Abstract
Background:
Short course radiotherapy with consolidation chemotherapy (SCRT-CCT) has emerged as a promising alternative to the long course chemoradiotherapy (LCRT) regimen in locally advanced rectal cancer management. The systematic review and meta-analysis is aimed at summarizing current evidence on SCRT-CCT and comparing it to LCRT.
Material and Methods:
Electronic databases of MEDLINE, Web of Science and Cochrane library were searched using a predefined search strategy returning 3314 articles. This review included 11 studies (6 randomized trials and 5 non-randomized studies) on SCRT-CCT regimen based on seven different cohorts. Weighted arithmetic means and forest plots were generated to determine summary estimates.
Results:
The probability of achieving pathological complete response (pCR) was higher with SCRT-CCT compared to LCRT (RR=1.75, 95% CI: 1.41-2.19). No statistically significant difference in 3-year OS was observed between the two groups (RR=1.06, 95% CI: 0.98-1.14). The weighted arithmetic mean of 3-year overall survival (OS) and pCR was 83.6% versus 80.9% and 24.5% versus 13.6% for SCRT-CCT and LCRT, respectively. R0 resection and T-downstaging rates ranged from 69.2-100% and 47-75% for SCRT-CCT, and 71%-92.3% and 41-75% for LCRT. The regimens had similar compliance, postoperative and late toxicity, however, acute toxicity rates varied primarily due to differences in treatment protocols.
Conclusions:
This review highlights the ability of SCRT-CCT to produce improved tumor response with comparable OS, R0 resection, and T-downstaging at the cost of increased acute toxicity. However, heterogeneity in treatment protocols across studies makes it difficult to provide definitive conclusions regarding the regimen. Several ongoing trials are expected to provide further confirm evidence provide by the RAPIDO trial and detail appropriate SCRT-CCT protocols to improve oncological outcomes, minimize toxicity and determine its effectiveness as the standard-of-care for LARC patients.
Keywords: Short-course radiotherapy, consolidation chemotherapy, neoadjuvant therapy, rectal cancer, pathologic complete response
Background
Globally, colorectal cancer (CRC) is the third most commonly diagnosed malignancy with over 30% arising in the rectum [1,2] . It is also the second most common cause of cancer-related deaths [1]. The molecular features of cancerous lesions in the colon and rectum are known to be different; therefore, the treatment of CRC also varies depending on the primary location [2].
Primary treatment of rectal cancer is surgery; however, neoadjuvant radiation therapy (RT) is indicated in patients with locally advanced disease, with the aim of reducing local-recurrence rates [3]. Currently, there are three regimens to deliver radiation in locally advanced rectal cancer (LARC): (i) short-course radiotherapy (SCRT; 25 Gy in 5 fractions) with immediate surgery (within 1 week) or (ii) with delayed surgery (after 4-8 weeks), and (iii) long-course chemoradiotherapy (LCRT; 45-50.4 Gy in 25-28 fractions with concurrent chemotherapy) with surgery after 6-8 weeks [4]. Generally, SCRT with immediate surgery is used for patients with intermediate risk factors for local recurrence, while LCRT for large tumors or N2 nodal disease. These approaches have similar surgical, long-term oncologic, late toxicity and overall quality of life outcomes [5-9]. The LCRT approach is associated with greater tumor response, as measured by downstaging and higher pathologic complete response (pCR) rates [10]. On the other hand, the SCRT regimen is an appealing option considering treatment cost, patient compliance and complications [11].
Tumor response to radiation takes time. Considering that surgery after SCRT is undertaken much earlier compared to LCRT, the question was asked whether extending the interval between end of SCRT and surgery will produce an equivalent tumor response. Recently, studies such as the Stockholm III trial have demonstrated that delayed surgery after SCRT provides an improved tumor response and a better safety profile compared to immediate surgery [12,13]. Historically, SCRT regimens did not include systemic chemotherapy, however, there is a growing interest in combining chemotherapy before, during or after SCRT, with the goal of further increasing tumor response [14-18]. Of these new approaches, SCRT followed by consolidation chemotherapy (CCT) seems to be the most promising alternative to the traditional LCRT regimen. Several studies including recently published RAPIDO trial have sought to understand the short-term and long-term effects of this novel SCRT-CCT regimen, where courses of chemotherapy are administered in the interval between SCRT and surgery. This regimen is believed to improve local control and response of the primary tumor due to the additive effects of a long waiting period and chemotherapy, while curbing adverse events.
The aim of this systematic review and meta-analysis of the literature is to summarize current data on the effectiveness of SCRT followed by CCT as neoadjuvant treatment for LARC, in terms of pCR, R0 resection rates and overall survival (OS). Additionally, evidence on the toxicity, compliance and other relevant information are also reviewed.
Methods and Material
Search strategy
The systematic review and meta-analysis was based on a PICO format (patients, interventions, comparisons, outcomes) question and performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. A search of the electronic databases MEDLINE, Web of Science and Cochrane Library was performed to identify relevant articles, without using any filters. Additionally, backward citation chaining of reference lists from all suitable full-text articles was performed to identify pertinent articles.
Evidence acquisition
On the 4th of January, 2021, two independent researchers (AP, PS) searched the abovementioned databases to identify eligible studies. The search strategy was as follows: (sequential OR consecutive OR concomitant OR consolidation) and (neoadjuvant OR preoperative) and (radiotherapy OR (radiation and therapy) OR (radiation and treatment) OR chemotherapy OR chemoradiotherapy OR radiochemotherapy) and (colon OR colorectal OR rectal OR rectum) and (cancer OR carcinoma OR adenocarcinoma). The initial search returned a total of 3314 studies. The screening protocol is shown as PRISMA flowchart. (Figure 1) Disagreements between researchers were resolved through discussion amongst them and consultation with another author (JK). The following data was retrieved from the included studies: first author, year of publication, enrollment period, center/country/clinical trial, study design, TNM stage of rectal cancer, number of patients, percentage of male patients, age, SCRT dose and modality, CCT regimen, treatment protocol, details on compliance with neoadjuvant treatment protocol, R0 resection, T-downstaging, pCR, OS and disease-free survival (DFS). Data on these parameters was also collected for the control groups if implemented. Additionally, information on acute toxicity, postoperative complication and late toxicities, as well as other relevant findings from each study was retrieved and described.
