Abstract
Objective:
The Attempted Suicide Short Intervention Program (ASSIP) was adapted for hospital delivery and to address substance use problems as well as evaluated for feasibility, acceptability, and therapist fidelity in a series of preparatory steps (n=28) and in a pilot randomized controlled trial, RCT (n=34).
Method:
In the RCT, patients with suicide attempts and substance use problem(s) with sufficient lengths of stay to deliver three ASSIP therapy sessions in hospital were randomized to adapted ASSIP or treatment as usual control. A blinded assessor identified suicide reattempts over 6-month follow-up with the Columbia-Suicide Severity Rating Scale (C-SSRS) and a comprehensive multi-source method. Treatment process measures and the Scale for Suicidal Ideation (SSI) were also administered.
Results:
Median hospital stay was 13 days. ASSIP subjects reported high satisfaction with the treatment and high therapeutic alliance. Study therapists showed high fidelity to the modified ASSIP intervention. Repetition of suicide attempt was common in both study groups including a combined 9 (26%) subjects with reattempt based on C-SSRS and 13 (38%) subjects with reattempt based on multiple sources.
Conclusions:
Adult suicide attempt patients with substance use problems who require lengthy hospitalizations are at exceptionally high risk and may require additional strategies to lower risk.
Keywords: suicide attempt, alcohol, substance use, RCT, intervention, hospital
Introduction
In 2019, there were 47,511 suicides in the United States, making it the tenth leading cause of death [1], along with more than 490,000 emergency department visits for suicide attempt [2]. Suicide attempts that lead to hospital admission are associated with dramatic risk for repetition of attempt and eventual suicide, with greatest risk in the initial months following discharge [3]. A hospital stay may provide a window of opportunity for openness to change, rapid intervention, and dramatic therapeutic progress which has been labeled “teachable moment” [4]. Indeed, teachable moment was used as the basis for the development of a promising, one session hospital-based intervention for suicide attempt patients [5]. Accordingly, the testing of methods to intervene with hospitalized suicide attempt patients to lower risk for subsequent suicidal behavior is of urgent public health significance.
Risk for suicidal behavior is increased by alcohol use disorder, a pattern of hazardous drinking, and by acute use of alcohol (drinking during the event), with risk rising steeply with the amount of alcohol consumed [6]. Drug use disorders also confer risk for suicidal behavior [7] including cocaine, amphetamine [8], and opioid use disorders [9]. Substance use disorder patients pose several challenges to suicide prevention including the potential for emergent risk for suicidal behavior during alcohol intoxication [10] and substance-induced depression [11]. Yet, patients with substance-related problems may receive less intensive treatment responses for suicidal risk such as reduced emergency department evaluation and referral efforts and shorter hospital stays [12–14].
Treatment Development Project
To address the need for evidence-based psychotherapy that may be delivered during hospitalization to lower risk for repetition of suicidal behavior, and addresses the challenges posed by substance use problems, we launched a three-year treatment development project. We based it on a seminal treatment development guide which outlined an iterative approach to development, refinement, and preliminary examination of the utility of a new or adapted intervention [15]. Specifically, we adapted the Attempted Suicide Short Intervention Program (ASSIP) [16] and piloted the adapted intervention. ASSIP is a manualized, patient-centered intervention that draws on narrative therapy, video playback, and cognitive-behavioral techniques and typically consists of three sessions. In session one, the clinician videotapes the narrative interview of the patient telling the “story” of their suicide attempt and what led up to it. In session two, the patient views segments of the video with the ASSIP therapist, leading to a shared understanding of the background, warning signs, triggers, and relevant vulnerabilities/threatened life goals that led up to the suicide attempt. In session three, the therapist presents a draft of a case formulation that is revised collaboratively with the patient in session, leading to a detailed suicide prevention plan. A homework task is also completed. Standardized follow-up letters signed by their ASSIP therapist are also sent to their patients as a means of support and to serve as a reminder that the therapist is available as a resource [16].
We chose ASSIP because it is brief, making it feasible to deliver it in hospital and thus take advantage of a teachable moment. Moreover, in a seminal randomized controlled trial (RCT), ASSIP showed efficacy to reduce risk for suicide reattempt in 120 adult suicide attempt patients with 24-month follow-up, showing a large effect size [17]. Specifically, the goals of the current project were to: adapt ASSIP for rapid administration during hospitalization to adult suicide attempt patients with substance use problems; establish feasibility and acceptability of the adapted ASSIP intervention among patients as well as therapist fidelity to the intervention; and to preliminarily describe suicide reattempt (primary outcome) and suicidal ideation (secondary outcome) in subjects who received the adapted ASSIP intervention and a control or treatment as usual (TAU) group who did not receive it. All subjects received TAU and enhanced safety measures.
Methods
Treatment Development Steps
We conducted the study at the University of Rochester Medical Center (URMC) in a series of six iterative steps including four a priori steps and two additional steps that became necessary. The study was approved by the IRB at URMC.
