Abstract
Objective:
Traumatic brain injury (TBI) is a leading cause of death and disability in the United States. While the impact of early multi-organ dysfunction (MODS) has been studied in many critical care paradigms, the clinical impact of early MODS in TBI is poorly understood. We examined the incidence and impact of early MODS on clinical, functional, and disability outcomes over the year following TBI.
Design:
Retrospective cohort study
Setting:
Patients enrolled in the TRACK-TBI study, an 18-center prospective cohort study of TBI patients evaluated in participating Level 1 trauma centers.
Subjects:
Adult (age > 17 years) patients with moderate-severe TBI [GCS < 13]. We excluded patients with major extracranial injury [AIS score ≥ 3].
Interventions:
Development of early MODS, defined as a maximum modified sequential organ failure assessment (mSOFA) score > 7 during the initial 72 hours following admission.
Measurements and Main Results:
The main outcomes were: hospital mortality, length of stay, 6-month functional and disability domains [Glasgow Outcome Scale -Extended (GOS-E) and Disability Rating Scale (DRS)], and 1-year mortality. Secondary outcomes included: intensive care unit (ICU) length of stay, 3-month GOS-E, 3-month DRS, 1-year GOS-E, 1-year DRS. We examined 373 subjects with moderate-severe TBI. The mean(SD) GCS in the emergency department was 5.8 (3.2), with 280 (75%) subjects classified as severe TBI (GCS 3-8). Among subjects with moderate-severe TBI, 252 (68%) developed early MODS. Subjects that developed early MODS had a 75% decreased odds of a favorable outcome (GOS-E 5-8) at 6 months [adjusted odds ratio (OR) 0.25, 95% CI 0.12 – 0.51] and increased disability (higher DRS score) at 6 months (adjusted mean difference 2.04, 95% CI 0.92 – 3.17). Subjects that developed early MODS experienced an increased hospital length of stay (adjusted mean difference 11.4 days, 95% CI 7.1 – 15.8), with a non-significantly decreased survival to hospital discharge (OR 0.47, 95% CI 0.18 – 1.2).
Conclusions:
Early multi-organ dysfunction following moderate-severe TBI is common and independently impacts multiple domains (mortality, function, and disability) over the year following injury. Further research is necessary to understand underlying mechanisms, improve early recognition, and optimize management strategies.
Keywords: trauma, traumatic brain injury, multi-organ dysfunction, shock, outcomes
Introduction:
Traumatic brain injury (TBI) is a leading cause of death and disability in the United States. In 2014, over 2.8 million emergency department visits and hospitalizations were attributed to TBI in the United States, resulting in over 56 thousand deaths1. Compared to mild TBI (GCS 13 – 15), moderate-severe TBI (GCS 3 - 9) has been associated with a greater incidence of hypotension [systolic blood pressure (SBP) < 90 mmHg]2, increased mortality, and worse functional outcomes.3 While significant research has examined the consequences and treatment of primary injury to the brain, comparatively little research has been undertaken to understand the impact of brain injury on extracranial organ dysfunction. Consequences of multi-organ dysfunction to the injured brain may include reduced cerebral blood flow, hypoxia, altered metabolism, acidosis, and bleeding, which contribute to secondary brain injury and poor clinical outcomes2,4. Therefore, understanding underlying mechanisms to inform optimal prevention and treatment strategies for multi-organ dysfunction could represent an important therapeutic paradigm to improve outcomes following moderate-severe TBI.
In recent years, there has been a greater recognition of extracranial organ dysfunction following TBI. For example, the consequences of circulatory shock, cardiac dysfunction, the acute respiratory distress syndrome (ARDS), and acute kidney injury (AKI) have been better understood following moderate-severe TBI.5-10 Unfortunately, most of this prior work has focused on the consequences of single organ dysfunction rather than multi-organ dysfunction, despite likely sharing common underlying mechanisms.11 In addition, there continue to be gaps in our understanding of multi-organ dysfunction following TBI, including the impact of early multi-organ dysfunction (when the brain is most vulnerable to secondary injury), the role of the injured brain itself on organ dysfunction (rather than the effect of concurrent polytrauma), and the examination of clinical and functional outcomes beyond hospital outcomes alone. To address these gaps, the goals of our study were: 1) To describe the epidemiology of early multi-organ dysfunction following isolated moderate-severe TBI; and 2) To examine the impact of early multi-organ dysfunction on clinical, functional, and disability outcomes over the year following injury.