Figure 1:
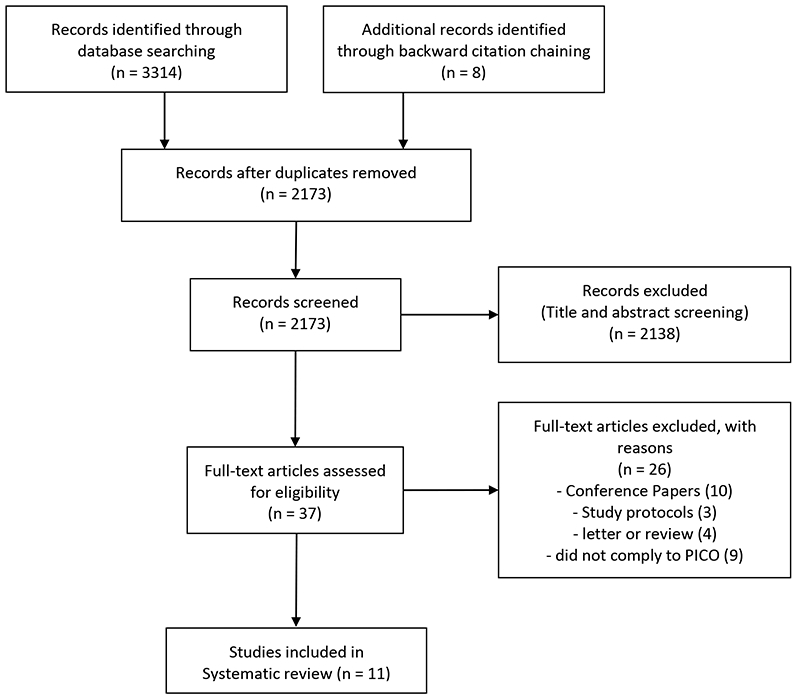
Prisma Flowchart
PICO-based inclusion and exclusion criteria
The PICO-based questions utilized to identify relevant studies for inclusion in analysis was as follows: Patients – patients with LARC (defined as WHO II or III or IV (if T3-4 or n+)); Intervention – SCRT followed by CCT as neoadjuvant therapy; Comparisons – LCRT-treated control groups (if present); Outcomes – pCR, OS, DFS, toxicity rates and compliance rates. The inclusion criteria were any full text, original study articles on SCRT-CCT reporting on pCR, with or without LCRT control groups. Articles were excluded if they were not original studies (editorials, reviews and clinical trial protocols) and if they did not comply to PICO (studies on other SCRT schemes such as with induction, concurrent or no additional chemotherapy). Additionally, data from conference papers on studies focusing on SCRT-CCT was also retrieved and are presented as supplementary material.
Evidence synthesis and Risk of Bias assessment
The details regarding evidence synthesis and risk of bias assessment are provided in the supplementary material.
Statistical Analysis
Weighted arithmetic mean for pCR and 3-year OS was calculated to obtain a summary estimate. It was calculated by multiplying the weight of each study (number of patients in the study/total number of patients) with the outcome (pCR/3-year OS) value [21]. If several studies reported findings from the same cohort over different time points, data from the latest available study was used. A random-effects model and Mantel-Haenszel (M-H) test were utilized to pool data in terms of relative risk (RR) for pCR and 3-year OS reported in randomized clinical trials, which included an LCRT control group and were the latest reports for their respective cohorts. A forest plot was created for visual interpretation of the summary estimates. Statistical heterogeneity across studies was assessed using the I2 coefficient. Excel version 19.11 and RevMan 5.4.1 were used for data abstraction and statistical analysis.
Results
After screening and full-text assessment (38 articles), 11 articles were included in this review. Of which, 4 were phase II trials, 4 phase III trials, 1 randomized controlled trials, 1 retrospective and 1 prospective cohort study. The characteristics of the included studies are presented in table 1.
Table 1:
Characteristics of included studies.
