Abstract
Monoclonal antibodies (mAb) are a major component of cancer therapy. In this review, we summarize the different therapeutic mAbs that have been successfully developed against various tumor-expressed antigens and examine our current understanding of their different mechanisms of anti-tumor action. These mechanisms of action (MOA) largely center on the stimulation of different innate immune effector processes, which appear to be principally responsible for the efficacy of most unconjugated mAb therapies against cancer. This is evident in studies of mAbs targeting antigens for hematologic cancers, with emerging data also demonstrating the critical nature of innate immune-mediated mechanisms in the efficacy of anti-HER2 mAbs against solid HER2+ cancers. While HER2-targeted mAbs were originally described as inhibitors of HER2-mediated signaling, multiple studies have since demonstrated these mAbs function largely through their engagement with Fc receptors to activate innate immune effector functions as well as complement activity. Next generation mAbs are capitalizing on these MOAs through improvements to enhance Fc-activity, although regulation of these mechanisms may vary in different tumor microenvironments. Additionally, novel antibody-drug conjugates (ADC) have emerged as an important means to activate different MOAs. Although many unknowns remain, an improved understanding of these immunologic MOAs will be essential for the future of mAb therapy and cancer immunotherapy.
Keywords: Monoclonal antibody, targeted therapy, cancer treatment, HER2, Trastuzumab, Antibody Dependent Phagocytosis
The idea of using antibodies as anti-cancer therapeutics has a long history dating back over fifty years when serologic techniques allowed assessments of cancer cells and foretold the possible use of antibodies as therapeutics for cancer. Since that time, there has been a revolution in the development of monoclonal antibodies (mAbs), which now comprise a major pharmaceutical therapeutic market. While mAbs have a variety of uses, significant efforts have been directed towards anti-tumor therapeutics, which began in 1997 with the development and approval of Rituximab. This mAb remains a standard-of-care (SOC) therapy for B-cell Non-Hodgkins’ Leukemia (NHL) and is now the basis for the development of two biosimilar mAbs (1). Therapeutic anti-tumor mAbs selectively target cell surface antigens in cancer. These antigens can represent proteins that are overexpressed or selectively expressed in cancer, and proteins that are mutated or post-translationally modified in a manner that is different in cancer cells than non-transformed cells. The functional effect of a mAb is related to a cancer antigen profile and the specific ability of the mAb to be internalized, activate Fcγ-receptors (FCGR) on innate immune cells, trigger the activation of complement, or block receptor-mediated oncogenic signaling. These therapeutic outcomes depend strongly on the isotype and the specific nature of the mAb (i.e., specific binding site, avidity of target binding, and particular conformation), as well as the nature of the target antigen. The long history of iterative clinical development of anti-tumor mAbs has demonstrated the importance of all of these factors, including the significance of appropriate monitoring of the pharmacokinetic and pharmacodynamics properties of mAbs in early clinical trials (2,3). An important consideration is the distribution of mAb target protein expression in non-malignant tissues, which has important implications for off-target toxicities. For instance, the respective expression of EGFR in epidermal tissue and HER2 in cardiac tissue generate a degree of toxicity using mAb against these targets, which is a critical concern in the development of anti-tumor mAbs and ADCs (4–6). These consideration thus stated, the purpose of this review is to summarize the types of mAbs that have been successfully developed against cancer expressed antigens and review our current understanding of their different mechanisms of anti-tumor action, with a particular emphasis on emerging studies of HER2-specific mAbs.
Current FDA approved monoclonal antibodies:
As a means of cancer therapy, mAbs in an idealized setting promise a potentially high degree of specificity and efficacy with a minimal degree of off-target toxicity. In cancers where mAbs have achieved considerable efficacy, such as in HER2+ breast cancer (BC), they have become the frontline standard-of-care, largely outperforming HER2-specifc small molecule inhibitors (such as Lapatinib and Neratinib) and have offered excellent response profiles with modest toxicities (7). However, only a small number of cancers have been successfully treated with mAbs, many of which are hematologic malignancies. Since the first approval of Rituximab in 1997 for NHL, >30 anti-tumor mAbs have received FDA approval for the treatment of cancer (Table 1), with many more being tested in clinical trials. To meet FDA approval, mAbs usually need to demonstrate an improvement of overall survival (OS) or a surrogate marker, such as progression free survival (PFS), in comparison to the current standard-of-care in phase III clinical trials. This high bar ensures that approved mAbs confer a significant clinical benefit for the intended population. Among the FDA approved tumor-targeting mAbs, it is notable that the majority of mAbs receiving approval in solid tumors have targeted either HER2 or EGFR (8 of 12), both members of the ERBB family. Additionally, a large class of these approved mAbs target more restricted immune markers (such as CD20 or CD52) of hematologic cancers, predominantly lymphomas and myelomas. Initially, the approvals of mAbs were for ‘naked’ non-conjugated mAbs against these targets (such as Rituximab in 1997 and Trastuzumab in 1998), which spawned the development of conjugated versions that generally incorporated a cytotoxic agent onto the mAb through a chemical linker. In general, non-conjugated mAbs can invoke several different mechanisms of action (MOA), while conjugated mAbs typically rely on the direct cytotoxic action of their payloads, ingested through endocytosis of receptor bound conjugated mAbs.
Table 1.
List of FDA-approved monoclonal antibodies targeting tumor antigens
| Antigen category | Antibody (INN) | Antibody (Trade name) | Target antigen | IgG Type | Year of FDA approval | Tumor disease | Major Mechanism of Action |
|---|---|---|---|---|---|---|---|
| Hematological cancer | Rituximab | Rituxan | CD20 | Chimeric IgG1 | 1997 | Non-Hodgkin’s lymphoma; chronic lymphocytic leukemia; | ADCP, ADCC, CDC |
