Abstract
Glucose-6-phosphate dehydrogenase (G6PD) maintains redox balance in a variety of cell types and is essential for erythrocyte resistance to oxidative stress. G6PD deficiency, caused by mutations in the G6PD gene, is present in ~400 million people worldwide, and can cause acute hemolytic anemia. Currently, there are no treatments for G6PD deficiency. We discuss the role of G6PD in hemolytic and non-hemolytic disorders, treatment strategies attempted over the years, and potential reasons for their failure. We also discuss potential pharmacological pathways, including glutathione metabolism, compensatory NADPH production routes, transcriptional upregulation of the G6PD gene, highlighting potential drug targets. The needs and opportunities described here may motivate the development of a therapeutic for hematological and other chronic diseases associated with G6PD deficiency.
Keywords: G6PD deficiency, therapeutic strategy, transcriptional regulators, enzyme activators, NAC
G6PD Deficiency as a Hematologic Disorder
Glucose-6-phosphate dehydrogenase (G6PD; see Glossary) regulates glycolytic flux through the pentose phosphate pathway (PPP) and plays a central role in redox homeostasis; it produces nicotinamide adenine dinucleotide phosphate (NADPH), an essential cofactor for glutathione (GSH/GSSG) regeneration. G6PD is a major source of NADPH and loss of G6PD function is detrimental in erythrocytes; it causes G6PD deficiency (G6PDdef), a potentially hemolytic disorder [1].
G6PDdef is an X-linked genetic disorder present in 300-400 million people worldwide [2]. To date, over 200 non-synonymous mutations in the G6PD gene have been reported [3] and in attempt to understand the genotype/phenotype correlation, the majority of mutations have been classified according to their residual enzymatic activity and clinical outcome as outlined by the WHO: Class I, <10% residual activity with chronic hemolytic anemia (CHA), Class II, <10% residual activity with intermittent acute hemolytic anemia (AHA), and Class III, 10-60% residual activity with AHA in response to an oxidative trigger [4]. Although Class I variants are the most severe, they are rare. Their low allele frequency is likely the result of G6PDs essential role in development; knock-down of G6PD is embryonic lethal in mice [5]. In contrast, Class II and III variants are more common, however they are largely asymptomatic throughout life when following preventative measures – dietary/treatment restrictions [1].
Although Class I mutations are rare and Class II/III largely asymptomatic, self-resolution from AHA is not guaranteed and blood transfusions not always viable. Additionally, treatment with 8-aminoquinolines, the only anti-malarial drugs capable of eliminating the dormant liver forms of Plasmodium Vivax, induces AHA in G6PDdef, with G6PDdef found in geographic areas with a high incidence of malaria [6,7]. This, combined with lack of reliable testing for G6PDdef, presents a major challenge in the eradication of malaria [8,9]. Thus, prophylactic or combination therapies may be attractive strategies for treatment of G6PDdef and the eradication of malaria.
Although hemolysis is the main human pathology associated with G6PDdef, G6PDdef has been implicated in other chronic diseases [10]. Additionally, G6PDdef has been thought to contribute to Coronavirus Disease 2019 (COVID-19) complications. Despite these health risks, G6PDdef is not routinely screened for and there are no active efforts to develop treatments to address it. The belief that common variants have a mild pathology that can be overcome by avoiding trigger foods and drugs or treated with blood transfusions during a hemolytic crisis hinders the development of a therapeutic. Here, we review past treatment strategies and discuss molecular pathways and potential drug targets that may motivate the development of therapies for G6PDdef-related disorders.
The effect of G6PDdef in Non-Hemolytic Disorders
The contribution of G6PDdef to the pathophysiology of chronic diseases will only be briefly discussed, as dedicated reviews exist for G6PDdef in viral infections [11], hyperbilirubinemia/kernicterus [12], diabetes [13], cardiovascular disease [14] and neurodegeneration [15].
Viral Infections, including COVID-19
G6PDdef may increase cell susceptibility to viral infections such as hepatitis B [16], dengue fever, enterovirus 71, and coronavirus 229E [11]. Additionally, viral infections may trigger hemolytic complications in G6PDdef patients [17-19]. Recently, two case-reports found that COVID-19 triggered hemolysis and methemoglobinemia in G6PDdef patients without an identifiable eliciting drug [20,21]. Methemoglobinemia has been reported in other COVID-19 cases [22] and anti-malarial drugs known to induce hemolysis in G6PDdef, hydroxychloroquine and chloroquine, have been used to treat COVID-19. To date, seven case studies reported hydroxychloroquine/chloroquine triggered hemolysis in G6PDdef COVID-19 patients [23-27].
Neonatal Jaundice, Kernicterus, Hyperbilirubinemia
G6PDdef is a major and well-known risk factor for jaundice and hyperbilirubinemia in newborns as hemolysis increases bilirubin levels in the serum [28]. In newborns, bilirubin can cross the blood brain barrier and cause neurological issues such as kernicterus or acute bilirubin encephalopathy [29]. Interestingly, newborns with kernicterus have a higher chance of developing sensorineural hearing loss [30], suggesting a role of G6PDdef in auditory disorders. Currently, the pathophysiology for kernicterus/hyperbilirubinemia-induced sensorineural hearing loss is unknown and only a few studies have explored the role of G6PDdef in auditory disorders [31,32]. Additionally, the role G6PD plays in kernicterus-induced brain damage remains elusive.
Diabetes
High glucose levels inhibit G6PD activity and increases oxidative stress [33]. Glucose-mediated G6PD inhibition reduced insulin secretion from ß-islets and induced ß-cells apoptosis, with overexpression of G6PD rescuing high glucose mediated dysfunction [34]. Additionally, glucose-mediated G6PD inhibition resulted in elevated ROS in podocytes resulting in podocyte injury [33], a complication of diabetes that can lead to diabetic kidney disease [35]. These findings suggest that glucose-mediated inhibition of G6PD and G6PDdef contribute to loss of ß-cell function and may increase susceptibility to diabetic complications. G6PDdef has been reported as a risk factor for diabetes [14].
Cardiovascular Diseases
Aldosterone has been found to inhibit G6PD activity, with G6PD inhibition reducing bioavailable nitric oxide, vascular reactivity, and the contractility of vascular smooth muscle cells, coronary arteries and cardiomyocytes [14]. Additionally, G6PD activity modulates genes related to thrombosis, atherosclerosis, contractility, and blood vessel calcification [36] and mice with reduced G6PD activity develop spontaneous pulmonary hypertension with pulmonary artery and right heart remodeling [37]. However, it remains unclear if dysfunctional reactivity and contractility caused by G6PDdef lead to a pathogenic phenotype in humans as G6PDdef has been reported to be both cardio-protective and cardio-damaging [14,38].
Neurodegenerative Disorders
Neurons are highly dependent on G6PD and the PPP to combat ROS [15]. Inhibiting G6PD in neurons increases ROS, induces apoptosis, and induces neurodegeneration in both aging and neurodegenerative disease models [39-41]. A recent genome-wide CRISPR screen identified loss of G6PD function as synthetic lethal when combined with mitochondria dysfunction [42], a known contributor to the pathogenesis and pathophysiology of neurodegenerative disorders [43].
Interventions to Address G6PD Deficiencies
Antioxidants
Ascorbate
Ascorbate, more commonly known as vitamin C, is a potent antioxidant; however, it has prooxidant effects in human erythrocytes in vivo [44] and high-dose ascorbate (e, g., ≥ 6mg/day [45]) induces AHA in G6PDdef individuals (Table 1). Despite this, lower doses have been used to treat methemoglobinemia in G6PDdef patients (Table 1) [23,46-48]; four case studies reported use of ascorbate to treat methemoglobinemia induced by rasburicase or other AHA triggers (Table 1). In all cases, ascorbate resolved methemoglobinemia. However, blood transfusions were used as co-treatments, masking the safety and efficacy profile of low-dose ascorbate.