| Author | Enrollment Years |
Design | Stage (TNM) |
Groups | N of Patients |
Male% | Age2 | RT dose (Gy) |
CT regimen | Interval Period7 |
Treatment Compliance%8 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Washington Cohort | Markovina et al. (2017) | 2009-2012 | P II | cT3-4 N0-2 M0 | Study | 69 | 71 | 57.2 | 25 | mFOLFOX-6 (4 cycles) | 11-17 | 96.7 |
| Control | 69 | 67 | 56.6 | 45 | 5FU or capecitabine | 6-8 | 100 | |||||
| Myerson et al. (2014) | 2009-2012 | P II (single arm) | cT3-4 N0-2 M0-1 | Study | 76 | 71 | 56.43 | 25 | mFOLFOX-6 (4 cycles) | 11-17 | 56 | |
| Control | ||||||||||||
| Polish Cohort | Ciseł et al. (2019) | 2008-2014 | P III | cT3-4 N0-2 | Study | 261 | 70 | 60 | 25 | FOLFOX-4 (3 cycles) | 12 | NR |
| Control | 254 | 67 | 60 | 50.4 | 5FU+Leucovorin plus Oxaliplatin (2 cycles)4 | 6 | NR | |||||
| Bujko et al. (2016) | 2008-2014 | P III | cT3-4 N0-2 | Study | 261 | 70 | 60 | 25 | FOLFOX-4 (3 cycles) | 12 | 63 | |
| Control | 254 | 67 | 60 | 50.4 | 5FU+Leucovorin plus Oxaliplatin (2 cycles)4 | 6 | 66 | |||||
| Bujko et al. (2013) | 2008-2010 | P III | cT3-4 N0-2 | Study | 49 | 67 | 60 | 25 | FOLFOX-4 (3 cycles) | 12 | 73.5 | |
| Control | 48 | 69 | 59 | 50.4 | 5FU+Leucovorin plus Oxaliplatin (2 cycles) | 6 | 72.9 | |||||
| Iranian Cohort | Aghili et al. (2020) | 2016-2020 | RCT | cT3-4 N0-2 M0 | Study | 33 | 55 | 56 | 25 | concurrent XELOX; Consolidative XELOX (3-4 cycles) | 15-20 | 87.9 |
| Control | 27 | 63 | 53 | 50-50.4 | concurrent Capecitabine; Consolidative XELOX (3-4 cycles) | 15-20 | 81.8 | |||||
| Aghili et al. (2018) | 2013-2015 | P II (single arm) | cT3-4 N0-2 M0 | Study | 33 | 73 | 61 | 25 | concurrent XELOX; Consolidative XELOX (1 cycle) | 7-9 | 87.9 | |
| Control | ||||||||||||
| Baltimore Cohort | Jia et al. (2019) | 2017-2019 | R | cT2-4 N0-2 | Study | 26 | 77 | 52 | 25 | mFOLFOX-6 (25 patients), CapeOX (1 patient)5 | 14.5 | 81 |
| Control | ||||||||||||
| Danish Cohort | Van Dijk et al. (2013) | 2006-2010 | P II (single arm) | cT2-4 N0-2 M1 | Study | 50 | 54 | 59 | 25 | CapeOX-Bevacizumab (6 cycles) | 26 | 84 |
| Control | ||||||||||||
| RAPIDO trial | Bahadoer et al. (2020) | 2011-2016 | P III | cT2-4 N0-2 M0 | Study | 462 | 65 | 62 | 25 | CapeOX (6 cycles) or FOLFOX4 (9 cycles)6 | 24 | 85 |
| Control | 450 | 69 | 62 | 50.4 or 50* | Capecitabine | 6-10 | 90 | |||||
| Indian Cohort | Thakur et al. (2020) 1 | 2015-2016 | Prospective | NR | Study | 14 | NR | NR | 25 | CapeOX (2 cycles) | 11-13 | 100 |
| Control | 13 | NR | NR | 45 | Capecitabine | 4-6 | 87 | |||||
(RCT = randomized clinical trial; P II/III = phase II/III; R = retrospective cohort, Pr = prospective cohort, N = number, NR = not reported, RT = radiotherapy; CT = chemotherapy; FOLFOX = fluorouracil, leucovorin, and oxaliplatin; 5FU = fluorouracil; XELOX/CapeOX = capecitabine and oxaliplatin)
Specific data on CRC stages, Male% and Age were not reported (NR) by Thakur et al., however, the authors state that the study and control groups were comparable in these regards.
Median Age reported in years.
Mean Age reported in years.
Oxaliplatin delivery left to local institution’s discretion after 2012.
Number of chemotherapy cycles were left at the treating physician’s discretion
Either chemotherapy regimen was used depending on the decision of the treating physician and hospital policy
Approximate intervals between completion of radiotherapy (SCRT or LCRT) and Surgery (in weeks)
Based on need for dose reduction and/or treatment delay due to toxicity or the proportion of patients completing the entire intended treatment
Two studies (Markovina et al., Myerson et al.) [22,23] reported findings from the Washington cohort, 3 on the Polish cohort (Cisel et al, Bujko et al. (2016), Bujko et al. (2013)) [24-26], 2 on the Iranian cohort (Aghili et al. (2020), Aghili et al. (2019)) [27,28] while each of remaining 4 reported on the Baltimore (Jia et al.) [29], Dutch (Van Dijk et al.) [30], RAPIDO trial (Bahadoer et al.) [31] and Indian (Thakur et al.) [32] cohorts. Eight studies included stage II or III rectal adenocarcinoma patients [22,24-29,31], while two studies additionally included stage IV patients [23,30]. Only one study (Thakur et al.) did not report tumor stage, but the authors mention that their groups were comparable in this regard [32].
A total of 1728 patients were examined over the seven cohorts including 915 SCRT-CCT treated patients (study group) and 813 LCRT treated patients (control group). Across studies, the percentage of male patients ranged from 54-77%, and median age ranged from 52-62 years.
Treatment Regimen
The information relating to the treatment regimen and patient compliance is presented in table 1 and supplementary table 1.
The neoadjuvant regimen for SCRT-CCT group comprised on 25 Gy radiotherapy (5 Gy x 5 days) followed by CCT. The FOLFOX regimen (either modified FOLFOX-6 or FOLFOX-4) was given as consolidative treatment in the Washington (4 cycles), Polish (3 cycles), Baltimore, and RAPIDO (9 cycles) cohorts. The RAPIDO cohort, additionally, included patients treated with the CapeOX regimen (6 cycles) [31]. The CapeOX regimen was also used in the Dutch (6 cycles, in addition to Bevacizumab) and Indian (2 cycles) cohorts [30,32]. The Iranian cohort (Aghili et al. 2020) utilized XELOX regimen which was given concurrently with SCRT as well as after SCRT as consolidative therapy (3-4 cycles) [27].
In the LCRT group, the radiotherapy dose ranged from 45-50.4 Gy with concurrent chemotherapy. One study from the Washington cohort included a LCRT control group, which received either 5-flourouracil (5-FU) or capecitabine chemotherapy [22]. In the Polish cohort, 5FU, leucovorin and oxaliplatin were used as chemotherapy during LCRT. However, delivery of oxaliplatin was made non-mandatory and left to the treating center’s discretion starting from 2012 [24,25]. The RAPIDO, Indian and Iranian (Aghili et al. 2020) cohorts used only capecitabine therapy [27,31,32]. Aghili et al., in addition to capecitabine therapy, also utilized consolidative XELOX (3-4 cycle) therapy [27].
