Abstract
The promising characteristics of the 7.2-h radiohalogen 211At have long been recognized; including having chemical properties suitable for labeling targeting vectors ranging from small organic molecules to proteins, and the emission of only one α-particle per decay, providing greater control over off-target effects. Unfortunately, the impact of 211At within the targeted α-particle therapy domain has been constrained by its limited availability. Paradoxically, the most commonly used production method – via the 209Bi(α,2n)211At reaction – utilizes a widely available natural material (bismuth) as the target and straightforward cyclotron irradiation methodology. On the other hand, the most significant impediment to widespread 211At availability is the need for an accelerator capable of generating ≥28 MeV α-particles with sufficient beam intensities to make clinically relevant levels of 211At. In this review, current methodologies for the production and purification of 211At – both by the direct production route noted above and via a 211Rn generator system – will be discussed. The capabilities of cyclotrons that currently produce 211At will be summarized and the characteristics of other accelerators that could be utilized for this purpose will be described. Finally, the logistics of networks, both academic and commercial, for facilitating 211At distribution to locations remote from production sites will be addressed.
Keywords: Astatine, alpha emitter, targeted alpha-particle therapy, cyclotron, radionuclide production
Graphical abstract
1. Introduction
The directions taken in the evolution of nuclear imaging and targeted radiotherapy have been influenced to a major degree by radionuclide availability. In the diagnostic realm, the inherent advantages of PET were recognized years before this modality became a practical reality. Although other factors certainly played a role, PET could not have had a significant impact in the clinical domain without the development of an effective infrastructure for 18F production and distribution. In analogous fashion, one might consider targeted α-particle therapy (TAT) to be the radionuclide therapy parallel of PET – an approach whose potential advantages were appreciated long before it could be applied to even early phase clinical studies. Likewise, a major impediment to the clinical translation of TAT has been the limited availability of the most promising radionuclides for this purpose.
As is the case for PET, the number of radionuclides with advantageous properties is small, perhaps five or less for TAT [1]. These can be divided into two classes – those that are sourced from natural decay chains and generating multiple α-particles per decay, exemplified by 225Ac, and those emitting a single α-particle per decay epitomized by 211At. Both 225Ac and 211At have promising properties for TAT and suffer from poor availability albeit for quite different reasons. With 225Ac, natural source material limitations would constrain its use to less than 1,000 patients per year leading to the pursuit of alternative production routes that are hindered by either radionuclidic purity (227Ac) issues or challenging targetry (226Ra) [2]. With 211At, significant hurdles also exist but they are quite different than those encountered with 225Ac. Production methods are available from abundant target material; however, only a few accelerators with the beam characteristics required for producing meaningful levels of 211At currently are available.
In this review, we shall first describe the nuclear and radiochemical properties of 211At and then discuss present and emerging approaches for the production and purification of this 7.2-h half-life α-emitter. Finally, we shall speculate on the prospects for the creation of network infrastructures for 211At supply.
2. Nuclear and Radiochemical Properties of 211At and their Implications for TAT
An important characteristic of 211At that is different than most other α-emitters of relevance to TAT is that it yields one α-particle per decay, which offers certain translational advantages including simplification of radiation dosimetry calculations for 211At-labeled TAT agents. The double-branched decay scheme for 211At is illustrated in Fig. 1. Fortuitously, about 58.2% of 211At decays occur via electron capture to 211Po, producing 77–92 keV polonium x-rays that permit counting 211At activity with conventional gamma detectors and quantification of 211At distributions in vivo by planar and SPECT imaging [3]. These x-rays allow measurement of 211At biokinetics in patients, which can be used for safety and stability monitoring, as well as organ-level assessment of radiation dosimetry of actual treatment doses. The 211Po then decays with a 0.516-s half-life by emission of a 7.45 MeV α-particle to 207Pb, which is stable. The second 211At decay branch (41.8%) involves emission of 5.87 MeV α-particles to 31.55-y 207Bi, which likewise decays (by electron capture) to 207Pb.
Figure 1.
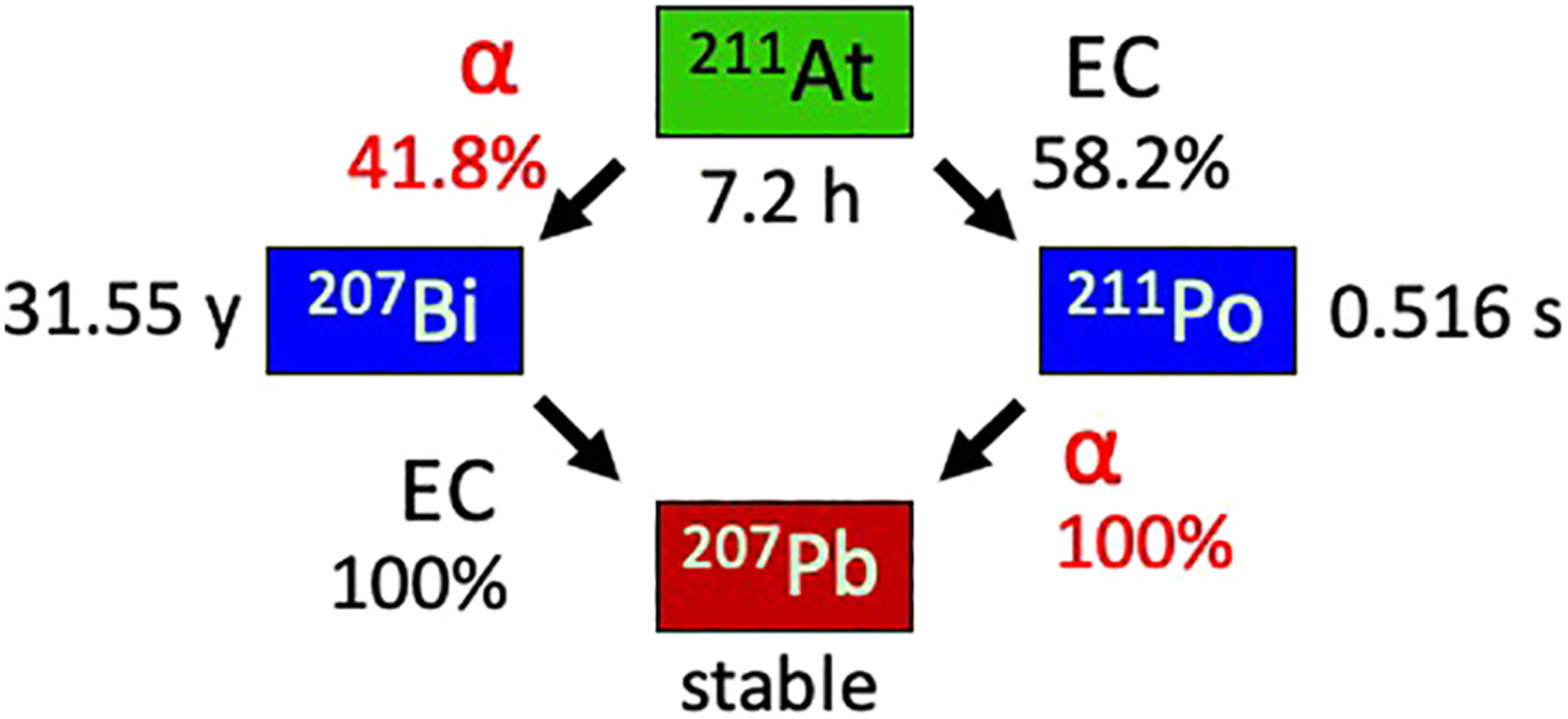
Simplified 211At decay scheme illustrating double-branched pathway; by direct alpha decay to 207Bi and by electron capture to 211Po followed by alpha decay to 207Pb.
It should be noted that there are two aspects of the 211At decay scheme that potentially could be problematic – the 211Po intermediate and the long-lived 207Bi intermediate noted above. With regard to the first, one must consider the effects of an initial nuclear decay event on the fate of a subsequent α-particle emission. If the first decay event is also by α-emission, then the daughters would not only undergo chemical transformation but also the α-particle recoil energy would lead to escape and migration from the original decay site [4]. On the other hand, in 211At decay, the 211Po intermediate is the progeny of electron capture decay, which involves chemical transformation but insignificant daughter recoil energy. Even with worst possible case assumptions – that this chemical transformation results in instantaneous release of 211Po from the cell surface and transport by unimpeded thermal diffusion - nearly 100% of 211Po atoms should decay within two cell diameters from the original cell surface [5]. Give that electron capture results in a highly charged daughter nucleus [6], which could impede diffusion [7, 8], the diffusion distance from the original 211At decay site might be even shorter. In any case, except in the rare instance where cancer presents for treatment as single-cell distributed disease, the diffusion of the 211Po daughter from the original decay site can be ignored. And second, with regard to the long half-life of the 207Bi daughter, this is also not of concern because nearly 100,000 decays of 211At are needed to produce a single decay of 207Bi [9]. Thus, a 370 MBq (10 mCi) hypothetical patient dose of an 211At-labeled TAT agent would generate ~0.1 μCi of 207Bi, a level that is only 0.1% of the 100 μCi Annual Limit of Intake (ALI) recommended for 207Bi by the Nuclear Regulatory Commission (US Nuclear Regulatory Commission Report NRC, 10CFR. https://www.nrc.gov/reading-rm/doc-collections/cfr/part020/appb/bismuth-207.html accessed 26 May 2021).