Table 1.
Summary of clinical cases reporting the use of ascorbate in G6PDdef.
| Hemolytic Trigger |
Dose | Treatment | Dose | Response to Trigger | Type of Study | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbate | 80g/day, IV | Blood transfusion | X | X | X | X | X | case study | [118] | ||
| Ascorbate | 40g/3x/week, IV + 20-40g/daily/oral | X | X | X | case study | [119] | |||||
| Increased to 80g/3x/week, IV | |||||||||||
| Ascorbate | 30g/day, IV | Blood transfusion | 8 days | X | X | case study | [120] | ||||
| 60g/day, IV | |||||||||||
| Fava Beans Ascorbate | ~10 beans | X | case study | [121] | |||||||
| 250-500mg/day, oral | |||||||||||
| Ascorbate | 4-6g/6hr, oral | Blood transfusion | X | X | case study | [122] | |||||
| Ascorbate | 30g/day, IV | Blood transfusion | X | X | X | X | case study | [123] | |||
| Ascorbate | 75g/day, IV | Blood transfusion | X | X | X | X | case study | [124] | |||
| Ascorbate | 2g/day, IV | - | X | X | case study | [125] | |||||
| Rasburicase | 6mg | Ascorbate | 5g/6hrs, IV | X | X | X | case study | [46] | |||
| Blood transfusion | |||||||||||
| Rasburicase | 6mg | Ascorbate | 1g/day, oral | X | X | X | case study | [47] | |||
| Blood transfusion | |||||||||||
| Chloroquine + Covid-19 | 600mg/day | Ascorbate | 1g/4x/day | X | X | case study | [23] | ||||
| Blood transfusion | |||||||||||
| Fava Beans Ciprofloxacin Acetaminophen | 500mg/2x/daily 1000mg/3x/daily |
Ascorbate | 1g/4x/day | X | X | X | X | case study | [48] | ||
| Blood transfusion | |||||||||||
| Hemolysis/Anemia | Dark Urine | Methemoglobinemia | Respiratory Distress /↓O2 | Death | Renal Failure | ||||||
A proposed mechanism for high-dose, ascorbate-induced, AHA in G6PDdef is described (Figure 1). Upon oxidant exposure, ascorbate is oxidized to dehydroascorbic acid (DHA) in the plasma and DHA is rapidly uptaken by erythrocyte glucose transporter 1 (GLUT1). Once inside, DHA is reduced to ascorbate by GSH, reducing intracellular GSH levels. Additionally, ascorbate participates in iron redox reactions; upon oxidation of the hemoglobin (Hb) iron, ascorbate can interact with the Fe3+ of methemoglobin, resulting in its oxidation to an ascorbate radical and concomitant reduction of MetHb (Fe+3) to OxyHb (Fe+2). Consequently, methemoglobinemia is inhibited; however, hyperoxy radicals, generated from the iron redox reaction, remain uncaptured [49]. Thus, both DHA reduction and hyperoxy radicals generated via iron redox reactions contribute to a hemolytic phenotype in G6PDdef patients. Additionally, ascorbate, DHA, and glucose are all internalized by GLUT1; therefore, ascorbate may alter glucose uptake and consequent PPP-mediated GSH recycling, especially in G6PDdef.
Figure 1.
A potential mechanism for ascorbate induced AHA in G6PDdef. a) Ascorbate concentration increases in the plasma as a result of IV or oral administration. b) In the presence of oxidative stress, ascorbate is oxidized to DHA in the plasma. c) Subsequently, DHA and ascorbate are imported into erythrocytes, with DHA having a higher preference for import. d) Once inside, DHA is reduced by GSH, depleting the already small GSH pool in G6PDdef. e) The ascorbate, imported from the plasma or generated via DHA reduction, can interact with oxidized iron to generate radicals. f) Both GSH depletion and iron-generated radicals likely contribute to cell damage and a hemolytic phenotype in G6PDdef.
Ascorbyl 6-palmitate (A6P), a lipid-soluble ester analog of ascorbate, acts as an antioxidant in erythrocytes in vitro [50,51] and increases ascorbate concentration in neuronal tissues, suggesting A6P crosses the blood brain barrier [52]. Other amphiphilic forms of ascorbate may have pharmacological value for the treatment of neurodegenerative and auditory disorders associated with G6PDdef, since they may cross tightly controlled barriers [53]. It remains unclear whether they will provide greater benefits than other lipid-soluble antioxidants such as vitamin E.
α-Tocopherol
α-Tocopherol, or vitamin E, is a lipid-soluble antioxidant which protects the cell membrane against oxidative stress. In G6PDdef patients, α-tocopherol improved hematological parameters (Table 2); it increased erythrocyte half-life (from 23 to 25 days), Hb levels, and reduced reticulocytosis in the most severe Class I and II G6PDdef [54-56]. However, challenging patient erythrocytes in vitro with H2O2 revealed no change in the degree of hemolysis, despite improvements in hematological parameters [55]. Additionally, three smaller clinical studies reported α-tocopherol did not improve hematological parameters or patient outcome [54,57,58] (Table 2). Although α-tocopherol efficacy remains inconclusive and studies have yet to be revisited, this is the first long-term preventive therapy attempted for G6PDdef. Additionally, treatment with α-tocopherol appeared safe at the indicated doses, suggesting that AHA is not a common feature of antioxidants.
Table 2.
Summary of clinical reports using antioxidants or NAC in G6PDdef.
| Treatment | Dose | Duration | Outcome | Type of Study | Reference |
|---|---|---|---|---|---|
| α-tocopherol | adults: 800IU/day, oral children: 400 IU/day, oral |
60 days | ↑ Hb levels ↑ erythrocyte number ↑ packed cell volume ↓ reticulocytosis ↓ serum bilirubin |
not registered | [126] |
| α-tocophero-acetate | 800 IU/day, oral | 12 weeks, 1 year | ↑ Hb levels ↑ erythrocyte half-life ↓ reticulocytosis |
not registered | [55] |
| α-tocophero-acetate | 1000 IU/day, oral | 24 weeks, 1 year | no improvements | [127] | |
| α-tocopherol | 800 IU/day, oral | 8 weeks | ↑ Hb levels ↑ erythrocyte half-life ↓ reticulocytosis |
not registered | [56] |
| α-tocopherol-acetate | 800 IU/day, oral | 16 weeks | ↑ erythrocyte survival ↓ reticulocytosis Failed to abolish hemolytic process |
case study | [54] |
| α-tocopherol | 400 IU/day, oral | 4 weeks | no improvements | case study | [58] |
| α-tocophero-acetate | 2000 IU/day, oral | 4 weeks | no improvements | case study | [57] |
| α-lipoic Acid | 600mg/day, oral | 28 days | ↑ GSH, TAC, and catalase activity ↓ lipid peroxidation in both groups |
not registered | [62] |
| α-lipoic Acid | 600mg/day, oral | 28 days | ↑ TAC at resting did not increase response to acute exercise stress | NCT02937363 | [63] |
| N-acetylcysteine | initial dose-10g, IV 5g/4hr, IV |
56 hrs | ↑ hemolysis, unclear if due to NAC | case study | [128] |
| N-acetylcysteine | 1200mg/x2, oral | 1 year | ↑ brief psychiatric rating score | case study | [129] |
| N-acetylcysteine | 10g/x3/day, IV 600mg/x2/day, IV 600mg/x2/day, IV |
1 day 7 days 5 days |
↓ hemolysis indices after each treatment | case study | [25] |
Astaxanthin and ß-Carotenoids
Astaxanthin, a lipid soluble carotenoid pigment, increased the activity of purified G6PDWT in a dose-dependent manner, with a maximum 30% activation achieved at 0.64 mM astaxanthin, suggesting a direct mechanism [59]. Other carotenoids have been shown to exert antioxidant effects in erythrocytes in vivo, though they did not protect against GSH depletion [60,61].
