer (61) and patients with HPV-associated high-grade vulvar intraepithelial neoplasia (VIN III) and high-grade vaginal intraepithelial neoplasia grade two (VAIN II) (63). Additionally, Tipapkinogen Sovacivec (TS) vaccine uses a Modified Vaccinia virus Ankara (MVA) vector encoded with genes for HPV16E6/E7 and IL-2 (64). A phase II clinical trial in women with high-risk HPV-associated CIN2/3 found that the TS vaccine course resulted in greater histologic clearance of CIN2/3 than in the placebo group, regardless of high-risk type (64). A few other viral vectors beyond vaccinia have additionally shown potential ability to target and clear HPV16 E6/E7-expressing tumors and increase HPV E6/E7-specific immunity in preclinical models, with some vaccines entering early phase of clinical trials (for review see (65)). For instance, a HPV16 E6/E7 arenavirus based vaccine is currently being evaluated in HPV-associated cancers in a Phase I/II clinical trial (NCT04180215, Table 1). Finally, Vvax001 is a Semliki Forest Virus based HPV vaccine that has been tested for the treatment of patients with CIN 2/3 or cervical cancer (NCT03141463, Table 1, (66)).
Despite advantages to live-vector based vaccines, native antiviral and antibacterial immune responses upon administration can neutralize the vaccine before it can express the target antigen, precluding repeated vaccination. When using a viral vector, there can be an immune response mounted against the vector itself, and there is possibility of causing immunity against the vector antigen rather than the encoded antigen, although this might be addressed with using a heterologous prime-boost strategy. Likewise, as a result of vector-specific immunity incited by a primary vaccination, it can be difficult to boost vaccination regimens using formulations that use the same vector. These problems with the viral vector itself can therefore limit HPV antigen-specific immune responses generated by repeated vaccination with the same viral vector-based vaccines. Furthermore, immunocompromised and immunosenescent individuals may also be at heightened risk for complications with live-vector based vaccines, which can pose a safety concern, although defective/single round infection vectors can mitigate this concern (for review see (67)).
2.1.2. Dendritic Cell Based Vaccine
A dendritic cell (DC) based vaccine is a variant of a whole-cell-based vaccine. Dendritic cells work as antigen-presenting cells (APCs) to serve as a bridge between innate and adaptive immunity (68). DC-based HPV vaccines require loading the DCs with HPV antigens then delivering the preloaded cells to the patient (69–75). DCs can enhance the potency of other antigen-specific therapeutic vaccines as they provide the patient with the key immune cells to initiate adaptive immune responses that are often limiting in advanced cancer (76). DC-based vaccines can be transfected with additional siRNAs to protect against apoptotic molecules in order to extend the life of the cell and maximize immune effect (73,74). A phase I clinical trial found that pulsing DCs with full-length HPV16/18 E7 and keyhole limpet hemocyanin (KLH) then subcutaneously injecting the cells back into the patient is safe and well tolerated in patients with stage Ib and IIa cervical cancer (77). The strategy also increased HPV-specific humoral and CD4+ T cell immune responses. However, a separate trial conducted in advanced cervical cancer found no consistent significant immune response (78). While progress has been made to improve the efficacy of DC-based vaccines, they have several limitations. DC-based vaccines are technically demanding to produce on a large-scale. Secondly, varying culture techniques may lead to inconsistencies in vaccine quality and standard evaluation criteria. Third, DC-based vaccines do not have the option of serving as an off-the-shelf vaccine. Lastly, the most effective route for DC vaccine administration to amplify the effects elicited by the vaccine has not yet been determined (70).
2.1.3. Peptide/Protein Based Vaccine
Peptide and protein based vaccines are safe, stable, and simple to produce at quantity. Peptides and proteins derived from HPV antigens are taken up by dendritic cells for presentation on MHC Class I or II. Peptide/protein based vaccines can have weaker immunogenicity than live vector-based vaccines, and therefore may need to be delivered with adjuvants or lipids for enhancement (for review see (30)). In contrast to peptide-based vaccines, protein-based vaccines may contain multiple human leukocyte antigen (HLA) restricted cytotoxic T lymphocyte epitopes suitable for general populations. In comparison, peptide-based vaccines may only be suitable for individuals with certain HLA haplotypes, which limits their usage in the general population (for review see (68)). However, one potential solution for this limitation in peptide-based vaccination is to generate overlapping long peptides encompassing the entire antigen(s) (79).
Currently, there are several peptide-based vaccines under investigation for potential use against cervical cancers. For example, ISA101 vaccine, a long peptide vaccine that targets HPV16 E6 and E7 has been used in a phase I/II clinical trial in recurrent and metastatic patients with HPV-associated cancers (NCT02128126, Table 1). While the early clinical trials on patients with HPV-associated vulvar and vaginal intraepithelial neoplasia using the ISA long peptide vaccines demonstrated encouraging results (80,81), it is not clear yet whether it works similarly well in patients with advanced cervical cancer.
Tissue Antigen-Cervical Intraepithelial Neoplasia vaccine (TA-CIN) is a protein-based vaccine made of a single fusion protein to trigger cellular toxicity against antigens HPV16 E6, E7, and L2 proteins (82). TA-CIN is being evaluated for efficacy for cervical dysplasia, including HSIL and LSIL. The vaccine has proven to be safe and immunogenic in several early phase clinical trials (83–85). Due to its proven safety and immunogenicity, TA-CIN has been used with imiquimod cream, a TLR7/8 agonist, for the treatment of grade 2 or 3 vulvar intraepithelial neoplasia (VIN) in hopes that TA-CIN would increase HPV16 E6 and E7 specific T effector cells (86). TA-CIN and imiquimod in combination produced lesion regression in a subset of patients, and increased CD8 and CD4 cell infiltration (86). Currently it is being investigated alongside a DNA vaccine for the treatment of ASC-US, ASC-H, and LSIL (NCT03911076, NCT03913117, Table 1), and for HPV16+ cervical cancer patients (NCT02405221 Table 1). Another protein vaccine named TVGV-1 can induce HPV16 E7CD8+ T cell responses in a preclinical model when delivered with CpG or GPI-0100 adjuvant (87). A phase II clinical trial using TVGV-1 is ongoing in patients with HSIL (NCT02576561, Table 1).
Tumor antigens other than HPV encoded antigens have also been explored for the control of cervical cancer. For example, a Universal Cancer Peptides peptide vaccine (UCPVax) is currently undergoing a clinical trial to determine its immunogenic efficacy in cervical cancer (NCT03946358, Table 1). UCPVax is a unique vaccine on trial for HPV-associated cancers because rather than targeting an immune response towards HPV antigen, UCPVax targets telomerases expressed by a variety of cancerous cells (88,89). UCPVax is used to stimulate CD4+ T cell activity against cancerous cells, with the goal of developing an immunotherapeutic vaccine that works against multiple cancer types (90).
2.1.4. Nucleic Acid Based Vaccine
Nucleic acid based vaccines use RNA or DNA plasmid backbones to deliver antigens to the immune system. Many candidate therapeutic HPV vaccines are DNA vaccines. DNA vaccines are constructed by inserting the target antigen into a mammalian expression vector, and once injected in the body the encoded antigen is transcribed in vivo, permitting uptake by antigen presenting cells to present the antigens through MHC Class I and II molecules. Similar to peptide and protein vaccines, DNA vaccines are safe, easy to produce, and stable. Individuals can be repeatedly vaccinated with the plasmid (for review see (68)). Although there is potential risk that administration of DNA encoding antigen oncogenes such as E6 and E7 may lead to cellular transformation, most of the therapeutic HPV DNA vaccines employ mutated or shuffled E6 and/or E7 antigens, which eliminates the concerns for oncogenicity while remaining immunogenic (91).
One of the major limitations for naked DNA vaccination is the low transfection efficiency in vivo. Electroporation has been used to enhance the DNA transfection efficiency in vivo to improve the efficacy of naked DNA vaccination. For example, GX-188E is a therapeutic HPV DNA vaccine, encoding a fusion protein consisting of an activator signal and FMS-like tyrosine kinase 3 ligand in addition to E6 and E7 of HPV16/18. Previous clinical trials have focused on the use of GX-188E for treatment of HPV-associated CIN (NCT02139267, NCT01634503, Table 1). To date, GX-188E has shown the ability to promote lesion and viral regression in a significant fraction of CIN3 patients. Although electroporation is transiently painful, this approach is well-tolerated by study participants (92), and produces a significant E6/E7 T cell response (93).
Another therapeutic HPV DNA vaccine delivered by electroporation is VGX-3100, which is a DNA vaccine encoding HPV16 and HPV18 E6 and E7 antigens. Phase I and II clinical trials in patients with HPV-positive CIN2/3 have demonstrated the vaccine’s safety, tolerability, and immunogenicity (94,95). VGX-3100 demonstrated regression of precancerous cervical lesions and viral clearance in 48% of vaccine treated patients compared to 30% of patients receiving the placebo control (94). Two phase III trials using VGX-3100 delivered via electroporation in cervical HSIL are currently underway (NCT03721978, NCT03185013, Table 1). Recently released endpoints from the REVEAL 1 trial (NCT03185013) showed that for the modified intention to treat (mITT) population (N=193) the primary endpoint of histopathological regression of HSIL combined with virologic clearance of HPV16 and/or HPV18 at week 36 was 23.7% (31/131) in the treatment group, versus 11.3% (7/62) in the placebo group (p=0.022; 12.4% difference in percentage, 95%CI: 0.4,22.5) (96). A similar vaccine, INO-3112, carries HPV16 and 18 E6 and E7 antigens but with the addition of IL-12 cytokine. INO-3112 vaccine has shown its tolerability and immunogenicity in clinical trial as an adjuvant for chemoradiation in cervical cancer patients (97) and in HPV-associated head and neck cancer (98).
Another strategy to enhance the potency of DNA vaccines is to employ an intracellular targeting strategy to improve antigen presentation through MHC class I molecule to CD8+ T cells (99–101). For example, pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine is a therapeutic HPV DNA vaccine that uses the mycobacteria heat shock protein 70 to improve DNA vaccine potency. The DNA vaccine encodes a fusion protein consisting of a signal peptide linked to a mutated HPV16 E7 protein and the mycobacteria heat shock protein 70 (HSP70). The linkage of HSP70 to the E7 protein leads to the targeting of the secreted E7 fusion protein to professional antigen presenting cells to enhance the cross presentation of the linked E7 antigens (102). Early phase trials in CIN3 patients have shown pNGVL4a-Sig/E7(detox)/HSP70 to be safe for use (99). A similar DNA vaccine, pNGVL4a-CRTE6E7L2, employs calreticulin to enhance MHC class I presentation (101). The DNA vaccine primes the immune system to generate HPV16 antigens (E6, E7, and L2) specific immune responses (101). This vaccine will enter clinical trial with TA-CIN vaccination as booster (NCT03913117, Table 1) and is currently being tested using electroporation (NCT04131413, Table 1).
VB10.16 DNA vaccine is another example of naked DNA vaccine that can lead to the targeted delivery of the encoded HPV antigens to professional antigen presenting cells to elicit immune responses to HPV16 E6 and E7 antigens. A phase I/II clinical trial in CIN2/3 patients found the vaccine to be safe and well tolerated, and the treatment elicited a CD8+ T cell immune response against HPV16 antigen (103).
RNA-based vaccines have emerged as a new form of vaccine for the control of infectious diseases and/or cancer. As opposed to naked DNA vaccines, RNA-based vaccines can be translated in the cytoplasm rather than needing to first be delivered to the nuclei for transcription. Naked RNA vaccines work by injecting the patient with RNA that encodes a specific antigen to be presented by APCs. The two recently licensed RNA-based vaccines for COVID-19 prevention represent a scientific triumph on the control of an infectious disease. The COVID-19 RNA based vaccines generate not only humoral immunity but also cellular immunity, which may be important for the control of cancers that require cellular immunity (104). There is one RNA-based vaccine candidate for the treatment of HPV-associated cancers named RNA-LPX that targets HPV16 E7 and has demonstrated the ability to induce long term antigen-specific CD8+ T cell responses in preclinical mouse models (105). A derivative of RNA-LPX, BNT113, is constructed using similar mRNA backbone and LPX technology, and it encodes both HPV16 E6 and E7. BNT113 is currently in a phase I/II clinical trial primarily for HPV16-associated head and neck cancer patients, with intent to test the vaccine in patients with HPV16-associated cervical cancer patients in the future (NCT03418480, Table 1).
RNA-based vaccines can also be derived from RNA viruses, commonly alphaviruses, including the Sindbis virus, Venezuelan Equine Encephalitis virus, and Semliki Forest virus (59,106,107). The RNA strand is inserted with the target antigen RNA, and it is capable of self-replication, which leads to sustained antigen presentation and increased immunogenicity over other forms of nucleic acid based vaccines (30). Crucially, RNA replicon vaccines lack structural genes, and thus do not elicit a neutralizing antibody immune response which permits repeated vaccinations, and there is a low risk of chromosomal integration with host DNA or reconstitution of infectious virus (108).
Despite advantages, RNA-based vaccines can have low stability. In attempt to stabilize the vaccine, RNA replicons and DNA vaccines can be combined into a DNA-launched RNA replicon vaccine, also termed “suicidal DNA” (30). “Suicidal DNA” induces apoptosis in cells that uptake the injected DNA to prevent potential integration and transformation of the infected cells (109). Current preclinical models using therapeutic HPV suicidal DNA vaccines and RNA replicons have shown poor immunogenicity; however, some strategies for boosting immunogenicity, such as the use of flavivirus Kunjin vector or inclusion of HSP70, VP22, or genes encoding anti-apoptotic proteins, have been explored with preliminary advantageous results in preclinical models. (30,106,109–112).
Another method of utilizing RNA for anti-cancer immunotherapy is to administer RNA to regulate APCs. DCs transfected with anti-apoptotic RNAs resisted cell death and presented antigens for longer than DCs from dendritic cell-based vaccines without the RNAs (112). Similarly, gene gun delivery of anti-apoptotic siRNAs alongside an HPV16 E7 DNA vaccine was able to extend the lives of antigen presenting cells and overall elicited stronger anti-tumor activity than DNA vaccine administered without siRNA in a preclinical model (113). Although siRNA strategies are not strictly RNA vaccines, as they do not encode target antigen, they can potentially be used to boost other immunotherapeutic vaccines for cancer treatment.