The α-particle emission characteristics of 211At are similar to those of other TAT-candidate radionuclides [1], exhibiting a mean linear energy transfer of about 100 keV μm.−1 This results in high relative biological effectiveness because they can create more than 10 ionizations in a 100 Å diameter × 3 nm column [10, 11], an ionization density close to the diameter of the DNA double helix [12]. Even though 211At emits fewer α-particles per decay than some other radionuclides under investigation for TAT, 211At-labeled targeted radiotherapeutics are exquisitely cytotoxic, with effective killing achievable with less than 10 atoms bound per cell [13]. Moreover, if uptake of 211At in the cell nucleus can be achieved, the fact that 211At also generates an average of 6.2 Auger electrons emitted per decay (comparable to 67Ga) [14] also might be of therapeutic benefit.
One of the most attractive properties of 211At that contributes to the emerging demand for this radionuclide is the spectrum of targeting agents that are compatible with its labeling chemistry and physical half-life. In contrast to other α-emitters, astatine is a halogen and shares similar chemical properties with iodine, albeit with more metalloid properties [15]. Astatine can exist in several oxidation states [16] providing multiple synthetic options but also contributing to its sometimes confounding, capricious behavior [17]. Although significant differences between astatine and iodine have been observed in labeling chemistry, carbon-halogen bond strength, lipophilicity and the in vivo stability of carbon-halogen bond, the most common strategy to develop 211At-labeled TAT agents is to build on previous studies with radioiodine. Provided that one can demonstrate similar in vivo behavior for the two labeled compounds, this suggests the possibility of using a radioiodinated analogue (124I for PET, 123I for SPECT) as an imaging theranostic partner for the corresponding 211At-labeled therapeutic. Astatine-211 can readily be incorporated by direct substitution into small organic molecules, a potential advantage compared with radiometals, which require somewhat bulky polydentate ligands for stable incorporation. Two of the widely investigated approaches for 211At labeling are the electrophilic demetallation of tin and silicon precursors [18], and the use of carboranyl precursors [19]. Recently, a novel approach for 211At labeling that involves Cu-catalysed astatination of boronic esters was demonstrated to have broad applicability, including to the labeling of a PARP inhibitor [20]. Another method used a sulfonyl precursor for labeling neopentyl derivatives, providing high in vivo stability against nucleophilic substitution or Cytochrome P450 (CYP) metabolism [21].
As is the case with radiometals, biomolecules including monoclonal antibodies, affibodies, diabodies and nanobodies can be labeled with 211At using a variety of procedures [22]. For example, this can be accomplished via either the prototypical acylation agent N-succinimidyl 3-[211At]astatobenzoate [23], using thiol-Michael addition for site-specific conjugation [24], or N-succinimidyl 3-[211At]astato-5-guanidinomethyl benzoate, a prosthetic agent that provides intracellular trapping of radioactivity after internalization of receptor-targeted vectors [25, 26]. Finally, the remarkable affinity of 211At for gold has permitted the direct and nearly quantitative 211At labeling of gold nanoparticles, which can be used either alone or with targeting vectors decorating their surface [27]. Additional information about many of the most promising 211At-labeled radiopharmaceuticals that have been investigated for TAT applications, including those that have been evaluated in patients, can be found in several reviews [13, 22].
3. Production and Availability of 211At: Current State of the Art
Although the potential advantages of 211At for TAT are well recognized, the availability of 211At has been compromised by the limited number of cyclotrons with the beam characteristics required for its efficient production. By far the most commonly used route for 211At production is to irradiate natural bismuth with α-particles via the the 209Bi(α,2n)211At nuclear reaction. The incident energy (Eα) threshold for this reaction is about 20 MeV, reaching a maximum of ca. 900 mb at 30 MeV [28]. Unfortunately, exploiting much of the energy range where 211At production is most efficient is not advisable because once Eα is above 28.1 MeV, production of 8.1-h 210At can occur via the 209Bi(α,3n)210At reaction (Fig. 2). Particularly when patient studies are envisioned, production of 210At must be avoided because it decays to 210Po, a 138-day α-emitter with well-documented toxicity to bone marrow at very low doses. For this reason, Eα needs to be carefully optimized to maximize the production yield of 211At while avoiding producing significant levels of 210At. With that goal in mind, Henriksen et al. [29] found that the content of co-produced 210At at Eα = 27.6 and 28.6 MeV was undetectable and <0.01% (210At activity relative to 211At; defined as the atomic ratio at the end of bombardment), respectively, while the 211At yield increased by about 40% from 27.6 to 28.6 MeV. Furthermore, the 211At yield increased 70% from 27.6 to 29.1 MeV with only 0.023% atomic ratio of 210At present. Increasing Eα further (29.6 MeV) increased the 210At atomic ratio almost tenfold, indicating an optimized Eα of 29 MeV. This was confirmed in a subsequent study reporting that 211At could be produced in high yield at 29 MeV with no evidence of co-produced 210At [30]. Nonetheless, most of the 13 accelerators believed to have produced 211At during the past 5 years (Table 1) have utilized an Eα ≤28.4 MeV, in some cases, because required activity levels were readily obtainable at a lower Eα [31, 32].
Figure 2.

Cross sections for the production of 211At and 210At by bombardment of natural bismuth targets via the 209Bi(α,2n)211At and 209Bi(α,3n)210At nuclear reactions, respectively, as a function of incident α-particle beam energy (Eα). Data from Experimental Nuclear Reaction Data (EXFOR https://www-nds.iaea.org/exfor/), reported by Hermanne et al., 2005) and Lambrecht and Mirzadeh, 1985.
Table 1.
Current 211At production sites. - Facilities that have reported production of 211At during the last 5 years.
| Location | Facility | CyclotronManufacturer | Model and Target | ProductionParameters | Current Production Status | |
|---|---|---|---|---|---|---|
| NorthAmerica | Durham,USA | Duke University Medical Center | CTI | CS-30 cyclotron, Internal target system | 28 MeV, 100 μA | Max 9.3 GBq in 4-h |
| Seattle, USA | University of Washington Medical Center | Scanditronix | MP-50, External target system | 29.0 MeV, 58 μA | Max 4.3 GBq in 4-h | |
| Philadelphia, USA | University of Pennsylvania | Japan Steel Works (JSW) | BC3015, External Target | 28.4 MeV, 10 μA | Max 395 MBq in 5-h | |
| Bethesda, USA | National Institutes of Health | CTI | CS-30 cyclotron, Internal target system | 29.8 MeV, 43 μA | Max 1.71 GBq in 1-h | |
| College Station, USA | Texas A&M University | In house | K150 variable energy cyclotron | 28.8 MeV, 7 μA | 1.5 GBq in 9-h | |
| Europe | Copenhagen, Denmark | Copenhagen University Hospital | Scanditronix | MC-32, Internal target system | 29 MeV, 20 μA | Max 3–4 GBq in 4-h |
| Nantes, France | Arronax | IBA | Cyclone 70 | 28 MeV, 15 μA | Production since 2020, 0.5 – 1 GBq capacity | |
| Asia | Osaka, Japan | RCNP-Osaka University | In house | K140 AVF cyclotron | 28.2 MeV | 3 GBq expected after upgrade |
| Chengdu, China | Sichuan University | CTI | CS-30 | 28 MeV, 15–20 μA | Max 200 MBq in 2-h | |
| Takasaki, Japan | QST-Takasaki, (TIARA) | In house | AVF (K110) | 28.1 MeV, 4.5 μA | 300 MBq in 3 h | |
| Chiba | QST-NIRS | In house | AVF-930 | 28.5 MeV, 10–13 μA | 0.74–1.11GBq in 5-h | |
| Wako Saitama, Japan | IPCR Riken | In house | AVF | 29 MeV, 40 μA | 1.3 GBq in 1-h | |
| Fukushima City, Japan | Fukushima Medical University | Sumitomo | CYPRIS MP-30, External target system | 29 MeV, 20 μA | Max 2 GBq in 4-h | |
The fact that this production route involves the irradiation of an inexpensive and abundant natural material is a significant advantage. This allows safe handling and fabrication of targets, and obviates the need for procedures to recover and purify the target material. However, another property of bismuth can limit efficient 211At production, particularly when scale up to high beam currents is envisioned. Bismuth has poor thermal conductivity (7.97 W·K−1·m−1) and a low melting point (272°C), making it essential to provide good target cooling, particularly for high-current production runs. Target preparation is straightforward - metallic bismuth is simply melted or vaporized onto an aluminum backing plate; the surface is often machined to provide a uniform surface. Copper backing plates also have been evaluated to improve thermal conductivity; however, this strategy was not effective because it lowered 211At isolation yields and led to the production of longer-lived radionuclidic impurities [33]. Thick target yields reported for 211At production after 28–29.5 MeV α-particle bombardments range from 16.3 to 41 MBq/μA-h (electric μA) [29, 33–37].
Several factors need to be considered to optimize 211At production yield with the thickness of the bismuth target perhaps being the most critical aspect. Assuming an α-particle beam of 28 MeV, its kinetic energy would be degraded to 20 MeV after penetrating 80 μm in a metallic Bi target at a perpendicular incident angle, and thus below the threshold for the 209Bi(α,2n)211At reaction (calculated using SRIM http://www.srim.org accessed 26 May 2021). In this case, the optimal thickness of the Bi target is 80 μm at a perpendicular incident angle because a thinner target will not fully utilize the productive energy window while a thicker target will generate excess heat in the target with no increase in usable cross section. Placing the Bi target at a tangential incident angle relative to the beam direction (grazing angle) will increase the contact area of beam on the target, allowing better heat dissipation. This also will reduce the effective thickness of the Bi, permitting use of a thinner target, thereby allowing higher heat dissipation on the Al backing plate. Moreover, use of a thinner target should facilitate subsequent extraction of the 211At from the target either by target distillation or dissolution (vide infra). Both the front of the bismuth target and the aluminum backing plate need to be cooled sufficiently to prevent the bismuth from melting during the irradiation, which can lead to 211At escape, compromising production yield and potentially complicating handling [30, 38]. Although not as effective as other potential backing materials like copper, the thermal conductivity of aluminum is σ=237 W/(m*K), much larger than that of bismuth. Thus, efficient heat transfer at the interface of both the Bi and Al, and the Al and the coolant, will be necessary to allow maximum bean current for high 211At production rates.
