Abstract
Objective:
Assess the influence of cochlear implant (CI) use on the perceived listening effort of adult and pediatric subjects with unilateral hearing loss (UHL) or asymmetric hearing loss (AHL)
Study Design:
Prospective cohort
Setting:
Tertiary referral center
Patients:
Adults and children with UHL or AHL
Intervention:
Cochlear implantation. Subjects received their CI as part of a clinical trial assessing the effectiveness of cochlear implantation in cases of UHL and AHL.
Main Outcome Measure(s):
Responses to the Listening Effort pragmatic subscale on the Speech, Spatial, and Qualities of Hearing (SSQ) scale or SSQ for Children with Impaired Hearing (SSQ-C) were compared over the study period. Subjects or their parents completed the questionnaires preoperatively and at pre-determined post-activation intervals. For the adult subjects, responses were compared to word recognition in quiet and sentence recognition in noise.
Results:
Forty adult subjects (n=20 UHL, n=20 AHL) and 16 pediatric subjects with UHL enrolled and underwent cochlear implantation. Subjects in all three groups reported a significant reduction in perceived listening effort within the initial months of CI use (p<0.001; η2 ≥0.351). The perceived benefit was significantly correlated with speech recognition in noise for the adult subjects with UHL at the 12-month interval (r(20)=.59, p=0.006).
Conclusions:
Adult and pediatric CI recipients with UHL or AHL report a reduction in listening effort with CI use as compared to their preoperative experiences. Use of the SSQ and SSQ-C Listening Effort pragmatic subscale may provide additional information about a CI recipient’s experience beyond the abilities measured in the sound booth.
INTRODUCTION
Listening effort, defined as the mental exertion required to attend to and understand an auditory message1, is a known handicap for patients with hearing loss and has a significant, negative impact on quality of life2–4. Patients with moderate-to-profound unilateral hearing loss (UHL) and asymmetric hearing loss (AHL) report increased listening effort likely due to limited access to binaural cues4–9. Binaural input improves speech recognition in dynamic listening conditions as compared to monaural listening10–12. Cochlear implantation of the poorer hearing ear and subsequent cochlear implant (CI) use may improve the spatial hearing abilities of patients with UHL and AHL due to bilateral auditory stimulation. Listening with a CI plus the acoustic-hearing ear may reduce perceived listening effort as compared to preoperative abilities in a monaural listening condition.
Emerging evidence from adult and pediatric CI recipients with UHL or AHL demonstrate cochlear implantation to be an effective treatment option on measures of speech recognition, spatial hearing, and quality of life as compared to an unaided condition or with technologies that route the signal to the better hearing ear, such as contralateral routing of signal (CROS) hearing aids (HA), bilateral CROS HAs (Bi-CROS HA), or bone conduction devices5,6,13–20. Little is known, however, about the influence of CI use on listening effort in subjects with UHL and AHL. One method to measure perceived listening effort before and after cochlear implantation is with a subjective questionnaire. The Speech, Spatial, and Qualities of Hearing Scale (SSQ)21, and The SSQ for Children with Impaired Hearing (SSQ-C)22 assess the subject’s perceived abilities in different listening situations. Scoring is typically calculated as the mean of all responses (total score) and the mean responses on the three subscales (i.e., Speech Hearing, Spatial Hearing, and Qualities of Hearing). The SSQ can also be scored as the mean responses on pragmatic subscales, which were defined and proposed by Gatehouse & Akeroyd10 to assess perceived binaural hearing abilities in dynamic listening conditions. For instance, Gatehouse and Akeroyd10 reported a strong correlation between binaural hearing abilities and the benefits of bilateral HA use as compared to unilateral HA use across all pragmatic subscales, which was most apparent in challenging listening environments. Listening Effort is one of the pragmatic subscales within the Qualities of Hearing subscale. Table 1 lists the specific questions from the SSQ and SSQ-C for the Listening Effort pragmatic subscale10,22.
Table 1:
Questions from the Qualities of Hearing subscale on the Speech, Spatial, and Qualities of Hearing Scale (SSQ) and the SSQ for Children with Impaired Hearing (SSQ-C) used to derive the Listening Effort pragmatic subscale (Gatehouse & Akeroyd, 2006; Galvin & Noble, 2013).