Step 1 (Adaptation)
We adapted ASSIP in two fundamental ways: 1) so the standard three ASSIP sessions could be completed during hospitalization including on consecutive days when necessary; 2) so the adapted ASSIP intervention could address patients’ substance use problems in addition to their suicide risk, for example, by integrating the role of substance use into the case formulation, homework task, and suicidal behavior prevention plan. We elaborated on the adaption in a prior report [18].
Step 2 (Training and Establishing Hospital Collaborations)
We established collaborations with hospital units and consult services, developing procedures to administer ASSIP in patient’s hospital rooms (e.g., taping the sessions using a camcorder on the patient’s nightstand), and doing so in a timely way to complete the therapy prior to discharge. Study therapists were a PhD graduate student in clinical psychology and the study principal investigator (PI) who received 16 hours of in-person training by a developer of ASSIP (this was the second such training for the PI). We also developed a fidelity checklist to assess therapist competence and adherence to the adapted ASSIP intervention [19].
Step 3 (First Open Trial)
We conducted an open trial in which all subjects received ASSIP (n = 12). This pilot study established the feasibility of recruiting adult suicide attempt patients with substance use problems hospitalized at URMC, completing the ASSIP therapy during hospitalization, demonstrating subject tolerance of and satisfaction with the therapy, and of retaining subjects over short-term follow-up. The study therapists also received intensive supervision of five cases each by the developers of ASSIP, leading to their certification as ASSIP therapists.
Step 4 (First RCT)
We launched a pilot RCT with parallel assignment and random allocation that compared ASSIP to a control group, along with enhanced safety measures. However, after enrolling eight subjects, two subjects died by suicide during the 6-month follow-up period (1 ASSIP, 1 control). Neither death was determined to be study related but these deaths underscored the high-risk nature of the study population and required us to modify the trial [20] in order to strengthen subject safety measures. Accordingly, we discontinued the trial, added a study data safety monitoring board (DSMB), and consulted with the DSMB, the developers of ASSIP, and two other experts.
Step 5 (Second Open Trial with Added Safety Procedures)
We revised the study in two ways. First, we added a fourth therapy session one week after discharge to aid ASSIP patients in making the transition from the hospital to the community. The major goals of this session were to assess the subject’s post-discharge adjustment and safety, to update the written suicide behavior prevention plan as needed, and to reinforce the importance of adherence to outpatient treatment. As time allowed and when it was clinically indicated, we also conducted a “mini-exposure,” deemed a fourth optional session in the ASSIP manual [16], whereby the therapist and patient watched segments of the narrative interview to identify additional lessons learned and prevention strategies now that the patient was seeking to apply the therapy in their home environment. Second, for all subjects, we added up to three standardized telephone check-ins conducted by study staff within the first month of discharge to screen for acute suicide risk, to promote safety as needed, and to reinforce adherence to keeping post-hospital treatment appointments. After making the aforementioned revisions, we piloted them in a second open trial (n = 8).
Step 6 (Second RCT)
We conducted a second RCT to provide a preliminary examination of the modified ASSIP treatment and a non-ASSIP control condition. We describe the methods and results of this second RCT for the remainder of this report.
Subjects.
The sample was made up of adults who were admitted to hospital at URMC following a suicide attempt. Subjects were identified by the research coordinator or PI through communications between researchers and hospital units and consultations services along with review of the electronic medical record. If deemed potentially eligible, and after obtaining permission of clinical staff to approach a patient, the research coordinator summarized the study briefly, answered questions, and administered a screen to determine eligibility.
There were four inclusion criteria (all needed to be met): 1) age 18-plus; 2) speaks and understands English; 3) positive screen for alcohol use problems in the past year on the 3-item Alcohol Use Disorders Identification Test – Consumption (females: score of 3-plus; males, score of 4-plus) [21] or recurrent use of “prescription drugs for non-medical reasons” or “illegal drugs” in the past year based on self-reported use of such drugs in response to standardized items on a “monthly” “weekly” or “daily or almost daily basis” (as opposed to “never” or “once or twice”); and 4) a suicide attempt with intent to die leading to hospitalization assessed with the item, “at the time you attempted suicide or tried to harm yourself, what percentage of you wanted to die?,” with percentages greater than zero satisfying the criterion [22]. We administered additional assessments of suicidal behavior, alcohol use problems, and drug use problems at the baseline assessment to characterize the sample, but we did not use these measures to conduct additional screening. We also did not require that suicide attempts occur in the context of alcohol or drug use. Exclusion criteria were: 1) residing outside a 40-mile radius of URMC which would create a burden for the subject to return for a fourth session post-discharge; 2) hospital stay that was anticipated to be too brief to deliver three sessions of ASSIP; 3) acute mania or psychosis; 4) impaired cognitive functioning or acute medical difficulties that would preclude participation; and 5) prisoner status.
Treatment Assignment and Masking.
