Abstract
Objectives:
Tumor HPV status is an established independent prognostic marker for oropharynx cancer (OPC). Recent studies have reported that tumor estrogen receptor alpha (ERα) positivity is also associated with prognosis independent of HPV. Little is known about the biologic and behavioral predictors of ERα positivity in head and neck squamous cell cancer (HNSCC). We therefore explored this in a multicenter prospective cohort study.
Materials and Methods:
Participants with HNSCC completed a survey and provided a blood sample. Tumor samples were tested for ERα using immunohistochemistry. ERα positivity was defined as ≥1%, standardized by the American Society of Clinical Oncology/College of American Pathologists in breast cancer. Characteristics were compared with χ2 and Fisher’s exact test. Odds ratios (OR) were calculated using logistic regression.
Results:
Of 318 patients with HNSCC, one third had ERα positive tumors (36.2%, n=115). Odds of ERα expression were significantly increased in those with HPV-positive tumors (OR=27.5, 95% confidence interval[CI] 12.1–62), smaller tumors (≤T2, OR=3.6, 95% CI 1.9–7.1), male sex (OR=2.0, 95% CI 1.1–3.6), overweight/obesity (BMI ≥25, OR=1.9, 95% CI 1.1–3.3), and those married/living with a partner (OR=1.7, 95% CI 1.0–3.0). In a multivariate model, HPV-positivity (aOR=27.5, 95% CI 11.4–66) and small tumor size (≤T2, aOR=2.2, 95% CI 1.0–4.8) remained independently associated with ERα status. When restricted to OPC (n=180), tumor HPV status (aOR=17.1, 95% CI 2.1–137) and small tumor size (≤T2, aOR=4.0 95% CI 1.4–11.3) remained independently associated with ERα expression.
Conclusion:
Tumor HPV status and small tumor size are independently associated with ERα expression in HNSCC.
Keywords: Estrogen receptor alpha, head and neck neoplasms, oropharyngeal neoplasms, tumor biomarkers, papillomaviridae
Introduction
Tumor HPV status is presently the only prognostic biomarker in oropharynx cancers included in the National Comprehensive Cancer Network (NCCN) guidelines which has been shown to be independent of other established risk factors including tobacco and age.1,2 Given the improved survival of those with HPV-positive tumors and numerous trials focused on de-escalation of treatment,3,4,5 identification of another prognostic biomarker to further refine risk categories fit for de-escalation is needed.
Recent novel studies have reported that tumor estrogen receptor alpha (ERα) positivity is associated with better overall and recurrence-free survival in patients with OPC, independent of HPV tumor status.6,7 Outside of the prognostic benefit of ERα positivity, the clinical or demographic predictors of ERα positivity in head and neck squamous cell cancer (HNSCC) are not well described.
Therefore, we investigated biologic and behavioral predictors of ERα positivity using a prospective multi-institutional study in HNSCC. As the detection of ERα in the context of HNSCC is novel and not yet standard of practice, we also explored how to define ERα positivity using varying immunohistochemical staining percentage and intensity cutoffs in relation to disease prognosis.
Materials and Methods
Study Population
Between 2013 and 2018, participants were enrolled in a study of head and neck squamous cell carcinomas entitled Papillomavirus Role in Oral cancer Viral Etiology study (PROVE). This study took place at three NCCN-designated Comprehensive Cancer Centers including the Sidney Kimmel Comprehensive Cancer Center at the Johns Hopkins Hospital (JHH, Baltimore, MD), the University of California, San Francisco Hellen Diller Family Comprehensive Cancer Center (UCSF, San Francisco, CA), and the Tisch Cancer Institute at the Mount Sinai Health System (MSHS, New York, NY). Cases with newly diagnosed, incident head and neck cancer with no prior history of malignancy (except skin cancer) were enrolled. The study was approved by the Institutional Review Board at each site and consent was obtained from all study participants.
Data Collection
Participants completed a survey upon enrollment. The survey was available in Mandarin, Spanish, and English, and was taken on a computer through computer assisted self-interview. The confidential survey included detailed questions on past medical history and past social history including substance use and specific sexual history questions such as total number of lifetime partners.8 Medical record abstraction (MRA) was performed at the time of diagnosis for tumor site, and tumor and nodal stage using the American Joint Committee on Cancer (AJCC) 7th Edition.9 Additional abstraction was completed to record primary treatment modality and for survival data. Patients who did not have updated oncologic surveillance documentation by an otolaryngologist, radiation oncologist, or medical oncologist within three months of MRA were contacted by phone to update survival and recurrence status.
To determine tumor HPV status, all tumors were centrally tested for p16 overexpression by immunohistochemistry (clone E6H4; Ventana Medical Systems, Tucson, AZ; prediluted) and HPV E6/E7 mRNA by RNA in situ hybridization (ISH; RNAscope®, Advanced Cell Diagnostics, Hayward, CA) using an HPV16 type-specific probe in all cases followed by a cocktail probe recognizing 18 high-risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) in cases that were p16-positive but HPV16-negative as previously described.10 Immunostaining for ERα (clone SP-1; Ventana; prediluted) was also performed on all cases on Ventana BenchMark XT autostainers (Ventana). Briefly, whole-slide sections of tumor were cut at 4 microns from formalin-fixed, paraffin-embedded tissue blocks. Slides were deparaffinized and steamed for 64 minutes at 95°C in 1X sodium citrate buffer and cooled for 5 minutes. Endogenous peroxidase activity was blocked by hydrogen peroxide treatment, and slides were incubated with the primary antibody at 36°C for 24 minutes. Signals were visualized using the Ultra view polymer detection kit (Ventana) with counterstaining with hematoxylin for 4 minutes.