α-Lipoic acid
Clinical studies find α-lipoic acid (ALA) improves erythrocyte total antioxidant capacity (TAC) in G6PDWT and G6PDdef subjects (NCT02937363i) [62,63]. However, although ALA improves TAC in the context of exercise induced oxidative stress, it does not change erythrocyte redox response to acute exercise (Table 2) [1]. Taken together, ALA improves TAC in G6PDWT and G6PDdef at baseline; however, like α-tocopherol, it may not improve response in the face of an acute stressor.
Lessons from Antioxidant Treatments
To date, the majority of treatments have focused on antioxidant therapies; however, these interventions have either triggered hemolysis (ascorbate) or have not prevented AHA in the face of a stressor (α-tocopherol, ALA), despite improvements in hematological parameters. It remains unclear if basal hematological indexes translate into meaningful clinical endpoints. A more appropriate measurement may be a surrogate endpoint; a carefully designed in vitro stress assay on blood withdrawn from human subjects starting and following a treatment regimen. Though, this may present challenging since drugs that cause AHA in vivo may not translate in vitro. A surrogate endpoint may reduce the duration length and cost of clinical trials and be a better indicator of drug efficacy with hematological indices better served as secondary endpoints. Additionally, animal models of G6PDdef may serve as good preclinical models for testing safety and efficacy of new therapeutics; zebrafish, mouse, and C. elegans [1].
GSH Metabolism and NAC Supplementation
Whereas NADPH-dependent GSH regeneration is one way by which the GSH pool can be restored, other metabolic pathways are capable of generating GSH. Studies reveal G6PDdef erythrocytes are metabolically different. At baseline, they have elevated levels of glycolytic metabolites related to NADH and ATP production, with a net increase in ATP levels [64]. However, when challenging G6PDdef erythrocytes with the oxidant diamide, GSH is depleted, and erythrocyte metabolism shifts towards GSH biosynthesis. Consequently, glutamate levels are elevated and metabolites related to ATP consumption increase, triggering glucose uptake by GLUT1 [65]. Yet, in G6PDdef, metabolic reprogramming is insufficient to restore GSH levels towards those seen in G6PDWT individuals [64].
Modeling GSH metabolism in erythrocytes predicts that a 50% increase in plasma L-cysteine (LC) will improve GSH levels when the GSH oxidation rate is increased by 20% [66]. However, a 50% increase in plasma LC levels may be difficult to achieve in vivo; high doses of LC are required to compensate for poor bioavailability but are limited by toxicity [67]. N-acetyl-cysteine (NAC), an LC prodrug, has been developed to improve LC bioavailability and can be delivered orally at high doses, with minimal side effects [68]. In the plasma, NAC undergoes redox exchange reactions with L-cystine, to generate plasma LC at a concentration capable of achieving the maximal rate of GSH synthesis in erythrocytes [69] and LC/NAC increase the rate of GSH biosynthesis in erythrocytes in vitro [70]. However, despite its reputable role as a therapeutic antioxidant, few cases report the use of NAC to treat G6PDdef (Table 2).
Recently, a case-study reported a G6PDdef patient with COVID-19 was treated with hydroxychloroquine, which triggered AHA, and NAC resolved hematological indexes and hemolysis on three different occasions [25]. The patient received 90 g of intravenous (IV) NAC, which immediately improved hematological indexes. One week after NAC treatment was discontinued, hematological indexes began to decline, and the patient received 1.2 g daily IV NAC for one week. Again, NAC quickly resolved hematological indexes. Upon discontinuation of the second round of NAC, hematological indexes declined with NAC treatment improving hematological indexes for a third time, suggesting NAC may be beneficial for the treatment of G6PDdef in AHA.
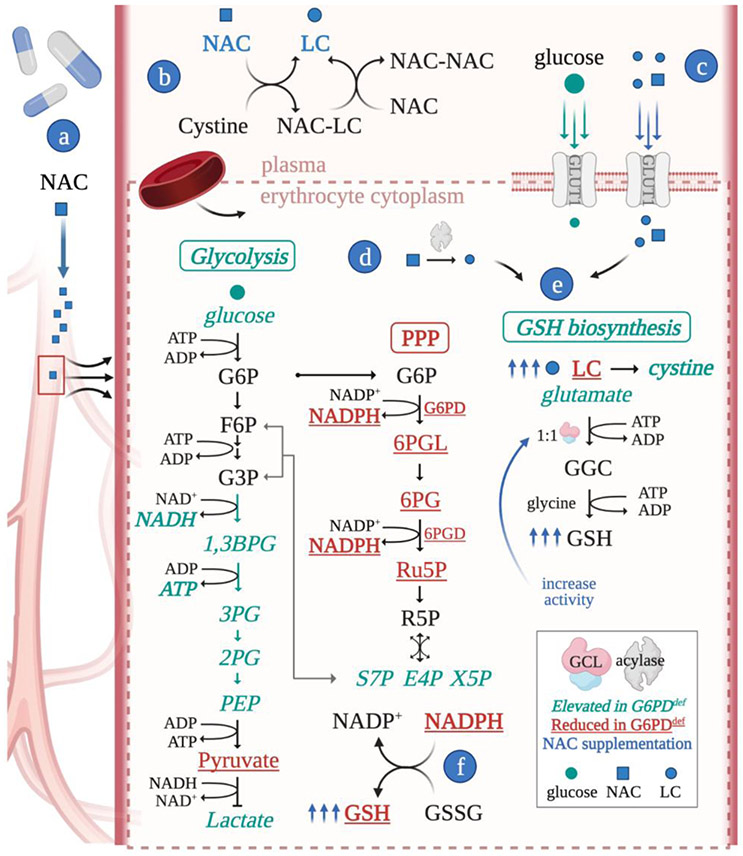
The use of LC or NAC as a therapeutic for G6PDdef may expand to other G6PDdef-related disorders as LC increases expression of G6PD, glutamate-cysteine ligase modifier subunit (GCLM), glutamate-cysteine ligase catalytic subunit (GCLC), in G6PDdef monocytes and endothelial cells [71,72]. A combination therapy including oral NAC and a GCL activator, the rate limiting enzyme of GSH biosynthesis, may improve GSH levels in erythrocytes, protecting them against oxidative stress. Mathematical modeling of erythrocyte metabolism estimates a 50% increase in GCL Vmax may reduce GSH pool depletion induced by an oxidative stressor [66]. A figure summarizing G6PDdef erythrocyte metabolism and potential modulation via NAC is presented (Figure 2).
Figure 2.
Metabolic rewiring in G6PDdef erythrocytes and NAC as a potential therapeutic route. G6PDdef erythrocytes have reduced (red) and elevated (teal) metabolites, favoring GSH production, however LC is limiting. a) NAC concentration increases in the plasma as a result of IV or oral administration. b) NAC undergoes redox exchange reactions in the plasma to generate LC. c) LC and NAC are imported into erythrocytes, where (d) NAC is deacylated to produce LC. (e) LC is used for GSH biosynthesis, (f) which may restore the GSH pool. NAC treatment combined with a GCL activator may maximize GSH biosynthesis and overcome GSH imbalance in G6PDdef erythrocytes. Abbreviations: 1,3 BPG, 1,3-bisphosphoglycerate; 2PG, 2-phosphoglycerate; 3PG, 3-phosphoglycerate; 6PG, 6-phosphogluconate; 6PGD, 6-phosphogluconate dehydrogenase; 6PGL, 6-phosphogluconolactone; E4P, erythrose 4-phosphate; F6P, fructose 6-phosphate; GGC, gamma-glutamylcysteine; G6P, glucose 6-phosphate; G3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; R5P, ribose 5-phosphate; Ru5P, ribulose 5-phosphate; S7P, sedoheptulose 7-phosphate; X5P, xylulose 5-phosphate.