After completing the baseline assessment, the research coordinator used a computer program to randomly assign subjects to treatment arm in blocks stratified by multiple attempt history to ensure a balance of multiple suicide attempt patients, a marker for risk for reattempt, between study conditions. The research coordinator did not know in advance which treatment the next subject would get in order to ensure proper allocation concealment. Subjects were informed of their assignment and those assigned to ASSIP typically had their first session the following day. A different assessor, who was masked to treatment assignment, performed the follow-up assessments that were planned for 1-, 3-, and 6-months after hospital discharge.
Experimental and Comparison Conditions.
The ASSIP group was assigned to a modified 4-session version of ASSIP, with the final session scheduled approximately one-week post-discharge (described above). Validated self-report measures assessed satisfaction with the therapy using the Client Satisfaction Questionnaire (CSQ) [23] and therapeutic alliance using the revised Working Alliance Inventory-Short Revised (WAI-SR) [24]. Fidelity to each therapy session was rated based on independent review of 50% of randomly selected ASSIP therapy cases using a measure developed for the project [19]. Informed by prior work in therapist fidelity measurement [25], both “adherence” (i.e., compliance with each technical procedure) and broader the concept of “competence” (i.e., therapeutic skill in recognizing distress, providing a therapeutic milieu, and executing the skills) were assessed. The content of these fidelity assessments closely aligned with the content of the first three ASSIP sessions [16]. Specifically, fidelity scores were derived by dividing the obtained score by the maximum possible score to generate a percentage (range 0% to 100%). The assessment of fidelity to the fourth therapy session was based on the extent to which the key agenda items for this study-specific meeting were met including assessment of the subject’s post-discharge adjustment, reinforcement of their connection with a community therapist, and updating their written prevention plan, along with facilitating a guided “mini-exposure” as described in the ASSIP manual.
All subjects were provided TAU during their hospitalization. TAU was provided on a variety of inpatient units including extended observation units, intensive care units, other medical wards, psychiatric inpatient units, and the “medicine in psychiatry unit” for patients with acute psychiatric needs who require medical stabilization. Subjects on a psychiatry unit and the medicine in psychiatry unit also completed a standard 6-step safety plan prior to discharge [26] that was administered by hospital staff as part of TAU. All subjects were also provided referral(s) for further treatment at discharge in line with TAU. The research team reinforced the importance of adhering to such treatment recommendations. Subjects also received enhanced safety measures (described below).
Outcome Measures.
The primary outcome was the number of subjects who made a suicide reattempt over 6-month follow-up, assessed in two ways. First, the Columbia-Suicide Severity Rating Scale (C-SSRS) was administered at baseline and at 1-, 3-, and 6-month follow-up to identify suicide attempts [27]. The dates of suicide attempts identified on the C-SSRS were obtained from the subject with the aid of a calendar. The C-SSRS has been validated for use with high-risk clinical populations [27] and for administration by telephone [28]. Second, we used a multi-source strategy [29] to identify and date suicide reattempts over follow-up including those identified on the C-SSRS or during telephone check-ins, review of the electronic medical record, or correspondence with patient’s consent with family or treatment providers.
The secondary outcome investigated changes in two measures of suicidal ideation (self-reported current and worst point) from baseline to 6-month follow-up as assessed by the Scale for Suicidal Ideation (SSI) [30]. The SSI is an interview measure with range 0–38 with two subscales (current, worst point). The SSI-current was used to assess suicidal ideation in the past week at baseline and suicidal ideation in the past week at 6-month follow-up. The SSI-worst point was used to assess suicidal ideation over one’s lifetime at its worst crisis point at baseline and to assess suicidal ideation at its worst crisis point during 6-month follow-up.
Safety Measures.
All subjects (ASSIP, control) received a safety procedures’ intervention in hospital in accordance with National Institute of Mental Health recommendations for research with high-risk individuals [31]. These procedures included sharing summary results of the research assessments regarding suicidal thoughts, suicidal behaviors, alcohol and drug use problems, and depressive symptoms with appropriate hospital clinical staff. This information was also shared, with subjects’ permission and a signed release of information, to their ongoing therapist or treatment program and, if none existed, to the therapist or program to which they were being referred. ASSIP therapy written case formulations were also shared with ongoing therapists with subjects’ permission. Control subjects also received general information about safety precautions and procedures to follow in the event of a crisis and emergency phone numbers.
All subjects were scheduled to receive up to three telephone check-ins conducted by study staff during the first month post-discharge to inquire about any difficulties with keeping scheduled treatment appointments, to problem solve as needed to keep such appointments, to confirm contact information, and to screen for acute risk for suicidal behavior. Study staff discussed the check-in results with the PI, typically while the subject was still on the phone, if the screening items suggested risk. In these instances, the PI would perform further assessment and take appropriate actions depending on the subject’s severity and acuity.