Interpretation of all stains was performed at Johns Hopkins Hospital by a single head and neck pathologist (L.M.R.). P16 overexpression was defined as nuclear and cytoplasmic staining in >70% of tumor cells, and RNA ISH positivity was defined as multiple punctate signals in the tumor cell cytoplasm and/or nuclei. For ERα staining, percentage of positive tumor cells, intensity of staining (weak, moderate, strong), and pattern of staining was noted (diffuse=staining most cells throughout tumor, patchy=staining subset of cells at same level throughout tumor, and block=staining only discrete areas of tumor).11
Participant serum was collected and tested for antibodies to oncogenic HPV types 16, 18, 31, 33, 35, 45, 52, and 58 and antibodies to HPV E1, E2, E6, and E7 proteins at the German Cancer Research Center (DKFZ, Heidelberg, Germany). Multiplex serology12 was used to detect antibodies based on glutathione S-transferase (GST) capture ELISA in combination with fluorescent bead-based technology.13 Standardized cutoff values for median fluorescence intensity (MFI) were used to determine if each antibody of interest was positive or negative.14
Statistical Analysis
Analysis was restricted to patients diagnosed with biopsy-proven head and neck squamous cell carcinoma and who had tumor sections available for ERα staining. Patients of all treatment modalities and tumor sites were included. HPV-positive tumors were defined as both p16- and ISH-positive. Patient data were compared based on estrogen receptor positivity (≥1%) as recommended by the American Society of Clinical Oncology and College of American Pathologists in breast cancer.15,16 Additional percentage cut-offs for positivity (≥10%, ≥20%, ≥35%) chosen to fit data distribution were also considered in our analysis. Chi-squared and Fisher’s exact test were used to compare demographic data and ERα status. Odds ratios and 95% confidence intervals were calculated with logistic regression. The Kaplan-Meier method was used to analyze survival data, and log-rank tests were performed to compare survival curves.
Logistic regression was utilized for univariate and multivariate analysis. Variables included in univariate analyses were assessed as both continuous and categorical variables, and several alternative cutoffs were explored for key categorical variables to ensure consistency of finding regardless of category selected (results not shown). As there were several patients with a low, non-meaningful amount of tobacco smoking history (e.g. ever smoker who smoked 1–2 cigarettes per week for a few months), never smoking was defined as <1 pack-year of tobacco smoking. For analysis, the following variables were treated as binary: age (≤60 years, >60 years), sex (female, male), BMI (<25, ≥25), tumor HPV status (negative, positive), T stage (≥T3, ≤T2), pack years (<10, ≥10), ever marijuana (no, yes), ever oral sex (no, yes). Multivariate models were created using variables significant in unadjusted analysis and those known to be important in the literature. Variables with no association by p-value magnitude were removed one at a time without strict p-value cutoffs to achieve a final multivariate model. Variables used in the model are listed in the first column of Table 3. Statistical significance was determined using a two-sided p-value of <0.05. The analysis was performed using STATA version 15.1 (College Station, TX).
Table 3:
Examining ERα IHC Staining Percentage and Intensity Categories In Relation to Overall Survival
| HNSCC (n=318) | |||
|---|---|---|---|
| ERα Staining Cutoffs | n | HR (95% CI) | aHR (95%CI)* |
| Percentage Positivity | |||
| ≥1% | 115 | 0.39 (0.18–0.85) | 0.81 (0.26–2.51) |
| ≥10% | 105 | 0.47 (0.22–1.0) | 1.1 (0.37–3.54) |
| ≥20% | 77 | 0.33 (0.12–0.93) | 0.53 (0.11–2.50) |
| ≥35% | 52 | 0.40 (0.12–1.30) | 0.59 (0.07–4.90) |
| Staining Intensity | |||
| 1–3 | 115 | 0.39 (0.18–0.85) | 0.81 (0.26–2.51) |
| 2–3 | 92 | 0.40 (0.17–0.95) | 0.77 (0.24–2.54) |
| 3 | 47 | 0.55 (0.19–1.53) | 1.07 (0.30–3.82) |
Adjusted for age, HPV tumor status and smoking
Abbreviations: HNSCC, head and neck squamous cell cancer; HR, hazard ratio; CI, confidence interval; Ref, reference group.
Bolding indicates statistical significance.
Results
Patient Characteristics
The study population consisted of 318 patients with HNSCC. The majority of participants were male (78.3%, n=249), under 60 years old (53.4%, n=170), and non-Hispanic white (81.8%, n=260). Oropharynx was the most common anatomic site of disease (n=180) followed by oral cavity (n=96). Among cases with OPC, the majority were male (84.4%, n=152), under 60 years old (60.5%, n=109), and Non-Hispanic white (85.5%, n=154). The median follow-up time for the entire cohort was 2.8 years (interquartile range, IQR 1.7–4.0) and 2.8 years (IQR 1.9–4.3) for OPC cases. Time to event (death) had a median of 1.4 years (IQR 0.7–2.2) for the entire cohort and 1.6 years (IQR 0.6–2.6) for OPC. There were 42 total deaths during the follow up period, including 13 in the OPC cohort.
Cohort Characteristics and Association with ERα
Demographic and behavioral characteristics of ERα positive and negative cases were compared in Table 1. Of the 318 patients with HNSCC 36.2% were ERα positive (n=115) and 63.8% were ERα negative (n=203). ERα positive cases were more likely to be male (85.2% vs. 74.4%, p=0.024), and overweight or obese (body mass index, BMI ≥25, 74.0% vs. 59.5%, p=0.015). While ERα positive cases were more likely to be married or living with a partner (p=0.048), ERα positive and negative cases had statistically similar alcohol use, history of smoking, and marijuana use. Those with ERα positive tumors were less likely to have a history of coronary artery disease (2.9% vs. 14.3%, p=0.002) and anemia (1.9% vs. 16.4%, p<0.001), but more likely to have a history of sexually transmitted infection (26.9% vs. 17.6%, p=0.04).