Methods:
Study Design and Database
We conducted a retrospective cohort study of patients enrolled in the Transforming Clinical Research and Knowledge in TBI (TRACK-TBI) study. TRACK-TBI is an 18-center prospective cohort study of patients evaluated in a participating American College of Surgeons Level 1 trauma center emergency department within 24 hours of suffering blunt TBI, and for whom a clinically indicated head CT scan was obtained by the treating clinician. In addition to the collection of detailed hospital encounter data, the study also collected a multidimensional outcome assessment battery across the year post-injury.12 Subjects were excluded if they: had significant extracranial injury or significant history of pre-existing conditions that would interfere with follow-up and outcome assessment. In addition, subjects were also excluded if they were: prisoners or patients in custody; pregnant; on psychiatric hold; had major debilitating baseline mental health disorders or major debilitating neurologic disease; participants in an interventional trial; or had penetrating TBI or spinal cord injury with ASIA score of C or worse. All data were obtained from participants by trained research coordinators who used structured data collection tools recommended as part of the National Institute of Neurological Disorders and Stroke (NINDS) Common Data Elements for TBI studies.13 The present study was approved by the Institutional Review Board at Duke University (Pro00100061).
Population
We examined adult (age > 17 years) moderate-severe TBI subjects from the TRACK-TBI cohort, defined as Glasgow Coma Scale (GCS) score < 13 after resuscitation. Because we aimed to examine the impact of the brain injury itself on the development of multi-organ dysfunction (rather than due to a concurrent extracranial injury), we excluded patients with major extracranial injury, defined as a non-head Abbreviated Injury Scale (AIS) score ≥ 3. Lastly, we excluded patients with missing data for variables that would allow calculation of at least one modified Sequential Organ Failure Assessment (mSOFA) score during the 72 hours following hospital admission.
Exposure, Outcomes, and Covariates
The primary exposure was the development of early multi-organ dysfunction syndrome (MODS) defined as a maximum mSOFA score > 7 during the initial 72 hours following hospital admission. Based on the available data and data structure in the TRACK-TBI database, we used a modification of the SOFA score (mSOFA score) to ascertain multi-organ dysfunction. Modifications in the SOFA score have been used in multiple prior studies, and has been shown to have similar predictive capabilities, as compared with the original SOFA score.14-17 We modified the SOFA score by omitting the bilirubin variable from the calculation as previously reported14 (as this was not consistently measured in our cohort of TBI subjects) and modifying the respiratory variable to include ventilation type and ARDS status, given lack of reliable fraction of inspired oxygen data within the TRACK-TBI database (Supplemental Table 1). Among the cohort, 20% of subjects did not have adequate variables for calculation of a complete mSOFA score each day. To address this, these components were imputed using the average of the last observation carried forward (LOCF) and next observation carried backward (NOCB) techniques, as previously described.18 The cut-point of SOFA > 7 to define multi-organ dysfunction was chosen to remain consistent with prior studies examining MODS following TBI.19
The main outcomes of interest were: in-hospital mortality, hospital length of stay (as a marker of health care utilization), 6-month functional and disability domains [Glasgow Outcome Scale -Extended (GOS-E) and Disability Rating Scale (DRS)], and 1-year mortality. To capture a broader spectrum of clinical and functional outcomes over the first year following injury, we also examined the following secondary outcomes: intensive care unit (ICU) length of stay, 3-month GOS-E, 3-month DRS, 1-year GOS-E, and 1-year DRS. The GOS-E (possible score 1-8) was considered as a binary outcome variable, with a score of 5-8 representing a favorable outcome and a score < 5 representing an unfavorable outcome; the DRS (possible score 0-29) was considered as a continuous outcome variable, with higher values representing greater levels of disability. Covariates included data on patient demographics (age, gender, race, ethnicity, and education), injury characteristics (cause of injury, body region abbreviated injury scores, and the injury severity score), and TBI characteristics (GCS score, presence of bleeding on head CT, and CT Rotterdam score20).
Statistical Analysis
Descriptive statistics were used to examine demographic, clinical, and injury characteristics among the entire cohort, as well as stratified by the presence/absence of early MODS. Mann-Whitney tests were used to examine differences between continuous variables, and Fisher’s exact tests were used to examine categorical variables. We performed multivariable logistic regression to assess the association of early MODS with primary and secondary binary outcomes (in-hospital mortality, 3-, 6-, and 12-month GOS-E). We performed multivariable linear regression to assess the association of early MODS with primary and secondary continuous outcomes (hospital length of stay, ICU length of stay, 3-, 6-, and 12-month DRS). Finally, we performed multivariable Cox proportional hazards regression to assess the association of early MODS with 1-year mortality. Covariates included in the statistical models were pre-specified based on literature review and expert opinion and included age, gender, non-head injury severity score, and CT Rotterdam score. The results of all models examining primary outcomes were adjusted for multiple testing using a false-discovery rate of 5% per the Benjamini-Hochberg method.
To confirm the robustness of the initial analytic approach, we performed additional sensitivity analyses. Because researchers have used differing cut-offs for favorable versus unfavorable outcome with the GOSE-E score, we also considered a GOS-E 4-8 as a favorable outcome in separate models. In addition, we performed an additional series of outcome analyses using multiple imputation (5 imputations) by chained equations to address missing covariate and outcome data, compared to our initial strategy of a complete case analysis. A p-value < 0.05 was considered statistically significant, and all analyses were performed using SPSS version 26 (Armonk, NY, USA).