| Ofatumumab | Arzerra | CD20 | Human IgG1 | 2009 | Chronic lymphocytic leukemia | ADCP, ADCC, CDC | |
| Ibritumomab tiuxetan | Zevalin | CD20 | Murine IgG1 /radioisotope conjugate | 2002 | Non-Hodgkin’s lymphoma | radionucleotide delivery | |
| Tositumomab-I131 | Bexxar | CD20 | Murine IgG2a /radioisotope conjugate | 2003* | Non-Hodgkin’s lymphoma | radionucleotide delivery | |
| Obinutuzumab | Gazyvaro | CD20 | Humanized IgG1 | 2013 | Chronic lymphocytic leukemia | ADCC, ADCP | |
| Alemtuzumab | Campath | CD52 | Humanized IgG1 | 2001*;2014 | B-cell chronic lymphocytic leukemia | ADCP, ADCC, CDC | |
| Tafasitamab | Monjuvi | CD19 | Humanized IgG1 | 2020 | Diffuse large B-cell lymphoma | ADCP, ADCC, CDC | |
| Loncastuximab tesirine | Zynlonta | CD19 | Humanized IgG1 | 2021 | Diffuse large B-cell lymphoma | cytotoxic drug delivery | |
| Polatuzumab vedotin | Polivy | CD79b | Humanized IgG1 ADC | 2019 | Diffuse large B-cell lymphoma | cytotoxic drug delivery | |
| Daratumumab | Darzalex | CD38 | Human IgG1/κ | 2015 | Multiple Myeloma | CDC, ADCC, ADCP, neutralization | |
| Isatuximab | Sarclisa | CD38 | Chimeric IgG1 | 2020 | Multiple Myeloma | ADCP, ADCC, CDC | |
| Mogamuizumab | Poteligeo | CCR4 | Humanized IgG1 | 2018 | Cutaneous T cell lymphoma | ADCP, ADCC, CDC | |
| Gemtuzumab ozogamicin | Mylotarg | CD33 | Humanized IgG4 / toxin conjugate | 2000*; 2017 | Acute myeloic leukemia (AML) | cytotoxic drug delivery | |
| Inotuzumab ozogamicin | BESPONSA | CD22 | Humanized IgG4 as ADC | 2017 | B-cell precursor acute lymphoblastic leukemia | cytotoxic drug delivery | |
| Moxetumomab pasudotox | Lumoxiti | CD22 | Murine IgG1 dsFv immunotoxin | 2018 | Hairy cell leukemia | cytotoxic drug delivery | |
| Belantamab mafodotin | BLENREP | BCMA | Humanized IgG1 ADC | 2020 | Multiple Myeloma | cytotoxic drug delivery | |
| Brentuximab vedotin | Adcetris | CD30 | Chimeric IgG1 as ADC | 2011 | Hodgkin lymphoma (HL), systemic anaplastic large cell lymphoma (ALCL) | cytotoxic drug delivery | |
| Elotuzumab | Elotuzumab | SLAMF7 | Humanized IgG1 | 2015 | Multiple Myeloma | ADCP, ADCC, CDC | |
| Solid cancer (ErbB family) | Trastuzumab | Herceptin | HER2 | Humanized IgG1 | 1998 | Breast cancer; metastatic gastric or gastroesophageal junction adenocarcinoma | ADCP, CDC** |
| Ado-Trastuzumab emtansine | Kadcyla | HER2 | Humanized IgG1 as ADC | 2013 | Breast cancer | cytotoxic drug delivery | |
| [fam]-trastuzumab deruxtecan | Enhertu | HER2 | Humanized IgG1 ADC | 2019 | Breast cancer | cytotoxic drug delivery | |
| Pertuzumab | Perjeta | HER2 | Humanized IgG1 | 2012 | Breast cancer | signal blockade, ADCP, CDC** | |
| Margetuximab | MARGENZA | HER2 | Chimeric IgG1 | 2020 | Breast cancer | ADCP, ADCC | |
| Cetuximab | Erbitux | EGFR | Chimeric IgG1 | 2004 | Head and neck cancer; colorectal cancer | signal blockade, ADCC, CDC | |
| Panitumumab | Vectibix | EGFR | Human IgG2 | 2006 | Metastatic colorectal carcinoma | signal blockade | |
| Necitumumab | Portrazza | EGFR | Human IgG1 | 2015 | Carcinoma, non-small-cell lung | signal blockade, ADCC | |
| Solid cancer (other targets) | Dinutuximab | Unituxin | GD2 | Chimeric IgG1 | 2015 | Neuroblastoma | ADCC, ADCP, CDC |
| Naxitamab | DANYELZA | GD2 | Humanized IgG1 | 2020 | Neuroblastoma | ADCC, ADCP, CDC | |
| Enfortumab vedotin | Padcev | Nectin-4 | Human IgG1 ADC | 2019 | Urothelial cancer | cytotoxic drug delivery | |
| Sacituzumab govitecan | Trodelvy | TROP-2 | Humanized IgG1 ADC | 2020 | Breast cancer | cytotoxic drug delivery | |
| Arcitumomab | CEA-scan | CEA | Murine Fab fragment | 1996 | colorectal cancer | Detection (non-therapeutic) | |
| Satumomab | OncoScint | TAG-72 | Murine MAb | 1992 | colorectal and ovarian cancers | Detection (non-therapeutic) | |
| Capromab | ProstaScint | PSMA | Murine MAb | 1996 | prostate adenocarcinoma | Detection (non-therapeutic) |
Footnotes:
indicates a mAb that was withdrawn from the market after initial FDA approval
major mechanism of action when Trastuzumab and Pertuzumab are used in combination
References:
Different mechanisms of action for non-conjugated mAbs:
Typically, direct antibody binding (for example to pathogens) causes immediate steric disruption, thereby suppressing pathogen entry into cells. This property is the most frequently sought after in the development of therapeutic antibodies to elicit blockade of specific signaling molecules. Binding of mAbs to specific receptors can also allow for their internalization through different processes, suggestive of a conserved regulatory mechanism. In anti-cancer therapies, this ability to block interactions and internalize receptors has provided a means to inhibit oncogenic cellular signaling.
In addition to this function, the majority of antibodies possess a conserved Fc (named from earlier studies, fragment crystallizable) domain that allows for their direct engagement with Fcγ-receptors (FCGR) on different types of immune cells (Figure 1A). This enables mAbs to directly trigger different immune responses, which are mediated by a difference in binding ratios to activating and inhibitory FCGRs (A:I ratio), which vary by antibody isotype (8). A major function of activating FCGR engagement allows for phagocytic engulfment of antibody-bound pathogens or cells. This process allows for target cell clearance and elimination, termed Antibody-Dependent-Cellular-Phagocytosis (ADCP). FCGR engagement and signaling activity also provokes cellular stimulation of different classes of immune cells (such as DCs, macrophages, or neutrophils), which can further alter adaptive immune responses through antigen presentation, cytokine production, and chemotaxis. Additionally, the Fc portion of bound antibodies can also stimulate other innate immune cells, such as Natural Killer (NK) cells, to directly lyse sufficiently opsonized targets, termed Antibody-Dependent-Cellular-Cytotoxicity (ADCC). The activation of FCGR is complex and is governed by the expression of different activating and inhibitory FCGRs on the surface of a given cell, as well as a collection of other signaling molecules (such as CD47-SIRPα in phagocytosis), which further govern cell signaling upon Fc-FCGR engagement. These dynamic signaling complexes directly modulate cellular activities (such as phagocytosis or degranulation), as well as indirectly affecting immunity through alterations of cellular activation and cytokine/chemokine secretion (9,10).
Figure 1. Immunologic mechanisms and potential of HER2 targeted mAb therapies.