| Question Number | Question | |
|---|---|---|
| SSQ | #14 | “Do you have to concentrate very much when listening to someone or something?” |
| #15 | “Do you have to put in a lot of effort to hear what is being said in a conversation with others?” | |
| #18 | “Can you easily ignore other sounds when trying to listen to something?” | |
| SSQ-C | #7 | “Do you have to try very hard when listening to someone or something?” |
| #9 | “Do you have to try hard to understand what other people are saying?” | |
| #10 | “Is it easy for you to ignore other sounds when trying to listen to something? |
The SSQ Listening Effort pragmatic subscale may be an effective measure of perceived abilities over time when listening with a CI plus the contralateral ear as compared to preoperative abilities in CI recipients with UHL and AHL. Dwyer et al4 reported greater perceived listening effort using the SSQ pragmatic subscale in adult subjects when listening in a monaural CI condition as compared to bilateral normal-hearing (NH) subjects. Subjects reported a significant reduction in perceived listening effort when listening with bilateral CIs as compared to the monaural CI condition. Similarly, children who met traditional candidacy criteria have experienced a reduction in listening effort with bilateral CIs as compared to the monaural listening condition8. Early significant reductions in perceived listening effort have been observed in adult CI recipients with UHL5 and AHL6 as compared to preoperative abilities. Twenty adult CI recipients with UHL5 and twenty adult CI recipients with AHL6 reported a significant reduction in perceived listening effort using the SSQ after one month of CI use as compared to preoperative perceptions. Of interest is whether there are differences in perceived listening effort over time between CI recipients with UHL and AHL, considering the hearing loss in the contralateral ear for the AHL recipients, which may influence initial performance in challenging listening situations. For instance, the CI recipients with UHL continued to report significant reductions in perceived listening effort through the 12-month interval5 whereas the CI recipients with AHL demonstrated no difference between the 1-month and 12-month intervals6. For pediatric CI recipients with UHL, the influence of CI use on perceived listening effort is unknown. It is hypothesized that pediatric CI recipients with UHL will experience significant reductions in perceived listening effort when listening with the CI plus the acoustic-hearing ear as compared to preoperative abilities.
The primary aim of the present report was to review the pattern of perceived listening effort during the first year of CI use in adult and pediatric subjects with UHL and AHL. Previous investigations demonstrate that adult CI recipients with UHL experience early, significant improvements in speech recognition and spatial hearing with the CI alone and with the CI plus the NH-ear14,15, which may underlie their perceptions of listening effort. Considering this, a secondary aim was to review whether reductions in perceived listening effort were associated with word recognition in quiet with the CI only and sentence recognition in noise with the CI plus the acoustic-hearing ear during the first year of CI use.
METHODS
Subjects received a CI as part of their participation in a clinical trial investigating the effectiveness of cochlear implantation in either adult or pediatric cases of UHL and AHL. The study procedures were approved by the study site IRB and the FDA as part of two Investigational Device Exemptions. Adult subjects provided consent, and pediatric subjects and/or their parents provided assent or consent to participate in the study procedures.
Adult Cohort
Adult subjects with UHL and AHL presented preoperatively with a pure tone average (PTA; 500, 1000 and 2000 Hz) ≥70 dB HL and aided CNC word recognition 23 of ≤60% in the ear to be implanted. Unaided thresholds in the contralateral ear were ≤35 dB HL from 125-8000 Hz for the UHL cohort and between 35-55 dB HL (PTA) for the AHL cohort. Subjects were implanted with the MED-EL Standard electrode array (31 mm) via round window approach and fit with an ear-level audio processor (MED-EL GmbH, Innsbruck, Austria). Activation of the CI occurred two to four weeks after cochlear implantation.
Subjects completed the SSQ (version 5.6) and speech recognition assessment (i.e., word recognition in quiet and sentence recognition in noise) at the preoperative interval and at 1-month, 3-months, 6-months, 9-months, and 12-months post-activation. At the preoperative interval, subjects responded to the SSQ based on their perceived abilities when listening with a traditional listening option for UHL or AHL (i.e., unaided, CROS HA, Bi-CROS HA, bone-conduction device, conventional HA). At the post-activation intervals, UHL subjects responded based on their perceived abilities when listening with the CI plus NH-ear and AHL subjects responded based on their perceived abilities when listening with the CI plus a HA on the acoustic-hearing ear (bimodal).
Speech recognition assessment was conducted in a sound booth, with recorded materials presented at 60 dB SPL. Word recognition in quiet was assessed with the CNC words test. Subjects were seated one meter from the loudspeaker. Subjects listened with a HA in the ear to be implanted at the preoperative interval and with the CI at the post-activation intervals. Masking was presented to the contralateral ear via an insert phone and a TDH headphone placed over the pinna. Sentence recognition in noise was assessed with the AzBio sentence test in a 10-talker masker24. Subjects were assessed in the unaided condition at the preoperative interval and with the CI plus the acoustic-hearing ear at the post-activation intervals. The assessment was conducted with subjects seated in the center of an 180° arc of loudspeakers, spanning +/−90°. Subjects were approximately one meter away from the loudspeakers and faced the center speaker at 0° azimuth. Subjects were assessed at either 10, 5, or 0 dB signal-to-noise ratio (SNR). An individual’s SNR was determined at the preoperative and 1-month intervals based on his or her performance when the target and the masker were co-located. Speech recognition was assessed initially at 10 dB SNR and the SNR was decreased in 5 dB steps until the subject scored ≤50% on a sentence list or reached 0 dB SNR to limit ceiling effects. The present analysis evaluated the sentence recognition in noise for the subjects who were evaluated at 0 dB SNR. The target was presented from the center loudspeaker, and the masker was presented 90° to the acoustic-hearing ear (SoNcontra). This target and masker condition assesses the head shadow effect and was selected as it may most likely reflect the benefit of bilateral auditory stimulation. For the word recognition in quiet and sentence recognition in noise measures, subjects were asked to repeat what they heard and encouraged to guess if unsure. Performance was calculated as the percent of correctly repeated words.