Despite the safety of many nucleic acid and peptide vaccines, most show limited immunogenicity in advanced cervical cancer. Although several cervical cancer vaccines, such as ISA101 or VGX-3100, do demonstrate immunogenicity as shown by their clinical efficacy in precancerous lesions (80,81,94), these vaccines alone may not be sufficient for the control of advanced cervical cancer due to primary or secondary cancer resistance mechanisms or an immunosuppressive tumor environment, which is discussed below. Those with low immunogenicity may require strategies to boost immunogenicity via adjuvants, co-treatments, multiple vaccinations, or delivery methods. Many DNA vaccines for HPV-associated precancerous lesions and malignancies are delivered via electroporation (NCT01634503, NCT04131413, Table 1). Alternatively, DNA prime followed by a protein or vaccinia vaccine boost strategies refers to the immunotherapeutic approach of an initial vaccination with a DNA plasmid, followed by a booster vaccination with a different type of immunotherapeutic vaccine. This combination strategy potentially elicits stronger immune responses than either vaccine alone (114). Currently clinical trials are investigating the strategy of combining a DNA vaccine with TA-CIN fusion protein vaccine for HPV16+ with abnormal cytology lower than high grade lesion (ASC-US/LSIL) (NCT03911076, NCT03913117, Table 1). Both plasmids and the TA-CIN protein boost vaccine have demonstrated safety profiles. Priming with DNA vaccine pNGVL4a-Sig/E7(detox)/HSP70 followed by boost with Live-vaccinia vector based vaccine TA-HPV are being investigated for potential therapeutic use against HPV16+ CIN3 (NCT00788164). Combined treatment with TA-HPV vaccinia vaccine and TA-CIN protein vaccine has additionally shown limited immunotherapeutic effects in HPV-associated VIN (84).
2.2. Adoptive T cell Therapies
Adoptive T cell therapies (ACT), or T-cell based vaccines, is the practice of removing T cells from the host and expanding, modifying or selecting T cells for tumor antigen reactivity ex vivo, then reinfusing them back into the host so that the T cells will target tumor antigens to promote tumor regression (for review see (115,116)). There are three major categories of ACT: tumor-infiltrating lymphocytes (TILs), engineered T-cell receptor T cells (TCRs), and chimeric antigen receptor (CAR) T cells (117). Current efforts to target HPV E6 and E7 with therapeutic vaccines for the treatment of cervical cancer have yet to demonstrate meaningful success in treating advanced cervical cancer. As the knowledge and understanding of tumor immunology has expanded exponentially over the last three decades, the complicated relationships between tumor cells, the tumor microenvironment, and immune cells, including cytotoxic T cells, helper T cells, and regulatory T cells, have only begun to become untangled. Recent research on the multitude of effects and heterogeneity of innate myeloid effector cells have suggested the importance of antitumor myeloid populations in cancer progression. Several studies have associated certain myeloid cell types with patient survival and responses, indicating that myeloid cells in the TME is still ripe for further investigation (for review see (118)). Despite this ongoing research, T cells are considered to be major effector cells of immune-mediated cancer regression. For this reason, ACT therapies are highly attractive. ACT has been shown to facilitate complete clinical responses in some patients with B-cell malignancies and metastatic melanomas; however, its success in epithelial malignancies has been limited and it remains to be seen whether ACT can mediate the regression of metastatic cervical cancer (119). Although ACT is a promising cancer treatment strategy and shows encouraging results in preclinical and clinical trials (117), there is still much work to be done for solid tumors such as cervical cancers.
HPV-associated cervical cancer is theoretically a strong candidate for ACT therapy. ACT requires a tumor-specific antigen for the T cells to target, and cervical cancers express HPV oncogenes. These HPV oncogenes can serve as targets for ACT therapies with significant tumor homing and low chance of the therapy targeting uninfected host tissue, a common toxicity related to ACT (115). Several ACTs have been tested in preclinical and clinical trials. ACTs bypass the immune reliance on functional host dendritic cells and APCs to activate T cells, and instead ACTs modify T cells directly. Additionally, ACT can be constructed to overcome immune tolerance and incorporate modified tumor-specific T cells, which may not result from conventional therapeutic vaccination (120). A further benefit of ACT is their ability to act as highly personalized therapeutic treatments with significant clinical potential (119). Despite potential advantages, ACTs come with drawbacks. These therapies come with risk of cytokine release syndrome (CRS), neurotoxicity, and off-tumor targeting toxicity (116,121). Prior to injecting the patient with these therapies, they may require temporary lymphodepletion (115). However, the individualized and immunogenic promises of ACT therapies ensure they remain one of the most intriguing immunotherapies for cancer research.
2.2.1. Tumor Infiltrating Lymphocytes
Tumor infiltrating lymphocytes (TILs) are T cells isolated from a tumor mass, expanded ex vivo, often cultured with IL-2 to permit reactive tumor antigen selection, then infused back into the patient (115,117,122). Since the late 1980s, TILs have been shown to control tumor regression in patients with metastatic melanoma (42,123). Recently, TIL therapy has been investigated for several other types of cancer such as gastrointestinal, lung, and HPV-associated malignancies (124). TILs selected for HPV oncogenes E6 and E7 have been shown to cause tumor regression in patients with metastatic cervical cancer (119).
Efficacy of TIL therapy can be improved by enhancing the reactivity toward defined cancer antigens (122). Unfortunately TILs are labor intensive, have low success rates, and have impaired functionality in tumor microenvironments that are overly immunosuppressive (44). TIL therapy is labor and time intensive as the treatment requires isolating tumor-reactive T cells from excised tumor and expanding them.
There have been several trials that have investigated the use of TILs in cervical cancer. One preclinical study isolated mononuclear cells from tumor draining lymph nodes from patients with HPV-associated cervical cancer. The resulting cells were cultured for HPV E6 specificity, then analyzed using assays, flow cytometry, and tested for CD25 and FoxP3 expression. The process resulted in many HPV16 E6 and E7 specific reactive T cells, which could be implicated for use in future clinical trials (125). A Phase II clinical trial investigated the use of TILs in HPV-associated cancers. After lymphocyte depleting chemotherapy, TILs were selected for HPV E6/E7 reactivity and administered to the patient with aldesleukin. Three of nine patients demonstrated an objective tumor response, two patients who had responses that lasted over a year, and additional toxicity was limited (119). However, there is a possibility that although the TILs were selected for HPV-specific antigen reactivity, cervical cancer cells do express other tumor antigens that could have also been targeted by the expanded T cells (44). Future research using TILs that are only HPV-specific could elucidate whether the regression was due to exclusively HPV-targeting (44).
An ACT approach similar to TIL administration is Cytokine induced killer cell (CIK) therapy. CIK therapy, rather than using immune cells located in tumor tissue, cultures and expands peripheral blood mononuclear cells (PBMCs), and then infuse the patient with the killer cells. This approach is simpler than other ACT methods, and still permits tumor homing and spares most non-cancerous host tissue, and has shown efficacy in several solid and hematologic malignancies (116). Within the context of cervical cancer, CIK therapy in conjunction with radiochemotherapy demonstrated short-term efficacy to treat cervical cancer (126) warranting further investigation.
2.2.2. Engineered T cell Receptor T cells
An alternate approach to ACT is the use of engineered T cell receptors (TCRs). TCR therapy is unique from TIL therapy as instead of expanding tumor-specific T cells that were already present in the body, host derived T cells are modified via genetic engineering to express a specific tumor-targeting T cell receptor (117). TCR cells can be additionally used to help bypass immune tolerance of the tumor. These cloned, tumor-specific TCR T cells that recognize specific antigen peptides bound to either MHC I or II are infused back into the host after expansion (124).
TCR therapy relies on generation of T cell receptor α and β chains that recognize tumor targets, and the expression of these engineered TCR molecules in autologous T cells (121). Therefore, the ability of engineered TCR cells to identify tumor cells depends on the cell surface abundance of alpha/beta heterodimer and receptor affinity of the target antigen (121). Modified promotors or mutations in the α and β chains are two possible strategies to optimize the efficacy of engineered TCR T cells (121). Engineered TCRs are then activated via the same signal transduction mechanisms as non-engineered T cells (124). Emerging evidence appears to demonstrate the ability of engineered TCR T cells to recognize E6 and E7 positive tumor cells (117).
A completed Phase I/II trial investigated the use of E6 engineered TCR T cells in HPV16+ cancers, delivered in conjunction with lymphocyte depletion and IL2. There were no major adverse events, and the results suggested that E6 TCR therapy may cause regression of HPV-associated epithelial cancers, and warrants further study (127). Several other clinical trials are investigating the use of HPV oncoprotein TCR T cells in cervical cancer, and are set to be finished over the next couple of years (NCT03578406, NCT04476251, NCT02858310, Table 2). Despite ongoing clinical trials, most success with TCR therapies have targeted hematologic malignancies, and overall TCR therapeutic effect on solid tumors as well as HPV associated malignancies is underexplored (65).
Table 2.
Summary of clinical trials using strategies to generate and enhance T-cell priming: T-cell therapies
| Immunotherapy Treatment(s) | Additional Therapy (chemo, radiation, chemical therapies) | Patient Population (n) | Phase of Clinical Trial | NCT Number |
|---|---|---|---|---|
| HPV E6-specific TCR-T Cells (engineered T Cells that recognize E6 HPV antigen, either including or excluding an Anti-PD-1 antibody element) | HPV+ metastatic or recurrent cervical or head and neck cancer patients (20 participants) | Phase 1 | NCT03578406 | |
| E7 TCRs (engineered T cells that recognize E7 HPV antigen) | HPV16+ IIB-IVA cervical cancer patients (180 participants) | Phase 1 | NCT04476251 | |
| E7 TCRs (Engineered T cells that recognize E7 HPV16 antigen) Aldesleukin (IL-2) |
Fludarabine, Cyclophosphamide | HPV16+ metastatic or refractory/recurrent cervical, vulvar, penile, anal, and oropharyngeal cancer patients (180 participants) | Phase 1/2 | NCT02858310 |
| Cervical cancer specific CAR-T Cells (PBMCs of patients who have GD2, PSMA, Muc1 or Mesothelin positive cervical cancer will be obtained through apheresis, and T cells will be activated and modified to cervical cancer-specific CAR-T cells) | Stage III, IV, or relapsed cervical cancer (20 participants) | Phase 1/ 2 | NCT03356795 |
2.2.3. Chimeric Antigen Receptor (CAR) T Cells
CAR T cell therapy is a branch of ACT that requires genetic redirection of T cell specificity via the introduction of a synthetic recognition structure to the host T cell called a chimeric antigen receptor (CAR) (124). A major benefit to CAR T cell therapy is that it does not require an intact MHC presentation system on cancer cells. This is significant as the immunosuppressive TME can downregulate of MHC presentation (115) (128,129), which renders other antigen specific immunotherapy that rely on MHC presentation ineffective. CAR T cells can therefore be used on tumors that are defective for antigen presentation and processing (124), unlike TILs, TCR therapy, or immunotherapeutic vaccines.
The CAR design aims to provide, therefore, an appropriate co-stimulatory signaling to activate effector T cells (128). CARs consist of an extracellular antigen recognition domain, a hinge domain, a transmembrane domain (TM), and an intracellular domain (130). The extracellular antigen recognition domain is derived from a single chain variable fragment (scFv) isolated from an antigen-specific monoclonal antibody (mAb), which enables the engineered T cell to bind to antigens expressed on tumor cells with preserved specificity and affinity. The hinge region permits CAR flexibility, ensures correct positioning of the binding domain during scFv-antigen interaction, and transduces crucial signals. The transmembrane domain influences CAR T cell function, and it is derived from CD3-z, CD4, CD8, OX40, or H2-Kb. The intracellular domain which delivers the signal is derived from lymphocyte signal initiating molecules. The signal then activates the effector function of the CAR T cells (128,130).
Currently four generations of CAR-T cells exist, and most have been used in hematological cancers rather than solid tumors (115). After the first generation showed poor persistence and clinical efficacy, one and two co-stimulatory molecules were added to the second and third generation, respectively (for review see (130)). The third generation CARs were built upon the increased T cell antitumor immunity of the second generation, with the addition of greater cytokine production and more tumor growth inhibition in mice (128). Despite these additions in the third generation, second generation CAR T cell therapy is used more widely in clinical trials because they have a greater activation threshold, which results in less on-target/off-tumor cytotoxicity in normal tissues (130). Finally, fourth generation CAR T cells include a cytokine expression cassette on the CAR construct vector, resulting in a chimeric T cell redirected for universal cytokine-mediated killing (TRUCKs) (128).
Clinical trials with second generation CD19 CAR T therapy has had promising effects for hematological malignancies, resulting in CD19 CAR T cell therapy (CTL019) being labeled a “breakthrough” therapy by the FDA (130). However, there are far fewer uses of CAR T therapy in solid tumors. There is currently one ongoing trial investigating the use of CAR T cells in cervical cancer. Unlike many other cervical cancer trials, rather than using an HPV antigen as a target for immunotherapy, the CAR T cells will be engineered for patients with GD2, PSMA, Muc1 or Mesothelin positive cervical cancer (NCT03356795, Table 2).
It is theorized that the histopathological structure and strong immunosuppressive environment of solid tumors limit the efficacy of CAR T cell therapy. These limitations have inspired several advancements in CAR-T cell therapy against solid tumors, including cytokine release, and target TAA modification (130). Nonetheless, until now CAR T cell therapy has not been shown to be an effective immunotherapeutic treatment for cervical cancer.
3. Strategies to Reverse Immunosuppression and Enhance Effector Immune Cells in the TME
Under normal, non-cancerous conditions, immunoregulatory factors such as immune checkpoint inhibitors, maintain self-tolerance, prevent autoimmunity, and protect healthy cells from immune attack or prolonged inflammation during infections. During this homeostasis, cellular immunity is regulated by activation signals (co-stimulatory molecules, as presented by APCs) as well as inhibition signals (immune checkpoints) (47). As tissues become cancerous, they undergo immune editing, the process by which cancerous cells are able to evade immune system elimination, permitting unchecked growth and spread. Cancer cells often exploit naturally occurring immune regulators to escape immune surveillance and create an immunosuppressive tumor microenvironment (TME), while downregulating anti-cancer activity by effector T cells. A major approach to immunotherapy is to augment and support extant immune cells within the TME. As opposed to enhancing T cell priming, which boosts the number of T cells, augmenting effector immune cells targets mechanisms that promote pre-existing cytotoxic activity and hinder mechanisms that limit effector cell activity. These strategies are not specific for any antigens, and do not involve in the de novo generation of cervical cancer-specific T cells.