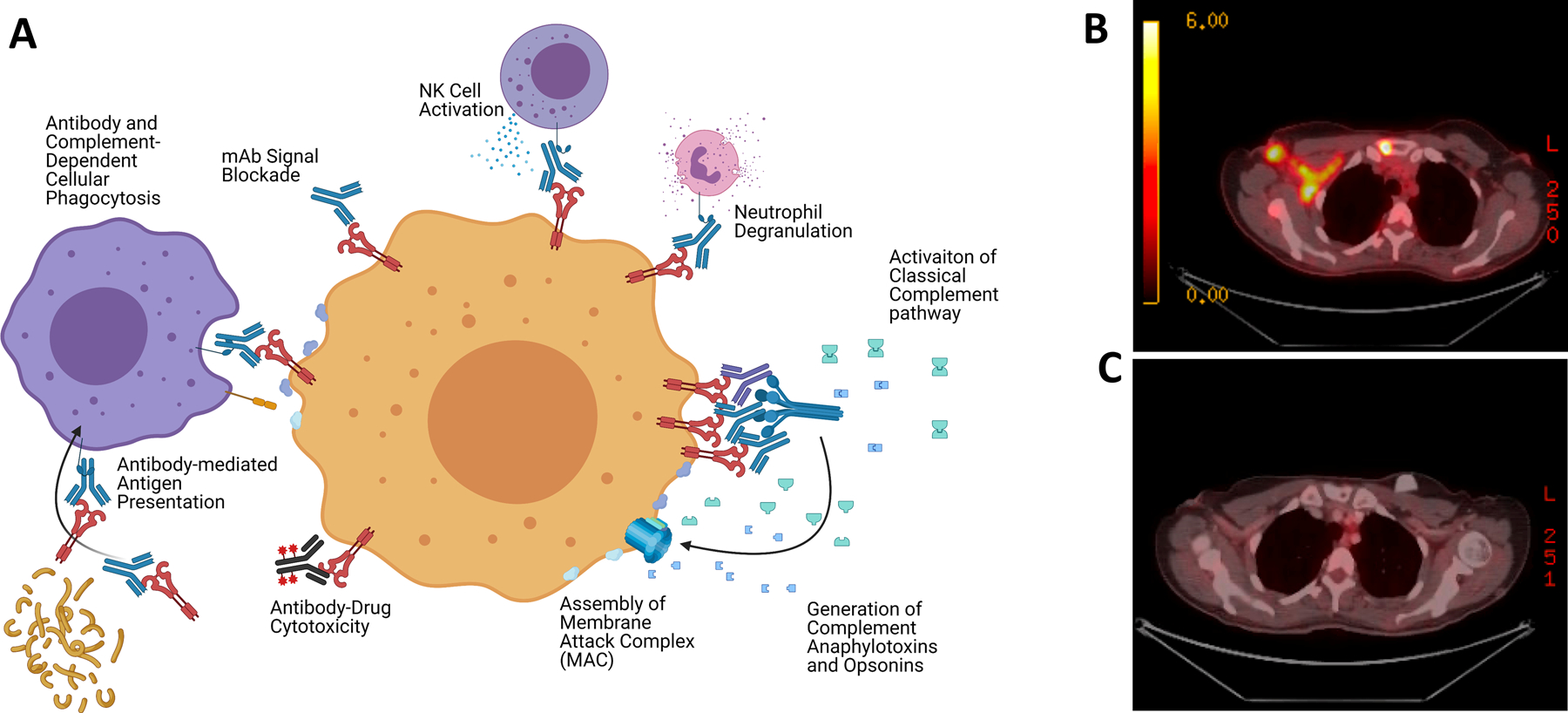
A) Diagram illustrating the potential MOAs involved in anti-tumor mAb directed therapies. B) A PET scan demonstrating FDG avid sites of disease in a patient initially diagnosed with metastatic HER2 positive breast cancer who had a complete response, or C) following induction docetaxel combined with trastuzumab and pertuzumab.
Finally, the Fc portions of mAbs can also trigger the activation of serum proteins, such as the complement family (Figure 1A) (11,12). When the Fc domains bind soluble C1q, they can facilitate the assembly of C1q hexamers. Once assembled, these complexes stimulate a proteolytic cascade that leads to the activation of complement, which produces a series of anaphylotoxins that can opsonize cells as well as stimulate or inhibit the activity of various C3a-receptor and C5a-receptor expressing immune cells. Moreover, this can result in the assembly of the Membrane-Attack-Complex (MAC), which allows for direct cellular target destruction (12). As with FCGR signaling, the activation of complement is highly regulated at the cell surface by a series of complement receptors, allowing for dynamic control of this process. In summary, the engagement of both FCGR and complement, while complex, can result in direct cellular destruction as well as the modulation of local and systemic immune responses, demonstrating the potency of mAbs as a critical arm of adaptive immunity and its potential as an anti-tumor therapeutic strategy (Figure 1B–C).
Given the importance of these functions, many efforts have been made to molecularly engineer the Fc region to optimize effector functions and antibody half-life. These interactions can be modulated through the introduction of point mutations, inserting/deleting amino acids, modifying glycan composition, or exchanging Fc domains. More detailed reviews on mAb Fc modifications has been discussed elsewhere (13,14). One important example of Fc-modified mAb that recently passed a large clinical trial is Margetuximab (15), which contains several optimization mutations and exhibits improved FCGR3A engagement and ADCC activity compared to the parental antibody Trastuzumab (16). Alternatively, strategies to increase hexamerization of mAbs to improve complement activation are being developed for clinical use (17)
Mechanisms of action for conjugated mAbs:
One of the fastest growing uses of mAb in cancer is through their conjugation to different cytotoxic payloads, termed antibody-drug conjugates (ADCs) (18,19). In this approach, a cytotoxic drug is conjugated to the heavy or light chain domain of a mAb through different types of chemical linkers. The ability of mAbs to internalize allows for a more specific delivery of the cytotoxic agent to tumor cells while simultaneously reducing systemic toxicity. This method is also exploited using alternatives to cytotoxic molecules, such as innate immune-stimulatory molecules, such as Toll-like receptor agonists, that allow for the activation of anti-tumor immunity (20). For conjugated approaches, the intended mechanism of actions straightforward: to enhance the delivery of a conjugated drug to the tumor. However, there are many parameters that affect the efficacy of this strategy, including the type of linker, the type of conjugated payload, the ratio of payload to mAb, the distribution of the antigen target, the ability of the mAb to bind and internalize, and the stability and biodistribution profile of the ADC. While complex, there have been great strides in the optimization of specific ADCs with a generally simpler MOA, which has resulted in their comprising a majority of recent FDA mAb approvals (7 of 12 since 2017, see Table 1). As such, ADCs may allow for a more direct means of eliciting therapeutic responses against different cancers.
Unconjugated therapeutic monoclonal antibodies in hematologic cancers:
In 1997, Rituximab became the first FDA approved mAb targeting cancer after demonstrating therapeutic activity in combination with chemotherapy against B-cell lymphomas. This mAb targets CD20, encoded by the MS4A1 gene (21), which was one of the first described markers of B-cells (22). CD20 is a highly restricted cell surface antigen with abundant, stable, and highly characterized expression in B-cells (23). The majority of studies to date support a major role for Fc-mediated myeloid cell activities as the dominant mediators of Rituximab efficacy, while other studies suggest that interruption of cell signaling and complement activity may also contribute substantially to efficacy (12,22,24–27). Clinical studies have demonstrated a synergy with different types of chemotherapy, typically cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), which may be due to sensitization of cell signaling interruption or enhanced immunogenicity (28). The reasons for potential synergy between different chemotherapies and Rituximab are not well studied; thus, it is unclear what MOAs are dominant in the clinical use of Rituximab, which is usually used in conjunction with chemotherapy.
Since the development of Rituximab, five other CD20-targeting mAbs (three murine mAbs and two ‘humanized’ mAbs) received FDA approval for the treatment of CD20+ B-cell cancers (Table 1). These mAbs differed in their binding sites, which altered the impact on CD20 signaling and growth, along with their ability to engage with FCGRs and activate complement (22,24,29,30). Despite these differences, Rituximab has remained a clinical mainstay of treatment, suggesting that slight alterations to these MOAs may not be sufficient to achieve significant differences in clinical outcomes for most patients. However, resistance to CD20-targeting mAbs can occur, which is thought to be potentially mediated by altering CD20 expression, upregulation of complement regulator genes, or elevated expression of suppressors of phagocytosis (27,30,31). In this last instance, the use of a CD47 blocking mAb has demonstrated early clinical indications of success in reversing resistance (32), potentially suggesting this strategy to enhance the phagocytosis MOA as a critical one in mitigating Rituximab-resistance in B-cell leukemias.
After the success of CD20-targeting mAbs, studies on other hematologic markers yielded FDA approval for mAbs targeting other immune cell antigens, including: CD52 (Alemtuzumab), CD38 (Daratumumab and Isatuximab), SLAMF7 (Elotuzumab), CCR4 (Mogamulizumab) and CD19 (Tafasitamab). Critically, these mAbs all contain human IgG1 isotypes (high A:I binding ratio), with multiple studies demonstrating their collective ability to elicit ADCC, ADCP, and some degree of CDC (3,12,33–35). While it is unclear which of these mechanisms mediate their impact, there is emerging consensus that the Fc portion of these mAbs is indispensable for their therapeutic activity, consistent with previous studies of CD20-targeting mAbs. As such, there is strong reason to believe that these mAbs may be similarly enhanced through strategies to potentiate Fc-based immune mechanisms.