The time interval between the completion of radiotherapy and surgical resection was considerably longer with SCRT-CCT (range: 7-26 weeks) than LCRT (range: 4-20 weeks).
The information on treatment compliance was available in all but one included study [24]. It ranged from 56-100% for the SCRT-CCT regimen across cohorts [22,23,25,27-31]. SCRT-CCT group was found to have better compliance than LCRT group in the Indian cohort (dose reduction: 0 vs 8%, delay: 0 vs 5%, p<0.0001 for both) [32]. All other studies found the two regimens to be comparable in this regard.
Outcomes
The details on the outcome measures retrieved is abstracted in table 2 and supplementary table 1.
Table 2:
Outcomes abstracted from included studies.
| Author | Groups | R0 resection% | T downstaging% | pCR% | OS% | DFS/DRTF% | |
|---|---|---|---|---|---|---|---|
| Washington Cohort | Markovina et al. (2017) | Study | NR | 75 | 28 | 96 (3-year) | 80 (3-year) |
| Control | NR | 41 | 16 | 88 (3-year) | 70 (3-year) | ||
| Myerson et al. (2014) | Study | NR | 71 | 28 | NR | NR | |
| Control | |||||||
| Polish Cohort | Ciseł et al. (2019) | Study | 77 | NR | 16 | 49 (8-year) | 43 (8-year) |
| Control | 71 | NR | 12 | 49 (8-year) | 41 (8-year) | ||
| Bujko et al. (2016) | Study | 77 | NR | 16 | 73 (3-year) | 53 (3-year) | |
| Control | 71 | NR | 12 | 65 (3-year) | 52 (3-year) | ||
| Bujko et al. (2013) | Study | 73 | 71 | 21 | NR | NR | |
| Control | 71 | 73 | 8 | NR | NR | ||
| Iranian Cohort | Aghili et al. (2020) | Study | 100 | 54.8 | 32.2 | NR | NR |
| Control | 96.2 | 53.8 | 23.1 | NR | NR | ||
| Aghili et al. (2018) | Study | NR | NR | 30.8 | 65 (3-year) | 55 (3-year) | |
| Control | |||||||
| Baltimore Cohort | Jia et al. (2019) | Study | 69.2 | NR | 35 | NR | NR |
| Control | |||||||
| Danish Cohort | Van Dijk et al. (2013) | Study | 72 | 47 | 26 | 80 (2-year) | NR |
| Control | |||||||
| RAPIDO trial | Bahadoer et al. (2020) | Study | 90 | NR | 28 | 89.1 (3-year) | 23.7 (3-year DRTF) |
| Control | 90 | NR | 14 | 88.8 (3-year) | 30.4 (3-year DRTF) | ||
| Indian Cohort | Thakur et al. (2020) | Study | 92.8 | NR | 0.071 | NR | NR |
| Control | 92.3 | NR | 0 | NR | NR | ||
(pCR = pathological complete response; OS = overall survival; DFS = disease free survival, DRTF = disease-related treatment failure, NR = not reported)
Data on R0 resection rates was provided by all included studies except for those originating from Washington [24-27,30-33]. Across studies, the R0 resection rates ranged from 69.2-100% for SCRT-CCT group and 71-92.3% for LCRT group. There were no statistically significant differences in R0 resection rates between the regimens in any of the comparative studies. The information on T-downstaging was available for the Washington, Polish, Iranian and Dutch cohorts [22,23,26,27,30]. It ranged from 47-75% and 41-75% for the SCRT-CCT and LCRT, respectively. Only Markovina et al. (Washington) reported a statistically significant difference in T-downstaging between the two regimens (75% vs 41%, p<0.001) [22].
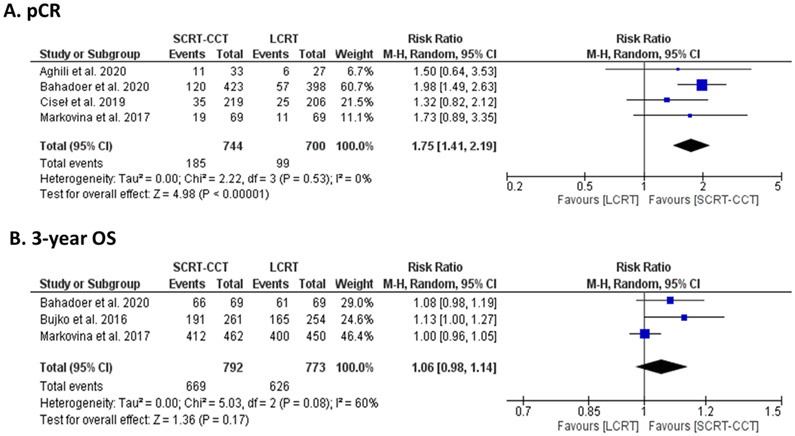
The data on pCR was published for all included cohorts, ranging from 7.1% to 35% and 0% to 23.1% for SCRT-CCT and LCRT regimens, respectively. In the RAPIDO trial, SCRT-CCT was found to result in higher pCR than LCRT (28% vs 14%, odds ratio=2.37, 95% CI:1.67-3.37) [31]. A prospective cohort study (Thakur et al.) found that 6.7% of SCRT-CCT- and 0% of the LCRT-treated patients achieved pCR. The weighted arithmetic mean of pCR were 24.5% and 13.6% for SCRT-CCT and LCRT regimen, respectively. On analysis for the 4 comparative trials, the probability of achieving pCR was found to be higher in the SCRT-CCT-treated patients compared to LCRT-treated patients (RR=1.75, 95% CI: 1.41-2.19, I2=0%). (Figure 2A)
Figure 2:
Forest Plot using the Mantel-Haenszel (M-H) analysis for A) pathological complete response (pCR) and B) 3-year overall survival (OS) based on parallel-arm clinical trials comparing SCRT-CCT to LCRT and reporting latest results for their respective cohorts. (SCRT-CCT = short course radiotherapy followed by consolidation chemotherapy, LCRT = long course radiochemotherapy)
The results of OS were available for the Washington, Polish, Iranian, Dutch and RAPIDO trial cohorts [24,25,28,30,31]. Van Dijk et al. (Dutch) reported an OS of 80% at 2-years after SCRT-CCT regimen [30]. Markovina et al. reported similar 3-year OS between SCRT-CCT and LCRT groups of the Washington cohort (96% vs 88%, p=0.67) [22]. Similarly, Bujko et al. (2016) (73% vs 65%, hazard ratio (HR)=0.73, 95% confidence interval (CI): 0.53-1.01) and Bahadoer et al. (89.1% vs 88.8%, HR=0.92, 95% CI: 0.67-1.25) reported comparable OS at 3-years between the two regimens in the Polish and RAPIDO cohorts [25,31]. Aghili et al. (2018) reported a 3-year OS of 65% with SCRT-CCT regimen in the Iranian cohort [28]. The weighted arithmetic mean of 3-year OS was 83.6% and 80.9% for SCRT-CCT and LCRT regimens, respectively. No statistically significant difference in 3-year OS was observed between the two groups (Risk Ratio (RR)=1.06, 95% CI: 0.98-1.14, I2=60%). (Figure 2B) Cisel et al. also reported OS at 8-years for the Polish cohort of 49% for both regimens [24]. Additionally, data on DFS and disease-related treatment failure (RAPIDO) were reported for 4 included cohorts [22,24,25,27,31]. (Table 2)