Generation of NADPH via Complementary Pathways
NADPH is regenerated in the cytosol via three metabolic routes: G6PD utilizes glucose to produce NADPH via the PPP, whereas malic enzyme 1 (ME1) and isocitrate dehydrogenase 1 (IDH1) use tricarboxylic acid (TCA) cycle intermediates; ME1 and IDH1 are reliant on mitochondrial redox balance and subsequent substrate trafficking to the cytosol [73]. The contribution and responsiveness of each pathway to NAPDH production varies according to tissue and cell type as well as genetic and environmental pressures. For example, erythrocytes and neurons strongly rely on G6PD whereas adipocytes and liver cells are more reliant on IDH1 and ME1 [14,74,75]. Furthermore, adipocytes rely more on G6PD under hypoxic conditions [74]. The lack of complimentary pathways to recycle NADPH increases cell susceptibility to oxidative stress.
In erythrocytes, the absence of mitochondria limits the contribution of IDH1 and ME1. Although erythrocytes lack mitochondria, they still express TCA cycle enzymes as well as IDH1 and ME1 [76] and are capable of metabolizing TCA intermediates to generate NADPH. Precursors of complementary routes have been found to accumulate in G6PDdef erythrocytes, suggesting ME1 and IDH1 pathways are activated to maintain NADPH levels [64]. However, this is insufficient to counteract erythrocytes vulnerability to oxidative stress in G6PDdef.
It remains to be determined whether boosting complimentary routes with exogenous substrates, such as citrate, or genetic/pharmacological interventions to increase ME1 or IDH1 levels and/or activity, is sufficient to counteract the excessive oxidative stress and hemolysis seen in G6PDdef erythrocytes. In this case, the development of small molecule activators of complimentary routes, focusing on regenerating NADPH, might become a feasible strategy to improve the antioxidant capacity of G6PDdef in erythrocytes as well as other cell types associated with chronic disorders.
G6PD Upregulation via Transcriptional Regulators
During erythropoiesis, erythroblasts undergo successive changes, including nuclear extrusion [77]. Although this process is essential for erythrocyte function, this presents a therapeutic challenge as erythrocytes are incapable of synthesizing new protein. However, targeting transcription in early-stage erythroblasts, before nuclei extrusion, or in other cell types with nuclei, is an attractive strategy for increasing G6PD protein levels and treating conditions associated with G6PDdef.
Histone deacetylase inhibitors (HDACi)s have gained attention for their ability to modulate protein transcription, and in the context of G6PD, have been shown to increase PPP flux by upregulating and downregulating G6PD expression in cancer cells [78,79]. Interestingly, treatment of patient derived erythroblasts and non-hemopoietic cells with HDACi sodium butyrate (NaBu) increased transcriptional upregulation of the G6PD gene, while having no effect on other genes in the glycolytic or PPP pathway. The specificity of G6PD induced upregulation was attributed to increased histone hyperacetylation by histone acetyltransferases (HAT)s and increased HDAC, HAT, DNA polymerase II, and SP1 (transcription factor) binding to the G6PD core promoter (Figure 3). SP1 binding induced G6PD transcription, mRNA production, and protein translation, which lead to a subsequent increase in Class I-III G6PDdef protein levels and activity, restoring it to that of WT or greater. Suberoylanilide hydroxamic acid (SAHA), another HDACi, produced similar results to NaBu, demonstrating transcriptional activation is not HDACi specific [80-82].
Figure 3.
The process and consequence of HDACi-mediated transcriptional activation of the G6PD gene in erythroid cells. a) HDACi exposes the G6PD core promoter; b) SP1, HAT, HDAC, and DNA polymerase localize to the core promoter to induce G6PD transcription in erythroid precursor cells and c,d) subsequently increase protein levels in new erythrocytes. However, HDAC5 and HDAC2 have been found to disrupt erythropoiesis and therefore, inhibition of these two HDACs may have adverse side effects. Other transcriptional modulators, such as a Nrf2/Keap1 protein-protein-interaction inhibitor, may also increase G6PD transcriptional output. e) G6PD variant biochemical properties will likely influence the efficacy of a transcriptional activator, with protein stability being a major determinant of residual protein levels in aging erythrocytes.
Although HDACis are capable of restoring Class I-III G6PD variant activity to that of WT or greater in erythroid precursor cells, histone deacetylation is important for DNA compression, nuclear extrusion, and erythroid maturation [83-85]. Therefore, it is not surprising that side-effects reported for multiple FDA approved HDACis include thrombocytopenia and anemia [86]. To date, two HDACs have been implicated in erythropoiesis: HDAC5 [84] and HDAC2 [87]. An HDACi that does not target HDAC5 or HDAC2 may limit side effects – if inhibition is not required for transcriptional upregulation of the G6PD gene. Improving HDAC isoform selectivity is a common strategy to reduce off-target effects and improve the safety profile of HDACis [88].
Due to their cytotoxic effects, the majority of HDACis are approved for use in hematological and neoplastic malignancies [89]. However, the use of HDACis in non-neoplastic indications is expanding; HDACis are in early stage clinical trials for Niemann-Pick type C1 (NCT02124083i), Alzheimer’s (NCT03056495i), epilepsy (NCT03894826i), and Crohn’s disease (NCT03167437i) [90], with Depakene® (valproic acid) being used as a first-line therapy for migraine prophylaxis and found to be well-tolerated [91,92]. The use of HDACis at their current stage may not be ideal for G6PDdef patients, however, the development of more selective HDACis may circumvent toxicity. Additionally, the use of HDACis provide a proof-of-concept; transcriptional upregulation increases G6PD activity in erythrocyte precursor cells. Therefore, other transcriptional targets, with improved target specificity, may reduce off target effects while providing benefit to G6PDdef patients.
A second molecular target of interest is transcription factor nuclear erythroid receptor 2 (Nrf2). Under basal conditions, Nrf2 levels are suppressed by Keap1-dependent-ubiquitination and subsequent proteasomal degradation. However, in the presence of electrophiles and oxidants, cysteine modifications deactivate Keap1 and in turn increase Nrf2 levels. Nrf2 exerts antioxidant effects by binding to antioxidant response elements and upregulating transcription of antioxidant defense enzymes, including G6PD [93].
Currently, Tecfidera® (dimethyl fumarate), indicated for use in multiple sclerosis, is the only FDA approved activator of Nrf2. Natural products such as curcumin, sulforaphane, and flavonoids have also been shown to activate Nrf2 [94,95]. Unfortunately, these molecules are electrophiles and lack target specificity, making them toxic [96,97]. A small molecule or peptide inhibitor of the Keap1/Nrf2 protein interaction may increase target selectivity and overcome toxicity and remains an attractive target [98]. As with all drugs, too much of anything can be a bad and chronic activation of Nrf2 may cause reductive stress [93].
AHA caused by G6PDdef is largely described by two biochemical enzyme parameters: catalytic activity and enzyme stability [99,100]. Protein stability is particularly important in erythrocytes since they are anucleate with a 120-day lifespan devoid of protein synthesis. We foresee transcriptional modifiers to be most beneficial for G6PD variants which maintain enzymatic stability, such as Class II and III variants; protein stability will ensure increased protein levels achieved by transcriptional upregulation will be maintained for significant period of time. Although Class I variants generally have reduced stability, it has yet to be determined whether a significant increase in G6PD activity for new erythrocytes, regardless of stability, will be sufficient to improve a CHA phenotype. Additionally, transcriptional regulators may be beneficial in cell types that have nuclei and are associated with G6PDdef related chronic disorders.
Small Molecule Activators of Clinical G6PD Variants
Recently, we sought a structural chaperone that could correct the most common variants and identified one lead compound, AG1. AG1 improved the activity and stability of several Class II and Class III variants, including the common Mediterranean and A− and Canton, by stabilizing dimeric G6PD in vitro, in cellulo, and in vivo models [101]. As a result, AG1 increased NADPH and GSH levels, reduced ROS, and protected cells from oxidative stress.
