Abstract
Introduction:
Labeling single domain antibody fragments (sdAbs) with 18F is an attractive strategy for immunoPET. Earlier, we developed a residualizing label, N-succinimidyl 3-((4-(4-fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate ([18F]RL-I), synthesized via a click reaction for labeling sdAbs with 18F, that has attractive features but suffered from modest radiochemical yields and suboptimal hydrophobicity. Herein, we have evaluated the potential utility of an analogous agent, N-succinimidyl 3-(1-(2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)-5-(guanidinomethyl)benzoate ([18F]SFETGMB; [18F]RL-III) designed to address these limitations.
Methods:
[18F]RL-III was synthesized by the click reaction between 3-((2,3-bis(tert-butoxycarbonyl)guanidino)methyl)-5-ethynylbenzoate and 1-azido-2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethane and subsequent deprotection. The anti-HER2 sdAbs 5F7 and 2Rs15d were labeled by conjugation with [18F]RL-III and compared in a paired-label fashion to the sdAbs labeled using N-succinimidyl 4-guanidinomethyl-3-[125I]iodobenzoate ([125I]SGMIB) or N-succinimidyl 3-guanidinomethyl-5-[125I]iodobenzoate (iso-[125I]SGMIB). The 18F-labeled sdAbs were evaluated in vitro using HER2-expressing breast and ovarian carcinoma cells (BT474/BT474M1 and SKOV-3) and in vivo in athymic mice bearing subcutaneous SKOV-3 or BT474 xenografts. PET imaging of athymic mice bearing either subcutaneous BT474 or intracranial BT474M1Br-Fluc xenografts after administration of [18F]RL-III-5F7 also was performed.
Results:
Radiochemical yields for the synthesis of protected Boc2-[18F]RL-III (21.5 ± 3.4%) were significantly higher than reported for protected Boc2-[18F]RL-I. The overall radiochemical yields for the synthesis of [18F]RL-III-2Rs15d and [18F]RL-III-5F7 from aqueous [18F]fluoride were 1.7 ± 0.7% and 3.8 ± 2.3%, respectively. Both sdAbs, labeled using [18F]RL-III, retained affinity and immunoreactivity to HER2. Uptake and internalization of [18F]RL-III-5F7 in HER2-expressing cells was higher than that seen for [18F]RL-III-2Rs15d. Although different xenograft models were used, [18F]RL-III-2Rs15d showed relatively high uptake in a number of normal tissues, while uptake of [18F]RL-III-5F7 was mainly in tumor and kidneys with minimal background activity. Concordant with the necropsy experiments, microPET imaging with [18F]RL-III-5F7 in the BT474 subcutaneous model demonstrated clear delineation of the tumor (12.2 ± 5.1% ID/g) with minimal background activity except in kidneys. A tumor uptake (max) of 0.98 %ID/g and a tumor-to-normal brain ratio of 9.8:1 were observed for [18F]RL-III-5F7 in the intracranial model.
Conclusions:
Although higher radiochemical yields than that reported for [18F]RL-I were obtained, considerable improvements are needed for this method to be of practical utility. Despite clear tumor delineation with [18F]RL-III-5F7 as early as 1 h, high activity levels in the kidneys remain a concern.
Keywords: Single domain antibody fragment, nanobody, HER2, fluorine-18, breast cancer
Graphical Abstract

Introduction
Because only about 20–25% of breast cancers express the tyrosine kinase receptor HER2, it is imperative to assess the HER2 expression level in a patient before administering a HER2-targeted therapy [1, 2]. Standard methods for determining HER2 status — immunohistochemistry and fluorescence in situ hybridization — are performed on biopsy samples [3] and do not provide global HER2 status assessment in real-time. Moreover, variability in HER2 expression between primary and metastatic cancers frequently occurs and changes with time [4, 5]. This has motivated the investigation of various PET-based and other molecular imaging methods as more informative alternatives for assessing HER2 status in tumors [6–11].
Single domain antibody fragments (sdAbs) are becoming an important tool kit for the diagnosis and therapy of cancers [12–14]. Unlike small molecules, sdAbs exhibit exquisite target specificity and show little off-target side effects [15]. Indeed, a case can be made that radiolabeling of small molecules, for example anti-HER2 tyrosine kinase inhibitors, is much more challenging and requires a specific labeling strategy for each molecule [16]. Lower metabolic stability of most labeled small molecules, compared with sdAbs, necessitates kinetic modeling for the labeled analogues in order to account for labeled metabolites. Earlier, we evaluated two anti-HER2 sdAbs labeled with 18F using a novel residualizing agent for assessment of HER2 status by PET imaging [17, 18]. This prosthetic agent was designed to incorporate a guanidine moiety based on the favorable residualization characteristics and in vivo behavior of sdAbs radioiodinated using N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate ([*I]SGMIB) and its isomer iso-SGMIB (Chart 1) [19, 20]. Because the radiochemical synthesis of an analogue wherein the iodine in SGMIB/iso-SGMIB is replaced with fluorine using the classical SNAr reaction would be challenging [21, 22], an alternative approach was utilized to synthesize the 18F residualizing agent [18F]RL-I [23].
Chart 1.

Structures of SGMIB, iso-SGMIB, RL-I and RL-III.
The critical step in the synthesis of [18F]RL-I involved a copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click reaction between an active ester prosthetic moiety containing both the guanidine and azido groups with 6-[18F]fluoro-1-hexyne. We chose 6-fluoro-1-hexyne for this purpose because of the difficulty in handling highly volatile fluoroalkynes of fewer carbons; even 6-fluoro-1-hexyne is very volatile. However, its high carbon content imparts unwanted hydrophobicity to the molecule. To counter this, we initially contemplated a click reaction between the guanidine-bearing prosthetic moiety containing an alkyne instead of an azido group as in RL-I and the commonly used two-carbon [18F]fluoro azido alkane, [18F]fluoroethyl azide [24]. Although its carbon content is low, this molecule also suffers from high volatility. An on-cartridge click reaction has been reported that makes [18F]fluoroethyl azide synthesis facile [25]; however, we obtained only very low radiochemical yields when this on-cartridge click reaction was adapted for our alkyne-containing agent noted above (see also below). Thus, we resorted to a nonvolatile and more hydrophilic fluoro-PEG-azide (1-azido-2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethane) in the hope that it might result in better radiochemical yields, higher tumor uptake and more favorable biodistribution of the final labeled sdAb.
In this work, we describe the synthesis a new 18F residualizing agent — N-succinimidyl 3-(1-(2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)-5-(guanidinomethyl)benzoate ([18F]SFETGMB; [18F]RL-III; Chart 1). It was derived by the click reaction of an active ester compound containing both guanidine and alkyne functions (4, Scheme 1) with 1-azido-2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethane [26, 27], a nonvolatile, hydrophilic azide. Two anti-HER2 sdAbs, 2Rs15d and 5F7, were labeled using [18F]RL-III and the labeled sdAb conjugates were evaluated in HER2 expressing cell and xenograft models.
Scheme 1.

a) Tributyl(ethynyl)stannane, (Ph3P)4Pd, THF b) i. MsCI, TEA, DCM ii. Bis-Boc-guanidine, KOtBu, DMF c) i. TBAF, THF ii. N-hydroxysuccinimide, EDC, DMAP, DCM d) 1-azido-2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethane, bathophenanthroline sulfonic acid di-sodium sait, CuSO4.5H2O, sodium ascorbate, DMF/H2O e) TFA
2. Materials and methods
2.1. General
All reagents were obtained from Sigma-Aldrich unless otherwise noted and used as such. Sodium [125I]iodide in 0.1 N NaOH with a molar activity of 81.4 TBq/mmol was purchased from Perkin-Elmer (Waltham, MA). The compound 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethan-1-ol was purchased from 1-Click Chemistry (Kendall Park, NJ). Compounds 1-azido-2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethane [28] and 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate [29] were synthesized as reported. Synthesis of Boc-protected tin precursors of SGMIB and iso-SGMIB, [125I]SGMIB and iso-[125I]SGMIB [19, 20, 30] as well as the labeling of the sdAbs using [125I]SGMIB and iso-[125I]SGMIB [17, 31] were performed following published methods. Synthesis of 2-(trimethylsilyl)ethyl 3-(hydroxymethyl)-5-iodobenzoate (1) was accomplished using the procedure described earlier [19]. Aluminum-backed silica gel sheets (Silica gel 60 F254) for analytical TLC were obtained from EM Science (Gibbstown, NJ). Chromatographic purification of nonpolar organic compounds was performed with the Biotage Isolera chromatography system (Charlotte, NC) using their pre-packed silica gel columns. Preparative thick layer chromatography, used for small-scale purification, was performed using thick layer silica gel plates obtained from Whatman (Clifton, NJ) or EM Science. High-performance liquid chromatography (HPLC) was conducted using an Agilent HPLC system equipped with a 1260 Infinity quaternary pump and a multiple wavelength detector (Agilent Technologies, Foster City, CA) interfaced with a ScanRam RadioTLC scanner/HPLC detector combination (LabLogic; Brandon, Fl). Normal phase HPLC, used for purification of Boc2-[18F]SFETGMB (Boc2-RL-III), was performed using a Zorbax RX-SIL (9.4 × 250 mm; 5 μm) semi-preparative column (Agilent Technologies, Santa Clara, CA). PD-10 desalting columns for gel filtration were purchased from GE Healthcare (Piscataway, NJ). SDS-PAGE was run under non-reducing conditions followed by phosphor imaging using the Storage Phosphor System Cyclone Plus phosphor imager (Perkin-Elmer, Downers Grove, IL). Activity in various samples was assessed using a CRC-7 dose calibrator (Capintec, Pittsburgh, PA) for higher activity levels and automated gamma counters — either an LKB 1282 (Wallac, Finland) or a Perkin Elmer Wizard II (Shelton, CT) — for lower activity levels. The NanoDrop™ Spectrophotometer (Thermofisher, Waltham, MA) was used to determine the concentration of unlabeled and radiolabeled sdAbs. Proton and carbon-13 NMR spectra of samples were obtained on a Varian/Agilent Inova (Palo Alto, CA) 400 MHz or 500 MHz spectrometers, and chemical shifts are reported in δ units using the residual solvent peaks as reference. Mass spectra of synthesized compounds were obtained using an Advion ExpressionL compact mass spectrometer for electrospray ionization (ESI) LC/MS and/or an Agilent LCMS-TOF (ESI) mass spectrometer; high-resolution mass spectra were obtained using the latter system.