Conjugated therapeutic monoclonal antibodies in hematologic cancers:
Early successes and failures of different ‘naked’ mAbs against hematologic antigens suggested the obvious potential of the conjugation of different cytotoxic agents to mAbs. This would provide a direct means to enhance tumor elimination and therapeutic efficacy, especially in comparison to systemic chemotherapies. The first successful use of this approach utilized radio-labeling (131I and 90Y-labeling) of both high and low A:I isotype CD20-targeting mAbs (Ibritumomab tiuxetan and tositumomab, respectively), which both demonstrated improved response rates and PFS in patients with NHL in 2002–2003. Notably, their comparable efficacy suggests their impact is due to their emission of beta particles to enhance tumor killing and not due to ADCP/ADCC mechanisms (36,37).
Aside from radiolabeling, efforts to directly conjugate cytotoxic agents to mAbs resulted in additional approvals for mAbs targeting CD30, CD33, CD22, BCMA and CD79b in the past decade. These antigens are highly restricted to different lymphoid and myeloid cell types, allowing a high degree of specificity of their different cytotoxic payloads, comprised of DNA toxins, microtubule inhibitors, and exotoxins (38–40). While many of these ADCs are used in isolation, Polatuzumab vedotin is used in combination with chemotherapy and Rituximab for the treatment of large B-cell lymphomas (40–42). This combinatorial approach demonstrates the potential of combining ADCs with unconjugated mAbs against different targets within a singular cancer to improve clinical outcomes and minimize toxicities.
Since the approval of Rituximab in 1997, there has been great progress in developing mAbs for the treatment of different hematologic cancers. These efforts have resulted in at least 16 (to date) different mAb drugs to treat a variety of myeloid and lymphoid carcinomas. Lacking complex stromal and extracellular matrix components, these cancers appear to be more sensitive to mAb-based depletion, whether through FCGR-interactions and complement activation or utilizing conjugated immunotoxins. The advent of agents to improve FCGR effector function suggests that blocking these types of innate immune checkpoints (e.g., blocking CD47-SIRPα interactions to improve phagocytosis) may enhance the activity of unconjugated mAbs (32), although it is unclear if there is a biological ceiling on the efficacy of this approach. Moreover, the interaction between unconjugated mAbs and chemotherapy, as well as the potential for combination usage of different unconjugated and/or conjugated mAbs, remains largely unexplored. The recent approval of multiple ADCs for different cancer antigens (e.g., CD79b, BCMA, and CD38) suggests the strong clinical potential for ADCs in the treatment of these malignancies. As such, the forefront of new treatments for hematologic cancers will more than likely consist of the development of new ADCs, as well as refined usage of unconjugated mAbs.
Monoclonal antibodies targeting ERBB family proteins in solid cancer:
In addition to their use against hematological tumor antigens, mAbs have also been extensively explored to target antigens in solid tumors (3). Notably, the majority of FDA approved mAbs target two members of the ERBB family, HER2 and EGFR (Table 1). Both EGFR and HER2 are cell surface membrane-spanning Type-I receptors that are highly expressed on different solid cancers and enable a broad repertoire of oncogenic signaling upon homo and hetero-dimerization (43). Ongoing clinical trials are also currently exploring therapeutic mAbs targeting HER3, a heterodimerization binding partner for both EGFR and HER2 (44). The combination of their elevated cell surface expression and their critical role in maintaining oncogenic signaling is thought to make these receptors ideal mAb-targets. While the goal of initial efforts to develop mAbs against these targets was to block their signaling, subsequent studies have revealed that the mAbs against HER2 have largely immunologic MOAs. In the following sections, we will discuss the latest preclinical and clinical developments on HER2 and EGFR mAbs therapy and the lessons learned from those studies, which is important for future mAbs developments of other solid tumor targets.
HER2-specific unconjugated mAbs:
In 1998, Trastuzumab became the first mAb approved for treatment of a solid cancer for use in HER2+ breast cancer (BC) and later in HER2+ gastric cancer. HER2 is amplified in 15–20% of breast cancers, which initially associated with poor prognosis and higher rates of recurrence (45). The use of Trastuzumab in combination with chemotherapy demonstrated impressive survival gains and quickly became the standard-of-care therapy for HER2+ BC (46,47). Initially, this mAb was thought to suppress HER2 signaling (48,49), with multiple studies have confirming the inhibition of downstream PI3K/Akt signaling pathway, resulting in the upregulation of p27 and inhibition of cellular proliferation (50–53). However, the parallel development of HER2-selective tyrosine-kinase inhibitors (TKIs, such as lapatinib, neratinib, and tucatinib) have proven to be far superior at suppressing HER2-mediated signaling (52,54–57). But despite being a weaker inhibitor of HER2 signaling, Trastuzumab has exhibited greater clinical efficacy in comparison to TKIs, becoming the backbone of therapy for HER2+ BC for more than 20 years (48,58–60). Clinically, this difference in efficacy suggests that the immunological engagement of antibody therapy, largely absent in TKIs, is likely critical for successful therapeutic outcome.
Indeed, pre-clinical and clinical studies have revealed that the anti-tumor effect of anti-HER2 mAbs depends on engagement with immune cells (61–63). Early studies using xenografts first demonstrated the requirement of FCGR host expression for Trastuzumab’s activity in vivo (64,65). Subsequent studies using a novel murinized version of Trastuzumab (4D5) in both syngeneic and HER2+ transgenic mouse models, demonstrated that the Trastuzumab principal therapeutic MOA occurs through ADCP by macrophages (54). These studies also found this effect was enhanced by the blockade of CD47, similar to findings observed with Rituxumab, suggesting that this strategy may overcome Trastuzumab resistance (54,66). These findings presaged the FDA approval in 2020 of Margetuximab, a HER2 mAb with an altered Fc domain that increases FCGR3A binding and activity (16). Its approval in metastatic HER2+ patients who had received two prior lines of HER2-specific therapies, highlights the importance and clinical need to improve the efficacy of FCGR engagement as dominant MOA in HER2 mAb therapies (15).
Aside from highlighting the importance of Fc activity, several studies have suggested that Trastuzumab may also be capable of activating HER2-specific adaptive immunity (67–70). This activation reportedly occurs through HER2 antibody-complexes that are engulfed by FCGR+ dendritic cells and macrophages (61,71–75). This phagocytic process is thought to trigger HMGB1-MyD88 and Type-I interferon signaling pathways, which are critical for the generation of adaptive anti-tumor T-cell responses (61,73,76–79). However, studies using a fully murine Trastuzumab mAb in an endogenous HER2+ transgenic model failed to demonstrate a significant stimulation of HER2-specific immune responses (54), which may relate to the lack of immunogenicity present in the treatment of an endogenous tumor with fully murine Trastuzumab. However, since its approval, Trastuzumab has been used in conjunction with immunogenic taxane-based chemotherapy as the standard-of-care regimen for HER2+ cancer. Taxane-based chemotherapy has been documented to induce immunogenic tumor cell death and augment phagocytosis (80), which can increase tumor antigen presentation to T-cells (9,81). Intriguingly, clinical studies have documented that lymphocytic infiltrates are positively correlated with clinical response rates and their transcriptional signatures predict lower recurrence in Trastuzumab treated patients (78,82,83). Collectively, these studies suggest that immunogenic chemotherapy could potentially stimulate adaptive immunity, which is likely enhanced by Trastuzumab-mediated ADCP, although further studies will be required to understand the relationship between HER2-targeted mAbs in combination with chemotherapy.