Subjects were scheduled to receive follow-up assessments at 1-month, 3-month, and 6-months post-discharge which also provided an opportunity to assess risk and promote safety. In instances of potential acute suicide risk, the PI reviewed the assessment before speaking with the subject to conduct further evaluation of safety and to make plans to promote safety. The measures routinely used to promote safety included assessment, refining and reinforcing the prevention plan, promoting adherence to treatment, and coordinating with family and/or treatment providers with subjects’ knowledge and consent. More rarely, we enlisted the assistance of a mobile crisis team to check on a patient in their home.
Analyses and Sample Size Calculation.
Data were screened for missingness, nonnormality, nonlinearity, and heteroscedasticity using statistics and graphic representations. Descriptive statistics were conducted to describe the sample and the outcome variables at baseline and over 6-month follow-up in the study groups. The planned sample size (n = 40) was based on guidelines for treatment development research [15] in order to adapt the therapy and to assess feasibility, safety, and patient acceptability. This sample size was assumed to be inadequate for the purpose of examining the efficacy of the modified ASSIP intervention or to generate stable effect size estimates [32]. Accordingly, and consistent with the approach taken by a recent treatment development study of a hospital-based intervention to lower risk for suicide reattempt [5], we did not compare the study groups on change in risk for suicide reattempt or other study outcomes.
Results
Second RCT
Enrollment
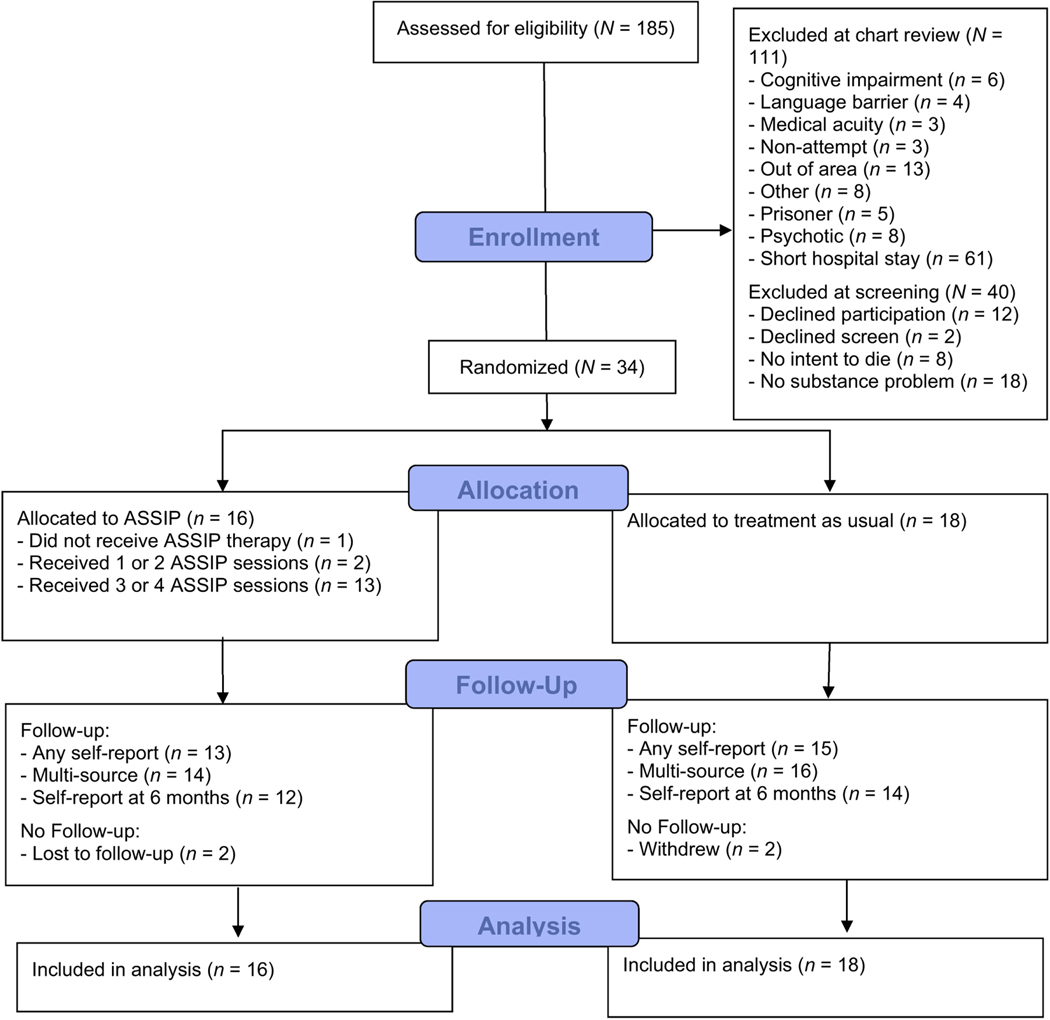
Over a 13-month period (February 2019-March 2020), 185 hospital patients were pre-screened for eligibility based on discussions with treatment providers and/or review of the electronic medical record, with 111 excluded, most commonly due to short hospital stays that would preclude delivery of the ASSIP treatment in hospital (n = 61), yielding 74 patients who were approached for screening (see Figure 1). Of these patients, 40 were excluded because they had no substance use problem based on the completed eligibility screen (n = 18). The remaining subjects (n = 34) were enrolled in the second RCT and completed the consent procedure and then a baseline assessment. Following baseline assessment, subjects were randomized to the ASSIP (n = 16) and control (n = 18) conditions. We did not reach our planned sample size of 40 because recruitment was discontinued in March 2020 as necessitated by the statewide executive order due to the COVID-19 pandemic.