As a frame of reference for the sections that follow, the irradiation parameters currently utilized at the three cyclotron facilities that have had the most active 211At production programs in recent years are compared in Fig. 3. In each case, 211At production runs have occurred on about a weekly basis in support of radiochemistry, preclinical and clinical studies. The target location, incident angle between the beam and the target, and the bismuth target thickness all vary among these cyclotron facilities. Nonetheless, each has been a reliable source of 211At production for more than 10 years.
Figure 3.

Irradiation parameters utilized at the 3 institutes currently having the highest 211At production capability.
3.1. Internal target systems for 211At production
At least three institutions have developed internal targets for 211At production [33–35]. One of these is at the leading 211At production facility in Europe - the PET and Cyclotron Unit at Copenhagen University Hospital in Denmark [32]. It is utilized on a Scanditronix MC32 cyclotron that was installed in the late 1980s. The internal target system consists of a bismuth target with an aluminum backing that is mounted on a water-cooled probe and placed at a 15° grazing angle for better heat dissipation (Fig. 4) [39]. An α-particle beam energy of 29 MeV is used for irradiation and GBq levels of 211At have been produced routinely, enabling many preclinical studies and at least one clinical trial [40].
Figure 4.

Production of 211At at the PET and Cyclotron Unit, Rigshospitalet, Copenhagen, Denmark. The Bi target is placed on a water-cooled probe at a grazing angle on beam contact. Following transport to Gothenberg, Sweden, a dry distillation apparatus is used for the purification of 211At.
The CS-30 cyclotron at Duke University Medical Center (DUMC) is equipped with an internal target system optimized for 211At production [33]. The MIT-1 internal target system was installed in the late 1990s and designed in collaboration with George Hendry (Cyclotron, Inc.) (Fig. 5) [33]. An aluminum target backing was fabricated with a curved face for bismuth target deposition to maximize the beam-target contact area and with fins machined into the back of the target to improve cooling efficiency. The target is placed at a 4.7° grazing angle, providing a higher surface area and reducing the heat density generated by the beam leading to improved heat dissipation. The target system also includes two graphite beam-current monitors located at the leading- and trailing-edge to optimize target positioning. These design elements have enabled the use of higher currents and longer irradiations without significantly increasing the surface temperature of the bismuth target. This internal target system has allowed weekly production of 211At at an α-beam energy of 28 MeV and greater than 65 μA beam current (electrical current), with a yield of 41 ± 7 MBq/μA-h; the saturation yield calculated based on this is 400 MBq/μA, close to the theoretical saturation yield - 420 MBq/μA [29, 34]. The highest published level of 211At produced with this system is 6.6 GBq at end of bombardment after a 4-h irradiation at 55 μAp (particle μA) [9]; although the maximum produced to date is 9.3 GBq. For recent clinical-level production runs, the irradiation parameters have been to run for 3 h at 48 μAp (particle μA), which has reliably provided an average of 6.8 GBq at the end of bombardment.
Figure 5.

Illustration of the 211At production and purification methods utilized at the Duke University Medical Center. The production of 211At is accomplished using an internal target system and 211At is isolated from the bismuth target by dry distillation. After elution from PTFE in the required solvent, the 211At is available for use in radiolabeling procedures.
It should be noted that there are significant hurdles in implementing internal target systems to existing cyclotrons because they require the design and manufacture of target rams that can accurately place the target inside the cyclotron with reliable and reproducible mechanical control. Beam simulations have to be implemented to ensure an accurate α-particle beam energy, particularly on the high end to avoid unwanted 210At production. Other optimization processes will be necessary to achieve high production rate and radionuclidic purity. Although current 211At activity level requirements have been readily achievable at DUMC using the internal target at an Eα=28 MeV, it seems likely that the system will be optimized in the near future for an Eα=29 MeV, which should increase 211At yields considerably [34].
3.2. External target systems for 211At production
Although lower 211At production yields have been reported using external targets at a few sites [33, 35], newer cyclotrons equipped with optimized external target systems are capable of producing 211At at levels comparable with those of internal target systems [30, 32, 41–43]. Notably, Gagnon et al. [30] at the University of Washington Medical Center reported a maximum 211At production of 4.3 GBq after a 4-h irradiation with a 29.0-MeV incident α-particle energy and a 58 μA beam current on a Scanditronix MC-50 cyclotron (Fig. 6). A slanted target geometry was used to optimize the incident angle at the beam-target contact to reduce beam heat density. By also utilizing a water cooling system, the peak target temperature was estimated to be only about 54°C when running at ~50 μA, well below the bismuth melting point [30]. This is the highest reported 211At production level for an external target system and has been sufficient to permit the clinical translation of an 211At-labeled ant-CD45 monoclonal antibody [44].
Figure 6.

The 211At production and purification process at the University of Washington Medical Center. An external target is placed at a slanted angle in the pneumatic target system. An automatic system was developed to dissolve the target material and purify 211At using a wet chemistry method.
4. Isolation of 211At from the Cyclotron Target
The efficiency of 211At isolation from the cyclotron target both in terms of yield and time depends in large part on the methods utilized for performing the cyclotron irradiation including the size, thickness and mass of the bismuth target and its backing plate, and the backing plate material. In addition, the length of the irradiation as well as the intensity of the beam current can also play a significant role [33]. Two strategies have been used successfully for 211At purification – dry distillation and wet harvesting – with the choice between the two depending on technical considerations such as target dimensions and subsequent labeling chemistry as well as on personal preference of the investigator [45].
4.1. Dry distillation
Currently the most commonly used approach, this procedure utilizes a distillation apparatus configured to accommodate the size and shape of the bismuth target/backing plate [39, 45, 46]. An exception is a method where the bismuth is separated from the backing by either scraping, cutting or heating before the beginning of the distillation [39, 45, 46]. Although this step allows the use of a still with a smaller footprint and can expedite the distillation process, the increased potential for contamination is a major concern particularly when high activity levels of 211At are present. All procedures involve heating the irradiated target above the melting point of bismuth (272°C) and the boiling point of astatine (302°C), with most using a temperature of 650–800°C for 30 min or less. A recent abstract reported that use of an induction heating method was able to achieve an average 211At recovery of 51% in 10 min [47]. Further refinements of this technology are expected and should provide a dry distillation procedure that will offer significant advantages compared with current methods in terms of convenience, speed and 211At recovery yields.
Effective trapping of 211At generally requires proper balance of carrier gas flow – either nitrogen [39], argon [9], or in some cases O2 [48] – with a modest vacuum on the distillation apparatus. Trapping the 211At after release from the target has been accomplished using several approaches including silica columns [28] and bubbler traps [49]. In current practice, a capillary tubing cryotrap fabricated from PEEK has become the favored approach [39]. A recent study has utilized PTFE tubing for this purpose due to its greater flexibility and translucency, facilitating the monitoring of extractant flow [50]. Distillation efficiencies of 80–90% have been reported at lower 211At activity levels [46]; however, lower and more variable 211At recovery (51–83%) was observed for therapy-level production runs [9]. Whether radiolytic factors are at play here remains to be elucidated in future studies. Although the potential merits of wet harvesting methods, described below, are recognized, and dry distillation methods can be capricious, they do offer several important advantageous including: a) limited handling of radioactive materials; b) minimal exposure of 211At to chemicals, including those that can alter its chemical form, frequently in unpredictable ways, c) the ability to trap the 211At activity in relatively small volumes and in different media to meet the needs of subsequent radiochemical procedures; and d) amenability to automation within systems that also can include the synthesis of the required 211At-labeled therapeutic [46].
4.2. Wet chemistry extraction methods
The separation of 211At from bismuth target materials in liquid form was first introduced in the 1950s [51, 52] during early studies of 211At radiochemistry. Balkin et al. [45] subsequently developed an optimized wet chemistry method that was both relatively easy to conduct and provided 78 ± 11% recovery after corrections for decay. More recently, this method was further optimized in order to meet the requirements for clinical translation [44]. Building on this advance, Li et al. developed a semi-automated process using a tellurium-packed column for isolating the 211At, reporting an 88–95% recovery yield after a 2-h separation process [53]. Wet chemistry approaches risk the formation of many solvated species of 211At, which can result in a lower radiochemical purity of the 211At product, potentially affecting the subsequent radiolabeling yield. On the other hand, this approach generally provides higher and more consistent 211At separation yields than dry distillation [45]. Additional refinements have improved the radiochemical purity of the isolated 211At. For example, Li et al. [54] optimized the tellurium-packed column separation method by neutralizing the HNO3 from the target dissolution process with aqueous NH2OH·HCl and achieved high separation yields (88–95%) and >99% radiochemical purity for sodium [211At]astatide. Others have addressed this issue by utilizing a solid-state support to permit control over 211At speciation at the end of separation [55]. Wet harvesting methods are advantageous because they can provide higher and more reproducible yields; however, the 211At speciation at the end of purification is limited to [211At]astatide, which is not directly useful for electrophilic demetallation radiolabelling chemistry. Finally, wet harvesting approaches avoid the formation of volatile 211At0, which is advantageous from a radiation safety perspective.
In summary, high 211At recovery from irradiated bismuth targets have been reported for both dry-distillation and wet-chemistry isolation methods. These two approaches are complementary and provide the researcher with the ability to choose the type of procedure that best suits local facility constraints and the type of chemistry for which the isolated 211At is intended. Moreover, new 211At purification methods are currently being developed [56]; however, it remains to be seen whether they will supplant existing procedures, particularly for use with high-activity level targets.
