Abstract
Background and purpose
A subset of ischemic stroke patients with atrial fibrillation (AF) have ischemic stroke despite anticoagulation. We sought to determine the association between pre-stroke anticoagulant therapy and recurrent ischemic events and symptomatic intracranial hemorrhage (sICH).
Methods
We included consecutive patients with acute ischemic stroke and AF from the Initiation of Anticoagulation after Cardioembolic stroke (IAC) study from 8 comprehensive stroke centers in the United States. We compared recurrent ischemic events and delayed sICH risk using adjusted cox-regression analyses between patients who were prescribed anticoagulation (ACp) versus patients who were naïve to anticoagulation therapy prior to the ischemic stroke (ACn).
Results
Among 2084 patients in IAC, 1518 had prior anticoagulation status recorded and were followed for 90 days. In adjusted Cox hazard models, ACp was associated with a non-significantly higher risk of 90-day recurrent ischemic events only in the fully adjusted model (adjusted HR 1.50 95% CI 0.99 – 2.28, p = 0.058) but not increased risk of 90-day sICH (adjusted HR 1.08 95% CI 0.46 – 2.51, p = 0.862). In addition, switching anticoagulation class was not associated with reduced risk of recurrent ischemic events (adjusted HR 0.41 95% CI 0.12 – 1.33, p = 0.136) nor sICH (adjusted HR 1.47 95% CI 0.29 – 7.50, p = 0.641).
Conclusion
AF patients with ischemic stroke despite anticoagulation may be higher risk for recurrent ischemic events compared to anticoagulation-naïve patients. This may suggest differing underlying stroke pathomechanisms requiring different stroke prevention measures and identifying these specific mechanisms may improve secondary stroke prevention strategies.
Keywords: stroke, atrial fibrillation, anticoagulation, recurrence
Introduction
Atrial fibrillation (AF) is associated with increased ischemic stroke risk and studies have shown that oral anticoagulation therapy is associated with reduced risk of ischemic stroke.1 The risk of ischemic stroke in patients with AF, however, remains elevated despite anticoagulation therapy. A patient-level data meta-analysis of the SPAF trials showed that the overall risk of ischemic stroke on warfarin was 2 per 100 patient-years. Importantly, this risk was almost double (nearly 4 per 100 patient-years) in patients with previous stroke or TIA.2 More recent studies comparing direct oral anticoagulants (DOACs) to warfarin3–5 and aspirin6 found a similar trend in that the rate of ischemic stroke/systemic embolism was up to 1.5% per year in patients treated with DOACs and up to 2.5% per year in patients with prior stroke/TIA.
Several potential explanations for this phenomenon have been suggested, including interruption of anticoagulation therapy, inappropriate anticoagulation dosing, atrial enlargement, presence of additional vascular risk factors as assessed by the CHA2DS2Vasc score, non-paroxysmal (versus paroxysmal) AF, as well as non-cardioembolic (versus cardioembolic) stroke mechanisms.7–9 Notably, switching to a different anticoagulant class10 did not mitigate the risk of stroke recurrence, suggesting that pharmacokinetic and dosing considerations insufficiently explain the persistent risk increase.10, 11 Yet, these studies did not adjust for important biomarkers of atrial disease as well as the presence/absence of a competing large artery atherosclerotic mechanism that portends a high risk of recurrence.12
In this study, we sought to assess the short-term risk of stroke recurrence to extend these findings in a large multicenter study by accounting for cardiac biomarkers as well as a competing large artery atherosclerotic stroke mechanism. Our primary hypothesis was that AF patients who were on anticoagulation prior to their ischemic stroke would be at higher risk of recurrence even in short-term when compared to anticoagulant naïve patients, regardless of post stroke preventive management.
Methods
De-identified data may be shared upon reasonable request to the corresponding author.