2.2. Single domain antibody fragments, cells, and culture conditions
The anti-HER2 sdAb 2Rs15d was obtained as a generous gift from Dr. Matthias D’Huyvetter, Vrije Universiteit Brussel, Brussels, Belgium. Details of its production, purification, and characterization have been described [32, 33]. The sdAb 5F7, recognizing a different domain on the HER2 molecule, was selected from phage libraries derived from llamas immunized with SKBR-3 human breast carcinoma cells [34] and was purchased from ATUM (Newark, CA). Both sdAbs were devoid of His-tags or other C-terminal modifications and were confirmed to be present exclusively as monomers by SDS-PAGE. All reagents used for cell studies were obtained from Invitrogen (Grand Island, NY). HER2-expressing SKOV-3 human ovarian carcinoma, BT474 and BT474M1 human breast carcinoma cells were used in this study [17, 31, 35]. McCoy’s 5A medium containing 10% fetal bovine serum and 1% penicillin-streptomycin was used for culturing SKOV-3 cells and RPMI 1640 medium containing 10% fetal bovine serum, 1% Penicillin-Streptomycin, 5 μg/ml plasmocin, 1% sodium pyruvate, 1% HEPES, and 0.4 μg/ml insulin was used for culturing BT474 and BT474M1 cells. Cells were cultured at 37°C in a humidified incubator under 5% CO2 with media changed every two days. When about 80% confluent, cells were sub-cultured by dissociation using TryLE Express Enzyme (Thermo Fisher Scientific, Waltham, MA).
2.3. Chemistry
2.3.1. Synthesis of 2-(trimethylsilyl)ethyl 3-ethynyl-5-(hydroxymethyl)benzoate (2)
A solution of 2-(trimethylsilyl)ethyl 3-(hydroxymethyl)-5-iodobenzoate [19] (1; 1.1 g, 2.91 mmol), tributyl(ethynyl)stannane (Matrix Scientific, Columbia, SC; 1.37 g, 4.36 mmol) in 50 mL of anhydrous THF was heated to reflux under argon. Tetrakis(triphenylphosphine)palladium(0) (0.3 g, 0.29 mmol) was added and the mixture was heated at reflux temperature for 4 h under argon. The reaction mixture was cooled to 0–5 °C, filtered through a Celite bed, which was washed with THF. The filtrate was evaporated to dryness, and the resultant crude oil purified by chromatography using a 25 g SNAP ULTRA column (Biotage) and 5:1 hexanes/ethyl acetate as the mobile phase to yield 665 mg (2.41 mmol, 83% yield) of a clear oil: 1H-NMR (400 MHz, C2HCl3) δH 8.06 (s,1H), 7.99 (s,1H), 7.68 (s, 1H), 4.73 (s, 2H), 4.45 – 4.38 (t, J = 8.6, 2H), 3.12 (s, 1H), 1.17 – 1.11 (t, J = 8.6, 2H), 0.90 (s, 9H). 13C-NMR (125 MHz, C2HCl3) δ 165.8, 141.5, 134.3, 132.3, 131.3, 128.0, 122.7, 82.5, 78.1, 64.3, 63.6, −1.5. HRMS (ESI): Calcd for C15H24NO3Si (M+NH4)+: 294.1525. Found: 294.1522.
2.3.2. Synthesis of 2-(trimethylsilyl)ethyl 3-((1,2-bis(tert-butoxycarbonyl)guanidino)methyl)-5-ethynylbenzoate (3)
A solution of 2 (660 mg, 2.39 mmol) and triethylamine (0.5 mL, 3.58 mmol) in 50 mL dichloromethane was cooled to 0–5°C, and methane sulfonyl chloride (0.28 mL, 3.58 mmol) was added. The mixture was stirred at 20°C for 4 h and then washed with brine. The dichloromethane layer was dried with anhydrous sodium sulfate, filtered, and the filtrate evaporated to dryness. The potassium salt of 1,3-bis(tert-butoxycarbonyl)guanidine was prepared as follows: a solution of potassium tert-butoxide in THF (1M; 3.58 mL, 3.58 mmol) was added to a solution of 1,3-bis(tert-butoxycarbonyl)guanidine (743 mg, 2.87 mmol) in 35 mL of anhydrous DMF. After 30 min, this solution was added to the crude mesylate prepared as described above, and the resultant mixture stirred at 20°C overnight under argon. The reaction mixture was quenched with saturated sodium bicarbonate solution, and the aqueous mixture was extracted with ethyl acetate. The ethyl acetate layer was washed with saturated sodium chloride, dried over anhydrous MgSO4, and concentrated to dryness to yield an oil. It was subjected to chromatography using a 25 g Biotage SNAP Ultra column and 5:1 hexanes/ethyl acetate as the mobile phase to afford 812 mg (1.57 mmol, 65.7 % yield) of a low melting solid. 1H-NMR (400 MHz, C2HCl3) 9.47 (bs, 1H), 9.29 (bs, 1H), 8.04 (s, 1H), 7.93 (s, 1H), 7.64 (s, 1H), 5.18 (s, 2H), 4.43 – 4.39 (t, J = 8.6, 2H), 3.10 (s, 1H), 1.50 (s, 9H), 1.37 (s, 9H), 1.48 – 1.11 (t, J = 8.6, 2H), 0.79 (s, 9H). 13C-NMR (125 MHz, C2HCl3) δ 165.8, 163.6, 160.5, 154.6, 139.5, 135.3, 131.9, 130.9, 128.9, 122.4, 84.6, 82.9, 79.0, 77.9, 63.6, 46.9, 28.3, 27.9, 17.4, −1.5. LRMS (ESI): m/z 518 (M+H)+. HRMS (ESI): Calcd for C26H40N3O6Si (M+H)+: 518.2686. Found: 518.2685.
2.3.3. Synthesis of N-succinimidyl 3-((2,3-bis(tert-butoxycarbonyl)guanidino)methyl)-5-ethynylbenzoate (4)
TBAF in THF (1M; 1.7 mL, 1.7 mmol) was added to a solution of 3 (800 mg; 1.55 mmol) in 30 mL of THF at 0–5°C, and the mixture stirred overnight under argon. THF was evaporated and the residual material was partitioned between ethyl acetate and water. The ethyl acetate layer was dried over anhydrous MgSO4, and the ethyl acetate from the filtrate was evaporated. The crude product was taken in dichloromethane (50 mL), and N-hydroxysuccinimide (267 mg, 2.32 mmol), DMAP (21 mg, 0.17 mmol), and EDC (444 mg, 2.32 mmol) were added in that order, and the mixture stirred at 20°C overnight. The reaction mixture was washed with water followed by saturated sodium bicarbonate. The organic layer was dried over MgSO4, filtered, and dichloromethane was evaporated from the filtrate. The crude material was chromatographed using a Biotage 25 g SNAP ULTRA column and 2:1 hexanes/ethyl acetate as the mobile phase to obtain 352 mg (0.68 mmol, 44.3% yield) of a tan solid: 1H-NMR (400 MHz, C2HCl3) 9.44 (bs, 1H), 9.25 (bs, 1H), 8.14 (s, 1H), 8.05 (s, 1H), 7.80 (s, 1H), 5.20 (s, 2H), 3.16 (s, 1H), 2.92 (s, 4H),1.50 (s, 9H), 1.41 (s, 9H). 13C-NMR (125 MHz, C2HCl3) δ 169.2, 161.3, 154.8, 140.4, 137.9, 133.0, 130.2, 125.6, 123.4, 85.2, 82.0, 79.2, 66.1, 47.0, 28.5, 28.2, 25.9. LRMS (ESI): m/z 537.2 (M+Na)+, 515.2 (M+H)+, 459.2, 403.1, 359.1, 315.1. HRMS (ESI): Calcd for C25H30N4O8 (M+H)+: 514.2064. Found: 514.2132 ± 0.0001 (n =4).