Table 1:
Demographic and Clinical Characteristics Comparing Tumor ERα Positivity
| Characteristic | HNSCC (n=318) | OPC (n=180) | ||||
|---|---|---|---|---|---|---|
| ERα negative (<1%) n=203, 63.8% | ERα positive (>=1%) n=115, 36.2% | p | ERα negative (<1%) n=77, 42.8% | ERα positive (>=1%) n=103, 57.2% |
p | |
| Study site | ||||||
| JHU/GBMC | 139 (68.5) | 77 (67.0) | 0.59 | 57 (74.0) | 69 (67.0) | 0.40 |
| UCSF | 40 (19.7) | 20 (17.4) | 8 (10.4) | 18 (17.5) | ||
| MSSM | 24 (11.8) | 19 (15.7) | 12 (15.6) | 16 (15.5) | ||
| Race | ||||||
| Non-Hispanic white | 164 (80.8) | 96 (83.5) | 0.74 | 67 (87.0) | 87 (84.5) | 0.77 |
| Non-Hispanic black | 21 (10.3) | 13 (11.3) | 6 (7.8) | 10 (9.7) | ||
| Hispanic | 8 (3.9) | 4 (3.5) | 1 (1.3) | 4 (3.9) | ||
| Asian/Pacific Islander | 7 (3.5) | 1 (0.9) | 1 (1.3) | 1 (1.0) | ||
| Other/multiracial | 3 (1.5) | 1 (0.9) | 2 (2.6) | 1 (1.0) | ||
| Age | ||||||
| <= 60 years | 110 (54.2) | 60 (52.2) | 0.73 | 50 (65.0) | 59 (57.3) | 0.30 |
| >60 years | 93 (45.8) | 55 (47.8) | 27 (35.1) | 44 (42.7) | ||
| Sex | ||||||
| Female | 52 (25.6) | 17 (14.8) | 0.024 | 15 (19.5) | 13 (12.6) | 0.21 |
| Male | 151 (74.4) | 98 (85.2) | 62 (80.5) | 90 (87.4) | ||
| Sexual orientation | ||||||
| Heterosexual | 168 (82.8) | 95 (82.6) | 0.40 | 68 (88.3) | 87 (84.5) | 0.48 |
| Homosexual/Bisexual/Other | 8 (3.9) | 8 (7.0) | 2 (2.6) | 7 (6.8) | ||
| BMI | ||||||
| Normal/Underweight (<25) | 70 (40.5) | 27 (26.0) | 0.01 | 27 (39.1) | 24 (25.5) | 0.07 |
| Overweight/Obese (>=25) | 103 (59.5) | 77 (74.0) | 42 (60.9) | 70 (74.5) | ||
| Marital Status | ||||||
| Married/living with partner | 63 (35.4) | 25 (24.0) | 0.05 | 55 (78.6) | 72 (75.8) | 0.68 |
| Widowed/Divorced/Other | 115 (64.6) | 79 (76.0) | 15 (21.4) | 23 (24.2) | ||
| Highest Degree | ||||||
| Less than high school | 12 (6.7) | 6 (5.8) | 0.99 | 4 (5.7) | 5 (5.3) | 0.94 |
| HS or GED | 39 (21.9) | 21 (20.2) | 11 (15.7) | 20 (21.1) | ||
| Some college | 32 (18.0) | 18 (17.3) | 12 (17.1) | 15 (15.8) | ||
| College grad | 55 (30.9) | 35 (33.7) | 26 (37.1) | 32 (33.7) | ||
| Graduate degree | 40 (22.5) | 24 (23.1) | 17 (24.3) | 23 (24.2) | ||
| >5 years in named “risky job” | ||||||
| No | 167 (82.3) | 97 (84.4) | 0.64 | 64 (83.1) | 85 (82.5) | 0.92 |
| Yes | 36 (17.7) | 18 (15.7) | 13 (16.9) | 18 (17.5) | ||
| Drinking status | ||||||
| Never | 2 (1.4) | 5 (5.9) | 0.10 | 0 (0) | 4 (5) | 0.22 |
| Current | 63 (44.7) | 42 (50.6) | 34 (56.7) | 40 (50.6) | ||
| Former | 76 (53.9) | 37 (43.5) | 26 (43.4) | 35 (44.3) | ||
| Ever smoke | ||||||
| No | 59 (33.7) | 30 (28.9) | 0.40 | 24 (34.3) | 29 (30.9) | 0.64 |
| Yes | 116 (66.3) | 74 (71.2) | 46 (65.7) | 65 (69.2) | ||
| Ever use drugs | ||||||
| No | 66 (37.5) | 27 (26.0) | 0.05 | 18 (25.7) | 23 (24.5) | 0.85 |
| Yes | 110 (62.5) | 77 (74.0) | 52 (74.3) | 71 (75.5) | ||
| Ever used marijuana | ||||||
| No | 67 (38.1) | 29 (27.6) | 0.074 | 19 (27.1) | 25 (26.3) | 0.90 |
| Yes | 109 (61.9) | 76 (72.4) | 51 (72.9) | 70 (73.7) | ||
| Comorbidities | ||||||
| Asthma | ||||||
| No | 157 (89.2) | 91 (85.6) | 0.73 | 66 (95.7) | 82 (87.2) | 0.16 |
| Yes | 16 (9.1) | 12 (11.5) | 3 (4.4) | 11 (11.7) | ||
| Diabetes | ||||||
| No | 149 (84.7) | 94 (90.4) | 0.17 | 67 (97.1) | 86 (91.5) | 0.19 |
| Yes | 27 (15.3) | 10 (9.6) | 2 (2.9) | 8 (8.5) | ||
| Coronary heart disease | ||||||
| No | 150 (85.2) | 100 (96.2) | 0.002 | 62 (89.9) | 90 (95.7) | 0.098 |
| Yes | 25 (14.2) | 3 (2.9) | 7 (10.1) | 3 (3.2) | ||
| Stroke | ||||||
| No | 167 (94.4) | 101 (97.1) | 0.21 | 68 (97.1) | 92 (97.9) | 0.76 |
| Yes | 8 (4.5) | 1 (1.0) | 2 (2.9) | 1 (1.1) | ||
| Anemia | ||||||
| No | 146 (82.5) | 102 (98.1) | <0.001 | 61 (87.1) | 92 (97.9) | 0.01 |
| Yes | 29 (16.4) | 2 (1.9) | 9 (12.9) | 2 (2.1) | ||
| HIV | ||||||
| No | 171 (97.1) | 102 (99.0) | 0.42 | 67 (97.1) | 92 (98.9) | 0.58 |