Patient population
The Initiation of Anticoagulation (IAC) study is a multi-center retrospective study that pooled ischemic stroke registry data from consecutive patients with acute ischemic stroke in the setting of AF treated at 8 comprehensive stroke centers in the United States within the years 2015 and 2018. Methodological details have been previously described in detail.13–15 For the purpose of this study, we included all patients with information about anticoagulation treatment status at the time of index stroke as well those who survived and were followed during the first 90-days. Institutional Review Board approval was obtained from each of the participating centers.
Primary predictor
Data on home medication use were collected by the sites. These included treatment with antiplatelets, statins, warfarin, and DOACs. The primary predictor was home anticoagulation or anticoagulation prior to stroke (ACp) defined as being on a DOAC or warfarin at the time of the ischemic stroke.
Primary outcomes
The co-primary outcomes were recurrent ischemic events and symptomatic intracranial hemorrhage (sICH) as defined in prior studies.13–15 All outcomes were abstracted from medical records by the study site research assistant and confirmed by the site principal investigator.
Co-variates
Demographic factors:
Age at the time of admission and sex.
Clinical variables:
Stroke risk factors (history of hypertension, history of diabetes, history of hyperlipidemia, history of prior stroke or TIA, active smoking), CHA2DS2Vasc score, and NIHSS score.
Medications prior to admission:
Antiplatelet agent and statin therapy.
Neuroimaging and vascular imaging variables:
Largest ischemic stroke lesion volume measured using the a*b*c/2 formula16 as well as the presence of intracranial or extracranial stenosis atherosclerosis with ≥ 50% luminal narrowing in the territory of the stroke abstracted from the vascular imaging report (ipsilateral atherosclerosis). The choice of brain imaging at baseline (CT vs. MRI) was at the discretion of the treating physician.
Echocardiographic variables:
Echocardiographic variables were abstracted by a transthoracic echocardiogram performed within 3 months from the ischemic stroke. For this analysis, we included left ventricular ejection fraction and left atrial enlargement determined by left atrial diameter or volume measruements.17
In-hospital treatments:
Intravenous alteplase, mechanical thrombectomy, bridging with heparin or low molecular weight heparin, and initiation or resumption of anticoagulation.
Analytical plan
Patients were divided into two groups: Treatment with anticoagulation prior to the index stroke (ACp) and anticoagulation naïve (ACn). We compared clinical characteristics, in-hospital treatments, and outcomes at 90-days between the two groups. To determine association between ACp and recurrent ischemic events, we built a priori determined Cox regression models adjusting for the following factors based on prior studies: model 1: adjusting for the CHA2DS2-Vasc score18, model 2 adjusting for the CHA2DS2-Vasc, presence of a small (< 10 mL) versus large (≥ 10mL) infarct18, and ipsilateral atherosclerosis19, and model 3 adjusting for variables in model 2 in addition to anticoagulation initiated. To determine the possible association between ACp and sICH, we built a priori determined Cox regression models adjusting for the following factors, model 1 adjusted for age sex, and small infarct size13, 18; model 2 adjusted for model 1 and bridging with low molecular weight heparin (LMWH)/heparin14; model 3 adjusted for model 2 plus anticoagulation initiated. Analyses were done using SPSS version 25 (IBM, Chicago, IL) and a two-sided p < 0.05 was considered statistically significant.
Results
Among 2084 patients included in the IAC study, 1518 subjects met the inclusion criteria. The reasons for exclusion were as follows: 195 patients were lost to follow up at 90 days, 369 patients had a non-outcome related death within 90 days, and 2 patients had missing information on anticoagulation treatment prior to stroke. The baseline demographics, median CHA2DS2Vasc score, and anticoagulation status prior to index stroke were not significantly different between those with vs. without follow-up were not significantly different between those with vs. without lost to follow up within 90-days.