3.1. PD-1 and CTLA-4 Immune Checkpoint Blockades in Cervical Cancer
Immune checkpoints may be modulated by either agonist or antagonist monoclonal antibodies used to enhance T cell activation and eliminate inhibition of T cell activation respectively in order to reactivate T cells to attack tumors (46). A common approach to many cancer immunotherapies is the use of antagonist antibodies that target programmed cell death receptor (PD-1) and cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), which have been extensively studied (131,132). The PD-1/PD-L1 axis is a major immune checkpoint mechanism. PD-1 is expressed on the surface of effector immune cells that binds programmed cell death receptor ligand (PD-L1) expressed by tumor cells or immunosuppressive cells in TME. The FDA has approved PD-1 inhibitory antibody pembrolizumab for cervical cancer following the KEYNOTE-158 clinical trial, a significant harbinger for its possible utility for metastatic/recurrent cases. In the KEYNOTE-158 trial, 98 patients with previously treated advanced cervical cancer were given 200mg of pembrolizumab every 3 weeks until progression, intolerable toxicity, patient/physician decision, or two years had passed. Of these patients, 82 had PD-L1 positive tumors. The overall response rate (ORR) was 12.2%, and all of the patients who experienced complete or partial responses had PD-L1 positive tumor. For patients who received at least one chemotherapy treatment, the ORR rose to 14% (48). In addition to demonstrating the therapeutic benefits of pembrolizumab alone, the increased ORR for patients who received standard-of-care chemotherapy suggests the potential of combining immunotherapy strategies with other cancer therapeutic modalities, further discussed in a later section.
PD-L1, the ligand for PD-1 that can be highly expressed on tumor cells, is another major target molecule for antagonist monoclonal antibody immune checkpoint inhibitor (ICI) therapy. When PD-L1 binds PD-1, T cells are deactivated. Binding of the PD-1/PD-L1 axis results in the inactivation of many immune cells, including CD8+ T cells (133). Under typical conditions, PD-L1 is induced by IFN-gamma and works to protect surrounding tissue from unwarranted and prolonged T cell mediated cytotoxicity (132,133). Several studies have indicated that virus-induced cancers, and more specifically, HPV-associated cervical cancers and cervical intraepithelial neoplasia, may upregulate PD-L1 (133–135). This upregulation was not found in non-HPV infected benign cervical tissues. Current FDA approved anti-PD-L1 antibodies include durvalumab, avelumab, and atezolizumab, although none have yet been approved for use in cervical cancer.
Several other trials to date have demonstrated the potential anti-tumor therapeutic benefit of using ICIs for the treatment of cervical cancer. PD-L1/PD-1 inhibitors have shown promising overall response rates in cervical cancer patients (For review see (133)). One case study suggested that chemotherapy in combination with pembrolizumab is well tolerated and potentially effective in stage IVB cervical cancer (136). This finding was corroborated by a study on human clinical samples before and after chemotherapy that found that cisplatin based chemotherapy can upregulate PD-L1 in cervical cancer, and that the use of checkpoint blockade immunotherapy may thereby aid tumor regression (137). Nivolumab and pembrolizumab have both demonstrated clinical benefit in clinical trials, and they are well tolerated in recurrent or metastatic cervical cancer patients (138,139).
Alternatively, CTLA-4 is expressed on the surface of T cells and, once bound to its ligands CD80 or CD86 that are found on APCs, downregulates T cell cytotoxicity (140). CTLA-4 contributes to the attenuation of activating T cells (141). This axis also plays a significant role in autoimmune diseases (142), but in the immunosuppressive TME the inhibition of CTLA-4 by ICI antibodies (Ipilimumab, tremelimumab) helps reverse immune system suppression and promotes tumor clearance (140,143). Whereas PD-1/PD-L1 blockade functions primarily by slowing CD8+ cell exhaustion, CTLA-4 ICI functions by activating new T cells and suppressing Tregs (46).
An ongoing phase I/II clinical trial in patients with metastatic/recurrent cervical cancer indicates that ipilimumab, a CTLA-4 inhibitor, is well tolerated, and can promote immune activation (144). Other ongoing clinical trials are investigating the use of different immune checkpoint inhibitors in cervical cancer (NCT03192059, NCT02257528, NCT03508570, NCT03833479, NCT04256213, Table 3) and in patients with cervical, vaginal, or vulvar HPV-associated lesions (NCT04211103, Table 3). Furthermore, ICIs can be used in tumors regardless of whether they are HPV-associated or not because they can potentiate both HPV-specific T cells and neoepitope-specific T cells. Therefore, several clinical trials using ICIs include cervical cancer, but do not require specific HPV-association or status. Clinical trials use the immunotherapeutic pembrolizumab in advanced solid tumors, including but not limited to cervical or gynecologic cancers (NCT02054806, NCT02628067, Table 3). One clinical trial aims to treat patients with advanced cancers using a combination of anti-PD-L1 monoclonal antibody (durvalumab) and anti-CTLA-4 monoclonal antibody (tremelimumab) (NCT01975831, Table 3).
Table 3.
Summary of clinical trials using strategies to reverse immunosuppression and enhance effector immune cells in TME
| Immunotherapy Treatment(s) | Additional Therapy (chemo, radiation, chemical therapies) | Patient Population (n) | Phase of Clinical Trial | NCT Number |
|---|---|---|---|---|
| Pembrolizumab (anti-PD-1 antibody) | Radiation, Vitamin D, Aspirin, Lansoprazole, Cyclophosphamide, Curcumin | Refractory or persistent endometrial, cervical, or uterine cancer patients (43 participants) | Phase 2 | NCT03192059 |
| Nivolumab (anti-PD-1 antibody) | Persistent, recurrent, metastatic cervical cancer (26 participants) | Phase 2 | NCT02257528 | |
| Ipilimumab (anti-CTLA-4 antibody) Nivolumab (anti-PD-1 antibody) |
Recurrent gynecologic cancer patients, including cervical (48 participants) | Phase 1 | NCT03508570 | |
| TSR-042 (anti-PD-1 antibody) | High risk locally advanced cervical cancer patients (132 participants) | Phase 2 | NCT03833479 | |
| Nivolumab (anti-PD-1 antibody) Ipilimumab (anti-CTLA4 antibody) |
Stages IBIII-IVA cervical cancer patients (40 participants) | N/A | NCT04256213 | |
| Pembrolizumab (anti-PD-1 antibody) | Cervical, vaginal, or vulvar HPV+ lesions (45 participants) | Phase 2 | NCT04211103 | |
| Pembrolizumab (anti-PD-1 antibody) | Solid tumor, including cervical (477 participants) | Phase 1 | NCT02054806 | |
| Pembrolizumab (anti-PD-1 antibody) | Advanced solid tumor patients, including cervical (1595 participants) | Phase 2 | NCT02628067 | |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) |
Ovarian, colorectal, breast, renal, or cervical cancer patients (104 participants) | Phase 1 | NCT01975831 | |
| INBRX-106 (OX40 agonist) Pemrolizumab (anti-PD-1 antibody) |
Solid tumor patients, including cervical for part 1 dose escalation cohort (150 participants) | Phase 1 | NCT04198766 | |
| mRNA-2752 (OX40 agonist, IL-23, Il-36γ) Durvalumab (anti-PD-L1 antibody) |
Solid tumor patients, including cervical cancer patients for dose escalation phase (126 participants) | Phase 1 | NCT03739931 | |
| INCAGN01949 (OX40 agonist) Ipilimumab (anti-CTLA-4 antibody) Nivolumab (anti-PD-1 antibody) |
Advanced malignancies patients, including cervical cancer patients (52 participants) | Phase 1/2 | NCT03241173 | |
| ASP1951 (GITR Agonist) Pembrolizumab (anti-PD-1 antibody) |
Advanced solid tumor patients, including cervical (435 participants) | Phase 1 | NCT03799003 | |
| INCAGN10876 (GITR Agonist) Nivolumab (anti-PD-1 antibody) Ipilimumab (anti-CTLA-4 antibody) |
Advanced or metastatic cancer patients, including cervical (45 participants) | Phase 1/2 | NCT03126110 | |
| Durvalumab (anti-PD-L1 antibody) Remelimumab (anti-CTLA-4 antibody) PolyICLC (TLR3 agonist) |
Advanced, measurable, biopsy-accessible cancers, including cervical (102 participants) | Phase 1/2 | NCT02643303 | |
| INCAGN02390 (anti-TIM3 antibody) | Advanced cancer patients, including cervical (40 participants) | Phase 1 | NCT03652077 | |
| INCAGN02385 (anti-LAG3 antibody) | Advanced cancer patients, including cervical (22 participants) | Phase 1 | NCT03538028 | |
| Daratumumab (anti-CD38 antibody) Relatlimab (anti-LAG3 antibody) Ipilimumab (anti-CTLA-4 antibody) Nivolumab (anti-PD-1 antibody) |
Patients with one of seven listed tumor types, including squamous cell carcinoma of the cervix and recurrent, metastatic cervical SCC patients (584 participants) | Phase 1/2 | NCT02488759 | |
| TTX-030 (CD39 enzymatic inhibitor) Budigalimab (anti-PD-1 antibody) |
MFOLFOX6, Docetaxel | Solid tumor patients, including cervical (152 participants) | Phase 1 | NCT04306900 |
| CPI-006 (CD73 inhibitor) Ciforadenant (anti-adenosine 2A receptor antibody) Pembrolizumab (anti-PD-1 antibody) |
Advanced cancers patients, including cervical cancer patients (378 participants) | Phase 1 | NCT03454451 | |
| XmAb22841 (Bifunctional CTLA-4 inhibitor and LAG3 inhibitor) Pembrolizumab (PD-1 inhibitor) |
Solid tumor patients, including cervical (242 participants) | Phase 1 | NCT03849469 | |
| AK104 (bispecific anti-PD-1/CTLA-4 antibody) | Recurrent, metastatic cervical cancer patients (40 participants) | Phase 2 | NCT04380805 | |
| RO7247669 (anti-PD-1 and anti-LAG3 bispecific inhibitory antibody) | Solid tumor patients (320 participants) | Phase 1 | NCT04140500 | |
| SL-279252 (Bispecific anti-PD-1 antibody and OX40 agonist) | Solid tumor or lymphoma patients, including cervical (87 participants) | Phase 1 | NCT03894618 | |
| Temsirolimus (mTOR inhibitor) | Cervical cancer patients (38 participants) | Phase 2 | NCT01026792 | |
| Celecoxib (COX2 inhibitor) | Cervical neoplasms (31 participants) | Phase 1/2 | NCT00152828 | |
| Celexoxib (COX2 inhibitor) | CIN 2/3, cervical carcinoma patients (130 participants) | Phase 2 | NCT00081263 | |
| INCB001158 (arginase inhibitor) Pembrolizumab (anti-PD-1 antibody) |
Solid tumor patients, including cervical cancer patients for dose determination (260 participants) | Phase 1/2 | NCT02903914 | |
| Bevacizumab (anti-VEGF antibody) | Cisplatin, Paclitaxel, Topotecan Hydrochloride | Recurrent, persistent, or stage IVB cervical cancer patients (452 participants) | Phase 3 | NCT00803062 |
| Atezolizumab (anti-PD-L1 antibody) Bevacizumab (anti-VEGF antibody) |
Recurrent, persistent, or metastatic cervical cancer patients (11 participants) | Phase 2 | NCT02921269 | |
| Atezolizumab (anti-PD-L1 antibody) Bevacizumab (anti-VEGF antibody) |
Solid tumor patients, including HPV+ cervical cancer patients (164 participants) | Phase 2 | NCT03074513 | |
| M7824 (drug that binds PD-L1 and neutralizes TGFβ) Bevacizumab (anti-VEGF antibody) |
Carboplatin, Paclitaxel | Cervical cancer patients (24 participants) | Phase 1 | NCT04551950 |
| Bevacizumab (anti-VEGF antibody) | Paclitazel Albumin-stabilized Nanoparticle Formulation | Stage IV gynecological cancers that cannot be removed, including cervical cancers (36 participants) | Phase 1 | NCT02020707 |
Although ICIs are generally well-tolerated, immune checkpoint inhibition can cause toxicities and immune related adverse events (irAEs), which are more specific to immunotherapy and are not associated with other forms of cancer therapy such as radiotherapy or chemotherapy. Common toxicities associated with immune checkpoint inhibition include diarrhea, colitis, rash, thyroid dysfunction, decreased appetite, fatigue, hepatitis, and pneumonitis (46,145). Other less common irAEs include arthritis, myositis, vasculitis, polymyalgia rheumatic-like syndrome, systemic sclerosis, sicca syndrome, and systemic lupus, but these conditions are more common in individuals with preexisting autoimmune disorders or autoantibodies (146).
3.2. Other Checkpoints to Augment Effector T Cells
In addition to antibodies targeting the PD-1/PD-L1 axis and CTLA-4, other therapeutic antibodies for the treatment of cervical cancer are also being actively investigated. Agonist antibodies to support functioning of OX40, GITR, and TLR3 have been explored for the treatment of cervical cancers, although no such treatment has yet been approved for use in cervical cancer (NCT04198766, NCT03739931, NCT03241173, NCT03799003, NCT03126110, NCT02643303, Table 3). OX40 is expressed by activated immune cells, and using an agonist antibody can facilitate its T cell stimulatory action and increase numbers of activated effector T cells (for review see (147)). Studies in a preclinical cervical cancer model (TC-1) demonstrated that OX40 agonists might have therapeutic benefit for HPV16-associated cancers, but that combination with PD-1 antagonist antibodies may be detrimental (148). Early results from a clinical trial of OX40 agonists in patients with solid tumors, including both HPV-positive and –negative cervical cancer patients, found no early clinical response, but did result in increases in Ki67+ CD4 and CD8 T cell populations and a 60% reduction in OX40+ FOXP3+ regulatory T cell populations (149). Enhanced expression of Glucocorticoid-Induced TNFR-Regulated protein (GITR) by immune cells in HPV-positive human cervical tissue samples indicates that GITR can serve as a disease progression biomarker. In addition, it is a target for GITR antibody immunotherapy (150), as GITR agonists can boost effector T cells by activating CD25, IL-2, IFN-γ, and inhibit suppressive Treg functioning (151). Several non-cervical cancer preclinical models have demonstrated the potential benefit of GITR immunotherapy as well (151).