4.3. Radiolysis and potential effects on 211At radiochemistry
The chemical form and oxidation state of 211At at the end of the purification process is perhaps the most important aspect in the production of 211At that has rarely been addressed. Nevertheless, these factors form a vital bridge between the chemical environment during purification and its effects on subsequent radiolabeling chemistry, sometimes confounding the later, particularly as the time interval between purification and radiolabeling increases. This behavior, as well as the sometimes erratic behavior of 211At at high activity concentrations [17, 32], suggest that radiolysis induced by the high-energy deposited by 211At α-particles in small volumes likely plays an important role. Radiolysis can generate free radicals, ions and other molecules including oxidants and reductants. These species can alter the oxidation state of the radionuclide and generate competitive species that either react with the radionuclide or the substrate, diminishing labeling yields and effective molar activity.
The dry distillation method is advantageous in this regard because during distillation atomic 211At0 is released from the target material in its gaseous phase and subsequently trapped by low temperature, minimizing exposure to chemicals and the likelihood of oxidation state change. On the other hand, the choice of solvent to elute 211At from the cold trap is critical because during the interaction between 211At and solvent, the radiation dose quickly exceeds 1000 Gy [9]. Chloroform frequently has been used for this purpose; however, chlorine radicals can be generated in a dose dependent manner after exposure to 211At. These radicals can then compete for precursor in electrophilic demetallation reactions, leading to a recommendation to use methanol as the solvent for 211At trapping [57]. Subsequent studies demonstrated that in methanol, exposure to increasing doses of 211At could alter pH and the oxidation state of the 211At to a species, presumed to be [211At]astatide, with poor reactivity for electrophilic astatodestannylation [58, 59]. This difficulty can be circumvented by adding a small amount of N-chlorosuccinimide (NCS) to the methanol used to elute the 211At from the cold trap, which is effective in preserving reactivity except at very high radiation doses and dose rates [60].
Although electrophilic approaches have been the mainstay of 211At labeling chemistry, promising alternative labeling strategies using nucleophilic astatine have emerged including those utilizing carboranyl and boronic ester precursors [19, 20, 45, 61]. The generation of [211At]astatide at elevated radiation doses suggests that these methods might be well suited to 211At labeling at very high activity concentrations. In addition, eluting the cold trap with NaOH solutions after dry distillation might be preferable with these chemistries. More research is needed to investigate the speciation of 211At, especially at high radioactivity levels, in potential trapping media.
Unfortunately, little information is available about potential radiolysis effects during the separation of 211At from cyclotron targets via wet chemistry approaches, and as noted above, on nucleophilic radioastatination. It is speculated that radiolysis could play a role in aqueous media but the effects will be different that those in organic solvents and that α-particles will generate different species that those from other types of radiation [62]. For example, the radiolysis of water can produce, hydrogen, hydrogen peroxide, as well as radicals that may be important at elevated 211At activity levels [63]. Studies of these phenomena might be aided by a recently reported radio-chromatography method using thin layer chromatography to identify different 211At species in aqueous solution in the presence of oxidation or reducing agents [64] although considering the volatility of the At0 species, an HPLC-based method might be preferable in the future.
4.4. Automation of 211At purification procedures
The radiation emitted by 211At is relatively benign from a shielding perspective. However, with >10 GBq activity levels anticipated and 2.5-min positron-emitting 30P (produced by α-irradiation of the aluminum target backing plate) delaying manual processing, automation of the 211At purification procedure needs to be considered. Unlike the case with PET radionuclides, there are no commercially available automated systems for the purification of 211At after cyclotron irradiation. At present, most institutions producing 211At use customized systems involving manual operations. A confounding factor for automation is the variety of target sizes and configurations and purification methods in use at the different sites. Nonetheless, efforts are ongoing to develop of automatic systems for 211At purification. Aneheim et al. [46, 65] reported an automatic process for dry distillation that involved software-controlled monitoring of 211At activity and the option of combining it with a synthesis module for radiolabeling. A scraping device was developed to remove the bismuth target material from the aluminum backing plate to be able to reduce the still footprint and shorten the distillation time. For use with wet chemistry approaches, Li et al. [53] developed an automatic system for 211At purification using a tellurium-packed column. In addition, O’Hara et al. [66] reported a similar system using wet chemistry method. Further research in the development of automated modules for 211At purification is needed to make 211At more amenable for routine availability.
5. Current 211At Production Capabilities
Although there is considerable interest in using 211At for TAT, the feasibility of translating these concepts into clinically impactful drugs is critically dependent on improving the supply of 211At. As a first step, we have attempted to provide a comprehensive overview of current capabilities worldwide. There is an ongoing effort to expand 211At production in the US, Europe and Japan either through the construction of new cyclotrons or by taking advantage of existing accelerators. With regard to the latter, several institutes stopped producing 211At during the last 10 years and their production capability could be revitalized, at least in principle, to meet a growing demand for this promising radionuclide. Herein, we have summarized the characteristics and capabilities of both the facilities that currently (defined as in the last 5 years) produce 211At (Table 1) as well as potential production sites for 211At that should/could be available in the future (Table 2).
Table 2.
Production sites for 211At that could be operational in the near future.
| Location | Facility | Accelerator Model | Irradiation Parameters | Current Production Status |
|---|---|---|---|---|
| Davis, USA | Crocker Nuclear Laboratory, UC Davis | Crocker Cyclotron | Currently 29 MeV α beam at 30 μA, internal target station | Retrofitting in progress |
| Lansing, USA | Ionetix | CS-30 cyclotron | Expected to produce 28 MeV α beam, 100 μA current | Cyclotron subsystem updating underway; should be operational in 2022 |
| Rez, Czech Republic | Nuclear Physics Institute of the CAS | U-120M | <40 MeV α beam 40/5 μA current at internal/external | Last production in 2003 but are ready and willing to restart |
| Warsaw, Poland | POLATOM | IBA Cyclone 30XP | 30 MeV α beam at 50 μA current, external target station | Building and installation begin at the end of 2021 |
| Birmingham, United Kingdom | University of Birmingham | Scanditronix MC-40 | 11.8–40 MeV α beam at >10 μA current, external target | Currently no production, but 1 GBq weekly could be expected on demand. Production can be optimized further. |
| Bucharest, Romania | IFIN-HH, Magurele | U-120 | 26 MeV α beam, 100/20 μA current at internal/external target stations | 3 MBq produced in 2015, cyclotron reinvigoration underway |
| Julich, Germany | Forschungszentrum Julich | IBA Cyclone 30 XP | 30 MeV α beam at 50 μA current, external target station | Production expected to start Q2 2021 |
| Seoul, South Korea | KIRMS | Scanditronix MC-50 | 29.2 MeV α beam at 17 μA current, external target station | 32 MBq/ μAh Production started Q1 2021 |
| Wako, Japan | RIKEN | Heavy-ion Linac | Estimated GBq level production | Optimization underway |
Before discussing institutional capabilities, it is worth noting efforts in several geographical areas to coordinate 211At production. Remarkably, during the last decade, five Japanese institutes have established routine production and purification of 211At, making a total of six 211At production sites, enabling many preclinical using 211At in Japan [48, 67–71]. In 2020, the European Cooperation in Science and Technology (COST) funded a multi-institutional program focused on 211At TAT including a goal to build an efficient 211At production network to address the needs of the European Union members and neighboring countries (COST Action CA19114, https://www.cost.eu/actions/CA19114/#tabs|Name:overview accessed 26 May 2021). In the US, the Department of Energy recently has provided funding for enhancing 211At production at existing cyclotrons with the objective of utilizing the DOE Isotope Program University Isotope Network (DOE IP UIN) as a conduit for 211At distribution. Hopefully, these cooperative efforts will expand the availability of 211At in the near future. Current production capabilities at selected cyclotrons in different geographical regions are presented below.
5.1. North America
Within North America, production of 211At using the most commonly used α-particle bombardment of bismuth approach has been limited to facilities within the US. At Duke University, high-level 211At is being produced weekly for radiochemistry and preclinical research. Although no clinical studies are currently ongoing, the typical production level has been about 8–10 GBq per month. With two 211At TAT agents expected to enter clinical trial in about a year, that level is expected to double, which is well within current capabilities. The cyclotron facility at the University of Washington is routinely producing high-level 211At in support of one clinical and several preclinical programs [44]. Since 2018, over 74 GBq of 211At has been produced on target per year. The University of Pennsylvania has recently enhanced their 211At production capability, reporting a maximum of 396 MBq produced from a single run on their JSW 3015 cyclotron [72]. In 2020, Texas A&M University reported the production of 890 ± 80 MBq of 211At after an 8 h run using a 28.8 MeV incident α-beam energy at 4–8 μA current [73]. Recently, they described a novel separation method for isolation of 211At from the target [56].
5.2. Europe
For more than a decade, the most active facility for 211At production in Europe has been the PET and Cyclotron Unit at the Copenhagen University Hospital. They are capable of producing a maximum of about 3.6 GBq of 211At after an 8-h run [32]. In Nantes, France, the ARRONAX facility has been operating since 2008 and is capable of accelerating 70 MeV α-particles with a beam current of 35 μA. The beam is degraded to about 28 MeV to avoid 210At production, which has permitted the production of up to ~1 GBq of 211At for basic research and eventually, clinical studies [74, 75].
5.3. Asia
Although the production of 211At in Asia has just recently gained momentum with multiple production facilities being established in Japan and South Korea, 211At has a deep-rooted history in Asia. The Research Center for Nuclear Physics (RCNP) at Osaka University is equipped with a K140 AVF cyclotron and has been producing 211At for more than two decades [76]. Even before that, Sichuan University in China installed a CS-30 cyclotron in the 1980s, contemporaneous with the CS-30 cyclotron installed at Duke. Astatine-211 production, separation and labeling chemistry have been investigated at Sichuan University since that time [77] and production of research level of 211At has been reported in recent years [78]. Since 2011, many new production sites have emerged across Japan including those at the Japan Atomic Energy Agency (JAEA), the National Institutes for Quantum and Radiological Science and Technology (QST), RIKEN and Fukushima Medical University (FMU). As summarized in Table 1, GBq levels of 211At are being produced in Chiba, Wako and Fukushima City.