Toxicity
The incidence of acute toxicity in SCRT-CCT and LCRT groups varied across studies. However, SCRT-CCT produced comparable postoperative complications and late toxicities in all included studies.
The incidence of acute toxicity was higher in the SCRT-CCT groups of the Washington and RADIPO trial cohorts than LCRT groups. In the Washington cohort, acute grade 3-4 hematological toxicities were higher with SCRT-CCT versus LCRT (22% vs 0%, p<0.001) likely due to use of mFOLFOX6 regimen (6 cycles), which is known to have higher rates of neutropenia [22,34]. Both groups were comparable in terms of any other form of acute toxicities. In the RAPIDO cohort, any acute grade 3+ toxicities occurred in 48% of SCRT-CCT and 25% of LCRT group, possibly due to greater number of CCT cycles (CapeOX (6 cycles) or FOLFOX4 (9 cycles)) given in the former group [31].
On the other hand, acute toxicity rates were lower after SCRT-CCT in the Polish (FOLFOX-4, 3 cycles) and Indian cohorts (CAPEOX, 2 cycles), with both using considerably lower number of CCT cycles than the abovementioned studies. Bujko et al. (2016) reported lower acute toxicities with SCRT-CCT versus LCRT (75% vs 83%, p=0.006) [25]. However, neutropenia was more common after SCRT-CCT regimen (p=0.032) likely due to treatment with FOLFOX-4 regimen [34]. Thakur et al. reported that incidence of any grade 3+ acute toxicities was lower in their SCRT-CCT group than in LCRT group (14.2% vs 61.5%, p=0.011) [32].
Lastly, Aghili et al. (2020) found grade 3+ acute toxicities rates to be similar in their SCRT-CCT and LCRT groups (24.2% vs 22.2%, p=0.55) [27]. These findings are likely related to the use of similar number of consolidation XELOX cycles (3-4 cycles) in both groups.
Risk of Bias assessment and Study quality
Overall, the quality of included studies was classified as low risk of bias. (Supplementary Figure 2) The details related to risk of bias assessment is presented in supplementary material.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis focusing on the sequential SCRT followed by CCT regimen for neoadjuvant treatment of LARC. The reviews performed to date have mainly focused on the standard SCRT regimen and its comparison with LCRT including only brief descriptions of different SCRT schemes such as with induction, consolidation, or concurrent chemotherapy [10,35-38]. This review summarizes the largest number of articles (n = 11) pertaining to the SCRT-CCT scheme. Our review highlights the ability of the SCRT-CCT regimen to achieve improved tumor response (pCR) with comparable R0 resection rates, T-downstaging and OS. Acute toxicity rates between SCRT-CCT and LCRT varied across studies, likely due to differences in CCT regimen and number of cycles given. However, SCRT-CCT appears to result in comparable postoperative complications and late toxicity. Furthermore, patient compliance with SCRT-CCT was also found to be similar to LCRT, with more than 80% of the patients completing the entire planned treatment course across studies. Recent data from the RAPIDO trial demonstrated extremely high compliance rates with the SCRT-CCT regimen [39].
pCR defined as absence of residual cancer in histopathological examination has been long investigated as a surrogate for long-term oncological outcomes. Maas et al. hypothesized that tumors responsive to neoadjuvant therapy possess a prognostically favorable biological profile, which in turn lowers the tendency of recurrence and metastasis, and improves survival [40]. Studies examining this concept have provided evidence supporting prognostication through pCR [41]. A systematic review on LCRT regimen concluded that achieving pCR was associated with statistically fewer local recurrences and distant failures, and better 5-year OS and DFS [42]. However, the rate of distant failure (8.7%) still remains considerably higher than local recurrence rate (0.7%). This presents a rationale for additional systemic therapy in either neoadjuvant or adjuvant setting. Furthermore, utilizing pCR as a prognostic indicator may in the future allow responsive patients to undergo a less radical surgery or a watch-and-wait approach. These less intensive pathways would allow for better organ preservation, an aspect becoming increasingly important in rectal cancer management [43]. Although most studies on non-operative management are related to LCRT, some reports suggest that it may be plausible with SCRT or SCRT-CCT regimens.[29,44].