2.3.4. Synthesis of N-succinimidyl 3-((1,3-bis(tert-butoxycarbonyl)guanidino)methyl)-5-(1-(2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)benzoate (Boc2-RL-III)
The click reaction reagent was prepared as follows: Solutions of copper (II) sulfate pentahydrate (10 mg, 0.04 mmol) and sodium ascorbate (30 mg, 0.15 mmol) each in 100 μL water were mixed. When the color of this mixture changed from black to yellow, a solution of bathophenanthrolinedisulfonic acid disodium salt hydrate (12 mg, 20.0 μmol) in 200 μL of 1:4 (v/v) DMF/H2O was added. A 100 μL aliquot of this click reaction reagent was added to a solution of 4 (30 mg, 0.06 mmol) and 1-azido-2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethane (51.6 mg, 0.23 mmol) in 1 mL of DMF and the mixture was stirred at 20°C overnight. The reaction mixture was partitioned between ethyl acetate and water. The ethyl acetate layer was dried with anhydrous MgSO4, and the ethyl acetate was evaporated from the filtrate. The crude product was purified by preparative TLC using 1:1 hexanes:ethyl acetate as the mobile phase to obtain 23 mg (0.03 mmol, 53.4%) of an off-white solid: 1H-NMR (400 MHz, C2HCl3) 9.48 (bs, 2H), 8.41 (s, 1H), 8.28 (s, 1H), 8.09 (s, 1H), 8.03 (s, 1H), 5.28 (s, 2H), 4.62 – 4.57 (m, 3H), 4.47 – 4.52 (m, 1H), 3.95 – 3.92 (m, 2H), 3.74 – 3.65 (m, 10H), 2.92 (s, 4H), 1.50 (s, 9H), 1.41 (s, 9H). 13C-NMR (125 MHz, C2HCl3) δ 171.3, 169.3, 163.8, 162.6, 161.8, 160.7, 154.9, 146.2, 140.8, 132.1, 131.5, 129.1, 126.4, 125.9, 121.9, 85.1, 84.0, 82.6, 79.2, 71.0, 70.8, 70.7, 69.6, 60.6, 50.7, 47.3, 29.9, 28.5, 28.1, 25.9. LRMS (ESI): m/z 774.3 (M+K)+, 758.3 (M+Na)+, 736.3 (M+H)+, 636.3 (M-Boc+H)+. HRMS (ESI): Calcd for C33H47FN7O11 (M+H)+ 736.3318; Found 736.3313 ± 0.0005 (n = 4).
2.3.5. Synthesis of N-succinimidyl 3-(1-(2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)-5-guanidinomethyl benzoate (RL-III)
TFA (200 μL) was added to a solution of Boc2-RL-III (50 mg, 68 μmol) in dichloromethane (5 mL), and the resultant solution was reacted at room temperature for 18 h. The volatiles were evaporated, and the residual solid was triturated with ether to obtain an orange solid. This solid was recrystallized using isopropanol to render 12 mg (27% - based on the trifluoroacetate salt) of RL-III as an orange-red solid: 1H-NMR (500 MHz, C2H3O2H) 8.60 (s,1H), 8.53 (s,1H), 8.28 (s,1H), 8.09 (s, 1H), 4.69 (m, 2H), 4.62 (bs, 2H), 4.45 (dm, 2H), 3.98 (m, 2H), 3.69–3.64 (m, 10H), 2.95 (s, 4H). 13C-NMR (125 MHz, C2H3O2H) δ 172.1, 163.2, 147.0, 140.7, 134.1, 131.6, 129.4, 128.3, 127.9, 124.5, 85.1, 83.7, 71.9, 71.8, 71.7, 70.5, 67.2, 52.0, 45.6, 26.9. HRMS (ESI): Calcd for C23H31FN7O7 (M+H)+ 536.2264; Found 536.2270.
2.4. Radiochemistry
2.4.1. Synthesis of N-succinimidyl 3-((1,3-bis(tert-butoxycarbonyl)guanidino)methyl)-5-(1-(2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)benzoate (Boc2-[18F]RL-III)
Fluorine-18 was produced in house as described before [36] or obtained from PET-NET (Durham, NC). The [18O]water containing 18F activity (~4 GBq) was passed through a QMA light cartridge (Waters) and the trapped activity was eluted with a solution of potassium carbonate (2.4 mg) and 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (13.2 mg) in 1 mL of 80% acetonitrile in water. The solvents were evaporated with a gentle stream of argon while heating the vial at 110°C. The activity was further dried by the addition and evaporation of 1 mL of acetonitrile three times. The precursor 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate (5 μL, ~8.0 mg, 0.02 mmol) and anhydrous acetonitrile (1 mL) were added to the vial containing the dried 18F-activity, and the vial was capped and heated at 100 °C for 20 min. The reaction mixture was cooled to 0–5°C, and 5 mL ice-cold water was added. The resultant mixture was extracted with 5 mL ether, and the ethereal layer was washed with 5 mL of water, and passed through an anhydrous Na2SO4 cartridge (BondElut, Agilent), and concentrated to dryness with a stream of argon. A solution of N-succinimidyl 3-((2,3-bis(tert-butoxycarbonyl)guanidino)methyl)-5-ethynylbenzoate (4; 4 mg, 7.8 μmol) in 0.2 mL of DMF was added to the above radioactivity. Freshly prepared click reaction reagent (vide supra; 0.1 mL) was added to the above vial, and the capped vial heated at 40–50°C for 20 min. Ethyl acetate (5 mL) was added to the reaction mixture, and the resultant solution was washed with brine (5 mL). The ethyl acetate layer was passed through an anhydrous Na2SO4 cartridge and concentrated to dryness using a gentle stream of argon. The product was purified by normal phase HPLC. For this, the Zorbax RX-SIL column was eluted in isocratic mode with 100% EtOAc at a flow rate of 3.0 mL/min. Under these conditions, Boc2-[18F]RL-III eluted with a retention time of 12.1 min. The HPLC fractions containing Boc2-[18F]RL-III were pooled, transferred to a 1-dram vial, and concentrated to dryness.
2.4.2. Synthesis of amino((3-(((2,5-dioxopyrrolidin-1-yl)oxy)carbonyl)-5-(1-(2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)benzyl)amino)methaniminium ([18F]RL-III)
Deprotection of Boc2-[18F]RL-III to provide [18F]RL-III was achieved by addition of trifluoroacetic acid (TFA; 100 μL) to the above residual activity and reacting at room temperature for 10 min. TFA was evaporated, and addition of 50 μL of ethyl acetate and its evaporation was repeated thrice to ensure complete removal of TFA.
2.4.3. Labeling sdAbs using [18F]RL-III
Having an active N-succinimidyl ester, [18F]RL-III was conjugated to sdAbs under the standard conditions used for this classic conjugation reaction [23, 37–39]. A solution of 2Rs15d or 5F7 sdAb in borate buffer, pH 8.5 (100 μg in 50 μL), was added to dried [18F]RL-III above (0.02 – 0.41 GBq), and the mixture was incubated at 20°C for 20 min. The [18F]RL-III-sdAb conjugates were isolated by gel filtration over a PD10 column that had been preconditioned with human serum albumin (HSA) to minimize nonspecific binding, and eluted with PBS as reported before [31, 40]. For determining the molar activity of labeled sdAbs, the radioactivity level in an aliquot of the purified sample was measured using a Capintec dose calibrator and the mass of sdAb in the sample was determined using the NanoDrop. By entering the molar extinction coefficient and molecular weight of the sdAb, the built-in program of the NanoDrop calculated the protein concentration. The molar extinction coefficient for 5F7 and 2Rs15d is 21555 M−1cm−1 and 25690 M−1cm−1, respectively. The molecular weight of the RL-III-conjugates of 5F7 and 2Rs15d is 12816 Da and 12630 Da, respectively. The molar activity, expressed as GBq/μmol, was obtained by simply dividing the radioactivity amount by the molar amount of the sdAb in the sample.
2.5. Radiochemical purity of 18F-labeled sdAbs
The purity of the 18F-labeled sdAbs was determined by SDS PAGE/phosphor imaging and in some cases, by trichloroacetic acid (TCA) precipitation as described before [18, 31, 40]. All supplies for SDS PAGE was obtained from Invitrogen/ThermoFisher Scientific (Waltham, MA). SDS PAGE was performed using NuPage 10% Bis-Tris gels and NuPage MES SDS running buffer (20X) in an Invitrogen mini gel tank. Labeled sdAbs in 25 μl PBS were mixed with 25 μl of NuPage LDS sample buffer (4X). After loading 20 μl (~11 kBq) of labeled sdAbs in duplicate lanes along with protein standards (PageRuler™ Plus Prestained Protein Ladder, 10 to 250 kDa), electrophoresis was performed at 250 V for 15 – 20 min. The gels were exposed to the phosphor plate for 1 min before imaging with Cyclone Plus scanner. The overall duration for SDS PAGE/phosphor imaging was ~30 min. A couple of batches of [18F]RL-III-2Rs15d were also analyzed by size-exclusion HPLC. For this, a TSKgel Super SW2000 4.6 mm x 30 cm column with a 4.6 mm × 3.5 cm guard column (Tosoh Bioscience, Montgomeryville, PA) was equilibrated and eluted with PBS, pH 7.0, at a flow rate of 0.3 mL/min with a run time of 25 min. Under these conditions, the labeled sdAbs eluted with a retention time of ~13 min.
2.6. Immunoreactivity and affinity of 18F-labeled sdAbs
Immunoreactivity of labeled sdAbs to HER2 was evaluated as reported [40, 41] following the Lindmo method [42] using magnetic beads attached with HER2 (positive) or HSA (negative) proteins. Saturation binding assays [40, 43] were performed to determine the binding affinity of the labeled sdAbs using HER2+ BT474M1 and/or SKOV3 cells. For this, cells seeded in 24-well plates at a density of 8 × 104 cells/well in 0.5 mL of medium were incubated at 37°C overnight. Cells were then incubated with a fresh cold medium containing increasing concentrations (0.1 to 100 nM; 0.6 mL total volume) of 18F-labeled sdAb at 4°C for 1 h. The cells were washed twice with a cold medium, lysed with 0.1% SDS, and the activity in cell lysates was counted using an automated gamma counter. Non-specific binding was determined in parallel assays performed by co-incubating cells with a 100-fold excess of unlabeled trastuzumab (for 5F7) or 2Rs15d (for 2Rs15d). The data were fitted using GraphPad Prism software (nonlinear regression, one-site binding) to determine Kd values.
