Abstract
Background:
Chemoradiotherapy for Esophageal cancer followed by Surgery (CROSS regimen) is standard of care for locally-advanced esophageal cancer. We evaluated CROSS completion rates, toxicity, and postoperative outcomes between older and younger adults receiving trimodality therapy.
Methods:
Retrospective analysis of patients with locally-advanced esophageal cancer who underwent CROSS regimen from May 2016 to January 2020 at a single academic center. Outcomes of those aged ≥70-years-old and <70 years-old were analyzed.
Results:
Of 201 patients, 136 were <70 and 65 were ≥70 years. Older adults were more likely to be male (91% vs. 79%; p = 0.045), have higher ECOG scores (median 1 vs. 0; p = 0.003), Charlson-comorbidity index (median 6 vs. 4; p < 0.001), and undergo open procedures (20% vs. 8% p = 0.008). Most completed CROSS regimen (78% vs. 84% respectively) with similar rates of treatment discontinuation and dose reduction (all p > 0.05). Time to surgery following neoadjuvant therapy was similar between age groups, except in those ≥80-years-old as compared to <70-years-old (p < 0.05). Overall toxicity rates were similar (68% vs. 71% respectively; p = 0.676). Only rates of delirium (19% vs. 5%) and urinary retention (9% vs. 0%) were higher in older adults (both p < 0.05). Length of stay, discharge disposition, mortality, and overall survival were similar. Age was not an independent risk factor for complication, neoadjuvant toxicity or completion, surgery timing, nor worse overall or recurrence-free survival (p > 0.05).
Conclusion:
Trimodality CROSS regimen for esophageal cancer in older adults is feasible, with similar completion rates and postoperative outcomes as compared to their younger counterparts.
Keywords: Esophageal cancer, CROSS, Esophagectomy, Older adults, Neoadjuvant toxicity
Introduction
Esophageal cancer is a disease of older adults with a median age of 68 and 25% of cases over the age of 75 [1]. Neoadjuvant chemoradiation (nCRT) therapy followed by surgery is the current mainstay of treatment for locally advanced (T1N1 or T2-T3/N0–N1) esophageal cancer for those who can tolerate major surgery [2]. Since the publication of the Dutch Chemoradiotherapy for Esophageal cancer followed by Surgery Study (CROSS) trial in 2012 (which we will refer to as the “CROSS regimen”) we have adopted it as our standard of care for locally advanced esophageal cancer [2,3]. This regimen uses neoadjuvant carboplatin (Area Under Curve (AUC) 2 mg/ml/min), paclitaxel)50 mg/m2) and radiation (minimum 41.4 Gy).
In CROSS [3], eligible patients were 18–75 years of age (median 60 years), had a WHO performance status of 0 or 1 and weight loss less than 10% body weight. It is unclear if similar results could be expected in older and frailer patients. Few studies have reported outcomes in patients older than 70 years with locally advanced esophageal cancer. Many were often missing baseline functional data and chemotherapy completion rates [4]. Age itself is not a sufficient predictor of surgical and multi-modality therapy outcomes [1,5].
We evaluate the differences in outcomes and completion rates of CROSS regimen for locally advanced esophageal cancer in patients aged 70 and older as compared to younger counterparts.
Methods
Study design and population
Retrospective cohort study of all patients aged 18 and above with esophageal cancer clinical stage T1N1 or T2-T3N0–1 [3] by either 7th or 8th edition AJCC TNM [6,7] who underwent neoadjuvant CROSS regimen followed by surgery between May 2016 and January 2020 at a large academic referral center. Patients were identified from the division of thoracic surgery's prospectively collected surgical database which includes only postoperative patients. We excluded all surgical cases without confirmed clinical staging (Tx or Nx), or those who received any alternative or unknown neoadjuvant regimen. The study was approved by the Institutional Review Board (IRB #2014P002478).
Treatment protocol
Planned neoadjuvant chemotherapy was carboplatin (AUC) 2 mg/ml/min and paclitaxel 50 mg/m2. Planned neoadjuvant radiation (XRT) was 50.4 Gy in the majority of patients. In our institution, planned time following neoadjuvant therapy completion and esophagectomy is 30–90 days. Patients underwent either a modified McKeown or Ivor-Lewis esophagectomy by open or minimally invasive technique as previously described [8–12]. Details of preoperative workup, perioperative care and routine follow up are described in Supplemental Appendix 1. Adjuvant therapy was not routinely utilized, and in our institution is largely given for recurrence.
Variables
Pre-treatment chemoradiotherapy and surgical variables were collected. Treatment completion was defined as completed prescribed cycles and doses of chemoradiation. Dose reduction was defined as decreased dose or cycle of chemoradiation. Treatment discontinuation was defined as early termination. Chemoradiation toxicities were scaled according to the Common Terminology Criteria for Adverse Events (CTCAE) 5th edition [13] and postoperative complications were graded by Clavian-Dindo classification [14] (appendix 2). We examined time to surgery after neoadjuvant within 8 weeks per CROSS protocol [3] and 30–90 days per our institutional best practices. Survival was calculated based on date of surgery to last known follow up or date of death. Recurrence-free survival was calculated from date of surgery to recurrence on imaging or biopsy when performed. Details of our quality assurance are presented as supplemental appendix 2. Variable definitions are presented as appendix 3.
Statistical analysis
Patients less than 70-years were compared to those aged 70-years old or greater. Categorical variables were analyzed using either chi-square or Fisher's exact test. Continuous variables were analyzed using medians and means with SD, and comparisons made with Wilcoxon rank-sum test.