| Yes | 5 (2.8) | 1 (1.0) | 2 (2.9) | 1 (1.1) | ||
| STI | ||||||
| No | 145 (82.4) | 75 (72.1) | 0.04 | 56 (81.2) | 67 (71.3) | 0.28 |
| Yes | 31 (17.6) | 28 (26.9) | 13 (18.8) | 26 (27.7) | ||
| Autoimmune disease | ||||||
| no | 161 (91.1) | 96 (92.3) | 0.94 | 67 (95.7) | 88 (93.6) | 0.87 |
| yes | 12 (6.8) | 6 (5.8) | 2 (2.9) | 4 (4.3) | ||
| Genital warts | ||||||
| No | 158 (90.0) | 90 (87.4) | 0.46 | 57 (81.4) | 80 (86.0) | 0.43 |
| Yes | 18 (10.2) | 12 (11.7) | 13 (18.6) | 12 (12.9) | ||
| History of any cancer | ||||||
| No | 138 (78.0) | 78 (74.3) | 0.48 | 56 (80) | 71 (74.7) | 0.43 |
| Yes | 39 (22.0) | 27 (25.7) | 14 (20) | 24 (25.3) | ||
| Oncologic Characteristics | ||||||
| Tumor site | ||||||
| Oropharynx | 77 (37.9) | 103 (89.6) | <0.001 | |||
| Oral Cavity | 92 (45.3) | 4 (3.5) | ||||
| Nasopharynx | 1 (0.5) | 1 (0.9) | ||||
| Larynx | 27 (13.3) | 1 (0.9) | ||||
| Other site | 6 (3.0) | 6 (5.2) | ||||
| Tumor site | ||||||
| Non-Oropharynx | 126 (62.1) | 12 (10.4) | <0.001 | |||
| Oropharynx | 77 (37.9) | 103 (89.6) | ||||
| HPV-related | ||||||
| Negative | 129 (64.5) | 7 (6.2) | <0.001 | 14 (18.7) | 1 (1.0) | <0.001 |
| Positive | 71 (35.5) | 106 (93.8) | 61 (81.3) | 100 (99.0) | ||
| p16 status | ||||||
| Negative | 123 (61.5) | 6 (5.3) | 15 (20) | 1 (1.0) | <0.001 | |
| Positive | 77 (38.5) | 107 (94.7) | <0.001 | 60 (80.0) | 100 (99.0) | |
| Treatment Modality | ||||||
| Surgery Only | 64 (31.5) | 19 (16.5) | 0.003 | 15 (19.5) | 17 (16.5) | 0.61 |
| Radiation Only | 7 (3.4) | 4 (3.4) | >.99 | 3 (3.9) | 4 (3.9) | 1 |
| Chemotherapy &radiation | 33 (16.3) | 23 (20) | 0.4 | 26 (33.8) | 23 (22.3) | 0.09 |
| Surgery & Chemoradiation | 39 (19.2) | 27 (23.4) | 0.37 | 14 (18.2) | 22 (21.4) | 0.71 |
| Serology Data (% positive) | ||||||
| HPV16 L1 | 62 (50) | 62 (50) | <0.001 | 47 (64.4) | 58 (62.4) | 0.79 |
| HPV16 E6 | 56 (28.9) | 88 (84.6) | <0.001 | 55 (75.3) | 82 (88.2) | 0.031 |
| HPV16 E7 | 46 (23.7) | 70 (67.3) | <0.001 | 41 (56.2) | 66 (71.0) | 0.048 |
| HPV16 E6 or E7 positive | 61 (31.4) | 90 (86.5) | <0.001 | 56 (76.7) | 84 (90.3) | 0.017 |
Abbreviations: HNSCC, Head & Neck Squamous Cell Carcinoma; OPC: Oropharynx cancer; JHU, Johns Hopkins University; UCSF, University of California; MSHS, Mount Sinai Health System; GED, General Educational Development; HIV human immunodeficiency virus; ISH, in-situ hybridization
Bolding indicates statistical significance.
There was a significant difference in anatomic site distribution by ERα status (p<0.001). The oropharynx was the predominant site (89.6%) for ERα positive patients, while oral cavity was the most common primary site among ERα negative cases (45.3%). ERα positivity was associated with HPV-positive tumor status (93.8% vs. 35.5%, p<0.001) and antibodies to HPV16 L1, E6, and E7 (p<0.001). When considering oropharynx only, HPV tumor status (p<0.001) and antibodies to HPV16 E6 and E7 oncoproteins were associated with ERα positivity (p=0.031, 0.048 respectively).
Univariate regression analysis was performed to identify factors associated with ERα status (Table 2). HPV-positivity (OR 27.5, 95% CI 12.1–62) and smaller tumor size (≤T2, OR 3.6, 95% CI 1.9–7.1) were associated with ERα positivity. Additionally, male sex (OR 2.0, 95% CI 1.1–3.6), obesity/overweight (BMI ≥25, OR 1.9, 95% CI 1.1–3.3), being married or living with a partner (OR 1.7, 95% CI 1.0–3.0), and ≥20 lifetime any sex partners (OR 2.3, 95% CI 1.4–3.7), Having ≥20 lifetime oral sex partners (OR 2.0, 95% CI 1.2–3.2), ever oral sex (OR 8.4, OR 2.0–36), and ≥ 1 cup of daily coffee consumption (OR 1.8, 95% CI 1.1–3.0) were each significantly associated with ERα expression, but associations were not significant when restricted to the OPC subgroup. There were no independent differences in oral hygiene factors, including gum disease (p=0.31) or use of mouthwash (p=0.91) by ERα status.