2.7. Uptake and internalization of labeled sdAbs in BT474M1 cells
These assays were performed in two formats. In the first, 8 × 105 cells/well were incubated with both 18F- and 125I-labeled sdAbs at 4°C for 1 h, and then the cells were washed and incubated at 37°C for 1, 2, and 4 h. In the second, only the 18F-labeled sdAb was incubated with 8 × 105 cells/well at 37°C for 1, 2, and 4 h. In both cases, as reported before [18, 31, 44], after incubation at 1, 2, and 4 h, cell culture supernatants were removed, surface-bound activity was stripped from the cells with an acidic buffer, and finally the cells were lysed with SDS. Fluorine-18 and 125I activity in various fractions was counted in an automated gamma counter either by a paired or single label protocol. Nonspecific uptake was determined in parallel assays by co-incubating cells with trastuzumab (for labeled 5F7) or unlabeled 2Rs15d (for labeled 2Rs15d). The results are expressed as the percentage of initially bound activity after the 4°C incubation for the first format and the percentage of input activity for the second format.
2.8. Biodistribution
These studies were performed following protocols approved by the Duke University Institutional Animal Care and Use Committee and as described previously [31, 40]. Subcutaneous SKOV-3 and BT474 xenografts were established in athymic mice, and two biodistribution experiments were conducted. In the first, groups of 5 animals bearing SKOV-3 xenografts were injected with 0.15 MBq (1.2 μg) of [125I]SGMIB-2Rs15d and 0.22 MBq (0.9 μg) of [18F]RL-III-2Rs15d and biodistribution was determined at 1, 2, and 3 h. In the second study, 5 animals with BT474 xenografts were injected with 0.19 MBq (1.7 μg) of iso-[125I]SGMIB-5F7 and 0.48 MBq (4.8 μg) of [18F]RL-III-5F7 and their biodistribution determined at 2 h. Statistical significance in the difference in uptake between the two tracers was determined by a paired t-test using Microsoft Excel software; a P-value of <0.05 was considered to be significant.
2.9. MicroPET/CT imaging
2.9.1. Subcutaneous model
Imaging of BT474 subcutaneous xenograft-bearing mice was performed on a Siemens Inveon microPET/CT system (Knoxville, TN). Three mice bearing BT474 subcutaneous xenografts were evaluated 1 h, 2 h, and 3 h after administration of 2.3 – 2.5 MBq (7.4 – 7.8 μg) of [18F]RL-III-5F7. Mice were anesthetized using 2–3% isoflurane in oxygen and placed prone in the scanner gantry for a 5-min static PET acquisition followed by a 5 min CT scan. List mode PET data were histogram-processed, and the images reconstructed using the standard OSEM3D/MAP algorithm—2 OSEM3D iterations, and 18 MAP iterations—with a cutoff (Nyquist) of 0.5. Images were corrected for attenuation (CT-based) and radioactive decay. Image analysis was performed using the Inveon Research Workplace software (Siemens).
2.9.2. Intracranial model
MicroPET/CT imaging was performed also on mice with intracranial HER2-expressing xenografts. This model was established at Northwestern University as described [45] by implanting ~6 × 105 BT474M1Br-Fluc cells in the brain of athymic mice and monitoring tumor growth by bioluminescence imaging. After about 8 weeks, 1.6 MBq (43 μCi; 12 μg) of [18F]RL-III-5F7 was injected i.v. and microPET/CT imaging was performed as described. Immediately after the imaging, mice were killed, their brains were excised and placed in optimal cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA), and frozen on dry ice. The frozen brains were cut into 20-μm sections, and autoradiography was performed by placing tissue sections on a phosphor screen in a film cassette for 1 h to determine radiotracer distribution. The phosphor imaging screen was read at 300 dpi resolution with a Cyclone Plus Storage Phosphor System. In addition, adjacent sections were stained with hematoxylin and eosin to confirm xenograft location.
3. Results
3.1. Chemistry
As shown in Scheme 1, Boc2-RL-III was synthesized starting with 2-(trimethylsilyl)ethyl 3-(hydroxymethyl)-5-iodobenzoate (1) [19]. Sonogashiara coupling of 1 with tributyl(ethynyl)stannane yielded 2 in 83% yield. Compound 2 was first converted to a mesylate derivative by treatment with methane sulfonyl chloride, and the crude intermediate was reacted with the potassium salt of bis-Boc-guanidine to render 3 in 66% overall yield for the two steps. For the transesterification of 3 to 4, the TMSE ester was deprotected by treatment with TBAF, and the resultant crude tetrabutyl ammonium salt subjected to EDC-mediated esterification with N-hydroxysuccinimide; 4 was obtained from 3 in an overall yield of 44%. Finally, 4 was converted to Boc2-RL-III via CuAAC with 1-azido-2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)ethane [28] in 53% yield. The final unlabeled prosthetic agent (RL-III) was synthesized by TFA treatment of Boc2-RL-III in 27% yield. NMR and mass spectral data of the novel compounds are consistent with their structures.
3.2. Radiochemistry
All radiochemical yields reported herein were decay-corrected to the start of the synthesis following the consensus nomenclature rules developed by the EANM Drug Development Committee [46]. The labeled reagent 1-azido-2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethane was synthesized from the tosylate precursor 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate via SN2 reaction in 69.8 ± 5.3% (n=9) radiochemical yield. It was then subjected to click reaction with 4 to derive Boc2-[18F]RL-III. The overall radiochemical yield for the synthesis of Boc2-[18F]RL-III from aqueous [18F]fluoride was 21.5 ± 3.4% (n = 9); 0.75 ± 0.12 GBq of Boc2-[18F]RL-III could be obtained from 3.7 GBq of aqueous [18F]fluoride in a total synthesis time of 100 min. Fig. S1 shows the analytical HPLC of isolated Boc2-[18F]RL-III. The radiochemical purity of Boc2-[18F]RL-III was 97.3% and the retention time corresponded to that of unlabeled Boc2-RL-III. The molar activity, determined for two batches of Boc2-[18F]RL-III as described in the Supplementary Materials (see Fig. S2 for the standard curve), was 24.1 TBq/mmol and 9.6 TBq/mmol, respectively.
Average radiochemical yields of 33.2 ± 13.8% (n = 5) and 47.1 ± 11.6% (n = 5) were obtained for the conjugation of [18F]RL-III to 2Rs15d and 5F7 sdAbs, respectively; the difference was not statistically significant (P=0.123). The total duration for the labeling of sdAbs with [18F]RL-III including deprotectection of Boc2-[18F]RL-III was 60 min, and the overall radiochemical yields for the synthesis of [18F]RL-III-2Rs15d and [18F]RL-III-5F7 from aqueous [18F]fluoride were 1.7 ± 0.7% and 3.8 ± 2.3%, respectively. Starting with 3.7 GBq of aqueous [18F]fluoride, 23 and 51 MBq of [18F]RL-III-2Rs15d and [18F]RL-III-5F7 could be obtained. The molar activity of [18F]RL-III-2Rs15d and [18F]RL-III-5F7 was in the range of 1.2 – 4.8 and 1.3 – 11.7 GBq/μmol, respectively.
3.3. Radiochemical purity, immunoreactivity and affinity of 18F-labeled sdAbs
The radiochemical purity of both [18F]RL-III-2Rs15d and [18F]RL-III-5F7, determined by SDS PAGE/phosphor imaging, TCA precipitation, and/or size-exclusion HPLC was greater than 96%. The immunoreactive fraction was 80–82% for [18F]RL-III-2Rs15d and 75–77% for [18F]RL-III-5F7. The affinity of both [18F]RL-III-2Rs15d and [18F]RL-III-5F7 was determined by saturation binding assays using HER2-expressing BT474M1 and SKOV-3 cell lines. A Kd value of 2.42 ± 0.6 nM was obtained for [18F]RL-III-2Rs15d using BT474M1 cells. Kd values of 2.4 ± 1.0 nM and 6.6 ± 1.8 nM were obtained for [18F]RL-III-5F7 using SKOV-3 and BT474M1 cells, respectively. Representative results of SDS-PAGE, affinity, and immunoreactivity for [18F]RL-III-5F7 are presented in Fig. 1 A–C. Results of SDS-PAGE, size-exclusion HPLC, immunoreactivity and affinity for [18F]RL-III-2Rs15d are presented in Fig. S3.
Figure 1.

Quality control of [18F]RL-III-5F7 by (A) SDS-PAGE/Phosphor imaging, (B) saturation binding assay on BT474M1 cells, and (C) immunoreactivity assay.
3.4. Uptake/internalization of labeled sdAbs
The data obtained from a paired-label internalization assay of [18F]RL-III-2Rs15d and [125I]SGMIB-2Rs15d performed with an initial incubation at 4°C using BT474M1 cells are shown in Fig. 2. The fraction of input activity that was cell-associated at 1, 2, and 4 h after the initial incubation was 8–9% and 9–10% for [125I]SGMIB-2Rs15d and of [18F]RL-III-2Rs15d, respectively; nonspecific uptake determined at 2 h was about 0.25% and 2.4%, respectively. The fraction of initially-bound activity that was trapped intracellularly for [18F]RL-III-2Rs15d at 1, 2, and 4 h was 22.51 ± 0.39%, 20.62 ± 1.18%, and 20.96 ± 0.84%, respectively (Fig. 2A) while total cell-associated activity (internalized plus surface-bound) was 37.91 ± 2.00%, 37.64 ± 1.27%, and 37.28 ± 1.28% (Fig. 2B). In comparison, the results for co-incubated [125I]SGMIB-2Rs15d were 24.17 ± 0.16%, 19.63 ± 0.32%, and 20.06 ± 1.20% for internalized and 38.81 ± 0.71%, 35.05 ± 0.82%, and 32.64 ± 1.32% for cell-associated. With a single-label assay for [18F]RL-III-2Rs15d on BT474M1 cells incubated directly at 37°C, internalized activity increased from 6.27 ± 0.38% at 1 h to 7.91 ± 0.53% of input activity at 4 h; likewise, total cell-associated activity increased from 13.77 ± 0.72% to 16.17 ± 0.61%. Co-incubated with a 100-fold excess of unlabeled 2Rs15d decreased total cell-associated activity at 2 h to 1.49 ± 0.12% of input activity.