Pediatric Cohort
Pediatric subjects with UHL presented preoperatively with a PTA ≥70 dB HL and aided CNC word recognition of ≤30% in the ear to be implanted, and a PTA ≤25 dB HL in the contralateral ear. Preoperative imaging was used to confirm an anatomically normal cochlea or no more significant malformation than Mondini malformation, and a present cochlear nerve. The majority of the pediatric subjects were implanted with the MED-EL Flex28 (28 mm) electrode array (MED-EL GmbH, Innsbruck, Austria). One subject was implanted with the Flex24 (24 mm) electrode array due to a Mondini malfolrmation. Subjects were implanted via a cochleostomy or round window approach. Activation of the CI occurred two to four weeks after cochlear implantation.
The parents of pediatric subjects completed the SSQ-C as a proxy for their child at the preoperative interval and at 3-months, 6-months, 9-months, and 12-months post-activation. At the preoperative interval, parents responded based on their perceptions of the child’s abilities in his or her typical listening condition. One subject used a bone conduction device and three utilized conventional amplification (i.e., behind-the-ear HA). The remaining subjects did not use any treatment. At the post-activation intervals, parents responded based on their perceptions of the child’s abilities when listening with his or her CI plus the NH-ear.
Data Analysis
Perceived listening effort was calculated as the mean responses to specific Qualities of Hearing subscale questions on the SSQ or SSQ-C, as defined in Table 1. Responses were assessed over the study period with a repeated-measures ANOVA using SPSS (IBM Corporation, Armonk, NY, version 26), with one model for the adult data and one for the pediatric data. The model for the adult data included cohort (i.e., UHL or AHL) as a between-subjects factor to assess potential differences related to the hearing sensitivity in the acoustic-hearing ear. There were missing data from one pediatric subject at the 6-month interval and two adult subjects with AHL at the 9-month interval. Also, one adult subject with AHL was withdrawn from the study after the 6-month interval due to moving out of state. Missing data were replaced with cohort mean data in the models. Linear Mixed Models using the R statistical software (R Core Team, 2019) with missing data and a random intercept for subject confirmed the results obtained with the repeated-measures ANOVA. Significance was defined as ∝ < 0.05.
For the adult subjects with UHL or AHL, responses on the Listening Effort subscale were compared to word recognition in quiet and sentence recognition in noise at the preoperative, 1-month, and 12-month intervals with a Bivariate Pearson Correlation using the SPSS software (version 26). The Bonferroni correction was applied to limit Type 1 error due to multiple comparisons. A rationalized arcsine transform was applied to percent correct data to normalize error variance 25 prior to statistical analysis.
RESULTS
Forty adult subjects (n=20 UHL, n=20 AHL) underwent cochlear implantation and participated in the study procedures. Demographic information is listed in Table 2. For the adult subjects (23 female), the age at implantation ranged from 23 to 79 years, with a mean of 60 years for the entire cohort (SD: 14 years). On average, subjects in the AHL cohort were older at the time of cochlear implantation (mean: 70 years; SD: 7 years) than subjects in the UHL cohort (mean: 50 years; SD: 11 years). The majority of subjects reported an unknown etiology of hearing loss (n=31). The remaining cases reported an etiology of Meniere’s disease (n=6), viral infection (n=1), noise-induced hearing loss (n=1), or trauma (n=1).
Table 2:
Demographic information for the adult subjects with UHL or AHL. Subjects are ordered by age at implantation within each cohort.
| Cohort/Subject | Gender | Etiology | Onset of hearing loss | Preoperative PTA | Preoperative Aided CNC | Age | SNR | |
|---|---|---|---|---|---|---|---|---|
| CI-ear | Contra | |||||||
| UHL1 | M | Trauma | Sudden | 110 | 7 | 0 | 22.6 | + |
| UHL2 | F | Unknown | Sudden | 82 | 3 | 0 | 28.6 | + |
| UHL3 | F | Unknown | Sudden | 83 | 2 | 0 | 33.5 | + |
| UHL4 | M | Meniere’s disease | Sudden | 115 | 0 | 0 | 43.7 | + |
| UHL5 | F | Unknown | Sudden | 107 | 2 | 0 | 44.2 | + |
| UHL6 | F | Unknown | Gradual | 113 | 10 | 10 | 45.0 | + |
| UHL7 | F | Meniere’s disease | Gradual | 120 | 8 | 0 | 46.4 | + |
| UHL8 | F | Meniere’s disease | Gradual | 70 | 7 | 0 | 49.9 | + |
| UHL9 | F | Unknown | Sudden | 80 | 8 | 0 | 50.3 | + |
| UHL10 | F | Unknown | Sudden | 73 | 22 | 0 | 50.4 | + |
| UHL11 | M | Unknown | Sudden | 90 | 15 | 6 | 53.2 | + |
| UHL12 | M | Unknown | Sudden | 80 | 8 | 0 | 54.1 | + |
| UHL13 | F | Unknown | Sudden | 87 | 15 | 18 | 56.0 | + |
| UHL14 | F | Unknown | Gradual | 103 | 12 | 12 | 56.6 | + |