6. Additional 211At Production Sites Potentially Available in the Near Term
In addition to the 13 production sites that are currently supplying 211At, we have identified 9 other accelerator facilities that are likely to contribute to worldwide 211At supply in the near future. With the exception of the IBA Cyclone 30XP that will be located at POLATOM (Warsaw) expected to be operational in 2022, the remaining sites are existing machines (Table 2). These cyclotrons, some of which have made 211At in the past, are undergoing a varying degree of modification, upgrading and/or target fabrication in order to produce 211At. Anticipated activity levels at these facilities range from about 3 MBq in Bucharest, sufficient for chemistry and cell studies, to more than 10 GBq in Lansing, enough to treat multiple patients. Of considerably less certainty is the possible contribution of the 10 cyclotrons listed in Table 3 to future 211At supply. Most of these machines have made 211At successfully in the past; however, some of these cyclotrons have either ceased operation or are scheduled for shutdown soon. At least hypothetically, some of these machines could be revived if demand for 211At increased to a level that would make a restart financially viable.
Table 3.
Facilities that were capable of 211At production but are either shut down, shutting down or of unknown availability/capability status.
| Location | Facility | Cyclotron Model | Cyclotron Parameters | Current Production Status |
|---|---|---|---|---|
| Oslo, Norway | University of Oslo | Scanditronix MC-35 | Unknown | Cyclotron under repair |
| Orleans, France | CNRS—CEMHTI laboratory | THOMSON-CSF | <50 MeV α beam at 15 μA current | Machine scheduled for shutdown in 2023 |
| Paris, France | Curium, Saclay | CGR-560 | <40 MeV | Appears to be operational as commercial machine |
| Dubna, Russia | JINR—FLNR | U-120 U-150 |
Unknown | Unknown |
| Tomsk, Russia | Tomsk polytechnic University | U-120 | 28 MeV α beam at 50 μA current | Producing 211At for research |
| Warsaw, Poland | Heavy Ion Laboratory, University of Warsaw | U200-P | ≤34 MeV α beam at ≤1 μA current | Last production in 2017; no plans for future 211 At because POLATOM will produce |
| Jyväskylä, Finland | University of Jyväskylä | Scanditronix K130 | Capable of producing α beam | No license for 211 At production, currently research purposed only |
| Ispra, Italy | JRC | Scanditronix MC-40 | Currently no production | Shut down in 2014 |
| Brussels, Belgium | VUB | CGR-MeV model 560 | <40 MeV α beam | Low level production in the past but cyclotron was shut down in 2020 |
| Essen, Germany | Universitätsklinikum Essen | Cyclotron Corp CV-28 | 28 MeV α beam at 50 μA current | Shut down in 2016 |
Of the cyclotrons listed in Table 2, several facilities are likely to begin 211At production during the next few years. In North America, Crocker Nuclear Laboratory at the University of California, Davis was recently funded by the US Department of Energy to establish 211At capability at their cyclotron. Significant progress has been made in target fabrication and beam current optimization, and production is estimated to begin within the year [79]. Ionetix has acquired a CS-30 cyclotron similar to the machine at Duke University and is in the process of updating the machine with the goal of producing at least 10 GBq batches of 211At by Q4 2022. In Europe, it is anticipated that the Network for Optimized Astatine labeled Radiopharmaceuticals (NOAR) under COST Action CA19114 will motivate and perhaps support the development of more accelerators for 211At production. Astatine-211 production is planned at the new IBA Cyclone 30XP cyclotron at the Radioisotope Centre POLATOM (Warsaw, Poland) and seems likely, pending demand, at existing cyclotrons at the Institute for Nuclear Chemistry (Jülich, Germany) and the University of Birmingham, UK. Finally, in Asia, 211At production began in Q1 2021 at the Korean Institute of Radiological and Medical Science (KIRMS) Scanditronix MC-50 cyclotron. It is anticipated that the activity levels required for clinical investigation of 211At-labeled TAT agents should be available in Seoul in about a year.
7. Current and Potential Future Commercially Available Accelerators for 211At Production
Unlike the case with other TAT radionuclides, 211At supply is not constrained by a dependency on nuclear stockpiles that are heavily regulated and in some cases, of limited availability, or on inconvenient target materials [2]. The situation with 211At is different - 211At can be produced at high activity levels using ordinary target material and existing technology. Currently, there are at least three commercially available cyclotrons suitable for 211At production and their characteristics are summarized in Table 4. Both the IBA Cyclone 70XP and the Sumitomo MP-30 cyclotrons have been used to make 211At and it is anticipated that the IBA Cyclone 30XP will be evaluated for this purpose soon. Also included in Table 4 are two accelerators at various stages of development. Nusano is working on a linear accelerator that is anticipated to produce 3 mA of 50 MeV α-particles. Ionetix is working on a superconducting cyclotron, conceptually similar to their [13N]ammonia unit-dose machines, with a design goal of 300-μA beam current at 29 MeV. Timelines for development of these accelerators are not available and it is also not clear whether these machines will be commercially available for purchase or operated by these companies as part of their own isotope supply networks. Either way, if they meet their design goals, these accelerators could have a major impact on worldwide 211At supply.
Table 4.
Current and potential future commercial sources of accelerators for 211At production.
| Company | Accelerator | Irradiation Parameters | Features | Status |
|---|---|---|---|---|
| IBA | Cyclone 30XP | 30 MeV, 50 μA current, External target station | Can produce proton and deuteron beams | Available |
| IBA | Cyclone 70XP | 67.4 MeV, 70 μA at internal target and 15 μA at external target station | Fixed energy so energy degrader required | Available |
| Sumitomo | MP-30 | 32 MeV, 30 μA current, External target station | Automatic target transport system | Available |
| Ionetix | Superconductingcyclotron | Design goal is 29 MeV, 300 μA current | Small footprint | Underdevelopment |
| Nusano | LINAC | Expected to produce 3 mA α beam current at 50 MeV | High beam current | Underdevelopment |
With regard to the 211At activity levels that might be available to meet future needs, as a first approximation, one could estimate the number of doses of a typical 211At-labeled TAT agent that could be produced given current and projected future accelerator capabilities. Based on a semi-automated procedure for the synthesis of 211At-labeled trastuzumab [32, 46], we assume decay-corrected radiochemical yields of 89% for 211At purification, 75% for 211At-TAT agent labeling and 1 h as the total time required for purification and synthesis (91% activity remaining after decay). Under these assumptions, 607 MBq of 211At-labeled TAT agent could be produced per GBq of 211At in the target. As noted in Section 3.1., we routinely produce 6.8 GBq of 211At from a 3-h irradiation with 28 MeV α-particles at our cyclotron; with a planned increase in α-particle beam energy to 29 MeV, production of at least 14 GBq is expected from a 3-h irradiation [29]. If one assumes that 370 MBq represents a typical patient dose, then a 3-h irradiation at 28 and 29 MeV could produce 11.2 and 23.0 doses, respectively. Thus, two 3-h 29-MeV production runs per day, 5 days a week, 50 weeks per year, could provide 11,500 patient doses per year with existing technology using one cyclotron, a level more than twice that estimated for existing 225Ac capabilities [2]. Under the admittedly optimistic assumptions that 1) next generation cyclotrons come close to meeting the design goals indicated in Table 4 2) purification and labeling chemistry is scalable, and 3) 211At accelerator production networks become financially viable, production of ≥100,00 patient doses per year might be possible.
8. The 211Rn→211At Generator
Although the 7.2-h half-life of 211At is an advantage for use in tandem with many of the most promising molecular vehicles for TAT, it presents a logistical hurdle for distribution of the radionuclide beyond its production site. An attractive approach to circumvent this problem is via the 211Rn/211At generator system. Radon-211 has a 14.6-h half-life and decays to 211At in transient equilibrium. A 211Rn/211At generator allows 211At to grow in and reach its maximum radioactivity level at 14.5 h. Moreover, over 80% of the maximum 211At activity level is still available on the generator after 24 h. This permits delivery of a generator to remote locations while 211At grows in during transportation, extending the time period for efficient 211At distribution significantly [80, 81].
Radon-211 can be produced via multiple nuclear reactions; however, the most relevant approaches are the bombardment of natural bismuth targets with 42-MeV 6Li ions via the 209Bi(6Li,4n)211Rn reaction [82], or with 60-MeV 7Li beams via the 209Bi(7Li,5n)211Rn reaction [83]. The main concern for these reactions is the co-production of 210Rn via the 209Bi(7Li,6n)210Rn reaction and 210At via a break-up process [84, 85] because these radionuclidic impurities, along with their daughter radionuclides (206Po, 210Po), complicate generator conditions and its utility for subsequent medical application. Maeda et al. [85] investigated excitation functions for these nuclear reactions and concluded that an incident energy between 50 and 60 MeV was optimal for the 209Bi(7Li,5n)211Rn nuclear reaction to maximize yield while minimizing radionuclidic impurities (Fig. 7). However, separation and purification of 211Rn from the target material, as well as astatine and polonium radionuclides is essential [85] and several purification methods for achieving this objective have been investigated [81, 82, 86, 87]. Nolen et al. [88] developed a porous bismuth oxide target material that would allow inline continuous release and trapping of 211Rn during the irradiation. The spallation of actinide targets is another approach for producing 211Rn with high yields [89]; however, it requires high energy protons (~480 MeV) so it is limited to a few facilities (e.g. TRIUMF) [90]. To date, the production of 211Rn and the development of the 211Rn/211At generator system are being investigated at only a few institutions (Table 5); however, the possibility of supplying 211At via this generator is an appealing strategy for helping meet a growing and more geographically disperse demand for this promising radionuclide.