Kaplan-Meier Curves were constructed to examine 3-year overall and recurrence-free survival by age group (<70 and 70+ years-old). Log-rank test was considered significant at a p-value < 0.05.
Univariable and multivariable logistic regressions tested the associations between age groups (<70 and ≥ 70 years, and <70 and ≥ 80 years-old) and preselected by clinical relevance variables with [1]: postoperative complications (baseline none) [2], completion of chemoradiation therapy (baseline either discontinuation or dose reduction) [3] overall neoadjuvant toxicity (baseline none) [4], performance of surgery within 8 weeks of neoadjuvant completion (baseline >8 weeks). Inclusion into the multivariable analysis was based on predetermined variables considered to be clinically relevant with the exclusion of variables that presented with a very low (<5) event rate, and consequently led to unstable estimates due to overestimated standard deviation. Correspondingly, univariable and multivariable Cox proportional hazard models were constructed to describe the possible association of age groups and clinically relevant-treatment variables with [1]: overall and [2] recurrence-free survival. Statistical analyses were performed using R 4.0.3 (R Core team 2020) [15].
Results
During the study period 393 esophagectomies were performed at our institution. Of those esophagectomies, 201 (51%) patients completed trimodality CROSS regimen and were included in our analysis:136 were <70-years and 65 patients were ≥70-years-old. Median overall age was 66.35 (range 26–83) with 84% adenocarcinoma and 13% squamous cell carcinoma. Patients in both age groups had similar sociodemographic factors, comorbidities (Table 1), and tumor characteristics (Table 2). However, older adults were more likely to be male (91% vs. 79%; p = 0.045), have a higher Eastern Cooperative Oncology (ECOG) score (median 1 vs. 0; p = 0.003), higher age-adjusted Charlson Comorbidity Index (median 6 vs. 4; p < 0.001), more hypertension (65% vs. 48%; p = 0.029) and moderate-to-severe chronic kidney disease (6% vs. 0.7%; p = 0.039).
Table 1.
Baseline characteristics of study population.
| Variables | Overall N = 201 |
<70 N = 136 |
70+ N = 65 |
p-value* |
|---|---|---|---|---|
|
| ||||
| Sociodemographic | ||||
| Age, years, median (range) | 66.35 (range 26–83) | 62.5 (26–70) | 73.7 (70–83) | <0.001 |
| Gender, male, n (%) | 167 (83) | 108 (79) | 59 (91) | 0.045 |
| BMI, median (range) | 27 (16–51) | 27 (16–51) | 27 (19–38) | 0.159 |
| ECOG, median (range) | 0 (0–2) | 0 (0–2) | 1 (0–2) | 0.003 |
| Any Smoking Hx, n (%) | 151 (75) | 104 (76) | 47 (72) | 0.601 |
| ETOH use (severe), n (%)a | 35 (17) | 23 (17) | 12 (18) | 0.843 |
| Comorbidities | ||||
| Age-adjusted Charlson Comorbidity Index, median (range) | 5 (1–12) | 4 (1–11) | 6 (5–12) | <0.001 |
| CHF, n (%) | 7 (3) | 3 (2) | 4 (6) | 0.216 |
| CAD, n (%) | 33 (17) | 18 (13) | 15 (23) | 0.082 |
| HTN, n (%) | 107 (54) | 65 (48) | 42 (65) | 0.029 |
| COPD, n (%) | 17 (8) | 8 (6) | 9 (14) | 0.058 |
| DVT, n (%)b | 10 (5) | 5 (4) | 5 (8) | 0.300 |
| PVD, n (%) | 30 (15) | 22 (16) | 8 (12) | 0.472 |
| CVA, n (%) | 13 (6) | 7 (5) | 6 (9) | 0.271 |
| Cognitive impairment, n (%) | 1 (0.5) | 0 (0) | 1 (1.5) | 0.323 |
| Moderate/Severe CKD, n (%) | 5 (2) | 1 (0.7) | 4 (6) | 0.039 |
| Diabetes, n (%)c | 40 (20) | 22 (16) | 18 (28) | 0.056 |
| Prior CT surgery (any) | 25 (12) | 19 (14) | 6 (9) | 0.341 |
| Immunosuppression, n (%) | 15 (7) | 12 (9) | 3 (5) | 0.395 |
| Anticoagulated, n (%) | 25 (12) | 11 (8) | 14 (22) | 0.007 |
| Home 02, n (%) | 1 (0.5) | 1 (0.74) | 0 (0) | 1.000 |
| Pulmonary function testd N = 180 N = 121 N = 59 | ||||
| FEV1, mean ± SD | 87.5± 21.2 | 89.1 ±20.4 | 94.3± 22.6 | 0.197 |
| FVC %, mean ± SD | 92.8±17.5 | 94.5±17.2 | 89.3±17.7 | 0.086 |
| FEV1/FVC, mean, +/− SD | 0.94±0.13 | 0.94± 0.12 | 0.94± 0.15 | 0.969 |
BMI= Body-Mass Index; CAD= Coronary Artery Disease; CHF= Congestive heart failure; CKD = chronic kidney disease; COPD= Chronic obstructive pulmonary disease; CT = cardiothoracic; CVA= Cerebrovascular disease; DVT = Deep vein thrombosis; ECOG = Eastern Cooperative Oncology Group; EtOH = Ethanol; FEV1% = Forced expiratory volume in 1 s; FVC= Forced vital capacity; HTN = hypertension; 02 = oxygen; PVD= Peripheral Vascular disease; SD= Standard Deviation.
17 drinks/week or more.
Pre-treatment.
Insulin and non-insulin dependent diabetes.