Figure 2.

Paired-label internalization of [18F]RL-III-2Rs15d (blue) and [125I]SGMIB-2Rs15d (red) in BT474M1 cells. Cells were incubated with the labeled sdAbs initially for 1 h at 4°C. After removing the unbound activity, fresh medium at 37°C was added and the cells were incubated at 37°C for 1 h, 2 h and 4h. The results are expressed as the percentage of cell-bound activity after the initial 1 h incubation at 4°C. A) Internalized activity B) total cell-associated activity.
Fig. 3 shows the results obtained from a paired-label assay comparing the internalization of [18F]RL-III-5F7 and iso-[125I]SGMIB-5F7 on BT474M1 cells. After an initial incubation at 4°C, the fraction of input activity that was cell-associated at 1, 2, and 4 h was 12–14% and 14–17% for [18F]RL-III-5F7 and iso-[125I]SGMIB-5F7, respectively; nonspecific uptake determined at 2 h was about 1.2% and 0.3%, respectively. The internalized activity for [18F]RL-III-5F7 at 1, 2, and 4 h was 37.26 ± 1.53%, 42.47 ± 0.47%, and 38.46 ± 1.51% of initially bound activity, respectively (Fig. 3A); likewise, total cell-associated fractions were 67.00 ± 2.16%, 68.11 ± 1.05%, and 62.62 ± 2.01% (Fig. 3B). In comparison, results for co-incubated iso-[125I]SGMIB-5F7 were 46.05 ± 2.86%, 51.16 ± 1.15%, and 44.83 ± 0.92% for internalized activity and 77.04 ± 2.96%, 77.71 ± 1.91% and 71.35 ± 1.04% for total cell-associated activity. A single-label assay conducted with [18F]RL-III-5F7 using BT474M1 cells incubated directly at 37°C gave internalized activity values of 8.71 ± 0.81%, 12.08 ± 0.22% and 15.72 ± 1.25% of input activity at 1, 2, and 4 h, respectively; total cell-associated activity was 18.40 ± 1.69%, 24.86 ± 0.91%, and 28.92 ± 1.34% of input activity at these time points. In a single label assay of [18F]RL-III-5F7 incubated directly at 37°C with SKOV-3 cells, the fraction of input activity that was internalized was 11.73 ± 0.48%, 17.22 ± 0.57%, and 16.8 ± 1.09% at 1, 2, and 4 h, respectively; at these time points, the total cell-associated activity was 17.88 ± 0.88%, 20.93 ± 0.64%, and 25.39 ± 1.11%. Total cell-associated activity at 2 h was reduced to 0.27 ± 0.02% of input activity when co-incubated with excess trastuzumab demonstrating that uptake was HER2-mediated. Another paired-label assay was performed on BT474M1 cells to compare [18F]RL-III-5F7 and [125I]SGMIB-5F7 following the 4°C format (Fig. 4). The percentage of input [18F]RL-III-5F7 activity that was taken up after the initial incubation at 4°C was 9.07 ± 0.83% compared with 11.26 ± 0.55% for [125I]SGMIB-5F7. Co-incubation with trastuzumab reduced the uptake to 1.87 ± 0.08% and 0.17 ± 0.01% for 18F and 125I, respectively. For [18F]RL-III-5F7, 31.15 ± 2.49%, 27.93 ± 2.25%, and 31.99 ± 2.40% of initially bound activity was internalized at 1 h, 2 h, and 4 h, respectively, while total cell-associated 18F activity was 60.91 ± 2.65%, 54.96 ± 2.38%, and 55.05 ± 2.52%, respectively. In comparison, values for co-incubated [125I]SGMIB-5F7 were 37.26 ± 1.54%, 35.13 ± 0.51%, and 39.59 ± 0.71% for internalized activity and 70.89 ± 2.92%, 65.06 ± 0.82%, and 65.55 ± 1.10%, respectively, for total cell-associated activity.
Figure 3.

Paired-label internalization of [18F]RL-III-5F7 (green) and iso-[125I]SGMIB-5F7 (purple) in BT474M1 cells. Cells were incubated with the labeled sdAbs initially for 1 h at 4°C. After removing the unbound activity, fresh medium at 37°C was added and the cells were incubated at 37°C for 1 h, 2 h and 4h. The results are expressed as the percentage of cell-bound activity after the initial 1 h incubation at 4°C. A) Internalized activity B) total cell-associated activity.
Figure 4.
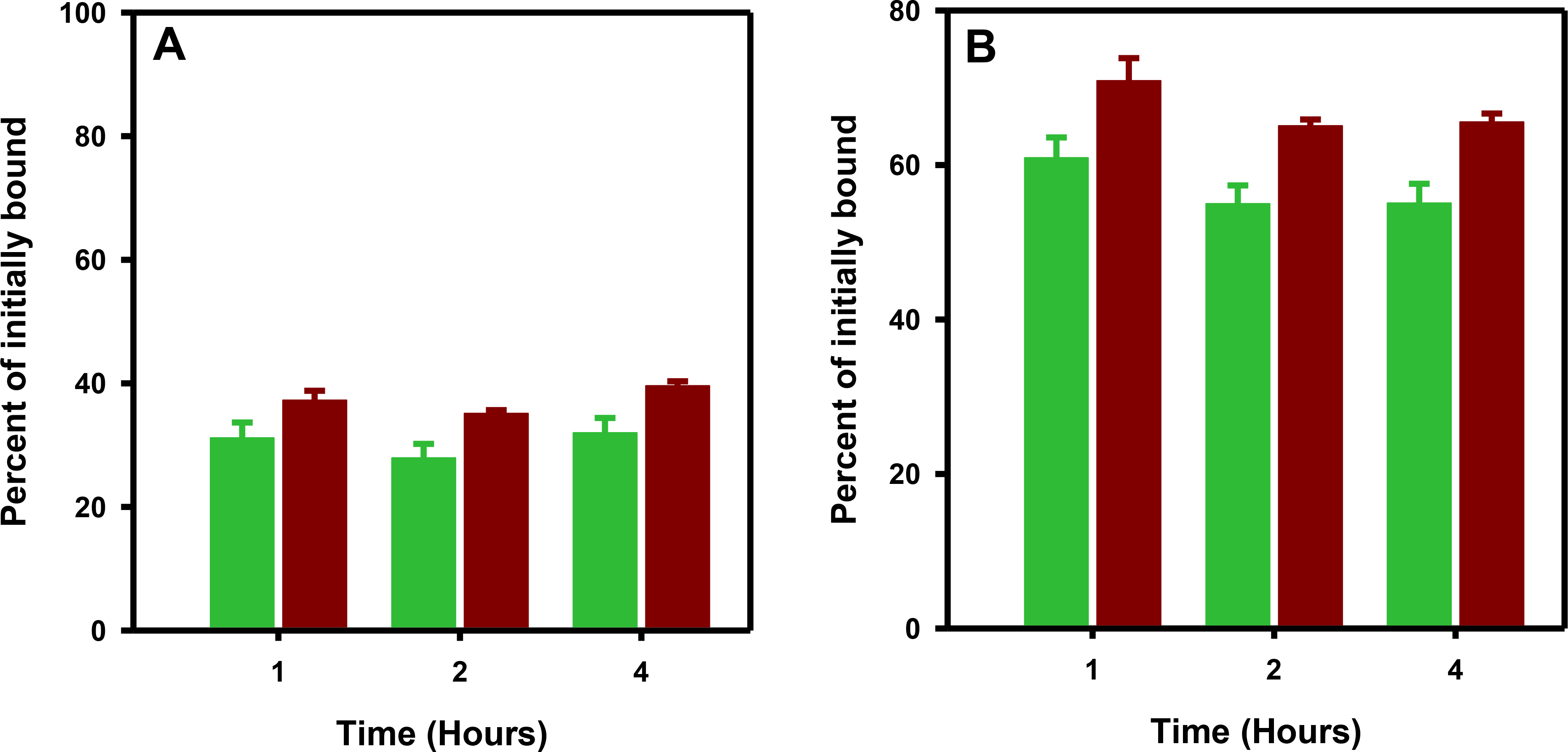
Paired-label internalization of [18F]RL-III-5F7 (green) and [125I]SGMIB-5F7 (brown) in BT474M1 cells. Cells were incubated with the labeled sdAbs initially for 1 h at 4°C. After removing the unbound activity, fresh medium at 37°C was added and the cells were incubated at 37°C for 1 h, 2 h and 4h. The results are expressed as the percentage of cell-bound activity after the initial 1 h incubation at 4°C. A) Internalized activity B) total cell-associated activity.
3.5. Biodistribution experiments
A paired-label study compared the biodistribution of [18F]RL-III-2Rs15d and [125I]SGMIB-2Rs15d in athymic mice bearing SKOV-3 xenografts at 1, 2, and 3 h (Table 1). Uptake of [18F]RL-III-2Rs15d in tumor was about 1.4–1.5-fold lower (P < 0.05) than that observed for [125I]SGMIB-2Rs15d. In a number of tissues, notably kidneys (3- to 4-fold), liver (25- to 42-fold), lungs (6- to 17-fold) and spleen (13– to 18-fold), [18F]RL-III-2Rs15d had significantly higher uptake than that seen for [125I]SGMIB-2Rs15d at all time points. Blood, muscle and bone uptake values were also higher albeit to a lesser extent except for bone at 3 h (3.5-fold).
Table 1.