Building upon the efficacy with Trastuzumab plus chemotherapy treatment, clinical studies demonstrated that the addition of another HER2 mAb (Pertuzumab) to this regimen significantly improved the outcomes of both metastatic HER2+ BC (84) and early-stage HER2+ BC (85), resulting in Pertuzumab FDA approval in 2012. Pertuzumab has the ability to block HER2 oncogenic signaling through its binding to domain II of HER2, which sterically inhibits HER2 heterodimerization with other ERBB family proteins (86). However, despite this ability to inhibit heterodimerization, Pertuzumab has demonstrated minimal efficacy as a monotherapy in pre-clinical and clinical studies (87,88). One possible explanation for this lack of efficacy is HER2 homodimerization (mediated by HER2Δ16 or HER2-p95), which allows for constitutive signaling while minimizing the requirement for ligand-driven heterodimerization (89–91). Nevertheless, pre-clinical and clinical studies have firmly documented the synergistic effects on tumor growth suppression by the combination of Pertuzumab and Trastuzumab, suggesting that emergent immune-mediated mechanisms may be responsible (88,92,93). Pertuzumab is a humanized IgG1 mAb capable of strongly activating innate responses such as ADCC, ADCP, and CDC (88,94–96). However, evidence that connects these immunologic mechanisms to Pertuzumab’s therapeutic synergy with Trastuzumab have been lacking. One intriguing hypothesis is the combination of these HER2 mAbs may allow for a more potent activation of complement, as has been suggested from in vitro studies (95), as well as with combinations of EGFR mAbs (97). This may allow for future strategies to exploit the activation of complement, which could be achieved by targeting complement regulators CD46, CD55, and CD59, which are often amplified in solid cancers (95,98). Intriguingly, these genes have been described as amplified in gastric cancers (99–102), where the addition of Pertuzumab did not enhance Trastuzumab treatment responses against HER2+ gastric carcinomas (7,103). But it is unknown how the activation of complement may impact other aspects of local immunity, such as modulation of cytokine secretion, DC maturation, and T-cell activation (104–106). Given the success of this dual HER2 targeting mAb combination with chemotherapy (Figure 1B–C), understanding the interplay between these therapies may be essential in the future success of mAb in other solid cancers.
HER2-specific conjugated mAbs:
The efficacy and ability to selectively target HER2+ tumors made HER2-specific mAbs an early candidate for cytotoxic conjugation. These efforts have resulted in two approved HER2-targeted ADCs in patients, Ado-Trastuzumab emtansine (T-DM1) and fam-trastuzumab deruxtecan (T-Dxd) in 2013 and 2020. These ADCs have been conjugated with cytotoxics (a microtubule inhibitor and a topoisomerase inhibitor, respectively) and approved as second-line therapies for patients who have developed resistance or progressed on Trastuzumab-based regimens (107) (108). Recently, TDM-1 was found to be superior to Trastuzumab in preventing recurrence in patients with early-stage HER2+ BC (109), while preclinical studies have demonstrated a T-Dxd impact against T-DM1 resistant HER2+ BC (110). However, multiple clinical studies have thus far failed to document notable improvements in survival, progression, or safety by using T-DM1 in place of Trastuzumab plus chemotherapy as front-line treatment in both early and advanced HER2+ BC (111–113). These findings suggest that improvements in the chemotherapeutic MOA (achieved by HER2-ADC targeting) are unlikely to shift the efficacy achieved by current treatment combinations. However, a plethora of additional conjugations to HER2 mAbs, many of which contain novel immune-stimulating agents, may allow for enhancements of additional therapeutic mechanisms. While early in development, if these agents could alter local anti-tumor immunity in substantial ways, they could offer the means to promote HER2-specific adaptive immunity that could translate into meaningful clinical therapeutic improvements.
EGFR-specific unconjugated mAbs:
Like HER2, EGFR is another member of the ERBB family responsible for the activation of multiple oncogenic signaling pathways. As a result, it is often overexpressed, dysregulated, or mutated in many cancers, including: colorectal carcinoma (CRC), head-and-neck squamous cell carcinoma (HNSCC), squamous non-small-cell-lung cancer (NSCLC) and Triple-Negative BC (TNBC) (114). This widespread tumor overexpression made EGFR an early mAb target, resulting in the approval of Cetuximab in 2004 for its use in metastatic CRC and HNSCC in combination with chemotherapy (115). However, retrospective analyses determined that efficacy occurred only in KRAS wild-type (non-mutated) cancers (116). And while Cetuximab has also demonstrated utility with radiotherapy in HNSCC, it has not been approved in many other EGFR expressing cancers (117). Two additional EGFR-targeting mAbs, Panitumumab and Necitumumab, subsequently gained FDA approval in metastatic CRC patients (Panitumumab) and NSCLC patients (Nectumumab) (118,119). While these mAbs all target different binding sites, they also differ in their isotype and degree of humanization. Cetuximab and Nectumimab are IgG1 mAbs (a high A:I isotype), while Pantumimab is an IgG2 (low A:I isotype) mAb. Given these differences, it is unsurprising that Cetuximab has demonstrated an enhanced ADCC and CDC capacity in multiple cancers, compared to Pantumimab (120–123). This may explain why Panitumumab has been less clinically effective than Cetuximab in both billary cancers and HNSCC (124,125). But despite these differences, both therapies appear relatively equivalent in CRC (126–128). Additionally, common resistance mechanisms to these therapies are often intrinsic, caused by mutations in KRAS, BRAF, and NRAS. These occur in about 50% of CRC, and are utilized as exclusion criteria for EGFR mAb treatment (129,130). The equivalent efficacy, side effect profile, and signaling-based resistance mechanisms of EGFR mAbs collectively suggest that EGFR signaling blockade is the dominant MOA for anti-EGFR mAbs. However, it is unknown if improvements of Fc activities of EGFR mAbs could offer enhanced therapeutic efficacy against CRC or other EGFR-expressing cancers. Additionally, strategies to enhance systemic adaptive anti-tumor immune responses of Cetuximab through combination with immunotherapy may be beneficial (121,131), which are also being investigated with other EGFR mAbs (132).
Other mAbs for non-ERBB family targets in solid cancers:
While initial approvals of mAbs in solid cancers have been against ERBB antigens, pre-clinical studies have evaluated a variety of different tumor targets (Table 1). This growing interest has now led to several FDA approvals for unconjugated and conjugated mAbs against multiple highly expressed antigens in solid cancers. The first such mAb to gain continual usage occurred in 2015, with the FDA approval of Dinutuximab for second-line treatment of children with high-risk neuroblastoma (133,134). This chimeric mAb targets GD2, a glycolipid found on cells of the neuroectoderm (135). An improved humanized version (Naxitamab) was later approved in 2020, with both allowing for Fc-mediated activation of ADCC, ADCP, and CDC activity (135–140). Notably, these mAbs were developed after extensive pre-clinical and clinical validation and utilized as part of an immunotherapeutic strategy, in combination with GM-CSF and chemotherapy. Recently, two other ADCs have been approved for solid cancers, Enfortumab vedotin for urothelial carcinoma and Sacituzumab govitecan for TNBC. These cytotoxic ADCs target two different tumor-associated antigens, Nectin-4 and TROP-2, respectively. Nectin-4 is a cell adhesion member of the Nectin family and is overexpressed in many cancers, including urothelial bladder cancer (141). TROP-2 is a transmembrane glycoprotein first identified in the trophoblast, with roles in growth, invasion, and spread. It is also highly expressed in many cancers, including Triple-Negative Breast Cancer (142,143). In both indications, these ADCs were approved as secondary therapies, but Enfortumab vedotin was also approved as a frontline therapy for urothelial carcinoma in combination with pembrolizumab (anti-PD1 mAb) as a first-line treatment for cisplatin ineligible patients (141,142,144,145). While both have direct anti-tumor effects through their cytotoxic payloads, this type of immunotherapeutic synergy suggests that ADCs targeting tumor-associated antigens may offer a viable therapeutic path as secondary therapies for resistant cancers, or in combination with immune-modulating agents as frontline therapies.