Results:
Demographic and Clinical Characteristics
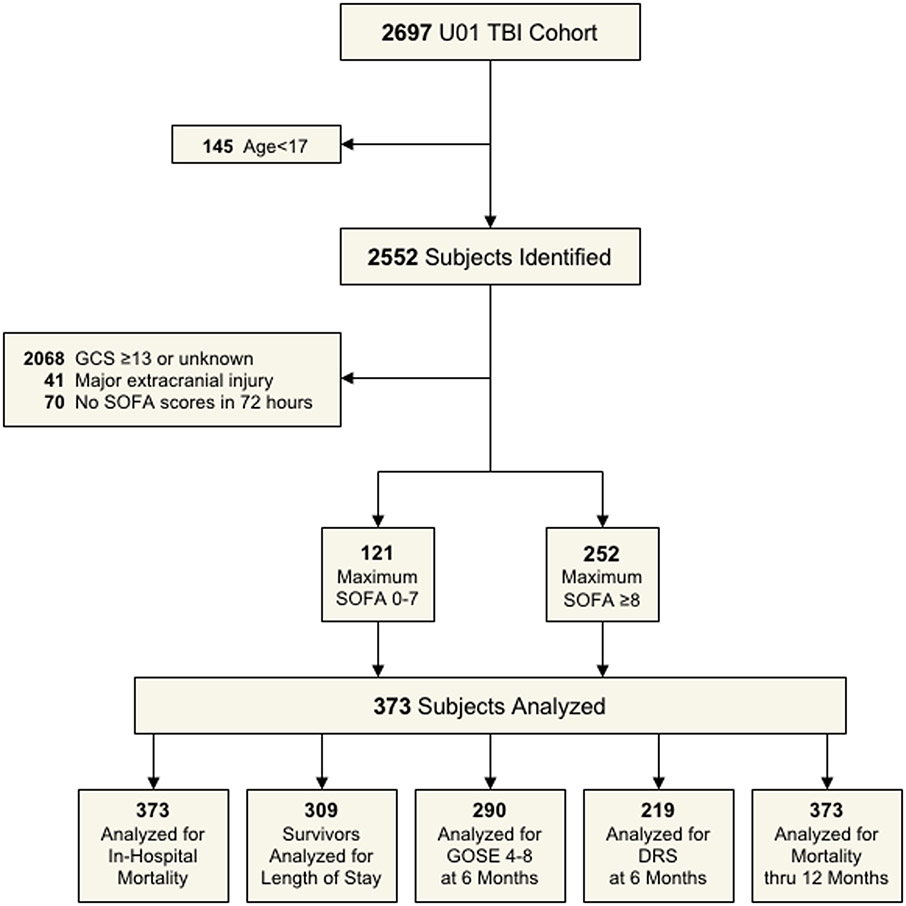
After applying all exclusion criteria to the cohort (Figure 1), we examined clinical and demographic characteristics in a final cohort of 373 subjects with moderate-severe TBI (Supplementary Table 1). The cohort was generally young (mean age 41 years), majority male (78%), and mostly of white race (80%). Among the moderate-severe TBI subjects in the cohort, 73 subjects (20%) identified as Hispanic ethnicity. The primary cause of injury in the cohort was motor vehicle collision (occupants and cyclist/pedestrian) (40%), followed by falls (24%), motorcycle collision (14%), and assault (8%). The mean(SD) GCS in the emergency department was 5.8 (3.2), with 280 (75%) subjects classified as severe TBI (GCS 3-8). Among the subjects in the cohort, 322 (92%) subjects had intracranial pathology consistent with TBI on the initial head CT (e.g., contusion, subarachnoid hemorrhage), with a mean(SD) CT Rotterdam score of 3.4 (1.3). The mean(SD) non-head ISS score was 6.0 (6.6), suggesting minimal extracranial traumatic injury.