Paired-label biodistribution of [18F]RL-III-2Rs15d and [125I]SGMIB-2Rs15d in athymic mice bearing HER2-expessing SKOV-3 xenografts.
| Tissue | Percent injected dose per grama | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 h | 2 h | 3 h | ||||
|
| ||||||
| 125I | 18F | 125I | 18F | 125I | 18F | |
|
| ||||||
| Liver | 0.47 ± 0.08 | 11.51 ± 0.83 | 0.32 ± 0.12 | 10.75 ± 1.50 | 0.21 ± 0.05 | 8.88 ± 1.59 |
| Spleen | 0.20 ± 0.02 | 2.96 ± 1.19 | 0.17 ± 0.07 | 2.25 ± 0.33 | 0.09 ± 0.02 | 1.62 ± 0.40 |
| Lungs | 0.67 ± 0.11 | 5.92 ± 0.48 | 0.33 ± 0.13 | 5.56 ± 1.61 | 0.79 ± 1.38 | 4.82 ± 2.08 |
| Heart | 0.25 ± 0.09 | 0.63 ± 0.16 | 0.14 ± 0.08 | 0.46 ± 0.22 | 0.07 ± 0.02 | 0.21 ± 0.06 |
| Kidneys | 58.68 ± 11.71 | 135.90 ± 11.36 | 20.40 ± 6.20 | 71.81 ± 14.92 | 12.15 ± 3.90 | 53.23 ± 17.31 |
| Stomach | 0.55 ± 0.22 | 0.81 ± 0.44b | 0.24 ± 0.07 | 0.28 ± 0.07 | 0.20 ± 0.08 | 0.30 ± 0.07 |
| Sm. Int. | 0.42 ± 0.08 | 1.66 ± 0.09 | 0.23 ± 0.10 | 1.15 ± 0.37 | 0.13 ± 0.03 | 0.75 ± 0.17 |
| Lg. Int. | 0.11 ± 0.02 | 0.44 ± 0.05 | 0.29 ± 0.13 | 1.29 ± 0.52 | 0.30 ± 0.20 | 1.37 ± 0.54 |
| Muscle | 0.21 ± 0.09 | 0.35 ± 0.04 | 0.13 ± 0.07 | 0.28 ± 0.09 | 0.05 ± 0.01 | 0.11 ± 0.03 |
| Blood | 0.44 ± 0.15 | 0.74 ± 0.19 | 0.23 ± 0.18 | 0.45 ± 0.28c | 0.14 ± 0.13 | 0.19 ± 0.04b,c |
| Bone | 0.27 ± 0.13 | 0.60 ± 0.11 | 0.12 ± 0.05 | 0.42 ± 0.17 | 0.06 ± 0.03 | 0.21 ± 0.08 |
| Brain | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Tumor | 5.52 ± 0.80 | 4.01 ± 0.47 | 6.43 ± 3.06 | 4.16 ± 1.39 | 3.79 ± 0.71 | 2.50 ± 0.32 |
%ID/gram, Mean ± SD (n=5)
Difference statistically NOT significant (P > 0.05)
n = 4.
The uptake in tumor and normal tissues from a paired-label biodistribution of [18F]RL-III-5F7 and iso-[125I]SGMIB-5F7 in athymic mice bearing BT474 xenografts at 2 h are presented in Fig. 5. Other than in tumor, kidneys, and bladder (not shown), activity levels in other tissues were <1.3% for both tracers. Tumor uptake of [18F]RL-III-5F7 in BT474 xenografts was 13.57 ± 4.53% ID/g, more than 3-fold that seen for [18F]RL-III-2Rs15d in SKOV-3 tumors at the same time point (4.16 ± 1.39% ID/g; P = 0.002 by unpaired t test). [18F]RL-III-5F7 exhibited about 7-fold higher activity levels than co-administered iso-[125I]SGMIB-5F7 in kidneys. Bone uptake of [18F]RL-III-5F7 was only 0.32 ± 0.12% ID/g, consistent with a low degree of defluorination in vivo. Expect in the kidneys, tumor-to-normal tissue ratios for [18F]RL-III-5F7 were high; for example, tumor-to-blood and tumor-to-muscle ratios were 39.0 ± 11.3 and 76.1 ± 32.6, respectively.
Figure 5.

Paired-label biodistribution of [18F]RL-III-5F7 and iso-[125I]SGMIB-5F7 in athymic mice bearing subcutaneous BT474 breast carcinoma xenografts at 2 h post injection.
3.6. MicroPET/CT imaging.
Maximum intensity projection images of a representative mouse receiving [18F]RL-III-5F7 are shown in Fig. 6. Concordant with the biodistribution data, most of the activity was found in the tumor, kidneys, and bladder even at 1 h. The activity values obtained from the imaging data (%ID/g; max) for tumor, kidneys, and liver are summarized in Table 2. The values at 2 h are in excellent agreement with those obtained from the necropsy experiment. Although the tumor uptake in Mouse 3 was considerably lower than that seen in the other two mice, the average for the three mice (12.2 ± 5.1%ID/g) was similar to that obtained from the biodistribution study. It should be noted that size of tumor in Mouse 3 (49.0 mm3) was considerably smaller than in the other two (180.0 and 137.4 mm3).
Figure 6.

MicroPET/CT imaging: Maximum intensity projection images obtained 1, 2 and 3 h after injection of [18F]RL-III-5F7 in athymic mice bearing subcutaneous BT474 xenografts. Tumor (T) and kidneys (K) are indicated by white arrows.
Table 2.
Uptake in selected tissues obtained from microPET/CT imaging of [18F]RL-III-5F7 in athymic mice bearing BT474 xenografts
| Mouse Number | Percent ID/gram (Max) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 h | 2 h | 3 h | |||||||
|
| |||||||||
| Tumor | Kidneys | Liver | Tumor | Kidneys | Liver | Tumor | Kidneys | Liver | |
|
| |||||||||
| 1 | 13.6 | 123.9 | 2.6 | 13.6 | 89.5 | 2.3 | 14.0 | 65.6 | 2.0 |
| 2 | 17.2 | 95.7 | 2.5 | 16.4 | 68.9 | 1.9 | 17.0 | 49.8 | 2.0 |
| 3 | 6.9 | 95.9 | 2.4 | 6.6 | 71.7 | 2.6 | 6.5 | 62.0 | 2.3 |
| Mean | 12.5 | 105.2 | 2.5 | 12.2 | 76.7 | 2.3 | 12.5 | 59.1 | 2.1 |
| SD | 5.2 | 16.2 | 0.1 | 5.1 | 11.2 | 0.4 | 5.4 | 8.3 | 0.2 |
Axial, coronal and sagittal images of the head of a mouse bearing an intracranial tumor and injected i.v. with [18F]RL-III-5F7 (Fig. 7A) demonstrate preferential uptake in the HER2-expressing tumor. The tumor uptake (max) was 0.98 %ID/g (SUV, 0.21) with a tumor-to-normal brain ratio of 9.8. As shown in Fig. 7 B&C, the presence of tumor was confirmed by H&E staining and autoradiography of brain sections harvested immediately after imaging.
Figure 7.

A) MicroPET/CT images obtained 2 h after administration of [18F]RL-III-5F7 in mouse bearing a BT474M1BrM3-Fluc intracranial xenograft. B) Brain section of the mouse in A stained with H&E. C) Autoradiography of an adjacent section. Size of the images in B and C are not the same scale.
4. Discussion
The residualizing prosthetic agent described herein was designed with the hope that it would offer improved radiochemical yields compared with those obtained for [18F]RL-I [23]. Having had no success with the radiochemistry involving the 2-carbon fluoroazide, we resorted to a fluoro-PEG-azide reported in the literature [28]. In addition to its nonvolatile nature, it was anticipated that the inclusion of a hydrophilic PEG linker might influence the tumor uptake and biodistribution of the final labeled sdAb favorably [47, 48]. The radiochemical yield for the synthesis of 1-azido-2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethane (69.8 ± 5.3%) was about 2-fold higher than reported by some investigators [27, 49] but lower than that obtained by Gill and Marik (86 ± 5%) [26]. The overall radiochemical yield for the synthesis of Boc2-[18F]RL-III (21.5 ± 3.4%) starting with aqueous [18F]fluoride was about 3-fold higher than that reported for Boc2-[18F]RL-I (8.5 ± 2.8%) [23]. In addition to loss of activity due to high volatility of [18F]fluorohexyne, an additional step was needed for the synthesis of Boc2-[18F]RL-I in order to remove a side product with a scavenging resin [23]. On the other hand, with Boc2-[18F]RL-III, previous issues due to the volatility of the 18F-labeled intermediate and the formation of the byproduct were eliminated. Yields for conjugation of [18F]RL-III with 5F7 (47.1 ± 11.6%) were higher (P = 0.0291) than those reported for coupling [18F]RL-I to the same sdAb (31.2 ± 6.7%). Consistent with this difference, from a stereoelectronic perspective, the carbonyl carbon of the active ester is expected to be slightly more positive in RL-III compared with that in RL-I, and thus the conjugation should be slightly more favorable with the new reagent. Another factor that may favor better conjugation of RL-III compared with RL-I is its potentially higher water solubility consistent with the calculated LogP values of −0.495 and −1.369 for RL-I and RL-III, respectively. Even though the overall radiochemical yield for the synthesis of [18F]RL-III-5F7 was ~1.5-fold higher than that obtained earlier for [18F]RL-I-5F7 [23], a considerable improvement would be needed for the method to be of practical utility. Using higher concentrations of sdAb, and optimizing temperature and/or pH likely would result in improved yields.
The HER2 binding affinity of the two sdAbs was not compromised by [18F]RL-III labeling as demonstrated by saturation binding assays with three cell lines. Although the Kd value obtained for [18F]RL-III-5F7 with BT474M1 cells (6.6 ± 1.8 nM) was higher than those seen with other combinations in this study, it was similar to that reported for [18F]RL-I-5F7 [23] and 5F7 labeled with 18F by a different method [50].