PFTs means are reported out of total N that was available for each cohort due to missing data or in cases were PFTs were not performed.
Table 2.
Tumor Characteristics in patients with locally advanced esophageal cancer undergoing CROSS trimodality therapy.
| Variables | Overall patient | <70 N = 136 |
70+ N = 65 |
p-value |
|---|---|---|---|---|
|
| ||||
| Tumor Characteristics | ||||
| Histology, n (%) | 0.277 | |||
| Adenocarcinoma | 168 (83.58) | 117 (86.03) | 51 (78.46) | |
| Squamous Cell Carcinoma | 26 (12.94) | 14 (10.29) | 12 (18.46) | |
| other | 7 (3.48) | 5 (3.68) | 2 (3.08) | |
| Grade, n (%) | 0.041 | |||
| Gx | 93 (46.27) | 54 (39.71) | 39 (60.00) | |
| G1 | 3 (1.49) | 2 (1.47) | 1 (1.54) | |
| G2 | 40 (19.90) | 32 (23.53) | 8 (12.31) | |
| G3 | 60 (29.85) | 43 (31.62) | 17 (26.15) | |
| Grade Missing | 5 (2.49) | 5 (3.68) | 0 (0.00) | |
| TNM | ||||
| cTStagea, n (%) | 0.578 | |||
| T1 | 4 (1.99) | 4 (2.94) | 0 | |
| T2 | 51 (25.37) | 33 (24.26) | 18 (27.69) | |
| T3 | 145 (72.14 | 98 (72.06) | 47 (72.31) | |
| cN Stage, n (%) | 0.427 | |||
| N0 | 104 (51.74) | 73 (53.68) | 31 (47.69) | |
| N1 | 97 (48.26) | 63 (46.32) | 34 (52.31) | |
| ypT stage, n (%) | 0.164 | |||
| T0 | 59 (29.35) | 33 (24.26) | 26 (40.00) | |
| T1 | 39 (19.40) | 29 (21.32) | 10 (15.38) | |
| T2 | 38 (18.91) | 27 (19.85) | 11 (16.92) | |
| T3 | 65 (32.34) | 47 (34.56) | 18 (27.69) | |
| T4 | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| ypN stage, n (%) | ||||
| Nx | 1 (0.50) | 1 (0.74) | 0 (0.00) | 0.235 |
| N0 | 130 (64.68) | 81 (59.56) | 49 (75.38) | |
| N1 | 56 (27.86) | 43 (6.62) | 13 (20.00) | |
| N2 | 12 (5.97) | 9 (6.62) | 3 (4.62) | |
| N3 | 2 (1.00) | 2 (1.47) | 0 (0.00) | |
| Pathologic Factors | ||||
| Positive margin, n % | 2 (1.01) | 1 (0.75) | 1 (1.54) | 0.684 |
| Tumor invade LN, n % | 62 (30.85) | 46 (33.82) | 16 (24.62) | 0.186 |
| Lymphovascular invasion n% | 35 (17.41) | 26 (19.12) | 9 (13.85) | 0.357 |
1 case of T2/T3 in <70 group C= Clinical; LN = Lymph node; P= Pathologic.
All patients received neoadjuvant chemoradiation. Median radiation dose was 50.4 Gy. Neoadjuvant treatment-related toxicity occurred in 140 patients (70%), which was most commonly gastrointestinal (GI) (Table 2). Toxicity rates were similar between age groups except for GI toxicity, which was more prevalent in the younger group (29% vs. 52%; p = 0.002). Most neoadjuvant treatment-related toxicities were mild (grade 2 or below), especially among younger patients (85% vs. 68% in the younger and older cohort respectively, p = 0.018). Overall, 14/65 (22%) of those 70 and older as compared to 22/165 (13%) of the younger cohort experienced a grade 3 toxicity or higher, requiring a dose reduction or discontinuation of therapy (p = 0.354).
Overall, 165 patients (82%) completed neoadjuvant chemoradiation, 17 patients (8%) discontinued therapy, and 19 (9%) required dose reduction. Treatment completion rates were not significantly different between age groups (p > 0.05).
Median time from neoadjuvant completion to esophagectomy was 49 days (range 12–241). One hundred and twenty-seven patients (65%) underwent surgery within 8 weeks of neoadjuvant completion and 173 (88%) underwent surgery within 30–90 days following neoadjuvant completion. There was no difference in these treatment intervals between the age groups (Table 3).
Table 3.
Neoadjuvant treatment factors and toxicity.