Fig. 1.
CONSORT Flow Diagram.
Baseline and Clinical Characteristics
ASSIP subjects and control subjects were similar and, therefore, we describe their characteristics for the combined sample (n = 34). Subjects were hospitalized a mean 16 days (median 13 days) and enrolled in the study a mean 5 days (median 4 days) following hospitalization. Mean age of subjects was 41 years and most were female (65%) and White, non-Hispanic (59%). A minority were employed (35%) or married or living with a partner (29%). Most subjects had a history of multiple suicide attempts (53%). Mean “percentage of you wanted to die” in the suicide attempt preceding hospitalization as reported by subjects was 84%. Alcohol-related problems (62%) were common as determined by a score of 8-plus on the 10-item AUDIT (range 0–40) and other drug use problems were common based on a score of 2-plus on the 10-item abbreviated version of the Drug Abuse Screening Test (range 0–10) [33, 34]. A discharge diagnosis of mood disorder was typical (85%). Mean depression scores based on the PHQ-9 [35] were in the severe range.
ASSIP Therapy
Of the 16 subjects assigned to ASSIP, 81% received at least three sessions, with 69% receiving a fourth therapy session post-discharge. Subjects reported high satisfaction with the ASSIP therapy, mean (SD) CSQ score = 29.5 (3.0) (scale range = 8–32) and high therapeutic alliance with study therapists, mean (SD) WAI-SR score = 53.6 (7.2) (scale range = 12–60). Therapist fidelity to ASSIP sessions was high, with mean adherence scores of 92% and above and mean competence scores of 89% and above to the first three ASSIP sessions. The goals of the fourth therapy session were introduced by the study therapist 100% of the time per the fidelity assessment which served to guide the session agenda. In 50% of the fourth therapy sessions, the therapist guided the subject in a “mini-exposure” as described in the ASSIP manual [16].
Attrition
Follow-up assessments were performed at (masked for anonymity) or by telephone, and were conducted exclusively by telephone following the COVID-19 executive order in March 2020. Subjects were reassessed over the 6-month follow-up period whenever possible and whether or not they reached the primary endpoint (suicide reattempt) earlier in the course of follow-up. Cumulative dropout due to subject withdrawal, suicide, or permanent loss to follow-up was 17.6% at 1-month follow-up, 23.5% at 3-month follow-up, and 23.5% at 6-month follow-up.
Study Outcomes
Suicide reattempt over 6-month follow-up was common in the sample. Based on C-SSRS results, 9 (26%) subjects across conditions made a total of 11 suicide attempts, with median (range) days to first attempt = 98 days (31, 148). Based on multi-source results, 13 (38%) subjects across conditions made a total of 19 attempts over 6-month follow-up, with median (range) days to first attempt = 53 days (7, 148). Descriptive suicide attempt results stratified by treatment condition (ASSIP, control) are presented in Table 2. One subject (assigned to ASSIP) died of suicide during follow-up. Descriptive results for suicidal ideation including current ideation (i.e., past week) and at the self-reported worst point, at baseline and at 6-month follow-up are presented in Table 3, stratified by treatment condition.
Table 2.
Suicide Attempt Results over 6-month Follow-up
| Self-report results1 | All source results2 | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Treatment condition (n) | No. with follow-up | No. making an attempt | Total no. attempts | No. with follow-up | No. making an attempt | Total no. attempts | |
|
| |||||||
| ASSIP, n = 16 | 12 | 5 | 7 | 14 | 7 | 113 | |
| Control, n = 18 | 14 | 4 | 4 | 16 | 6 | 8 | |
Note. ASSIP = Attempted Suicide Short Intervention Program.
Self-report results = based on administration of Columbia-Suicide Severity Rating Scale at follow-up assessment(s).
Multi-source results = based on self-report, review of electronic medical record, and communications with study team (e.g., family, community provider) with subjects’ written consent.
Includes one fatal suicide attempt (i.e., suicide death).
Table 3.
Scale for Suicidal Ideation (SSI) – Current and Worst Point Results
| Treatment Condition | n | Mean (SD) | n | Mean (SD) |
|---|---|---|---|---|
|
| ||||
| SSI-Current at Baseline 1 | SSI-Current at 6-month follow-up 2 | |||
|
| ||||
| ASSIP | 16 | 18.75 (9.81) | 12 | 1.42 (3.40) |
| Control | 18 | 16.67 (12.93) | 14 | 6.43 (8.41) |
|
| ||||
| SSI-Worst Point at Baseline 3 | SSI-Worst Point at 6-month follow-up 4 | |||
|
| ||||
| ASSIP | 16 | 21.12 (9.32) | 12 | 7.50 (9.66) |
| Control | 18 | 21.78 (7.61) | 14 | 13.28 (11.31) |
Note. ASSIP = Attempted Suicide Short Intervention Program; SSI = Scale for Suicidal Ideation.