Figure 7.

Optimization of 7Li beam energy and cooling period for the production of 211Rn. A) Estimated yields of 211Rn and 210Rn in a thick Bi target after a 1-h irradiation at a beam intensity of 1 μAp. B) The radioactivity ratio of 211At and 210At during the cooling period. Data kindly provided by Professor Akihiko Yokoyama (Kanazawa University, Japan). C) Possible impurities.
Table 5.
Facilities investigating the production of 211At via a 211Rn/211At generator.
| Location | Institute | Production Means | Current Production Status |
|---|---|---|---|
| Lemont, USA | Argonne National Laboratory | ATLAS | Generator in development |
| Vancouver, Canada | TRIUMF | 500 MeV TRIUMF cyclotron | 40 MBq 211At per production |
| Caen, France | GANIL | Superconducting linear accelerator and Synchrocyclotrons | Production of 211Rn in development |
| Tokai, Japan | JAEA | Tandem accelerator facility | Generator in development |
9. Future 211At Distribution Networks: Prospects and Considerations
If 211At-labeled TAT agents are to have any realistic prospect for evaluation in multicenter clinical trials and subsequent regulatory approval, methods must be devised for achieving their efficient distribution. As noted earlier, the challenge has similarities to that encountered by [18F]FDG, which was overcome by the installation of cyclotrons in multiple medical centers, and then when PET was on firmer financial footing, by commercial entities. Compared with 18F, 211At has a longer half-life and significantly less penetrating radiation requiring less shielding during transport; however, as discussed in Section 4.3., radiolysis can be a complicating issue.
With a 211At-labeled TAT agent, there are at least 3 choices for distribution – the irradiated bismuth target, 211At after isolation from the target, and the final 211At-labeled TAT agent. Shipping the target has been the approach taken to permit 211At produced in Copenhagen to be utilized in Gothenburg where it is distilled and labeling of the final product is performed [32, 40]. On the other hand, 211At was isolated from the cyclotron target before shipment from Duke University to Baltimore, MD in support of preclinical therapy studies. Although anecdotal, lower yields were obtained when 211At labeling was performed after the 6–8 h trip rather than before transport [91]. Further work needs to be done to optimize this strategy, which relieves the recipient institution of the burden of having the facilities and personnel required to perform 211At purification on site. On the other hand, shipping the target is attractive because it minimizes radiolysis issues by minimizing the time interval between exposure of 211At to solvents and initiation of the labeling reaction.
In one distribution model, the formulation of the 211At labeled TAT agent is centralized for use at treatment centers within a few hours’ drive of the production center (Fig. 8). This centralized distribution model is suitable for highly populated areas. In some cases, patients would travel to a hospital near the cyclotron not unlike what is done with patients undergoing proton therapy. In the de-centralized distribution model, irradiated bismuth targets could be transported to formulation hubs that have GMP-level radiochemistry labs staffed with skilled technicians to perform onsite 211At purification, labeling, formulation and quality control (Fig. 8). The formulated 211At-labeled TAT agents could then be delivered to locations around the multiple hubs, extending the coverage of the 211At production centers. Irradiated Bi targets are shipped instead of purified 211At or 211At-labeled compounds to avoid radiolysis issues and additional quality control steps, and also to allow different TAT agents or combinations to be synthesized at each site as needed. This de-centralized model would benefit by the development of automatic purification and synthesis systems to streamline radiopharmaceutical production at the distribution hubs.
Figure 8.

Two potential distribution models for 211At: A) Centralized distribution where formulated radiopharmaceuticals are distributed to hospitals within a feasible distance radius; B) De-centralized distribution where irradiated targets containing 211At are distributed to hub-facilities with a GMP radiochemistry lab and 211At radiopharmaceuticals are formulated there before distribution to hospitals.
Various combinations of the centralized and de-centralized models are possible depending on population density and available transport options. In the US, the delivery of 211At-labeled TAT agents to more than 90% of the population could be feasible with about 7–10 optimized 211At production centers. One could envision eventual involvement of entities such as PETNET Solutions Inc. and Cardinal Health because they already have countrywide networks of GMP distribution hubs and transport infrastructure for supplying PET radiopharmaceuticals. Moreover, the de-centralized distribution network model has been used in China for many years. China Isotope & Radiation Corporation, the largest radiopharmaceutical supplier in China, has built a network of GMP distribution hubs. Instead of distributing 99Mo/99mTc generators to end users, they are sent to distribution hubs (i.e. “milk stations”) and formulated 99mTc radiopharmaceuticals are distributed to hospitals for patient use. With the emerging interest in 211At TAT and progress in 211At production and purification methodologies described herein, such initiatives for 211At distribution networks are not beyond the realm of reality. As a first step in that direction, Ionetix recently announced plans for an 211At distribution network (https://ionetix.com/why-alpha-therapy accessed 26 May 2021). Taking advantage of their existing GMP radiochemistry labs across the country, their CS-30 cyclotron production center in Lansing, Michigan will be able to send irradiated bismuth targets to these distribution hubs while also providing 211At for use within a 400 km radius of the cyclotron. It is anticipated that the first phase of this network – production of 211At for use locally - will be operational in 2022.
10. Conclusions
Astatine-211, with its one α-particle per decay and flexible chemistry, remains an attractive option for TAT, particularly for small molecular scaffolds. Despite the fact that 211At is produced from an abundant target material using straightforward irradiation and purification methods, its availability remains limited. It is encouraging that the need for improved accelerator infrastructure for 211At production recently has led to tangible action by government agencies in several parts of the world. In the US, the Department of Energy has funded enhancements and updates of five existing cyclotrons to improve capabilities for 211At production. Meanwhile, an EU funded COST Program was funded in 2020 and an accelerator network has been established in Japan with the same overall goal. Finally, recent interest in the commercial sector in 211At supply is an encouraging development. Hopefully, these initiatives will lead to substantive near-term improvements in 211At availability so the potential benefits of this radionuclide for TAT can be evaluated.
Acknowledgements
It is a pleasure to acknowledge Drs. Kohshin Washiyama (Fukushima Medical University), D. Scott Wilbur and Yawen Li (University of Washington), Sture Lindegren (University of Gothenburg) and Holger Jensen (Copenhagen University Hospital) for sharing pictures and unpublished information, as well as for very helpful discussions. We also wish to thank Professor Akihiko Yokoyama (Kanazawa University) for sharing cross section data for 211Rn production. In addition, the graphical consultation of Marek Krasivi is greatly appreciated. Work performed in the authors’ laboratory was funded in part by Grants CA42324 and CA184228 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
M.R.Z. is a co-inventor on patents and patent applications for high-level 211At labeling as well as 211Atlabeled compounds of potential utility for targeted radiotherapy. The authors declare no other known potential competing interests.