| Combined chemoradiation | Overall Patient N = 201 |
<70 N = 136 |
70+ N = 65 |
p-value |
|---|---|---|---|---|
|
| ||||
| Treatment Type | ||||
| Combined chemoradiation, n (%) | 201 (100) | 136 (100) | 65 (100) | – |
| Radiation dose Grey, median (range)a | 50.4 (36–54) | 50.4 (36–54) | 50.4 (41.4–50.4) | 0.263 |
| Treatment Completion | ||||
| Completed, n (%) | 165 (82) | 114 (84) | 51 (78) | 0.441 |
| discontinued, n (%) | 17 (8) | 13 (10) | 4 (6) | 0.4589 |
| Dose Reduced, n (%) | 19 (9) | 9 (7) | 10 (15) | 0.00.069 |
| Surgery Timeline | ||||
| Time from neoadjuvant completion to Esophagectomy, days, median (range) | 49 (12–241) | 49 (12–149) | 50 (25–241) | 0.204 |
| Patients undergoing esophagectomy within 8-weeks of nCRT, n (%)b | 127 (64) | 90 (67) | 37 (59) | 0.249 |
| Patients undergoing esophagectomy 30–90 days after nCRT, n (%)c | 173 (88) | 119 (89) | 54 (86) | 0.536 |
| Neoadjuvant Treatment Toxicity | ||||
| Overall neoadjuvant toxicity rate, n (%) | 140 (70) | 96 (71) | 44 (68) | 0.676 |
| Neoadjuvant chemoradiation toxicity by CTCAE groupd, n (%) | ||||
| Cardiac | 5 (2) | 3 (2) | 2 (3) | 0.659 |
| Gastrointestinal | 90 (45) | 71 (52) | 19 (29) | 0.002 |
| Skin and Subcutaneous | 6 (0.03) | 5 (3.7) | 1 (1.5) | 0.666 |
| General disorderse | 27 (13) | 21 (15) | 6 (9) | 0.227 |
| Nervous system disorders | 6 (3) | 5 (4) | 1 (1.5) | 0.666 |
| Metabolism and Nutrition disorders | 35 (17) | 28 (21) | 7 (11) | 0.086 |
| Injury, Poisoning and Procedural Complication | 4 (2) | 3 (2) | 1 (1.5) | 1.000 |
| Vascular Disorders | 2 (1) | 0 (0) | 2 (3) | 0.103 |
| Respiratory, thoracic and Mediastinal Disorder | 3 (1.5) | 3 (2) | 0 (0) | 0.552 |
| Infection/Infestation | 10 (5) | 8 (6) | 2 (3) | 0.505 |
| Blood and Lymphatic system disorders | 9 (4) | 6 (4) | 3 (5) | 1.000 |
| Investigation/lab | 41 (20) | 26 (19) | 15 (23) | 0.515 |
nCRT = Neoadjuvant chemoradiation.
Dose known for 148 patients.
N = 134 and N = 63.
30–90-day goal determined by institutional practice (N = 134 for <70 and N = 63 for 70+ years of age).
Toxicity groups defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
General disorders such as chills, injection site reaction, fatigue, malaise, etc.
Operative details are described in Supplemental Table 1. Ivor-Lewis was the most common surgical technique overall (66% vs. 68% in those <70 and ≥ 70 years old respectively; p = 0.831). The most common approach for either group was Video Assisted thoracoscopic surgery (VATS) (76% vs. 65% in those <70 and ≥ 70 years old respectively) followed by robotic in the younger cohort (16%) and open procedure in the older patients (20%). Older patients were likelier to undergo an open procedure (p = 0.008) and less likely to undergo VATS approach than the younger cohort (p = 0.045). Higher rates of open procedure in older adults were due to surgeon preference, as rates of conversion were similar (7.7% vs. 5.1% for older and younger respectively; p = 0.467). Overall R0 resection was achieved in 99% of patients. There was no difference in rate of robotic assisted surgery between the groups (p = 0.886). Breakdown of pT and pN stage, as well as rates of positive margins, lymphovascular invasion, and lymph node involvement were similar between the two cohorts (Table 2).
The overall 30-day postoperative complication rate was 52% (Table 4). While those aged 70 years or older tended to have more overall complications (58% vs. 49%), this did not achieve statistical significance (p = 0.222). Rates of grade II complications (55% vs. 38%) including delirium (18% vs. 5%) and urinary retention (9% vs. 0%) were more common in those 70 years or older (all p < 0.05). However, there was no difference in grade III-V complications. One hospital death occurred in the older group and none in the younger group. Hospital length of stay (LOS) and discharge disposition were not statistically different between groups.
Table 4.
Postoperative complications.
| Variables | All patient N = 201 |
<70 years-old N = 136 |
70+ years-old N = 65 |
p-value |
|---|---|---|---|---|
|
| ||||
| Overall postoperative complications, n (%) | 105 (52) | 67 (49) | 38 (58) | 0.222 |
| Complications by Grade | ||||
| Grade II, n (%) | 87 (43) | 51 (38) | 36 (55) | 0.017 |
| Grade III, n (%) | 50 (25) | 35 (26) | 15 (23) | 0.683 |
| Grade IV, n (%) | 10 (5) | 5 (4) | 5 (8) | 0.298 |
| Grade V, n (%) | 1 (0.5) | 0 (0) | 1 (1.5) | 0.323 |
| Complications by Organ System | ||||
| Neurologic, n (%) | ||||
| delirium | 19 (9) | 7 (5) | 12 (18) | 0.004 |
| Cardiovascular, n (%) | ||||
| Atrial Fibrillation | 40 (20) | 25 (18) | 15 (23) | 0.436 |
| Pulmonary, n (%) | ||||
| Pneumonia | 18 (9) | 13 (10) | 5 (8) | 0.795 |
| Respiratory Failure | 8 (4) | 5 (4) | 3 (5) | 0.715 |
| Gastrointestinal, n (%) | ||||
| Esophageal Leak | 10 (5) | 6 (4) | 4 (6) | 0.595 |
| Chyle Leak, n (%) | 14 (7) | 9 (7) | 7 (8) | 0.773 |
| Genitourinary, n (%) | ||||
| Urinary Retention | 6 (3) | 0 | 6 (9) | 0.001 |
| Acute kidney injury/Renal Failure | 21 (10) | 15 (3) | 6 (9) | 0.696 |
| Sepsis, n (%) | 2 (1) | 0 | 2 (3) | 0.103 |
| Transfusion, n (%) | 17 (8) | 8 (6) | 9 (14) | 0.100 |
| Return to operating room | 31 (15) | 21 (15) | 10 (15) | 1.000 |
| Hospital LOS, days, median (range) | 9 (6–56) | 9 (6–47) | 10 (6–56) | 0.155 |
| Disposition | 0.491 | |||
| Home, n (%) | 7 (3.5) | 4 (3) | 3 (5) | |
| Home with services, n (%) | 168 (84) | 117 (86) | 51 (80) | |
| Rehabilitation facility, n (%) | 25 (12.5) | 15 (11) | 10 (16) | |
| Overall Survival (from time of surgery to death or last known follow up)a | ||||
| 30-day mortality (n = 201) | 1 (0.5) | 0 (0) | 1 (1.5) | 0.1 |
| 90-day mortality (n = 199) | 4 (2.0) | 2 (1.5) | 2 (3.1) | 0.4 |
| 1-year mortality (n = 187) | 22 (12.0) | 12 (9.6) | 10 (17.3) | 0.2 |
LOS = Length of stay.