These results demonstrate that a single small molecule can partially correct the catalytic activity and stability of multiple common G6PD variants. Although AG1’s effect on catalytic activity is important, stabilization of dimeric G6PD is likely an important feature; enzyme stability is a major determinant of residual activity in erythrocytes [99,100]. Therefore, a small molecule that increases the stability of G6PD may be an effective therapeutic, though its efficacy may not be evident until tested in an in vivo model.
The discovery of AG1 has inspired the pursuit of other G6PD structural chaperones. In recent computational studies, AG1 was used as a scaffold for a structure-based pharmacophore screen where five compounds were identified as potential activators of G6PD [102]. In addition, several AG1 analogs were developed; although none activated G6PD better than AG1, several displayed AG1-like activation and are better suited for in vivo studies – they lack a disulfide bond [103].
Recently, the structure of the most severe Class I variants revealed a common structural defect [104]. This common defect in Class I variants presents an opportunity for a single molecular chaperone that corrects this common defect. Future work focuses on search for activators of most severe Class I variants and identifying a small molecule that stabilizes the tetrameric state; it has been shown tetrameric G6PD has increased catalytic efficiency over dimeric G6PD [105]. Such compounds may also be beneficial in chronic diseases that are associated with the downregulation of G6PD [106], reduced GSH levels [107], or increased ROS [108], such as neurodegenerative disorders [15], but do not have G6PDdef, as demonstrated by overexpression studies [109,31].
Gene Therapy
Gene therapy is gaining recognition for its use in inherited genetic disorders and gene therapies for cystic fibrosisii, spinal muscular atrophyiii, and retinal dystrophyiv have already received FDA approval, with therapies for sickle cell anemia and ß-thalassemia in clinical trials [110]. Recently, Zynteglo® (betibeglogene autotemcel), a gene therapy for ß-thalassemia, received conditional market approval in Europe [111,112]. Approval was granted after Zynteglo® reversed blood transfusion dependency in 12 of 13 patients in early phase clinical trials [113]: a remarkable feat for genetic hematological therapies.
A gene therapy for G6PDdef will likely overcome the therapeutic challenges that arise from the genetic diversity of the disorder. However, the development of a gene therapy for G6PDdef presents a major challenge; the cost per Zynteglo® treatment course is set at $1.8 million with high costs common in gene therapy [114]. Although rare, G6PDdef Class I variants have a severe phenotype, impacting the patient’s quality of life [115,116]. Early studies find retroviral transduction of the human G6PD gene into mouse and human hematopoietic stem cells resulted in stable lifelong expression of human G6PD in primary and secondary bone marrow transplant recipient mice, increasing G6PD activity two-fold [117]. suggesting gene therapy for the most severe variants of G6PDdef is warranted.
Concluding Remarks and Future Perspectives
Although G6PD-associated enzymopathy has been discovered more than 60 years ago, there are still no therapies for the treatment of G6PDdef. The fact that the most severe form of G6PDdef is rare and although Class II and III variants are common, they are often asymptomatic, unless exposed to an oxidative stressor, reduces interest by pharmaceutical companies. Additionally, the large number of mutations and divergent consequences on protein structure, make a “one-size fits all” therapy unlikely. Even with a single point mutation, correcting enzyme dysfunction is inherently challenging.
Past failures with antioxidant therapies make this strategy less attractive with the majority of explored therapies not having reached the stage of clinical trials. Therapeutic routes warranting further investigation include: 1) NAC with the potential for a combination therapy; a GCL activator or transcriptional regulator; 2) modulation of NADPH pathways; 3) transcriptional regulators of G6PD such as HDACi or Nrf2; 4) small molecule chaperones that activate G6PD directly and 5) gene therapy. Due to high treatment costs, gene therapy is likely a good candidate for the most severe Class I variants, with non-genetic approaches best for less severe variants.
Therapeutic routes and molecular targets are summarized (Figure 4, Key Figure) and questions regarding these routes as drug targets remain largely unanswered (see Outstanding Questions). We present potential treatment strategies for G6PDdef in hopes of motivating the development of new therapies. The interest of the pharmaceutical industry will increase only after more epidemiological studies confirm that G6PDdef is an important unmet need for a variety of common acute and chronic diseases.
Figure 4.
Summary of some of the chronic diseases associated with G6PDdef and strategies to treat G6PD related disorders. Treatment strategies include increasing GSH biosynthesis by activating GCL and co-supplementing with LC/NAC; activating NADPH compensatory enzymes ME1, IDH1; upregulating G6PD protein levels via HDAC inhibitors (HDACi) or Nrf2; small molecule chaperones increasing catalytic activity and stability of G6PD; antioxidants; and gene therapy (this strategy is likely for most severe Class I variants).
Outstanding Questions.
Although antioxidants do not alleviate a G6PD deficient hematologic phenotype, are they effective for treatment of chronic diseases associated with G6PD deficiency?
Are hematological indexes suitable clinical endpoints for clinical trials in G6PD deficient patients? If so, how should improvements in hematological indexes be translated to a clinical phenotype?
Should a surrogate endpoint, subjecting patient blood to oxidative stressors, be used for patient selection in a trial and/or a primary endpoint to establish efficacy for clinical trials in G6PD deficient patients?
Can improving GSH production via targeting GSH biosynthesis prevent hemolysis induced by oxidative stress?
Can other NADPH producing pathways, such as IDH1 and ME1, be targeted to compensate for G6PD deficiency in chronic diseases or in a hemolytic context?
Is improving G6PD protein levels in erythroblast sufficient to restore enzymatic activity? If so, is variant stability a major predictor of efficacy?
What HDACs are important for erythropoiesis and G6PD transcription? Are HDAC2 and HDAC5 required for G6PD transcription?
Can NRF2 upregulate G6PD transcription in erythroblasts? Are benefits comparable to an HDAC inhibitor?
Is stabilization of G6PD protein sufficient to improve clinical phenotype?
Does cost/benefit ratio for gene therapy deem it as practical for treatment of G6PD deficiency?
Highlights.
G6PD deficiency is better known as a hematologic disorder. However, G6PD plays a role in other chronic disorders such as diabetes, cardiovascular disease, viral infectivity and complications, and neurodegeneration.
Antioxidant therapy is the most commonly explored treatment strategy for hemolysis caused by G6PD deficiency. However, it is largely ineffective, with ascorbate triggering AHA in erythrocytes.
Therapeutic opportunities for G6PD deficiency include improved glutathione biosynthesis via NAC administration, exploiting NADPH compensatory pathways, small molecules which correct enzyme dysfunction by directly binding to G6PD, small molecules that increase the transcriptional output of G6PD, and gene therapy.
Acknowledgements
A.A.G is supported by National Science Foundation Graduate Research Fellowship (NSF-GRFP) [Grant DGE - 1656518] and National Institute of Health (NIH) training grant [Grant 5T32GM113854]. A.K and D.M-R are supported by NIH R01 HD08442 awarded to D.M-R; J.C.B.F is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) #2018/18627-3, #2019/25049-9, #2013/07937-8, #2015/22814-5, Conselho Nacional de Pesquisa e Desenvolvimento – Brasil (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) Finance Code 001. The authors thank Dr. Kate Samardzic for thoughtful comments and proofreading of the manuscript. Figures were created using BioRender.com.
Glossary
- Bilirubin
a byproduct of heme catabolism, often noted for its color (yellow pigment), found to have antioxidant and oxidant properties and to have pathological consequences.
- Glucose-6-phosphate dehydrogenase (G6PD)
A housekeeping enzyme responsible for catalyzing the conversion of glucose-6-phosphate and NADP+ into 6-phosphoglucono-δ-lactone and NADPH.
- G6PD deficiency (G6PDdef)
A genetic disorder, caused by mutations in the G6PD gene, which leads to the hemolysis of red blood cells.