Figure 1:
STROBE diagram/flow
Epidemiology of Early Multi-Organ Dysfunction
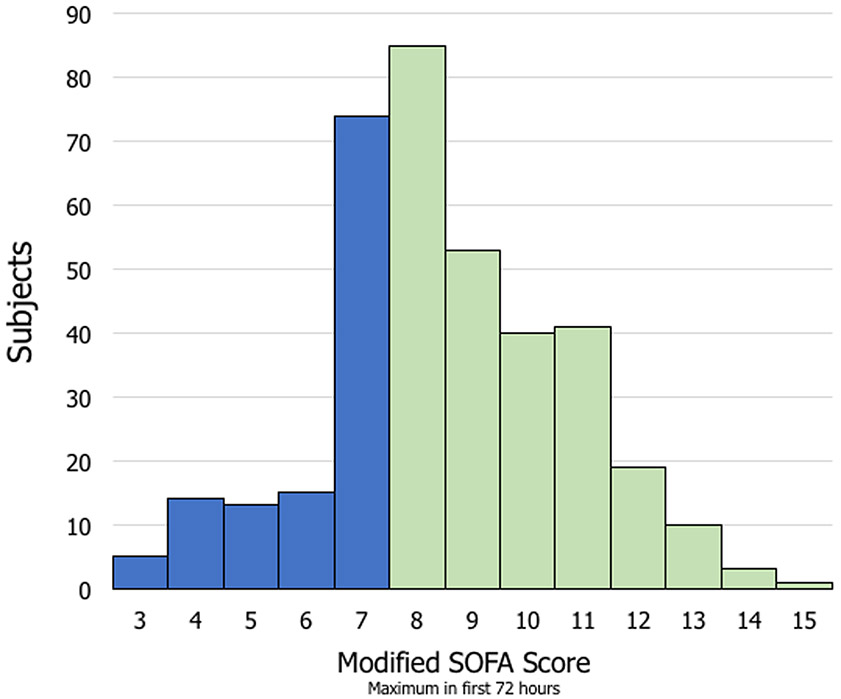
The distribution of maximum SOFA score within the first 72 hours following admission is shown in Figure 2. Among subjects with moderate-severe TBI, 252 (68%) developed early MODS. Compared with subjects who did not develop early MODS, subjects with early MODS were slightly older (42.5 versus 38.9 years), had a lower mean(SD) GCS in the emergency department [5.0 (2.8) versus 7.5 (3.3)], and had a higher mean(SD) CT Rotterdam score [3.6 (1.3) versus 2.9 (1.0)]. The distribution of organ dysfunction contributing to early MODS is shown in Supplemental Figure 1.
Figure 2:
Distribution of SOFA scores over first 72 hours
Association of Early Multi-Organ Dysfunction with Clinical and Functional Outcomes
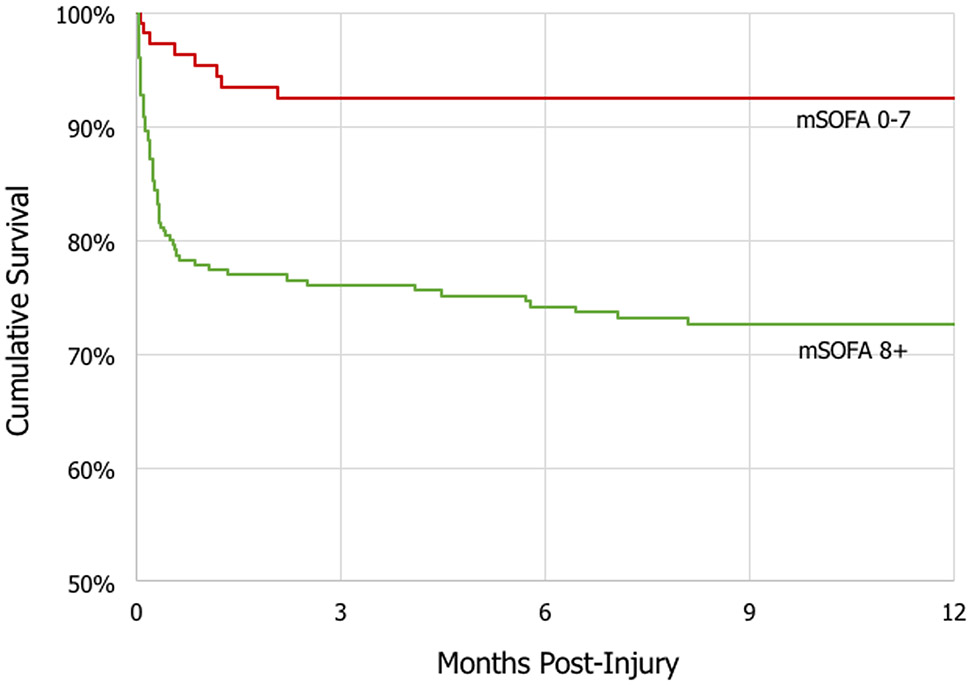
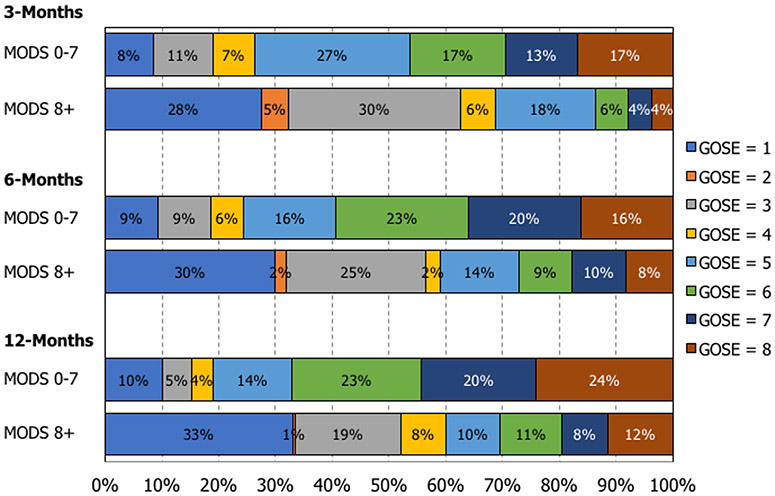
Table 1 shows clinical and functional outcomes among moderate-severe TBI subjects who developed early MODS, compared with subjects who did not develop early MODS. With regard to primary outcomes, subjects that developed early MODS experienced an increased hospital length of stay (adjusted mean difference 11.4 days, 95% CI 7.1 – 15.8, p<0.001), had a 75% decreased odds of a favorable outcome (GOS-E 5-8) at 6 months (adjusted odds ratio 0.25, 95% CI 0.12 – 0.51, p<0.001), had increased disability (higher DRS score) at 6 months (adjusted mean difference 2.04, 95% CI 0.92 – 3.17, p=0.001), and increased mortality at 1 year (adjusted hazard ratio 2.59, 95% CI 1.07 – 6.28, p=0.04) [Figure 3]. Subjects that developed early MODS had a trend for decreased odds for being discharged alive from the hospital, which was non-statistically significant (adjusted odds ratio 0.47, 95% CI 0.18 – 1.20, p=0.11). With regard to secondary outcomes, subjects that developed early MODS experienced increased ICU length of stay (p<0.001), decreased odds of a favorable outcome at 3 months (p<0.001) and 12 months (p<0.001), and increased disability at 3 months (p<0.001) and 12 months (p<0.001). Figure 4 describes the GOS-E distribution at 3 months, 6 months, and 12 months following injury, stratified by the development of early MODS. Sensitivity analyses (Supplementary Table 2) demonstrated stable and robust risk estimates following application of varying definitions for a favorable outcome, as well as following application of multiple imputation to account for missing covariates and outcome data.
Table 1:
Demographic, clinical characteristics (stratified by MODS)
| Outcome | N | MODS3 | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0-7 | 8+ | Effect | p | Effect | p1 | 95% CI | p2 | |||
| Primary Outcomes | ||||||||||