| UHL15 | F | Unknown | Sudden | 78 | 12 | 24 | 58.1 | + |
| UHL16 | M | Unknown | Sudden | 72 | 3 | 0 | 58.3 | + |
| UHL17 | F | Unknown | Gradual | 78 | 3 | 0 | 60.6 | + |
| UHL18 | M | Unknown | Sudden | 118 | 12 | 2 | 61.7 | + |
| UHL19 | M | Unknown | Sudden | 78 | 13 | 0 | 62.6 | + |
| UHL20 | M | Unknown | Sudden | 77 | 2 | 14 | 66.0 | + |
| AHL1 | M | Unknown | Sudden | 102 | 40 | 4 | 52.1 | − |
| AHL2 | M | Unknown | Sudden | 75 | 43 | 0 | 58.0 | + |
| AHL3 | M | Viral infection | Sudden | 70 | 35 | 0 | 59.5 | − |
| AHL4 | F | Unknown | Sudden | 83 | 35 | 12 | 67.7 | + |
| AHL5 | M | Unknown | Sudden | 68 | 35 | 12 | 67.8 | + |
| AHL6 | F | Unknown | Sudden | 120 | 37 | 14 | 68.2 | + |
| AHL7 | F | Unknown | Sudden | 72 | 35 | 18 | 68.3 | + |
| AHL8 | F | Unknown | Gradual | 90 | 45 | 0 | 70.3 | − |
| AHL9 | F | Meniere’s disease | Gradual | 83 | 37 | 14 | 70.5 | − |
| AHL10 | F | Meniere’s disease | Sudden | 70 | 40 | 0 | 70.7 | + |
| AHL11 | F | Unknown | Sudden | 70 | 35 | 24 | 71.4 | + |
| AHL12 | M | NIHL | Gradual | 107 | 35 | 0 | 72.6 | + |
| AHL13 | F | Unknown | Gradual | 102 | 45 | 2 | 74.5 | − |
| AHL14 | M | Meniere’s disease | Gradual/Sudden | 70 | 35 | 0 | 74.5 | + |
| AHL15 | F | Unknown | Gradual | 95 | 50 | 0 | 75.2 | + |
| AHL16 | M | Unknown | Sudden | 82 | 35 | 0 | 75.3 | + |
| AHL17 | M | Unknown | Gradual/Sudden | 115 | 42 | 20 | 75.7 | − |
| AHL18 | F | Unknown | Sudden | 82 | 35 | 12 | 76.7 | + |
| AHL19 | M | Unknown | Sudden | 120 | 42 | 0 | 76.8 | + |
| AHL20 | F | Unknown | Gradual | 90 | 37 | 4 | 79.1 | − |
Preoperative PTA reported in dB HL, rounded to nearest whole number. Preoperative aided CNC for the ear-to-be implanted reported in percent correct. Age at cochlear implantation reported in years.
The signal-to-noise ratio (SNR) column indicates the subjects who were (+) versus who were not (−) assessed at 0 dB SNR on the sentence recognition in noise task.
NIHL: noise-induced hearing loss; PTA: pure tone average.
Sixteen pediatric subjects (8 female) underwent cochlear implantation and completed the reviewed study intervals at the time of analysis. Demographic information is listed in Table 3. The majority had an unknown etiology of hearing loss (n=9). The remaining cases reported an etiology of congenital Cytomegalovirus (cCMV) infection (n=2), Mondini malformation (n=2), Waardenberg syndrome (n=1), other infection (n=1), or trauma (n=1). Four subjects failed their newborn hearing screening and were subsequently diagnosed with UHL. For the remaining subjects, three passed a follow-up hearing screening, one was not screened, and the remainder passed the initial newborn hearing screening (n=8); considering this, there was not documentation available for the onset of hearing loss for the majority of subjects. Seven subjects provided medical records with evidence of sudden (n=2), progressive (n=3), or congenital (n=2) moderate-to-profound UHL. For the remaining nine subjects, their parents reported the UHL as sudden (n=4), progressive (n=2), or congenital (n=3). The age at implantation ranged from 3.7 to 12.7 years, with a mean of 5.9 years (SD: 2.1 years). Fifteen subjects were implanted with the Flex28 electrode array and one subject with the Flex24 electrode array (PED#3).
Table 3:
Demographic information for the pediatric subjects with UHL. Subjects are ordered by age at implantation.
| Cohort/Subject | Gender | Etiology | Hearing Stability | Age |
|---|---|---|---|---|
| PED1 | F | Unknown | Reported Congenital | 3.7 |
| PED2 | F | cCMV | Known Progressive | 3.9 |
| PED3 | M | Mondini malformation | Known Congenital | 4.0 |
| PED4 | F | Trauma | Known Sudden | 4.5 |
| PED5 | M | Syndromic (Waardenberg) | Known Congenital | 4.7 |
| PED6 | M | Unknown | Known Progressive | 4.8 |
| PED7 | M | Unknown | Reported Sudden | 5.4 |
| PED8 | M | Unknown | Known Progressive | 5.5 |
| PED9 | F | Mondini malformation | Reported Congenital | 6.1 |
| PED10 | F | Unknown | Reported Progressive | 6.1 |
| PED11 | F | Unknown | Reported Sudden | 6.3 |
| PED12 | M | Infection | Known Sudden | 6.4 |
| PED13 | F | Unknown | Reported Congenital | 6.5 |
| PED14 | M | Unknown | Reported Progressive | 6.5 |
| PED15 | F | cCMV | Reported Sudden | 7.0 |
| PED16 | M | Unknown | Reported Sudden | 12.7 |
Age is reported as age at cochlear implantation in years.
cCMV: congenital Cytomegalovirus
Figure 1 plots the responses on the Listening Effort pragmatic subscale for the adult and pediatric cohorts over the study period. A higher value indicates better perceived abilities, which reflects a reduction in listening effort. Responses are indicated with open boxes for adult subjects with UHL, filled boxes for adult subjects with AHL, and diagonal hatching boxes for pediatric subjects with UHL. All cohorts demonstrated a reduction in perceived listening effort in the post-activation period as compared to preoperative perceptions.