- Glutathione (GSH)
An endogenous antioxidant that protects the cell from oxidative stress by neutralizing ROS; upon reaction with ROS, GSH is converted to GSSH, where it is recycled back into GSH by glutathione reductase, using NADPH as a cofactor.
- Histone Deacetylase Inhibitor (HDACi)
A class of anti-cancer small molecules that target histone deacetylases.
- Methemoglobinemia
a disorder characterized by excessive levels of methemoglobin. Due to loss of hemoglobin O2 binding, symptoms are largely related to O2 deficiency.
- Nicotinamide adenine dinucleotide phosphate (NADPH)
A cofactor that is used for biosynthetic reactions and plays a major role in protecting the cell against oxidative stress.
- Pentose phosphate pathway (PPP)
A branch of glucose metabolism, parallel to glycolysis, where glucose-6-phosphate is diverted to produce cellular NADPH and ribose-5-phosphate; metabolites important in redox homeostasis, nucleotide and fatty acid synthesis.
Footnotes
Resources
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luzzatto L et al. (2020) Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 136, 1225–1240 [DOI] [PubMed] [Google Scholar]
- 2.Jamerson BD et al. (2020) Glucose-6-Phosphate Dehydrogenase Deficiency: An Actionable Risk Factor for Patients with COVID-19? Arch. Med. Res 51, 743–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gómez-Manzo S et al. (2016) Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. Int. J. Mol. Sci 17, 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancone G and Chu CS (2021) G6PD Variants and Haemolytic Sensitivity to Primaquine and Other Drugs. Front. Pharmacol 12, 638885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H-C et al. (2019) The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 8, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbanefo EC et al. (2017) Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci. Rep 7, 45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JK (2019) 8-Aminoquinoline Therapy for Latent Malaria. Clin. Microbiol. Rev 32, e00011–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird JK (2015) Point-of-care G6PD diagnostics for Plasmodium vivax malaria is a clinical and public health urgency. BMC Med. 13, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avalos S et al. (2018) G6PD deficiency, primaquine treatment, and risk of haemolysis in malaria-infected patients. Malar. J 17, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan K and Tekwani BL (2021) Current investigations on clinical pharmacology and therapeutics of Glucose-6-phosphate dehydrogenase deficiency. Pharmacol. Ther 222, 107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H-C et al. (2021) G6PD deficiency, redox homeostasis, and viral infections: implications for SARS-CoV-2 (COVID-19). Free Radic. Res DOI: 10.1080/10715762.2020.1866757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham AD et al. (2016) Glucose-6-Phosphate Dehydrogenase Deficiency and the Need for a Novel Treatment to Prevent Kernicterus. Clin. Perinatol 43, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge T et al. (2020) The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol 11, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dore MP et al. (2021) The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxid. Med. Cell. Longev 2021, 5529256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang BL (2019) Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway. J. Cell. Biochem 120, 14285–14295 [DOI] [PubMed] [Google Scholar]
- 16.Zhao J et al. (2019) The association between low glucose-6-phosphate dehydrogenase activity level and hepatitis B virus infection among pre-pregnant reproductive-age Chinese females. Sci. Rep 9, 3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma D et al. (2018) Hepatitis A Virus-induced Severe Hemolysis Complicated by Severe Glucose-6-Phosphate Dehydrogenase Deficiency. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med 22, 670–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad BS et al. (2018) Severe haemolysis and renal failure precipitated by hepatitis E virus in G6PD Deficient patient: A case report. J Pak Med Assoc 68, 1397–1399 [PubMed] [Google Scholar]
- 19.Araujo T et al. (2018) Acute Retroviral Syndrome Presenting with Hemolytic Anemia Induced by G6PD Deficiency. Trop. Med. Infect. Dis 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer K et al. (2020) Methemoglobinemia in Patient with G6PD Deficiency and SARS-CoV-2 Infection. Emerg. Infect. Dis 26, 2279–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes DV et al. (2020) Methemoglobinemia and hemolytic anemia after COVID-19 infection without identifiable eliciting drug: A case-report. IDCases 23, e01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naymagon L et al. (2020) The emergence of methemoglobinemia amidst the COVID- 19 pandemic. Am. J. Hematol 95, E196–E197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuipers MT et al. (2020) G6PD deficiency-associated hemolysis and methemoglobinemia in a COVID-19 patient treated with chloroquine. Am. J. Hematol 95, E194–E196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastroianni F et al. (2020) Hydroxychloroquine in a G6PD-Deficient Patient with COVID-19 Complicated by Haemolytic Anaemia: Culprit or Innocent Bystander? Intern. Med 7, DOI: 10.12890/2020_001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim H et al. (2020) Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol 219, 108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaney S et al. (2020) COVID-19 & Hydroxychloroquine side-effects: Glucose 6-phosphate dehydrogenase deficiency (G6PD) and acute haemolytic anaemia. QJM Int. J. Med 113, 890–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar J and Averbukh Y (2020) Hemolytic Anemia in a Glucose-6-Phosphate Dehydrogenase-Deficient Patient Receiving Hydroxychloroquine for COVID-19: A Case Report. Perm. J 24, 20.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Omran A et al. (2017) Readmission for neonatal hyperbilirubinemia in an area with a high prevalence of glucose-6-phosphate dehydrogenase deficiency: A hospital-based retrospective study. J. Neonatal-Perinat. Med 10, 181–189 [DOI] [PubMed] [Google Scholar]
- 29.Amini N et al. (2017) A new rat model of neonatal bilirubin encephalopathy (kernicterus). J. Pharmacol. Toxicol. Methods 84, 44–50 [DOI] [PubMed] [Google Scholar]
- 30.Boskabadi H et al. (2018) Risk Factors for Sensorineural Hearing Loss in Neonatal Hyperbilirubinemia. Iran J Otorhinolaryngol 30, 195–202 [PMC free article] [PubMed] [Google Scholar]
- 31.Bermúdez- Muñoz JM et al. (2020) G6PD overexpression protects from oxidative stress and age-related hearing loss. Aging Cell 00, e13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White K et al. (2017) G6PD Deficiency Does Not Affect the Cytosolic Glutathione or Thioredoxin Antioxidant Defense in Mouse Cochlea. J. Neurosci 37, 5770–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M et al. (2019) High glucose-induced ubiquitination of G6PD leads to the injury of podocytes. FASEB J. 33, 6296–6310 [DOI] [PubMed] [Google Scholar]
- 34.Ježek P et al. (2021) The Pancreatic β-Cell: The Perfect Redox System. Antioxidants 10, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JS and Susztak K (2016) Podocytes: The Weakest Link in Diabetic Kidney Disease? Curr. Diab. Rep 16, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhagia V et al. (2021) G6PD activity contributes to the regulation of histone acetylation and gene expression in smooth muscle cells and to the pathogenesis of vascular diseases. Am. J. Physiol.-Heart Circ. Physiol 320, H999–H1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valuparampil Varghese M et al. (2021) Glucose-6 Phosphate dehydrogenase deficiency contributes to metabolic abnormality and pulmonary hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol 320, L508–L521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagawa A et al. (2021) Inhibition of G6PD activity attenuates right ventricle pressure and hypertrophy elicited by VEGFR inhibitor + hypoxia. J. Pharmacol. Exp. Ther 377, 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeng W et al. (2013) Brain Glucose-6-phosphate Dehydrogenase Protects against Endogenous Oxidative DNA Damage and Neurodegeneration in Aged Mice. ACS Chem. Neurosci 4, 1123–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besson MT et al. (2015) Enhanced Neuronal Glucose Transporter Expression Reveals Metabolic Choice in a HD Drosophila Model. PLoS ONE 10, e0118765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loniewska MM et al. (2019) DNA damage and synaptic and behavioural disorders in glucose-6-phosphate dehydrogenase-deficient mice. Redox Biol. 28, 101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To T-L et al. (2019) A Compendium of Genetic Modifiers of Mitochondrial Dysfunction Reveals Intra-organelle Buffering. Cell 179, 1222–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobore TO (2019) On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurol. Sci 40, 1527–1540 [DOI] [PubMed] [Google Scholar]