| Discharged Alive A | 373 | 93% | 78% | Odds Ratio 0.25 | <.001 | Odds Ratio 0.47 | .113 | Odds Ratio 0.18 | Odds Ratio 1.20 | .113 |
| Length of StayB (survivors only) | 309 | 10.7 | 24.2 | Diff.in Means 13.5 | <.001 | Diff.in Means 11.4 | <.001 | Diff.in Means 7.1 | Diff.in Means 15.8 | <.001 |
| 6mo GOSE 5-8 A | 293 | 76% | 41% | Odds Ratio 0.23 | <.001 | Odds Ratio 0.25 | <.001 | Odds Ratio 0.12 | Odds Ratio 0.51 | <.001 |
| 6mo DRS B | 228 | 1.58 | 4.08 | Diff.in Means 2.50 | <.001 | Diff.in Means 2.04 | <.001 | Diff.in Means 0.92 | Diff.in Means 3.17 | .001 |
| Mortality thru 12mo C | 373 | 9% | 33% | Hazard Ratio 4.19 | <.001 | Hazard Ratio 2.59 | .035 | Hazard Ratio 1.07 | Hazard Ratio 6.28 | .043 |
| Secondary Outcomes | ||||||||||
| ICU Length of Stay B | 309 | 4.2 | 14.3 | Diff.in Means 10.1 | <.001 | Diff.in Means 10.0 | <.001 | Diff.in Means 7.0 | Diff.in Means 12.9 | --- |
| 3mo GOSE 5-8 A | 309 | 74% | 31% | Odds Ratio 0.16 | <.001 | Odds Ratio 0.19 | <.001 | Odds Ratio 0.10 | Odds Ratio 0.37 | --- |
| 3mo DRS B | 244 | 1.79 | 5.59 | Diff.in Means 3.79 | <.001 | Diff.in Means 2.97 | <.001 | Diff.in Means 1.72 | Diff.in Means 4.22 | --- |
| 12mo GOSE 5-8 A | 279 | 81% | 40% | Odds Ratio 0.16 | <.001 | Odds Ratio 0.14 | <.001 | Odds Ratio 0.06 | Odds Ratio 0.32 | --- |
| 12mo DRS B | 205 | 1.01 | 3.61 | Diff.in Means 2.60 | <.001 | Diff.in Means 2.08 | <.001 | Diff.in Means 1.00 | Diff.in Means 3.16 | --- |
Dichotomous outcome, analyzed using logistic regression, reporting odds ratios
Continuous outcome, analyzed using linear regression, reporting difference in means
Survival outcome, analyzed using Cox regression, reporting hazard ratio
Dichotomous outcome, analyzed using logistic regression with multiple imputation to account for missing covariates and outcome
Analysis adjusted for age, sex, Rotterdam score, ISS non-head
Significance values further adjusted for multiple comparisons (Benjamini-Hochberg, m=5)
Presence/absence of MODS based on mSOFA score (mSOFA 0-7 versus mSOFA 8+)
Figure 3:
Kaplan-Meier Curve for 1-year cumulative survival
Figure 4:
GOSE distribution (3-month, 6-month, 1-year)
Discussion:
In this study examining the epidemiology and outcomes associated with the development of early MODS following isolated moderate-severe TBI, we found that: 1) The development of early extracranial multi-organ dysfunction following moderate-severe TBI is common; and 2) The development of early MODS is associated with unfavorable clinical outcomes and greater disability over the year following injury. Our data suggests that early MODS is an important manifestation following acute brain injury, with significant impact on patient outcomes in multiple domains over the year following brain injury.