Among included patients, the mean age was 76.2 ± 11.7 years and 49.9% (757/1518) were men; 546 patients (36.0%) were on anticoagulation at home (ACp) [278 (50.9%) were on a DOAC] and 972 patients (64.0%) were not on home anticoagulation (ACn). After the ischemic stroke, 92 patients were switched to a different anticoagulant class: DOAC to warfarin (n = 81 patients) and warfarin to DOAC (n = 11 patients). In addition, 16.4% (245/1492) had evidence of atherosclerosis with 50% or more luminal narrowing in a vessel supplying the territory of the infarct.
Univariate analyses
Table 1 summarizes the patient characteristics stratified according to pre-stroke anticoagulant status. In brief, when compared to patients in the ACn group, patients in the ACp group had a lower admission NIHSS score (median 6 vs. 9, p = 0.001), were more likely to have diabetes (39.7% vs. 30.2%, p < 0.001), hyperlipidemia (59.9% vs. 54.0%, p = 0.027), prior stroke or TIA (39.6% vs. 26.8%, p < 0.001), congestive heart failure (29.7% vs. 22.0%, p = 0.001), coronary artery disease (38.1% vs. 27.5%, p, 0.001), severe left atrial enlargement (43.9% vs. 34.9%, p = 0.002), and were more frequently on a statin at home (64.7% vs. 49.5%, p < 0.001), placed on anticoagulation after the ischemic stroke (95.6% vs. 80.2%, p < 0.001), and less likely on antiplatelet medication at home (32.1% vs. 58.9%, p < 0.001).
Table 1.
Baseline characteristics and outcomes of patients stratified by anticoagulation treatment status prior to the stroke.
| Anticoagulation at time of index stroke (n = 546) | No anticoagulation prior to index stroke (n = 972) | |
|---|---|---|
| Age (median, interquartile range) | 77 (68 – 84) | 78 (69 – 86) |
| Sex (% men) | 51.6% (282) | 48.9% (475) |
| Hypertension (%) | 84.4% (461) | 81.8% (95) |
| Diabetes (%) | 39.7% (217) | 30.2% (293/971) |
| Hyperlipidemia (%) | 59.9% (327) | 54.0% (525) |
| Prior Stroke or TIA (%) | 39.6% (216) | 26.8% (260) |
| Congestive Heart Failure (%) | 29.7% (159/535) | 22.1% (212/961) |
| Coronary Artery Disease (%) | 38.1% (208) | 27.5% (267) |
| CHADS2 Vasc score (median, interquartile range) | 5 (4 – 6) | 4 (3 – 6) |
| CHADS2Vasc 0–1 (%) | 3.8% (21) | 6.0% (58) |
| Antiplatelet use (%) | 32.1% (175) | 58.8% (572) |
| Statin use (%) | 64.7% (353) | 49.5% (481) |
| Ipsilateral atherosclerosis ≥ 50% (%) | 18.0% (97/538) | 15.5% (148/954) |
| Ischemic lesion size > 10 mL (%) | 57.0% (286/502) | 60.0% (540/900) |
| NIHSS score (median, interquartile range) | 6 (2 – 14) | 9 (3 – 16) |
| Ejection fraction (median, interquartile range) | 55 (50 – 65) | 60 (50 – 65) |
| Severe left atrial enlargement (%) | 43.9% (189/431) | 34.9% (279/800) |
| Alteplase treatment (%) | 14.7% (80) | 36.7% (357) |
| Mechanical thrombectomy (%) | 22.5% (123) | 26.7% (261) |
| Anticoagulation not initiated within 90 days (%) | 4.4% (24) | 19.6% (192) |
| Symptomatic intracranial hemorrhage at 90 days (%) | 1.6% (9) | 1.3% (13) |
| Recurrent ischemic event at 90 days (%) | 8.1% (44) | 6.2% (60/972) |
Association between anticoagulation prior to the index stroke and 90-day outcomes
Recurrent ischemic events occurred in 104 patients (6.9%): recurrent stroke in 91 patients, recurrent TIA in 6 patients, and recurrent symptomatic systemic embolism in 9 patients. Furthermore, 23 subjects had a sICH, with 91.3% (21/23) occurring after initiation of anticoagulation. Predictors of ischemic recurrence and hemorrhagic events in the IAC study were previously reported.13
Association between anticoagulation prior and recurrent ischemic events
In minimally adjusted pre-specified models (models 1 and 2), associations between ACp and recurrent ischemic events were not statistically significant (Table 2). In the fully adjusted model (model 3) adjusting for pre-specified potential confounders including initiation of anticoagulation and CHA2DS2-Vasc score, Cox-regression analyses indicated a non-significantly higher risk for recurrent ischemic events among subjects with ACp (adjusted HR 1.50 95% CI 0.99 – 2.28, p = 0.058) (Table 2) (Figure 1).