References
- [1].Poty S, Francesconi LC, McDevitt MR, Morris MJ, and Lewis JS. α-Emitters for radiotherapy: From basic radiochemistry to clinical studies—Part 1. J. Nucl. Med 2018;59:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Robertson AK, Ramogida CF, Schaffer P, and Radchenko V. Development of 225Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Current Radiopharm. 2018;11:156–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson E, Turkington T, Jaszczak R, Gilland D, Vaidyanathan G, Greer K, et al. Quantitation of 211At in small volumes for evaluation of targeted radiotherapy in animal models. Nucl Med Biol 1995;22:45–54. [DOI] [PubMed] [Google Scholar]
- [4].De Kruijff RM, Wolterbeek HT, and Denkova AG. A critical review of alpha radionuclide therapy—how to deal with recoiling daughters? Pharmaceuticals 2015;8:321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Palm S, Humm JL, Rundqvist R, and Jacobsson L. Microdosimetry of Astatine‐211 single‐cell irradiation: Role of daughter Polonium‐211 diffusion. Med. Phys 2004;31:218–25. [DOI] [PubMed] [Google Scholar]
- [6].Krohn KA, Moerlein SM, Link JM, and Welch MJ. Hot atom chemistry and radiopharmaceuticals. AIP Conf. Proc; 2012, p. 3–15. [Google Scholar]
- [7].Vanysek P Ionic conductivity and diffusion at infinite dilution. CRC handbook of chemistry and physics 2000;83:76–8. [Google Scholar]
- [8].Sato H, Yui M, and Yoshikawa H. Ionic diffusion coefficients of Cs+, Pb2+, Sm3+, Ni2+, SeO2−4 and TcO−4 in free water determined from conductivity measurements. J Nucl. Sci. Technol 1996;33:950–5. [Google Scholar]
- [9].Zalutsky MR, Zhao X-G, Alston KL, and Bigner D. High-level production of α-particle–emitting 211At and preparation of 211At-labeled antibodies for clinical use. J. Nucl. Med 2001;42:1508–15. [PubMed] [Google Scholar]
- [10].Goodhead D, Munson R, Thacker J, and Cox R. Mutation and inactivation of cultured mammalian cells exposed to beams of accelerated heavy ions IV. Biophysical interpretation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med 1980;37:135–67. [DOI] [PubMed] [Google Scholar]
- [11].Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J. Nucl. Med 2010;51:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hall EJ and Giaccia AJ. Radiobiology for the Radiologist: Philadelphia,2006. [Google Scholar]
- [13].Radiobiology Sgouros G. and Dosimetry for Radiopharmaceutical Therapy with Alpha-Particle Emitters. Society of Nuclear Medicine and Molecular Imaging, Reston VA, 2015:pp.24–29. [Google Scholar]
- [14].Stepanek J, Larsson B, and Weinreich R. Auger-electron spectra of radionuclides for therapy and diagnostics. Acta Oncol. 1996;35:863–8. [DOI] [PubMed] [Google Scholar]
- [15].Molina B, Soto J, and Castro J. Halogen-like properties of the Al 13 cluster mimicking astatine. Phys. Chem. Chem. Phys 2018;20:11549–53. [DOI] [PubMed] [Google Scholar]
- [16].Meyer GJ. Astatine. J. Labelled. Comp. Radiopharm 2018;61:154–64. [DOI] [PubMed] [Google Scholar]
- [17].Wilbur DS. Enigmatic astatine. Nat. Chem 2013;5:246–246. [DOI] [PubMed] [Google Scholar]
- [18].Zalutsky MR and Vaidyanathan G. Astatine-211-labeled radiotherapeutics an emerging approach to targeted alpha-particle radiotherapy. Curr. Pharm. Des 2000;6:1433–55. [DOI] [PubMed] [Google Scholar]
- [19].Wilbur DS, Chyan M-K, Hamlin DK, Kegley BB, Risler R, Pathare PM, et al. Reagents for astatination of biomolecules: comparison of the in vivo distribution and stability of some radioiodinated/astatinated benzamidyl and nido-carboranyl compounds. Bioconjug. Chem 2004;15:203–23. [DOI] [PubMed] [Google Scholar]
- [20].Reilly SW, Makvandi M, Xu K, and Mach RH. Rapid Cu-catalyzed [211At] astatination and [125I] iodination of boronic esters at room temperature. Org. Lett 2018;20:1752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaizuka Y, Suzuki H, Tanaka H, Washiya N, Tatsuta M, Sato Y, et al. Metabolic studies of astatine-and radioiodine-labeled neopentyl derivatives. J. Nucl. Med 2020;61:1100–1100. [Google Scholar]
- [22].Guérard F, Gestin J-F, and Brechbiel MW. Production of [211At]-astatinated radiopharmaceuticals and applications in targeted α-particle therapy. Cancer. Biother. Radiopharm 2013;28:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zalutsky MR and Narula AS. Astatination of proteins using an N-succinimidyl tri-n-butylstannyl benzoate intermediate. Int. J. Rad. Appl. Instr. A 1988;39:227–32. [DOI] [PubMed] [Google Scholar]
- [24].Dekempeneer, Bäck T, Aneheim E, Jensen H, Puttemans J, avier C, et al. Labeling of anti-HER2 nanobodies with Astatine-211: optimization and the effect of different coupling reagents on their in vivo behavior. Mol. Pharm 2019;16:3524–33. [DOI] [PubMed] [Google Scholar]
- [25].Vaidyanathan G, Affleck DJ, Bigner DD, and Zalutsky MR. N-succinimidyl 3-[211At]astato-4-guanidinomethylbenzoate: an acylation agent for labeling internalizing antibodies with α-particle emitting 211At. Nucl. Med. Biol 2003;30:351–9. [DOI] [PubMed] [Google Scholar]
- [26].Choi J, Vaidyanathan G, Koumarianou E, Kang CM, and Zalutsky MR. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol 2018;56:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dziawer Ł, Majkowska-Pilip A, Gaweł D, Godlewska M, Pruszyński M, Jastrzębski J, et al. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lambrecht RM and Mirzadeh S. Cyclotron isotopes and radiopharmaceuticals—XXXV Astatine-211. Int. J. Appl. Radiat. Isot 1985;36:443–50. [Google Scholar]
- [29].Henriksen G, Messelt S, Olsen E, and Larsen RH. Optimisation of cyclotron production parameters for the 209Bi(α, 2n)211At reaction related to biomedical use of 211At. Appl. Radiat. Isot 2001;54:839–44. [DOI] [PubMed] [Google Scholar]
- [30].Gagnon K, Risler R, Pal S, Hamlin D, Orzechowski J, Pavan R, et al. Design and evaluation of an external high‐current target for production of 211At. J. Labelled. Comp. Radiopharm 2012;55:436–40. [Google Scholar]
- [31].Zalutsky MR and Pruszynski M. Astatine-211: production and availability. Curr. Radiopharm 2011;4:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lindegren S, Albertsson P, Bäck T, Jensen H, Palm S, and Aneheim E. Realizing clinical trials with Astatine-211: the chemistry infrastructure. Cancer Biother. Radiopharm 2020;35:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Larsen RH, Wieland BW, and Zalutsky MR. Evaluation of an internal cyclotron target for the production of 211At via the 209Bi(α, 2n)211At reaction. Appl. Radiat. Isot 1996;47:135–43. [DOI] [PubMed] [Google Scholar]
- [34].Lebeda O, Jiran R, Ráliš J, and Štursa J. A new internal target system for production of 211At on the cyclotron U-120M. Appl. Radiat. Isot 2005;63:49–53. [DOI] [PubMed] [Google Scholar]
- [35].Schwarz U, Plascjak P, Beitzel M, Gansow O, Eckelman W, and Waldmann T. Preparation of 211At-labeled humanized anti-Tac using 211At produced in disposable internal and external bismuth targets. Nucl. Med. Biol 1998;25:89–93. [DOI] [PubMed] [Google Scholar]
- [36].Hermanne A, Tárkányi F, Takács S, Szücs Z, Shubin YN, and Dityuk A. Experimental study of the cross-sections of α-particle induced reactions on 209Bi. Appl. Radiat. Isot 2005;63:1–9. [DOI] [PubMed] [Google Scholar]
- [37].Alfarano A, Abbas K, Holzwarth U, Bonardi M, Groppi F, Alfassi Z, et al. Thick target yield measurement of 211At through the nuclear reaction 209Bi(α, 2n). J. Phys. Conf. Ser. IOP Publishing; 2006, p. 009. [Google Scholar]
- [38].Lindencrona U, Sillfors-Elverby L, Nilsson M, and Forssell-Aronsson E. Adsorption and volatility of free 211At and 125I. Appl. Radiat. Isot 2005;62:395–403. [DOI] [PubMed] [Google Scholar]
- [39].Lindegren S, Bäck T, and Jensen HJ. Dry-distillation of Astatine-211 from irradiated bismuth targets: a time-saving procedure with high recovery yields. Appl. Radiat. Isot 2001;55:157–60. [DOI] [PubMed] [Google Scholar]
- [40].Hallqvist A, Bergmark K, Bäck T, Andersson H, Dahm-Kähler P, Johansson M, et al. Intraperitoneal α-emitting radioimmunotherapy with 211At in relapsed ovarian cancer: long-term follow-up with individual absorbed dose estimations. J. Nucl. Med 2019;60:1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Washiyama K, Oda T, Sasaki S, Aoki M, Gomez FLG, Taniguchi M, et al. At-211 production using the CYPRIS MP-30. J. Med. Imaging. Radiat. Sci 2019;50:S42. [Google Scholar]
- [42].Wang Y, Sato N, Komori Y, Yokokita T, Mori D, Usuda S, et al. Present status of 211At production at the RIKEN AVF cyclotron. RIKEN Accel. Prog. Rep 2020;53:192. [Google Scholar]
- [43].Watabe T, Kaneda-Nakashima K, Liu Y, Shirakami Y, Ooe K, Toyoshima A, et al. Enhancement of 211At uptake via the sodium iodide symporter by the addition of ascorbic acid in targeted α-therapy of thyroid cancer. J. Nucl. Med 2019;60:1301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li Y, Hamlin DK, Chyan M-K, Wong R, Dorman EF, Emery RC, et al. cGMP production of Astatine-211-labeled anti-CD45 antibodies for use in allogeneic hematopoietic cell transplantation for treatment of advanced hematopoietic malignancies. PLoS One 2018;13:e0205135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Balkin ER, Hamlin DK, Gagnon K, Chyan M-K, Pal S, Watanabe S, et al. Evaluation of a wet chemistry method for isolation of cyclotron produced [211At]astatine. Appl. Sci 2013;3:636–55. [Google Scholar]
- [46].Aneheim E, Albertsson P, Bäck T, Jensen H, Palm S, and Lindegren S. Automated astatination of biomolecules–a stepping stone towards multicenter clinical trials. Sci. Rep 2015;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Martorano P, Taghvaee T, Schaub D, Toto L, Lee H, MaKvandi M, et al. Dry distillation of Astatine-211 by electromagnetic induction. J. Nucl. Med 2020;61:518. [Google Scholar]