Conclusion and Future Directions:
The advent and development of mAbs directly targeting cancers has fundamentally altered the landscape of targeted therapy in oncology, having become a frontline standard-of-care for many hematologic and some solid cancers. In combination with chemotherapy, these mAbs have generated success against advanced-stage cancers, which previously had poor outcomes. This is due to the induction of multiple, mostly immunologic MOAs, which collectively serve to engage innate immune cells and stimulate anti-tumor immunity, likely in combination with chemotherapy. These same MOAs appear to be present in mAbs against hematologic cancers (e.g. Rituximab), as well as in other solid cancers (e.g. HER2 mAbs). In cases where these immunologic MOAs are not strongly induced (potentially with EGFR mAbs), responses have been more muted. As such, the selection of new mAbs should likely prioritize the ability to elicit robust immune stimulation, in addition to ensuring adequate binding affinity, bio-distribution, and tumor penetrance. Additionally, these responses are likely to vary by tissue type in the regulation of these MOAs, potentially evidenced by a lack of Pertuzumab additive efficacy in HER2+ gastric carcinomas. This also may explain why greater mAb efficacy has been observed against hematologic cancers, which have less stromal influence. Strategies to enhance these MOAs, such as CD47 blockade to enhance ADCP (54) or reducing mAbs fucosylation to enhance Fc activity (146), have demonstrated promising clinical signals and suggest the potential for these types of approaches. Moreover, the use of ADCs and novel conjugations may also serve to enable existing MOAs or elicit novel MOAs (e.g., cytotoxicity and immune stimulation) against different cancers. This may be particularly pertinent given the success of PD-1/PD-L1 immune checkpoint blockade mAbs, which have demonstrated the potential to enable adaptive anti-tumor immunity. Given their ability to elicit innate anti-tumor immunity, it may only be a matter of time before the full potential of anti-tumor mAbs are realized, as a means to cooperatively marshal both innate and adaptive immune responses against cancer.
Footnotes
COI Statement:
The authors declare no potential conflicts of interest.
References
- 1.Nupur N, Chhabra N, Dash R, Rathore AS. Assessment of structural and functional similarity of biosimilar products: Rituximab as a case study. MAbs 2018;10:143–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012;12:278–87 [DOI] [PubMed] [Google Scholar]
- 3.Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel) 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert JM, Morris CQ. Antibody-Drug Conjugates (ADCs) for Personalized Treatment of Solid Tumors: A Review. Advances in therapy 2017;34:1015–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewer MS, O’Shaughnessy JA. Cardiac toxicity of trastuzumab-related regimens in HER2-overexpressing breast cancer. Clinical breast cancer 2007;7:600–7 [PubMed] [Google Scholar]
- 6.Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic Toxicity Occurring During Anti-EGFR Monoclonal Inhibitor Therapy in Patients With Metastatic Colorectal Cancer: A Systematic Review. Clinical colorectal cancer 2018;17:85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020;17:33–48 [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Divergent Immunoglobulin G Subclass Activity Through Selective Fc Receptor Binding. Science 2005;310:1510. [DOI] [PubMed] [Google Scholar]
- 9.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol 2014;14:94–108 [DOI] [PubMed] [Google Scholar]
- 10.Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. Journal for ImmunoTherapy of Cancer 2014;2:1–1024829758 [Google Scholar]
- 11.Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol 2018;18:5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golay J, Taylor RP. The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer mAbs. Antibodies (Basel) 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein & cell 2018;9:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders KO. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Frontiers in Immunology 2019;10:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rugo HS, Im SA, Cardoso F, Cortés J, Curigliano G, Musolino A, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Research 2011;13:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oostindie SC, van der Horst HJ, Kil LP, Strumane K, Overdijk MB, van den Brink EN, et al. DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer Journal 2020;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grilo AL, Mantalaris A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol 2019;37:9–16 [DOI] [PubMed] [Google Scholar]
- 20.Ackerman SE, Pearson CI, Gregorio JD, Gonzalez JC, Kenkel JA, Hartmann FJ, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nature Cancer 2021;2:18–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Tedder TF. Identification of a CD20-, FcepsilonRIbeta-, and HTm4-related gene family: sixteen new MS4A family members expressed in human and mouse. Genomics 2001;72:119–27 [DOI] [PubMed] [Google Scholar]
- 22.Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther 2017;34:2232–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica 2020;105:1494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res 2012;2:676–90 [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor RP, Lindorfer MA. Analyses of CD20 monoclonal antibody-mediated tumor cell killing mechanisms: rational design of dosing strategies. Mol Pharmacol 2014;86:485–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol 2008;20:444–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavanello F, Zucca E, Ghielmini M. Rituximab: 13 open questions after 20years of clinical use. Cancer Treat Rev 2017;53:38–46 [DOI] [PubMed] [Google Scholar]
- 28.Nowakowski GS, Blum KA, Kahl BS, Friedberg JW, Baizer L, Little RF, et al. Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research. J Natl Cancer Inst 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer S, Evers M, Jansen JHM, Buijs J, Broek B, Reitsma SE, et al. New insights in Type I and II CD20 antibody mechanisms-of-action with a panel of novel CD20 antibodies. Br J Haematol 2018;180:808–20 [DOI] [PubMed] [Google Scholar]
- 30.Perez-Callejo D, Gonzalez-Rincon J, Sanchez A, Provencio M, Sanchez-Beato M. Action and resistance of monoclonal CD20 antibodies therapy in B-cell Non-Hodgkin Lymphomas. Cancer Treat Rev 2015;41:680–9 [DOI] [PubMed] [Google Scholar]
- 31.Johnson NA, Leach S, Woolcock B, deLeeuw RJ, Bashashati A, Sehn LH, et al. CD20 mutations involving the rituximab epitope are rare in diffuse large B-cell lymphomas and are not a significant cause of R-CHOP failure. Haematologica 2009;94:423–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 2018;379:1711–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touzeau C, Moreau P. Daratumumab for the treatment of multiple myeloma. Expert Opin Biol Ther 2017;17:887–93 [DOI] [PubMed] [Google Scholar]
- 34.Weisel K Spotlight on elotuzumab in the treatment of multiple myeloma: the evidence to date. Onco Targets Ther 2016;9:6037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ 2020;370:m3176. [DOI] [PubMed] [Google Scholar]
- 36.Pohlman B, Sweetenham J, Macklis RM. Review of clinical radioimmunotherapy. Expert Rev Anticancer Ther 2006;6:445–61 [DOI] [PubMed] [Google Scholar]
- 37.Cheson BD. Radioimmunotherapy of non-Hodgkin lymphomas. Blood 2003;101:391–8 [DOI] [PubMed] [Google Scholar]
- 38.Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 2019;393:229–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, et al. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin Cancer Res 2019;25:5441–8 [DOI] [PubMed] [Google Scholar]
- 40.Camus V, Tilly H. Polatuzumab vedotin, an anti-CD79b antibody-drug conjugate for the treatment of relapsed/refractory diffuse large B-cell lymphoma. Future Oncol 2021;17:127–35 [DOI] [PubMed] [Google Scholar]
- 41.Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38:155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilly H, Morschhauser F, Bartlett NL, Mehta A, Salles G, Haioun C, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. Lancet Oncol 2019;20:998–1010 [DOI] [PubMed] [Google Scholar]