Autonomic dysfunction and systemic inflammation have been implicated as central to the pathophysiology of the development of early MODS following TBI. Moderate-severe TBI results in alterations of underlying central and peripheral autonomic tone, as well as significant central and peripheral catecholamine release through the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system.21-23 This resulting activation leads to direct and indirect injuries to organ systems throughout the body.24 While the prior TBI literature has focused on individual extracranial organ dysfunction, fewer studies have considered the clinical impact of the development of multi-organ dysfunction following TBI.
A significant issue in examining the epidemiology of MODS is the heterogeneity of definitions used in the TBI literature. While prior studies have used individual parameters of organ system dysfunction or clinical syndromes (such as shock, acute respiratory distress syndrome, pneumonia, and sepsis) to describe organ dysfunction following acute brain injury,2 recent studies have used clinical scoring systems, such as the Denver score, Multiple Organ Dysfunction Syndrome (MODS) score, and SOFA score.11,25 Among these scoring systems, the SOFA score strikes an improved balance between sensitivity/specificity characteristics in the trauma population,25 and it is widely used for describing organ dysfunction in critical care syndromes, such as sepsis.26 While examination of the SOFA score to describe MODS has been used in prior studies of TBI,19 our study fills important gaps by examining early MODS (when the brain is most susceptible to secondary brain injuries), examining the effect of primarily isolated brain injury on organ systems (rather than the effects of polytrauma), and examining the impact of MODS on long-term clinical, functional, and disability outcomes beyond hospital discharge. While our findings of a high burden of extracranial organ dysfunction and impact on poor hospital outcomes are consistent with prior literature, we show that the impact of early MODS on poor functional outcomes is consistent over the year following injury. Furthermore, we show that the brain injury itself may be responsible for MODS (rather than concurrent polytrauma), as our cohort was comprised of primarily isolated TBI. Therefore, further research aimed at understanding the underlying mechanisms of brain-organ interactions is needed in order to develop targeted therapies for prevention and treatment of post-TBI MODS.
In this study, we observed that 68% of subjects developed early MODS following moderate-severe TBI, and this was largely driven by cardiopulmonary dysfunction. The development of shock and cardiac dysfunction are particularly relevant, because they can have significant impact on early cerebral perfusion following injury, especially in the setting of impaired autoregulation capacity after brain injury.27,28 Further, widespread autonomic dysfunction and systemic inflammation29 following injury can lead to a cascade of additional organ dysfunction that can synergistically increase the burden of secondary brain injuries, such as hypoxia in the setting of respiratory failure, acidosis from acute kidney injury, and bleeding from hematologic dysfunction. While MODS can have an impact early after brain injury, we found that early MODS was associated with worse clinical, functional, and disability domains throughout the year following injury. Given the significant impact of early MODS on patient outcomes, treatment strategies to help improve clinical outcomes in this population may need to consider the effects of extracranial organ dysfunction on cerebral physiology and metabolism.
There are several limitations to our analysis. First, given the acute nature of moderate-severe TBI data collection, there is a risk of decreased ascertainment of baseline factors – such as co-morbidities – that may have influenced the development of early organ dysfunction; despite this risk, the subjects in our cohort were relatively young (mean age 41 years) and would not be expected to have severe underlying co-morbidities. Second, we focused on the development of early MODS (MODS likely impacted by the brain injury itself) rather than late MODS (MODS likely impacted by the development of hospital complications); therefore, we were not able to tease apart the differential impact of MODS (early versus late) on outcomes. In addition to late MODS, there may be additional events that could contribute to the development of bias in the ascertainment of 6-month outcomes. Despite this limitation, our outcomes across the spectrum of time points over the first year of injury (hospital discharge, 3 months, 6 months, and 1 year) were generally consistent. Third, we used a modification of the SOFA score (based on the available data) to derive a definition of MODS, rather than the SOFA score as originally described, although we were reassured that modified SOFA scores have been shown to have similar test characteristics to the original SOFA score and have been used in multiple studies, including as clinical endpoints in randomized controlled trials.14 Despite this, SOFA score modification may lead to overestimation or underestimation of MODS and possibly lead to misclassification. Fourth, given that we examined outcomes across the first year after injury (and that some subjects died during this period), there is a potential for survivorship bias. Fifth, despite the examination of a broad moderate-severe TBI population, generalizability may be limited, as our sample was biased toward male gender, white race, and severe TBI. Last, despite the granular data available in the TRACK-TBI database, the observational nature of our study puts the results at risk of residual confounding.