- 44.Pearson AG et al. (2021) Peroxiredoxin 2 oxidation reveals hydrogen peroxide generation within erythrocytes during high-dose vitamin C administration. Redox Biol. 43, 101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii T et al. (2019) Vitamin C therapy for patients with sepsis or septic shock: a protocol for a systematic review and a network meta-analysis. BMJ Open 9, e033458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reeves DJ et al. (2016) I.V. ascorbic acid for treatment of apparent rasburicase-induced methemoglobinemia in a patient with acute kidney injury and assumed glucose-6-phosphate dehydrogenase deficiency. Am. J. Health. Syst. Pharm 73, e238–e242 [DOI] [PubMed] [Google Scholar]
- 47.Sonbol MB et al. (2013) Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am. J. Hematol 88, 152–154 [DOI] [PubMed] [Google Scholar]
- 48.Rehman A et al. (2018) Severe acute haemolytic anaemia associated with severe methaemoglobinaemia in a G6PD-deficient man. BMJ Case Rep. 2018, DOI: 10.1136/bcr-2017-223369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padayatty SJ and Levine M (2016) Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 22, 463–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.May JM et al. (1996) Accessibility and reactivity of ascorbate 6-palmitate bound to erythrocyte membranes. Free Radic. Biol. Med 21, 471–480 [DOI] [PubMed] [Google Scholar]
- 51.Ross D et al. (1999) Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic. Biol. Med 26, 81–89 [DOI] [PubMed] [Google Scholar]
- 52.Veurink G et al. (2020) Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol. 10, DOI: 10.1098/rsob.200084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rybak LP et al. (2019) Local Drug Delivery for Prevention of Hearing Loss. Front. Cell. Neurosci 13, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spielberg SP (1979) Improved Erythrocyte Survival with High-Dose Vitamin E in Chronic Hemolyzing G6PD and Glutathione Synthetase Deficiencies. Ann. Intern. Med 90, 53. [DOI] [PubMed] [Google Scholar]
- 55.Corash L et al. (1980) Reduced chronic hemolysis during high-dose vitamin E administration in Mediterranean-type glucose-6-phosphate dehydrogenase deficiency. N. Engl. J. Med 303, 416–420 [DOI] [PubMed] [Google Scholar]
- 56.Hafez M et al. (1986) Improved erythrocyte survival with combined vitamin E and selenium therapy in children with glucose-6-phosphate dehydrogenase deficiency and mild chronic hemolysis. J. Pediatr 108, 558–561 [DOI] [PubMed] [Google Scholar]
- 57.Johnson GJ et al. (1983) High-Dose Vitamin E does not decrease the rate of chronic hemolysis in glucose-6-phosphate dehydrogenase deficiency. N. Engl. J. Med 308, 1014–1017 [DOI] [PubMed] [Google Scholar]
- 58.Newman JG et al. (1979) An examination of the role of vitamin E in glucose-6-phosphate dehydrogenase. Clin. Biochem 12, 149–151 [DOI] [PubMed] [Google Scholar]
- 59.Temel Y et al. (2017) Effect of astaxanthin and aluminum chloride on erythrocyte G6PD and 6PGD enzyme activities in vivo and on erythrocyte G6PD in vitro in rats. J. Biochem. Mol. Toxicol 31, e21954. [DOI] [PubMed] [Google Scholar]
- 60.Chisté RC et al. (2014) Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 99, 52–60 [DOI] [PubMed] [Google Scholar]
- 61.Koriem KMM and Arbid MS (2018) Evaluating of β-carotene role in ameliorating of favism-induced disturbances in blood and testis. J. Complement. Integr. Med 15, DOI: 10.1515/jcim-2017-0164 [DOI] [PubMed] [Google Scholar]
- 62.Georgakouli K et al. (2013) α-Lipoic acid supplementation up-regulates antioxidant capacity in adults with G6PD deficiency. Food Chem. Toxicol 61, 69–73 [DOI] [PubMed] [Google Scholar]
- 63.Georgakouli K et al. (2018) Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals. Antioxidants 7, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francis RO et al. (2020) Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Invest 130, 2270–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang H et al. (2015) Inability to Maintain GSH Pool in G6PD-Deficient Red Cells Causes Futile AMPK Activation and Irreversible Metabolic Disturbance. Antioxid. Redox Signal 22, 744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raftos JE et al. (2010) Glutathione Synthesis and Turnover in the Human Erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J. Biol. Chem 285, 23557–23567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clemente Plaza N et al. (2018) Effects of the Usage of L-Cysteine (L-Cys) on Human Health. Mol. J. Synth. Chem. Nat. Prod. Chem 23, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pei Y et al. (2018) Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid. Med. Cell. Longev 2018, 2835787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fazary AE et al. (2020) Protonation Equilibria of N-Acetylcysteine. ACS Omega 5, 19598–19605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raftos JE et al. (2007) Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int. J. Biochem. Cell Biol 39, 1698–1706 [DOI] [PubMed] [Google Scholar]
- 71.Parsanathan R and Jain SK (2018) l-Cysteine in vitro can restore cellular glutathione and inhibits the expression of cell adhesion molecules in G6PD-deficient monocytes. Amino Acids 50, 909–921 [DOI] [PubMed] [Google Scholar]
- 72.Parsanathan R and Jain SK (2019) Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: Protective role of l-cysteine. Arch. Biochem. Biophys 663, 11–21 [DOI] [PubMed] [Google Scholar]
- 73.Chen L et al. (2019) NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab 1, 404–415 [PMC free article] [PubMed] [Google Scholar]
- 74.Liu L et al. (2016) Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat. Chem. Biol 12, 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrero-Mendez A et al. (2009) The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat. Cell Biol 11, 747–752 [DOI] [PubMed] [Google Scholar]
- 76.D’Alessandro A et al. (2017) Red blood cell proteomics update: is there more to discover? Blood Transfus. 15, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menon V and Ghaffari S (2021) Erythroid enucleation: a gateway into a “bloody” world. Exp. Hematol 95, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Debeb BG et al. (2016) Histone deacetylase inhibitor-induced cancer stem cells exhibit high pentose phosphate pathway metabolism. Oncotarget 7, 28329–28339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El- Naggar AM et al. (2019) Class I HDAC inhibitors enhance YB- 1 acetylation and oxidative stress to block sarcoma metastasis. EMBO Rep. 20, e48375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Makarona K et al. (2012) Gene-Selective Histone Hyperacetylation and Enhanced Sp1 Occupancy Underpin Transcriptional Modulation of Genes of the Glycolytic-Pentose Phosphate Pathway in Response to Histone Deacetylase Inhibitors - Therapeutic Implications. Blood 120, 977–977 [Google Scholar]
- 81.Makarona K et al. (2014) Transcriptional and epigenetic basis for restoration of G6PD enzymatic activity in human G6PD-deficient cells. Blood 124, 134–141 [DOI] [PubMed] [Google Scholar]
- 82.Bautista JM (2014) Epigenetic therapy reprograms hereditary disease. Blood 124, 7–8 [DOI] [PubMed] [Google Scholar]