HER2-specific uptake was seen for [18F]RL-III-2Rs15d in BT474M1 cells and was similar to that for co-incubated [125I]SGMIB-2Rs15d. Total cell-associated and internalized activity observed previously for both [18F]RL-I-2Rs15d and [125I]SGMIB-2Rs15d in BT474M1 cells were higher than that observed in the current study [18]; but, unlike observed herein, they declined with time. Consistent with the results we have seen before [31], because of its higher internalization rate, sdAb 5F7 demonstrated higher initial uptake, higher internalized activity as well as higher total cell-associated activity than that obtained for 2Rs15d from assays performed with BT474M1 cells after initial incubation at 4°C. The intracellularly trapped activity from [18F]RL-III-5F7 was about 76–85% (P < 0.05) of that seen for [18F]RL-I-5F7 in the same cell line under the same assay conditions [17]. To facilitate a direct comparison of RL-III with RL-I, a paired-label internalization assay was performed by incubating BT474M1 cells with [18F]RL-III-5F7 and 5F7 labeled with 1,3,4-[125I]SGMIB, the agent that was used earlier in tandem with [18F]RL-I-5F7 [17]. The 18F/125I ratios calculated from the data obtained from this assay and those from the earlier study [17] indicate that the internalized 18F activity was about 90% of that for 125I in the prior study compared with about 80% in the current study suggesting that RL-I is slightly more residualizing than RL-III.
Although biodistribution studies were performed in different xenograft models, the co-administration of the [125I]SGMIB-2Rs15d conjugate in both cases allows comparison of the tumor localizing properties of 2Rs15d labeled using [18F]RL-I [18] and [18F]RL-III relative to their radioiodinated counterparts. With [18F]RL-III-2Rs15d, tumor levels were 64–72% those for [125I]SGMIB-2Rs15d compared with 80–84% of [125I]SGMIB-2Rs15d levels for [18F]RL-I-2Rs15d [18]. This is consistent with the in vitro data and suggests that RL-III may be less residualizing than RL-I. It should be pointed out that the position of attachment of the triazole moiety had been shown to influence tumor uptake in the case of small-molecule PSMA inhibitors [51]. Likewise, it may be possible that a different arrangement of the triazole moiety in RL-III compared with RL-I could influence the tumor uptake. Nonetheless, the tumor uptake observed for [18F]RL-III-2Rs15d was similar to that reported for 2Rs15d labeled with metallic radionuclides in the same SKOV-3 model [33, 52–55]. Consistent with that seen for [18F]RL-I-2Rs15d as well as the aforementioned radiometal conjugates, the renal activity levels for [18F]RL-III-2Rs15d were very high, presumably due to the presence of highly polar guanidine, triazole [56] and PEG functionalities. In addition, compared with [125I]SGMIB-2Rs15d, [18F]RL-III-2Rs15d exhibited several-fold higher uptake in liver, spleen, and lungs as was observed previously for [18F]RL-I-2Rs15d in athymic mice with BT474M1 xenografts [18]. The presence of aggregates could account for this behavior [55]; however, SDS-PAGE analysis of the [18F]RL-III-2Rs15d preparation used for the biodistribution study did not indicate the presence of aggregates. Mass of sdAb can influence uptake in normal tissues, especially liver [33]; however, given that the hepatic uptake of co-administered [125I]SGMIB-2Rs15d was lower, higher uptake of [18F]RL-III-2Rs15d in liver cannot be due to lack of sufficient mass of sdAb. It should be noted that liver uptake for [18F]RL-III-5F7 in this study and for [18F]RL-I-5F7 earlier [17] was significantly lower. This suggests that the higher liver uptake seen for [18F]RL-III-2Rs15d herein and [18F]RL-I-2Rs15d previously [18] might be due to either the intrinsic properties of 2Rs15d or more likely, its modification by RL-I/RL-III prosthetic groups. Adapting strategies such as N-terminal modification [57] with hydrophilic moieties as employed for affibodies [58] may ameliorate high hepatic uptake.
Unlike the biodistribution of [18F]RL-III-2Rs15d in athymic mice with SKOV-3 xenografts, the biodistribution of [18F]RL-III-5F7 in the BT474 tumor model showed minimal uptake in normal tissues except the kidneys and bladder. However, the tumor uptake was more than 2.5-fold lower than that obtained earlier for [18F]RL-I-5F7 [17], which could be in part due to differences in mouse strain (athymic vs. SCID), xenograft source (BT474 vs. metastatic BT474M1 used previously), and differences in residualizing ability between RL-III and RL-I. On the other hand, tumor uptake of [18F]RL-III-5F7 was about 1.5-fold higher than for 5F7 labeled using [18F]TFPFN, a less residualizing prosthetic agent, in the same BT474 xenograft model [31]. Tumor uptake was more than sufficient after intravenous administration of [18F]RL-III-5F7 for microPET imaging with noticeable background activity confined to kidneys and bladder. Moreover, it was possible to visualize intracranial HER2-expressing tumors using [18F]RL-III-5F7, an important first step in developing PET probes for monitoring brain metastases, an increasingly prevalent population in patients with HER2+ breast cancer [59].
5. Conclusion
Using a PEG-bearing fluoroazide in lieu of 6-fluorohexyne utilized earlier for RL-I, improvements in radiochemical yields have been achieved. However, to be practically useful, significantly higher overall radiochemical yields are needed. As seen previously with [18F]RL-I-2Rs15d, although biodistribution of [18F]RL-III-2Rs15d was not favorable, excellent tumor targeting and high tumor-to-normal tissue ratios (except kidneys and bladder) were achieved with [18F]RL-III-5F7 albeit in a different HER2-expressing xenograft model. We note that 2Rs15d does not internalize appreciably into tumor cells after receptor binding in contrast to 5F7, which does internalize extensively. While this difference may be a factor influencing tumor uptake of the two sdAbs, its effect on normal tissue uptake is not clear — perhaps membrane shedding of the HER2 sdAb complex could play a role here. Finally, our results suggest that including a guanidine-bearing moiety rather than fluoronicotinoyl moiety [31] is a design element worth further development particularly with rapidly internalizing sdAbs.
Supplementary Material
Acknowledgements
This work was supported by NIH Grants CA188177 and CA42324, and the Lynn Sage Research Foundation. The authors thank Dr. Matthias D’Huyvetter (Free University of Brussels) for providing the 2Rs15d sdAb, and Dr. Xiao-Guang Zhao and Thomas Hawk for excellent technical assistance with the animal biodistribution studies and micro-PET/CT imaging, respectively.
Footnotes
Declaration of competing interest
Michael R. Zalutsky holds ownership interest and is a board member for Cereius, Inc. Ganesan Vaidyanathan is a consultant and shareholder for Cereius, Inc. There are no other potential conflicts of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22. [DOI] [PubMed] [Google Scholar]
- [2].Nicolini A, Ferrari P, and Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018;52:56–73. [DOI] [PubMed] [Google Scholar]
- [3].Nitta H, Kelly BD, Allred C, Jewell S, Banks P, Dennis E, et al. The assessment of HER2 status in breast cancer: the past, the present, and the future. Pathol Int. 2016;66:313–24. [DOI] [PubMed] [Google Scholar]
- [4].Thangarajah F, Vogel C, Pahmeyer C, Eichler C, Holtschmidt J, Ratiu D, et al. Profile and outcome of supraclavicular metastases in patients with metastatic breast cancer: Discordance of receptor status between primary and metastatic site. Anticancer Res. 2018;38:6023–6. [DOI] [PubMed] [Google Scholar]
- [5].Schrijver W, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, and van Diest PJ. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J Natl Cancer Inst. 2018;110:568–80. [DOI] [PubMed] [Google Scholar]
- [6].Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pereira PMR, Abma L, Henry KE, and Lewis JS. Imaging of human epidermal growth factor receptors for patient selection and response monitoring - From PET imaging and beyond. Cancer Lett. 2018;419:139–51. [DOI] [PubMed] [Google Scholar]
- [8].Ducharme M and Lapi SE. Peptide based imaging agents for HER2 Imaging in oncology. Mol Imaging. 2020;19:1536012120960258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lorusso M, Scolozzi V, Taralli S, Caldarella C, Altini C, Rubini G, et al. Radiolabelled Trastuzumab PET/CT imaging: a promising non-invasive tool for the in vivo assessment of HER2 status in breast cancer patients. Clin Trans Imaging. 2020;8:95–105. [Google Scholar]
- [10].Capala J and Bouchelouche K. Molecular imaging of HER2-positive breast cancer: a step toward an individualized ‘image and treat’ strategy. Curr Opin Oncol. 2010;22:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tolmachev V, Orlova A, and Sorensen J. The emerging role of radionuclide molecular imaging of HER2 expression in breast cancer. Semin Cancer Biol. 2021; ahead of print. [DOI] [PubMed] [Google Scholar]
- [12].Bao G, Tang M, Zhao J, and Zhu X. Nanobody: a promising toolkit for molecular imaging and disease therapy. Eur J Nucl Med Mol Imaging Res. 2021;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu M, Li L, Jin D, and Liu Y. Nanobody-A versatile tool for cancer diagnosis and therapeutics. WIREs Nanomed Nanobiotechnol. 2021:e1697. [DOI] [PubMed] [Google Scholar]