Table 2.
Cox regression models showing associations between anticoagulation status before the stroke and recurrent ischemic events and symptomatic intracranial hemorrhage
| Recurrent ischemic event | Symptomatic intracranial hemorrhage | |
|---|---|---|
| Unadjusted | 1.31 (0.89 – 1.94) p = 0.171 |
1.15 (0.50 – 2.66) p = 0.744 |
| Model 1 | 1.29 (0.87 – 1.90) p = 0.208 |
1.16 (0.50 – 2.68) p = 0.731 |
| Model 2 | 1.41 (0.94 – 2.11) p = 0.102 |
1.14 (0.50 – 2.65) p = 0.748 |
| Model 3 | 1.50 (0.99 – 2.28) p = 0.058 |
1.08 (0.46 – 2.51) P = 0.862 |
Recurrent ischemic event: model 1 adjusted for CHADS2Vasc and model 2 adjusted for CHADS2Vasc, ipsilateral large artery disease with 50% or more luminal narrowing, and ischemic lesion < 10 mL, model 3 adjusted for model 2 plus anticoagulation initiated or continued (vs. not)
sICH: model 1 adjusted for age, sex, and ischemic lesion < 10 mL; model 2 adjusted for model 1 and bridging with LMWH/heparin; model 3 adjusted for model 2 plus anticoagulation initiated; LMWH, low-molecular-weight heparin; sICH, symptomatic intracranial haemorrhage.
Figure 1.
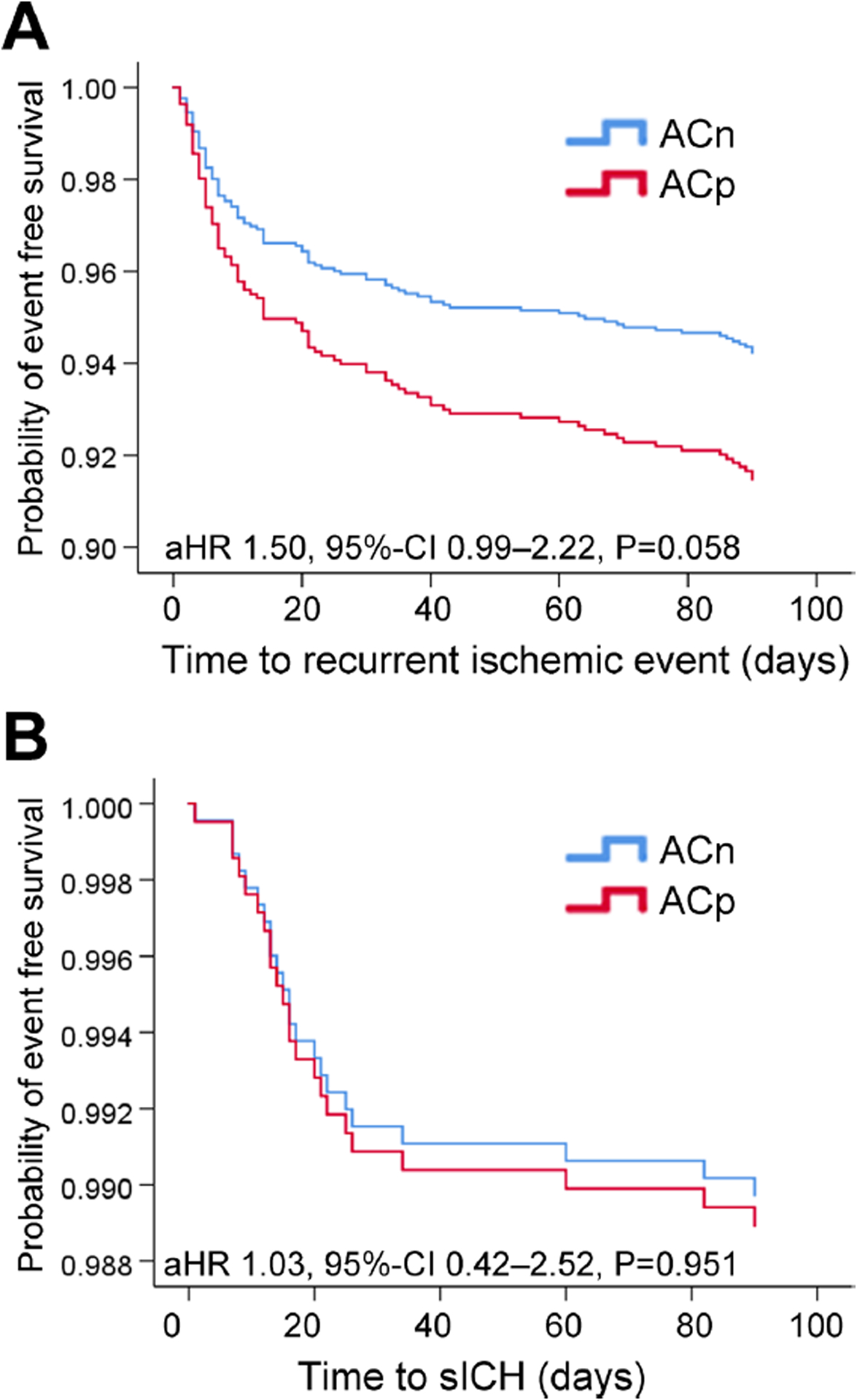
Figure 1 A. Kaplan-Meyer survival analysis showing the risk of recurrent ischemic events within 90-days stratified by home anticoagulation status: anticoagulation prior (ACp) vs. anticoagulation naïve (ACn).
Figure 1 B. Kaplan-Meyer survival analysis showing the risk of symptomatic intracranial hemorrhage within 90-days stratified by home anticoagulation status: anticoagulation prior (ACp) vs. anticoagulation naïve (ACn). sICH, symptomaticintracranial haemorrhage.