- [48].Sato N, Yano S, Toyoshima A, Haba H, Komori Y, Shibata S, et al. Development of a production technology of 211At at the RIKEN AVF cyclotron:(i) Production of 211At from the 209Bi (α, 2n) 211At reaction. RIKEN Accel. Prog. Rep 2017;50:262. [Google Scholar]
- [49].Friedman A, Zalutsky M, Wung W, Buckinham F, Harper P Jr Scherr GH, et al. Preparation of a biologically stable and immunogenically competent astatinated protein. Int. J. Nucl. Med. Biol 1977;4:219–24. [DOI] [PubMed] [Google Scholar]
- [50].Vaidyanathan G, Pozzi OR, Choi J, Zhao X-G, Murphy S, and Zalutsky MR. Labeling Monoclonal Antibody with α-emitting 211At at High Activity Levels via a Tin Precursor. Cancer Biother. Radiopharm 2020;35:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Neumann H Solvent distribution studies of the chemistry of astatine. J. Inorg. Nucl. Chem 1957;4:349–53. [Google Scholar]
- [52].Neirinckx R and Smit J. Separation of Astatine-211 from bismuth metal. Anal. Chim. Acta 1973;63:201–4. [DOI] [PubMed] [Google Scholar]
- [53].Li Y, Chyan M-K, Hamlin D, and Wilbur DS. An automated process for Astatine-211 isolation from irradiated bismuth targets using a tellurium-packed column. J. Nucl. Med 2018;59:666. [Google Scholar]
- [54].Li Y, Hamlin DK, Chyan M-K, Morscheck TM, Ferrier MG, Wong R, et al. Investigation of a tellurium-packed column for isolation of Astatine-211 from irradiated bismuth targets and demonstration of a semi-automated system. Sci. Rep 2019;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Woen DH, Eiroa-Lledo C, Akin AC, Anderson NH, Bennett KT, Birnbaum ER, et al. A solid-state support for separating astatine-211 from bismuth. Inorg. Chem 2020;59:6137–46. [DOI] [PubMed] [Google Scholar]
- [56].Burns JD, Tereshatov EE, Avila G, Glennon KJ, Hannaman A, Lofton KN, et al. Rapid recovery of At-211 by extraction chromatography. Sep. Purif. Technol 2021;256:117794. [Google Scholar]
- [57].Pozzi OR and Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 1: effects of solvent on the degradation of radiohalogenation precursors by 211At α-particles. J. Nucl. Med 2005;46:700–6. [PubMed] [Google Scholar]
- [58].Pozzi OR and Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 2: radiolytic effects of 211At α-particles influence N-succinimidyl 3-211At-astatobenzoate synthesis. J. Nucl. Med 2005;46:1393–400. [PubMed] [Google Scholar]
- [59].Pozzi OR and Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 3: α-particle–induced radiolytic effects on the chemical behavior of 211At. J. Nucl. Med 2007;48:1190–6. [DOI] [PubMed] [Google Scholar]
- [60].Pozzi OR and Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 4: Strategies for 211At labeling at high activities and radiation doses of 211At α-particles. Nucl. Med. Biol 2017;46:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wilbur DS, Chyan M-K, Hamlin DK, Vessella RL, Wedge TJ, and Hawthorne MF. Reagents for astatination of biomolecules. 2. Conjugation of anionic boron cage pendant groups to a protein provides a method for direct labeling that is stable to in vivo deastatination. Bioconjug. Chem 2007;18:1226–40. [DOI] [PubMed] [Google Scholar]
- [62].Dzaugis ME, Spivack AJ, and D’Hondt S. A quantitative model of water radiolysis and chemical production rates near radionuclide-containing solids. Radiat. Phys. Chem 2015;115:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Le Caër S Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 2011;3:235–53. [Google Scholar]
- [64].Nishinaka I, Hashimoto K, and Suzuki H. Speciation of astatine reacted with oxidizing and reducing reagents by thin layer chromatography: formation of volatile astatine. J. Radioanal. Nucl. Chem 2019;322:2003–9. [Google Scholar]
- [65].Aneheim E, Jensen H, Albertsson P, and Lindegren S. Astatine-211 labeling: a study towards automatic production of astatinated antibodies. J. Radioanal. Nucl. Chem 2015;303:979–83. [Google Scholar]
- [66].O’Hara MJ, Krzysko AJ, Niver CM, Morrison SS, Owsley SL Jr, Hamlin DK, et al. An automated flow system incorporating in-line acid dissolution of bismuth metal from a cyclotron irradiated target assembly for use in the isolation of Astatine-211. Appl. Radiat. Isot 2017;122:202–10. [DOI] [PubMed] [Google Scholar]
- [67].Toyoshima A, Kanda A, Ikeda T, Ichimura S, Ooe YK, Yoshimura T, et al. Production and isolation of At-211 for targeted alpha therapy at Osaka University. International Workshop on the Biological Effects of Radiation. Osaka University, Japan; 2018. [Google Scholar]
- [68].Toyoshima A, Zhang Z, Kanda A, Ikeda T, Ichimura S, Ooe K, et al. Isolation of At-211 by Dry-distillation under oxidative conditions for targeted alpha therapy in Osaka University. J. Med. Imaging Radiat. Sci, 2019;50:S76–S7. [Google Scholar]
- [69].Nagatsu K, Minegishi K, Fukada M, Suzuki H, Hasegawa S, and Zhang M-R. Production of 211At by a vertical beam irradiation method. Appl. Radiat. Isot 2014;94:363–71. [DOI] [PubMed] [Google Scholar]
- [70].Ogawa K, Mizuno Y, Washiyama K, Shiba K, Takahashi N, Kozaka T, et al. Preparation and evaluation of an Astatine-211-labeled sigma receptor ligand for alpha radionuclide therapy. Nucl. Med. Biol 2015;42:875–9. [DOI] [PubMed] [Google Scholar]
- [71].Ukon N, Zhao S, Washiyama K, Oriuchi N, Tan C, Shimoyama S, et al. Human dosimetry of free 211At and meta-[211At] astatobenzylguanidine (211At-MABG) estimated using preclinical biodistribution from normal mice. EJNMMI Phys. 2020;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Freifelder R, Kachur A, LeGeyt B, Schmitz A, and Toto L. Production of 211At using the JSW BC3015 at the University of Pennsylvania. AIP Conf. Proc; 2012, p. 129–34. [Google Scholar]
- [73].Burns JD, Tereshatov EE, McCarthy MA, McIntosh LA, Tabacaru GC, Yang X, et al. Astatine partitioning between nitric acid and conventional solvents: indication of covalency in ketone complexation of AtO+. Chem. Comm 2020;56:9004–7. [DOI] [PubMed] [Google Scholar]
- [74].Haddad F, Ferrer L, Guertin A, Carlier T, Michel N, Barbet J, et al. ARRONAX, a high-energy and high-intensity cyclotron for nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2008;35:1377–87. [DOI] [PubMed] [Google Scholar]
- [75].Teze D, Sergentu D-C, Kalichuk V, Barbet J, Deniaud D, Galland N, et al. Targeted radionuclide therapy with Astatine-211: Oxidative dehalogenation of astatobenzoate conjugates. Sci. Rep 2017;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Takahashi N, Yano D, Ishikuro M, and Baba H. Formation and decomposition of astatine molecules: one-atom-based chemistry of astatine. Radiochim. Acta 1993;61:35–40. [Google Scholar]
- [77].Cheng L, Qunxia D, Jimin Z, Yanyan Z, Lun X, Boli L, … & Zhigang T Preparation of heavy halogen nuclide 211At and fast labelling. Heh Hua Hsueh Yu Fang She Hua Hsueh;(China) 1984., 6(4). [Google Scholar]
- [78].Liu W, Ma H, Tang Y, Chen Q, Peng S, Yang J, et al. One-step labelling of a novel small-molecule peptide with Astatine-211: preliminary evaluation in vitro and in vivo. J. Radioanal. Nucl. Chem 2018;316:451–6. [Google Scholar]
- [79].Prebys E, Casey W, Cebra D, and Abergel R. Development of Astatine-211 production in the Crocker Nuclear Laboratory cyclotron. 10th International Particle Accelerator Conference, Melbourne, Australia: 2019; doi: 10.18429/JACoW-IPAC2019-THPMP051. [DOI] [Google Scholar]
- [80].Mirzadeh S Generator-produced alpha-emitters. Appl. Radiat. Isot 1998;49:345–9. [Google Scholar]
- [81].Crawford JR, Yang H, Kunz P, Wilbur DS, Schaffer P, and Ruth TJ. Development of a preclinical 211Rn/211At generator system for targeted alpha therapy research with 211At. Nucl. Med. Biol 2017;48:31–5. [DOI] [PubMed] [Google Scholar]
- [82].Greene JP, Nolen J, and Baker S. Nickel-backed Bi targets for the production of 211At. J. Radioanal. Nucl. Chem 2015;305:943–6. [Google Scholar]
- [83].Meyer G-J and Lambrecht R. Excitation function for the 209Bi(7Li, 5n)211Rn nuclear reaction as a route to the 211Rn-211At generator. J. Labelled. Comp. Radiopharm 1981;18:233. [Google Scholar]
- [84].Dasgupta M, Gomes P, Hinde D, Moraes S, Anjos R, Berriman A, et al. Effect of breakup on the fusion of 6Li, 7Li, and 9Be with heavy nuclei. Phys. Rev. C 2004;70:024606. [Google Scholar]
- [85].Maeda E, Yokoyama A, Taniguchi T, Washiyama K, and Nishinaka I. Measurements of the excitation functions of radon and astatine isotopes from 7Li-induced reactions with 209Bi for development of a 211Rn–211At generator. J. Radioanal. Nucl. Chem 2020;323:921–6. [Google Scholar]
- [86].Maeda E, Yokoyama A, Taniguchi T, Washiyama K, and Nishinaka I. Extraction of astatine isotopes for development of radiopharmaceuticals using a 211Rn–211At generator. J. Radioanal. Nucl. Chem 2015;303:1465–8. [Google Scholar]
- [87].Aoi K, Kawasaki K, Maruyama S, Higashi M, Washiyama K, Yokoyama A, et al. Decontamination of Po in the 211Rn/211At generator system. RIKEN Accel. Prog. Rep 2020;53:193. [Google Scholar]
- [88].Nolen J, Mustapha B, Gott M, de Kruijff R, Song J, Gampa R, et al. Development of 211At production via continuous extraction of 211Rn. J. Med. Imaging Radiat. Sci 2019;50:S35–S6. [Google Scholar]
- [89].Nolen J, and Gomes I, Production of isotopes using high power proton beams. U.S. Patent No.10,249,399. 2April. 2019.
- [90].Crawford JR, Kunz P, Yang H, Schaffer P, and Ruth TJ. 211Rn/211At and 209At production with intense mass separated Fr ion beams for preclinical 211At-based α-therapy research. Appl. Radiat. Isot 2017;122:222–8. [DOI] [PubMed] [Google Scholar]
- [91].Mease RC, Kang CM, Kumar V, Banerjee SR, Minn I, Brummet M et al. An improved 211At-labeled agent for PSMA-targeted alpha therapy. J Nucl. Med, 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