Table 2 :
Univariate and Multivariate Regression Analysis Associated with ERα Positivity
| HNSCC (n=318) | OPC (n=180) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | |
| Tumor HPV Status | ||||||||
| Negative | Ref | Ref | Ref | Ref | ||||
| Positive | 27.5 (12.1–62) | <0.001 | 27.5 (11.4–66) | <0.001 | 23.0 (2.9–179) | 0.003 | 17.1 (2.1–137) | 0.007 |
| T Stage | ||||||||
| ≥T3 | Ref | Ref | Ref | Ref | ||||
| ≤T2 | 3.6 (1.9–7.1) | <0.001 | 2.2 (1.0–4.8) | 0.05 | 5.7 (2.1–14.9) | <0.001 | 4.0 (1.4–11.3) | 0.008 |
| Sex | ||||||||
| Female | Ref | Ref | ||||||
| Male | 2.0 (1.1–3.6) | 0.03 | 1.7 (0.74–3.8) | 0.21 | ||||
| Age | ||||||||
| ≤60 | Ref | Ref | ||||||
| >60 | 1.1 (0.7–1.7) | 0.73 | 1.4 (0.8–2.5) | 0.30 | ||||
| BMI | ||||||||
| <25 | Ref | Ref | ||||||
| ≥25 | 1.9 (1.1–3.3) | 0.015 | 1.9 (1.0–3.7) | 0.066 | ||||
| Pack-Years | ||||||||
| <10 | Ref | Ref | ||||||
| ≥10 | 1.4 (0.9–2.4) | 0.16 | 1.6 (0.8–3.0) | 0.19 | ||||
| Ever marijuana | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.6 (1.0–2.7) | 0.075 | 1.0 (0.5–2.1) | 0.12 | ||||
| Married/living with partner | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.7 (1.0–3.0) | 0.048 | 0.9 (0.4–1.8) | 0.68 | ||||
| # Any Sex Partners | ||||||||
| <20 | Ref | Ref | ||||||
| ≥20 | 2.3 (1.4–3.7) | 0.003 | 1.2 (0.6–2.2) | 0.58 | ||||
| # Oral Sex Partners | ||||||||
| <20 | Ref | 0.005 | Ref | |||||
| ≥20 | 2.0 (1.2–3.2) | 1.77 (0.9–3.3) | 0.064 | |||||
| Ever Oral Sex | ||||||||
| No | Ref | 0.004 | Ref | |||||
| Yes | 8.4 (2.0–36) | 4.1 (0.4–40) | 0.23 | |||||
| Daily Coffee Consumption | ||||||||
| <1 cup | Ref | 0.03 | Ref | |||||
| ≥ 1 cup | 1.8 (1.1–3.0) | 1.8 (0.9–3.4) | 0.08 | |||||
Abbreviations: HNSCC, head and neck squamous cell cancer; OPC, oropharynx cancer; OR, odds ratio; BMI, body mass index
Bolding indicates statistical significance.
After adjusting for tumor HPV status, sex, BMI, marital status and sexual behavior were no longer associated with odds of ERα positivity. Both HPV-positive tumor status (aOR 27.5, 95% CI 11.4–66) and small tumor size (≤T2, aOR 2.2, 95% CI 1.0–4.8) remained independently associated with odds of ERα positivity. Results were the same when restricted to OPC (n=180; Table 2).
ERα IHC Positivity Cutoffs and Staining Intensity
ERα positivity (defined as ≥1%) was associated with improved overall survival (hazard ratio [HR]=0.39 95%=0.18–0.85) (Table 3). While ≥1% is the established definition for IHC ERα positivity in breast cancer literature,15 this has not been standardized in head and neck cancer literature. Therefore, we explored additional cutoffs of ≥10%, ≥20%, and ≥35%. When using ERα cutoffs of ≥10% (HR 0.47, 95% CI 0.22–1.0) and ≥20% (HR 0.33, 95% CI 0.12–0.93) overall survival remained similarly improved. However, after adjusting for age, HPV status, and smoking, level of ERα staining was no longer associated with overall survival.
We also examined differences in ERα IHC staining intensity and its relationship to survival (Table 3). Any staining (≥1 vs. 0) and high level of staining intensity (≥2 vs 0–1) were each associated with improved overall survival, but similarly to ERα staining percentage, the associations were no longer significant after adjusting for age, HPV status, and smoking. Variance inflation factor (VIF) between ERα and HPV status was 1.46 which suggests that collinearity is not a major factor in our calculations.
As shown in Figure 1, in addition to staining percentage and intensity, the pattern of staining was another tumor IHC characteristic that was described. Of the 115 tumor samples that demonstrated ERα positivity, 38.2% (n=44) were noted to have a “block” pattern, 30.4% (n=35) had a “diffuse” pattern, and 33.4% (n=39) were described as a “patchy” pattern.