Association between anticoagulation prior and sICH
There was no association between ACp and sICH on unadjusted (HR 1.15 95% CI 0.50 – 2.66, p = 0.744) and adjusted Cox-regression (adjusted HR 1.08 95% CI 0.46 – 2.51, p = 0.862) (Table 2) (Figure 1).
Sensitivity analysis
We performed additional analyses restricted to patients with ACp, to determine whether switching anticoagulation had an impact on early recurrence and major bleeding in patients with ACp. In Cox regression analyses, there was no association between switching anticoagulant class and sICH unadjusted (HR 1.40 95% CI 0.29 – 6.75, p = 0.674) and after adjusting for potential confounders (HR 1.47 95% CI 0.29 – 7.50, p = 0.641). In addition, switching anticoagulation class was associated with non-significantly lower risk of early recurrent ischemic events in unadjusted models (HR 0.35 95% CI 0.11 – 1.13, p = 0.079) but this association was not significant after adjusting for potential confounders (adjusted HR 0.41 95% CI 0.12 – 1.33, p = 0.136).
In addition, we performed a sensitivity analysis including patients with non-outcome related and when the exact time of death was not recorded, the time of death was imputed as day 90. In these analyses and if the fully adjusted models ACp was associated with a non-significantly lower risk of recurrent ischemic events persisted (adjusted HR 1.46 95%CI 0.96–2.21, p = 0.076) with a similar risk of sICH (adjusted HR 1.08 95% CI 0.47–2.52, p = 0.857).