Figure 1:
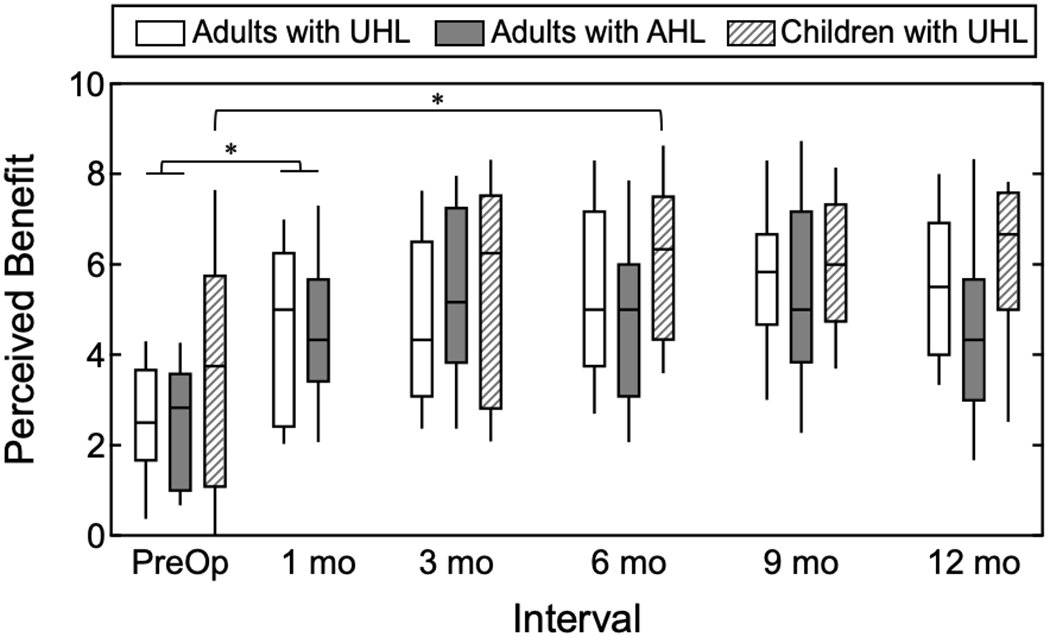
Responses on the Listening Effort pragmatic subscale over time for adults with UHL, adults with AHL, and children with UHL. Boxes indicate the distribution of values for each cohort at each test interval. The horizontal line indicates the median, boxes the 25th and 75th percentile, and the tails the 10th and 90th percentiles. Subjects responded with perceived abilities with a traditional listening option for UHL or AHL at the preoperative interval and with the CI plus the acoustic-hearing ear at the post-activation intervals. A significant difference in perceived listening effort across the study period was observed for the adult subjects and the pediatric subjects. For the adult subjects, a significant reduction in perceived listening effort was observed between the preoperative and 1-month intervals. For the pediatric subjects, a significant reduction in perceived listening effort was observed between the preoperative and 6-month intervals.
For the adult subjects, there was a significant difference in the responses provided across the study period (F(5,190)=22.47, p<0.001, η2 =0.372). There was a non-significant interaction between cohort and interval (F(5,190)=1.65, p=0.149, η2 =0.042), indicating similar responses between the UHL and AHL cohorts over time. Review of the simple effects revealed a significant reduction in perceived listening effort between the preoperative and 1-month intervals (p<0.001), with non-significant changes between all other adjacent intervals (p≥0.05).
The parent responses for the pediatric subjects also demonstrated a significant difference in scores on the Listening Effort pragmatic subscale over the study period (F(4,60)=8.13, p<0.001, η2 =0.351). Review of the simple effects revealed that perceived listening effort did not change significantly between the preoperative and 3-month interval (p=0.173). There was a significant reduction in perceived listening effort between the preoperative and 6-month intervals (p=0.025).
Responses on the Listening Effort pragmatic subscale at the preoperative, 1-month, and 12-month intervals were compared to the performance on the speech recognition tasks (i.e., word recognition in quiet and sentence recognition in noise) for the adult subjects with UHL or AHL. Figure 2 plots the CNC word scores (top row) and AzBio sentences in spatially-separated noise scores (bottom row) as a function of perceived listening effort (perceived benefit) at each interval. Symbols indicate results for adults with UHL (open circles) and AHL (filled diamonds). Speech recognition scores are reported in RAUs, where a higher value indicates better performance. Thirty-three adults were evaluated at 0 dB SNR on the sentence recognition in noise task.