In addition to PD-1 and CTLA-4, other antagonist antibodies under investigation include those for LAG3, TIM3, CD39, and A2AR (NCT03652077, NCT03538028, NCT02488759, NCT04306900, NCT03454451, Table 3). High LAG3 can inhibit cytotoxic T cell functioning and promote Treg immunosuppression, in addition to a myriad of other functions that decrease cytotoxic T cell functioning, and preclinical models have demonstrated increased tumor specific immune responses to anti-LAG3 antibodies (for review see (152). LAG3 is a promising target for immunotherapy because cervical cancer tissue samples, especially HPV-associated cervical cancer samples, have demonstrated high LAG3 expression (153). Likewise, cervical cancer samples have high TIM3 expression, and TIM3 may promote metastasis of cervical cancer (154). TIM3 is expressed on multiple T cell types, and its expression is associated with highly suppressive Tregs and can signal exhaustion of cytotoxic T cells (for review see (155)). Several preclinical non-cervical cancer models have demonstrated the potential benefit of anti-TIM3 immunotherapy in cancers with high TIM3 expression (155). Finally, CD39/CD73 and A2AR molecules are closely related: transmembrane CD39 facilitates adenosine production, which in turn signals Adenosine 2A Receptor (A2AR) to downregulate immune system functioning. Therefore, inhibiting a step in this pathway could enhance T cell functioning. HPV16 positive CIN patient samples have high CD39 and CD73 expression (156), and cervical cancer cell lines similarly displayed CD39 and CD73 expression (157). CD39 expression can be upregulated on tumor cells or expressed on T cells, including Tregs, to downregulate immune system function. Anti-CD39 immunotherapy is still under development, and clinical trials have begun exploring its use for antitumor immunity (158). However, recent findings suggest that CD39 is not always a marker for immunosuppression. CD39 has been associated with tumor-infiltrating CD8 and CD4 effector T cells that can recognize overexpressed self-antigen or neoantigens (159,160). CD39 expression has been associated with improved clinical prognosis in colorectal, lung, and head and neck cancer, and more recently, HPV-reactive CD39+/CD4+ T cells have been associated with improved clinical prognosis in HPV-associated vulvar, oropharynx, and cervical cancers (161). CD39 may not solely be a marker of immune exhaustion. Monoclonal antibodies for CD39 may thus have a myriad of anti-tumor mechanisms to reflect its wide array of functioning across categories of immune cells, from B cells, Tregs, innate immune cells, and tumor-infiltrating non-Treg CD4 and CD8 cells (160). Likewise, preclinical cervical cancer models have suggested that A2AR may influence the immunosuppressive cervical environment (162), suggesting that A2AR antibodies may be beneficial immunotherapy (for review see (163).
3.3. Suppressive Immune Cells
An additional immunotherapeutic approach is to target immunosuppressive and cancer-promoting factors arising from the cervical cancer cells, specifically inhibiting suppressive immune cells and limiting certain immunosuppressive metabolites and stroma molecules. Immunotherapies that target immunosuppressive factors within the tumor microenvironment can reverse immunosuppressive environments, prevent further tumor growth, and permit immune cells to recognize cervical cancer cells. Targeting immunosuppressive factors in the TME does not rely upon cervical cancer specific antigen presentation. Although the majority of cervical cancers are associated with hrHPV types, this type of immunotherapy does not necessitate the generation of HPV antigen-specific T cells and can work on a broader range of cancers. Nevertheless, such immunotherapeutic strategy potentially can be used in conjunction with a strategy to boost tumor-specific immunity to further improve therapeutic antitumor effect (see section 4).
Some cervical immunotherapies can target suppressive immune cells that exist within the TME, including Tregs, tumor associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) (164). Strategies to prevent suppressive immune cells from gathering near tumor include c-MET inhibitors (165), IL-23 inhibitors (164) and PI3K receptor inhibition (166). The c-MET/HGF pathway has been associated with poor prognosis in cervical cancer (167). IL-23 has been associated with pro-tumor environments, specifically through the induction of Th17 cells (168), and cervical malignancies have been associated with increased levels of the cytokine (168–170). The PI3K/Akt/mTOR pathway additionally has been identified as a potential therapeutic target for cervical cancer (171–175). Several clinical trials targeting this aspect of the tumor microenvironment are being explored (Table 3).
Cervical cancer cells also manipulate metabolites within the TME, for example via arginases and IDO. Arginase inhibits T cell and NK cell proliferation within the TME, and its upregulation has been associated with cervical cancer immunosuppressive environments (176,177). INCB0011158 is being used in a clinical trial for solid tumors, including cervical tumors, to determine whether inhibiting arginase enzymatic activity can reverse immunosuppression (NCT02903914, Table 3). Another enzyme, indoleamine dioxygenase 2,3 (IDO), suppresses anti-cancer immune response via several downstream effects (178). In a preclinical cervical cancer model, the downregulation of IDO suppressed tumor growth and boosted NK cell activation (179). In a study of human cervical cancers, a significant number expressed IDO or had co-expression of PD-L1 and IDO (180). Taken with the preclinical model data, it appears that targeting the immunosuppressive metabolite IDO within the TME could potentially impact cervical cancer malignancies.
3.4. Angiogenesis Inhibition
Preventing vascularization of tumor tissues slows growth of tumors, which gives the immune system a greater chance of bringing the cervical cancer cells under control. Bevacizumab is an antiangiogenic molecule that inhibits vascular endothelial growth factors (VEGF), thereby preventing the growth and maintenance of new blood vessels. Fast growing cancerous cells that require vascularization can be inhibited by the use of bevacizumab. In 2014, bevacizumab was approved by several countries for its use in advanced cervical cancer following a successful clinical trial, GOG 240 ((181), NCT00803062, Table 3). Currently, chemotherapy in combination with bevacizumab is the first-line therapy for treatment of recurrent/metastatic cervical cancer (181,182). Given the efficacy of bevacizumab for cervical cancers, anti-angiogenetic drugs are still being explored and in combinations with other anti-immunosuppression treatments, such as atezolizumab (NCT02921269, NCT03074513, Table 3), or M7824, which is a PD-L1 and TGFβ inhibitor (NCT04551950, Table 3). Clinical trials are also examining the potential of anti-VEGF treatments in combination with chemotherapies: a phase I clinical trial using Bevacizumab and paclitaxel-albumin stabilized nanoparticle formulation chemotherapy is underway in stage IV gynecological cancers (NCT02020707, Table 3).
4. Combinational Therapy
Combining immunotherapeutic strategies can be advantageous. Rather than solely enhancing antigen specific T cells, targeting immunosuppression in the TME, or supporting effector immune cells, selecting multiple strategies can result in a broader, more effective approach. Combinational immunotherapy can comprise of one or more immunotherapeutic strategy, or an immunotherapeutic strategy in combination with standard-of-care therapies such as chemotherapy or radiation therapy. Since some standard of care treatments may result in heightened immunosuppression (183,184), combining immunotherapies to reverse the side effect may have a synergistic antitumor effect.
As mentioned, antigen specific immunotherapeutic strategies for cancer treatment can present a challenge because they require an intact and robust immune system to manipulate, which advanced cervical cancer patients may lack. Thus, therapeutic HPV vaccines targeting established HPV infections and/or precancerous lesions to prevent malignant progression may represent an easier endeavor than targeting advanced cervical cancer. Alternatively, combinatorial strategies with other immune modulation agents may become important approaches to boost the immune system in order to best combat advanced cervical cancer.
Combinational therapies can include combination of different immunotherapeutic approaches, combination of immunotherapy with chemotherapy, and combination of immunotherapy with radiotherapy. We will discuss these combinational therapies in the following.
4.1. Combination of Immunotherapeutic Approaches:
Antigen specific immunotherapeutic vaccines for cancer treatment can present a challenge because they require an intact and robust immune system to manipulate, and each can come with respective drawbacks, for instance DNA vaccines can have low immunogenicity, or vector-based vaccines may not be able to be administered more than once. Thus, combining antigen specific therapeutic vaccinations with antibodies to reverse immunosuppression and support effector T cells may result in a booster immune response.
In general, antibody-based immunotherapy can help reverse an immunosuppressive TME which clears a path for a vaccine to generate a robust antitumor effect and enhance the immunogenicity of otherwise weakly effective treatments (185,186). Clinical trials in multiple cancers have found that combination treatment can boost immune responses while retaining acceptable safety and tolerability (185). Within HPV-associated tumors, several preclinical studies have indicated that combining an anti-PD-1 antibody with therapeutic HPV16 E6/E7 vaccines results in greater tumor clearance and larger number of CD8+ T cells (187,188). Multiple clinical trials combining HPV antigen-specific vaccines with anti-PD-1/anti-PD-L1 have been initiated. Priming with Ad/E6E7 and boosting with MG1-E6E7 virus-based vaccines administered in combination with atezolizumab is currently being evaluated in a Phase I clinical trial for HPV-associated cancers (NCT03618953, Table 4). Another clinical trial conducted in 24 patients with HPV-positive cancers found that ISA101 vaccine in combination with nivolumab had acceptable overall response rates and durability (189). Notably, this trial demonstrated the combination treatment had a median overall survival (OS) of 17.5 months, and a 12-month OS of 70%, which is higher than PD-1 antibody treatment alone by nearly a factor of two (189). GX-188E, an HPV16/18 E6/E7 DNA vaccine delivered with electroporation, is being tested in a phase I/II trial alongside Keytruda (pembrolizumab) in HPV-associated advanced cervical cancer (NCT03444376, Table 4). At the interim report, patients reported manageable side effects and demonstrated encouraging antitumor activity, corroborating the hypothesis that combining therapeutic HPV vaccines and ICIs may be a potentially promising treatment (190). Other ongoing trials combining peptide vaccines and ICIs include Universal Cancer Peptide Vaccine (UCPVax) and atezolizumab (NCT03946358, Table 4) and PDS0101 multi-peptide HPV16 vaccine, IL-12, and M7824, a PD-L1 antibody (NCT04287868, Table 4). INO-3112 is currently being used in a clinical trial alongside Durvalumab for HPV-associated cancers, including cervical cancer (NCT03439085, Table 4). VB10.16, a therapeutic HPV DNA vaccine, is also in a clinical trial for patients with metastatic, recurrent, and persistent cervical cancer using atezolizumab as an adjuvant due to the upregulation of PD-L1 in cervical cancer (NCT04405349, Table 4).
Table 4.