Not all patients had 90-days or 1-year of follow up data available at time of the study completion. Rates are reported out of those with sufficient follow enough time (n = 201 for 30-day, n = 199 for 90-day, n = 187 for 1-year follow up).
Thirty-day, 90-day, and 1-year overall mortality were 0.50%, 2.02% and 12.0% respectively for the overall patient population. Mortality rates were not statistically different between age-groups (Table 4). Kaplan-Meier survival curves showed no significant differences in overall survival (Fig. 1A) (log-rank p-value = 0.24) nor 1-year survival (90.4% (95% CI 85.4%–95.7%) vs. 82.7% (95% CI 73.4%–93.1%) for <70 and ≥ 70 years old respectively). Median survival was not achieved for either age group at 3 years.
Fig. 1.
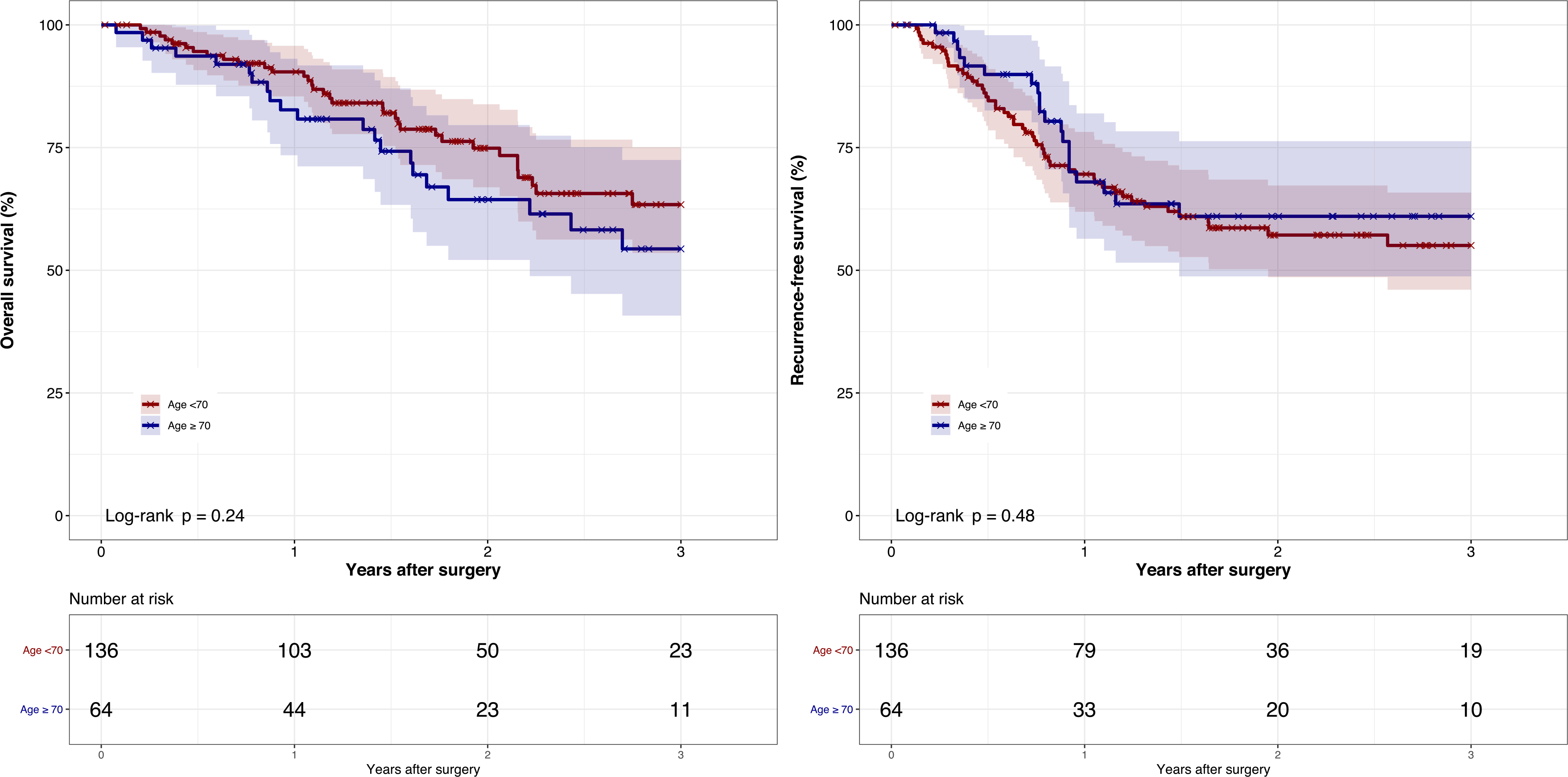
Kaplan-Meier curve of 3-year overall survival and recurrence-free survival by age group Figure legend: (A) Kaplan-Meier curve of 3-year overall survival by age group (B) Kaplan-Meier curve of 3-year recurrence-free survival by age group.