- 43.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12 [DOI] [PubMed] [Google Scholar]
- 44.Jacob W, James I, Hasmann M, Weisser M. Clinical development of HER3-targeting monoclonal antibodies: Perils and progress. Cancer Treat Rev 2018;68:111–23 [DOI] [PubMed] [Google Scholar]
- 45.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY) 1987;235:177–82 [DOI] [PubMed] [Google Scholar]
- 46.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92 [DOI] [PubMed] [Google Scholar]
- 47.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97 [DOI] [PubMed] [Google Scholar]
- 48.Moasser MM, Krop IE. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncology 2015;1:1154–61 [DOI] [PubMed] [Google Scholar]
- 49.Moasser MM. Two dimensions in targeting HER2. Journal of Clinical Oncology 2014;32:2074–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 2002;62:4132–41 [PubMed] [Google Scholar]
- 51.Le XF, Pruefer F, Bast RC, Jr. HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle 2005;4:87–95 [DOI] [PubMed] [Google Scholar]
- 52.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast cancer research and treatment 2010;122:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Meng Y, Zheng L, Zhang X, Tong Q, Tan W, et al. Bispecific antibody to ErbB2 overcomes trastuzumab resistance through comprehensive blockade of ErbB2 heterodimerization. Cancer Res 2013;73:6471–83 [DOI] [PubMed] [Google Scholar]
- 54.Tsao LC, Crosby EJ, Trotter TN, Agarwal P, Hwang BJ, Acharya C, et al. CD47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 2001;1:85–94 [PubMed] [Google Scholar]
- 56.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 2006;66:1630–9 [DOI] [PubMed] [Google Scholar]
- 57.Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation. Breast cancer research : BCR 2011;13:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin Y, Guo WW, Huang Q, Zhang P, Zhang L-Z, Jiang G, et al. Effects of lapatinib or trastuzumab, alone and in combination, in human epidermal growth factor receptor 2-positive breast cancer: a meta-analysis of randomized controlled trials. Cancer medicine 2016;5:3454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurvitz SA, Caswell-Jin JL, McNamara KL, Zoeller JJ, Bean GR, Dichmann R, et al. Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07). Nat Commun 2020;11:5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelmon KA, Boyle FM, Kaufman B, Huntsman DG, Manikhas A, Di Leo A, et al. Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2-Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J Clin Oncol 2015;33:1574–83 [DOI] [PubMed] [Google Scholar]
- 61.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proceedings of the National Academy of Sciences of the United States of America 2011;108:7142–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer cell 2010;18:160–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu MM, Pu Y, Zhang Y, Fu YX. The Role of Adaptive Immunity in the Efficacy of Targeted Cancer Therapies. Trends in immunology 2016;37:141–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Medicine 2000;6:443. [DOI] [PubMed] [Google Scholar]
- 65.Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, et al. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast cancer research : BCR 2012;14:R116–R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Candas-Green D, Xie B, Huang J, Fan M, Wang A, Menaa C, et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun 2020;11:4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moasser MM, Krop IE. The Evolving Landscape of HER2 Targeting in Breast Cancer. JAMA Oncol 2015;1:1154–61 [DOI] [PubMed] [Google Scholar]
- 68.Moasser MM. Two dimensions in targeting HER2. J Clin Oncol 2014;32:2074–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rakha EA, Ellis IO. Breast cancer: updated guideline recommendations for HER2 testing. Nature reviews Clinical oncology 2014;11:8–9 [DOI] [PubMed] [Google Scholar]
- 70.Gradishar WJ. Emerging approaches for treating HER2-positive metastatic breast cancer beyond trastuzumab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2013;24:2492–500 [DOI] [PubMed] [Google Scholar]
- 71.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. Journal of translational medicine 2013;11:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. The Journal of clinical investigation 2008;118:1700–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? British journal of cancer 2006;94:259–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. The Journal of clinical investigation 2012;122:1066–75 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. Journal of immunology 2011;186:3401–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 2007;13:5133–43 [DOI] [PubMed] [Google Scholar]
- 77.Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast cancer research and treatment 2011;125:65–72 [DOI] [PubMed] [Google Scholar]
- 78.Ladoire S, Arnould L, Mignot G, Apetoh L, Rebe C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. British journal of cancer 2011;105:366–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mortenson ED, Park S, Jiang Z, Wang S, Fu YX. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19:1476–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang YJ, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes & diseases 2018;5:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng M, Jiang W, Kim BYS, Zhang CC, Fu Y-X, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature reviews Cancer 2019;19:568–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varadan V, Gilmore H, Miskimen KL, Tuck D, Parsai S, Awadallah A, et al. Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to PreoperativeTrastuzumab and Chemotherapy in HER2-Positive Early Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt WD, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant treated HER2-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2016 [DOI] [PubMed] [Google Scholar]
- 84.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519–30 [DOI] [PubMed] [Google Scholar]
- 85.Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. The Lancet Oncology 2016;17:791–800 [DOI] [PubMed] [Google Scholar]
- 86.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004;5:317–28 [DOI] [PubMed] [Google Scholar]
- 87.Cortés J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K, Pivot X, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:1594–600 [DOI] [PubMed] [Google Scholar]
- 88.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330–6 [DOI] [PubMed] [Google Scholar]
- 89.Turpin J, Ling C, Crosby EJ, Hartman ZC, Simond AM, Chodosh LA, et al. The ErbB2ΔEx16 splice variant is a major oncogenic driver in breast cancer that promotes a pro-metastatic tumor microenvironment. Oncogene 2016;35:6053–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pedersen K, Angelini PD, Laos S, Bach-Faig A, Cunningham MP, Ferrer-Ramón C, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol 2009;29:3319–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res 2011;71:1515–9 [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Chen J, Weng Z, Li Q, Zhao L, Yu N, et al. A new anti-HER2 antibody that enhances the anti-tumor efficacy of trastuzumab and pertuzumab with a distinct mechanism of action. Molecular immunology 2020;119:48–58 [DOI] [PubMed] [Google Scholar]