Summary of clinical trials using a combination of immunotherapeutic strategies
| Immunotherapy Treatment(s) | Additional Therapy (chemo, radiation, chemical therapies) | Patient Population (n) | Phase of Clinical Trial | NCT Number |
|---|---|---|---|---|
| Ad/E6E7 (Adenovirus vaccine encoding HPVE6/E7) MG1-E6E7 (Maraba oncolytic virus encoding HPV E6 and E7) Atezolizumab (anti-PD-L1 antibody) |
HPV+ cancers, including cervical cancer (75 participants) | Phase 1 | NCT03618953 | |
| Electroporation of GX-188E (DNA vaccine that encodes HPV16/18 E6 and E7 antigen), Keytruda® (anti-PD-1 antibody) | HPV16/18+ advanced, non-resectable cervical cancer patients (60 participants) | Phase 1/2 | NCT03444376 | |
| Subcutaneous UCPVax (Universal Cancer Peptide Vaccine, induces DC4 T cells) Atezolizumab (anti-PD-L1 antibody) |
Advanced or metastatic HPV+ head and neck, cervical, or anal cancer patients (47 participants) | Phase 2 | NCT03946358 | |
| Subcutaneous PDS0101 (multi-peptide T-cell stimulating vaccine for targeting HPV16 E6 and E7 peptides) M7824 (PD-L1 antibody and TGF-beta) NHS-IL12 (IL-12) |
Advanced or metastatic HPV+ malignancies, including cervical cancer (40 participants) | Phase 1/2 | NCT04287868 | |
| Electroporation of INO-3112 (DNA vaccine encoding IL-12, HPV16/18 E6 and E7) Durvalumab (anti-PD-L1 antibody) |
HPV16/18+ cancer patients, including cervical (77 participants) | Phase 2 | NCT03439085 | |
| Intramuscular VB10.16 Vaccine (DNA vaccine encoding HPV18 E6 and E7) Atezolizumab (anti-PD-L1 antibody) |
Advanced, recurrent HPV16+ cervical cancer patients (50 participants) | Phase 2 | NCT04405349 | |
| LN-145 (adoptive cell therapy with autologous tumor infiltrating lymphocyte infusion followed by IL-2); Pembrolizumab (anti-PD-1 antibody) | Recurrent, metastatic, persistent cervical cancer (138 participants) | Phase 2 | NCT03108495 | |
| Vigil (GM-CSF to block furin and TGFβ production) Atezolizumab (anti-PD-L1 antibody) |
Advanced gynecological cancer, ovarian cancer, cervical cancer, and uterine cancer patients (25 participants) | Phase 2 | NCT03073525 | |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) |
Ovarian, colorectal, breast, renal, or cervical cancer patients (104 participants) | Phase 1 | NCT01975831 | |
| Daratumumab (anti-CD38 antibody) Relatlimab (anti-LAG3 antibody) Ipilimumab (anti-CTLA-4 antibody) Nivolumab (anti-PD-1 antibody) |
Patients with one of seven listed tumor types, including squamous cell carcinoma of the cervix and recurrent, metastatic cervical SCC patients (584 participants) | Phase 1/2 | NCT02488759 | |
| Young TIL (Tumor will be biopsied from the patient to generate autologous Tumor Infiltrating Lymphocytes, which will be isolated, grown and multiplied, and re-administered to the patient) Aldesleukin (IL-2) |
Fludarabine, Cyclophosphamide, | HPV16/18+ metastatic or locally advanced cancer patients, including cervical (29 participants) | Phase 2 | NCT01585428 |
| HPV16 E6 TCRs (Injection of autologous cells transduced with anti-HPV16 E6 T Cell Receptor) Aldesleukin (IL-2) |
Fludarabine, Cyclophosphamide | HPV16+ cancer patients, including cervical (12 participants) | Phase 1/2 | NCT02280811 |
| E7 TCRs (Engineered T cells that recognize E7 HPV16 antigen) Aldesleukin (IL-2) |
Fludarabine, Cyclophosphamide | HPV16+ cancer patients, including cervical (180 participants) | Phase 1/2 | NCT02858310 |
| Atezolizumab (anti-PD-L1 antibody) | Carboplatin, Cyclophosphamide | Advanced breast, cervical, ovarian, or endometrial cancer patients (12 participants) | Phase 1 | NCT02914470 |
| Camrelizumab (anti-PD-1 antibody) | Albumin-bound Paclitaxel | Recurrent uterine cervical cancer patients (34 participants) | Phase 2 | NCT04188860 |
| Pembrolizumab (anti-PD-1 antibody) | Carboplatin, Taxol | Stage IB2-IIB cervical cancer patients (45 participants) | Phase 2 | NCT04238988 |
| BCD-100 (anti-PD-1 antibody) Bevacizumab (Anti-VEGF antibody) |
Paclitaxel, Cisplatin, Carboplatin | Cervical cancer patients (316 participants) | Phase 3 | NCT03912415 |
| BCD-100 (anti-PD-1 antibody) Bevacizumab (Anti-VEGF antibody) |
Paclitaxel, Cisplatin, Carboplatin | Recurrent, metastatic cervical cancer patients (49 participants) | Phase 2 | NCT03912402 |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) |
Vinorelbine | Advanced solid tumor patients, including cervical cancer (150 participants) | Phase 1/2 | NCT03518606 |
| Pembrolizumab (anti-PD-1 antibody) Bevacizumab (anti-VEGF antibody) |
Cisplatin, Carboplatin, Paclitaxel | Cervical cancer patients (40 participants) | Phase 2 | NCT03367871 |
| Pembrolizumab (anti-PD-1 antibody) | Cabozantinib | Recurrent, metastatic, or persistent cervical cancer patients (39 participants) | Phase 2 | NCT04230954 |
| Atezolizumab (anti-PD-L1 antibody) | Stereotactic Body Radiation therapy | Advanced, recurrent, metastatic cervical cancer patients (26 participants) | Phase 2 | NCT03614949 |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) |
Radiosurgery | Recurrent, metastatic cervical, vaginal, or vulvar cancer patients (18 participants) | Phase 1 | NCT03452332 |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) |
Radiation therapy | Metastatic or recurrent gynecologic cancer patients, including cervical (32 participants) | Phase 1 | NCT03277482 |
| Pembrolizumab (anti-PD-1 antibody) | Radiation, Vitamin D, Aspirin, Lansoprazole, cyclophosphamide, curcumin | Refractory or persistent endometrial, cervical, or uterine cancer patients (43 participants) | Phase 2 | NCT03192059 |
| Pembrolizumab (anti-PD-1 antibody) | Brachytherapy Radiation, Cisplatin | Locally advanced cervical cancer patients (88 participants) | Phase 2 | NCT02635360 |
| Copanlisib (PI3K inhibitor): Copanlisib Hydrochloride (PI3K inhibitor) Nivolumab (anti-PD-1 antibody: PI3K-beta Inhibitor GSK2636771 Sunitinib Malate (anti-VEGF receptor 2): |
Adavosertib; Afatinib; Afatinib Dimaleate; Binimetinib; Capivasertib; Crizotinib; Dabrafenib; Dabrafenib Mesylate; Dasatinib; Defactinib; Defactinib Hydrochloride; Ipatasertib Erdafitinib; Taselisib; FGFR Inhibitor AZD4547; Larotrectinib; Larotrectinib Sulfate; Osimertinib; Palbociclib; Pertuzumab; Sapanisertib; Trametinib; Trastuzumab; Trastuzumab Emtansine; Ulixertinib; Vismodegib | Solid tumor, lymphomas, and myelomas patients, including cervical cancer (6452 participants) | Phase 2 | NCT02465060 |
| RO6874281 (Fibroblast activation protein-alpha (FAPα) inhibitor, IL-2) Atezolizumab (anti-PD-L1 antibody) |
Gemcitabine, Vinorelbine | Advanced, metastatic head and neck, oesophageal, or cervical cancer patients (256 participants) | Phase 2 | NCT03386721 |
| N-803 (IL-15 super agonist) Pembrolizumab (anti-PD-1 antibody) Nivolumab (anti-PD-1 antibody) Atezolizumab (anti-PD-L1 antibody) Durvalumab (anti-PD-L1 antibody) PD-L1 targeting high-affinity NK cells (thANKs) |
Solid cancer patients, including cervical (636 participants) | Phase 2 | NCT03228667 | |
| Durvalumab (anti-PD-L1 antibody) Tremelimumab (anti-CTLA-4 antibody) PolyICLC (TLR3 agonist) |
Advanced, measurable, biopsy-accessible cancers, including cervical (102 participants) | Phase 1/2 | NCT02643303 | |
| Electroporation of GX-188E (DNA vaccine that encodes HPV16/18 E6 and E7 antigen), Keytruda® (anti-PD-1 antibody) | HPV16/18+ metastatic cervical cancer patients (60 participants) | Phase 1/2 | NCT03444376 | |
| Electroporation of INO-3112 (DNA vaccine encoding IL-12, HPV16/18 E6 and E7) Durvalumab (anti-PD-L1 antibody) |
HPV16/18+ cancer patients, including cervical (77 participants) | Phase 2 | NCT03439085 | |
| Filgrastim (Activates G-CSF receptor, promotes neutrophilic granulocytes) Pegfilgrastim (long acting filgrastim) Veliparib (PARP inhibitor) |
Persistent or recurrent cervical cancer patients (27 participants) | Phase 2 | NCT01266447 | |
| Vigil (reduces TGF-β) Durvalumab (anti-PD-L1 antibody |
Breast, ovarian, fallopian tube, primary peritoneal, uterine, cervical, or endometrial cancer patients (13 participants) | Phase 2 | NCT02725489 |
Few ACT therapies have shown significant efficacy against solid tumors, such as cervical cancer. Some ACT therapies require immunodepletion of naturally circulating lymphocytes prior to infusion and administration of IL-2. An ongoing phase II trial of TIL infusion therapy and IL-2 following pembrolizumab in patients with recurrent, metastatic, or persistent recurrent cervical cancer is underway (NCT03108495, Table 4). Alternatively, Vigil is a form of ACT that amplifies T cells extracted from patient tumor, transfected with an immune-stimulatory cytokine, GM-CSF, and a hairpin RNA that knocks down the expression of several growth factors (TGFβ-1 and TGFβ-2), before being reinfused in the patient. The combination of Vigil and atezolizumab is being investigated for gynecological cancers (NCT03073525, Table 4).
Combining ICI antibodies that aim to inhibit different immunosuppressive factors and to enhance T cell functioning for the treatment of cervical cancer is currently ongoing, as it is possible to modulate the TME and increase antitumor immunity without consideration HPV target antigens. For example, a phase I trial in patients with breast, ovarian, colorectal, renal, or cervical cancers is investigating the potential benefits of combining durvalumab (anti-PD-L1) with tremelimumab (anti-CTLA-4) (NCT01975831, Table 4). A clinical phase I/II study is exploring the use of different combinations of monoclonal antibodies in virus-associated cancer, including cervical cancers (NCT02488759). The study will use nivolumab (a PD-1 inhibitor) in combination with either ipilimumab (CTLA-4 inhibitor), relatilmab (anti-LAG-3 antibody), or daratumumab (anti-CD38).
4.2. Combination of Immunotherapy with Chemotherapy
To date, there have been limited studies on the potential benefit of combining chemotherapy and vaccines for cervical cancer. Combination of therapeutic HPV vaccine with chemotherapy has been shown to improve the therapeutic HPV effect by reducing the unfavorable immunosuppressive TME in a preclinical model (191). A clinical and a separate preclinical study found that chemotherapy, including carboplatin, paclitaxel, and cisplatin, can reduce immunosuppressant myeloid cells and promote inflammatory myeloid cells in HPV-associated tumor regions (192,193). This chemotherapy-induced reduction of immunosuppressant cells and promotion of effector cells can foster an environment for a vaccine to boost antigen-specific T cells. One virus based vaccine, TA-HPV, has been successfully used in conjunction with chemotherapy (cisplatin) in a preclinical TC-1 mouse model for HPV-associated cancers (194). Furthermore, one clinical trial administered ISA101 peptide vaccine with carboplatin and paclitaxel in women with advanced, recurrent, or metastatic cervical cancer in a phase II study. Overall, 77 patients received treatment. Nearly 43% of patients demonstrated tumor regression, and patients mounted a HPV16-specific T cell response (192). The combination was found to be tolerable, and the observation that chemotherapy reduced myeloid cells was confirmed (192). Despite these successes, a phase II clinical trial combining live-attenuated listeria vaccine ADXS11–001 with chemotherapy (cisplatin) did not find the combination treatment to be any more effective than therapeutic HPV vaccine treatment alone (195).
ACT therapies coupled with chemotherapy is additionally being tested for the treatment of HPV-associated cancers, including cervical cancer cervical cancer. Metastatic cervical cancer patients who had previously been treated with platinum-based chemotherapy or chemoradiotherapy were given one infusion of IL-2 and HPV16/18 E6/E7 TILs. Of nine patients, 3 had objective tumor responses, with two patients that experienced complete responses for 22 and 15 months (119) (NCT01585428, Table 4). However, no patients experienced a complete tumor response and only 2 patients experienced a partial response after treatment with E6 TCRs, aldesleukin, fludarabine, and cyclophosphamide (NCT02280811, Table 4). An ongoing trial in patients with HPV-associated cancers is using HPV E7 TCR cells with fludarabine, clyclophosmphamide, and aldesleukin, and results are expected in 2026 (NCT02858310, Table 4).
As mentioned above, chemotherapy can lead to an unfavorable immunosuppressive TME, and therefore ICIs can potentially reverse immunosuppression when used in combination with chemotherapy to improve therapeutic antitumor effect. Investigators are also researching combining antibody immunotherapies with only chemotherapy (NCT02914470, NCT04188860, NCT04238988, NCT03912415, NCT03912402, NCT03518606, NCT03367871, Table 4). For example, an ongoing phase II clinical study is investigating the use of pembrolizumab and cabozantinib in 39 patients with recurrent/metastatic/persistent cervical cancer (NCT04230954, Table 4).
4.3. Combination of Immunotherapy with Radiotherapy
Radiotherapy in combination with ICIs can be beneficial because radiation can cause cellular inflammatory damage, which prompts activation of tumor specific effector T cells. The inclusion of ICIs on top of this inflammation may enhance the effects of these T cells by reducing the inhibitory effects of the TME for a synergistic antitumor immune effect (For review see (196)). One phase II clinical trial uses radiation therapy in combination with atezolizumab in patients with advanced, recurrent, or metastatic cervical cancer (NCT03614949, Table 4). Several other clinical trials are underway to investigate the use of immunotherapeutic antibodies targeting the TME for cervical cancer with radiation therapy (NCT03452332, NCT03277482, Table 4). As immune system activation, suppression, and the TME are complicated, combinational approaches that target several immune checkpoints and/or enhance T cell activation are likely needed to improve control of cervical cancer. Additionally, the therapeutic benefits of combining ICIs, radiotherapy, and chemotherapy for the treatment of cervical cancer are also under investigation (136) in several clinical trials are evaluating such tri-modal treatment (NCT03192059, NCT02635360, Table 4).
In addition, immune checkpoint inhibitors in combination with radiation therapy have been explored in patients with cervical cancer, and the combination of strategies do not result in increased toxicity (136,196). For a full account of currently active clinical trials using combination of treatments, see Table 4.
5. Future Directions
In the United States, cervical cancer is one of only two cancers for which survival has not significantly improved since the 1970s (5). For individuals diagnosed with metastatic, recurrent, or persistent cervical cancer, five-year survival is <20%, and there are few treatment options (197,198). Therefore, there is a clear need for improved cervical cancer therapeutics. With the increasing understanding of strategies to generate cervical cancer antigen-specific T cells and approaches to reverse immunosuppression and support effector immune cells in the TME, we have opportunity to create better, safer treatments for the control of cervical cancer. Clearly there are many opportunities to improve treatment by combining different immunotherapeutic strategies in order to find synergistic therapeutic effects. Furthermore, immunotherapy can also be used in conjunction with conventional standard-of-care treatments, such as chemoradiation, to improve outcome.
One of the major limitations for the control of cervical cancer by immunotherapy is the delivery of immune cells, antibodies and drugs throughout the tumor microenvironment, thereby limiting efficacy. For example, CAR-T cells have been most effective in “liquid” hematological cancers and less effective in solid tumors, where their access to the entire tumor may be limited. Another example is so called “cold tumors”, which have low lymphocyte infiltration and immune activation. As a result, ICI therapy may be less effective in cold tumors than in tumors with some ongoing antitumor response and tumor-infiltrating immune cells (199,200). Without local immune cells expressing the relevant markers, antibodies such as anti-PD-L1 cannot significantly help clear tumors. Therefore, future research to address this limited access of key immune cells, antibodies and drugs to the tumor microenvironment can potentially lead to better control of cervical cancer.
HPV infection is a major etiological factor in the development of cervical cancer and is present in 95% of cases (1,15). Because of this, immunotherapeutic options have a clear target antigen that is ubiquitous in infected cells, foreign, and absent from healthy tissue (201). This simplifies antigen-specific immunotherapeutic development for cervical cancer relative to most other cancers, which have no well-defined, consistently expressed antigens. Cervical intraepithelial neoplasia is the immediate precursor of cervical cancer. Current standard of care surgical treatment prevents the majority of CIN2/3 from progressing to cervical cancer; however, there are potential reproductive ramifications and drawbacks (for review see (202,203)). Most therapeutic HPV vaccines target either high-grade intraepithelial lesions and/or cervical cancer. However, persistent high-risk HPV infection precedes the development of high-grade lesions and cancer, and currently there is no medical treatment to eliminate persistent HPV infection. Few vaccines in development focus on the elimination of high risk persistent HPV infection, although there is currently a phase II clinical trial underway for the control of persistent HPV16 infection (NCT03911076, Table 1). If a treatment for persistent HPV infection is developed, it would limit progression of HPV infection to high grade lesions and cervical cancer. Future development of immunotherapy that could better clear persistent high-risk HPV infections and prevent progression to cervical cancer remains of significant interest.