One year recurrence-free survival for the overall cohort was 69.3% (95% CI 62.8%–76.4%), which was similar between age groups (69.6% (95% CI 61.9%–78.2%) vs. 68.0% (95% CI 56.4%–82.0%) for <70 and ≥ 70 years old respectively). Kaplan-Meier recurrence-free survival curves (Fig. 1B) showed no difference between age groups (log-rank p-value = 0.48). Median recurrence-free survival was not reached at 3 years for either age group.
In both our univariable and multivariable logistic regression, age ≥70 years (reference <70) was not associated with increased odds of neoadjuvant toxicity, overall complication, performance of surgery later than 8-weeks after CROSS completion, nor lower odds of neoadjuvant completion (all p > 0.05) (Supplemental Tables 2–5). All patients on immunosuppression except 1 experienced a complication. This led to overestimated standard deviation of the corresponding coefficient in our regression model for complications and was therefore excluded.
Similarly, our univariable and multivariable cox models showed age was not associated with reduced overall survival (Supplemental Table 6) and recurrence free survival (Table 5). For overall survival, only increasing pathologic T stage (either pT1/T2 or T3) (baseline pT0) and presence of pathologic nodal disease (pN1-N3) (baseline pN0) were significantly associated with increased hazard ratio of death (all p < 0.05). Similarly, advanced pathologic T stage (pT3) (baseline pT0) and presence of pathologic nodal disease (pN1-N3) (baseline pN0) were associated with increased hazard ratio for recurrence. Additionally, presence of lymphovascular invasion was found to be associated with increased risk of recurrence. Our results showed only performance of surgery within 8-weeks of neoadjuvant completion was associated with lower risk of recurrence (HR 0.44 95% CI 0.23–0.85).
Table 5.
Univariable and Multivariable Cox models for recurrence-free survival.
| Univariable | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| variable | Hazard Ratio [95% CI] | p-value | Hazard Ratio [95% CI] | p-value |
| Age ≥ 70 years (ref. <70) | 0.832 [0.496, 1.396] | 0.4869 | 1.594 [0.812, 3.130] | 0.163 |
| Neoadjuvant, completion | Reference | Reference | – | |
| Neoadjuvant, dose reduced | 0.811 [0.325, 2.020] | 0.6525 | 0.697 [0.204, 2.379] | 0.5646 |
| Neoadjuvant, discontinued | 1.196 [0.546, 2.621] | 0.6542 | 2.784 [0.939, 8.258] | 0.0649 |
| Radiation dose, less than or equal 4500 g y | Reference | Reference | – | |
| Radiation dose >4500–5000 g y | 1.781 [0.378, 8.393] | 0.4653 | 0.895 [0.163, 4.918] | 0.8983 |
| Radiation dose >5000 g y | 1.296 [0.603, 2.783] | 0.5066 | 0.992 [0.436, 2.258] | 0.9843 |
| Surgery within 8 weeks of neoadjuvant (ref >8 weeks) | 0.753 [0.466, 1.218] | 0.2479 | 0.443 [0.230, 0.854] | 0.015 |
| Squamous cell Carcinoma (ref. Adenocarcinoma) | 0.534 [0.231, 1.235] | 0.1427 | 1.129 [0.382, 3.332] | 0.8263 |
| Pathological Stage T1/T2 (ref. pT0) | 2.649 [1.241, 5.658] | 0.0118 | 2.485 [0.772, 8.000] | 0.1269 |
| Pathological stage T3/T4 (ref. pT0) | 5.180 [2.486, 10.796] | <0.0001 | 4.947 [1.481, 16.531] | 0.0094 |
| Pathologic nodal disease N1–N3 (ref. pN0) | 3.075 [1.924, 4.915] | <0.0001 | 2.308 [1.130, 4.716] | 0.0217 |
| Lymphovascular invasion (ref. no) | 2.673 [1.574, 4.539] | 0.0003 | 2.229 [1.057, 4.699] | 0.0352 |
CI = confidence interval; Gy = Grey; N=Node descriptor; P = pathologic; T = tumor descriptor.
Subanalysis of patients older than or equal to 80-years
Eight patients (12%) were ≥80 years-old (median 81.7 years). Compared to the patients <70 years, these patients had a higher median Charlson comorbidity index (8.5 versus 4; p < 0.001) and median ECOG score (1 versus 0, p = 0.05).
Of the 8 patients, 5 completed therapy, 2 required dose reduction and 1 discontinued neoadjuvant therapy. None of these rates were statistically different compared to those aged less than 70. In all cases, the reason for dose reduction or discontinuation was due to grade 3 hematologic toxicity (2 cases of thrombocytopenia, 1 case of neutropenia).
Those aged 80 or older were less likely to undergo surgery within 8 weeks compared to those less than 70 (38% vs. 67%, p < 0.001) but the rates of surgery within 30–90 days were similar (75% vs. 89%; p = 0.245). No difference in overall, hematologic or non-hematologic toxicity was observed. Furthermore, as compared to less than 70 years old, those aged 80 or greater underwent fewer VATS (50% vs. 78%), and more open (25% vs. 7%) or robotic procedures (25% vs. 16%), but this did not achieve statistical significance.
Five of 8 (63%) patients aged 80 or older experienced a postoperative adverse event, but there was no significant difference in rate of overall, grade II, III, or V complications as compared to age < 70 years. Grade IV complications were statistically more common (p = 0.049). Only 1 perioperative mortality occurred within 30-days in the eldest group (p = 0.056).