The pCR rates for SCRT with immediate surgery, with delayed surgery, and LCRT have been reported to be approximately 1.7-7%, 2.7-14%, and 5-25%, respectively [42,45,46]. In this review, the pCR rates for SCRT-CCT regimen ranged from 7.1% to 35% (summary=24.5%) and LCRT regiment from 0% to 23.1% (summary=13.62%). The lack of pCR achievement in LCRT group of Thakur et al. should be interpreted with caution considering the very small sample size (28 patients) and observational design [32]. There are two aspects contributing to improved pCR with SCRT-CCT: 1) longer interval between neoadjuvant treatment and surgery, and 2) additional systemic CCT [47]. Delayed surgery after neoadjuvant treatment has previously been proven to result in superior pCR rates, with similar complications and outcomes, in comparison to immediate surgery [12]. This can be explained by the phenomenon wherein DNA damage of tumor cells occurs during radiotherapy but cell lysis occurs with delay after few weeks [48]. Preclinical studies also suggest that radiotherapy elicits T-cell immune response, which peaks within 8-10 days after irradiation [49]. A recent review suggested that optimal interval between radiotherapy and surgery may be around 11-16 days after SCRT [50]. The interval between completion of SCRT and surgery in the included studies ranged from 7-26 weeks. Furthermore, the addition of neoadjuvant CCT is known to induce local tumor response while also serving as systemic treatment of disseminated disease [51]. A phase II, multicenter trial has shown that pCR rates increased proportionally with longer interval to surgery and increasing number of CCT cycles [52].
Data regarding another important endpoint assessed by major LARC trials, DFS, was reported for three included cohorts [22,24,25,27,31]. In the Washington trials, SCRT-CCT resulted in superior 3-year DFS than LCRT regimen, which was possibly a result of two factors: 1) greater number and earlier (neoadjuvant) delivery of systemic CCT cycles compared to LCRT and 2) better patient compliance with neoadjuvant chemotherapy rather than with adjuvant chemotherapy after a major surgical resection [22]. In this cohort, SCRT-CCT also resulted in greater T-downstaging and pCR, and lower recurrence rates than LCRT. Similar explanation can be given for the lower 3-year disease-related treatment failure with SCRT-CCT noticed in RAPIDO trial [31]. However, this benefit was not noticed in the Polish trials, which reported similar 3-year DFS and other outcomes between the two regimens [24,25]. The relatively fewer CCT cycles (compared to RAPIDO and Washington trials) confounded by a lack of planned adjuvant chemotherapy (left at the local institution’s discretion) might explain these findings [22,25,31]. Additionally, it must be highlighted that, although greater CCT cycles may improve oncological outcomes, they also lead to greater acute toxicities as noticed in RAPIDO and Washington trials and vice-versa in Polish trials.
This review has several limitations. Firstly, the number of patients studied in the included cohorts was small, with only two cohorts comprising of more than 100 patients. Secondly, the treatment protocols for SCRT-CCT and LCRT regimens varied across studies. The effect of various chemotherapy regimens was not taken into consideration while calculating summary estimates. Thirdly, the tumor characteristics of included patients differed slightly across studies, with two studies additionally including patients with metastatic disease. However, the rate of pCR achieved in these studies was comparable with the rest of the included studies. Fourthly, the period of follow-up also varied across studies (range: 1.5 to 7 years). These considerations limits our ability to make precise conclusions in favor or against the SCRT-CCT regimen. Regardless, this review is the first to provide an overview of the current scientific evidence on this topic.
Several clinical trials on this regimen are either ongoing or planned such as STELLAR, LARCT-US, ESCORT, SUNRISE and seven others [16,53-57]. These are likely to shed light on several questions regarding SCRT-CCT such as the optimal CCT regimen and dose, role of adjuvant chemotherapy, its role in replacing traditional LCRT, impact on quality of life, utility in metastatic rectal cancer, and concomitant immunotherapeutic agent use. Preliminary data from the STELLAR trial have been published reporting greater acute toxicities and pCR rates with SCRT-CCT than LCRT, with comparable postoperative complications, compliance rates and R0 resection rates [58-60]. The conference abstracts of included full-text studies covered in the review and from STELLAR trial are summarized in supplementary table.
In conclusion, this systematic review presents encouraging evidence regarding the role of SCRT-CCT in the neoadjuvant treatment of LARC. Current evidence suggests that SCRT-CCT regimen improves pCR and possibly DFS, while having comparable other oncological outcomes, at the cost of increased, but acceptable, acute toxicities. Our findings are in line with the RAPIDO trial, which concluded that SCRT-CCT may now be regarded as a standard of care for LARC patients. This regimen is already gaining acceptance, or is being currently introduced in centers globally. Results from currently ongoing clinical trials are expected to provide confirm its effectiveness and inform regarding its efficient use to improve oncological outcomes and minimize related toxicities, consolidating its position as the standard of care for LARC patients.
Supplementary Material
Acknowledgement:
The authors would like to thank Arthur Gelmis for editorial assistance.