Although there are many clinical trials and preclinical investigations regarding the use of immunotherapy for the control of cervical cancer, so far only pembrolizumab has been approved for clinical use for advanced cervical cancer. With the FDA approval of pembrolizumab for the treatment of metastatic/recurrent cervical cancer, we expect there will be other similar immunotherapeutic approaches targeting different aspects of the immune environment as described in Figure 1 that can improve control of cervical cancer. We are hopeful for the future of cervical cancer immunotherapy given the breadth of novel treatments under development that focus on the generation of HPV antigen specific T cells and the modulation of tumor microenvironment to improve overall disease control and outcomes for cervical cancer patients with generally tolerable side effects.
Figure 1: Schematic overview of cancer immunotherapies to target cervical cancer.
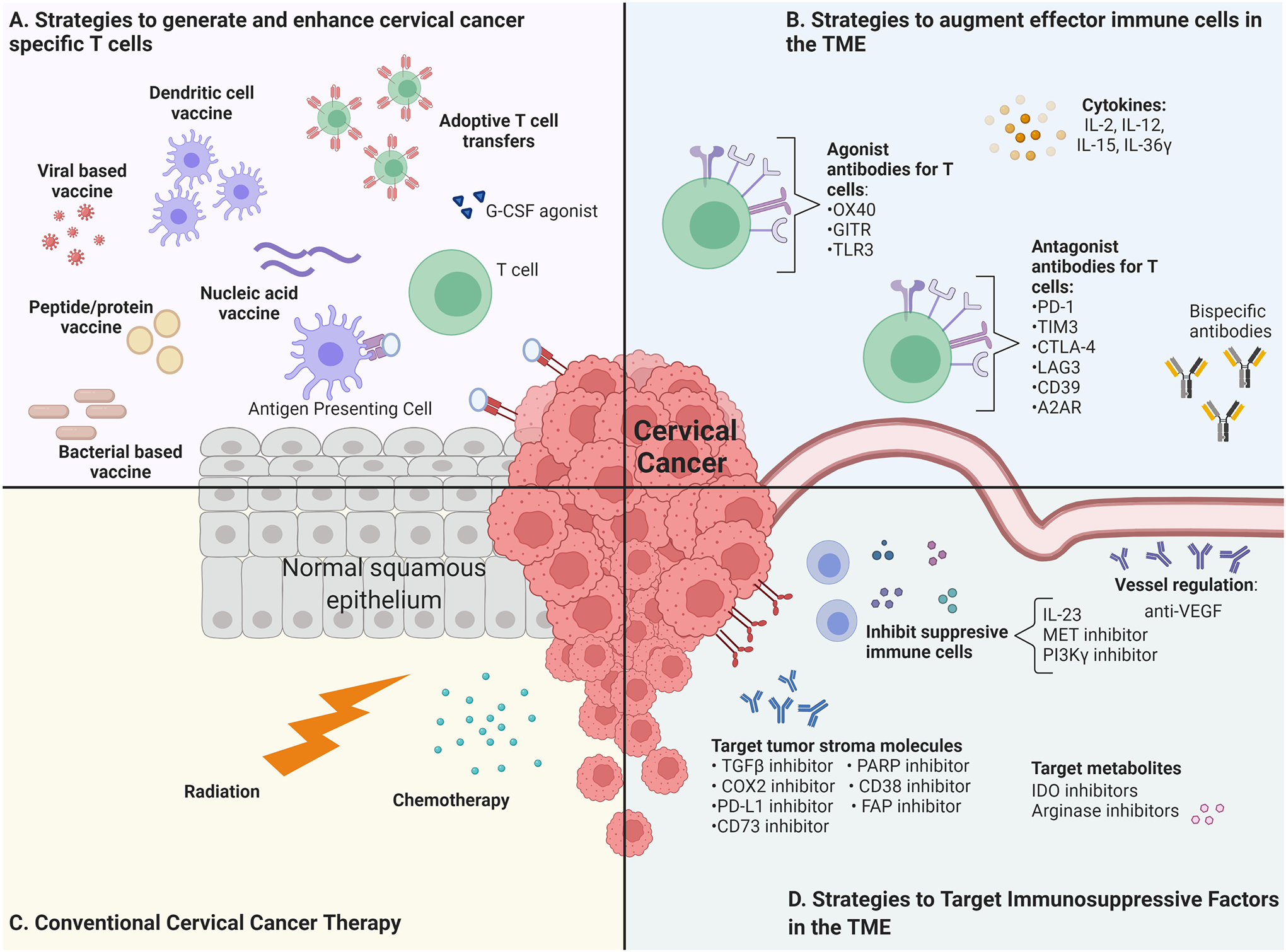
A). Strategies to generate and enhance cervical cancer specific T Cells. G-CSF: Granulocyte Colony Stimulating Factor. B). Strategies to augment effector immune cells in the TME. GITR: Glucocorticoid-Induced TNFR-Related protein; TLR3: Toll-Like Receptor 3; PD-1: Programmed cell Death protein 1; TIM3: T cell Immunoglobulin and Mucin-domain containing 3; CTLA-4: Cytotoxic T Lymphocyte Associated Protein 4; LAG3: Lymphocyte Activation Gene 3; A2AR: Adenosine 2A Receptor. C). Conventional cervical cancer therapies. D) Strategies to target immunosuppressive factors in the TME. MET: Mesenchymal Epithelial Transition factor; PI3K: Phosphoinositide 3-kinase; COX: Cyclooxygenase; PD-L1: Programmed cell Death protein Ligand; PARP: Poly ADP Ribose Polymerase; FAP: Fibroblast Activation Protein; VEGF: Vascular Endothelial Growth Factor; IDO: Indoleamine 2,3-dioxygenase. Figure created with BioRender.com.
Acknowledgments
Financial Support:
R.B.S. Roden, C.F Hung, and T.-C. Wu were supported by the National Institute of Health and the National Cancer Institute under award numbers R01CA237067 and P50CA098252.
Abbreviations list:
- HPV
Human PapillomaVirus
- hrHPV
high risk Human PapillomaVirus
- HDI
Human Development Index
- CIN 1/2/3
Cervical Intraepithelial Neoplasia 1/2/3
- LSIL
Low-grade Squamous Intraepithelial Lesion
- HSIL
High-grade Squamous Intraepithelial Lesion
- ACT
Adoptive Cell Therapy
- TIL
Tumor Infiltrating Lymphocytes
- TME
Tumor MicroEnvironment
- LLO
ListerioLysin O
- VIN
Vulvar Intraepithelial Neoplasia
- VAIN
Vaginal Intraepithelial Neoplasia
- MVA
Modified Vaccinia virus Ankara
- DC
Dendritic Cell
- MDSC
Myeloid-Derived Suppressor Cell
- IDO
Indoleamine DiOxygenase
- OS
Overall Survival
- APC
Antigen Presenting Cell
- HLA
Human Leukocyte Antigen
- ASC-US
Atypical Squamous Cells of Undetermined Significance
- ASC-H
Atypical Squamous Cells, cannot exclude High-Grade
- UCPVax
Universal Cancer Peptide Vaccine
- HSP70
Heat Shock Protein 70
- TA-CIN
Tissue Antigen-Cervical Intraepithelial Neoplasia vaccine
- TCR
engineered T-Cell Receptor T cells
- CAR
Chimeric Antigen Receptor
- CTLA-4
Cytotoxic T-Lymphocyte associated Antigen 4
- PD-1
Programmed cell Death receptor
- PD-L1
Programmed cell Death receptor Ligand
- ICI
Immune Checkpoint Inhibitor
- irAE
immune related Adverse Event
- GITR
Glucocorticoid-Induced TNFR-Regulated protein
- TAM
Tumor Associated Macrophages
Footnotes
Conflict of Interest Statement: Drs. Wu and Roden are co-founders of and have an equity ownership interest in Papivax LLC. In addition, Drs. Wu and Roden own Papivax Biotech Inc. stock and are a member of Papivax Biotech Inc.'s Scientific Advisory Board.
References
- 1.Small W Jr., Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, et al. Cervical cancer: A global health crisis. Cancer 2017;123(13):2404–12 doi 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 2.Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep 2014;16(9):402 doi 10.1007/s11912-014-0402-4. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8(2):e191–e203 doi 10.1016/s2214-109x(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30Suppl 5:F12–23 doi 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 6.Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol 2011;29:111–38 doi 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]
- 7.Board PDQATE. Cervical Cancer Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. [Google Scholar]
- 8.2020July31, 2020.Survival Rates for Cervical Cancer. American Cancer Society; <https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html#references>. Accessed 2020 July 31, 2020. [Google Scholar]
- 9.Petignat P, Roy M. Diagnosis and management of cervical cancer. Bmj 2007;335(7623):765–8 doi 10.1136/bmj.39337.615197.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Šarenac T, Mikov M. Cervical Cancer, Different Treatments and Importance of Bile Acids as Therapeutic Agents in This Disease. Front Pharmacol 2019;10:484 doi 10.3389/fphar.2019.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay HJ, Wenzel L, Mileshkin L. Nonsurgical management of cervical cancer: locally advanced, recurrent, and metastatic disease, survivorship, and beyond. Am Soc Clin Oncol Educ Book 2015:e299–309 doi 10.14694/EdBook_AM.2015.35.e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serkies K, Jassem J. Systemic therapy for cervical carcinoma - current status. Chin J Cancer Res 2018;30(2):209–21 doi 10.21147/j.issn.1000-9604.2018.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16(1):1–17 doi 10.1128/cmr.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015;7(7):3863–90 doi 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol 2017;40(2):80–5. [PubMed] [Google Scholar]
- 16.Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: A growing global problem. Int J Appl Basic Med Res 2016;6(2):84–9 doi 10.4103/2229-516x.179027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015;25Suppl 1(Suppl Suppl 1):2–23 doi 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther 2011;11(3):295–306 doi 10.4161/cbt.11.3.14686. [DOI] [PubMed] [Google Scholar]
- 19.Sanclemente G, Gill DK. Human papillomavirus molecular biology and pathogenesis. J Eur Acad Dermatol Venereol 2002;16(3):231–40 doi 10.1046/j.1473-2165.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 20.Ljubojevic S, Skerlev M. HPV-associated diseases. Clin Dermatol 2014;32(2):227–34 doi 10.1016/j.clindermatol.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 2012;131(9):1969–82 doi 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham SV. Human papillomavirus: gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol 2010;5(10):1493–506 doi 10.2217/fmb.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kash N, Lee MA, Kollipara R, Downing C, Guidry J, Tyring SK. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. J Clin Med 2015;4(4):614–33 doi 10.3390/jcm4040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372(8):711–23 doi 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 25.Gardasil Cuzick J. 9 joins the fight against cervix cancer. Expert Rev Vaccines 2015;14(8):1047–9 doi 10.1586/14760584.2015.1051470. [DOI] [PubMed] [Google Scholar]
- 26.Schiller JT, Müller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol 2015;16(5):e217–25 doi 10.1016/s1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 27.Signorelli C, Odone A, Ciorba V, Cella P, Audisio RA, Lombardi A, et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect 2017;145(10):1962–82 doi 10.1017/s0950268817000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B, Maraj B, Tran NP, Knoff J, Chen A, Alvarez RD, et al. Emerging human papillomavirus vaccines. Expert Opin Emerg Drugs 2012;17(4):469–92 doi 10.1517/14728214.2012.744393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies. Discov Med 2010;10(50):7–17. [PubMed] [Google Scholar]
- 30.Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines 2016;15(8):989–1007 doi 10.1586/14760584.2016.1157477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, Wang Y, Du J. Human Papillomavirus Vaccines: An Updated Review. Vaccines (Basel) 2020;8(3) doi 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lott BE, Okusanya BO, Anderson EJ, Kram NA, Rodriguez M, Thomson CA, et al. Interventions to increase uptake of Human Papillomavirus (HPV) vaccination in minority populations: A systematic review. Prev Med Rep 2020;19:101163 doi 10.1016/j.pmedr.2020.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014;168(1):76–82 doi 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MS, Savoy M. Impact of Human Papillomavirus Vaccination in Reducing Cancer. Prim Care 2020;47(3):529–37 doi 10.1016/j.pop.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Announcement FP. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. 2018.