- 93.Huang S, Li F, Liu H, Ye P, Fan X, Yuan X, et al. Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody. MAbs 2018;10:864–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tóth G, Szöőr Á, Simon L, Yarden Y, Szöllősi J, Vereb G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. MAbs 2016;8:1361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol 2013;7:580–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. British journal of cancer 2010;102:134–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klausz K, Berger S, Lammerts van Bueren JJ, Derer S, Lohse S, Dechant M, et al. Complement-mediated tumor-specific cell lysis by antibody combinations targeting epidermal growth factor receptor (EGFR) and its variant III (EGFRvIII). Cancer Sci 2011;102:1761–8 [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Yang YJ, Wang Z, Liao J, Liu M, Zhong XR, et al. CD55 and CD59 expression protects HER2-overexpressing breast cancer cells from trastuzumab-induced complement-dependent cytotoxicity. Oncol Lett 2017;14:2961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inoue T, Yamakawa M, Takahashi T. Expression of complement regulating factors in gastric cancer cells. Mol Pathol 2002;55:193–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiso T, Mizuno M, Nasu J, Shimo K, Uesu T, Yamamoto K, et al. Enhanced expression of decay-accelerating factor and CD59/homologous restriction factor 20 in intestinal metaplasia, gastric adenomas and intestinal-type gastric carcinomas but not in diffuse-type carcinomas. Histopathology 2002;40:339–47 [DOI] [PubMed] [Google Scholar]
- 101.Schmitt CA, Schwaeble W, Wittig BM, Meyer zum Buschenfelde KH, Dippold WG. Expression and regulation by interferon-gamma of the membrane-bound complement regulators CD46 (MCP), CD55 (DAF) and CD59 in gastrointestinal tumours. Eur J Cancer 1999;35:117–24 [DOI] [PubMed] [Google Scholar]
- 102.Juhl H, Helmig F, Baltzer K, Kalthoff H, Henne-Bruns D, Kremer B. Frequent expression of complement resistance factors CD46, CD55, and CD59 on gastrointestinal cancer cells limits the therapeutic potential of monoclonal antibody 17–1A. J Surg Oncol 1997;64:222–30 [DOI] [PubMed] [Google Scholar]
- 103.Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19:1372–84 [DOI] [PubMed] [Google Scholar]
- 104.Kandasamy M, Ying PC, Ho AWS, Sumatoh HR, Schlitzer A, Hughes TR, et al. Complement mediated signaling on pulmonary CD103(+) dendritic cells is critical for their migratory function in response to influenza infection. PLoS pathogens 2013;9:e1003115–e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S, Wu J, Zhang T, Qian B, Wu P, Li L, et al. Complement C1q chemoattracts human dendritic cells and enhances migration of mature dendritic cells to CCL19 via activation of AKT and MAPK pathways. Molecular immunology 2008;46:242–9 [DOI] [PubMed] [Google Scholar]
- 106.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008;28:425–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keam SJ. Trastuzumab Deruxtecan: First Approval. Drugs 2020;80:501–8 [DOI] [PubMed] [Google Scholar]
- 109.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108 [DOI] [PubMed] [Google Scholar]
- 111.Harbeck N, Im S-A, Barrios CH, Bonnefoi HR, Gralow J, Toi M, et al. Primary analysis of KAITLIN: A phase III study of trastuzumab emtansine (T-DM1) + pertuzumab versus trastuzumab + pertuzumab + taxane, after anthracyclines as adjuvant therapy for high-risk HER2-positive early breast cancer (EBC). Journal of Clinical Oncology 2020;38:500- [Google Scholar]
- 112.Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol 2017;35:141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tolaney SM, Trippa L, Barry W, Hu J, Dang C, Yardley D, et al. Abstract GS1–05: TBCRC 033: A randomized phase II study of adjuvant trastuzumab emtansine (T-DM1) vs paclitaxel (T) in combination with trastuzumab (H) for stage I HER2-positive breast cancer (BC) (ATEMPT). Cancer Research 2020;80:GS1–05-GS1- [Google Scholar]
- 114.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer cell 2014;25:282–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040–8 [DOI] [PubMed] [Google Scholar]
- 116.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65 [DOI] [PubMed] [Google Scholar]
- 117.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8 [DOI] [PubMed] [Google Scholar]
- 118.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705 [DOI] [PubMed] [Google Scholar]
- 119.Ciuleanu T, Socinski MA, Obasaju C, Luft AV, Szczesna A, Szafrański W, et al. Efficacy and Safety of Necitumumab Continuation Therapy in the Phase III SQUIRE Study of Patients With Stage IV Squamous Non-Small-Cell Lung Cancer. Clinical lung cancer 2018;19:130–8.e2 [DOI] [PubMed] [Google Scholar]
- 120.Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin Cancer Res 2016;22:5229–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P, et al. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front Oncol 2019;9:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.López-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother 2009;58:1853–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hsu Y-F, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Molecular Cancer 2010;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vogel A, Kasper S, Bitzer M, Block A, Sinn M, Schulze-Bergkamen H, et al. PICCA study: panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer-a randomised biomarker-driven clinical phase II AIO study. Eur J Cancer 2018;92:11–9 [DOI] [PubMed] [Google Scholar]
- 125.Mesía R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol 2015;16:208–20 [DOI] [PubMed] [Google Scholar]
- 126.Tomkova H, Kohoutek M, Zabojnikova M, Pospiskova M, Ostrizkova L, Gharibyar M. Cetuximab-induced cutaneous toxicity. J Eur Acad Dermatol Venereol 2010;24:692–6 [DOI] [PubMed] [Google Scholar]
- 127.Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 2009;4:107–19 [DOI] [PubMed] [Google Scholar]
- 128.Sipples R Common side effects of anti-EGFR therapy: acneform rash. Semin Oncol Nurs 2006;22:28–34 [DOI] [PubMed] [Google Scholar]
- 129.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. The Lancet Oncology 2010;11:753–62 [DOI] [PubMed] [Google Scholar]
- 130.Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992–5 [DOI] [PubMed] [Google Scholar]
- 131.Sacco AG, Chen R, Ghosh D, Worden F, Wong DJ, Adkins D, et al. An open-label, non-randomized, multi-arm, phase II trial evaluating pembrolizumab combined with cetuximab in patients (pts) with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): updated results of cohort 1 analysis. International Journal of Radiation Oncology*Biology*Physics 2020;106:1121–2 [Google Scholar]
- 132.Besse B, Garrido P, Puente J, Cortot A, Olmedo ME, Pérol M, et al. MA09.11 Efficacy and Safety of Necitumumab and Pembrolizumab Combination Therapy in Stage IV Nonsquamous Non-Small Cell Lung Cancer (NSCLC). Journal of Thoracic Oncology 2017;12:S397 [Google Scholar]
- 133.Mora J Dinutuximab for the treatment of pediatric patients with high-risk neuroblastoma. Expert Rev Clin Pharmacol 2016;9:647–53 [DOI] [PubMed] [Google Scholar]
- 134.Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma--the role of monoclonal antibodies. Ann Pharmacother 2013;47:210–8 [DOI] [PubMed] [Google Scholar]
- 135.Perez Horta Z, Goldberg JL, Sondel PM. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 2016;8:1097–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miraldi F Monoclonal antibodies and neuroblastoma. Semin Nucl Med 1989;19:282–94 [DOI] [PubMed] [Google Scholar]
- 137.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 1989;73:1936–41 [PubMed] [Google Scholar]
- 138.Cheung NK, Walter EI, Smith-Mensah WH, Ratnoff WD, Tykocinski ML, Medof ME. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest 1988;81:1122–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doronin II, Vishnyakova PA, Kholodenko IV, Ponomarev ED, Ryazantsev DY, Molotkovskaya IM, et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014;14:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Honeychurch J, Alduaij W, Azizyan M, Cheadle EJ, Pelicano H, Ivanov A, et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood 2012;119:3523–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heath EI, Rosenberg JE. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol 2021;18:93–103 [DOI] [PubMed] [Google Scholar]
- 142.Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019;380:741–51 [DOI] [PubMed] [Google Scholar]
- 143.Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018;9:28989–9006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang E, Weinstock C, Zhang L, Charlab R, Dorff SE, Gong Y, et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res 2021;27:922–7 [DOI] [PubMed] [Google Scholar]
- 146.Liu SD, Chalouni C, Young JC, Junttila TT, Sliwkowski MX, Lowe JB. Afucosylated antibodies increase activation of FcγRIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer Immunol Res 2015;3:173–83 [DOI] [PubMed] [Google Scholar]