Footnotes
Disclosure of interest: The authors report no conflicts of interest
References:
- [1].Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89–103. 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019;394:1467–80. 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- [3].Gaertner WB, Kwaan MR, Madoff RD, et al. Rectal cancer: An evidence-based update for primary care providers. World J Gastroenterol 2015;21:7659–71. 10.3748/wjg.v21.i25.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Panagiotopoulou IG, Parashar D, Qasem E, et al. Neoadjuvant long-course chemoradiotherapy for rectal cancer: Does time to surgery matter? Int Surg 2015;100:968–73. 10.9738/INTSURG-D-14-00192.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15–24. 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- [6].Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–23. 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- [7].Pietrzak L, Bujko K, Nowacki MP, et al. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: Report of a randomised trial. Radiother Oncol 2007;84:217–25. 10.1016/j.radonc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [8].Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol 2012;30:3827–33. 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- [9].Wiltink LM, Nout RA, van der Voort van Zyp JRN, et al. Long-Term Health-Related Quality of Life in Patients With Rectal Cancer After Preoperative Short-Course and Long-Course (Chemo) Radiotherapy. Clin Colorectal Cancer 2016;15:e93–9. 10.1016/j.clcc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- [10].Kane C, Glynne-Jones R. Should we favour the use of 5 × 5 preoperative radiation in rectal cancer. Cancer Treat Rev 2019;81:101908. 10.1016/j.ctrv.2019.101908. [DOI] [PubMed] [Google Scholar]
- [11].Wang X, Zheng B, Lu X, et al. Preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer: Meta-analysis with trial sequential analysis of long-term survival data. PLoS One 2018;13. 10.1371/journal.pone.0200142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–46. 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- [13].Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 2015;102:972–8. 10.1002/bjs.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fokas E, Allgauer M, Polat B, et al. Randomized phase 2 trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. Ann Oncol 2019;30:JCO1900308. 10.1200/JCO.19.00308. [DOI] [PubMed] [Google Scholar]
- [15].A Prospective Phase II Randomized Clinical Trial of Preoperative Chemotherapy Combined With Short-course Radiotherapy Versus Conventional Neo-adjuvant Therapy for Locally Advanced Rectal Cancer Implemented by MDT - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02941562?term=short+course+radiotherapy&cond=Rectal+Cancer&draw=2&rank=8.
- [16].Evaluation of Efficacy, Quality of Life and Cost Effectiveness of Short-course Radiotherapy Followed by Capecitabine Plus Oxaliplatin chemotheRapy and TME for High-risk Rectal Cancer (ESCORT Trial) - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03676517?term=short+course+radiotherapy&recrs=abdf&cond=Rectal+Cancer&draw=2&rank=2.
- [17].Totally Neoadjuvant FOLFOXIRI + Short-course Radiation + XELOX in Patients With Locally Advanced Rectal Cancer - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03484221?term=short+course+radiotherapy&recrs=abdf&cond=Rectal+Cancer&draw=3&rank=17.
- [18].Phase II Study of Up-front Chemotherapy and Neo-adjuvant Short-course Radiotherapy for Resectable Rectal Carcinoma (COLORE) - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02000050?term=short+course+radiotherapy&cond=Rectal+Cancer&draw=2&rank=9
- [19].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- [21].9.4.2 Principles of meta-analysis. Https://Handbook-5-1CochraneOrg/2011. https://handbook-5-1.cochrane.org/chapter_9/9_4_2_principles_of_meta_analysis.htm.
- [22].Markovina S, Youssef F, Roy A, et al. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int J Radiat Oncol Biol Phys 2017;99:417–26. 10.1016/j.ijrobp.2017.05.048. [DOI] [PubMed] [Google Scholar]
- [23].Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys 2014. 10.1016/j.ijrobp.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ciseł B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation vs. 5 x 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann Oncol 2019;30:1298–1303. 10.1093/annonc/mdz186. [DOI] [PubMed] [Google Scholar]
- [25].Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016;27:834–42. 10.1093/annonc/mdw062. [DOI] [PubMed] [Google Scholar]
- [26].Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, et al. Neoadjuvant treatment for unresectable rectal cancer: an interim analysis of a multicentre randomized study. Radiother Oncol 2013;107:171–177. 10.1016/j.radonc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- [27].Aghili M, Khalili NN, Khalili NN, et al. Short-course versus long-course neoadjuvant chemoradiotherapy in patients with rectal cancer: preliminary results of a randomized controlled trial. Radiat Oncol J 2020;38:119–128. 10.3857/roj.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aghili M, Sotoudeh S, Ghalehtaki R, et al. Preoperative short course radiotherapy with concurrent and consolidation chemotherapies followed by delayed surgery in locally advanced rectal cancer: preliminary results. Radiat Oncol J 2018;36:17–24. 10.3857/roj.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jia AY, Narang A, Safar B, et al. Sequential short-course radiation therapy and chemotherapy in the neoadjuvant treatment of rectal adenocarcinoma. Radiat Oncol 2019;14:147. 10.1186/s13014-019-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762–9. 10.1093/annonc/mdt124. [DOI] [PubMed] [Google Scholar]
- [31].Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2020;22:1:29–42. 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- [32].Thakur N, Seam RK, Gupta MK, et al. A Prospective Observational Study Comparing Long-Course Conventional Neoadjuvant Chemoradiotherapy with Short-Course Radiotherapy Followed by Consolidation Chemotherapy with Delayed Surgery in Locally Advanced Rectal Cancer. South Asian J Cancer 2020;9:80–5. 10.1055/s-0040-1721220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jia AY, Narang A, Safar B, et al. Sequential Short-Course Radiation Therapy and Chemotherapy in the Neoadjuvant Treatment of Rectal Cancer. Int J Radiat Oncol Biol Phys 2019;105:E166. 10.1016/j.ijrobp.2019.06.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grenon NN, Chan J. Managing toxicities associated with colorectal cancer chemotherapy and targeted therapy: A new guide for nurses. Clin J Oncol Nurs 2009;13:285–96. 10.1188/09.CJON.285-296. [DOI] [PubMed] [Google Scholar]
- [35].Rödel C, Trojan J, Bechstein WO, et al. Neoadjuvant short-or long-term radio(chemo)therapy for rectal cancer: How and who should be treated? Dig. Dis 2012;30(e2):102–108. 10.1159/000342038. [DOI] [PubMed] [Google Scholar]
- [36].Mullen TD, Kim EY, Apisarnthanarax S. Short-Course Radiation Therapy Versus Long-Course Chemoradiation in the Neoadjuvant Treatment of Locally Advanced Rectal Cancer: New Insights from Randomized Trials. Curr Colorectal Cancer Rep 2017;13:165–74. 10.1007/s11888-017-0359-4. [DOI] [Google Scholar]
- [37].Qiaoli W, Yongping H, Wei X, et al. Preoperative short-course radiotherapy (5 × 5 Gy) with delayed surgery versus preoperative long-course radiotherapy for locally resectable rectal cancer: a meta-analysis. Int J Colorectal Dis 2019;34:2171–83. 10.1007/s00384-019-03433-9. [DOI] [PubMed] [Google Scholar]
- [38].Ma B, Gao P, Song Y, et al. Short-Course Radiotherapy in Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-analysis. Clin Colorectal Cancer 2018;17:320–330.e5. 10.1016/j.clcc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- [39].van der Valk M, van Etten B, Marijnen C, et al. Compliance, acute toxicity and postoperative complications of short-course radiotherapy followed by chemotherapy and surgery for high-risk rectal cancer. results of the randomized RAPIDO-trial. Eur J Surg Oncol 2020;46:e20. 10.1016/j.ejso.2019.11.472. [DOI] [PubMed] [Google Scholar]
- [40].Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer : a pooled analysis of individual patient data. Lancet Oncol n.d.;11:835–44. 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- [41].Capirci C, Valentini V, Cionini L, et al. Prognostic Value of Pathologic Complete Response After Neoadjuvant Therapy in Locally Advanced Rectal Cancer: Long-Term Analysis of 566 ypCR Patients. Int J Radiat Oncol Biol Phys 2008;72:99–107. 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- [42].Martin ST, Heneghan HM, Winter DC. Systematic review and meta-Analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012;99:918–28. 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- [43].Non-Operative Radiation Management of Adenocarcinoma of the Lower Rectum - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT02641691.