Furthermore, to account for patient clustering by study site, we fit mixed-effects logistic regression models to our outcomes and our findings were not meaningfully different; in full adjusted models, ACp was associated with a non-significantly higher risk of recurrent ischemic events (adjusted OR 1.42 95% CI 0.93 – 2.19, p = 0.106) but no increased risk of sICH (adjusted OR 1.05 95% CI 0.49 – 2.25, p = 0.809).
Discussion
The results gained from our real-world study is in line with prior multi-center studies.10, 11 We found that AF patients with ischemic stroke on anticoagulation therapy had a heightened risk of recurrent ischemic stroke even after adjustment for pertinent confounders. Switching anticoagulation class in AF patients who had an ischemic stroke while using anticoagulation was not associated with reduced risk of recurrent ischemic events, again a finding reported in a previous large multicenter study.11 Contrary to prior studies, our findings did not achieve statistical significance likely due to lower events rates, shorter follow up duration, as well as small sample size than that in prior studies.
Possible reasons for ischemic stroke recurrence despite anticoagulation therapy might include suboptimal medication adherence, anticoagulation interruption, and inappropriate dosing. Concomitant stroke mechanisms such as small vessel disease, large artery atherosclerosis, and cancer related hypercoagulability in patients with AF may augment recurrent stroke risk despite anticoagulation therapy. For many of these non-AF related stroke mechanisms, anticoagulation is not superior to aspirin. For instance, the Warfarin Aspirin Recurrent Stroke Study (WARSS) showed no benefit of warfarin over aspirin in patients with non-cardioembolic stroke.20 This was also shown in the Warfarin Aspirin Symptomatic intracranial Disease (WASID) trial where warfarin was not superior to aspirin in secondary stroke prevention in patients with intracranial atherosclerosis.21 In addition, symptomatic large artery atherosclerosis and cancer related hypercoagulability are associated with increased risk of early recurrence regardless of antithrombotic regimen.12, 22 Furthermore, patients who have more vascular risk factors, more severe atrial disease, and higher AF burden, remain at an increased risk of stroke despite anticoagulation therapy.7 Consistent with this notion, we found that in our study these patients with stroke recurrence despite oral anticoagulant therapy had a higher CHA2DS2Vasc scores and more frequently had a history of prior stroke, underlying coronary artery disease, and diabetes.
It is noteworthy that 19.6% of patients who were not on anticoagulation prior to the index event were not started on anticoagulation in the 90-days following the index event as compared to 4.4% of those who were on anticoagulation prior to the index event. There are several reasons that could account for this difference including more severe strokes in the ACn group or those who were not on anticoagulation prior to the index event having a contraindication to anticoagulation. In addition, it is also important to note that the percentage of patients on DOACs was higher than that reported in prior studies10 and may at least partially explain the relatively low bleeding complication events, line with a prior study.23
Our findings have several therapeutic implications. It suggests that anticoagulation therapy alone may not be sufficient in reducing stroke risk in patients with AF thus highlighting the importance of improving stroke prevention beyond anticoagulation therapy.24 First, risk factor and lifestyle modification are the corner stone for prevention. Indeed, in patients with AF, risk factors such as hypertension and diabetes have been associated with increased stroke risk,25 thus controlling modifiable risk factors is an essential step in reducing the residual risk of ischemic stroke in patients with AF started on anticoagulation. Second, AF frequently co-exists with cerebrovascular atherosclerotic disease19, 26, 27 and small vessel disease28 and thus in patients with ischemic stroke despite anticoagulation, it is essential to assess for a competing non-cardioembolic mechanism and ensure that stroke prevention strategies are targeted. For instance, finding severe carotid stenosis in a patients whose infarct is in its territory may lead to carotid revascularization, which has been shown to reduce the risk of recurrent stroke.29 Third, left atrial appendage occlusion has recently emerged as a stroke prevention strategy in patients with AF and an alternative to long-term anticoagulation.30 The Novel Anticoagulation Agents in Atrial Fibrillation (PRAGUE-17) trial compared left atrial appendage occlusion to DOACs and showed that left atrial appendage occlusion is non-inferior to DOACs in reducing the risk of stroke and systemic embolism in patients with AF.31 The risk of ischemic stroke however was 2% in each group suggesting that left atrial appendage occlusion may not superior to DOAC therapy in reducing the risk of ischemic stroke31 and may not be effective by itself in reducing the residual risk of recurrence in patients with breakthrough strokes despite anticoagulation. Whether combining left atrial appendage occlusion with anticoagulation would reduce risk more than either alone is uncertain and has not yet been tested in trials.