- [14].Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, et al. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging 2020. 10.1007/s00259-020-05094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Menzel S, Schwarz N, Haag F, and Koch-Nolte F. Nanobody-based biologics for modulating purinergic signaling in inflammation and immunity. Front Pharmacol 2018;9:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Dongen GA, Poot AJ, and Vugts DJ. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumour Biol 2012;33:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vaidyanathan G, McDougald D, Choi J, Koumarianou E, Weitzel D, Osada T, et al. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J Nucl Med. 2016;57:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou Z, Vaidyanathan G, McDougald D, Kang CM, Balyasnikova I, Devoogdt N, et al. Fluorine-18 labeling of the HER2-targeting single-domain antibody 2Rs15d using a residualizing label and preclinical evaluation. Mol Imaging Biol. 2017;19:867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi J, Vaidyanathan G, Koumarianou E, McDougald D, Pruszynski M, Osada T, et al. N-Succinimidyl guanidinomethyl iodobenzoate protein radiohalogenation agents: influence of isomeric substitution on radiolabeling and target cell residualization. Nucl Med Biol. 2014;41:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vaidyanathan G and Zalutsky MR. Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: a radioiodination agent for labeling internalizing proteins and peptides. Nat Protoc. 2007;2:282–6. [DOI] [PubMed] [Google Scholar]
- [21].Jacobson O, Kiesewetter DO, and Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem 2015;26:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Preshlock S, Tredwell M, and Gouverneur V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev 2016;116:719–66. [DOI] [PubMed] [Google Scholar]
- [23].Vaidyanathan G, McDougald D, Choi J, Pruszynski M, Koumarianou E, Zhou Z, et al. N-Succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)ben zoate ([18F]SFBTMGMB): a residualizing label for 18F-labeling of internalizing biomolecules. Org Biomol Chem. 2016;14:1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Galante E, Schoultz BW, Koepp M, and Arstad E. Chelator-accelerated one-pot ‘click’ labeling of small molecule tracers with 2-[18F]fluoroethyl azide. Molecules. 2013;18:5335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou D, Chu WH, Peng X, McConathy J, Mach RH, and Katzenellenbogen JA. Facile purification and click labeling with 2-[F-18]fluoroethyl azide using solid phase extraction cartridges. Tetrahedron Lett. 2015;56:952–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gill HS and Marik J. Preparation of 18F-labeled peptides using the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition. Nat Protoc. 2011;6:1718–25. [DOI] [PubMed] [Google Scholar]
- [27].Park JY, Lee TS, Song IH, Cho YL, Chae JR, Yun M, et al. Hybridization-based aptamer labeling using complementary oligonucleotide platform for PET and optical imaging. Biomaterials. 2016;100:143–51. [DOI] [PubMed] [Google Scholar]
- [28].Steen EJL, Shalgunov V, Denk C, Mikula H, Kjaer A, Kristensen JL, et al. Convenient entry to 18F-labeled amines through the Staudinger reduction. European J Org Chem. 2019;2019:1722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Merz V, Lenhart J, Vonhausen Y, Ortiz-Soto ME, Seibel J, and Krueger A. Zwitterion-functionalized detonation nanodiamond with superior protein repulsion and colloidal stability in physiological media. Small 2019;15:e1901551. [DOI] [PubMed] [Google Scholar]
- [30].Vaidyanathan G, Affleck DJ, Li J, Welsh P, and Zalutsky MR. A polar substituent-containing acylation agent for the radioiodination of internalizing monoclonal antibodies: N-succinimidyl 4-guanidinomethyl-3-[131I]iodobenzoate ([131I]SGMIB). Bioconjug Chem. 2001;12:428–38. [DOI] [PubMed] [Google Scholar]
- [31].Zhou Z, McDougald D, Devoogdt N, Zalutsky MR, and Vaidyanathan G. Labeling single domain antibody fragments with fluorine-18 using 2,3,5,6-Tetrafluorophenyl 6-[18F]fluoronicotinate resulting in high tumor-to-kidney ratios. Mol Pharm. 2019;16:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vaneycken I, Devoogdt N, Van Gassen N, Vincke C, Xavier C, Wernery U, et al. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. Faseb J. 2011;25:2433–46. [DOI] [PubMed] [Google Scholar]
- [33].Xavier C, Vaneycken I, D’Huyvetter M, Heemskerk J, Keyaerts M, Vincke C, et al. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013;54:776–84. [DOI] [PubMed] [Google Scholar]
- [34].Pruszynski M, Koumarianou E, Vaidyanathan G, Revets H, Devoogdt N, Lahoutte T, et al. Targeting breast carcinoma with radioiodinated anti-HER2 Nanobody. Nucl Med Biol. 2013;40:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yu Z, Xia W, Wang HY, Wang SC, Pan Y, Kwong KY, et al. Antitumor activity of an Ets protein, PEA3, in breast cancer cell lines MDA-MB-361DYT2 and BT474M1. Mol Carcinog. 2006;45:667–75. [DOI] [PubMed] [Google Scholar]
- [36].Vaidyanathan G, White BJ, and Zalutsky MR. Propargyl 4-[18F]fluorobenzoate: A putatively more stable prosthetic group for the fluorine-18 labeling of biomolecules via click chemistry. Curr Radiopharm. 2009;2:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Olafsen T, Sirk SJ, Olma S, Shen CK, and Wu AM. ImmunoPET using engineered antibody fragments: fluorine-18 labeled diabodies for same-day imaging. Tumour Biol 2012;33:669–77. [DOI] [PubMed] [Google Scholar]
- [38].Ward CC, Kleinman JI, and Nomura DK. NHS-esters as versatile reactivity-based probes for mapping proteome-wide ligandable hotspots. ACS Chem Biol 2017;12:1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Koniev O and Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem Soc Rev 2015;44:5495–551. [DOI] [PubMed] [Google Scholar]
- [40].Zhou Z, Devoogdt N, Zalutsky MR, and Vaidyanathan G. An efficient method for labeling single domain antibody fragments with 18F using tetrazine- trans-cyclooctene ligation and a renal brush border enzyme-cleavable linker. Bioconjug Chem. 2018;29:4090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Foulon CF, Reist CJ, Bigner DD, and Zalutsky MR. Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 2000;60:4453–60. [PubMed] [Google Scholar]
- [42].Lindmo T, Boven E, Cuttitta F, Fedorko J, and Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89. [DOI] [PubMed] [Google Scholar]
- [43].Zhou Z, Chitneni SK, Devoogdt N, Zalutsky MR, and Vaidyanathan G. Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: Chemistry and preliminary evaluation. Bioorg Med Chem. 2018;26:1939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Choi J, Vaidyanathan G, Koumarianou E, Kang CM, and Zalutsky MR. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: radiolabeling and preliminary evaluation. Nucl Med Biol. 2018;56:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kanojia D, Balyasnikova IV, Morshed RA, Frank RT, Yu D, Zhang L, et al. Neural stem cells secreting anti-HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells. 2015;33:2985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Coenen HH, Gee AD, Adam M, Antoni G, Cutler CS, Fujibayashi Y, et al. Status of the ‘consensus nomenclature rules in radiopharmaceutical sciences’ initiative. Nucl Med Biol 2019;71:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vegt E, de Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51:1049–58. [DOI] [PubMed] [Google Scholar]
- [48].Narayanam MK, Toutov AA, and Murphy JM. Rapid one-step 18F-labeling of peptides via heteroaromatic silicon-fluoride acceptors. Org Lett. 2020;22:804–8. [DOI] [PubMed] [Google Scholar]
- [49].Collins J, Waldmann CM, Drake C, Slavik R, Ha NS, Sergeev M, et al. Production of diverse PET probes with limited resources: 24 18F-labeled compounds prepared with a single radiosynthesizer. Proc Natl Acad Sci U S A. 2017;114:11309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhou Z, Zalutsky MR and Vaidyanathan G Labeling a TCO-functionalized single domain antibody fragment with 18F via inverse electron demand Diels Alder cycloaddition using a fluoronicotinyl moiety-bearing tetrazine derivative. Bioorg Med Chem. 2020;28:ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kelly J, Amor-Coarasa A, Nikolopoulou A, Kim D, Williams C Jr., Ponnala S, et al. Synthesis and pre-clinical evaluation of a new class of high-affinity 18F-labeled PSMA ligands for detection of prostate cancer by PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dekempeneer Y, Caveliers V, Ooms M, Maertens D, Gysemans M, Lahoutte T, et al. Therapeutic efficacy of 213Bi-labeled sdAbs in a preclinical model of ovarian cancer. Mol Pharm. 2020;17:3553–66. [DOI] [PubMed] [Google Scholar]
- [53].D’Huyvetter M, Vincke C, Xavier C, Aerts A, Impens N, Baatout S, et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4:708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Massa S, Xavier C, De Vos J, Caveliers V, Lahoutte T, Muyldermans S, et al. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug Chem. 2014;25:979–88. [DOI] [PubMed] [Google Scholar]
- [55].Pruszynski M, D’Huyvetter M, Bruchertseifer F, Morgenstern A, and Lahoutte T. Evaluation of an anti-HER2 nanobody labeled with 225Ac for targeted alpha-particle therapy of cancer. Mol Pharm. 2018;15:1457–66. [DOI] [PubMed] [Google Scholar]
- [56].Nguyen ML, Shin TJ, Kim H-J and Cho B-K Oriented columnar films of a polar 1,2,3-triazole-based liquid crystal prepared by applying an electric field. J Mat Chem C. 2017;5:8256–65. [Google Scholar]
- [57].Tang KC and Raj M. One-step azolation strategy for site- and chemo-selective labeling of proteins with mass-sensitive probes. Angew Chem Int Ed Engl. 2021;60:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Westerlund K, Honarvar H, Norrstrom E, Strand J, Mitran B, Orlova A, et al. Increasing the net negative charge by replacement of DOTA chelator with DOTAGA improves the biodistribution of radiolabeled second-generation synthetic affibody molecules. Mol Pharm. 2016;13:1668–78. [DOI] [PubMed] [Google Scholar]
- [59].Zimmer AS, Van Swearingen AED, and Anders CK. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Rep (Hoboken). 2020:e1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