Logistic regression and survival analysis were not performed due to very low number of patients in this sub-group.
Discussion
In this study we examined the outcomes of trimodality CROSS regimen in older adults with locally advanced esophageal cancer which in our practice is offered to all eligible patients with adenocarcinoma or squamous cell carcinoma. We focused not only on surgical outcomes but also on neoadjuvant toxicity and therapy completion rates, which have not been extensively reported in older adults. Our results show that completion of treatment, neoadjuvant toxicity, time to surgery, rate R0 resection, and most postoperative complications were not different among age groups despite higher comorbidities and slightly worse performance status in the older age group. There was only a significant difference in terms of delirium and urinary retention compared to their younger counterparts. These findings are aligned with other studies, showing that urinary retention and delirium are both substantial and prevalent in older adults who undergo esophagectomies [16,17]. Furthermore, hospital LOS and discharge disposition were similar among age groups, with most patients going home with services postoperatively.
When we separately examined patients aged 80 years or older, we did not identify an age-cutoff where a clinically relevant difference in morbidity was observed, except for grade IV complications. Importantly, 75% of patients aged 80 or older were able to complete CROSS regimen and most had surgery in the defined time interval of 30–90 days.
We additionally found that overall and recurrence-free survival were similar between the two age groups and aligned with outcomes from the original CROSS trial [3] which showed improved survival in those undergoing trimodality regimen compared to surgery alone regardless of age. These findings are consistent with other studies. Verma et al. [18] retrospectively compared outcomes of trimodality therapy to surgery alone and definitive chemoradiotherapy in patients older than 75 years. They showed that overall survival was significantly improved with trimodality therapy in this age group. However, in their study patients who could not tolerate combined CRT or received reduced doses were excluded. Furthermore, most of their study population (>70%) had no reported comorbidities, which raises concern about the generalizability to “real life” populations. In contrast, we analyzed outcomes in patients who required neoadjuvant dose reduction or discontinuation with diverse comorbidities. Comorbidities are an important aspect of risk stratification in older adults prior to surgery and even more specifically in esophageal cancer, as they were shown to predict the treatment completion rate prior to surgery [19]. While advanced age (>80 years) and increased comorbidity score are associated with reduced likelihood of receiving trimodality treatment, in high volume centers this approach is more frequently utilized with improved survival rates, including in older adults [20].
Our study showed that neoadjuvant toxicity rates were similar among age groups, except for GI toxicity rates that were higher in the younger age group. One possible explanation may be that older adults who experienced high rates of GI toxicity may subsequently not undergone surgery, thus were not identifiable in our surgical database. Other studies showed that CRT in older adults may cause considerable toxicity and worse outcomes [21]. Some have even advocated to consider avoiding resection in high-risk older patients with squamous cell carcinoma and complete clinical response since surgery in these patients had less survival benefit [22,23]. Overall, only a small percentage of older adults with locally advanced esophageal cancer receive trimodality treatment, with socioeconomic status and age being most predictive of withholding surgery [24]. However, the results of our study did not identify age alone as associated with increased toxicity or overall complications nor worse survival. Furthermore, we did not identify tumor histology to be associated with differences in overall or recurrence-free survival.
Finally, performance of surgery within 8-weeks of neoadjuvant completion consistent with CROSS regimen [3], led to improvement in recurrence-free survival which aligned with previous studies as well [25]. We did not find timing of surgery to be associated with increased overall complication rate.
The strength of our study is the large number of older adults who underwent what is now considered standard of care in locally advanced esophageal cancer with the trimodality CROSS regimen. Furthermore, given the higher ECOG and Charlson scores in our study, we believe our cohort is typical of the older adult population with esophageal cancer. Apart from this, our study had similar patient characteristics (majority male) and tumor histology (primarily adenocarcinoma) and stage (majority T3) as the original CROSS trial by Van Hagen et al. [3]. Furthermore, we report more granular detail about operative techniques than other studies [3,20], notably the higher rate of open procedures performed in older adults in our series. However, unlike one recent clinical trial [26], the operative method was not associated with worse overall complication rates in a univariate analysis.
Limitations
The present study reflects a retrospective analysis of a prospective database in a single large volume center in the United States. Second, our study cohort included only those patients who have completed surgery in our institution, therefore, our results may be confounded by potential selection bias. On the other hand, in our population there were high numbers of comorbidities and elevated ECOG scores as compared to CROSS trial [3]. Yet, due to the retrospective nature of this study we cannot report on frailty measures as these have not been routinely assessed and uniformly reported, nor do we report patient-reported outcomes which are relevant to older adults. We have not prospectively catalogued mortality cause, so we can only report all-cause mortality rate and overall recurrence-free survival. Finally, our limited sub-group analysis of patients who are 80 years or older was hampered by the small size of this group. Future analysis with larger sample size is warranted to determine whether a higher age cutoff may exist where CROSS regimen should not be the preferred treatment.
Conclusion
Trimodality CROSS regimen for esophageal cancer in selected older adults in a high-volume surgical center is safe, with similar completion rates and postoperative outcomes as compared to younger counterparts. Chronologic age should not be the primary consideration for limiting treatments in older adults with locally advanced esophageal cancer. Further studies should focus on patient reported outcomes and pre-treatment geriatric assessment, to identify high-risk older adults who may benefit from additional intervention and modified treatment plans.
Supplementary Material
Acknowledgment
A. Dezube is supported in part through the generous donations of Bruce Bartlett and family and the Jack Mitchell Thoracic Oncology Fellowship.