- 36.Lin K, Roosinovich E, Ma B, Hung CF, Wu TC. Therapeutic HPV DNA vaccines. Immunol Res 2010;47(1–3):86–112 doi 10.1007/s12026-009-8141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res 1999;59(24):6132–6. [PubMed] [Google Scholar]
- 38.Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines 2016;15(10):1327–36 doi 10.1080/14760584.2016.1176533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol 2011;23(2):252–7 doi 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008;8(5):351–60 doi 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 41.Hung CF, Monie A, Alvarez RD, Wu TC. DNA vaccines for cervical cancer: from bench to bedside. Exp Mol Med 2007;39(6):679–89 doi 10.1038/emm.2007.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348(6230):62–8 doi 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchi V, Harari A, Coukos G. Neoantigen-Specific Adoptive Cell Therapies for Cancer: Making T-Cell Products More Personal. Front Immunol 2020;11:1215 doi 10.3389/fimmu.2020.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zsiros E, Tsuji T, Odunsi K. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J Clin Oncol 2015;33(14):1521–2 doi 10.1200/jco.2014.60.6566. [DOI] [PubMed] [Google Scholar]
- 45.Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017;356(6334):200–5 doi 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortezaee K Immune escape: A critical hallmark in solid tumors. Life Sci 2020:118110 doi 10.1016/j.lfs.2020.118110. [DOI] [PubMed] [Google Scholar]
- 47.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–64 doi 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37(17):1470–8 doi 10.1200/jco.18.01265. [DOI] [PubMed] [Google Scholar]
- 49.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine 2008;26(41):5315–20 doi 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, Alcocer-Gonzalez JM, Tamez-Guerra RS, de Oca-Luna RM, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. Journal of medical microbiology 2004;53(Pt 5):427–33 doi 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 51.Cortes-Perez NG, Azevedo V, Alcocer-Gonzalez JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, et al. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. Journal of drug targeting 2005;13(2):89–98 doi 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- 52.Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 2010;28(16):2810–7 doi 10.1016/j.vaccine.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes and infection / Institut Pasteur 2007;9(10):1176–87 doi 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Peters C, Paterson Y. Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts. Vaccine 2003;21(11–12):1187–94. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Ozbun L, Chong N, Wallecha A, Berzofsky JA, Khleif SN. Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3- and CD8+ T cells. Cancer Immunol Res 2014;2(9):911–22 doi 10.1158/2326-6066.Cir-13-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huh WK, Brady WE, Fracasso PM, Dizon DS, Powell MA, Monk BJ, et al. Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study. Gynecol Oncol 2020. doi 10.1016/j.ygyno.2020.06.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, McMasters KM. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother 2007;56(7):997–1007 doi 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol 2000;74(6):2888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassetti MC, McElhiney SP, Shahabi V, Pullen JK, Le Poole IC, Eiben GL, et al. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine 2004;22(3–4):520–7. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz M, Boyd DA, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine 2004;22(29–30):3993–4001 doi 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 61.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996;347(9014):1523–7. [DOI] [PubMed] [Google Scholar]
- 62.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol 2001;75(20):9654–64 doi 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res 2003;9(14):5205–13. [PubMed] [Google Scholar]
- 64.Harper DM, Nieminen P, Donders G, Einstein MH, Garcia F, Huh WK, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol Oncol 2019;153(3):521–9 doi 10.1016/j.ygyno.2019.03.250. [DOI] [PubMed] [Google Scholar]
- 65.Yang A, Farmer E, Lin J, Wu TC, Hung CF. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res 2017;231:148–65 doi 10.1016/j.virusres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Komdeur FL, Singh A, van de Wall S, Meulenberg JJM, Boerma A, Hoogeboom BN, et al. First-in-Human Phase I Clinical Trial of an SFV-Based RNA Replicon Cancer Vaccine against HPV-Induced Cancers. Mol Ther 2020. doi 10.1016/j.ymthe.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes and infection / Institut Pasteur 2001;3(10):851–7. [DOI] [PubMed] [Google Scholar]
- 68.Lee SJ, Yang A, Wu TC, Hung CF. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol 2016;27(5):e51 doi 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackova J, Kutinova L, Hainz P, Krystofova J, Sroller V, Otahal P, et al. Adjuvant effect of dendritic cells transduced with recombinant vaccinia virus expressing HPV16-E7 is inhibited by co-expression of IL12. International journal of oncology 2004;24(6):1581–8. [PubMed] [Google Scholar]
- 70.Wang TL, Ling M, Shih IM, Pham T, Pai SI, Lu Z, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene therapy 2000;7(9):726–33 doi 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 71.Benencia F, Courreges MC, Coukos G. Whole tumor antigen vaccination using dendritic cells: comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. Journal of translational medicine 2008;6:21 doi 10.1186/1479-5876-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murakami M, Gurski KJ, Marincola FM, Ackland J, Steller MA. Induction of specific CD8+ T-lymphocyte responses using a human papillomavirus-16 E6/E7 fusion protein and autologous dendritic cells. Cancer Res 1999;59(6):1184–7. [PubMed] [Google Scholar]
- 73.Peng S, Kim TW, Lee JH, Yang M, He L, Hung CF, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Human gene therapy 2005;16(5):584–93 doi 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JH, Kang TH, Noh KH, Bae HC, Kim SH, Yoo YD, et al. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunology letters 2009;122(1):58–67 doi 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, et al. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). Vaccine 2003;21(7–8):787–90. [DOI] [PubMed] [Google Scholar]
- 76.Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des 2005;11(27):3485–500. [DOI] [PubMed] [Google Scholar]
- 77.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, et al. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol 2008;82(4):1968–79 doi 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramanathan P, Ganeshrajah S, Raghanvan RK, Singh SS, Thangarajan R. Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer--a feasibility study. Asian Pacific journal of cancer prevention : APJCP 2014;15(14):5909–16. [DOI] [PubMed] [Google Scholar]
- 79.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008;14(1):169–77 doi 10.1158/1078-0432.Ccr-07-1881. [DOI] [PubMed] [Google Scholar]
- 80.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361(19):1838–47 doi 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 81.van Poelgeest MI, Welters MJ, Vermeij R, Stynenbosch LF, Loof NM, Berends-van der Meer DM, et al. Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. Clin Cancer Res 2016;22(10):2342–50 doi 10.1158/1078-0432.Ccr-15-2594. [DOI] [PubMed] [Google Scholar]
- 82.van der Burg SH, Kwappenberg KM, O'Neill T, Brandt RM, Melief CJ, Hickling JK, et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 2001;19(27):3652–60. [DOI] [PubMed] [Google Scholar]
- 83.de Jong A, O'Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002;20(29–30):3456–64. [DOI] [PubMed] [Google Scholar]
- 84.Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJ, Burt DJ, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 2004;22(21–22):2722–9 doi 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 85.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res 2004;10(9):2954–61. [DOI] [PubMed] [Google Scholar]
- 86.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. British journal of cancer 2010;102(7):1129–36 doi 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Da Silva DM, Skeate JG, Chavez-Juan E, Lühen KP, Wu JM, Wu CM, et al. Therapeutic efficacy of a human papillomavirus type 16 E7 bacterial exotoxin fusion protein adjuvanted with CpG or GPI-0100 in a preclinical mouse model for HPV-associated disease. Vaccine 2019;37(22):2915–24 doi 10.1016/j.vaccine.2019.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dosset M, Vauchy C, Beziaud L, Adotevi O, Godet Y. Universal tumor-reactive helper peptides from telomerase as new tools for anticancer vaccination. Oncoimmunology 2013;2(3):e23430 doi 10.4161/onci.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galaine J, Kellermann G, Guillaume Y, Boidot R, Picard E, Loyon R, et al. Heparan Sulfate Proteoglycans Promote Telomerase Internalization and MHC Class II Presentation on Dendritic Cells. J Immunol 2016;197(5):1597–608 doi 10.4049/jimmunol.1502633. [DOI] [PubMed] [Google Scholar]
- 90.Dosset M, Godet Y, Vauchy C, Beziaud L, Lone YC, Sedlik C, et al. Universal cancer peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin Cancer Res 2012;18(22):6284–95 doi 10.1158/1078-0432.Ccr-12-0896. [DOI] [PubMed] [Google Scholar]
- 91.Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene therapy 2004;11(12):1011–8 doi 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 92.Choi YJ, Hur SY, Kim T-J, Hong SR, Lee JK, Cho C-H, et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clinical Cancer Research 2020;26(7):1616–23 doi 10.1158/1078-0432.Ccr-19-1513. [DOI] [PubMed] [Google Scholar]
- 93.Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun 2014;5:5317 doi 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386(10008):2078–88 doi 10.1016/s0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012;4(155):155ra38 doi 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inovio Pharmaceuticals I. INOVIO Announces Positive Results from REVEAL 1, a Phase 3 Pivotal Trial Evaluating VGX-3100, its DNA-based HPV Immunotherapy for the Treatment of High-grade Precancerous Cervical Dysplasia Caused by HPV-16 and/or HPV-18. Web: Cision PR Newswire; 3/1/2021.
- 97.Hasan Y, Furtado L, Tergas A, Lee N, Brooks R, McCall A, et al. A Phase 1 Trial Assessing the Safety and Tolerability of a Therapeutic DNA Vaccination Against HPV16 and HPV18 E6/E7 Oncogenes After Chemoradiation for Cervical Cancer. Int J Radiat Oncol Biol Phys 2020;107(3):487–98 doi 10.1016/j.ijrobp.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin Cancer Res 2019;25(1):110–24 doi 10.1158/1078-0432.Ccr-18-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res 2009;15(1):361–7 doi 10.1158/1078-0432.Ccr-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alvarez RD, Huh WK, Bae S, Lamb LS Jr., Conner MG, Boyer J, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol 2016;140(2):245–52 doi 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim D, Gambhira R, Karanam B, Monie A, Hung CF, Roden R, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine 2008;26(3):351–60 doi 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol 1999;162(7):3757–60. [PubMed] [Google Scholar]
- 103.Hillemanns P, Petry KU, Woelber L, Böhmer G, Stubsrud E, Skjørestad I, et al. Abstract CT209: Safety, efficacy and immunogenicity of VB10.16, a therapeutic DNA vaccine targeting human papillomavirus (HPV) 16 E6 and E7 proteins for high grade cervical intraepithelial neoplasia (CIN 2/3): 6-month data from an exploratory open-label phase I/2a trial. Cancer Research 2019;79(13 Supplement):CT209–CT doi 10.1158/1538-7445.Am2019-ct209. [DOI] [Google Scholar]
- 104.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020;586(7830):594–9 doi 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 105.Grunwitz C, Salomon N, Vascotto F, Selmi A, Bukur T, Diken M, et al. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology 2019;8(9):e1629259 doi 10.1080/2162402x.2019.1629259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Polo JM, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Human gene therapy 2002;13(4):553–68 doi 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 107.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses 1997;13(17):1487–95. [DOI] [PubMed] [Google Scholar]
- 108.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther 2008;8(4):421–39 doi 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu KF, Hung CF, Cheng WF, He L, Slater LA, Ling M, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene therapy 2001;8(5):376–83 doi 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 110.Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Human gene therapy 2001;12(3):235–52 doi 10.1089/10430340150218387. [DOI] [PubMed] [Google Scholar]
- 111.van de Wall S, Ljungberg K, Ip PP, Boerma A, Knudsen ML, Nijman HW, et al. Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens. Oncoimmunology 2018;7(10):e1487913 doi 10.1080/2162402x.2018.1487913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim TW, Hung CF, Juang J, He L, Hardwick JM, Wu TC. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene therapy 2004;11(3):336–42 doi 10.1038/sj.gt.3302164. [DOI] [PubMed] [Google Scholar]
- 113.Kim TW, Lee JH, He L, Boyd DA, Hardwick JM, Hung CF, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res 2005;65(1):309–16. [PubMed] [Google Scholar]
- 114.Peng S, Qiu J, Yang A, Yang B, Jeang J, Wang JW, et al. Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus-associated disease. Cell Biosci 2016;6:16 doi 10.1186/s13578-016-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rohaan MW, Wilgenhof S, Haanen J. Adoptive cellular therapies: the current landscape. Virchows Arch 2019;474(4):449–61 doi 10.1007/s00428-018-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sukari A, Abdallah N, Nagasaka M. Unleash the power of the mighty T cells-basis of adoptive cellular therapy. Crit Rev Oncol Hematol 2019;136:1–12 doi 10.1016/j.critrevonc.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 117.Draper LM, Kwong ML, Gros A, Stevanovic S, Tran E, Kerkar S, et al. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin Cancer Res 2015;21(19):4431–9 doi 10.1158/1078-0432.CCR-14-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mantovani A, Marchesi F, Jaillon S, Garlanda C, Allavena P. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cellular & Molecular Immunology 2021. doi 10.1038/s41423-020-00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33(14):1543–50 doi 10.1200/jco.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeanbart L, Swartz MA. Engineering opportunities in cancer immunotherapy. Proc Natl Acad Sci U S A 2015;112(47):14467–72 doi 10.1073/pnas.1508516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hossain NM, Chapuis AG, Walter RB. T-Cell Receptor-Engineered Cells for the Treatment of Hematologic Malignancies. Curr Hematol Malig Rep 2016;11(4):311–7 doi 10.1007/s11899-016-0327-0. [DOI] [PubMed] [Google Scholar]
- 122.Kelderman S, Heemskerk B, Fanchi L, Philips D, Toebes M, Kvistborg P, et al. Antigen-specific TIL therapy for melanoma: A flexible platform for personalized cancer immunotherapy. Eur J Immunol 2016;46(6):1351–60 doi 10.1002/eji.201545849. [DOI] [PubMed] [Google Scholar]
- 123.Schober K, Busch DH. TIL 2.0: More effective and predictive T-cell products by enrichment for defined antigen specificities. Eur J Immunol 2016;46(6):1335–9 doi 10.1002/eji.201646436. [DOI] [PubMed] [Google Scholar]