Figure 2:
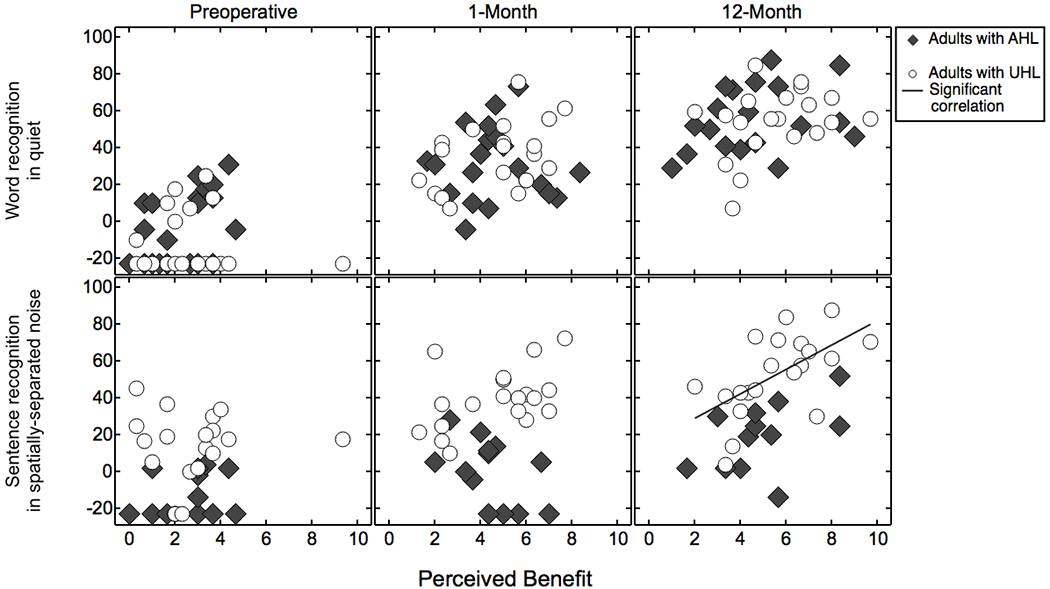
Speech recognition as a function of perceived benefit, shown separately for word recognition in quiet with the CI alone (top row) and sentence recognition in spatially-separated noise with the CI and the acoustic-hearing ear (bottom). Subjects are adults with UHL or AHL who underwent testing at the preoperative, 1-month, and 12-month intervals. Speech recognition scores are reported in rationalized arcsine units (RAUs). Higher values indicate greater perceived benefit and better speech recognition. The solid line indicates significant correlation between sentence recognition in spatially-separated noise (SoNcontra) and perceived listening effort at the 12-month interval for the UHL cohort.
Table 4 lists the results of the comparisons between speech recognition and perceived listening effort for the adult UHL and AHL cohorts at each interval, with significant correlations indicated in bold italic. There was a significant correlation between responses on the Listening Effort pragmatic subscale and sentence recognition in noise for the UHL cohort at the 12-month interval (r(20)=.59, p=0.006). All other comparisons were non-significant.
Table 4:
Results of the correlations between perceived listening effort and speech recognition (i.e., word recognition in quiet with the CI alone and masked sentence recognition with the CI plus acoustic-hearing ear) for adult subjects with UHL or AHL at the preoperative, 1-month, and 12-month intervals. The Bonferroni corrected p values are reported, with bold italic indicating significant correlation.
| Adults with UHL | Adults with AHL | |||||
|---|---|---|---|---|---|---|
| Preop | 1-mo | 12-mo | Preop | 1-mo | 12-mo | |
| Word Recognition | r = −0.09, p = 0.999 | r = 0.43, p = 0.366 | r = 0.32, p = 0.984 | r = 0.46, p = 0.246 | r = −0.03, p = 0.999 | r = 0.30, p = 0.999 |
| Masked Sentence Recognition | r = 0.07, p = 0.999 | r = 0.47, p = 0.222 | r = 0.59, p = 0.036 | r = 0.15, p = 0.999 | r = −0.50, p = 0.498 | r = 0.49, p = 0.648 |
DISCUSSION
Adult and pediatric subjects with UHL or AHL experienced significant reductions in perceived listening effort with CI use as compared to preoperative abilities. The early significant reductions in perceived listening effort observed here were maintained over the 12-month post-activation study period. These results indicate that providing bilateral input with a CI in cases of UHL and AHL reduces perceived listening effort, even while the listener is continuing to acclimate to electric stimulation.
A positive correlation between responses on the Listening Effort pragmatic subscale and speech recognition in noise was observed for the adult subjects with UHL at the 12-month interval, but not for the subjects with AHL. This discrepancy could be due to differences in demographics or hearing history between the UHL and AHL cohorts, including the hearing sensitivity in the acoustic-hearing ear and the age at implantation. First, access to sound from the NH-ear in the UHL cohort may support more rapid acclimatization in the combined condition as compared to subjects with AHL listening in a bimodal configuration. In previously published data, the present UHL cohort experienced significantly improved sentence recognition in spatially-separated noise after 1 month of CI use, with asymptotic performance demonstrated after 3 months15. Subjects in the AHL cohort may require additional time to acclimate to the combination of a CI and contralateral HA as compared to CI recipients with UHL16,19, thus a correlation between performance in noise and perceived listening effort may not be observed within the initial months of CI use. Additionally, the advanced age at implantation of the AHL cohort compared to the UHL cohort (mean AHL: 70 years; mean UHL: 50 years) may influence the acclimatization with the CI, and thus the listening effort needed in challenging listening situations. Advanced age at implantation has been shown to be associated with poorer speech recognition26–29. It is possible that the older adult CI recipients require additional listening experience before a relationship between sentence recognition in spatially-separated noise and perceived listening effort is observed.