Disclosure
C. Dumontier is supported by the Harvard Translational Research in Aging Training Program (NIH/NIA T32AG023480). P Enzinger is a consultant for: Astellas, Astra-Zeneca, Celgene, Daiichi-Sankyo, Five-Prime, Lilly, Loxo, Merck, Taiho, Takeda, Zymeworks. The remainder of the authors have no conflicts of interest or financial disclosures.
Abbreviations
- AUC
Area Under Curve
- AJCC
American Joint Committee on Cancer
- CTCAE
Common Terminology Criteria for Adverse Events
- ECOG
Eastern Cooperative Oncology
- Gy
Grey
- IRB
Institutional review board
- KM
Kaplan-Meier
- LOS
Length of Stay
- nCRT
Neoadjuvant chemoradiation
- pN
pathologic nodal descriptor
- pT
pathologic tumor descriptor
- TNM
Tumor, node, metastasis
- VATS
Video Assisted Thoracoscopic Surgery
- XRT
Radiation Therapy
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejso.2021.04.013.
References
- [1].Cancer facts and figures 2020. https://www.cancer.org/content/dam/cancerorg/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. [Accessed 11 November 2020].
- [2].National Comprehensive Cancer Network. Esophageal and esophagogastric junction (version 4.2020). https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. [Accessed 11 November 2020].
- [3].Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366(22): 2074–84. [DOI] [PubMed] [Google Scholar]
- [4].Guttmann DM, Mitra N, Metz JM, Plastaras J, Feng W, Swisher-McClure S. Neoadjuvant chemoradiation is associated with improved overall survival in older patients with esophageal cancer. J Geriatr Oncol 2018;9(1):40–6. [DOI] [PubMed] [Google Scholar]
- [5].Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur J Canc 2004;40(7):926–38. [DOI] [PubMed] [Google Scholar]
- [6].Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17: 1721–4. [DOI] [PubMed] [Google Scholar]
- [7].Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6(2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Swanson SJ, Sugarbaker DJ. The three-hole esophagectomy. The Brigham and Women's Hospital approach (modified McKeown technique). Chest Surg Clin 2000;10(3):531–52. [PubMed] [Google Scholar]
- [9].Wee JO, Bueno R, Swanson SJ. Minimally invasive esophagectomy: the Brigham and Women's Hospital experience. Ann Cardiothorac Surg 2017;6: 175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spector R, Zheng Y. Yeap BY, et al. The 3-Hole Minimally Invasive Esophagectomy: a Safe Procedure Following Neoadjuvant Chemotherapy and Radiation. Semin Thorac Cardiovasc Surg 2015;27:205–15. [DOI] [PubMed] [Google Scholar]
- [11].Patel S, Bakhos C, Abbas A, Bakhos C. Robotic-assisted McKeown esophagectomy. J Vis Surg 2019;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wee JO, Bravo-Iñiguez CE, Jaklitsch MT. Early experience of robot-assisted esophagectomy with circular end-to-end stapled anastomosis. Ann Thorac Surg 2016July;102(1):253–9. [DOI] [PubMed] [Google Scholar]
- [13].Common Terminology Criteria for Adverse. Events (CTCAE). July6th, 2020, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- [14].Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].R: the R project for statistical computing. https://www.r-project.org/.
- [16].Kanda M, Koike M, Tanaka C, et al. Feasibility of subtotal esophagectomy with systematic lymphadenectomy in selected elderly patients with esophageal cancer; a propensity score matching analysis. BMC Surg 2019;19(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hii MW, Smithers BM, Gotley DC, et al. Impact of postoperative morbidity on long-term survival after oesophagectomy. Br J Surg 2013;100(1):95–104. [DOI] [PubMed] [Google Scholar]
- [18].Verma V, Haque W, Zheng D, Osayande F, Lin C. Patterns of care and outcomes of elderly esophageal cancer patients not meeting age-based Criteria of the CROSS trial. Am J Clin Oncol 2019;42(1):67–74. [DOI] [PubMed] [Google Scholar]
- [19].Bernardi D, Asti E, Aiolfi A, Bonitta G, Luporini A, Bonavina L. Outcome of trimodal therapy in elderly patients with esophageal cancer: prognostic value of the Charlson comorbidity index. Anticancer Res 2018;38(3):1815–20. [DOI] [PubMed] [Google Scholar]
- [20].Vlacich G, Samson PP, Perkins SM, et al. Treatment utilization and outcomes in elderly patients with locally advanced esophageal carcinoma: a review of the National Cancer Database. Cancer Med 2017;6(12):2886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walter F, Böckle D, Schmidt-Hegemann NS, et al. Clinical outcome of elderly patients (≥ 70 years) with esophageal cancer undergoing definitive or neoadjuvant radio(chemo)therapy: a retrospective single center analysis. Radiat Oncol 2018;13(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Furlong H, Bass G, Breathnach O, O'Neill B, Leen E, Walsh TN. Targeting therapy for esophageal cancer in patients aged 70 and over. J Geriatr Oncol 2013;4(2):107–13. [DOI] [PubMed] [Google Scholar]
- [23].Schlottmann F, Strassle PD, Nayyar A, Herbella FAM, Cairns BA, Patti MG. Postoperative outcomes of esophagectomy for cancer in elderly patients. J Surg Res 2018;229:9–14. [DOI] [PubMed] [Google Scholar]
- [24].Molena D, Stem M, Blackford AL, Lidor AO. Esophageal cancer treatment is underutilized among elderly patients in the USA. J Gastrointest Surg 2017;21(1):126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim Jae Y, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93(1):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. J Clin Oncol 2018;36(4):6–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