- 124.Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med 2016;22(1):26–36 doi 10.1038/nm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Poelgeest MI, Visconti VV, Aghai Z, van Ham VJ, Heusinkveld M, Zandvliet ML, et al. Potential use of lymph node-derived HPV-specific T cells for adoptive cell therapy of cervical cancer. Cancer Immunol Immunother 2016;65(12):1451–63 doi 10.1007/s00262-016-1892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li N, Tian YW, Xu Y, Meng DD, Gao L, Shen WJ, et al. Combined Treatment with Autologous CIK Cells, Radiotherapy and Chemotherapy in Advanced Cervical Cancer. Pathol Oncol Res 2019;25(2):691–6 doi 10.1007/s12253-018-0541-2. [DOI] [PubMed] [Google Scholar]
- 127.Doran SL, Stevanovic S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, et al. Genetically engineered T-cell therapy for HPV-associated epithelial cancers: A first in human, phase I/II clinical trial. Journal of Clinical Oncology 2018;36(15_suppl):3019- doi 10.1200/JCO.2018.36.15_suppl.3019. [DOI] [Google Scholar]
- 128.Dai H, Wang Y, Lu X, Han W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J Natl Cancer Inst 2016;108(7) doi 10.1093/jnci/djv439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haji-Fatahaliha M, Hosseini M, Akbarian A, Sadreddini S, Jadidi-Niaragh F, Yousefi M. CAR-modified T-cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol 2016;44(6):1339–49 doi 10.3109/21691401.2015.1052465. [DOI] [PubMed] [Google Scholar]
- 130.Zhang H, Ye ZL, Yuan ZG, Luo ZQ, Jin HJ, Qian QJ. New Strategies for the Treatment of Solid Tumors with CAR-T Cells. Int J Biol Sci 2016;12(6):718–29 doi 10.7150/ijbs.14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Callahan MK, Flaherty CR, Postow MA. Checkpoint Blockade for the Treatment of Advanced Melanoma. Cancer treatment and research 2016;167:231–50 doi 10.1007/978-3-319-22539-5_9. [DOI] [PubMed] [Google Scholar]
- 132.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013;73(6):1733–41 doi 10.1158/0008-5472.can-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Allouch S, Malki A, Allouch A, Gupta I, Vranic S, Al Moustafa AE. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front Oncol 2020;10:914 doi 10.3389/fonc.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol 2015;28(12):1594–602 doi 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 135.Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol 2017;9(6):431–9 doi 10.1177/1758834017708742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lyu M, Shen Y, Beharee N, Lu J, Deng F, Wang J. The Combined Use of Chemotherapy and Radiotherapy with PD-1 Inhibitor, Pembrolizumab, in Advanced Cervical Cancer: A Case Report. Onco Targets Ther 2020;13:4465–71 doi 10.2147/ott.S245190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang Y, Yu M, Zhou C, Zhu X. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagn Pathol 2020;15(1):67 doi 10.1186/s13000-020-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hollebecque A, Meyer T, Moore KN, Machiels J-PH, Greve JD, López-Picazo JM, et al. An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. Journal of Clinical Oncology 2017;35(15_suppl):5504- doi 10.1200/JCO.2017.35.15_suppl.5504. [DOI] [Google Scholar]
- 139.Frenel J-S, Tourneau CL, O'Neil BH, Ott PA, Piha-Paul SA, Gomez-Roca CA, et al. Pembrolizumab in patients with advanced cervical squamous cell cancer: Preliminary results from the phase Ib KEYNOTE-028 study. Journal of Clinical Oncology 2016;34(15_suppl):5515- doi 10.1200/JCO.2016.34.15_suppl.5515. [DOI] [Google Scholar]
- 140.Van Coillie S, Wiernicki B, Xu J. Molecular and Cellular Functions of CTLA-4. Adv Exp Med Biol 2020;1248:7–32 doi 10.1007/978-981-15-3266-5_2. [DOI] [PubMed] [Google Scholar]
- 141.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363(8):711–23 doi 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: From mechanism to autoimmune therapy. Int Immunopharmacol 2020;80:106221 doi 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 143.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunology 2002;3(7):611–8 doi 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 144.Lheureux S, Butler MO, Clarke B, Cristea MC, Martin LP, Tonkin KS, et al. A phase I/II study of ipilimumab in women with metastatic or recurrent cervical carcinoma: A study of the Princess Margaret and Chicago N01 Consortia. Journal of Clinical Oncology 2015;33(15_suppl):3061- doi 10.1200/jco.2015.33.15_suppl.3061. [DOI] [Google Scholar]
- 145.Guo L, Zhang H, Chen B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J Cancer 2017;8(3):410–6 doi 10.7150/jca.17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong H, Zhou J, Xu D, Zeng X. Rheumatic immune-related adverse events induced by immune checkpoint inhibitors. Asia Pac J Clin Oncol 2020. doi 10.1111/ajco.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52:50–66 doi 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 148.Shrimali RK, Ahmad S, Verma V, Zeng P, Ananth S, Gaur P, et al. Concurrent PD-1 Blockade Negates the Effects of OX40 Agonist Antibody in Combination Immunotherapy through Inducing T-cell Apoptosis. Cancer Immunol Res 2017;5(9):755–66 doi 10.1158/2326-6066.Cir-17-0292. [DOI] [PubMed] [Google Scholar]
- 149.Glisson BS, Leidner RS, Ferris RL, Powderly J, Rizvi NA, Keam B, et al. Safety and Clinical Activity of MEDI0562, a Humanized OX40 Agonist Monoclonal Antibody, in Adult Patients with Advanced Solid Tumors. Clin Cancer Res 2020;26(20):5358–67 doi 10.1158/1078-0432.Ccr-19-3070. [DOI] [PubMed] [Google Scholar]
- 150.Padovani CT, Bonin CM, Tozetti IA, Ferreira AM, Fernandes CE, Costa IP. Glucocorticoid-induced tumor necrosis factor receptor expression in patients with cervical human papillomavirus infection. Rev Soc Bras Med Trop 2013;46(3):288–92 doi 10.1590/0037-8682-0029-2013. [DOI] [PubMed] [Google Scholar]
- 151.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer 2016;67:1–10 doi 10.1016/j.ejca.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 152.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017;276(1):80–96 doi 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panda A, Rosenfeld JA, Singer EA, Bhanot G, Ganesan S. Genomic and immunologic correlates of LAG-3 expression in cancer. Oncoimmunology 2020;9(1):1756116 doi 10.1080/2162402x.2020.1756116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, et al. Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS One 2013;8(1):e53834 doi 10.1371/journal.pone.0053834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Solinas C, De Silva P, Bron D, Willard-Gallo K, Sangiolo D. Significance of TIM3 expression in cancer: From biology to the clinic. Semin Oncol 2019;46(4–5):372–9 doi 10.1053/j.seminoncol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 156.de Lourdes Mora-García M, López-Cisneros S, Gutiérrez-Serrano V, García-Rocha R, Weiss-Steider B, Hernández-Montes J, et al. HPV-16 Infection Is Associated with a High Content of CD39 and CD73 Ectonucleotidases in Cervical Samples from Patients with CIN-1. Mediators Inflamm 2019;2019:4651627 doi 10.1155/2019/4651627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, et al. Cervical Cancer Cells Express Markers Associated with Immunosurveillance. J Immunol Res 2019;2019:1242979 doi 10.1155/2019/1242979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol 2020;20(12):739–55 doi 10.1038/s41577-020-0376-4. [DOI] [PubMed] [Google Scholar]
- 159.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557(7706):575–9 doi 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 160.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nature Communications 2018;9(1):2724 doi 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kortekaas KE, Santegoets SJ, Sturm G, Ehsan I, van Egmond SL, Finotello F, et al. CD39 Identifies the CD4(+) Tumor-Specific T-cell Population in Human Cancer. Cancer Immunol Res 2020;8(10):1311–21 doi 10.1158/2326-6066.Cir-20-0270. [DOI] [PubMed] [Google Scholar]
- 162.García-Rocha R, Monroy-García A, Hernández-Montes J, Weiss-Steider B, Gutiérrez-Serrano V, Del Carmen Fuentes-Castañeda M, et al. Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine 2019;118:71–9 doi 10.1016/j.cyto.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 163.Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015;13:265–72 doi 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16(3):151–67 doi 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 165.Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O'Donnell JS, Szczepanski S, et al. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 2017;47(4):789–802.e9 doi 10.1016/j.immuni.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 166.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016;539(7629):443–7 doi 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boromand N, Hasanzadeh M, ShahidSales S, Farazestanian M, Gharib M, Fiuji H, et al. Clinical and prognostic value of the C-Met/HGF signaling pathway in cervical cancer. J Cell Physiol 2018;233(6):4490–6 doi 10.1002/jcp.26232. [DOI] [PubMed] [Google Scholar]
- 168.Walch-Rückheim B, Ströder R, Theobald L, Pahne-Zeppenfeld J, Hegde S, Kim YJ, et al. Cervical Cancer-Instructed Stromal Fibroblasts Enhance IL23 Expression in Dendritic Cells to Support Expansion of Th17 Cells. Cancer Res 2019;79(7):1573–86 doi 10.1158/0008-5472.Can-18-1913. [DOI] [PubMed] [Google Scholar]
- 169.Lin W, Niu Z, Zhang H, Kong Y, Wang Z, Yang X, et al. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int J Clin Exp Pathol 2019;12(9):3604–12. [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang ZS, Gu Y, Liu BG, Tang H, Hua Y, Wang J. Oncogenic role of Tc17 cells in cervical cancer development. World J Clin Cases 2020;8(1):11–9 doi 10.12998/wjcc.v8.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bahrami A, Hasanzadeh M, Hassanian SM, ShahidSales S, Ghayour-Mobarhan M, Ferns GA, et al. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J Cell Biochem 2017;118(12):4163–9 doi 10.1002/jcb.26118. [DOI] [PubMed] [Google Scholar]
- 172.Cao P, Wang Y, Lv Y, Jiang N, Zhong L, Ma X, et al. PI3K p110α inhibition sensitizes cervical cancer cells with aberrant PI3K signaling activation to PARP inhibitor BMN673. Oncol Rep 2019;42(5):2097–107 doi 10.3892/or.2019.7313. [DOI] [PubMed] [Google Scholar]
- 173.Li YJ, Wang Y, Wang YY. MicroRNA-99b suppresses human cervical cancer cell activity by inhibiting the PI3K/AKT/mTOR signaling pathway. J Cell Physiol 2019;234(6):9577–91 doi 10.1002/jcp.27645. [DOI] [PubMed] [Google Scholar]
- 174.Zhang W, Zhou Q, Wei Y, Da M, Zhang C, Zhong J, et al. The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol 2019;12(7):2474–84. [PMC free article] [PubMed] [Google Scholar]
- 175.Bossler F, Hoppe-Seyler K, Hoppe-Seyler F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int J Mol Sci 2019;20(9) doi 10.3390/ijms20092188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bedoya AM, Tate DJ, Baena A, Córdoba CM, Borrero M, Pareja R, et al. Immunosuppression in cervical cancer with special reference to arginase activity. Gynecol Oncol 2014;135(1):74–80 doi 10.1016/j.ygyno.2014.07.096. [DOI] [PubMed] [Google Scholar]
- 177.Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer 2017;5(1):101 doi 10.1186/s40425-017-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends Pharmacol Sci 2018;39(3):307–25 doi 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 179.Sato N, Saga Y, Mizukami H, Wang D, Takahashi S, Nonaka H, et al. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol Rep 2012;28(5):1574–8 doi 10.3892/or.2012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chinn Z, Stoler MH, Mills AM. PD-L1 and IDO expression in cervical and vulvar invasive and intraepithelial squamous neoplasias: implications for combination immunotherapy. Histopathology 2019;74(2):256–68 doi 10.1111/his.13723. [DOI] [PubMed] [Google Scholar]
- 181.Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370(8):734–43 doi 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Minion LE, Tewari KS. Cervical cancer - State of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol Oncol 2018;148(3):609–21 doi 10.1016/j.ygyno.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shevtsov M, Sato H, Multhoff G, Shibata A. Novel Approaches to Improve the Efficacy of Immuno-Radiotherapy. Front Oncol 2019;9:156 doi 10.3389/fonc.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Meir H, Nout RA, Welters MJ, Loof NM, de Kam ML, van Ham JJ, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017;6(2):e1267095 doi 10.1080/2162402x.2016.1267095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao J, Chen Y, Ding ZY, Liu JY. Safety and Efficacy of Therapeutic Cancer Vaccines Alone or in Combination With Immune Checkpoint Inhibitors in Cancer Treatment. Front Pharmacol 2019;10:1184 doi 10.3389/fphar.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mougel A, Terme M, Tanchot C. Therapeutic Cancer Vaccine and Combinations With Antiangiogenic Therapies and Immune Checkpoint Blockade. Front Immunol 2019;10:467 doi 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rice AE, Latchman YE, Balint JP, Lee JH, Gabitzsch ES, Jones FR. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther 2015;22(9):454–62 doi 10.1038/cgt.2015.40. [DOI] [PubMed] [Google Scholar]
- 188.Peng S, Ferrall L, Gaillard S, Wang C, Chi WY, Huang CH, et al. Development of a DNA vaccine targeting E6 and E7 proteins of HPV16 and HPV18 for immunotherapy in combination with recombinant vaccinia boost and PD-1 antibody. mBio (in press) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5(1):67–73 doi 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Youn JW, Hur S-Y, Woo JW, Kim Y-M, Lim MC, Park SY, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. The Lancet Oncology 2020;21(12):1653–60 doi 10.1016/S1470-2045(20)30486-1. [DOI] [PubMed] [Google Scholar]
- 191.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res 2008;14(10):3185–92 doi 10.1158/1078-0432.Ccr-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Melief CJM, Welters MJP, Vergote I, Kroep JR, Kenter GG, Ottevanger PB, et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med 2020;12(535) doi 10.1126/scitranslmed.aaz8235. [DOI] [PubMed] [Google Scholar]
- 193.Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, van Veelen PA, et al. Tumor Eradication by Cisplatin Is Sustained by CD80/86-Mediated Costimulation of CD8+ T Cells. Cancer Res 2016;76(20):6017–29 doi 10.1158/0008-5472.Can-16-0881. [DOI] [PubMed] [Google Scholar]
- 194.Lee SY, Kang TH, Knoff J, Huang Z, Soong RS, Alvarez RD, et al. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer Immunol Immunother 2013;62(7):1175–85 doi 10.1007/s00262-013-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Basu P, Mehta AO, Jain MM, Gupta S, Nagarkar RV, Kumar V, et al. ADXS11–001 immunotherapy targeting HPV-E7: Final results from a phase 2 study in Indian women with recurrent cervical cancer. Journal of Clinical Oncology 2014;32(15_suppl):5610- doi 10.1200/jco.2014.32.15_suppl.5610. [DOI] [Google Scholar]
- 196.Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol 2020. doi 10.1016/j.radonc.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 197.Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol 2016;214(1):22–30 doi 10.1016/j.ajog.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol 2016;27(4):e43 doi 10.3802/jgo.2016.27.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine 2018;24(5):541–50 doi 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li B, Cui Y, Nambiar DK, Sunwoo JB, Li R. The Immune Subtypes and Landscape of Squamous Cell Carcinoma. Clin Cancer Res 2019;25(12):3528–37 doi 10.1158/1078-0432.Ccr-18-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barra F, Della Corte L, Noberasco G, Foreste V, Riemma G, Di Filippo C, et al. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J Obstet Gynaecol Res 2020;46(7):989–1006 doi 10.1111/jog.14276. [DOI] [PubMed] [Google Scholar]
- 202.Desravines N, Miele K, Carlson R, Chibwesha C, Rahangdale L. Topical therapies for the treatment of cervical intraepithelial neoplasia (CIN) 2–3: A narrative review. Gynecol Oncol Rep 2020;33:100608 doi 10.1016/j.gore.2020.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Castle PE, Murokora D, Perez C, Alvarez M, Quek SC, Campbell C. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstet 2017;138Suppl 1:20–5 doi 10.1002/ijgo.12191. [DOI] [PubMed] [Google Scholar]