Neither cohort experienced a significant correlation between word recognition in quiet with the CI only and perceived listening effort. One interpretation of this result is that the word recognition with the CI only (a monaural condition) is not strongly associated with perceived listening difficulties with bilateral input, as measured with the Listening Effort subscale. Ongoing work is assessing whether performance with the CI only is associated with performance on spatial hearing measures for CI recipients with UHL and AHL. This finding also highlights the limitation of assessing the benefits associated with CI use in a monaural listening condition alone. Expanding test batteries to include subjective questionnaires and the assessment of spatial hearing abilities in the bilateral condition may provide a fuller representation of CI recipient performance.
Our findings substantiate previous investigations of improved quality of life with CI use in adult recipients with UHL and AHL5,6,13–19, and add to the emerging literature on the effectiveness of CI use in pediatric recipients with UHL. Children with UHL are at risk for increased fatigue compared to their NH peers, despite having a NH-ear7. This observation has been interpreted as indicating that listening with two ears reduces listening effort for children in challenging listening situations. For example, traditional pediatric CI recipients demonstrate reductions in listening effort after second CI, with results comparable to their NH peers8. The present report found pediatric CI recipients with UHL experienced significantly reduced perceived listening effort with bilateral input as compared to preoperative perceptions. Ongoing work is assessing the development of spatial hearing abilities in this cohort and the potential association with perceived listening effort.
While the present findings support a reduction in perceived listening effort with CI use for adult and pediatric cases of UHL or AHL, there are limitations that warrant discussion. The study design used subjective questionnaires to measure the perceived listening effort of adults with UHL or AHL and children with UHL. Though subjective questionnaires assess the perception of listening effort, they do not capture the magnitude of the effort needed for specific tasks. The amount of listening effort expended on a given task is influenced by the individual’s hearing abilities, available cognitive energy, motivation, and the task itself30. Future work should include objective measures to assess listening effort in this patient population, including assessment of response time31, pupil dilation32–34, or performance on dual-task paradigms35,36. Also, assessment of the relationship between performance on objective measures and perceived listening effort may provide additional support of the utility of subjective questionnaires as part of the preoperative and postoperative test battery for CI recipients.
CONCLUSION
Adult and pediatric CI recipients with UHL and AHL experience early, significant improvements in perceived listening effort as measured with the SSQ or SSQ-C. Incorporating subjective measures, such as the pragmatic subscales with the SSQ and SSQ-C, provides additional insight into the benefits of CI use on the quality of life and daily abilities for patients with UHL and AHL, and provides further evidence of the effectiveness of cochlear implantation in these patient populations.
Acknowledgements:
The authors thank Emily Buss, PhD for her insight on this work. We acknowledge the regulatory assistance of the North Carolina Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The clinical trials were supported by research grants from MED-EL Corporation.
Disclosures:
MTD, MAR, LRP, and MER are supported by a research grant provided to their university. KDB serves on the surgical advisory board for MED-EL Corporation. HCP is a consultant for MED-EL Corporation. BPO is a consultant for Advanced Bionics and Johnson & Johnson.
References:
- 1.McGarrigle R, Munro KJ, Dawes P, et al. Listening effort and fatigue: what exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘white paper’. International journal of audiology. 2014;53(7):433–440. [DOI] [PubMed] [Google Scholar]
- 2.Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. The Annals of otology, rhinology, and laryngology. 2010;119(11):772–781. [PubMed] [Google Scholar]
- 3.Ohlenforst B, Zekveld AA, Jansma EP, et al. Effects of Hearing Impairment and Hearing Aid Amplification on Listening Effort: A Systematic Review. Ear and hearing. 2017;38(3):267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwyer NY, Firszt JB, Reeder RM. Effects of unilateral input and mode of hearing in the better ear: self-reported performance using the speech, spatial and qualities of hearing scale. Ear and hearing. 2014;35(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon MT, Buss E, Rooth MA, et al. Effect of Cochlear Implantation on Quality of Life in Adults with Unilateral Hearing Loss. Audiology & neuro-otology. 2017;22(4-5):259–271. [DOI] [PubMed] [Google Scholar]
- 6.Thompson NJ, Dillon MT, Buss E, et al. Subjective Benefits of Bimodal Listening in Cochlear Implant Recipients with Asymmetric Hearing Loss. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2020:194599820911716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bess FH, Davis H, Camarata S, et al. Listening-Related Fatigue in Children With Unilateral Hearing Loss. Language, speech, and hearing services in schools. 2020;51(1):84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes KC, Galvin KL. Measuring listening effort expended by adolescents and young adults with unilateral or bilateral cochlear implants or normal hearing. Cochlear implants international. 2013;14(3):121–129. [DOI] [PubMed] [Google Scholar]