Figure 1:
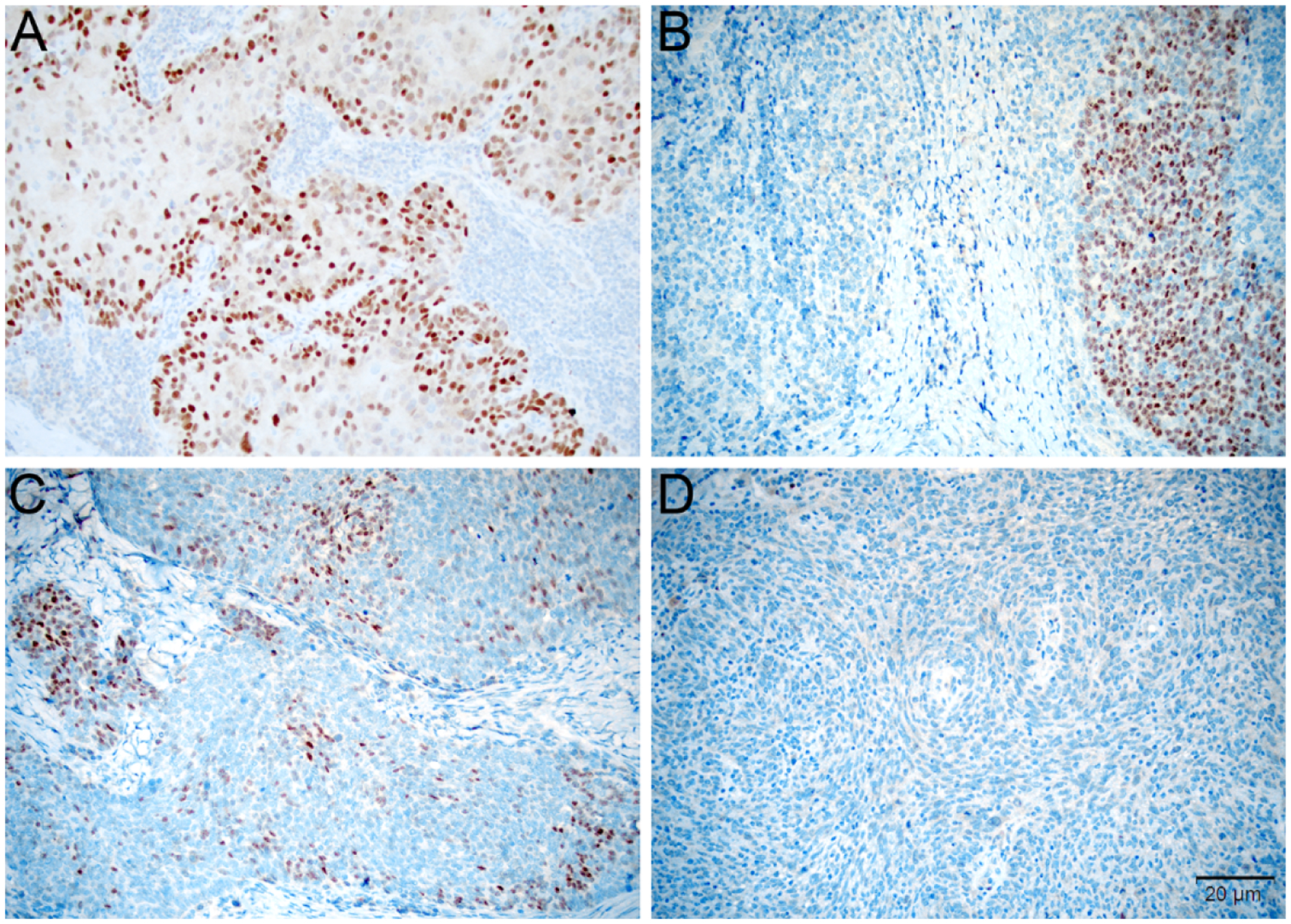
Tumors were scored for ERα based on the percentage of positive tumor cells, intensity of staining, and pattern of staining. Tumors were considered diffusely positive if most cells were uniformly positive throughout the tumor (A, 200x), to have block-like staining if discrete positive areas alternated with negative areas (B, 200x), and patchy if a subset of cells were stained at a similar level throughout the tumor (C, 200x). Based on the thresholds developed in breast carcinoma, staining was considered negative if <1% of cells showed reactivity (D, 200x).
Discussion
While the biological and clinical profile of ERα positivity in HNSCC is not yet well-established, this study provides a comprehensive investigation of clinical and demographic factors associated with tumor ERα positivity. This study builds upon prior studies which have established a role for ERα in HNSCC. HPV is a cause of cervical and oropharyngeal oncogenesis, and the finding of ERα predominance in OPC supports the possibility that the two are co-factors in a potentially hormone-dependent process.6,7,17,18
The link between HPV and estrogen has been investigated in cervical cancer oncogenesis. Estrogen is essential for cervical metaplasia and dysplasia.2 Cervical cancer cells treated with estrogen demonstrate increased HPV-16 and 18 oncogene transcription and expression,20,21,22 suggesting that HPV and estrogen may be synergistic in cervical carcinogenesis. Therefore, it is possible that the interplay of estrogen and HPV is also relevant in the etiology of the predominant subtype of head and neck cancers.
In this study we found that tumor ERα positivity was common in oropharyngeal cancer and that ERα was strongly associated with tumor HPV status, consistent with prior studies.6,7 Predictors of ERα positivity included tumor characteristics such as p16 positivity, smaller tumor, and presence of antibodies to the HPV16 oncoproteins E6 and E7, all of which are descriptors of HPV-OPC.25–30 The interaction between HPV and estrogen in oropharyngeal oncogenesis is still largely undefined, but recent studies have focused on characterizing their role in OPC. Kano et al. recently reported that in HPV-OPC, estrogen induces apolipoprotein B, a protein that promotes HPV integration into the genome,6,31 similar to what has been observed in the pathogenesis of cervical cancer. They found ERα expression to be upregulated in HPV-positive HNSCC. Additionally, treatment of HPV16-positive cell lines with estrogen resulted in growth attenuation and reduction of early RNA transcripts of E6 and E7, an effect which was not seen in HPV-negative cell lines.18 The presence of HPV16 in HNSCC cell lines resulted in estrogen sensitization and ERα upregulation.18 While it is clear that there is an interplay between HPV and estrogen, further investigation into mechanisms of action and potential therapeutic targets for HPV-OPC are necessary.
In both HNSCC and OPC cohorts, small tumor size was independently associated with increased odds of tumor ERα expression. In breast cancer, the association between early tumor stage and ERα has been described.32,33,34 While there are many postulated mechanisms of estrogen in tumorigenesis, there is no evidence that ERα expression drives tumor size.35 Compared to HPV-negative oropharyngeal cancer, it is recognized that HPV-OPC tends to present with smaller tumor stage.36 This finding may further underscore the overlap of clinical characteristics between ERα tumor positivity and HPV-OPC.