Our study has several important limitations. First, our study is a multicenter retrospective study of comprehensive stroke centers which introduces bias and limits generalizability. Second, we lack data on anticoagulation adherence and risk factor control prior to presentation and therefore it is possible that some of the strokes occurred in the setting of poor risk factor control or non-compliance with anticoagulation. Third, nearly 9% of patients were lost to follow up within 90 days. Fourth, while we collected data on infarct size and competing large artery atherosclerotic mechanisms, we lack data on other mechanisms such as dissection, small vessel disease, and hypercoagulability from cancer. Fifth, we lack data on the imaging characteristics and stroke subtyping of recurrent events which in some cases could have been related to a competing non-cardioembolic stroke mechanism. Sixth, our study is an observational study and thus subject to treatment by indication bias. Seventh, we lack information on whether patients who were in the ACn whether they had new AF or known AF but not on anticoagulation although we suspect that most patients had known AF and were not prescribed anticoagulation as as evidenced by a) much higher antiplatelet use despite no difference in coronary artery disease and b) 20% of these patient were still not anticoagulated at 90 days. Finally, the follow up period in our study is only 90-days and thus it is unclear if the risk of recurrence remains elevated beyond the first 90-days as other studies suggested.
Notable strengths of our study include being a large multicenter study, collecting a wide range of variables and outcomes, and providing real world data on the risk of early recurrence in patients with AF.
Conclusion
AF patients with ischemic stroke on anticoagulation may be at higher risk for recurrent stroke compared to anticoagulation-naïve patients, even after adjusting for estimated risk and switching the anticoagulant type does not decrease such recurrent stroke risk. This raises the possibility that pathologic mechanisms either not amenable to anticoagulation or not covered by anticoagulation alone are at play in these patients. Future research should seek to identify these mechanisms and test additional prevention strategies.
Funding Sources
This study is partially funded by the National Institutes of Health grant K08NS091499 (Dr. Henninger).
Disclosures
Dr. Yaghi has a non-funded research collaboration with Medtronic. Dr. Gurol reports grants from AVID (Eli Lilly), grants from Boston Scientific, and grants from Pfizer outside the submitted work. Dr Henninger reports grants from NINDS during the conduct of the study; grants from NICHD of the NIH, grants from NINDS of the NIH, grants from CDMRP of the DoD, and personal fees from Astrocyte Pharmaceuticals, Inc. outside the submitted work. Dr Mistry reports grants from NIH/NINDS outside the submitted work. Dr. de Havenon reports research support from AMAG and Regeneron.