- 83.Jayapal SR et al. (2010) Down-regulation of Myc Is Essential for Terminal Erythroid Maturation. J. Biol Chem 285, 40252–40265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Popova EY et al. (2009) Chromatin condensation in terminally differentiating mouse erythroblasts does not involve special architectural proteins but depends on histone deacetylation. Chromosome Res. 17, 47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delehanty LL et al. (2012) Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1. Blood 120, 4219–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McClure JJ et al. (2018) Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. In Advances in Cancer Research 138pp. 183–211, Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 87.Ji P et al. (2010) Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica 95, 2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L et al. (2019) Therapeutic potential of selective histone deacetylase 3 inhibition. Eur. J. Med. Chem 162, 534–542 [DOI] [PubMed] [Google Scholar]
- 89.Ho TCS et al. (2020) Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem 63, 12460–12484 [DOI] [PubMed] [Google Scholar]
- 90.Bondarev AD et al. (2021) Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol 10, DOI: 10.1111/bcp.14889 [DOI] [PubMed] [Google Scholar]
- 91.Modi S and Lowder DM (2006) Medications for Migraine Prophylaxis. Am. Fam. Physician 73, 72–78 [PubMed] [Google Scholar]
- 92.Cui X-Y et al. (2020) The efficacy and safety of valproate medications for migraine in adults: a meta-analysis. Eur. Rev. Med. Pharmacol. Sci 24, 5734–5741 [DOI] [PubMed] [Google Scholar]
- 93.Bellezza I et al. (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta BBA - Mol. Cell Res 1865, 721–733 [DOI] [PubMed] [Google Scholar]
- 94.Matzinger M et al. (2018) Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv 36, 1738–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gazaryan IG and Thomas B (2016) The status of Nrf2-based therapeutics: current perspectives and future prospects. Neural Regen. Res 11, 1708–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chin MP et al. (2018) Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am. J. Nephrol 47, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reata Pharmaceuticals, Inc. 20-October-(2012), Company statement: Termination of the BEACON trial. [Google Scholar]
- 98.Rojo de la Vega M et al. (2016) NRF2-targeted therapeutics: New targets and modes of NRF2 regulation. Curr. Opin. Toxicol 1, 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunningham A et al. (2017) Coupling between Protein Stability and Catalytic Activity Determines Pathogenicity of G6PD Variants. Cell Rep. 18, 2592–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boonyuen U et al. (2017) A trade off between catalytic activity and protein stability determines the clinical manifestations of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Int. J. Biol. Macromol 104, 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang S et al. (2018) Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat. Commum 9, 4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saddala MS et al. (2020) Discovery of Small-Molecule Activators for Glucose-6-Phosphate Dehydrogenase (G6PD) Using Machine Learning Approaches. Int. J. Mol. Sci 21, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raub A et al. (2019) Small-Molecule Activators of Glucose-6-phosephate Dehydrogenase (G6PD) Bridging the Dimer Interface. ChemMedChem 14, 1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horikoshi N et al. (2021) Long-range structural defects by pathogenic mutations in most severe glucose-6-phosphate dehydrogenase deficiency. Proc. Natl. Acad. Sci 118, e2022790118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ranzani AT and Cordeiro AT (2017) Mutations in the tetramer interface of human glucose-6-phosphate dehydrogenase reveals kinetic differences between oligomeric states. FEBS Lett. 591, 1278–1284 [DOI] [PubMed] [Google Scholar]
- 106.Sun Q et al. (2019) Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats. Mol. Pain 15, DOI: 10.1177/1744806919838659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teskey G et al. (2018) Glutathione as a Marker for Human Disease. In Advances in Clinical Chemistry 87pp. 141–159, Elsevier; [DOI] [PubMed] [Google Scholar]
- 108.Juan CA et al. (2021) The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci 22, 4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao L et al. (2017) G6PD plays a neuroprotective role in brain ischemia through promoting pentose phosphate pathway. Free Radic. Biol. Med 112, 433–444 [DOI] [PubMed] [Google Scholar]
- 110.High KA and Roncarolo MG (2019) Gene Therapy. N. Engl. J. Med 381, 455–464 [DOI] [PubMed] [Google Scholar]
- 111.Harrison C (2019) First gene therapy for β-thalassemia approved. Nat. Biotechnol 37, 1102–1103 [DOI] [PubMed] [Google Scholar]
- 112.European Medicines Agency, S.M.H. May-(2019), Zynteglo (autologous CD34+ cells encoding βA-T87Q-globin gene): An overview of Zynteglo and why it is authorised in the EU. [Google Scholar]
- 113.Thompson AA et al. (2018) Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med 378, 1479–1493 [DOI] [PubMed] [Google Scholar]
- 114.Carr DR and Bradshaw SE (2016) Gene therapies: the challenge of super-high-cost treatments and how to pay for them. Regen. Med 11, 381–393 [DOI] [PubMed] [Google Scholar]
- 115.Couronné L et al. (2017) A somatic mosaicism in the G6PD gene inducing a late onset chronic non-spherocytic hemolytic anemia. Am. J. Hematol 92, E153–E155 [DOI] [PubMed] [Google Scholar]
- 116.Watanabe M et al. (2019) Laparoscopic cholecystectomy and postoperative pain control in a patient with chronic non-spherocytic hemolytic anemia from glucose-6-phosphate dehydrogenase deficiency. J. Clin. Anesth 54, 128–129 [DOI] [PubMed] [Google Scholar]
- 117.Ludwig LS et al. (2016) Emerging Cellular and Gene Therapies for Congenital Anemias. Am. J. Med. Genet. C Semin. Med. Genet 172, 332–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campbell GD (1975) Ascorbic Acid-Induced Hemolysis in G-6-PD Deficiency. Ann. Intern. Med 82, 810. [DOI] [PubMed] [Google Scholar]
- 119.Rees DC et al. (1993) Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 306, 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rees MJ et al. (2018) Massive oxidative haemolysis and renal failure caused by high dose vitamin C. Med. J. Aust 209, 248–249 [DOI] [PubMed] [Google Scholar]
- 121.Mentzer WC and Collier E (1975) Hydrops fetalis associated with erythrocyte G-6-PD deficiency and maternal ingestion of fava beans and ascorbic acid. J. Pediatr 86, 565–567 [DOI] [PubMed] [Google Scholar]
- 122.Mehta JB et al. (1990) Ascorbic-Acid-Induced haemolysis in G-6-P-D deficiency. The Lancet 336, 944. [DOI] [PubMed] [Google Scholar]
- 123.Lo YH and Mok KL (2020) High dose vitamin C induced methemoglobinemia and hemolytic anemia in glucose-6-phosphate dehydrogenase deficiency. Am. J. Emerg. Med 38, 2488.e3–2488.e5 [DOI] [PubMed] [Google Scholar]
- 124.Quinn J et al. (2017) Effect of High-Dose Vitamin C Infusion in a Glucose-6-Phosphate Dehydrogenase-Deficient Patient. Case Rep. Med 2017, 5202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang Y-C et al. (2014) C for colored urine: Acute hemolysis induced by high-dose ascorbic acid. Clin. Toxicol 52, 984–984 [DOI] [PubMed] [Google Scholar]
- 126.Sultana N et al. (1970) Effects of vitamin E supplementation on some aspects of hematological variables in patients of hemolytic anemia with glucose 6 phosphate dehydrogenase (G6PD) deficiency. Bangladesh J. Physiol. Pharmacol DOI: 10.3329/bjpp.v22i1.3563 [DOI] [Google Scholar]
- 127.Corash LM et al. (1982) Chronic hemolytic anemia due to glucose-6-phosphate dehydrogenase deficiency or glutathione synthetase deficiency: the role of vitamin E in its treatment. Ann. N. Y. Acad. Sci 393, 348–360 [DOI] [PubMed] [Google Scholar]
- 128.Wright RO et al. (1996) Hemolysis After Acetaminophen Overdose in a Patient with Glucose-6-phosphate Dehydrogenase Deficiency. Clin. Toxicol 34, 731–734 [DOI] [PubMed] [Google Scholar]
- 129.Monsivais SR et al. (2016) N-Acetylcysteine Supplementation in an Individual with Glucose-6-Phosphate Dehydrogenase Deficiency–Associated Psychosis. Biol. Psychiatry 80, e71–e72 [DOI] [PubMed] [Google Scholar]