Another explanation for this association may be differential timing of ERα expression and that ERα signaling predominates in early stages of tumor growth, although further studies are needed to evaluate this in OPC. It is worth noting that prior studies did not find an independent association between tumor size and ERα expression. Kano et al. found that ERα was more prevalent in higher tumor T stage (T3-T4 vs. T1-T2), although it was not statistically significant (p=0.075).6 Koenigs et al. also did not find this independent association between tumor size and ERα expression. In their HNSCC cohort however, there was an association with smaller tumor size and ERα (T0–2 vs T3–4, p=0.03) and their OPC cohort trended toward significance for association with smaller tumor classification, T0–2 (p=0.09).
Male sex, sexual behavior, and marital status were all found to be associated with ERα expression in univariate analysis, although these associations were no longer statistically significant after adjustment for HPV. This is not surprising, as they are all variables known to be related HPV-associated oropharyngeal cancer, suggesting that this association is likely via the known relationship between male sex6 and marital status37 and HPV status rather than an independent association with ERα expression itself.
In addition to clinical and biologic predictors of tumor estrogen receptor status, intensity of ERα staining and pattern of cell expression was examined. While ERα staining pattern is well-characterized and a mandatory practice in breast cancer pathology,15,38,39 it is not yet standard of practice in HNSCC. In breast cancer ERα staining is described as “diffuse” 92% of the time, and “focal” staining is only seen in 8% of samples, with minimal intensity differences.40 We found that ERα staining pattern in HNSCC tumor samples was widely heterogenous, which may indicate etiologic differences in receptor expression compared to breast cancer and requires further study. While the homogenous staining observed in breast cancer suggests a potentially monoclonal origin, the heterogeneity observed in HNSCC illustrates the complex carcinogenic patterns that may be at hand.40 As pattern of staining in HNSCC differs greatly from breast cancer, we propose that other classifications may need to be considered when defining ERα positivity in HNSCC or that descriptive quantification of ERα staining may allow for subtype categorization. We explored effects of applying other percentage cutoffs for ERα positivity and classification of staining intensity which may be worth addressing in future studies, as the cellular expression and patterns in HNSCC seem to differ from that of breast cancer.
This study has strengths and limitations. While previous studies on ERα in HNSCC reported on a patient population who had only received chemoradiation,7 the treatment regimens of our participants represent the full spectrum of therapeutic options for HNSCC patients. Using detailed survey and demographic data we expand the understanding of novel clinical predictors of ERα status and identify possible associations that merit further study. While we explored many variables, only small tumor size and HPV status remained statistically significant in multivariate analysis. We recognize that with all self-reported behavioral data, recall bias may present.
Several studies have shown that ER portends better prognosis in OPC.6,7 While our data trended in the same direction, ERα was no longer a statistically significant predictor of survival after adjusting for HPV. However, we had a limited number of deaths among OPC (n=13) which may have limited our power to observe this difference. Additionally, patients were treated with heterogeneous modalities (surgery, radiation, and/or chemotherapy) which may have influenced the inability to identify an association between ERα and survival after adjustment for other predictors.
Conclusions
Here we report detailed clinical predictors of estrogen receptor status in HNSCC. Tumor HPV status and small tumor size were independently associated with ERα tumor expression. These results suggest a potential role in HNSCC tumor biology and warrant further investigation of ERα as a clinically significant prognostic biomarker.
Highlights:
Predictors of estrogen receptor alpha status in head and neck squamous cell carcinoma are explored.
Tumor HPV status and small tumor size were independently associated with ERα tumor expression.
ERα may have a potential role in HNSCC tumor biology. Further investigation of ERα as a clinically significant prognostic biomarker is warranted.
Funding
This work was supported by the National Institute of Dental and Craniofacial Research (P50 DE019032).
Abbreviations:
- OPC
oropharynx cancer
- HPV
human papilloma virus
- HPV-OPC
HPV-related oropharyngeal cancer
- HNSCC
head and neck squamous cell carcinoma
- ERα
Estrogen receptor alpha
- OR
odds ratio
- CI
confidence interval
- BMI
body mass index
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Waterboer serves on advisory boards for MSD (Merck Sharp & Dohme)
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. The New England journal of medicine. 2010;363(1):24. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Westra WH, Li S, et al. Improved Survival of Patients With Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 3.Bigelow EO, Seiwert TY, Fakhry C. Deintensification of treatment for human papillomavirus-related oropharyngeal cancer: Current state and future directions. Oral Oncology. 2020;105:104652. doi: 10.1016/j.oraloncology.2020.104652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: Summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D.C. Head & Neck. 2009;31(11):1393–1422. doi: 10.1002/hed.21269 [DOI] [PubMed] [Google Scholar]
- 5.Deschuymer S, Mehanna H, Nuyts S. Toxicity Reduction in the Treatment of HPV Positive Oropharyngeal Cancer: Emerging Combined Modality Approaches. Front Oncol. 2018;8. doi: 10.3389/fonc.2018.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano M, Kondo S, Wakisaka N, et al. Expression of estrogen receptor alpha is associated with pathogenesis and prognosis of human papillomavirus‐positive oropharyngeal cancer. International Journal of Cancer. 2019;145(6):1547–1557. doi: 10.1002/ijc.32500 [DOI] [PubMed] [Google Scholar]