References
- 1.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:3754–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. Jama. 2002;288:2441–2448 [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2011;365:981–992 [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009;361:1139–1151 [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. The New England journal of medicine. 2011;365:883–891 [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. The New England journal of medicine. 2011;364:806–817 [DOI] [PubMed] [Google Scholar]
- 7.Paciaroni M, Agnelli G, Caso V, Silvestrelli G, Seiffge DJ, Engelter S, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin k antagonist oral anticoagulants for stroke prevention. Stroke. 2019;50:2168–2174 [DOI] [PubMed] [Google Scholar]
- 8.Dakay K, Chang AD, Hemendinger M, Cutting S, McTaggart RA, Jayaraman MV, et al. Left atrial enlargement and anticoagulation status in patients with acute ischemic stroke and atrial fibrillation. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2018;27:192–197 [DOI] [PubMed] [Google Scholar]
- 9.Yaghi S, Liberman AL, Henninger N, Grory BM, Nouh A, Scher E, et al. Factors associated with therapeutic anticoagulation status in patients with ischemic stroke and atrial fibrillation. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2020;29:104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K, Koga M, Lee KJ, Kim BJ, Park EL, Lee J, et al. Atrial fibrillation-associated ischemic stroke patients with prior anticoagulation have higher risk for recurrent stroke. Stroke. 2020;51:1150–1157 [DOI] [PubMed] [Google Scholar]
- 12.Yaghi S, Rostanski SK, Boehme AK, Martin-Schild S, Samai A, Silver B, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA neurology. 2016;73:572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaghi S, Henninger N, Scher E, Giles J, Liu A, Nagy M, et al. Early ischaemic and haemorrhagic complications after atrial fibrillation-related ischaemic stroke: Analysis of the iac study. Journal of neurology, neurosurgery, and psychiatry. 2020;91:750–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaghi S, Mistry E, Liberman AL, Giles J, Asad SD, Liu A, et al. Anticoagulation type and early recurrence in cardioembolic stroke: The iac study. Stroke. 2020;51:2724–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghi S, Trivedi T, Henninger N, Giles J, Liu A, Nagy M, et al. Anticoagulation timing in cardioembolic stroke and recurrent event risk. Annals of neurology. 2020;88:807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. Abc/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2005;18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 18.Paciaroni M, Agnelli G, Caso V, Tsivgoulis G, Furie KL, Tadi P, et al. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: The alessa score study. Stroke. 2017;48:726–732 [DOI] [PubMed] [Google Scholar]
- 19.Kanter MC, Tegeler CH, Pearce LA, Weinberger J, Feinberg WM, Anderson DC, et al. Carotid stenosis in patients with atrial fibrillation. Prevalence, risk factors, and relationship to stroke in the stroke prevention in atrial fibrillation study. Archives of internal medicine. 1994;154:1372–1377 [PubMed] [Google Scholar]
- 20.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. The New England journal of medicine. 2001;345:1444–1451 [DOI] [PubMed] [Google Scholar]
- 21.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. The New England journal of medicine. 2005;352:1305–1316 [DOI] [PubMed] [Google Scholar]
- 22.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-k oral anticoagulants (raf-noacs) study. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association of cardio-thoracic surgery (eacts). European heart journal. 2020 [DOI] [PubMed] [Google Scholar]
- 25.Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: Stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292 [DOI] [PubMed] [Google Scholar]
- 26.Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O’Fallon WM, et al. Association of atrial fibrillation and aortic atherosclerosis: A population-based study. Mayo Clinic proceedings. 2001;76:252–259 [DOI] [PubMed] [Google Scholar]
- 27.Proietti M, Farcomeni A. Association between peripheral artery disease and incident risk of atrial fibrillation: Strong evidence coming from population-based cohort studies. Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayasi Y, Helenius J, McManus DD, Goddeau RP Jr., Jun-O’Connell AH, Moonis M, et al. Atrial fibrillation is associated with anterior predominant white matter lesions in patients presenting with embolic stroke. Journal of neurology, neurosurgery, and psychiatry. 2018;89:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. The New England journal of medicine. 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 30.Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, et al. 5-year outcomes after left atrial appendage closure: From the prevail and protect af trials. Journal of the American College of Cardiology. 2017;70:2964–2975 [DOI] [PubMed] [Google Scholar]
- 31.Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. Journal of the American College of Cardiology. 2020;75:3122–3135 [DOI] [PubMed] [Google Scholar]