SSI-Current at Baseline = SSI scores in past week at baseline.
SSI-Current at 6-month follow-up = SSI scores in past week at 6-month follow-up assessment.
SSI-Worst Point at Baseline = SSI scores for worst lifetime suicidal crisis at baseline prior to past week.
SSI-Worst Point at 6-month follow-up = SSI scores for worst suicidal crisis prior to past week as assessed at 6-month follow-up.
Discussion
This three-year treatment development study achieved its goals of adapting the evidence based ASSIP therapy for delivery in hospital to adult suicide attempt patients with alcohol or drug use problems. We were able to establish the feasibility of recruiting such patients, delivering three sessions of ASSIP in their hospital rooms along with a fourth session post-discharge within an established academic medical center setting, and doing so with fidelity to the therapy. ASSIP subjects also reported high satisfaction and working alliance with their study therapist. The feasibility of retaining subjects over 6-mont follow-up was also demonstrated. Study outcomes were assessed using gold standard measures of suicide reattempt (C-SSRS) and suicidal ideation (SSI), along with the innovative use of a multi-source method to identify suicide reattempts missed by self-report. Suicide reattempt over 6-month follow-up was common in the study sample including in the ASSIP group (see Table 2). However, the descriptive results for change in suicidal ideation in the ASSIP therapy group at 6- month follow-up appear more encouraging (see Table 3). There is precedence for lowering suicidal ideation using brief, suicide-focused interventions [36], and further study of ASSIP in reducing suicidal ideation seems warranted.
There were limitations of the study. As a small, underpowered RCT with short period of follow-up, formal tests of comparison between the study groups were not performed. Accordingly, the results concerning the study outcomes are descriptive and should be interpreted with caution. The added safety features including the use of telephone check-ins during the first month post-discharge provided added support to both controls and ASSIP patients, potentially reducing group differences. Although we delivered the bulk of the therapy in hospital to take advantage of a teachable moment, it could be that delivering it in such a short period of time reduced its potency. The large number of suicide reattempts in the sample, along with suicide deaths over the course of the project, warrant further comment. First and foremost, it was a high-risk sample of suicide attempt patients. Our goal to complete the first three sessions of ASSIP in hospital necessitated recruiting subjects with sufficient length of stay to fulfill this agenda, complicated by the need for stabilization and uncertainty early in the hospital course about projected length of stay. Accordingly, subjects typically had lengthy periods of hospitalization and a signal that clinical staff had significant concerns about patients’ safety. These safety concerns included risk for suicidal behavior related to: 1) history of trauma and/or interpersonal violence, ongoing housing instability, legal charges, unemployment, or interpersonal tensions; 2) fallout from the suicide attempts including shame, disruptions to one’s interpersonal network, or lingering cognitive and other medical effects of large overdoses; 3) histories of accessing information about lethal agents and ordering such agents through the internet; and 4) challenges accessing appropriate treatment or in adhering to such treatment. For example, the suicide decedent in the RCT had marked difficulties in all four of these areas of safety. Our eligibility requirement of alcohol- or drug-related problems also increased risk.
The high-risk nature of the study population suggests that additional measures beyond a brief intervention may be needed to lower the risk for repetition of suicidal behavior. Additionally, the most common reason for exclusion was brief lengths of stays because the 3-session ASSIP intervention could not be completed in hospital, a limiting factor for the purpose of dissemination. An alternative strategy would be to complete the first ASSIP session in hospital, in line with a teachable moment, but to continue the therapy following discharge on an outpatient basis which is consistent with the original ASSIP and its weekly sessions. This approach could accommodate most short stay patients and was used with a subset of ASSIP cases in the seminal RCT which showed a large effect in reducing risk for suicide reattempt [17]. However, disseminating such a strategy is challenging in the U.S. context where there is typically a hard distinction between staff providing inpatient care and those providing outpatient care, and it would require more flexible approaches because continuity of ASSIP with the same therapist is essential to the therapeutic alliance, a likely mechanism of the therapy [37].