- 9.Hick CB, Tharpe AM. Listening effort and fatigue in school-age children with and without hearing loss. Journal of speech, language, and hearing research : JSLHR. 2002;45(3):573–584. [DOI] [PubMed] [Google Scholar]
- 10.Gatehouse S, Akeroyd M. Two-eared listening in dynamic situations. International journal of audiology. 2006;45Suppl 1:S120–124. [DOI] [PubMed] [Google Scholar]
- 11.Welsh LW, Welsh JJ, Rosen LF, et al. Functional impairments due to unilateral deafness. The Annals of otology, rhinology, and laryngology. 2004;113(12):987–993. [DOI] [PubMed] [Google Scholar]
- 12.Rothpletz AM, Wightman FL, Kistler DJ. Informational masking and spatial hearing in listeners with and without unilateral hearing loss. Journal of speech, language, and hearing research : JSLHR. 2012;55(2):511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011;32(1):39–47. [DOI] [PubMed] [Google Scholar]
- 14.Dillon MT, Buss E, Anderson ML, et al. Cochlear Implantation in Cases of Unilateral Hearing Loss: Initial Localization Abilities. Ear and hearing. 2017;38(5):611–619. [DOI] [PubMed] [Google Scholar]
- 15.Buss E, Dillon MT, Rooth MA, et al. Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends in hearing. 2018;22:2331216518771173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firszt JB, Reeder RM, Holden LK, et al. Results in Adult Cochlear Implant Recipients With Varied Asymmetric Hearing: A Prospective Longitudinal Study of Speech Recognition, Localization, and Participant Report. Ear and hearing. 2018;39(5):845–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeitler DM, Sladen DP, DeJong MD, et al. Cochlear implantation for single-sided deafness in children and adolescents. International journal of pediatric otorhinolaryngology. 2019;118:128–133. [DOI] [PubMed] [Google Scholar]
- 18.Deep NL, Gordon SA, Shapiro WH, et al. Cochlear Implantation in Children with Single-Sided Deafness. The Laryngoscope. 2020. [DOI] [PubMed] [Google Scholar]
- 19.Dillon MT, Buss E, Rooth MA, et al. Cochlear Implantation in Cases of Asymmetric Hearing Loss: Subjective Benefit, Word Recognition, and Spatial Hearing. Trends in hearing. 2020;24:2331216520945524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvin JJ 3rd, Fu QJ, Wilkinson EP, et al. Benefits of Cochlear Implantation for Single-Sided Deafness: Data From the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear and hearing. 2019;40(4):766–781. [DOI] [PubMed] [Google Scholar]
- 21.Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). International journal of audiology. 2004;43(2):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvin KL, Noble W. Adaptation of the speech, spatial, and qualities of hearing scale for use with children, parents, and teachers. Cochlear implants international. 2013;14(3):135–141. [DOI] [PubMed] [Google Scholar]
- 23.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. The Journal of speech and hearing disorders. 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 24.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear and hearing. 2012;33(1):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Studebaker GA. A “rationalized” arcsine transform. Journal of speech and hearing research. 1985;28(3):455–462. [DOI] [PubMed] [Google Scholar]
- 26.Füllgrabe C, Moore BC. Effects of age and hearing loss on stream segregation based on interaural time differences. The Journal of the Acoustical Society of America. 2014;136(2):El185–191. [DOI] [PubMed] [Google Scholar]
- 27.Eddins AC, Ozmeral EJ, Eddins DA. How aging impacts the encoding of binaural cues and the perception of auditory space. Hearing research. 2018;369:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2004;25(3):298–301. [DOI] [PubMed] [Google Scholar]
- 29.Waltzman SB, Fisher SG, Niparko JK, et al. Predictors of postoperative performance with cochlear implants. The Annals of otology, rhinology & laryngology Supplement. 1995;165:15–18. [PubMed] [Google Scholar]
- 30.Pichora-Fuller MK, Kramer SE, Eckert MA, et al. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear and hearing. 2016;37Suppl 1:5s–27s. [DOI] [PubMed] [Google Scholar]
- 31.Brennan MA, Lewis D, McCreery R, et al. Listening Effort and Speech Recognition with Frequency Compression Amplification for Children and Adults with Hearing Loss. Journal of the American Academy of Audiology. 2017;28(9):823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winn MB, Moore AN. Pupillometry Reveals That Context Benefit in Speech Perception Can Be Disrupted by Later-Occurring Sounds, Especially in Listeners With Cochlear Implants. Trends in hearing. 2018;22:2331216518808962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winn MB, Edwards JR, Litovsky RY. The Impact of Auditory Spectral Resolution on Listening Effort Revealed by Pupil Dilation. Ear and hearing. 2015;36(4):e153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner AE, Nagels L, Toffanin P, et al. Individual Variations in Effort: Assessing Pupillometry for the Hearing Impaired. Trends in hearing. 2019;23:2331216519845596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picou EM, Ricketts TA, Hornsby BW. How hearing aids, background noise, and visual cues influence objective listening effort. Ear and hearing. 2013;34(5):e52–64. [DOI] [PubMed] [Google Scholar]
- 36.Hornsby BW. The effects of hearing aid use on listening effort and mental fatigue associated with sustained speech processing demands. Ear and hearing. 2013;34(5):523–534. [DOI] [PubMed] [Google Scholar]