In conclusion, early multi-organ dysfunction following isolated moderate-severe TBI is common and independently impacts multiple domains (mortality, function, and disability) over the year following injury. Further research is necessary to understand underlying mechanisms, improve early recognition and prevention, and optimize management strategies for extracranial multi-organ dysfunction following injury.
Supplementary Material
Supplement Figure 1: Distribution of individual organ dysfunction over first 72 hours
Supplement Table 1: Modified SOFA Score definitions
Supplement Table 2: Sensitivity Analyses
Acknowledgments
Source of Funding: NIH (NINDS): K23NS109274 (Krishnamoorthy)
Appendix
The TRACK-TBI Investigators
Opeolu Adeoye, MD, University of Cincinnati; Neeraj Badjatia, MD, University of Maryland; Kim Boase, University of Washington; Yelena Bodien, PhD, Massachusetts General Hospital; M. Ross Bullock, MD PhD, University of Miami; Randall Chesnut, MD, University of Washington; John D. Corrigan, PhD, ABPP, Ohio State University; Karen Crawford, University of Southern California; Ramon Diaz-Arrastia, MD PhD, University of Pennsylvania; Sureyya Dikmen, PhD, University of Washington; Ann-Christine Duhaime, MD, MassGeneral Hospital for Children; Richard Ellenbogen, MD, University of Washington; V Ramana Feeser, MD, Virginia Commonwealth University; Adam R. Ferguson, PhD, University of California, San Francisco; Raquel Gardner, University of California, San Francisco; Etienne Gaudette, PhD, University of Southern California; Joseph Giacino, PhD, Spaulding Rehabilitation Hospital; Dana Goldman, PhD, University of Southern California; Luis Gonzalez, TIRR Memorial Hermann; Shankar Gopinath, MD, Baylor College of Medicine; Rao Gullapalli, PhD, University of Maryland; J Claude Hemphill, MD, University of California, San Francisco; Gillian Hotz, PhD, University of Miami; Sonia Jain, PhD, University of California, San Diego; C. Dirk Keene, MD PhD, University of Washington; Joel Kramer, PsyD, University of California, San Francisco; Natalie Kreitzer, MD, University of Cincinnati; Harvey Levin, MD, Baylor College of Medicine; Chris Lindsell, PhD, Vanderbilt University; Joan Machamer, MA, University of Washington; Christopher Madden, MD, UT Southwestern; Geoffrey T Manley, MD PhD, University of California, San Francisco; Alastair Martin, PhD, University of California, San Francisco; Thomas McAllister, MD, Indiana University; Michael McCrea, PhD, Medical College of Wisconsin; Randall Merchant, PhD, Virginia Commonwealth University; Pratik Mukherjee, MD PhD, University of California, San Francisco; Lindsay Nelson, PhD, Medical College of Wisconsin; Laura B. Ngwenya, MD, PhD, University of Cincinnati; Florence Noel, PhD, Baylor College of Medicine; Amber Nolan, MD PhD, University of California, San Francisco; David Okonkwo, MD PhD, University of Pittsburgh; Eva Palacios, PhD, University of California, San Francisco; Daniel Perl, MD, Uniformed Services University; Ava Puccio, PhD, University of Pittsburgh; Miri Rabinowitz, PhD, University of Pittsburgh; Claudia Robertson, MD, Baylor College of Medicine; Jonathan Rosand, MD, MSc, Massachusetts General Hospital; Angelle Sander, PhD, Baylor College of Medicine; Gabriella Satris, University of California, San Francisco; David Schnyer, PhD, UT Austin; Seth Seabury, PhD, University of Southern California; Murray Stein, MD MPH, University of California, San Diego; Sabrina Taylor, PhD, University of California, San Francisco; Arthur Toga, PhD, University of Southern California; Alex Valadka, MD, Virginia Commonwealth University; Mary Vassar, RN MS, University of California, San Francisco; Paul Vespa, MD, University of California, Los Angeles; Kevin Wang, PhD, University of Florida; John K. Yue, MD, University of California, San Francisco; Esther Yuh, MD PhD, University of California, San Francisco; Ross Zafonte, Harvard Medical School
Footnotes
The authors disclose no relevant conflicts of interest.