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | ASSIP (n = 16) | Control (n = 18) |
|---|---|---|
|
| ||
| Female1 | 12 (75%) | 10 (63%) |
| Minority race or ethnicity2 | 6 (38%) | 8 (44%) |
| Age, mean (SD) | 42.8 (15.2) | 38.4 (17.8) |
| Any post-high school education | 10 (63%) | 6 (33%) |
| Employed full-time or part-time | 5 (31%) | 7 (39%) |
| Married or living with partner | 5 (31%) | 5 (28%) |
| One or more children | 8 (50%) | 10 (63%) |
| Veteran of the military | 1 (6%) | 3 (17%) |
| Multiple (2+) prior suicide attempts3 | 9 (56%) | 9 (50%) |
| What percentage of you wanted to die (in attempt)? mean (SD) | 89.4 (16.4) | 79.4 (26.2) |
| AUDIT score, mean (SD) | 15.3 (12.6) | 12.4 (10.3) |
| Alcohol use related problems4 | 10 (63%) | 11 (61%) |
| DAST-105 score, mean (SD) | 3.2 (3.2) | 4.2 (3.7) |
| Non-medical drug use related problems5 | 10 (63%) | 12 (67%) |
| PHQ-9 score, mean (SD) | 21.1 (2.3) | 17.4 (6.3) |
| Days hospitalized, mean (SD) | 15.9 (10.7) | 15.6 (8.1) |
| Days to study enrollment, mean (SD) | 4.2 (3.1) | 5.6 (4.5) |
| Recruited on a medical floor (vs. psychiatric unit) | 7 (44%) | 8 (44%) |
| Mood disorder diagnosis at hospital discharge | 14 (88%) | 15 (83%) |
| Anxiety disorder diagnosis at hospital discharge | 3 (19%) | 7 (39%) |
Notes. n (%) shown unless otherwise indicated. ASSIP = Attempted Suicide Short Intervention Program; AUDIT = Alcohol Use Disorders Identification Test; DAST-10 = Drug Abuse Screening Test (10-item); PHQ-9 = Patient Health Questionnaire (9-item).
All subjects self-identified as female or male.
Minority race or ethnicity includes: 8 Black subjects (4 ASSIP, 4 Control), 4 Multiracial (2 ASSIP, 2 Control), 1 American Indian/Alaskan Native (Control), and 2 Hispanic/Latinx (1 ASSIP, 1 Control).
Multiple attempt history (2+) was a stratification variable for treatment assignment.
Alcohol-related problems based on AUDIT score ≥8.
Non-medical drug use related problems based on DAST-10 score ≥2.
Acknowledgments
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (1R34AA02601601; Conner, PI). Clinical Trial Registration – Registry: ClinicalTrials.gov, Identifier: NCT03300596, Title: Suicidal Adults with Alcohol or Drug Use Problems: A New Hospital-based Treatment, URL: https://clinicaltrials.gov/ct2/show/NCT03300596
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Centers for Disease Control. Reporting System (WISQARS). Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. 2021. [Google Scholar]
- [2].Centers for Disease Control. WISQARS Self-harm All Injury Causes Nonfatal Emergency Department Visits and Rates per 100,000, 2019., 2021. [Google Scholar]
- [3].Chung DT, Ryan CJ, Hadzi-Pavlovic D, Singh SP, Stanton C and Large MM. Suicide rates after discharge from psychiatric facilities: a systematic review and meta-analysis. JAMA psychiatry 2017;74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boudreaux ED, Bock B and O’Hea E. When an event sparks behavior change: an introduction to the sentinel event method of dynamic model building and its application to emergency medicine. Academic Emergency Medicine 2012;19:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Connor SS, Mcclay MM, Choudhry S, Shields AD, Carlson R, Alonso Y, et al. Pilot randomized clinical trial of the Teachable Moment Brief Intervention for hospitalized suicide attempt survivors. General hospital psychiatry 2020;63:111–118. [DOI] [PubMed] [Google Scholar]
- [6].Conner KR and Bagge CL. Suicidal behavior: links between alcohol use disorder and acute use of alcohol. Alcohol research: current reviews 2019;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Conner KR, Bridge JA, Davidson DJ, Pilcher C and Brent DA. Metaanalysis of mood and substance use disorders in proximal risk for suicide deaths. Suicide and Life-Threatening Behavior 2019;49:278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilcox HC, Conner KR and Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug and alcohol dependence 2004;76:S11–S19. [DOI] [PubMed] [Google Scholar]
- [9].Bohnert AS and Ilgen MA. Understanding links among opioid use, overdose, and suicide. New England journal of medicine 2019;380:71–79. [DOI] [PubMed] [Google Scholar]
- [10].Bryan CJ, Garland EL and Rudd MD. From impulse to action among military personnel hospitalized for suicide risk: Alcohol consumption and the reported transition from suicidal thought to behavior. General hospital psychiatry 2016;41:13–19. [DOI] [PubMed] [Google Scholar]
- [11].Conner KR, Gamble SA, Bagge CL, He H, Swogger MT, Watts A, et al. Substance-induced depression and independent depression in proximal risk for suicidal behavior. Journal of studies on alcohol and drugs 2014;75:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ries RK, Yuodelis-Flores C, Comtois KA, Roy-Byrne PP and Russo JE. Substance-induced suicidal admissions to an acute psychiatric service: characteristics and outcomes. Journal of substance abuse treatment 2008;34:72–79. [DOI] [PubMed] [Google Scholar]