- [44].Duraes LC, Efron J, Gearhart S, et al. When less is more: neoadjuvant short-course intensity-modulated radiation therapy followed by consolidation chemotherapy for rectal cancer is associated with high complete response rate. Dis Colon Rectum 2019;62:E58. [Google Scholar]
- [45].Zhou ZR, Liu SX, Zhang TS, et al. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: A systematic review and meta-analysis. Surg Oncol 2014;23:211–21. 10.1016/j.suronc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [46].Jalilian M, Davis S, Mohebbi M, et al. Pathologic response to neoadjuvant treatment in locally advanced rectal cancer and impact on outcome. J Gastrointest Oncol 2016;7:603–8. 10.21037/jgo.2016.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- [48].Bujko K Timing of surgery following preoperative therapy in rectal cancer: There is no need for a prospective randomized trial. Dis Colon Rectum 2012;55:2012. 10.1097/DCR.0b013e31823f86cb. [DOI] [PubMed] [Google Scholar]
- [49].Frey B, Rückert M, Weber J, et al. Hypofractionated Irradiation Has Immune Stimulatory Potential and Induces a Timely Restricted Infiltration of Immune Cells in Colon Cancer Tumors. Front Immunol 2017;8:231. 10.3389/fimmu.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Glynne-Jones R, Hall M, Nagtegaal ID. The optimal timing for the interval to surgery after short course preoperative radiotherapy (5 ×5 Gy) in rectal cancer - are we too eager for surgery? Cancer Treat Rev 2020;90:102104. 10.1016/j.ctrv.2020.102104. [DOI] [PubMed] [Google Scholar]
- [51].Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 Chemotherapy After Chemoradiotherapy Improves Survival in Patients With Locally Advanced Rectal Cancer: Final Results of a Multicenter Phase II Trial. Dis Colon Rectum 2018;61:1146–55. 10.1097/DCR.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957–66. 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Short Course Radiation Therapy Followed by Pre-operative Chemotherapy and Surgery in High-risk Rectal Cancer - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03729687.
- [54].Zhang MX, Li XB, Guan BJ, et al. Dose escalation of preoperative short-course radiotherapy followed by neoadjuvant chemotherapy in locally advanced rectal cancer: Protocol for an open-label, single-centre, phase i clinical trial. BMJ Open 2019;9. 10.1136/bmjopen-2018-025944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Short Course Radiotherapy Followed Intensive Chemotherapy With Delayed Surgery for Rectal Cancer With Synchronous Distant Metastasis - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01923987?term=short+course+radiotherapy&cond=Rectal+Cancer&draw=2&rank=7.
- [56].Short-course Radiotherapy (5×6Gy/7Gy/8Gy) Followed by Neo-adjuvant Chemotherapy for Locally Advanced Rectal Cancer - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03466424?term=%28short+course+radiotherapy%29+AND+%28consolidation+OR+preoperative+OR+chemotherapy%29&recrs=abdf&draw=3&rank=1. [DOI] [PMC free article] [PubMed]
- [57].Short Course Radiotherapy Combined With Chemotherapy in Stage IV Rectal Cancer With Resectable Liver Metastases - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02510378?term=%28short+course+radiotherapy%29+AND+%28consolidation+OR+preoperative+OR+chemotherapy%29&recrs=abdf&draw=3&rank=5.
- [58].Jin J, Liu S, Zhu Y, et al. 5 x 5 Gy and consolidation chemotherapy vs long-course preoperative chemoradiation for locally advanced rectal cancer: An interim analysis of a randomized phase III study. J Clin Oncol 2017;35. 10.1200/JCO.2017.35.15_suppl.e15006. [DOI] [Google Scholar]
- [59].Jin J, Jin J, Tang Y, et al. The initial results for a phase III study of short-term radiotherapy plus chemotherapy vs long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR trial). J Clin Oncol 2016;34:e15000–e15000. 10.1200/jco.2016.34.15_suppl.e15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jin J, Liu S, Zhu Y, et al. The Updated Results for the Phase 3 Study of 5×5 Gy Followed By Chemotherapy in Locally Advanced Rectal Cancer (STELLAR trial). Int J Radiat Oncol 2017;99:E157. 10.1016/j.ijrobp.2017.06.976. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.