- 7.Koenigs MB, Lefranc-Torres A, Bonilla-Velez J, et al. Association of Estrogen Receptor Alpha Expression With Survival in Oropharyngeal Cancer Following Chemoradiation Therapy. JNCI: Journal of the National Cancer Institute. 2019;111(9):933–942. doi: 10.1093/jnci/djy224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windon MJ, Waterboer T, Hillel AT, et al. Sex differences in HPV immunity among adults without cancer. Hum Vaccin Immunother. 2019;15(7–8):1935–1941. doi: 10.1080/21645515.2019.1568157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge SB, American Joint Committee on Cancer, eds. AJCC Cancer Staging Manual. 7th ed.Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 10.Rooper LM, Windon MJ, Hernandez T, et al. HPV-positive Squamous Cell Carcinoma of the Larynx, Oral Cavity, and Hypopharynx: Clinicopathologic Characterization With Recognition of a Novel Warty Variant. Am J Surg Pathol. 2020;44(5):691–702. doi: 10.1097/PAS.0000000000001433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Alqatari M, Sultan K, et al. Using P16 Immunohistochemistry to Classify Morphologic Cervical Intraepithelial Neoplasia 2: Correlation of Ambiguous Staining Patterns with HPV Subtypes and Clinical Outcome. Hum Pathol. 2017;66:144–151. doi: 10.1016/j.humpath.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. doi: 10.1002/ijc.30697 [DOI] [PubMed] [Google Scholar]
- 13.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381 [DOI] [PubMed] [Google Scholar]
- 14.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version). Archives of Pathology & Laboratory Medicine. 2010;134(7):e48–e72. doi: 10.1043/1543-2165-134.7.e48 [DOI] [PubMed] [Google Scholar]
- 16.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen Receptor Status by Immunohistochemistry Is Superior to the Ligand-Binding Assay for Predicting Response to Adjuvant Endocrine Therapy in Breast Cancer. JCO. 1999;17(5):1474–1474. doi: 10.1200/JCO.1999.17.5.1474 [DOI] [PubMed] [Google Scholar]
- 17.Spurgeon ME, den Boon JA, Horswill M, et al. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc Natl Acad Sci U S A. 2017;114(43):E9076–E9085. doi: 10.1073/pnas.1712018114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristol ML, James CD, Wang X, Fontan CT, Morgan IM. Estrogen Attenuates the Growth of Human Papillomavirus-Positive Epithelial Cells. mSphere. 2020;5(2). doi: 10.1128/mSphere.00049-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung S-H, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for Estrogen Receptor α in a Mouse Model for Human Papillomavirus–Associated Cervical Cancer. Cancer Res. 2008;68(23):9928–9934. doi: 10.1158/0008-5472.CAN-08-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitrani-Rosenbaum S, Tsvieli R, Tur-Kaspa R. Oestrogen Stimulates Differential Transcription of Human Papillomavirus Type 16 in SiHa Cervical Carcinoma Cells. Journal of General Virology,. 1989;70(8):2227–2232. doi: 10.1099/0022-1317-70-8-2227 [DOI] [PubMed] [Google Scholar]
- 21.Kim CJ, Um SJ, Kim TY, et al. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells. Int J Gynecol Cancer. 2000;10(2):157–164. doi: 10.1046/j.1525-1438.2000.00016.x [DOI] [PubMed] [Google Scholar]
- 22.Chung S-H, Lambert PF. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc Natl Acad Sci USA. 2009;106(46):19467–19472. doi: 10.1073/pnas.0911436106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;93(7):2930–2935. doi: 10.1073/pnas.93.7.2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. PNAS. 2005;102(7):2490–2495. doi: 10.1073/pnas.0409883102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Souza G, Kreimer AR, Viscidi R, et al. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. New England Journal of Medicine. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 26.Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565–574. doi: 10.1016/j.oraloncology.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–1849. doi: 10.1002/cncr.20998 [DOI] [PubMed] [Google Scholar]
- 28.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104(3):336–344. doi: 10.1002/ijc.10960 [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025 [DOI] [PubMed] [Google Scholar]
- 31.Kondo S, Wakae K, Wakisaka N, et al. APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene. 2017;36(12):1687–1697. doi: 10.1038/onc.2016.335 [DOI] [PubMed] [Google Scholar]
- 32.Alqaisi A, Chen L, Romond E, et al. Impact of estrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) co-expression on breast cancer disease characteristics: implications for tumor biology and research. Breast Cancer Res Treat. 2014;148(2):437–444. doi: 10.1007/s10549-014-3145-x [DOI] [PubMed] [Google Scholar]
- 33.Bulut N, Altundag K. Does estrogen receptor determination affect prognosis in early stage breast cancers? Int J Clin Exp Med. 2015;8(11):21454–21459. [PMC free article] [PubMed] [Google Scholar]
- 34.Sofi GN, Sofi JN, Nadeem R, et al. Estrogen receptor and progesterone receptor status in breast cancer in relation to age, histological grade, size of lesion and lymph node involvement. Asian Pac J Cancer Prev. 2012;13(10):5047–5052. doi: 10.7314/apjcp.2012.13.10.5047 [DOI] [PubMed] [Google Scholar]
- 35.Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Experimental Hematology & Oncology. 2018;7(1):24. doi: 10.1186/s40164-018-0116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.BOSCOLO-RIZZO P, DEL MISTRO A, BUSSU F, et al. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33(2):77–87. [PMC free article] [PubMed] [Google Scholar]
- 37.Drake VE, Fakhry C, Windon MJ, et al. Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer. Cancer. Published onlineJanuary11, 2021. doi: 10.1002/cncr.33346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa Y, Moriya T, Kato Y, et al. Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: analysis for a cut-off point as the predictor for endocrine therapy. Breast Cancer. 2004;11(3):267–275. doi: 10.1007/bf02984548 [DOI] [PubMed] [Google Scholar]
- 39.Dekker TJA, ter Borg S, Hooijer GKJ, et al. Quality assessment of estrogen receptor and progesterone receptor testing in breast cancer using a tissue microarray-based approach. Breast Cancer Res Treat. 2015;152(2):247–252. doi: 10.1007/s10549-015-3444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of Estrogen and Progesterone Receptors ReconsideredExperience With 5,993 Breast Cancers. Am J Clin Pathol. 2005;123(1):21–27. doi: 10.1309/4WV79N2GHJ3X1841 [DOI] [PubMed] [Google Scholar]