Copyright Form Disclosure: Drs. Krishnamoorthy and Foreman’s institutions received funding from the National Institutes of Health (NIH). Drs. Krishnamoorthy, Temkin, Foreman, Goldstein, and Vavilala received support for article research from the NIH. Dr. Temkin’s institution also received funding from the U.S. Federal Government; she received funding from several pharmaceutical companies and the University of Southern California. Dr. Foreman’s institution also institution received funding from the National Institute of Neurological Disorders and Stroke, the Department of Defense, and the National Science Foundation; he received funding from UCB Pharma. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References:
- 1.Centers for Disease Control and Prevention (2019). Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. [Google Scholar]
- 2.Mascia L, Sakr Y, Pasero D, Payen D, Reinhart K, Vincent JL. Extracranial complications in patients with acute brain injury: a post-hoc analysis of the SOAP study. Intensive Care Med. 2008;34(4):720–727. [DOI] [PubMed] [Google Scholar]
- 3.Zafar SN, Millham FH, Chang Y, et al. Presenting blood pressure in traumatic brain injury: a bimodal distribution of death. J Trauma. 2011;71(5):1179–1184. [DOI] [PubMed] [Google Scholar]
- 4.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54(2):312–319. [DOI] [PubMed] [Google Scholar]
- 5.Brenner M, Stein DM, Hu PF, Aarabi B, Sheth K, Scalea TM. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. J Trauma Acute Care Surg. 2012;72(5):1135–1139. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Ley EJ, Bukur M, et al. Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–1837. [DOI] [PubMed] [Google Scholar]
- 7.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–337. [DOI] [PubMed] [Google Scholar]
- 8.Aisiku IP, Yamal JM, Doshi P, et al. The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg. 2016;80(2):308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010;32(9):1060–1065. [DOI] [PubMed] [Google Scholar]
- 10.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care. 2012;16(2):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramtinfar S, Chabok SY, Chari AJ, Reihanian Z, Leili EK, Alizadeh A. Early detection of nonneurologic organ failure in patients with severe traumatic brain injury: Multiple organ dysfunction score or sequential organ failure assessment? Indian J Crit Care Med. 2016;20(10):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodien YG, McCrea M, Dikmen S, et al. Optimizing Outcome Assessment in Multicenter TBI Trials: Perspectives From TRACK-TBI and the TBI Endpoints Development Initiative. J Head Trauma Rehabil. 2018;33(3):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010;91(11):1650–1660 e1617. [DOI] [PubMed] [Google Scholar]
- 14.Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javed A, Guirgis FW, Sterling SA, et al. Clinical predictors of early death from sepsis. J Crit Care. 2017;42:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasilevskis EE, Pandharipande PP, Graves AJ, et al. Validity of a Modified Sequential Organ Failure Assessment Score Using the Richmond Agitation-Sedation Scale. Crit Care Med. 2016;44(1):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmatinejad Z, Reihani H, Tohidinezhad F, et al. Predictive performance of the SOFA and mSOFA scoring systems for predicting in-hospital mortality in the emergency department. Am J Emerg Med. 2019;37(7):1237–1241. [DOI] [PubMed] [Google Scholar]
- 18.Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Hwang H, Yamal JM, et al. IMPACT probability of poor outcome and plasma cytokine concentrations are associated with multiple organ dysfunction syndrome following traumatic brain injury. J Neurosurg. 2019;131(6):1931–1937. [DOI] [PubMed] [Google Scholar]
- 20.Yeole U, Krishnakumar M, Gopalakrishna KN, Sekar A, Sadashiva N, Shukla D. Surgical outcomes in traumatic brain injuries with bilateral mass occupying lesions. Analysis of prognostic factors. Clin Neurol Neurosurg. 2020;196:106017. [DOI] [PubMed] [Google Scholar]
- 21.Rosner MJ, Newsome HH, Becker DP. Mechanical brain injury: the sympathoadrenal response. J Neurosurg. 1984;61(1):76–86. [DOI] [PubMed] [Google Scholar]
- 22.Koiv L, Merisalu E, Zilmer K, Tomberg T, Kaasik AE. Changes of sympatho-adrenal and hypothalamo-pituitary-adrenocortical system in patients with head injury. Acta Neurol Scand. 1997;96(1):52–58. [DOI] [PubMed] [Google Scholar]
- 23.Rizoli SB, Jaja BN, Di Battista AP, et al. Catecholamines as outcome markers in isolated traumatic brain injury: the COMA-TBI study. Crit Care. 2017;21(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald SJ, Sharkey JM, Sun M, et al. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J Neurotrauma. 2020;37(5):770–781. [DOI] [PubMed] [Google Scholar]
- 25.Frohlich M, Wafaisade A, Mansuri A, et al. Which score should be used for posttraumatic multiple organ failure? - Comparison of the MODS, Denver- and SOFA- Scores. Scand J Trauma Resusc Emerg Med. 2016;24(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–17; discussion 17-19. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt B, Klingelhofer J, Perkes I, Czosnyka M. Cerebral autoregulatory response depends on the direction of change in perfusion pressure. J Neurotrauma. 2009;26(5):651–656. [DOI] [PubMed] [Google Scholar]
- 29.Casault C, Al Sultan AS, Banoei M, Couillard P, Kramer A, Winston BW. Cytokine Responses in Severe Traumatic Brain Injury: Where There Is Smoke, Is There Fire? Neurocrit Care. 2019;30(1):22–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: Distribution of individual organ dysfunction over first 72 hours
Supplement Table 1: Modified SOFA Score definitions
Supplement Table 2: Sensitivity Analyses