- [13].Suokas J and Lönnqvist J. Suicide attempts in which alcohol is involved: a special group in general hospital emergency rooms. Acta Psychiatrica Scandinavica 1995;91:36–40. [DOI] [PubMed] [Google Scholar]
- [14].Suominen K and Lönnqvist J. Determinants of psychiatric hospitalization after attempted suicide. General hospital psychiatry 2006;28:424–430. [DOI] [PubMed] [Google Scholar]
- [15].Rounsaville BJ, Carroll KM and Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice 2001;8:133–142. [Google Scholar]
- [16].Michel K and Gysin-Maillart A. ASSIP–Attempted Suicide Short Intervention Program: A Manual for Clinicians. Hogrefe Publishing, 2015. [Google Scholar]
- [17].Gysin-Maillart A, Schwab S, Soravia L, Megert M and Michel K. A novel brief therapy for patients who attempt suicide: A 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS medicine 2016;13:e1001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Conner KR, Wiegand TJ and Goldston DB. A hospital-based treatment of suicide attempt patients with problematic alcohol use: Rationale and treatment development. General hospital psychiatry 2020;63:30–32. [DOI] [PubMed] [Google Scholar]
- [19].Conner KR, Goldston DA, Kearns JC, Pizzarello E and Gysin-Maillart A. Therapist fidelity rating procedure for the Attempted Suicide Short Intervention Program (ASSIP). Poster presented at the International Aeschi Suicide Research and Practice Meeting. Vail, CO, 2019. [Google Scholar]
- [20].Britton PC, Conner KR and Maisto SA. A Procedure for Changing a Behavioral Health Treatment During a Trial, with Case Example in Suicide Prevention. Archives of Suicide Research 2020;24:285–300. [DOI] [PubMed] [Google Scholar]
- [21].Reinert DF and Allen JP. The alcohol use disorders identification test: an update of research findings. Alcoholism: Clinical and Experimental Research 2007;31:185–199. [DOI] [PubMed] [Google Scholar]
- [22].Bagge CL, Glenn CR and Lee H-J. Quantifying the impact of recent negative life events on suicide attempts. Journal of abnormal psychology 2013;122:359. [DOI] [PubMed] [Google Scholar]
- [23].Corcoran K and Fischer J. Client Satisfaction Questionnaire (CSQ-8). Measures for Clinical Practice. New York, NY: The Free Press, 1987. [Google Scholar]
- [24].Munder T, Wilmers F, Leonhart R, Linster HW and Barth J. Working Alliance Inventory-Short Revised (WAI-SR): psychometric properties in outpatients and inpatients. Clinical Psychology & Psychotherapy: An International Journal of Theory & Practice 2010;17:231–239. [DOI] [PubMed] [Google Scholar]
- [25].Shaw BF, Elkin I, Yamaguchi J, Olmsted M, Vallis TM, Dobson KS, et al. Therapist competence ratings in relation to clinical outcome in cognitive therapy of depression. Journal of consulting and clinical psychology 1999;67:837. [DOI] [PubMed] [Google Scholar]
- [26].Stanley B and Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cognitive and behavioral practice 2012;19:256–264. [Google Scholar]
- [27].Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. American journal of psychiatry 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mundt JC, Greist JH, Gelenberg AJ, Katzelnick DJ, Jefferson JW and Modell JG. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. Journal of psychiatric research 2010;44:1224–1228. [DOI] [PubMed] [Google Scholar]
- [29].Swain RS, Taylor LG, Braver ER, Liu W, Pinheiro SP and Mosholder AD. A systematic review of validated suicide outcome classification in observational studies. International journal of epidemiology 2019;48:1636–1649. [DOI] [PubMed] [Google Scholar]
- [30].Beck AT, Kovacs M and Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology 1979;47:343. [DOI] [PubMed] [Google Scholar]
- [31].Pearson JL, Stanley B, King CA and Fisher CB. Intervention research with persons at high risk for suicidality: safety and ethical considerations. Journal of Clinical Psychiatry 2001;62:17–26. [PubMed] [Google Scholar]
- [32].Kraemer HC, Mintz J, Noda A, Tinklenberg J and Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of general psychiatry 2006;63:484–489. [DOI] [PubMed] [Google Scholar]
- [33].Cocco KM and Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychological Assessment 1998;10:408. [Google Scholar]
- [34].Saunders JB, Aasland OG, Babor TF, De La Fuente JR and Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88:791804. [DOI] [PubMed] [Google Scholar]
- [35].Kroenke K, Spitzer RL and Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Britton PC, Conner KR, Chapman BP and Maisto SA. Motivational interviewing to address suicidal ideation: a randomized controlled trial in veterans. Suicide and Life-Threatening Behavior 2020;50:233–248. [DOI] [PubMed] [Google Scholar]
- [37].Ring M and Gysin-Maillart A. Patients’ satisfaction with the therapeutic relationship and therapeutic outcome is related to suicidal ideation in the Attempted Suicide Short Intervention Program (ASSIP). Crisis: The Journal of Crisis Intervention and Suicide Prevention 2020. [DOI] [PubMed] [Google Scholar]